Abstract
Phospholipase D (PLD) is a key facilitator of multiple types of membrane vesicle trafficking events. Two PLD isoforms, PLD1 and PLD2, exist in mammals. Initial studies based on overexpression studies suggested that in resting cells, human PLD1 localized primarily to the Golgi and perinuclear vesicles in multiple cell types. In contrast, overexpressed mouse PLD2 was observed to localize primarily to the plasma membrane, although internalization on membrane vesicles was observed subsequent to serum stimulation. A recent report has suggested that the assignment of PLD2 to the plasma membrane is in error, because the endogenous isoform in rat secretory cells was imaged and found to be present primarily in the Golgi apparatus. We have reexamined this issue by using a monoclonal antibody specific for mouse PLD2, and find, as reported initially using overexpression studies, that endogenous mouse PLD2 is detected most readily at the plasma membrane in multiple cell types. In addition, we report that mouse, rat, and human PLD2 when overexpressed all similarly localize to the plasma membrane in cell lines from all three species. Finally, studies conducted using overexpression of wild-type active or dominant-negative isoforms of PLD2 and RNA interference-mediated targeting of PLD2 suggest that PLD2 functions at the plasma membrane to facilitate endocytosis of the angiotensin II type 1 receptor.
INTRODUCTION
Phospholipase D (PLD), which hydrolyzes phosphatidylcholine to generate choline and the bioactive lipid phosphatidic acid, has been implicated in signal transduction, membrane trafficking, transformation, and cytoskeletal reorganization (reviewed in Frohman et al., 1999; Jones et al., 1999; Liscovitch et al., 2000). There are two mammalian PLDs, PLD1 (Hammond et al., 1995) and PLD2 (Colley et al., 1997c), which are expressed in a wide but selective variety of tissues and cells (Colley et al., 1997a; reviewed in Gibbs and Meier, 2000). Although frequently expressed in the same cell types, PLD1 and PLD2 have generally been proposed to mediate isoform-specific functions, based on their selective abilities, when overexpressed as wild-type or catalytically inactive alleles, to facilitate or hinder different steps in cytoskeletal reorganization (Colley et al., 1997b; O'Luanaigh et al., 2002) and regulated exocytosis from neuroendocrine cells (Vitale et al., 2001) and mast cells (Choi et al., 2002).
In addition, numerous reports based on overexpression have described localization of the isoforms to separate regions of the cell—PLD1 to perinuclear vesicles (Colley et al., 1997b; Toda et al., 1999; Lucocq et al., 2001)—and PLD2 to the plasma membrane (Colley et al., 1997b; Park et al., 2000; O'Luanaigh et al., 2002). Localization of the isoforms is clearly complex though. In some cells, PLD1 preferentially localizes to the plasma membrane (Vitale et al., 2001), and more generally, it has been shown to cycle between perinuclear regions and the plasma membrane (Brown et al., 1998; Emoto et al., 2000; Du et al., 2003). Similarly, internalization of PLD2 subsequent to serum (Colley et al., 1997b) or insulin (Rizzo et al., 1999) stimulation and its localization to early endosomes when one of its several membrane targeting motifs is altered (Sciorra et al., 2002) suggests that it too cycles regularly from the plasma membrane through subcellular membrane vesicle compartments.
In a recent study, it was reported that use of anti-PLD2 peptide antisera to image endogenous PLD2 in rat normal rat kidney (NRK) and GH3 cells yielded a different observation, namely, that PLD2 instead localizes primarily to the rim of the Golgi apparatus (Freyberg et al., 2002). One conclusion of this study was that prior studies demonstrating localization of PLD2 to the plasma membrane based on overexpression of tagged isoforms were accordingly artifactual. Several other possibilities occurred to us, though, and in this study, we examine which, if any, account for the different sets of findings and then explore potential roles for PLD2 at the plasma membrane.
One such role would be to regulate endocytosis of the angiotensin II (Ang II) receptor (AT1R). Ang II triggers numerous types of physiological actions, primarily through stimulation of AT1R (Touyz and Schiffrin, 1999). Initial models for AT1R regulation proposed that signaling occurred only while the agonist-bound receptor was at the plasma membrane and that receptor endocytosis served to sequester and down-regulate the receptor. However, more recent evidence has suggested that the endocytosed receptor also mediates specific signaling pathways (Ferguson, 2001). Although numerous proteins have been identified that function in the endocytic recycling of AT1R, little is known about the role of phospholipid dynamics during this process. The involvement we report here for PLD2 in AT1R endocytosis suggests that regulated production of phosphatidic acid, PLD2's enzymatic product, will turn out to be important.
MATERIALS AND METHODS
General Reagents
Cell culture media, DMEM, Opti-MEM-I, and LipofectAMINE Plus were from Invitrogen (Carlsbad, CA). Enhanced chemiluminescence reagent was purchased from Pierce Chemical (Rockford, IL). All other reagents were of analytical grade unless otherwise specified.
Antibodies
Two rat monoclonal antibodies generated against the N terminus of mouse PLD2 (expressed and purified from bacteria) were kindly provided by Y. Kanaho (University of Tokyo, Tokyo, Japan. Identical images were obtained with each monoclonal. 3F10 antibody (rat monoclonal anti-hemagglutinin [HA] tag antibody) was from Roche Diagnostics (Indianapolis, IN). Early endosome antigen 1 (EEA1) and GM130 were from BD Transduction Laboratories (Lexington, KY). Monoclonal anti-α-tubulin was from Sigma-Aldrich (St. Louis, MO). Goat anti-mouse or anti-rabbit IgG conjugated with Alexa488, 568, 647, or 680 was from Molecular Probes (Eugene, OR). Goat anti-mouse IgG conjugated with IRDye 800 was from Rockland Immunochemicals (Gilbertsville, PA). Goat anti-rat and anti-mouse IgG conjugated with horseradish peroxidase or Cy3 were from Jackson ImmunoResearch Laboratories (West Grove, PA).
Western Blotting
Twenty micrograms of total cell lysates was subjected to 8% SDS-polyacrylamide gels and transferred to nitrocellulose membranes. The blot in Figure 7 was probed at 4°C overnight with a rat anti-PLD2 monoclonal antibody (mAb) (1:50), washed, and incubated with secondary antibody conjugated to horseradish peroxidase and developed with SuperSignal West Pico trial kit (Pierce Chemical). The blot was stripped at 55°C for 30 min and reprobed with mouse anti-tubulin antibody to confirm that equal amounts of protein had been loaded. Other Western blotting was performed with the indicated primary antibodies, followed by secondary antibodies conjugated with Alexa 680 or IRDye 800. Fluorescent signals were detected with an Odyssey infrared imaging system.
Figure 7.
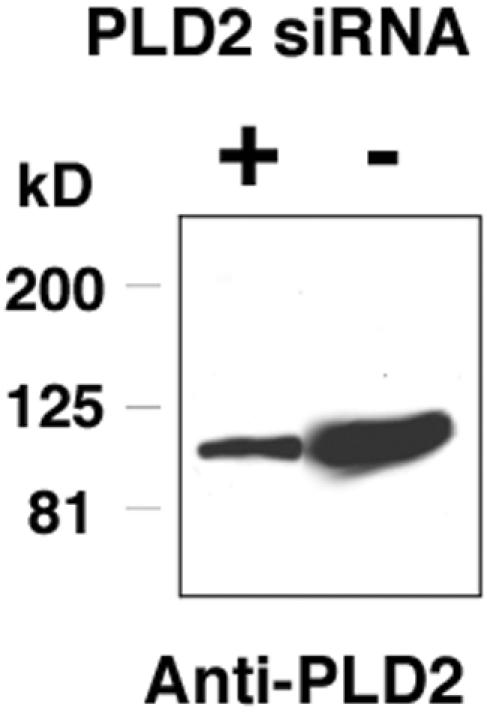
A mAb directed against mouse PLD2 recognizes a single band on Western blot analysis that decreases when PLD2 is targeted by siRNA. Western blot analysis of 3T3-L1 adipocytes by using the anti-PLD2 mAb #2. A single band is detected at the expected size (106 kDa). siRNA was transfected into 3T3-L1 fibroblasts as described in MATERIALS AND METHODS. No changes in the levels of tubulin or PLD1 were observed (our unpublished data).
Cell Culture and Transfection
Cells were maintained in DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. 3T3-L1 fibroblasts were differentiated into adipocytes as described previously (Harrison et al., 1990). Tetracycline-inducible Chinese hamster ovary (CHO) stable cells expressing HA-tagged PLD2 were generated using the T-Rex system from Invitrogen and were grown in F12 medium supplemented with 10% tetracycline-free fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin.
For transfections, cells were grown on coverslips in 35-mm dishes (3–4 × 105 cells/dish) and then switched into Opti-MEM I media before being transfected with 1 μg of DNA/dish by using LipofectAMINE Plus. Four hours posttransfection, the media were replaced with fresh growth medium, and the cells were incubated a further 24 h.
Dispersed mouse adult cardiomyocytes were prepared as described previously (Hu et al., 2001). In brief, after retrograde perfusion, hearts were treated with liberase (Roche Diagnostics) in 25 μM CaCl2-containing modified Tyrode's solution consisting of 126 mM NaCl, 4.4 mM KCl, 1.0 mM MgCl2, 18 mM NaHCO3, 11 mM glucose, 4 mM HEPES, and 30 mM 2,3-butanedione monoxime. The cells released were then plated out on coverslips, cultured until attached at pH 7.4 in 5% CO2, 95% O2, and fixed by 4% paraformaldehyde before processing for immunohistochemistry.
Imunofluorescence Staining
Unless stated, cells were fixed with 2% paraformaldehyde for 10 min, permeabilized with 0.1% Triton X-100, and blocked with 5% bovine serum albumin and normal goat serum. The cells were then immunostained using primary antibodies against the specific proteins, followed by fluorescent dye-conjugated secondary antibodies. Stained cells were visualized using a Leica TCS SP2 confocal microscope. Images were processed using Adobe Photoshop 6.0, and quantitative analyses were performed using the ImageJ analysis software package (National Institutes of Health). All experiments were performed at least three times with similar results.
Small Interference RNA (siRNA) Synthesis and Transfection
A 19-nt sequence matching mouse, rat, and human PLD2 (at mouse PLD2 open-reading frame nt 347–365; GACACAAAGTCTTGATGAG) was chosen for performing PLD2 RNAi targeting. Sense and antisense RNA oligonucleotides were synthesized by Oligos, etc. (Wilsonville, OR). Then 25 nM of siRNA duplex was transiently transfected into 3T3-L1 fibroblasts by using TransIT-TKO from Mirus (Madison, WI) or LipofectAMINE 2000 (Carlsbad, CA), and 2 d later the cells were harvested for Western blotting and AT1R receptor internalization analysis. ShRNA (small hairpin)-mediated targeting of PLD was performed using pSuper (Brummelkamp et al., 2002).
RESULTS
Localization of Overexpressed Human PLD1 and Mouse PLD2 in COS-7 (Monkey) Cells
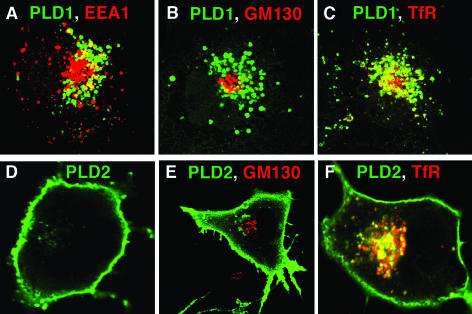
A typical pattern of subcellular localization for overexpressed human PLD1 and mouse PLD2 is shown in Figure 1. PLD1 localizes to endosomal vesicles and as well to the Golgi apparatus (Figure 1, A–C), as reported previously by multiple groups (Colley et al., 1997a; Toda et al., 1999; Lucocq et al., 2001; Du et al., 2003). In contrast, overexpressed mouse PLD2 localizes to the plasma membrane and vesicles close to plasma membrane (Figure 1, D–F), as reported previously (Colley et al., 1997a). In most cells, essentially complete localization to the plasma membrane is observed (Figure 1D). In others, there are variable amounts of perinuclear vesicular localization that do not colocalize with the Golgi marker GM130 (Figure 1E) but do colocalize with recycling endosomes (as evidenced by coinciding with the transferrin receptor; Figure 1F).
Figure 1.
Localization of overexpressed human PLD1 and mouse PLD2 in COS-7 cells. COS-7 cells were transiently transfected with HA-tagged PLD1 or PLD2 expression plasmids. Twenty-four hours later, the cells were fixed and immunostained using anti-HA mAb. Images were captured using a Leica TCS SP2 confocal microscope with an Alexa 488-labeled secondary antibody. Colocalization with EEA1, GM130, and TfR were performed using Alexa 647 as the second fluorophore. In B, two cells are shown.
Mouse, Rat, and Human PLD2 Homologues Localize Similarly to the Plasma Membrane When Overexpressed
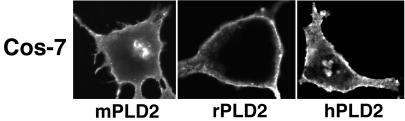
An interesting possibility for the unexpected localization of rat PLD2 to the Golgi apparatus as reported by Freyberg et al. (2002) was raised by a specific instance of lack of sequence conservation for mouse PLD2 versus rat and human PLD2 in the region of the pleckstrin homology (PH) domain (Figure 2). Ktistakis and coworkers had reported that the PH domain in human PLD1 becomes palmitoylated at the pair of cysteines shown in Figure 2 (top line) and that loss of the palmitoylation through mutagenesis alters PLD1 subcellular targeting, causing it to become localized instead to the plasma membrane (Sugars et al., 1999). With respect to PLD2, Sciorra et al. (2002) reported recently that an intact PH domain is also required for overexpressed mouse PLD2 to remain localized to the plasma membrane (mutations to other residues involved in phosphoinositide binding caused relocation of the mutant PLD2 allele to early endosomes). As shown in Figure 2, rat and human PLD2 conserve both cysteine residues at the site shown to be critical for PLD1 localization to perinuclear regions, whereas mouse PLD2 encodes only one of the cysteines. This posed the question of whether overexpressed mouse PLD2 would localize differently from rat and human PLD2, that is, it would be retained at the plasma membrane due to decreased palmitoylation, whereas the rat and human isoforms would preferentially internalize. Most overexpression studies have been carried out with mouse PLD2 because it was the isoform first cloned and most widely distributed and for which the largest series of mutant alleles exists. To address this possibility, mouse, rat, and human PLD2 were transfected in parallel into COS-7 cells. All three isoforms localized similarly to the plasma membrane (Figure 3). Thus, although there are interesting sequence differences between the isoforms, this does not seem to affect their pattern of subcellular localization.
Figure 2.
Mouse PLD2 lacks a potentially key cysteine present in mammalian PLD1 and human and rat PLD2. Mouse PLD2 encodes only one cysteine at the hypothetically critical site for palmitoylation in its PH domain, whereas PLD1 in all three species, and PLD2 in rat and human, encodes two. Two palmitates are believed to be necessary to provide sufficient force to affect protein trafficking (Zacharias et al., 2002). In the case of PLD1, the palmitates have been proposed to mediate internalization by facilitating PLD1's entry into plasma membrane lipid rafts (Du et al., 2003). PLD2 is less well studied, although there has been one report that rat PLD2 does become palmitoylated on both of the cysteine residues and that this alters its membrane association (Xie et al., 2002).
Figure 3.
Mouse, rat, and human PLD2 nonetheless localize similarly when overexpressed. COS-7 cells were transiently transfected with HA-tagged mouse, rat, or human PLD2 expression plasmids. Twenty-four hours later, the cells were fixed and immunostained using anti-HA mAb. Images were captured using a Leica TCS SP2 confocal microscope.
PLD2 Localizes to the Plasma Membrane in Cell Lines from Four Different Mammalian Species
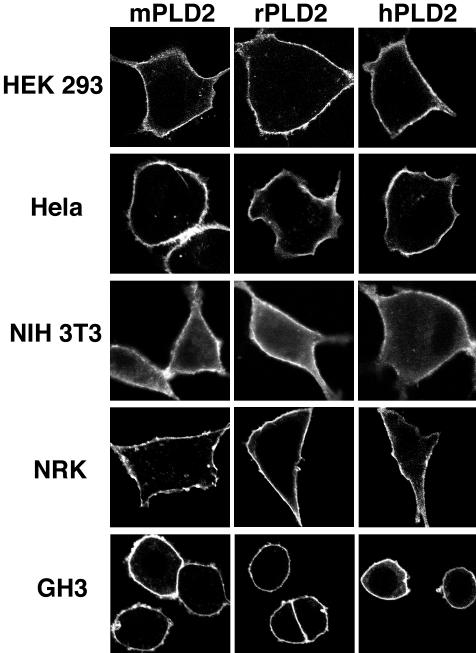
Another possibility was that PLD2 would behave differently for unknown reasons in cell types originating from different species. Accordingly, the PLD2 cDNAs were transfected into HeLa (human), human embryonic kidney (HEK)293 (human) NIH3T3 (mouse), NRK (rat), and GH3 (rat) cells and imaged. PLD2 was found to localize to the plasma membrane regardless of origin of species (Figure 4). These findings are consistent with prior reports that overexpressed mouse PLD2 localizes to the plasma membrane in rat mast (Choi et al., 2002; O'Luanaigh et al., 2002) and PC12 cells (Vitale et al., 2001). Thus, neither species origin of the PLD2 isoform nor the species of the transfected cell seems to play a determining role in the localization of PLD2 to the plasma membrane.
Figure 4.
Mouse PLD2 localizes similarly to the plasma membrane in mouse, rat and human cell lines. HeLa (human), HEK293 (human), NIH3T3 (mouse), NRK (rat), and GH3 (rat) cells were transiently transfected with HA-tagged mouse, rat, or human PLD2 expression plasmids and processed as described above.
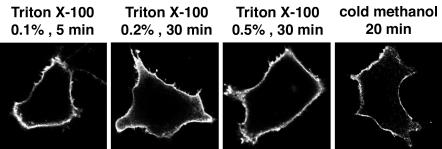
In some instances, protein relocalization can occur during fixation and permeabilization, depending on the specific protocol used. To explore this possibility, HeLa cells transfected with HA-tagged PLD2 were fixed with paraformaldehyde and permeabilized with differing concentrations of Triton X-100, or were fixed and permeabilized with cold methanol. Regardless of the method used, PLD2 was localized to the plasma membrane (Figure 5).
Figure 5.
Plasma membrane localization of PLD2 is not affected by different sample processing methods. HeLa cells were transfected with pCGN-PLD2 for 20 h. The cells were then either fixed with 2% paraformaldehyde for 10 min and permeabilized with different concentrations of Triton X-100 for the indicated times, or fixed and permeabilized with cold methanol for 20 min. The HA-PLD2 was visualized using an anti-HA mAb.
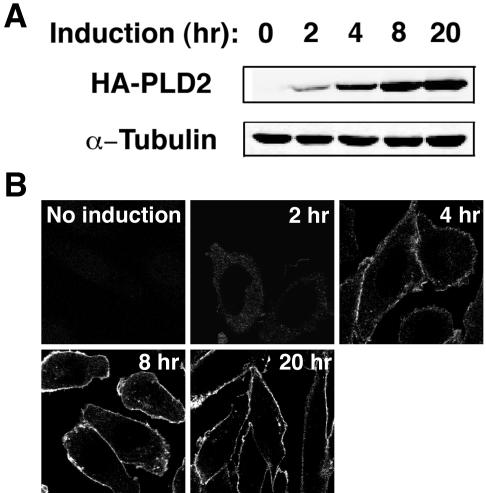
Alternatively, as proposed by Freyberg et al. (2002), consequences of transfection conditions or overexpression of PLD2 might cause it to mislocalize to the plasma membrane. To address this, we examined PLD2 localization using a tetracycline-inducible PLD2-expressing CHO stable cell line. Expression levels of recombinant PLD2 were controlled by incubation of the cells with doxycycline for different periods of time (Figure 6A). PLD2 was consistently found to localize to the plasma membrane regardless of its level of expression (Figure 6B). As well, because the cells were induced with doxycycline rather than transiently transfected with an expression plasmid, this also rules out the possibility that transient transfection using LipofectAMINE Plus (a cationic lipid) alters endogenous membrane properties in some manner that results in artifactual localization of PLD2.
Figure 6.
Plasma membrane localization of PLD2 is not affected by PLD2's level of expression. Tetracycline-inducible PLD2-expressing CHO stable cells were incubated with 1 μg/ml doxycycline (Dox) to elicit expression of PLD2, which was detected by either Western blotting (A) or immunofluorescent staining (B) by using a rat anti-HA mAb (3F10). α-Tubulin was used as a loading control in A. Expression and plasma membrane localization of PLD2 was detected 2 h after Dox addition.
Endogenous Mouse PLD2 Localizes to the Plasma Membrane in Multiple Cell Types
Finally, we used a pair of rat monoclonal antibodies generated against the N-terminal third of mouse PLD2 to examine the localization of endogenous PLD2. Both antibodies seem to recognize epitopes poorly conserved in rat and human PLD2, because the monoclonal antibodies detect recombinant rat and human PLD2 with much less sensitivity than mouse PLD2 (our unpublished data). The monoclonal anti-PLD2 antibodies detect a single band of the appropriate size (∼106 kDa) in mouse 3T3-L1 fibroblasts in Western blots (Figure 7), and the band detected becomes reduced in intensity when the cells are transfected with siRNA directed against PLD2, confirming the specificity of the antibodies. Finally, the monoclonal anti-PLD2 antibodies detect overexpressed HA-tagged mouse PLD2 in the same pattern of expression revealed using monoclonal anti-HA antibody (our unpublished data).
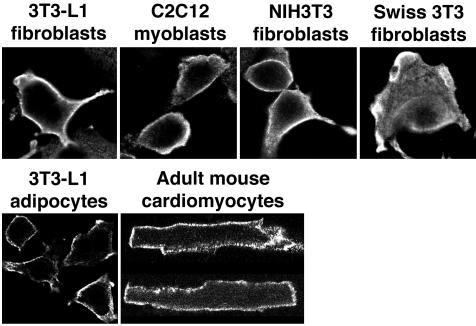
A panel of mouse cells—3T3-L1 fibroblasts and adipocytes, C2C12 myoblasts, NIH3T3 fibroblasts, Swiss 3T3 fibroblasts, and dispersed adult cardiomyocytes—were fixed and immunostained using the monoclonal anti-PLD2 antibody. In each case, staining was evident on the plasma membrane (Figure 8), although in some cells some cytosolic staining was observed as well. In no instance was staining observed in a pattern consistent with Golgi rim localization. The pattern of localization was similar to that shown above and reported previously for overexpressed PLD2, suggesting that the plasma membrane localization of overexpressed protein does not ensue from artifactual mislocalization.
Figure 8.
Endogenous mouse PLD2 is found on the plasma membrane in multiple types of cell lines and primary cells. Mouse cell lines and dispersed adult cardiomyocytes were fixed and immunostained using monoclonal anti-mouse PLD2 antibody #2 followed by labeled secondary. Confocal images were collected at the midcell level. PLD2 localization to membrane ruffles is observed in the Swiss 3T3 fibroblasts. The dispersed adult cardiomyocytes exhibit a characteristic rectangular morphology.
PLD2 Regulates Endocytosis of the Angiotensin II Receptor
PLD2 is activated by Ang II through the plasma membrane-localized AT1R (Touyz and Schiffrin, 1999; Ushio-Fukai et al., 1999; Andresen et al., 2001; Parmentier et al., 2001). Binding of Ang II to AT1R causes the receptor to internalize into perinuclear endosomes (Hein et al., 1997; Hunyady et al., 2002). Because PLD2 has been proposed to function in membrane trafficking processes, including endocytosis (Shen et al., 2001; Koch et al., 2003), we set out to examine whether alterations in the level of PLD2 activity affect AT1R endocytosis by using RNAi and overexpression of dominant-negative PLD2 to eliminate or interfere with PLD2 function, and overexpression of wild-type PLD2 to increase PLD2 activity/function.
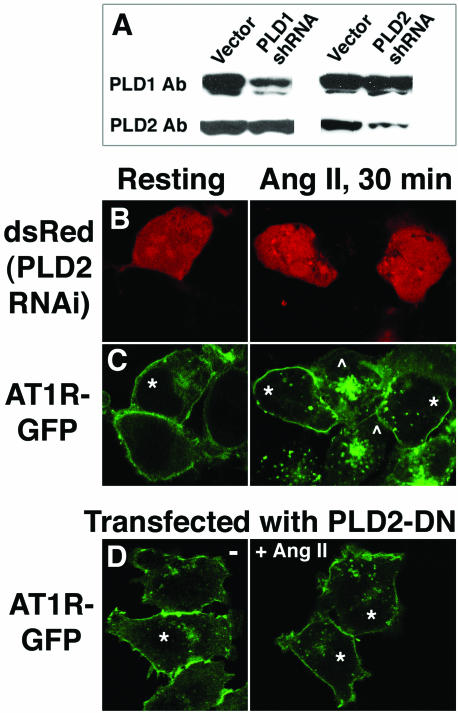
HeLa cells were transfected with PLD2 shRNA and assessed by Western blot analysis (Figure 9A). shRNA directed against endogenous PLD2 mRNA efficiently decreased the level of PLD2 protein detectable 2 d later. The decreased expression was specific because the level of expression of the related gene PLD1 was not affected (Figure 9A), nor was that of α-tubulin (our unpublished data). The shRNA treatment did not completely eliminate PLD2 expression, but the degree to which it was diminished was consistent with the efficiency of transfection of these cells. Similar results consistent with the efficiency of transfection were observed with other cell types. HEK293 cells stably expressing GFP-tagged AT1R (AT1R-GFP) were cotransfected with PLD2 shRNA and with pdsRed, cultured for 48 h, and stimulated with Ang II (Figure 9, B and C). Expression of dsRed served to identify the cells that had been successfully transfected (Figure 9, B and C, asterisks). AT1R-GFP localized primarily to the plasma membrane in both transfected and nontransfected resting cells (Figure 9C, left). Ang II stimulation for 30 min caused the AT1R-GFP in nontransfected cells to endocytose and acumulate in perinuclear recycling endosomes (Figure 9C, right, cells marked with *). In contrast, the AT1R-GFP underwent very limited if any endocytosis in dsRed/PLD2 shRNA transfected cells (Figure 9C, right, cells marked with *). No effect on AT1R endocytosis was observed with transfection of control shRNAs. ImageJ analysis was used to quantitate the relative intensity of AT1R-GFP at the plasma membrane of transfected and nontransfected cells after Ang II stimulation. After subtraction of average cytoplasmic fluorescence, the fluorescent signal from AT1R-GFP at the plasma membrane of transfected cells was on average 12.0-fold (+/- 2.5) more intense than the fluorescent signal at the plasma membrane of nontransfected cells (n = 6). In contrast, the respective plasma membrane fluorescence signals were not significantly different before Ang II stimulation.
Figure 9.
Inhibition of AT1R endocytosis by PLD2 inhibition. (A) HeLa cells were transfected with shRNA plasmids directed against PLD1 or PLD2, cultured for 48 h, and assessed using Western blot analysis and isoform-specific antibodies. PLD1 and PLD2 were detected with rabbit antipeptide antibodies by using Western blotting analysis (this PLD2 antibody is unable to generate a satisfactory signal for immunofluorescent staining). (B and C) HEK293 cells stably expressing GFP-AT1R were cotransfected with a shRNA plasmid directed against PLD2 and with an expression plasmid for dsRed, a red-emitting fluorescent protein, in a 15:1 M ratio. After 48 h of additional culture, some wells were stimulated with 100 nM Ang II for 30 min. Cells successfully transfected were identified in B by expression of dsRed and are marked in C by asterisks. In C, GFP fluorescence was used to determine the subcellular localization of the GFP-tagged AT1R isoform. Nontransfected cells are indicated by the symbol ^. (D) Inhibition of AT1R internalization by the inactive allele of PLD2 K758R. HEK293 cells stably expressing GFP-AT1R were transfected with (HA)-PLD2-K758R and cultured for 24 h, after which some wells were stimulated with 100 nM Ang II for 30 min. The cells successfully transfected were visualized using an anti-HA antibody and are indicated by asterisks.
Transfection of catalytically-inactive (K758R) PLD2 into the AT1R-GFP-expressing HEK293 cells to act as a dominant negative was examined as another approach to assess loss-of-function. Ang II-triggered AT1R internalization was again inhibited (Figure 9D). Inhibition of endocytosis was not observed in cells overexpressing catalytically inactive PLD1 (our unpublished data).
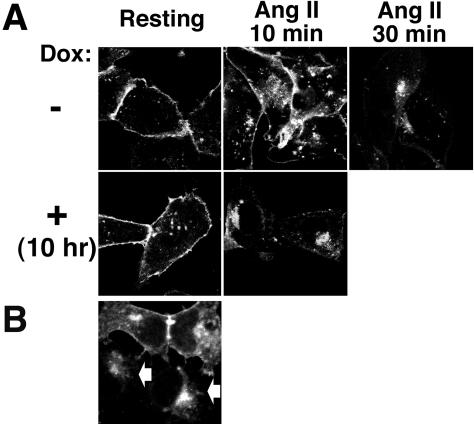
To examine the consequences of increased levels of PLD2 expression and activity, CHO stable cell lines inducibly expressing wild-type PLD2 under the control of tetracycline were transfected with AT1R-GFP and cultured for 48 h. AT1R-GFP localized primarily to the plasma membrane in resting cells, and induction of PLD2 during the final 10 h of culture did not alter this (Figure 10A, left; PLD2 localized to the plasma membrane as described above). Noninduced cells required 30 min of Ang II stimulation to accumulate the majority of the AT1R into the perinuclear endosomes (Figure 10A, top right); at 10 min poststimulation, it remained primarily on the plasma membrane or in dispersed vesicles (presumably early endosomes) in the these cells (Figure 10A, top middle). In contrast, PLD2 induction accelerated Ang II-triggered AT1R internalization: by 10 min poststimulation, AT1R-GFP had accumulated in the perinuclear region in the PLD2-induced cells (Figure 10A, bottom middle). Moreover, in cells in which PLD2 had been induced for 48 h, AT1R internalization occurred even in the absence of Ang II addition (our unpublished data). Similar results were observed in HEK293 cells transiently transfected with PLD2 and cultured for 2 d (Figure 10B), suggesting the generality of the phenomenon. This result is very interesting because agonist-independent AT1R internalization has also been reported for cells overexpressing β-arrestin2, which is a well-characterized mediator of G protein-coupled receptor endocytosis (Anborgh et al., 2000).
Figure 10.
Facilitation of AT1R endocytosis by PLD2. (A) Acceleration of Ang II-triggered AT1R endocytosis after brief PLD2 induction. PLD2-inducible CHO cells were transfected with GFP-AT1R and cultured for 48 h. PLD2 expression was then induced using doxycycline (Dox) during the final 10 h of culture. (B) Loss of surface AT1R by long-term PLD2 overexpression. HEK293 cells expressing GFP-AT1R were transfected with HA-tagged PLD2 and cultured for 48 h. The cells transfected with PLD2 were visualized using an anti-HA antibody and are indicated by arrows.
Together, the inhibition of endocytosis by RNAi and dominant negative approaches and the acceleration of endocytosis by overexpression of wild-type PLD2 indicate that PLD2 facilitates agonist-induced AT1R internalization.
DISCUSSION
PLD2 has been proposed to play roles at the plasma membrane to regulate cortical cytoskeletal reorganization, endocytosis, and receptor tyrosine kinase signaling. In this report, we demonstrate that the plasma membrane localization of PLD2 reported by many laboratories using overexpression of PLD2 cDNAs does not result from artifactual mislocalization of the protein, and describe a functional role for PLD2 in facilitating agonist-induced AT1R endocytosis. Thus, these findings support a functional role for PLD2 at the plasma membrane. But how then to account for the differing results between this study and the report by Freyberg et al. (2002) that found that endogenous PLD2 localizes to the rim of the Golgi?
One possibility could be technical, because the antisera used by Freyberg et al. (2002) was noted in a prior publication to exhibit some reactivity against bands other than PLD2 by Western blot analysis (Houle et al., 1999). However, the demonstration in Freyberg et al. (2002) that preincubation of the antisera with immunizing peptide eliminated the Golgi rim signal and that the same pattern of localization was observed with a second antisera directed against a different region of PLD2, makes it relatively unlikely that the pattern of localization they observed derived from nonspecific immunoreactivity.
An alternate approach to assess localization involves biochemical analysis. But here again, conflicting results have been reported. Sweeney et al. (2002) reported by subcellular fractionation that endogenous PLD2 in liver was evident mainly in fractions corresponding to Golgi and light membranes. In contrast, Czarny et al. (1999) reported using similar approaches that endogenous PLD2 from HaCaT human keratinocytes, Chinese hamster ovary cells, and mouse NIH3T3 cells colocalized with markers of the plasma membrane and with overexpressed PLD2. Park et al. (2000) reported similar findings for cardiac muscle cells, and recently, Sarri et al. (2003) and Chahdi et al. (2003) observed the same outcome using the RBL-2H3 rat mast cell line.
The most obvious remaining untested possibility is whether there are unique aspects to the NRK and GH3 endocrine secretory cells used by Freyberg et al. (2002) (or the culture conditions in which they were maintained) that lead PLD2 to be localized differently in these cell types. PLD2 was similarly reported to localize to a crescent-shaped perinuclear region colocalizing with Golgi markers (ARF1 and 58K Golgi protein) in the polarized secretory cell HT29-cl19A by using three different anti-PLD2 antisera, including the one used in this report and a nonpeptide antisera directed against the N-terminal 124 amino acids of PLD2 (Denmat-Ouisse et al., 2001). The nonpeptide anti-PLD2 antisera detected only PLD2 by Western blot analysis, and transfection of GFP-PLD2 into these cells again yielded the crescentshaped perinuclear Golgi pattern of localization. In further support of this hypothesis, PLD1 exhibits altered patterns of localization in different cell types (e.g., on the plasma membrane in PC12 cells Vitale et al., 2001); thus, there is clear precedence for this hypothesis based on the other PLD isoform. Further characterization of PLD localization in the NRK and GH3 cell lines described in Freyberg et al. (2002) by using additional, nonpeptide-directed antisera in their culture system should help to resolve the issue.
Multiple groups have described internalization of PLD2 from the plasma membrane upon agonist stimulation (Colley et al., 1997; Slaaby et al., 1998; Rizzo et al., 2000; O'Luanaigh et al., 2002). Given that PLD2 seems to cycle in the cell between the plasma membrane and intracellular vesicles, it is unclear whether variations in the relative amounts of PLD2 observed at each site are functionally relevant. More importantly, there is presently a key need to develop model systems in which the role of endogenous PLD2 can be examined, after which the significance of the localization may become more obvious. One such system is described here for the role of PLD2 in AT1R endocytosis. Questions to be addressed include determining which step(s) is regulated by PLD2 activity and what the mechanism of action is, and whether PLD2 functions in concert with β-arrestin2, which can also mediate agonist-independent endocytosis when overexpressed (Anborgh et al., 2000).
Acknowledgments
We thank Yasunori Kanaho for providing the monoclonal anti-PLD2 antibodies and Sylvain Bourgoin for the rabbit polyclonal antibody. We also thank Drs. Tamas Balla and Kevin J. Catt for the HEK293 cell line stably expressing AT1R and the AT1R-EGFP plasmid. This work was supported by grants from the American Diabetes Society and National Institutes of Health (RO1 DK64166) to M.A.F., and the American Heart Association Scientist Development Grant to G.D.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–09–0673. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-09-0673.
Abbreviations used: Ang II, angiotensin II; AT1R, type I angiotensin II receptor; EEA1, early endosome antigen 1; PLD, Phospholipase D; TfR, transferrin receptor.
References
- Anborgh, P.H., Seachrist, J.L., Dale, L.B., and Ferguson, S.S. (2000). Receptor/beta-arrestin complex formation and the differential trafficking and resensitization of beta2-adrenergic and angiotensin II type 1A receptors. Mol. Endocrinol. 14, 2040-2053. [DOI] [PubMed] [Google Scholar]
- Andresen, B.T., Jackson, E.K., and Romero, G.G. (2001). Angiotensin II signaling to phospholipase D in renal microvascular smooth muscle cells in SHR. Hypertension 37, 635-639. [DOI] [PubMed] [Google Scholar]
- Brown, F.D., Thompson, N., Saqib, K.M., Clark, J.M., Powner, D., Thompson, N.T., Solari, R., and Wakelam, M.J. (1998). Phospholipase D1 localises to secretory granules and lysosomes and is plasma-membrane translocated on cellular stimulation. Curr. Biol. 8, 835-838. [DOI] [PubMed] [Google Scholar]
- Brummelkamp, T.R., Bernards, R., and Agami, R. (2002). A system for stable expression of short interfering RNAs in mammalian cells. Science 296, 550-553. [DOI] [PubMed] [Google Scholar]
- Chahdi, A., Choi, W.S., Kim, Y.M., and Beaven, M.A. (2003). Mastoparan selectively activates phospholipase D2 in cell membranes. J. Biol. Chem. 278, 12039-12045. [DOI] [PubMed] [Google Scholar]
- Choi, W.S., Kim, Y.M., Combs, C., Frohman, M.A., and Beaven, M.A. (2002). Phospholipase D1 and 2 regulate different phases of exocytosis in mast cells. J. Immunol. 168, 5682-5689. [DOI] [PubMed] [Google Scholar]
- Colley, W.C., Altshuller, Y.M., Sue-Ling, C.K., Copeland, N.G., Gilbert, D.J., Jenkins, N.A., Branch, K.D., Bollag, R.J., Bollag, W.B., and Frohman, M.A. (1997b). Cloning and expression analysis of murine phospholipase D1. Biochem. J. 326, 745-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley, W., Sung, T.C., Roll, R., Hammond, S.M., Altshuller, Y.M., Bar-Sagi, D., Morris, A.J., and Frohman, M.A. (1997a). Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Curr. Biol. 7, 191-201. [DOI] [PubMed] [Google Scholar]
- Colley, W.C., Sung, T.C., Roll, R., Jenco, J., Hammond, S.M., Altshuller, Y., Bar-Sagi, D., Morris, A.J., and Frohman, M.A. (1997c). Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Curr. Biol. 7, 191-201. [DOI] [PubMed] [Google Scholar]
- Czarny, M., Lavie, Y., Fiucci, G., and Liscovitch, M. (1999). Localization of phospholipase D in detergent-insoluble, caveolin-rich membrane domains. Modulation by caveolin-1 expression and caveolin-182-101. J. Biol. Chem. 274, 2717-2724. [DOI] [PubMed] [Google Scholar]
- Denmat-Ouisse, L.A., Phebidias, C., Honkavaara, P., Robin, P., Geny, B., Min do, S., Bourgoin, S., Frohman, M.A., and Raymond, M.N. (2001). Regulation of constitutive protein transit by phospholipase D in HT29-cl19A cells. J. Biol. Chem. 276, 48840-48846. [DOI] [PubMed] [Google Scholar]
- Du, G., Vitale, N., Chasserot-Golaz, S., Huang, P., Altshuller, Y.M., Morris, A.J., Bader, M.-F., and Frohman, M.A. (2003). Regulation of phospholipase D subcellular cycling through coordination of multiple membrane association motifs. J. Cell Biol. 62, 305-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emoto, M., Klarlund, J., Waters, S., Hu, V., Buxton, J., Chawla, A., and Czech, M. (2000). A role for phospholipase D in GLUT4 glucose transporter translocation. J. Biol. Chem. 275, 7144-7151. [DOI] [PubMed] [Google Scholar]
- Ferguson, S.S. (2001). Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol. Rev. 53, 1-24. [PubMed] [Google Scholar]
- Freyberg, Z., Bourgoin, S., and Shields, D. (2002). Phospholipase D2 is localized to the rims of the Golgi apparatus in mammalian cells. Mol. Biol. Cell 13, 3930-3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman, M.A., Engebrecht, J., and Morris, A.J. (1999). Molecular and cell biological roles for the phospholipase D genes. Chem. Physiol. Lipids 98, 127-140. [Google Scholar]
- Gibbs, T.C., and Meier, K. (2000). Expression and regulation of phospholipase D isoforms in mammalian cell lines. J. Cell. Physiol. 182, 77-87. [DOI] [PubMed] [Google Scholar]
- Hammond, S.M., Altshuller, Y.M., Sung, T.C., Rudge, S.A., Rose, K., Engebrecht, J., Morris, A.J., and Frohman, M.A. (1995). Human ADP-ribosylation factor-activated phosphatidylcholine-specific phospholipase D defines a new and highly conserved gene family. J. Biol. Chem. 270, 29640-29643. [DOI] [PubMed] [Google Scholar]
- Harrison, S.A., Buxton, J.M., Clancy, B.M., and Czech, M.P. (1990). Insulin regulation of hexose transport in mouse 3T3-L1 cells expressing the human HepG2 glucose transporter. J. Biol. Chem. 265, 20106-20116. [PubMed] [Google Scholar]
- Hein, L., Meinel, L., Pratt, R.E., Dzau, V.J., and Kobilka, B.K. (1997). Intracellular trafficking of angiotensin II and its AT1 and AT2 receptors: evidence for selective sorting of receptor and ligand. Mol. Endocrinol. 11, 1266-1277. [DOI] [PubMed] [Google Scholar]
- Houle, M.G., and Bourgoin, S. (1999). Regulation of phospholipase D by phosphorylation-dependent mechanisms. Biochimica Biophysica Act. 1439, 135-150. [DOI] [PubMed] [Google Scholar]
- Hu, B., Mei, Q.B., Yao, X.J., Smith, E., Barry, W.H., and Liang, B.T. (2001). A novel contractile phenotype with cardiac transgenic expression of the human P2X4 receptor. FASEB J. 15, 2739-2741. [DOI] [PubMed] [Google Scholar]
- Hunyady, L., Baukal, A.J., Gaborik, Z., Olivares-Reyes, J.A., Bor, M., Szaszak, M., Lodge, R., Catt, K.J., and Balla, T. (2002). Differential PI 3-kinase dependence of early and late phases of recycling of the internalized AT1 angiotensin receptor. J. Cell Biol. 157, 1211-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, D., Morgan, C., and Cockcroft, S. (1999). Phospholipase D and membrane traffic. Biochem. Biophys. Acta 1439, 229-244. [DOI] [PubMed] [Google Scholar]
- Koch, T., Brandenburg, L.O., Schulz, S., Liang, Y., Klein, J., and Hollt, V. (2003). ADP-ribosylation factor-dependent phospholipase D2 activation is required for agonist-induced mu-opioid receptor endocytosis. J. Biol. Chem. 278, 9979-9985. [DOI] [PubMed] [Google Scholar]
- Liscovitch, M., Czarny, M., Fiucci, G., and Tang, X. (2000). Phospholipase D: molecular and cell biology of a novel gene family. Biochem. J. 345, 401-415. [PMC free article] [PubMed] [Google Scholar]
- Lucocq, J., Manifava, M., Bi, K., Roth, M.G., and Ktistakis, N.T. (2001). Immunolocalisation of phospholipase D1 on tubular vesicular membranes of endocytic and secretory origin. Eur. J. Cell Biol. 80, 508-520. [DOI] [PubMed] [Google Scholar]
- O'Luanaigh, N., Pardo, R., Fensome, A., Allen-Baume, V., Jones, D., Holt, M.R., and Cockcroft, S. (2002). Continual production of phosphatidic acid by phospholipase D is essential for antigen-stimulated membrane ruffling in cultured mast cells. Mol. Biol. Cell 13, 3730-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J.B., Kim, J.H., Kim, J., Yoo, J.-S., Suh, P.-G., Du, G., Frohman, M.A., and Ryu, S.H. (2000). Phospholipase D2 is localized in sarcolemmal membrane and inhibited by a-actinin through direct interaction. J. Biol. Chem. 275, 21295-21301. [DOI] [PubMed] [Google Scholar]
- Parmentier, J.H., Muthalif, M.M., Nishimoto, A.T., and Malik, K.U. (2001). 20-Hydroxyeicosatetraenoic acid mediates angiotensin ii-induced phospholipase d activation in vascular smooth muscle cells. Hypertension 37, 623-629. [DOI] [PubMed] [Google Scholar]
- Rizzo, M.A., Shome, K., Watkins, S.C., and Romero, G. (2000). The recruitment of Raf-1 to membranes is mediated by direct interaction with phosphatidic acid and is independent of association with Ras. J. Biol. Chem. 275, 23911-23918. [DOI] [PubMed] [Google Scholar]
- Rizzo, M.A., Shome, K., Vasudevan, C., Stolz, D.B., Sung, T.-C., Frohman, M.A., Watkins, S.C., and Romero, G. (1999). Phospholipase D and its product, phosphatidic acid, mediate agonist-dependent Raf-1 translocation to the plasma membrane and the activation of the MAP kinase pathway. J. Biol. Chem. 274, 1131-1139. [DOI] [PubMed] [Google Scholar]
- Sarri, E., Pardo, R., Fensome-Green, A., and Cockcroft, S. (2003). Endogenous phospholipase D2 localizes to the plasma membrane of RBL-2H3 mast cells and can be distinguished from ADP ribosylation factor-stimulated phospholipase D1 activity by its specific sensitivity to oleic acid. Biochem J. 369, 319-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciorra, V.A., Rudge, S.A., Wang, J., McLaughlin, S., Engebrecht, J., and Morris, A.J. (2002). Dual role for phosphoinositides in regulation of yeast and mammalian phospholipase D enzymes. J. Cell Biol. 159, 1039-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, Y., Xu, L., and Foster, D. (2001). Role for phospholipase D in receptormediated endocytosis. Mol. Cell. Biol. 21, 595-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaaby, R., Jensen, T., Hansen, H.S., Frohman, M.A., and Seedorf, K. (1998). PLD2 complexes with the EFG receptor and undergoes tyrosine phosphorylation at a single site upon agonist stimulation. J. Biol. Chem. 273, 33722-33727. [DOI] [PubMed] [Google Scholar]
- Sugars, J., Cellek, S., Manifava, M., Coadwell, J., and Ktistakis, N. (1999). Fatty acylation of phospholipase D1 on cysteine residues 240 and 241 determines localization on intracellular membranes. J. Biol. Chem. 274, 30023-30027. [DOI] [PubMed] [Google Scholar]
- Sweeney, D.A., Siddhanta, A., and Shields, D. (2002). Fragmentation and re-assembly of the Golgi apparatus in vitro. A requirement for phosphatidylinositol 4,5-bisphosphate synthesis. J. Biol. Chem. 277, 3030-3039. [DOI] [PubMed] [Google Scholar]
- Toda, K., Nogami, M., Murakami, K., Kanaho, Y., and Nakayama, K. (1999). Colocalization of phospholipase D1 and GTP-binding-defective mutant of ADP-ribosylation factor 6 to endosomes and lysosomes. FEBS Lett. 442, 221-225. [DOI] [PubMed] [Google Scholar]
- Touyz, R.M., and Schiffrin, E.L. (1999). Ang II-stimulated superoxide production is mediated via phospholipase D in human vascular smooth muscle cells. Hypertension 34, 976-982. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai, M., Alexander, R.W., Akers, M., Lyons, P.R., Lassegue, B., and Griendling, K.K. (1999). Angiotensin II receptor coupling to phospholipase D is mediated by the betagamma subunits of heterotrimeric G proteins in vascular smooth muscle cells. Mol. Pharmacol. 55, 142-149. [DOI] [PubMed] [Google Scholar]
- Vitale, N., Caumont-Primus, A.-S., Chasserot-Golaz, S., Du, G., Wu, S., Sciorra, V.A., Morris, A.J., Frohman, M.A., and Bader, M.-F. (2001). Phospholipase D1: a key factor for the exocytotic machinery in neuroendocrine cells. EMBO J. 20, 2424-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Z., Ho, W.T., and Exton, J.H. (2002). Functional implications of posttranslational modifications of phospholipases D1 and D2. Biochim. Biophys. Acta 1580, 9-21. [DOI] [PubMed] [Google Scholar]
- Zacharias, D.A., Violin, J.D., Newton, A.C., and Tsien, R.Y. (2002). Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296, 913-916. [DOI] [PubMed] [Google Scholar]