Abstract
Significance: The hemoglobin (Hb) scavenger receptor, CD163, is a macrophage-specific protein and the upregulated expression of this receptor is one of the major changes in the macrophage switch to alternative activated phenotypes in inflammation. Accordingly, a high CD163 expression in macrophages is a characteristic of tissues responding to inflammation. The scavenging of the oxidative and proinflammatory Hb leading to stimulation of the heme-oxygenase-1 and production of anti-inflammatory heme metabolites indicates that CD163 thereby indirectly contributes to the anti-inflammatory response. Recent Advances: In addition to this biological role in inflammation, CD163 is a potential inflammation biomarker and a therapeutic target. The biomarker form of CD163 is the soluble plasma CD163 that arises from the increased shedding of CD163 mediated by the tumor necrosis factor-α (TNF-α) cleaving enzyme. This explains that a steadily increasing literature documents that the plasma level of soluble CD163 is increased in a large spectrum of acute and chronic inflammatory disorders. The nonshed membrane form of CD163 in macrophages constitutes a target for drugs to be directed to macrophages in inflammation. This approach has been used in an animal inflammation model to highly increase the apparent therapeutic index of anti-inflammatory glucocorticoid drug that was coupled to an anti-CD163 antibody. Furthermore, other recent animal data, which indirectly involve CD163 in macrophages, demonstrate that injections of haptoglobin attenuate Hb-induced damages after blood transfusion. Critical Issues and Future Directions: The diagnostic and therapeutic properties of CD163 await further clinical studies and regulatory approval before implementation in the clinic. Antioxid. Redox Signal. 18, 2352–2363.
Introduction
CD163 was originally identified in two independent reports (75, 123) using monoclonal antibodies as an unknown monocyte–macrophage-specific antigen associated with the anti-inflammatory process. Several other groups identified the same protein named as the antigen of different monocyte–macrophage-specific antibodies, such as Ki-M8 (95), GHI/61 and SM4 (94), Ber-MAc3 (6), AM-3K (121), and 2A10 (100). The Leukocyte Typing Workshop V defined the common antigen of the different antibodies leading to the CD163 designation (52, 93) The function of the protein was for long unknown, but its expression, regulation, and primary structure (59) suggested an immunological receptor function (52, 75, 123). In 2001, CD163 was identified as the “hemoglobin (Hb) scavenger receptor” HbSR (57) for the uptake of Hb released into the plasma and complexed to haptoglobin (Hp) during intravascular hemolysis. Although this function appeared distinct from a direct immunological role, it was fully in accordance with the structure, macrophage-specific expression, and regulation (69). In recent years, the receptor has also been reported to bind the tumor necrosis factor-α (TNF-α)-like weak inducer of the apoptosis (TWEAK) protein (15), and some pathogenic bacteria (30) and virus (101, 113).
In addition to providing the basic information about CD163 in terms of structure, function, and expression, the present review will describe the many links between CD163 and human inflammation. This includes the regulated expression in macrophages, direct removal of proinflammatory ligands (in particular Hb), fueling of the pathway for generation of anti-inflammatory heme metabolites, inflammation-induced shedding of CD163, the use of soluble CD163 as a biomarker for inflammatory diseases, and the development of CD163-targed anti-inflammatory therapy.
CD163 Structure
CD163 is a 130-kDa membrane protein with a short cytoplasmic tail, a single transmembrane segment, and a large ectodomain consisting of nine scavenger receptor cysteine-rich (SRCR) scavenger receptor class B domains (59). Different isoforms of human CD163 have been described, including three variants with different length of the cytoplasmic tail (59), with the short tail form (42 amino acids) being the most abundant. All variants contain common internalization motifs and exhibit endocytic activity (83).
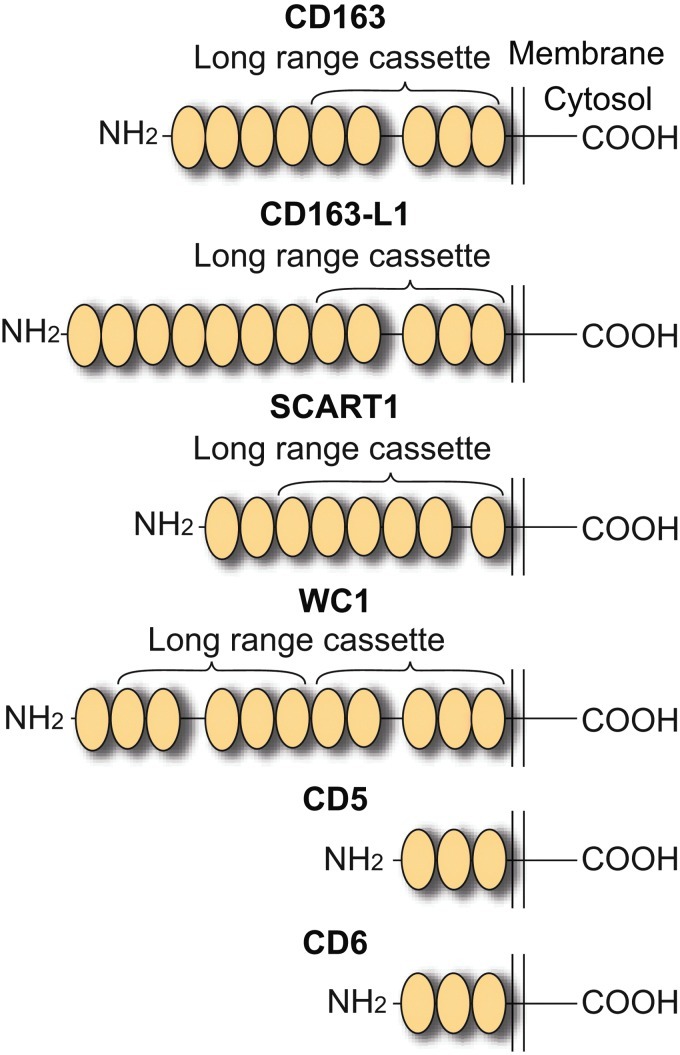
The SRCR domain is a common 100–110 amino acid domain for molecular interactions (65) and with a determined structural fold of six or seven β-sheets cradling an α-helix (34, 45, 98). The SRCR class A and class B domains share a similar fold (34, 98) and differ only by the presence of an additional disulfide bond in the class B domains. Whereas SRCR class A domains largely are present as single domains in different mosaic domain proteins, including the scavenger receptor, AI and MARCO. The class B domains are largely present as tandem repeat membrane proteins that only contain this type structural motif in the ectodomain (65). Figure 1 shows the human SRCR class B family membrane proteins CD163, CD163b, CD5, CD6, and Scart1. CD163b, which has its encoding gene located close (107) to the CD163 gene on chromosome 12 (97) and represents the closest homologue to CD163 (41). It exhibits similarities in terms of tissue distribution, regulation, and endocytic capability although it seems to have another not yet defined ligand repertoire (67).
FIG. 1.
Scavenger receptor cysteine-rich (SRCR) class B family membrane protein members. All members contain multiple extracellular repeats of the SRCR class B domain. A conserved repeat, known as the long-range cassette, is defined by a cassette of five SRCR domains separated by a 31 amino acid spacer between repeat 2 and 3. All the proteins, except for WC1, which was identified in the pig, are encoded by the human genome. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.
Some of the CD163 SRCR domains contain consensus sites (88) for calcium binding, and the binding of Hp-Hb complexes, as well as several antibodies (62, 63) exhibit calcium-dependent binding. The calcium binding is pH-sensitive and the calcium-binding structure is therefore suggested to be an essential structural component for the uncoupling of ligand upon internalization (62). Accordingly, the calcium-binding SRCR domain 3 of CD163 has been shown to be essential for the binding of Hb-Hp to CD163 (62).
CD163 Expression and Regulation
Human CD163 expression is restricted to the monocytic–macrophage linage with high expression in, for example, red pulp macrophages, bone marrow macrophages, liver macrophages (Kupffer cells), lung macrophages, and in macrophages of several other tissues (93). A similar expression pattern is observed in the Lewis rat (10, 91). Monocytes have a modest expression of CD163, but the expression level highly increases in culture along with other macrophage characteristics (2). Previous conflicting data on the monocyte expression have been explained by a receptor-based study revealing that the cellular staining in flow cytometry protocols is very sensitive to the antibody used and the incubation conditions, which impacts reproducibility of results (63).
Low or absent CD163 expression is seen in other monocyte-derived cells, such as dendritic cells (64), Langerhans cells (55), and white pulp macrophages in the spleen (93). A number of factors (Table 1) regulate CD163 expression in vitro. The most potent stimulators of CD163 expression known are glucocorticoid, interleukin (IL)-6, IL-10, and heme/Hb, whereas IL-4, lipopolysaccharide (LPS), TNF-α and interferon γ, CXC-chemokine ligand 4 (CXCL4), and granulocyte–macrophage colony-stimulating factor downregulate CD163 expression (17, 19, 36, 49, 108, 111). The effect of glucocorticoids in vitro (75, 123) has been confirmed in vivo by analyzing human monocytes after administration of glucocorticoids to human volunteers (124). The glucocorticoid-mediated regulation of CD163 is further evidenced by the identification of three glucocorticoid receptor-binding sites in the promoter region of the CD163 gene. Furthermore, binding sites for several transcription factors important for myeloid differentiation have been identified. Altogether, the observations on the regulation of CD163 conclude that CD163 is a feature of macrophages that differentiate into the “alternatively activated” macrophages that contrast the classical activated M1-type macrophages (37). Accordingly, CD163-expressing macrophages have been detected in sites of inflammation, such as chronically inflamed arthritis joints (8, 33), atherosclerotic plaques (96), and the vicinity of tumor cells (tumor-associated macrophages) (18).
Table 1.
Substances Regulating CD163 Expression in Monocytes/Macrophages In Vitro
| Compound | Up- or downregulation |
|---|---|
| Glucocorticoid | Up |
| IL-4 | Down |
| IL-6 | Up |
| IL-10 | Up |
| IFN-γ | Down |
| LPS | Down |
| TNFα | Down |
| CXCL4 | Down |
| GM-CSF | Down |
| Hb | Up |
The CD163-positive macrophage may originate from extravasation of monocytes or may represent macrophage activation switching (92) of already present M1 proinflammatory macrophages. Studies of atherosclerosis (16) and Hb (49) suggest that CD163 and Hb from local microbleedings (plaque hemorrhage) have an atheroprotective role via the metabolism of Hb leading to polarization of macrophages. These studies have led to a definition of a new class of CD163-positive atheroprotective and anti-inflammatory macrophages in atherosclerotic lesions (16). These macrophages, now designated Mhem macrophages, are characterized by a high iron load and heme-oxygenase-1 (HO-1) activity in contrast to the low content of those in M1, M2, and Mox macrophages (16). This further underscores the plasticity of macrophages and their multiple and overlapping phenotypes that may be regarded as a pronounced tendency to adapt to the local environment.
Future studies of atherosclerosis and other types of inflammation in CD163 knockout animals should further define the protective role of CD163 in site of acute and chronic inflammation. CD163 knockout animals may better define a recent hypothesis that atherogenesis is reduced in mice with a knock out of the gene encoding the platelet chemokine, CXCL4, might relate to an absent CXCL4-mediated polarization of macrophages with low CD163 expression in these animals (36).
The present literature on CD163 expression is largely based on work on human material and to some extent the rat and pig systems, in vivo data are limited. Unfortunately, most of the comprehensive characterization of macrophage differentiation in animal models is based on the mouse system, where a suitable anti-CD163 antibody for tracking CD163 expression until recently has been missing. By implementing CD163 expression in future studies of the many mouse inflammation models, new information on macrophage differentiation and CD163 expression during inflammation will hopefully become available.
CD163- and Hp-Mediated Hb Scavenging
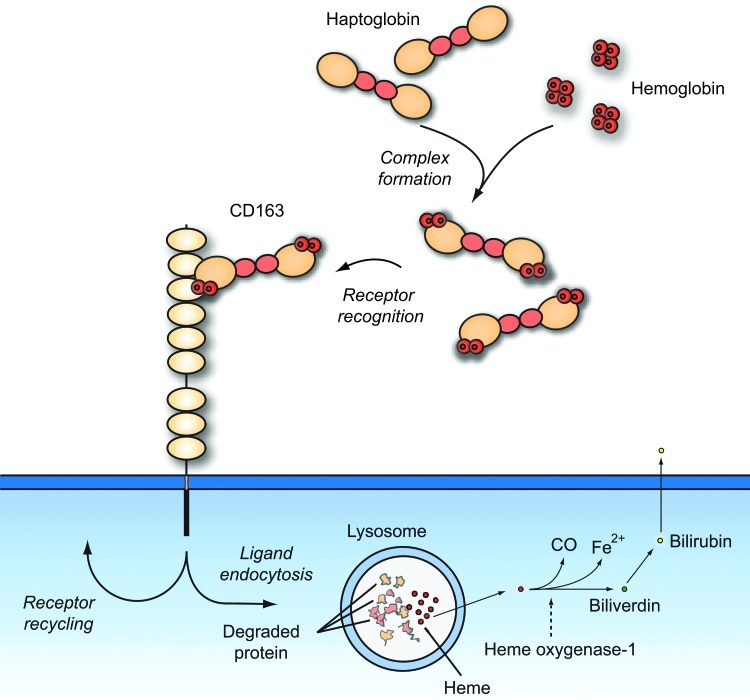
CD163 is a high-affinity receptor of human Hp-Hb complexes (57, 62) that instantly form when Hb is released from erythrocytes during physiological or pathological hemolysis (Fig. 2). Moreover, free Hb can bind to low affinity to CD163 (102) and this may have importance after depletion of Hp during excessive hemolysis. Binding of Hp to Hb is one the strongest protein–protein interactions occurring in plasma (48). The high-resolution structure of the porcine complex is now known (3) and it shows how a previously identified loop region important for CD163 recognition (87) pertrudes from the complex in the proximity of Hb. Surprisingly, studies of mice did not reveal a clearance-promoting effect of Hp (26) suggesting that in this and other species, the Hp role in relation to Hb may be limited to protective functions, such as avoiding peroxidative modification of Hb (14, 20) and impairing filtration of the relatively small Hb molecule by the kidney (31, 51). The binding of the complex to CD163 macrophages leads to a fast degradation of the complex, and in case of an increased intravascular hemolysis as seen in many pathological conditions, such as malaria, hemoglobinopathies (e.g., sickle cell disease), autoimmune hemolyses, and drug-induced hemolysis, Hp may virtually disappear from the human plasma. Hp and the protein moiety of Hb are degraded in lysosomes (57). The fate of Hb is most likely identical to that of Hb present in macrophages upon erythrophagocytosis of outdated red cells. In the macrophage, the heme-oxygenases convert heme to biliverdin, carbon monoxide (CO), and iron. Biliverdin is further converted to bilirubin, which is released and transported by albumin to the liver for conjugation and excretion in the bile. Overall, the removal of Hb and the generation of heme metabolites result in a localized anti-inflammatory response (99).
FIG. 2.
CD163-mediated scavenging of Hb upon intravascular hemolysis. CD163 is highly expressed on phagocytic macrophages. Upon hemolysis, released hemoglobin (Hb) is rapidly bound by the acute-phase protein Hp forming the haptoglobin–hemoglobin (Hp-Hb) complex. The complex is subsequently bound and removed from the circulation by CD163-positive macrophages in the liver, spleen,and bone marrow. The uptake of Hb by macrophages contributes to the recycling of iron and also to the inflammatory response. The uptake of Hb in macrophages and subsequent degradation of heme by heme oxygenase-1 (HO-1), produce the anti-inflammatory metabolites, Fe2+, CO, and biliverdin. Biliverdin reductase converts biliverdin to bilirubin, which is secreted to the cell exterior. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.
The anti-inflammatory effects of the heme metabolites CO and biliverdin/bilirubin are the outcome of multiple biological mechanisms not fully elucidated and reviewed in detail elsewhere (40). Among the many effects of CO, where some overlap those of NO, this well-known, but potentially very toxic CO gas, is a potent inhibitor of proinflammatory cytokines, such as IL1 and TNF-α and a stimulator of IL-10 (61). In malaria, where the plasmodium parasites may cause severe hemolysis and heme intoxication, CO is reported to have a specific cytoprotective role because it binds to free Hb and thereby prevents heme release from Hb (32). Biliverdin and bilirubin are antioxidants and their main cytoprotective function is based on the inhibition of lipid and protein peroxidation, but they apparently also exhibit direct anti-inflammatory activity, such as inhibition of the complement cascade, P/E-selectin expression, and attenuation of leukocyte rolling (9, 42, 105). Finally, the release of Fe2+ may indirectly lead to protection of cells from oxidative stress even though this atom is indirectly pro-oxidative. The mechanism is that Fe2+ stimulates the expression of ferritin, the iron storage protein, which has antioxidative properties (89).
Interestingly, HO-1 may also have cytoprotective effects independent of the effect mediated by the heme metabolites. Elegant studies of cells transfected with catalytic inactive HO-1 have shown that HO-1 itself is involved in cell signaling and different changes in phenotype, including an upregulated expression of catalase, glutathione peroxidase, and GSH (46). However, much seems still to be learned about the HO-1 protein that also may have an adverse affect in the central nervous system, where HO-1 is reported to exacerbate early brain injury after intracerebral bleeding (117).
Altogether, the stimulation of the Hp-CD163-HO-1 metabolic pathway owing to an increased Hb metabolism leads to a counter response of the pro-oxidative effects of Hb. In case of consumption of Hp, the protective effect of the system may be weakened leading to heme-induced damage of tissues, in particular, the kidney. However, CD163 may still play a role because in vitro studies have shown a low-affinity binding of Hb to the receptor. Moreover, plasma contains hemopexin, which acts as a kind of back-up protein (110) that binds the heme leading to the uptake of hemopexin–heme complexes by CD91 (47, 85) expressed in macrophages, hepatocytes, and several other cell types (68). The backup by hemopexin has been demonstrated in knock models (116). These data show that mice with a combined hemopexin and Hp knockout are far more sensitive to Hb-induced inflammation than mice with knockout of Hp or hemopexon alone. (109). In line with these data, the knockout of CD163 as described in an accompanying article of this issue seems to have no obvious effect on the mouse health (26). On the other hand, the same study shows striking differences between mouse and man in terms of the role of Hp in Hb metabolism. Most important, mouse Hp, which has less than a tenth of the Hp plasma concentration in humans, does not promote high-affinity binding of the complex to CD163. Interestingly, free mouse Hb binds with higher affinity to CD163 compared to human Hb. In view of these differences, it seems essential to take precautions when translating rodent findings in the Hb degradation pathway to human settings.
Humans also differ from other species by having two different Hp gene alleles encoding Hp1 and Hp2, where the Hp2 protein contains a duplicated α-chain that bridge to two other Hp α-chains. This provides the property of forming disulfide-linked Hp multimers (see (60) for review) in individuals heterozygous or homozygous for the Hp2 gene (the Hp2-1 and Hp2-2 phenotypes, respectively). Individuals with the Hp1-1 phenotype have dimeric Hp that is the basic Hp form in most mammalian species. All the human Hp phenotypes bind with high affinity to CD163 when complex Hb. In vitro experiments have shown that the multimeric Hp2-2 binds with a higher functional affinity to the immobilized receptor than the Hp1-1 form (57). The multivalency in terms receptor-binding sites leading to crosslinking of multiple receptors by the multimers may explain this observation.
Human and other old monkey primates also express the Hp-related protein (Hpr), which has only 23–30 amino acids in difference compared to Hp1-1. Hpr binds Hb with high affinity, but the Hpr-Hb complex does not bind to CD163 (84–86, 115). Hpr is instead associated to apolipoprotein L-containing lipoprotein particles that constitute a subspecies of high-density lipoprotein (HDL) particles. Hb binds to Hpr in the particles, which then are activated as an innate defense weapon against certain trypanosome parasites (Trypanosoma brucei brucei) causing animal sleeping sickness (Nagana) (84, 114). To sequester heme for enzymes, the parasites have a Hp-Hb receptor, but in contrast to CD163, this receptor also binds Hpr-Hb (84, 114, 115). Consequently, the parasites take up circulating HDL particles also containing Hp-Hb and apolipoprotein L. The latter has a strong membrane pore-forming effect that leads to rupture of the lysosomal membrane and to self-digestion of the parasites (12). Primates, including humans, therefore have an innate immunity against the trypanosoma brucei brucei infection, which is a great threat against domestic animals in a large part of the African continent (115). However, humans have no resistance against the human pathogenic Trypanosoma brucei rhodiense, and Trypanosoma brucei gambiense parasites are apparently accounted for by the presence of an apolipoprotein L inhibitor (119) and reduced haptoglobin–hemoglobin complex receptor expression (50), respectively.
Other Functions of CD163
In addition to the established role of CD163 in Hb metabolism, other ligands for CD163 have been identified as reviewed in detail recently (112).
Early data on the rat ED2 antigen, which later have been identified as CD163, identified binding to rat erythroblast (11). A later follow-up study reported that CD163 has regulatory role during rat erythropoiesis (29).
Several reports (15, 74) have shown that TWEAK, a secreted cytokine belonging to the TNF-α superfamily, binds to CD163. CD163 is proposed as a scavenger receptor for TWEAK, preventing TWEAK from exerting its biological functions by sequestering it from the physiological environment.
Some bacteria and virus have been reported to bind human CD163. The first study on binding of the bacteria to CD163 reports that CD163 functions as sensor rather than an endocytic receptor for Streptococcus mutans, Eschericia coli, and Staphylococcus aureus. The expression of CD163 in monocytic cells promoted bacteria-induced production of proinflammatory cytokines, like TNF-α. Another study has reported binding of soluble CD163 to Staphylococcus aureus via binding of specific fibronectin peptides, which promotes recognition, phagocytosis, and killing of the bacteria (53).
African swine fever virus (ASFV) and the porcine reproductive and respiratory syndrome virus (PRRSV) are the two virus reported to bind CD163 (21, 101, 113). The binding of virus to CD163 seems important for the virus infection, but in different ways. ASFV is proposed to exploit CD163 for attachment and internalization (101), whereas PRRSV data suggest a role for CD163 during virus uncoating (113). Minor envelope glycoproteins GP2a and GP4 of PRRSV are reported to interact with CD163 (24).
Among the new ligands reported during the last decade, only the virus–CD163 interaction and TWEAK interactions have been reported by more than one laboratory. Further investigation should establish the physiological role and the consequences in human disease.
Finally, it is intriguing to speculate that CD163 might have other not yet identified ligands. Many endocytic scavenging receptors are multiligand receptors (56) and it would make sense if, for instance, other intracellular components, such as Hb, liberated to the plasma during cell rupture or extracellular waste products generated in the resolution phase of inflammation take advantage of the CD163 (or CD163b)-mediated scavenging in macrophages.
CD163 As Clinical Biomarker in Inflammation
A soluble form of CD163 ectodomain is present in normal plasma (81) and an increased plasma concentration of sCD163 is seen in diseases relating to macrophage activity, including acute and chronic inflammations (71). The physiological role of sCD163 is not defined. Soluble CD163 binds Hp-Hb complexes (81), but it is a poor competitor of CD163-mediated uptake of this ligand (80), probably because the ligand has no affinity gain in crosslinking to soluble receptors. It is possible that the soluble receptor has functions not related to Hb, such as the proposed role in opsonization of bacteria described above (53).
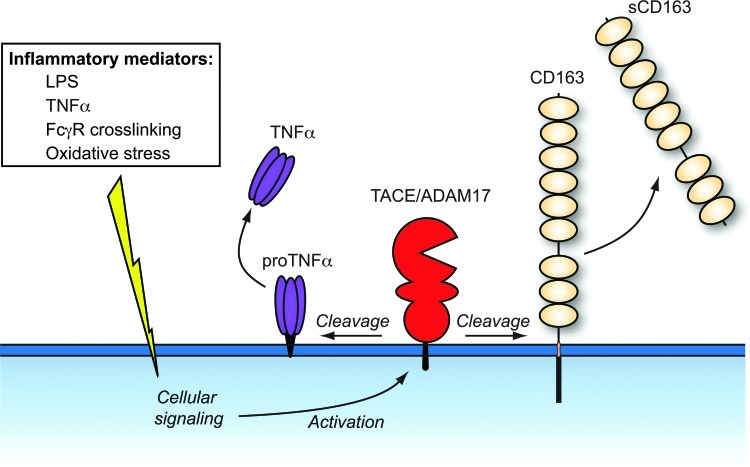
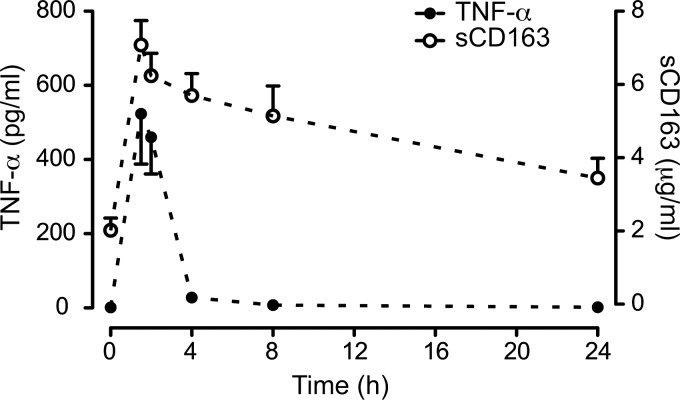
Soluble CD163 is a homogenous protein (80) spanning at least 94% of the entire CD163. Several studies (43, 66, 118) have pointed on a metalloproteinase as the enzyme responsible for cleavage of CD163 in macrophages, and recently the inflammation regulated a disintegrin and metalloproteinase 17 (ADAM17)/TNF-α–cleaving enzyme (TACE) was identified as the responsible enzyme using inhibitor and siRNA knockdown analyses (28) (Fig. 3). In view of the TNF-α-mediated inflammatory symptoms during conditions, such as sepsis and chronic inflammations (1), it is likely that the increased levels of sCD163 measured under these conditions are the outcome of concomitant ADAM17/TACE-induced release of CD163 and TNF-α in macrophages. Figure 4 shows the effect on the LPS and TNF-α levels in healthy subjects after an LPS injection, which instantly increases the plasma levels of TNF-α and CD163. In contrast to the fast clearance of TNF-α, the soluble CD163 level remains increased for days. In this context, soluble CD163 may be regarded as a long-circulating surrogate marker for TNF-α in conditions where LPS leads to shedding of CD163. Probably, this may extend to other inflammatory conditions, since several other stimuli, such as Fc receptor crosslinking via activation of toll-like receptors, cause TACE/ADAM17 activation and release of TNF-α and CD163 (104, 118).
FIG. 3.
A disintegrin and metalloproteinase 17 (ADAM17)/tumor necrosis factor (TNF)-α converting enzyme (TACE)-mediated shedding of CD163 and TNF-α upon stimulation by proinflammatory stimuli. CD163 and proTNF-α are rapidly cleaved from the surface of activated macrophages by an ADAM17/TACE-dependent mechanism. A number of proinflammatory substances induce ADAM17/TACE activation. The exact site of cleavage in CD163 awaits identification. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.
FIG. 4.
Soluble CD163 is a long-circulating surrogate marker of TNF-α in experimental endotoxemia. Serum levels of sCD163 and TNF-α in healthy volunteers (n=8) after receiving a bolus-injection of endotoxin. Serum analysis shows a fast increase in both markers, whereas TNF-α rapidly cleared increased levels sCD163 are still measured after 24 h. The figure is reproduced from (28) with permission from J. Leuk. Biology.
Although the levels of soluble CD163 may increase many-fold by ADAM17/TACE activation, the amount of soluble CD163 in plasma is probably low compared to the amount of the membrane-bound form in macrophages. There are no data with exact comparison of the two forms in humans, which so far is the only species, where the soluble CD163 has been reported. However, comparison of the levels of receptor purified from human tissues (57) with the measured amount in plasma suggests that most of the body's CD163 is membrane-associated even when the soluble CD163 concentration is upregulated. The major pool of CD163 seems to be localized intracellularly (83) as seen for other endocytic receptors trafficking between the surface and endosomes (70), and it is therefore likely that a temporary shedding of CD163 will be followed by a fast mobilization of CD163 from the intracellular pool.
As reviewed in detail elsewhere (71), the level of CD163 has been thoroughly characterized in normal and diseased individuals (Table 2). The range in normal individual is 1–4 mg/l based on a widely used enzyme-linked immunosorbent assay (77) and there is a low intraindividual variation (78). Increased levels are seen in many diseases (71) involving macrophages with the highest levels measured in patients with hemophagocytosis (103) and the related macrophage activation syndrome (13). These conditions are characterized by an abnormal lymphohistiocytic activation of complex and partially unknown etiology leading to the systemic inflammatory response syndrome. Macrophage accumulation in the bone marrow, liver, or lymphoid tissues and overt phagocytosis of blood cells and their precursors are a pathognomonic feature of the syndrome, which has a high mortality. The high significant level of sCD163 in hemophagocytosis and macrophage activation syndrome has led to the proposal of including soluble CD163 as one of the diagnostic criteria (22, 25).
Table 2.
Reported CD163 Binding Substances
| Hp-Hb complexes (57) |
| Hb (102) |
| TWEAK protein (15, 74) |
| Porcine-pathogenic viruses (the African swine fever virus (ASFV) (101) |
| Porcine reproductive and respiratory syndrome virus (PRRSV) (21, 113) |
| Various gram-positive and gram-negative bacteria (30, 53) |
| Erythroblasts (11, 29) |
Hp-Hb, haptoglobin–hemoglobin complex; TWEAK, TNF-like weak inducer of apoptosis.
Several other diseases, where inflammation is an important component of the pathogenesis have increased levels of soluble CD163. This includes acute diseases, such as bacterial sepsis/infection (54, 76, 79), hepatitis (44), and malaria (58) as well chronic inflammation, such as rheumatoid arthritis (39, 66), Crohn's disease (76), scleroderma (82), coeliac disease (23), and atherosclerosis (4, 73) (Table 3). Generally, the increase in the CD163 level is much more pronounced in acute inflammations, such as bacteremia/sepsis, where the CD163 level also has negative correlation to survival (79).
Table 3.
Examples of Inflammatory Diseases with Increased CD163 Plasma Levels
| Acute inflammations (infections) | ||
| Hepatitis | High increase | (44) |
| Bacteriemia/sepsis | High increase | (54, 76, 79) |
| Malaria | High increase | (58) |
| Chronic inflammations | ||
| Rheumatoid arthritis | Moderate increase | (39, 66) |
| Mb Crohn | Low increase | (76) |
| Scleroderma | Low increase | (82) |
| Celiac disease | Low increase | (23) |
| Atherosclerosis | Low increase | (4, 73) |
| Low-grade chronic inflammation | ||
| Diabetes II | Low increase | (71, 90, 120) |
| Other type of inflammation | ||
| Macrophage activation syndrome/ | ||
| Hemophagocytosis | Very high increase | (13, 103) |
The measurement of soluble CD163 in a general population cohort encompassing 8849 individuals followed for 18 years revealed that soluble CD163 is a risk marker for the development of type 2 diabetes and it correlates with indices of the metabolic syndrome (72). Previous data have shown that soluble CD163 correlates with the body fat mass (5, 106), which probably reflects the fat-induced low-grade inflammation in these individuals. Interestingly, the cohort study shows that soluble CD163 predicts diabetes, independently of body mass index and age, suggesting that soluble CD163 may be used to identify persons genetically predisposed for low-grade inflammation diabetes 2 (72). Two recent studies following up on the large cohort study have shown that soluble CD163 positively correlates to insulin resistance, which is the fundamental problem in diabetes 2 (90, 120).
In conclusion, soluble CD163 is increased in a number of diseases (and in particular, inflammatory diseases) with increased macrophage activity. The receptor will probably not be used as a single diagnostic marker of a disease, but it may gain use as a macrophage activity marker complementary to clinical findings and other laboratory tests. Furthermore, it may be used as a monitoring marker of single individuals during disease progression and treatment.
CD163 As a Receptor for Drug-Targeting Macrophages
The specific expression of CD163 in monocytes/macrophages makes the receptor an interesting gate for drug delivery. This may apply to different kinds of disease, including inflammatory disorders, certain cancers of myeolo-monocytic origin, infections, where the macrophages harbor the pathogen (e.g., mycobacterium, HIV, or leishmania parasites), and rare genetic disorders (e.g., Gaucher's disease affecting macrophages).
The pathological mechanisms of inflammation includes several types of leukocyte effector cells (monocytes–macrophages, T-lymphocytes, and granulocytes) and complex immune cell interplays such as those between macrophages and T helper cells. There are therefore many potential drug attack points in treatment of inflammation. Cytostatics (e.g., methotrexate), glucocorticoids (e.g., prednisolone and dexamethasone), and different biological drugs (e.g., antibodies against TNF-α ad other cytokines) are potent drugs widely used in rheumatoid arthritis and other chronic inflammatory disease. However, serious side effects (e.g., by glucorticoids) or loss of efficacy (e.g., by methotrexate and biological drugs) keep up the need for development of new anti-inflammatory drugs.
The CD163-expressing macrophage is an interesting therapeutic target because CD163 macrophages are present at the site of inflammation where they, despite an overall anti-inflammatory function, also produce inflammatory cytokines, such as TNF-α. This cytokine is largely produced by macrophages and the efficacy of anti-TNF-α biological drugs (35) suggests that the macrophage is an obvious target for anti-inflammatory therapy. The proof of concept of this CD163-targeting therapy has recently been established in a recent study in rats using an antibody drug conjugate (ADC) consisting of average four glucocorticoid drugs (dexamethasone) linked to an anti-rat CD163 antibody (38). This glucocorticoid conjugate exhibited high affinity to CD163 comparable with that of nonconjugated antibody, and cell experiments revealed that the receptor mediates the endocytosis and transport of the drug to the cell interior. A rat model of acute sepsis-induced inflammation (LPS-induced TNF-α release) revealed about a 50-fold higher efficacy of the intravenously injected conjugate compared to free dexamethasone. Holding promise for the use of this new strategy in glucocorticoid treatment, the conjugate showed no major systemic effects/side effects measured as suppression of endogenous cortisol production and weight loss of whole body and thymus (indicates lymphocyte apoptosis). In contrast, the equipotent amount of free dexamethasone induced complete suppression of endogenous cortisol production and a substantial loss in body and thymus weight.
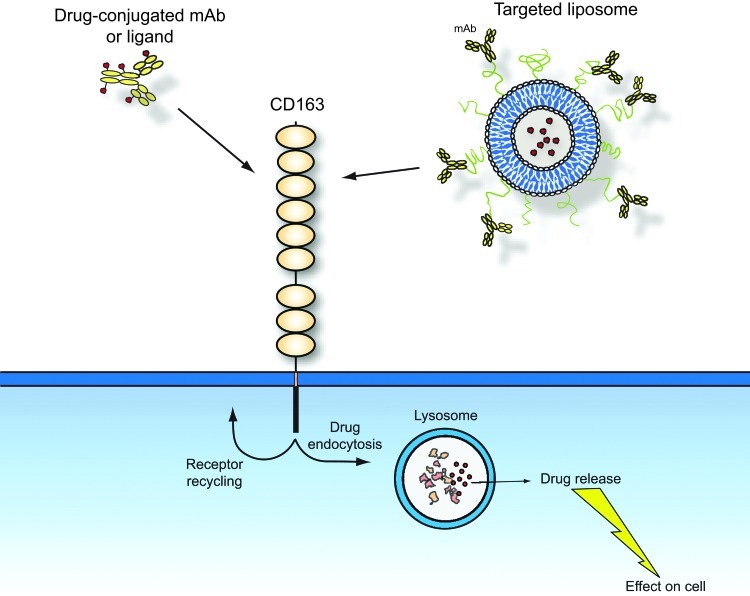
CD163 targeting may also be used for other type of conjugates, such as drugs directed linked to a physiological CD163 ligand (e.g., Hp-Hb) or liposome-encapsulated liposomes with a CD163 targeting moiety in the membrane (Fig. 5). A recent rodent study has, for instance, designed liposomes encapsulating the fluorescent dye calcein in the interior and integrated Hb in the phospholipid layer (122). In vitro uptake analysis showed that they are taken by cultured macrophages. The CD163 specificity in terms of targeting CD163-positive macrophages of these Hb-liposomes remains to be investigated though. The potential alternative clearance mechanism of Hb and the apparent absent Hp-promoting of Hb clearance in the mouse (25) suggest that mice, and perhaps also other rodents, should be used as the only test animals. An alternative type of conjugate vehicles targeting CD163 has recently been constructed and analyzed in vivo and in vitro (27). This type of conjugate vehicle is a pegylated liposome (stealth liposome) protected with polyethylene glycol that prevents nonspecific targeting and intravascular rupture. Anti-mouse CD163 is linked to the phospholipid layer by a hydrophobic linker. Loading the liposomes with the cytotoxic agent doxorubicin revealed strong CD163-dependent cytotoxic effects in cultured CD163-expressing cells. In vivo analysis of calcein-loaded anti-CD163 stealth liposomes showed accumulation in mainly the liver that contains the majority of the body macrophages. A much lower uptake was seen with nontargeting liposomes (27).
FIG. 5.
Using CD163 as a target for directed drug delivery. The endocytic property of CD163 allows either ligand- or antibody-associated drugs to have an easy and specific access to cytosolic compartment of macrophages. The figure shows examples of two types of conjugates: CD163 antibody- or ligand-drug conjugates and drugs encapsulated in pegylated liposomes with a CD163-binding antibody or ligand linked to the phospholipid layer. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.
Using CD163 for drug delivery to macrophages could suggest an inhibitory effect of circulating soluble CD163 that may bind to the therapeutic ADC intended for targeting CD163 in macrophages. However, the dose for use in human therapy of ADC is far higher than the plasma amount of soluble CD163, thus suggesting that the soluble receptor is competed out in the first dose. Furthermore, soluble CD163 is a poor competitor for macrophage uptake of Hp-Hb complexes probably because the ligand gains functional affinity by crosslinking two CD163s (or more for the Hp2-1 and Hp2-2 phenotypes) in the membrane. The IgG moiety of the therapeutic ADC is also divalent and therefore likely also to have preference for crosslinking membrane-associated CD163.
Finally, CD163 may also indirectly be involved in therapy using Hp that may be administered during excessive hemolysis, where the body's Hp store has been consumed. A recent study in guinea pigs nicely demonstrates that such a Hp therapy attenuates the tissue damaging effects by Hb released during blood transfusion (7).
Conclusion
The present review summarizes information on the Hb scavenger receptor CD163 with focus on its pamphlet of relations to inflammation. Besides being an important biological link between Hb metabolism and the anti-inflammatory response elicited by HO-1 and the heme metabolites in macrophages, an increasing body of evidence from many laboratories have now evidenced that the level of soluble CD163 increases in several acute and chronic inflammatory disorders. Novel data have documented that this increased presence in plasma is owing to the stimulated activation of the ADAM 17/TACE metalloproteinase (27). The high concentration of soluble CD163 in systemic inflammatory hemophagocytosis, has led to a proposal of including soluble CD163 as a marker in the diagnosis of this disorder. Further studies and documentation may reveal whether soluble CD163 in the future may be included in clinical risk prediction, diagnosis, and disease monitoring in relation to other inflammatory diseases. The latest novel connection between CD163 and inflammation concerns the use of CD163 and a target for drug delivery to macrophages. By the use of targeting antibodies or ligands directly coupled to drugs or to liposomes encapsulating drugs, it is now possible to direct in principle any kind of drug, such as cystostatic and anti-inflammatory compounds, directly to macrophages in the inflammatory process. The first reports (26,37) using this technology seem rather promising for the development of conjugate glucocorticiods with far lower side effects and higher potency than the nonconjugated glucocorticoid used in therapy today.
Abbreviations Used
- ADAM17
a disintegrin and metalloproteinase 17
- ADC
antibody drug conjugate
- ASFV
African swine fever virus
- CO
carbon monoxide
- CXCL4
CXC-chemokine ligand 4
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- Hb
hemoglobin
- HDL
high-density lipoprotein
- HO-1
heme oxygenase-1
- Hp
haptoglobin
- Hp-Hb
haptoglobin–hemoglobin complex
- Hpr
haptoglobin-related protein
- IL
interleukin
- LPS
lipopolysaccharide
- PRRSV
porcine reproductive and respiratory syndrome virus
- SRCR
scavenger receptor cysteine-rich
- TACE
TNF-α converting enzyme
- TNF-α
tumor necrosis factor-α
- TWEAK
TNF-like weak inducer of apoptosis
Acknowledgment
This review was supported by the TROJA ERC advanced grant 233312.
References
- 1.Aggarwal BB. Gupta SC. Kim JH. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood. 2012;119:651–665. doi: 10.1182/blood-2011-04-325225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambarus CA. Krausz S. van Eijk M. Hamann J. Radstake TRDJ. Reedquist KA. Tak PP. Baeten DLP. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J Immunol Methods. 2012;375:196–206. doi: 10.1016/j.jim.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Andersen CBF. Torvund-Jensen M. Nielsen MJ. Oliveira CLP. Hersleth H-P. Andersen NH. Pedersen JS. Andersen GR. Moestrup SK. Structure of the haptoglobin-hemoglobin complex. Nature. 2012;489:456–459. doi: 10.1038/nature11369. [DOI] [PubMed] [Google Scholar]
- 4.Aristoteli LP. Møller HJ. Bailey B. Moestrup SK. Kritharides L. The monocytic lineage specific soluble CD163 is a plasma marker of coronary atherosclerosis. Atherosclerosis. 2006;184:342–347. doi: 10.1016/j.atherosclerosis.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Axelsson J. Møller HJ. Witasp A. Qureshi AR. Carrero JJ. Heimbürger O. Bárány P. Alvestrand A. Lindholm B. Moestrup SK. Stenvinkel P. Changes in fat mass correlate with changes in soluble sCD163, a marker of mature macrophages, in patients with CKD. Am J Kidney Dis. 2006;48:916–925. doi: 10.1053/j.ajkd.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Backé E. Schwarting R. Gerdes J. Ernst M. Stein H. Ber-MAC3: new monoclonal antibody that defines human monocyte/macrophage differentiation antigen. J Clin Pathol. 1991;44:936–945. doi: 10.1136/jcp.44.11.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baek JH. D'Agnillo F. Vallelian F. Pereira CP. Williams MC. Jia Y. Schaer DJ. Buehler PW. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest. 2012;122:1444–1458. doi: 10.1172/JCI59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baeten D. Møller HJ. Delanghe J. Veys EM. Moestrup SK. de Keyser F. Association of CD163+ macrophages and local production of soluble CD163 with decreased lymphocyte activation in spondylarthropathy synovitis. Arthritis Rheum. 2004;50:1611–1623. doi: 10.1002/art.20174. [DOI] [PubMed] [Google Scholar]
- 9.Baranano DE. Rao M. Ferris CD. Snyder SH. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci USA. 2002;99:16093–16098. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbé E. Damoiseaux JG. Döpp EA. Dijkstra CD. Characterization and expression of the antigen present on resident rat macrophages recognized by monoclonal antibody ED2. Immunobiology. 1990;182:88–99. doi: 10.1016/S0171-2985(11)80586-3. [DOI] [PubMed] [Google Scholar]
- 11.Barbé E. Huitinga I. Döpp EA. Bauer J. Dijkstra CD. A novel bone marrow frozen section assay for studying hematopoietic interactions in situ: the role of stromal bone marrow macrophages in erythroblast binding. J Cell Sci. 1996;109(Pt 12):2937–2945. doi: 10.1242/jcs.109.12.2937. [DOI] [PubMed] [Google Scholar]
- 12.Barrett MP. Gilbert IH. Targeting of toxic compounds to the trypanosome's interior. Advances in parasitology. 2006;63:125–183. doi: 10.1016/S0065-308X(06)63002-9. [DOI] [PubMed] [Google Scholar]
- 13.Bleesing J. Prada A. Siegel DM. Villanueva J. Olson J. Ilowite NT. Brunner HI. Griffin T. Graham TB. Sherry DD. Passo MH. Ramanan AV. Filipovich A. Grom AA. The diagnostic significance of soluble CD163 and soluble interleukin-2 receptor alpha-chain in macrophage activation syndrome and untreated new-onset systemic juvenile idiopathic arthritis. Arthritis Rheum. 2007;56:965–971. doi: 10.1002/art.22416. [DOI] [PubMed] [Google Scholar]
- 14.Boretti FS. Buehler PW. D'Agnillo F. Kluge K. Glaus T. Butt OI. Jia Y. Goede J. Pereira CP. Maggiorini M. Schoedon G. Alayash AI. Schaer DJ. Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs. J Clin Invest. 2009;119:2271–2280. doi: 10.1172/JCI39115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bover LC. Cardó-Vila M. Kuniyasu A. Sun J. Rangel R. Takeya M. Aggarwal BB. Arap W. Pasqualini R. A previously unrecognized protein-protein interaction between TWEAK and CD163: potential biological implications. J Immunol. 2007;178:8183–8194. doi: 10.4049/jimmunol.178.12.8183. [DOI] [PubMed] [Google Scholar]
- 16.Boyle JJ. Harrington HA. Piper E. Elderfield K. Stark J. Landis RC. Haskard DO. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am J Pathol. 2009;174:1097–1108. doi: 10.2353/ajpath.2009.080431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyle JJ. Johns M. Lo J. Chiodini A. Ambrose N. Evans PC. Mason JC. Haskard DO. Heme induces heme oxygenase 1 via Nrf2: role in the homeostatic macrophage response to intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2011;31:2685–2691. doi: 10.1161/ATVBAHA.111.225813. [DOI] [PubMed] [Google Scholar]
- 18.Bronkhorst IHG. Ly LV. Jordanova ES. Vrolijk J. Versluis M. Luyten GPM. Jager MJ. Detection of M2-macrophages in uveal melanoma and relation with survival. Invest Ophthalmol Vis Sci. 2011;52:643–650. doi: 10.1167/iovs.10-5979. [DOI] [PubMed] [Google Scholar]
- 19.Buechler C. Ritter M. Orsó E. Langmann T. Klucken J. Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000;67:97–103. [PubMed] [Google Scholar]
- 20.Buehler PW. Abraham B. Vallelian F. Linnemayr C. Pereira CP. Cipollo JF. Jia Y. Mikolajczyk M. Boretti FS. Schoedon G. Alayash AI. Schaer DJ. Haptoglobin preserves the CD163 hemoglobin scavenger pathway by shielding hemoglobin from peroxidative modification. Blood. 2009;113:2578–2586. doi: 10.1182/blood-2008-08-174466. [DOI] [PubMed] [Google Scholar]
- 21.Calvert JG. Slade DE. Shields SL. Jolie R. Mannan RM. Ankenbauer RG. Welch S-KW. CD163 Expression Confers Susceptibility to Porcine Reproductive and Respiratory Syndrome Viruses. J Virol. 2007;81:7371–7379. doi: 10.1128/JVI.00513-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coca A. Bundy KW. Marston B. Huggins J. Looney RJ. Macrophage activation syndrome: serological markers and treatment with anti-thymocyte globulin. Clin Immunol. 2009;132:10–18. doi: 10.1016/j.clim.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Daly A. Walsh C. Feighery C. O'Shea U. Jackson J. Whelan A. Serum levels of soluble CD163 correlate with the inflammatory process in coeliac disease. Aliment Pharmacol Ther. 2006;24:553–559. doi: 10.1111/j.1365-2036.2006.03012.x. [DOI] [PubMed] [Google Scholar]
- 24.Das PB. Dinh PX. Ansari IH. de Lima M. Osorio FA. Pattnaik AK. The minor envelope glycoproteins GP2a and GP4 of porcine reproductive and respiratory syndrome virus interact with the receptor CD163. J Virol. 2010;84:1731–1740. doi: 10.1128/JVI.01774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emmenegger U. Schaer DJ. Larroche C. Neftel KA. Haemophagocytic syndromes in adults: current concepts and challenges ahead. Swiss Med Wkly. 2005;135:299–314. doi: 10.4414/smw.2005.10976. [DOI] [PubMed] [Google Scholar]
- 26.Etzerodt A. Kjolby M. Nielsen MJ. Maniecki M. Svendsen P. Moestrup SK. Plasma clearance of hemoglobin and haptoglobin in mice and effect of CD163 gene targeting disruption. Antioxid Redox Signal. 2013;18:2254–2263. doi: 10.1089/ars.2012.4605. [DOI] [PubMed] [Google Scholar]
- 27.Etzerodt A. Maniecki MB. Graversen JH. Møller HJ. Torchilin VP. Moestrup SK. Efficient intracellular drug-targeting of macrophages using stealth liposomes directed to the hemoglobin scavenger receptor CD163. J Control Release. 2012;160:72–80. doi: 10.1016/j.jconrel.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 28.Etzerodt A. Maniecki MB. Møller K. Møller HJ. Moestrup SK. Tumor necrosis factor α-converting enzyme (TACE/ADAM17) mediates ectodomain shedding of the scavenger receptor CD163. J Leukoc Biol. 2010;88:1201–1205. doi: 10.1189/jlb.0410235. [DOI] [PubMed] [Google Scholar]
- 29.Fabriek BO. Polfliet MMJ. Vloet RPM. van der Schors RC. Ligtenberg AJM. Weaver LK. Geest C. Matsuno K. Moestrup SK. Dijkstra CD. van den Berg TK. The macrophage CD163 surface glycoprotein is an erythroblast adhesion receptor. Blood. 2007;109:5223–5229. doi: 10.1182/blood-2006-08-036467. [DOI] [PubMed] [Google Scholar]
- 30.Fabriek BO. van Bruggen R. Deng DM. Ligtenberg AJM. Nazmi K. Schornagel K. Vloet RPM. Dijkstra CD. van den Berg TK. The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood. 2009;113:887–892. doi: 10.1182/blood-2008-07-167064. [DOI] [PubMed] [Google Scholar]
- 31.Fagoonee S. Gburek J. Hirsch E. Marro S. Moestrup SK. Laurberg JM. Christensen EI. Silengo L. Altruda F. Tolosano E. Plasma protein haptoglobin modulates renal iron loading. Am J Pathol. 2005;166:973–983. doi: 10.1016/S0002-9440(10)62319-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferreira A. Balla J. Jeney V. Balla G. Soares MP. A central role for free heme in the pathogenesis of severe malaria: the missing link? J Mol Med. 2008;86:1097–1111. doi: 10.1007/s00109-008-0368-5. [DOI] [PubMed] [Google Scholar]
- 33.Fonseca JE. Edwards JCW. Blades S. Goulding NJ. Macrophage subpopulations in rheumatoid synovium: reduced CD163 expression in CD4+ T lymphocyte-rich microenvironments. Arthritis Rheum. 2002;46:1210–1216. doi: 10.1002/art.10207. [DOI] [PubMed] [Google Scholar]
- 34.Garza-Garcia A. Esposito D. Rieping W. Harris R. Briggs C. Brown MH. Driscoll PC. Three-dimensional solution structure and conformational plasticity of the N-terminal scavenger receptor cysteine-rich domain of human CD5. J Mol Biol. 2008;378:129–144. doi: 10.1016/j.jmb.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Geiler J. Buch M. McDermott MF. Anti-TNF Treatment in Rheumatoid Arthritis. Curr Pharm Des. 2011;17:3141–3151. doi: 10.2174/138161211798157658. [DOI] [PubMed] [Google Scholar]
- 36.Gleissner CA. Shaked I. Erbel C. Bockler D. Katus HA. Ley K. CXCL4 downregulates the atheroprotective hemoglobin receptor CD163 in human macrophages. Circ Res. 2010;106:203. doi: 10.1161/CIRCRESAHA.109.199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gordon S. Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Graversen JH. Svendsen P. Dagnæs-Hansen F. Dal J. Anton G. Etzerodt A. Petersen MD. Christensen PA. Moller HJ. Moestrup SK. Targeting the hemoglobin scavenger receptor CD163 in macrophages highly increases the anti-inflammatory potency of dexamethasone. Mol Ther. 2012;20:1550–1558. doi: 10.1038/mt.2012.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greisen SR. Møller HJ. Stengaard-Pedersen K. Hetland ML. Hørslev-Petersen K. Jørgensen A. Hvid M. Deleuran B. Soluble macrophage-derived CD163 is a marker of disease activity and progression in early rheumatoid arthritis. Clin Exp Rheumatol. 2011;29:689–692. [PubMed] [Google Scholar]
- 40.Grochot-Przeczek A. Dulak J. Jozkowicz A. Haem oxygenase-1: non-canonical roles in physiology and pathology. Clin Sci. 2012;122:93–103. doi: 10.1042/CS20110147. [DOI] [PubMed] [Google Scholar]
- 41.Gronlund J. Vitved L. Lausen M. Skjodt K. Holmskov U. Cloning of a novel scavenger receptor cysteine-rich type I transmembrane molecule (M160) expressed by human macrophages. J Immunol. 2000;165:6406–6415. doi: 10.4049/jimmunol.165.11.6406. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi S. Takamiya R. Yamaguchi T. Matsumoto K. Tojo SJ. Tamatani T. Kitajima M. Makino N. Ishimura Y. Suematsu M. Induction of heme oxygenase-1 suppresses venular leukocyte adhesion elicited by oxidative stress: role of bilirubin generated by the enzyme. Circ Res. 1999;85:663–671. doi: 10.1161/01.res.85.8.663. [DOI] [PubMed] [Google Scholar]
- 43.Hintz KA. Rassias AJ. Wardwell K. Moss ML. Morganelli PM. Pioli PA. Givan AL. Wallace PK. Yeager MP. Guyre PM. Endotoxin induces rapid metalloproteinase-mediated shedding followed by up-regulation of the monocyte hemoglobin scavenger receptor CD163. J Leukoc Biol. 2002;72:711–717. [PubMed] [Google Scholar]
- 44.Hiraoka A. Horiike N. Akbar SMF. Michitaka K. Matsuyama T. Onji M. Soluble CD163 in patients with liver diseases: very high levels of soluble CD163 in patients with fulminant hepatic failure. J Gastroenterol. 2005;40:52–56. doi: 10.1007/s00535-004-1493-8. [DOI] [PubMed] [Google Scholar]
- 45.Hohenester E. Sasaki T. Timpl R. Crystal structure of a scavenger receptor cysteine-rich domain sheds light on an ancient superfamily. Nat Struct Biol. 1999;6:228–232. doi: 10.1038/6669. [DOI] [PubMed] [Google Scholar]
- 46.Hori R. Kashiba M. Toma T. Yachie A. Goda N. Makino N. Soejima A. Nagasawa T. Nakabayashi K. Suematsu M. Gene transfection of H25A mutant heme oxygenase-1 protects cells against hydroperoxide-induced cytotoxicity. J Biol Chem. 2002;277:10712–10718. doi: 10.1074/jbc.M107749200. [DOI] [PubMed] [Google Scholar]
- 47.Hvidberg V. Maniecki MB. Jacobsen C. Højrup P. Møller HJ. Moestrup SK. Identification of the receptor scavenging hemopexin-heme complexes. Blood. 2005;106:2572–2579. doi: 10.1182/blood-2005-03-1185. [DOI] [PubMed] [Google Scholar]
- 48.Hwang PK. Greer J. Interaction between hemoglobin subunits in the hemoglobin. haptoglobin complex. J Biol Chem. 1980;255:3038–3041. [PubMed] [Google Scholar]
- 49.Kaempfer T. Duerst E. Gehrig P. Roschitzki B. Rutishauser D. Grossmann J. Schoedon G. Vallelian F. Schaer DJ. Extracellular hemoglobin polarizes the macrophage proteome toward Hb-Clearance, enhanced antioxidant capacity and suppressed HLA class 2 expression. J Proteome Res. 2011;10:2397–2408. doi: 10.1021/pr101230y. [DOI] [PubMed] [Google Scholar]
- 50.Kieft R. Capewell P. Turner CMR. Veitch NJ. MacLeod A. Hajduk S. Mechanism of Trypanosoma brucei gambiense (group 1) resistance to human trypanosome lytic factor. Proc Natl Acad Sci. 2010;107:16137–16141. doi: 10.1073/pnas.1007074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kino K. Mizumoto K. Watanabe J. Tsunoo H. Immunohistochemical studies on hemoglobin-haptoglobin and hemoglobin catabolism sites. J Histochem Cytochem. 1987;35:381–386. doi: 10.1177/35.3.3546486. [DOI] [PubMed] [Google Scholar]
- 52.Kishimoto T. Goyert S. Kikutani H. Mason D. Miyasaka M. Moretta L. Ohno T. Okumura K. Shaw S. Springer TA. Sugamura K. Sugawara H. Borne von dem AE. Zola H. CD antigens 1996. Blood. 1997;89:3502. [PubMed] [Google Scholar]
- 53.Kneidl J. Löffler B. Erat MC. Kalinka J. Peters G. Roth J. Barczyk K. Soluble CD163 promotes recognition, phagocytosis and killing of Staphylococcus aureus via binding of specific fibronectin peptides. Cell Microbiol. 2012;14:914–936. doi: 10.1111/j.1462-5822.2012.01766.x. [DOI] [PubMed] [Google Scholar]
- 54.Knudsen TB. Gustafson P. Kronborg G. Kristiansen TB. Moestrup SK. Nielsen JO. Gomes V. Aaby P. Lisse I. Møller HJ. Eugen-Olsen J. Predictive value of soluble haemoglobin scavenger receptor CD163 serum levels for survival in verified tuberculosis patients. Clin Microbiol Infect. 2005;11:730–735. doi: 10.1111/j.1469-0691.2005.01229.x. [DOI] [PubMed] [Google Scholar]
- 55.Kodelja V. Goerdt S. Dissection of macrophage differentiation pathways in cutaneous macrophage disorders and in vitro. Exp Dermatol. 1994;3:257–268. doi: 10.1111/j.1600-0625.1994.tb00287.x. [DOI] [PubMed] [Google Scholar]
- 56.Krieger M. Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP) Annu Rev Biochem. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- 57.Kristiansen M. Graversen JH. Jacobsen C. Sonne O. Hoffman HJ. Law SK. Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 58.Kusi KA. Gyan BA. Goka BQ. Dodoo D. Obeng-Adjei G. Troye-Blomberg M. Akanmori BD. Adjimani JP. Levels of soluble CD163 and severity of malaria in children in Ghana. Clin Vaccine Immunol. 2008;15:1456–1460. doi: 10.1128/CVI.00506-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Law SK. Micklem KJ. Shaw JM. Zhang XP. Dong Y. Willis AC. Mason DY. A new macrophage differentiation antigen which is a member of the scavenger receptor superfamily. Eur J Immunol. 1993;23:2320–2325. doi: 10.1002/eji.1830230940. [DOI] [PubMed] [Google Scholar]
- 60.Levy AP. Asleh R. Blum S. Levy NS. Miller-Lotan R. Kalet-Litman S. Anbinder Y. Lache O. Nakhoul FM. Asaf R. Farbstein D. Pollak M. Soloveichik YZ. Strauss M. Alshiek J. Livshits A. Schwartz A. Awad H. Jad K. Goldenstein H. Haptoglobin: basic and clinical aspects. Antioxid Redox Signal. 2010;12:293–304. doi: 10.1089/ars.2009.2793. [DOI] [PubMed] [Google Scholar]
- 61.Loboda A. Jazwa A. Grochot-Przeczek A. Rutkowski AJ. Cisowski J. Agarwal A. Jozkowicz A. Dulak J. Heme oxygenase-1 and the vascular bed: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2008;10:1767–1812. doi: 10.1089/ars.2008.2043. [DOI] [PubMed] [Google Scholar]
- 62.Madsen M. Møller HJ. Nielsen MJ. Jacobsen C. Graversen JH. van den Berg T. Moestrup SK. Molecular characterization of the haptoglobin.hemoglobin receptor CD163. Ligand binding properties of the scavenger receptor cysteine-rich domain region. J Biol Chem. 2004;279:51561–51567. doi: 10.1074/jbc.M409629200. [DOI] [PubMed] [Google Scholar]
- 63.Maniecki MB. Etzerodt A. Moestrup SK. Møller HJ. Graversen JH. Comparative assessment of the recognition of domain-specific CD163 monoclonal antibodies in human monocytes explains wide discrepancy in reported levels of cellular surface CD163 expression. Immunobiology. 2011;216:882–890. doi: 10.1016/j.imbio.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 64.Maniecki MB. Møller HJ. Moestrup SK. Møller BK. CD163 positive subsets of blood dendritic cells: the scavenging macrophage receptors CD163 and CD91 are coexpressed on human dendritic cells and monocytes. Immunobiology. 2006;211:407–417. doi: 10.1016/j.imbio.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 65.Martínez VG. Moestrup SK. Holmskov U. Mollenhauer J. Lozano F. The conserved scavenger receptor cysteine-rich superfamily in therapy and diagnosis. Pharmacol Rev. 2011;63:967–1000. doi: 10.1124/pr.111.004523. [DOI] [PubMed] [Google Scholar]
- 66.Matsushita N. Kashiwagi M. Wait R. Nagayoshi R. Nakamura M. Matsuda T. Hogger P. Guyre PM. Nagase H. Matsuyama T. Elevated levels of soluble CD163 in sera and fluids from rheumatoid arthritis patients and inhibition of the shedding of CD163 by TIMP-3. Clin Exp Immunol. 2002;130:156–161. doi: 10.1046/j.1365-2249.2002.01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moeller JB. Nielsen MJ. Reichhardt MP. Schlosser A. Sorensen GL. Nielsen O. Tornøe I. Grønlund J. Nielsen ME. Jørgensen JS. Jensen ON. Mollenhauer J. Moestrup SK. Holmskov U. CD163-L1 is an Endocytic macrophage protein strongly regulated by mediators in the inflammatory response. J Immunol. 2012;188:2399–2409. doi: 10.4049/jimmunol.1103150. [DOI] [PubMed] [Google Scholar]
- 68.Moestrup SK. Gliemann J. Pallesen G. Distribution of the alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein in human tissues. Cell Tissue Res. 1992;269:375–382. doi: 10.1007/BF00353892. [DOI] [PubMed] [Google Scholar]
- 69.Moestrup SK. Møller HJ. CD163: a regulated hemoglobin scavenger receptor with a role in the anti-inflammatory response. Ann Med. 2004;36:347–354. doi: 10.1080/07853890410033171. [DOI] [PubMed] [Google Scholar]
- 70.Moestrup SK. The alpha 2-macroglobulin receptor and epithelial glycoprotein-330: two giant receptors mediating endocytosis of multiple ligands. Biochim Biophys Acta. 1994;1197:197. doi: 10.1016/0304-4157(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 71.Moller HJ. Soluble CD163. Scand J Clin Lab Invest. 2012;72:1–13. doi: 10.3109/00365513.2011.626868. [DOI] [PubMed] [Google Scholar]
- 72.Moller HJ. Frikke-Schmidt R. Moestrup SK. Nordestgaard BG. Tybjærg-Hansen A. Serum soluble CD163 predicts risk of type 2 diabetes in the general population. Clin Chem. 2011;57:291–297. doi: 10.1373/clinchem.2010.154724. [DOI] [PubMed] [Google Scholar]
- 73.Moreno JA. Dejouvencel T. Labreuche J. Smadja DM. Dussiot M. Martín-Ventura JL. Egido J. Gaussem P. Emmerich J. Michel J-B. Blanco-Colio LM. Meilhac O. Peripheral artery disease is associated with a high CD163/TWEAK plasma ratio. Arterioscler Thromb Vasc Biol. 2010;30:1253–1262. doi: 10.1161/ATVBAHA.110.203364. [DOI] [PubMed] [Google Scholar]
- 74.Moreno JA. Muñoz-García B. Martín-Ventura JL. Madrigal-Matute J. Orbe J. Páramo JA. Ortega L. Egido J. Blanco-Colio LM. The CD163-expressing macrophages recognize and internalize TWEAK: potential consequences in atherosclerosis. Atherosclerosis. 2009;207:103–110. doi: 10.1016/j.atherosclerosis.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 75.Morganelli PM. Guyre PM. IFN-gamma plus glucocorticoids stimulate the expression of a newly identified human mononuclear phagocyte-specific antigen. J Immunol. 1988;140:2296–2304. [PubMed] [Google Scholar]
- 76.Møller HJ. Aerts H. Grønbaek H. Peterslund NA. Hyltoft Petersen P. Hornung N. Rejnmark L. Jabbarpour E. Moestrup SK. Soluble CD163: a marker molecule for monocyte/macrophage activity in disease. Scand J Clin Lab Invest Suppl. 2002;237:29–33. doi: 10.1080/003655102762377466. [DOI] [PubMed] [Google Scholar]
- 77.Møller HJ. Hald K. Moestrup SK. Characterization of an enzyme-linked immunosorbent assay for soluble CD163. Scand J Clin Lab Invest. 2002;62:293–299. doi: 10.1080/003655102760145852. [DOI] [PubMed] [Google Scholar]
- 78.Møller HJ. Hyltoft Petersen P. Rejnmark L. Moestrup SK. Biological variation of soluble CD163. Scand J Clin Lab Invest. 2003;63:15–21. doi: 10.1080/00365510310000439. [DOI] [PubMed] [Google Scholar]
- 79.Møller HJ. Moestrup SK. Weis N. Wejse C. Nielsen H. Pedersen SS. Attermann J. Nexø E. Kronborg G. Macrophage serum markers in pneumococcal bacteremia: prediction of survival by soluble CD163. Crit Care Med. 2006;34:2561–2566. doi: 10.1097/01.CCM.0000239120.32490.AB. [DOI] [PubMed] [Google Scholar]
- 80.Møller HJ. Nielsen MJ. Maniecki MB. Madsen M. Moestrup SK. Soluble macrophage-derived CD163: a homogenous ectodomain protein with a dissociable haptoglobin-hemoglobin binding. Immunobiology. 2010;215:406–412. doi: 10.1016/j.imbio.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 81.Møller HJ. Peterslund NA. Graversen JH. Moestrup SK. Identification of the hemoglobin scavenger receptor/CD163 as a natural soluble protein in plasma. Blood. 2002;99:378–380. doi: 10.1182/blood.v99.1.378. [DOI] [PubMed] [Google Scholar]
- 82.Nakayama W. Jinnin M. Makino K. Kajihara I. Makino T. Fukushima S. Inoue Y. Ihn H. Serum levels of soluble CD163 in patients with systemic sclerosis. Rheumatol Int. 2012;32:403–407. doi: 10.1007/s00296-010-1691-z. [DOI] [PubMed] [Google Scholar]
- 83.Nielsen MJ. Madsen M. Møller HJ. Moestrup SK. The macrophage scavenger receptor CD163: endocytic properties of cytoplasmic tail variants. J Leukoc Biol. 2006;79:837–845. doi: 10.1189/jlb.1005602. [DOI] [PubMed] [Google Scholar]
- 84.Nielsen MJ. Moestrup SK. Receptor targeting of hemoglobin mediated by the haptoglobins: roles beyond heme scavenging. Blood. 2009;114:764–771. doi: 10.1182/blood-2009-01-198309. [DOI] [PubMed] [Google Scholar]
- 85.Nielsen MJ. Møller HJ. Moestrup SK. Hemoglobin and heme scavenger receptors. Antioxid Redox Signal. 2010;12:261–273. doi: 10.1089/ars.2009.2792. [DOI] [PubMed] [Google Scholar]
- 86.Nielsen MJ. Petersen SV. Jacobsen C. Oxvig C. Rees D. Møller HJ. Moestrup SK. Haptoglobin-related protein is a high-affinity hemoglobin-binding plasma protein. Blood. 2006;108:2846–2849. doi: 10.1182/blood-2006-05-022327. [DOI] [PubMed] [Google Scholar]
- 87.Nielsen MJ. Petersen SV. Jacobsen C. Thirup S. Enghild JJ. Graversen JH. Moestrup SK. A unique loop extension in the serine protease domain of haptoglobin is essential for CD163 recognition of the haptoglobin-hemoglobin complex. J Biol Chem. 2007;282:1072–1079. doi: 10.1074/jbc.M605684200. [DOI] [PubMed] [Google Scholar]
- 88.Ojala JRM. Pikkarainen T. Tuuttila A. Sandalova T. Tryggvason K. Crystal structure of the cysteine-rich domain of scavenger receptor MARCO reveals the presence of a basic and an acidic cluster that both contribute to ligand recognition. J Biol Chem. 2007;282:16654–16666. doi: 10.1074/jbc.M701750200. [DOI] [PubMed] [Google Scholar]
- 89.Otterbein L. Soares M. Yamashita K. Bach F. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 90.Parkner T. Sørensen L. Nielsen A. Fischer C. Bibby C. Nielsen S. Pedersen B. Møller H. Soluble CD163- a biomarker linking macrophages and insulin resistance. Diabetologia. 2012;55:1856–1862. doi: 10.1007/s00125-012-2533-1. [DOI] [PubMed] [Google Scholar]
- 91.Polfliet M. Fabriek B. Daniėls W. Dijkstra C. van den Berg T. The rat macrophage scavenger receptor CD163: expression, regulation and role in inflammatory mediator production. Immunobiology. 2006;211:419–425. doi: 10.1016/j.imbio.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 92.Porcheray F. Viaud S. Rimaniol A-C. Léone C. Samah B. Dereuddre-Bosquet N. Dormont D. Gras G. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142:481–489. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pulford K. Micklem K. Law ASK. Mason DY. CD163 (M130 antigen) Workshop Panel report. In: Kishimoto T, editor; Kikutani H, editor; Borne von dem AEGK, editor; Goyert AM, editor; Miyasaka M, editor; Moretta L, editor; Okumura K, editor; Shaw S, editor; Springer TA, editor; Sugamura K, editor; Zola H, editor. Leukocyte Typing IV: White Cell Differentiation Antigens. New York: Garland Publishing, Inc.; 1997. pp. 1089–1091. [Google Scholar]
- 94.Pulford K. Micklem K. McCarthy S. Cordell J. Jones M. Mason DY. A monocyte/macrophage antigen recognized by the four antibodies GHI/61, Ber-MAC3, Ki-M8 and SM4. Immunology. 1992;75:588–595. [PMC free article] [PubMed] [Google Scholar]
- 95.Radzun HJ. Kreipe H. Bödewadt S. Hansmann ML. Barth J. Parwaresch MR. Ki-M8 monoclonal antibody reactive with an intracytoplasmic antigen of monocyte/macrophage lineage. Blood. 1987;69:1320–1327. [PubMed] [Google Scholar]
- 96.Ratcliffe NR. Kennedy SM. Morganelli PM. Immunocytochemical detection of Fcgamma receptors in human atherosclerotic lesions. Immunol Lett. 2001;77:169–174. doi: 10.1016/s0165-2478(01)00217-6. [DOI] [PubMed] [Google Scholar]
- 97.Ritter M. Genomic organization and chromosomal localization of the human cd163 (m130) gene: a member of the scavenger receptor cysteine-rich superfamily. Biochem Biophys Res Commun. 1999;260:466–474. doi: 10.1006/bbrc.1999.0866. [DOI] [PubMed] [Google Scholar]
- 98.Rodamilans B. Muñoz IG. Bragado-Nilsson E. Sarrias MR. Padilla O. Blanco FJ. Lozano F. Montoya G. Crystal structure of the third extracellular domain of CD5 reveals the fold of a group B scavenger cysteine-rich receptor domain. J Biol Chem. 2007;282:12669–12677. doi: 10.1074/jbc.M611699200. [DOI] [PubMed] [Google Scholar]
- 99.Rother RP. Bell L. Hillmen P. Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 100.Sánchez C. Doménech N. Vázquez J. Alonso F. Ezquerra A. Domínguez J. The porcine 2A10 antigen is homologous to human CD163 and related to macrophage differentiation. J Immunol. 1999;162:5230–5237. [PubMed] [Google Scholar]
- 101.Sánchez-Torres C. Gómez-Puertas P. Gómez-del-Moral M. Alonso F. Escribano JM. Ezquerra A. Domínguez J. Expression of porcine CD163 on monocytes/macrophages correlates with permissiveness to African swine fever infection. Arch Virol. 2003;148:2307–2323. doi: 10.1007/s00705-003-0188-4. [DOI] [PubMed] [Google Scholar]
- 102.Schaer DJ. Schaer CA. Buehler PW. Boykins RA. Schoedon G. Alayash AI. Schaffner A. CD163 is the macrophage scavenger receptor for native and chemically modified hemoglobins in the absence of haptoglobin. Blood. 2006;107:373–380. doi: 10.1182/blood-2005-03-1014. [DOI] [PubMed] [Google Scholar]
- 103.Schaer DJ. Schleiffenbaum B. Kurrer M. Imhof A. Bächli E. Fehr J. Moller HJ. Moestrup SK. Schaffner A. Soluble hemoglobin-haptoglobin scavenger receptor CD163 as a lineage-specific marker in the reactive hemophagocytic syndrome. Eur J Haematol. 2005;74:6–10. doi: 10.1111/j.1600-0609.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- 104.Scheller J. Chalaris A. Garbers C. Rose-John S. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 2011;32:380–387. doi: 10.1016/j.it.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 105.Snyder SH. Barañano DE. Heme oxygenase: a font of multiple messengers. Neuropsychopharmacology. 2001;25:294–298. doi: 10.1016/S0893-133X(01)00275-5. [DOI] [PubMed] [Google Scholar]
- 106.Sporrer D. Weber M. Wanninger J. Weigert J. Neumeier M. Stögbauer F. Lieberer E. Bala M. Kopp A. Schäffler A. Adiponectin downregulates CD163 whose cellular and soluble forms are elevated in obesity. Eur J Clin Invest. 2009;39:671–679. doi: 10.1111/j.1365-2362.2009.02170.x. [DOI] [PubMed] [Google Scholar]
- 107.Stover CM. Schleypen J. Gronlund J. Speicher MR. Schwaeble WJ. Holmskov U. Assignment of CD163B, the gene encoding M160, a novel scavenger receptor, to human chromosome 12p13.3 by in situ hybridization and somatic cell hybrid analysis. Cytogenet Cell Genet. 2000;90:246–247. doi: 10.1159/000056781. [DOI] [PubMed] [Google Scholar]
- 108.Sulahian TH. Högger P. Wahner AE. Wardwell K. Goulding NJ. Sorg C. Droste A. Stehling M. Wallace PK. Morganelli PM. Guyre PM. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine. 2000;12:1312–1321. doi: 10.1006/cyto.2000.0720. [DOI] [PubMed] [Google Scholar]
- 109.Tolosano E. Fagoonee S. Hirsch E. Berger FG. Baumann H. Silengo L. Altruda F. Enhanced splenomegaly and severe liver inflammation in haptoglobin/hemopexin double-null mice after acute hemolysis. Blood. 2002;100:4201–4208. doi: 10.1182/blood-2002-04-1270. [DOI] [PubMed] [Google Scholar]
- 110.Tolosano E. Fagoonee S. Morello N. Vinchi F. Fiorito V. Heme scavenging and the other facets of hemopexin. Antioxid Redox Signal. 2010;12:305–320. doi: 10.1089/ars.2009.2787. [DOI] [PubMed] [Google Scholar]
- 111.van den Heuvel MM. Tensen CP. van As JH. van den Berg TK. Fluitsma DM. Dijkstra CD. Döpp EA. Droste A. van Gaalen FA. Sorg C. Högger P. Beelen RH. Regulation of CD163 on human macrophages: cross-linking of CD163 induces signaling and activation. J Leukoc Biol. 1999;66:858–866. doi: 10.1002/jlb.66.5.858. [DOI] [PubMed] [Google Scholar]
- 112.van Gorp H. Delputte PL. Nauwynck HJ. Scavenger receptor CD163, a Jack-of-all-trades and potential target for cell-directed therapy. Mol Immunol. 2010;47:1650–1660. doi: 10.1016/j.molimm.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 113.van Gorp H. Van Breedam W. Delputte PL. Nauwynck HJ. Sialoadhesin and CD163 join forces during entry of the porcine reproductive and respiratory syndrome virus. J Gen Virol. 2008;89:2943–2953. doi: 10.1099/vir.0.2008/005009-0. [DOI] [PubMed] [Google Scholar]
- 114.Vanhollebeke B. de Muylder G. Nielsen MJ. Pays A. Tebabi P. Dieu M. Raes M. Moestrup SK. Pays E. A haptoglobin-hemoglobin receptor conveys innate immunity to Trypanosoma brucei in humans. Science. 2008;320:677–681. doi: 10.1126/science.1156296. [DOI] [PubMed] [Google Scholar]
- 115.Vanhollebeke B. Pays E. The trypanolytic factor of human serum: many ways to enter the parasite, a single way to kill. Mol Microbiol. 2010;76:806–814. doi: 10.1111/j.1365-2958.2010.07156.x. [DOI] [PubMed] [Google Scholar]
- 116.Vinchi F. Gastaldi S. Silengo L. Altruda F. Tolosano E. Hemopexin prevents endothelial damage and liver congestion in a mouse model of heme overload. Am J Pathol. 2008;173:289–299. doi: 10.2353/ajpath.2008.071130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang J. Doré S. Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain. 2007;130:1643–1652. doi: 10.1093/brain/awm095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weaver LK. Hintz-Goldstein KA. Pioli PA. Wardwell K. Qureshi N. Vogel SN. Guyre PM. Pivotal advance: activation of cell surface Toll-like receptors causes shedding of the hemoglobin scavenger receptor CD163. J Leukoc Biol. 2006;80:26–35. doi: 10.1189/jlb.1205756. [DOI] [PubMed] [Google Scholar]
- 119.Xong HV. Vanhamme L. Chamekh M. Chimfwembe CE. Van Den Abbeele J. Pays A. Van Meirvenne N. Hamers R. De Baetselier P. Pays E. A VSG expression site-associated gene confers resistance to human serum in Trypanosoma rhodesiense. Cell. 1998;95:839–846. doi: 10.1016/s0092-8674(00)81706-7. [DOI] [PubMed] [Google Scholar]
- 120.Zanni MV. Burdo TH. Makimura H. Williams KC. Grinspoon SK. Relationship between monocyte/macrophage activation marker soluble cd163 and insulin resistance in obese and normal‐weight subjects. Clin Endocrinol. 2011;77:385–390. doi: 10.1111/j.1365-2265.2011.04284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zeng L. Takeya M. Takahashi K. AM-3K, a novel monoclonal antibody specific for tissue macrophages and its application to pathological investigation. J Pathol. 1996;178:207–214. doi: 10.1002/(SICI)1096-9896(199602)178:2<207::AID-PATH427>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 122.Zhang N. Palmer AF. Liposomes surface conjugated with human hemoglobin target delivery to macrophages. Biotechnol Bioeng. 2012;109:823–829. doi: 10.1002/bit.24340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zwadlo G. Voegeli R. Osthoff KS. Sorg C. A monoclonal antibody to a novel differentiation antigen on human macrophages associated with the down-regulatory phase of the inflammatory process. Exp Cell Biol. 1987;55:295–304. doi: 10.1159/000163432. [DOI] [PubMed] [Google Scholar]
- 124.Zwadlo-Klarwasser G. Bent S. Haubeck HD. Sorg C. Schmutzler W. Glucocorticoid-induced appearance of the macrophage subtype RM 3/1 in peripheral blood of man. Int Arch Allergy Appl Immunol. 1990;91:175–180. doi: 10.1159/000235111. [DOI] [PubMed] [Google Scholar]