Abstract
The TRPM4 channel is a Ca2+-activated, monovalent cation-selective channel of the melastatin transient receptor potential (TRPM) family. The TRPM4 channel is implicated in the regulation of many cellular processes including the immune response, insulin secretion, and pressure-induced vasoconstriction of cerebral arteries. However, the expression and function of the TRPM4 channels in detrusor smooth muscle (DSM) have not yet been explored. Here, we provide the first molecular, electrophysiological, and functional evidence for the presence of TRPM4 channels in rat DSM. We detected the expression of TRPM4 channels at mRNA and protein levels in freshly isolated DSM single cells and DSM tissue using RT-PCR, Western blotting, immunohistochemistry, and immunocytochemistry. 9-Hydroxyphenanthrene (9-phenanthrol), a novel selective inhibitor of TRPM4 channels, was used to examine their role in DSM function. In perforated patch-clamp recordings using freshly isolated rat DSM cells, 9-phenanthrol (30 μM) decreased the spontaneous inward current activity at −70 mV. Real-time DSM live-cell Ca2+ imaging showed that selective inhibition of TRPM4 channels with 9-phenanthrol (30 μM) significantly reduced the intracellular Ca2+ levels. Isometric DSM tension recordings revealed that 9-phenanthrol (0.1–30 μM) significantly inhibited the amplitude, muscle force integral, and frequency of the spontaneous phasic and pharmacologically induced contractions of rat DSM isolated strips. 9-Phenanthrol also decreased the amplitude and muscle force integral of electrical field stimulation-induced contractions. In conclusion, this is the first study to examine the expression and provide evidence for TRPM4 channels as critical regulators of rat DSM excitability and contractility.
Keywords: TRPM4, detrusor smooth muscle, 9-phenanthrol
increased detrusor smooth muscle (DSM) contractility is often associated with overactive bladder (OAB), a pathophysiological condition that affects millions of individuals by disturbing their sleep, work, quality of life, and social interactions (1, 2). The primary pharmacotherapy for OAB is based on antimuscarinics; however, their clinical use is limited by the lack of efficacy and many adverse side effects including dry mouth, tachycardia, ataxia, constipation, and blurred vision (1, 2). Therefore, the current research is directed toward targeting alternative pathways with the potential to reduce DSM contractility and thus identify novel therapeutic targets for the treatment of OAB.
The resting membrane potential and contractility of DSM cells are regulated by various types of K+, Ca2+, and nonselective Na+-permeable cation channels (1, 2, 16, 17, 23, 25, 27, 31). The activation of nonselective cation channels leads to depolarization of the cell membrane, activation of L-type voltage-dependent Ca2+ channels (VDCC), an increase in intracellular Ca2+ concentration, and thus contraction of the DSM. Blockade of these cation channels or activation of K+ channels has the opposite effect, evoking DSM relaxation (25, 31). The depolarized membrane potential or altered DSM excitability contributes to the enhanced contractility by increasing the generation of spontaneous phasic contractions.
The TRPM4 channel is a Ca2+-activated channel of the melastatin transient receptor potential (TRPM) family, and it is highly selective for monovalent cations such as Na+ and K+ but impermeable to anions and divalent cations including Ca2+ (12). The TRPM4 channel contains six transmembrane domains tetramerizing to form a functional channel, with the pore-forming loop and selectivity filter located between transmembrane segments 5 and 6 (12). In many types of cells and tissues, TRPM4 channels play various physiological roles. For example, TRPM4 channels modulate immune responses via T cells (21), insulin secretion via pancreatic β-cells (5), and the myogenic tone of cerebral arteries (7). A recent report has also identified a pathological condition associated with a mutation in the TRPM4 channel in the His-Purkinje system (20).
Since the resting membrane potential in DSM cells is about −40 mV (13, 15, 28), the K+ equilibrium potential is about −85 mV, and the Na+ equilibrium potential is about +70 mV, nonselective ion channels permeable to Na+, perhaps including the TRPM4 channel, contribute a depolarizing influence to the DSM resting membrane potential (24, 25, 31). The physiological significance of TRPM4 channels in the DSM of the urinary bladder, however, has not yet been studied. Recent studies have shown in non-DSM cells that TRPM4 channels are activated by an increase in intracellular Ca2+ and phosphatidylinositol-4, 5-bisphosphate (22, 35). A mechanism for the activation of TRPM4 channels in cerebral artery smooth muscle cells has been proposed in a recent study by Gonzales et al. (8). Using patch-clamp recordings, the authors found that the generation of transient inward cation currents (TICCs) results from the activation of TRPM4 channels by Ca2+ released via inositol triphosphate (IP3) receptors. Activation of TRPM4 channels increases the net influx of Na+, which depolarizes the cell membrane and subsequently activates VDCC (8). We propose that this mechanism may also operate in DSM cells.
In a recent study, it was demonstrated that the hydroxytricyclic compound 9-hydroxyphenanthrene (9-phenanthrol) selectively inhibited human TRPM4 but not TRPM5, its close family member (11). In human embryonic kidney-293 (HEK-293) cells expressing recombinant human TRPM4 channel cDNA, 9-phenanthrol effectively blocked these channels with the potency (IC50 value) of 16.7 ± 4.5 μM under conventional whole cell patch-clamp conditions (11). Furthermore, 9-phenanthrol did not alter the activity of the canonical (C) TRP channels TRPC3, and TRPC6, voltage-gated K+ (KV), large-conductance Ca2+-activated K+ (BK), inwardly rectifying K+ (KIR), or VDCC (10). More importantly these findings showed that TRPM4-dependent cation currents have a primary depolarizing influence in cerebral artery smooth muscle cells. Blockade of the TRPM4 channels by 9-phenanthrol in intact cerebral vessels at physiological intraluminal pressure resulted in substantial hyperpolarization of smooth muscle cell membrane potential (10). These findings confirm that 9-phenanthrol is a selective inhibitor of the TRPM4 channels and can be used as a pharmacological tool to investigate the physiological roles of TRPM4 channels in DSM excitability and contractility.
In recent studies (7–10), the TRPM4 channel has been shown to be an important determinant of pressure-induced vascular smooth muscle cell depolarization and vasoconstriction, thus suggesting a key role for this channel in the control of cerebral blood flow. Since the TRPM4 channels have been previously identified in other types of smooth muscle cells (vascular), we hypothesized that these channels may be expressed and functional in DSM cells as well. Thus the present study was commissioned to explore the expression and potential functional role of the TRPM4 channel in rat DSM excitability and contractility and investigate how it pertains to rat DSM contractility using the TRPM4 channel-selective inhibitor 9-phenanthrol. To achieve this aim, we applied a multidisciplinary approach using molecular biology, immunohistochemistry, immunocytochemistry, live-cell Ca2+ imaging, patch-clamp electrophysiology, and isometric DSM tension recordings of rat DSM.
MATERIALS AND METHODS
Tissue preparation.
Fifty-three male Sprague-Dawley rats, weighing on average 353.0 ± 43.8 g, were used in this study. All animals were kept under standard laboratory conditions (12:12-h light-dark). Rats were euthanized with CO2 followed by a thoracotomy according to the protocol reviewed and approved by the Institutional Animal Care and Use Committee of the University of South Carolina (Animal Use Protocol no. 1747). The urinary bladders were removed and placed in ice-cold Ca2+-free dissection solution (DS). Each bladder was sliced open and rinsed free of urine. The urothelial layer and lamina propria were removed by careful dissection, and only the DSM tissue was used in this study.
DSM single-cell isolation.
Rat DSM single cells were isolated based on the protocol described previously (3, 4, 18). Briefly, small DSM strips (5–10 mm long; 2–4 mm wide) were cut from the rat DSM specimens. Next, two to three DSM strips were incubated in 2 ml of DS supplemented with 1 mg/ml BSA, 1 mg/ml papain (Worthington, Lakewood, NJ), and 1 mg/ml dithiothreitol for 25 min at 37°C. Then, the DSM strips were transferred to prewarmed 2 ml of DS containing 1 mg/ml BSA, 0.5 mg/ml collagenase type II (Sigma-Aldrich, St. Louis, MO), 0.5 mg/ml trypsin inhibitor (Sigma-Aldrich), and 100 μM CaCl2 for 7–14 min at 37°C. Enzyme-treated DSM strips were then washed two to three times with fresh DS containing BSA. Individual cells were released from the DSM tissue by passing the strips through the tip of a Pasteur pipette. Several drops of the isolated DSM cell suspension were placed onto a recording chamber and allowed to adhere to the glass bottom for ∼20 min. Freshly isolated elongated, distinct, bright, and shiny DSM cells, when viewed illuminated under an Axiovert 40CFL microscope (Carl Zeiss, Thornwood, NY), were selected for patch-clamp recordings, live-cell intracellular Ca2+ imaging, single-cell RT-PCR, and immunocytochemistry.
RNA extraction, RT-PCR, and sequencing.
For RT-PCR experiments, mucosa-free whole DSM tissue or freshly isolated, pooled single DSM cells were used as previously described (3, 4). Briefly, freshly isolated DSM cells were left to settle at the bottom of a chamber for ∼5 min and washed several times before individual single cell selection by suction into a glass pipette. Approximately 50–100 DSM single cells were collected and expelled into a 1.5-ml centrifuge tube with RNAlater (Qiagen, Hilden, Germany) and then pelleted at 7,000 g for 5 min. Total RNA was isolated from whole DSM tissue kept in RNAlater, as well as from freshly isolated, pooled single DSM cells using the RNeasy Mini Kit (Qiagen). Extracted RNA was reverse-transcribed into cDNA using Moloney murine leukemia virus reverse transcriptase (Promega) and oligo (dT) primers. The TRPM4 channel specific primer pair sequence was selected based on a previous publication (7) and synthesized by Integrated DNA Technologies (IDT, Coralville, IA). The sense and antisense primers were 5′-GTCATCGTGAGCAAGATGATGAA-3′ and 3′-GTCCACCTTCTGGGACGTGC-5′, respectively. The cDNA produced was PCR amplified using GoTaq Green Master Mix (Promega) and specific primers for the TRPM4 channel. The PCR annealing temperature for the primer pair was optimized using a mastercycler gradient thermocycler (Eppendorf, Hamburg, Germany). Rat prostate tissue was used as a positive control based on a previous report (33). Negative control experiments were performed in the absence of reverse transcriptase (−RT). PCR products were purified using the GenElute PCR Clean-Up Kit (Sigma-Aldrich) and sequenced at the University of South Carolina Environmental Genomics facility for sequence conformation.
Western blotting.
Mucosa-free DSM isolated tissue strips (30–50 mg) were placed in Tris-buffered saline (TBS) and sonicated using a model 100 Dismembrator (Thermo Fisher Scientific, Pittsburgh, PA). The mixture was centrifuged at 10,000 g for 5 min at 4°C. The mixture was then placed in a 37°C water bath for 10 min to enhance phase separation. The supernatant was collected, and the protein concentration was determined using Nano Drop 2000 (Thermo Fisher Scientific). Protein was mixed with 2× Laemmli buffer (1:1) and put on a rotator for 5–10 min to homogenize. The mix was then denatured in a water bath at 100°C for 5 min. Subsequently, equal amounts of protein (∼50 μg) were loaded into adjacent lanes subjected to 4–20% precast SDS-PAGE for 50 min at 150 V (Bio-Rad Mini-PROTEAN Tetra Cell) and transferred to Immobilon-P transfer membranes (Millipore, Bedford, MA) at 100 mA for 50 min using a semidry blot. The membrane was blocked with 5% dry milk/Tris-buffered saline-Tween 20 buffer for 1 h at room temperature. The membrane was incubated with the primary antibody, anti-TRPM4 (1:300 dilution; ab63080, Abcam), overnight at 4°C. The membrane was washed with Tris-buffered saline-Tween-20 three to four times and incubated with goat anti-rabbit IgG conjugated with horseradish peroxidase (1:3,000) in blocking buffer for 1 h at room temperature. Bound antibodies were detected by a Pierce Fast echochemiluminescence substrate kit (Thermo Fisher Scientific) according to the manufacturer's instructions. The antibody specificity was verified by preincubation of the antibody with the respective competing peptide.
Immunohistochemistry and immunocytochemistry.
For immunocytochemical studies, freshly isolated DSM cells were dropped on a poly-l-lysine-coated slide and allowed to settle for 30 min at room temperature. The DSM cells were fixed with 4% paraformaldehyde for 10 min. DSM cells were then washed three times for 15 min each with PBS/0.01 M glycine/0.1% Triton X-100 solution and blocked in 1% BSA/PBS containing 5% normal donkey serum for 10 min. DSM cells were then incubated with the rabbit polyclonal primary antibody, anti-TRPM4 (1:500 dilution; ab63080, Abcam), diluted in 1% BSA/PBS at 37°C for 1 h. After incubation with the primary antibody, cells were rinsed two times for 15 min with 1% BSA in PBS and were again blocked in 1% BSA/PBS containing 5% normal donkey serum for 10 min. DSM cells were then labeled with the secondary antibody [Cy3-conjugated anti-rabbit IgG, at 1:500, 1% BSA/PBS (Jackson ImmunoResearch, West Grove, PA)] overnight in the dark at 4°C. After labeling, cells were washed three times for 15 min with 1% BSA/PBS and then washed with PBS for 15 min. After washing, the cells were incubated with Alexa Fluor 488 phalloidin (diluted in PBS, 1:50) for 2 h in the dark. After incubation with Alexa Fluor 488 phalloidin, DSM cells were then washed two more times with PBS and incubated with 4′,6-diamidino-2-phenylindole (DAPI; diluted in PBS 1:5,000) for 15 min and washed again with PBS. 1,4-Diazabicyclo[2.2.2]octane (DABCO) was placed onto the slides, and the slides were coverslipped. Images at ×63 oil objective were acquired with a LSM 510 META confocal microscope (Carl Zeiss, Göttingen, Germany). The cells for each group were imaged with the same laser power, gain settings, and pinhole for the controls and antibody treatment.
Immunohistochemical experiments were performed using freshly isolated mucosa-free native rat DSM tissue. Rat DSM tissue was sliced into 120-μm-thick sections using a vibratome model G tissue slicer (Oxford Laboratories, Foster City, CA). Sections were then stained with primary antibodies directed toward TRPM4 (1:100; ab63080, Abcam) and α-smooth muscle actin (1:100, ab21027). Nuclear staining was achieved with DAPI. Secondary antibodies were tagged with Cy3-conjugated anti-rabbit IgG at 1:100 (used to stain for TRPM4) and Alexa Fluor 488 donkey anti-goat IgG (Life Technologies, A11055) at 1:100 (used to stain for α-smooth muscle actin).
Electrophysiological (patch-clamp) recordings.
We employed the amphotericin B perforated whole cell configuration of the patch-clamp technique, which preserves the native environment and the signal transduction pathways of the cells (16, 18, 26). Spontaneous inward whole cell currents were recorded using an Axopatch 200B amplifier, Digidata 1440A, and pCLAMP version 10.2 software (Molecular Devices, Union City, CA). An eight-pole Bessel filter 900CT/9L8L (Frequency Devices, Ottawa, IL) was used to filter the recorded currents. The patch-clamp pipettes were prepared from borosilicate glass (Sutter Instruments, Novato, CA) and pulled using a Narishige PP-830 vertical puller (Narishige Group, Tokyo, Japan). The patch-clamp pipettes were coated with dental wax to reduce capacitance and polished with a Micro Forge MF-830 fire polisher to give a final tip resistance of ∼4–6 MΩ. DSM cells were held at −70 mV, and spontaneous inward currents were recorded. At this potential, the activity of BK channels is negligible, and thus we did not detect any transient BK currents (TBKCs) (16, 18). After obtaining a stable perforated patch-clamp recording, measurements were made for at least 5–10 min before and after the addition of 9-phenanthrol. All patch-clamp experiments were conducted at room temperature (22–23°C).
Live-cell Ca2+ imaging.
Measurement of DSM cell intracellular Ca2+ levels was performed as previously described (19). Briefly, suspension of freshly isolated single DSM cells (500 μl) was added into a poly-l-lysine-coated dish and allowed to settle for at least 30 min to adhere to the glass bottom. Fura 2-AM solution was added to the cell suspension at a final concentration of 2 μM and incubated for 30 min in the dark at room temperature. DSM cells were washed three times with a fresh bath solution. Ca2+ imaging was acquired with a ×40/1.35 oil Iris objective and an OLYMPUS IX81 motorized inverted research microscope. The light was generated by a TILL LB LS/30 300 Walt Xenon Arc Lamp and then filtered into monochromatic light (340 and 380 nm) with an emission wavelength of 510 nm. A region without any cells was selected as a background, which was subtracted from the measured values. The elicited light was detected with a digital CCD camera (Hamamatsu Photonics C10600–10B-H), with an exposure time of 200 ms. Image acquisition and hardware control were performed with MetaFluor 7.7.2.0 software (Molecular Devices, Sunnyvale, CA).
Isometric DSM tension recordings.
Isometric DSM tension recordings were conducted as previously described (3, 18, 30). Briefly, small mucosa-free DSM strips (∼2–4 mm wide × 5–10 mm long) were cut from the bladder and transferred to a small petri dish containing Ca2+-free DS. Each strip was attached to an isometric force-displacement transducer and suspended in a 10-ml temperature-controlled (37°C) water bath containing physiological saline solution (PSS) and aerated with 95% O2-5% CO2 (pH 7.4). The DSM strips were initially stretched to 1 g of tension and washed with fresh PSS solution every 15 min during an equilibration period of 45–60 min. After the equilibration period, DSM strips were separated into four experimental groups. The first group consisted of DSM isolated strips that exhibited substantial spontaneous phasic contractions after the initial stretch of tension following the equilibration period. In the second, third, and fourth group, DSM contractions were induced by carbachol (0.1 μM), a cholinergic agonist; by KCl (20 or 60 mM), a depolarizing agent; or by electrical field stimulation (EFS), respectively. To minimize the effects of neurotransmitter release, the DSM strips exhibiting spontaneous phasic contractions, carbachol-induced, 20 or 60 mM KCl-induced contractions were performed in the presence of 1 μM tetrodotoxin (TTX), a selective blocker of the neuronal Na+ channels. The spontaneous, carbachol-induced, and KCl-induced phasic and tonic contractions were allowed to stabilize for ∼30 min. Then increasing concentrations of 9-phenanthrol (0.1–30 μM) were applied at 10-min intervals directly into the bath solution. In the fourth experimental group, nerve-evoked contractions were induced by EFS using a pair of platinum electrodes mounted in the tissue bath in parallel to the DSM strip. The EFS pulses were generated using a PHM-1521 stimulator (MED Associates, Georgia, VT), and the EFS pulse parameters were 0.75-ms pulse width, 20-V pulse amplitude, 3-s stimulus duration, and polarity was reversed for alternating pulses. For EFS studies, after the equilibration period, DSM strips were subjected to either continuous repetitive stimulation with a frequency of 10 Hz at 1-min intervals or increasing frequencies from 0.5 to 50 Hz at 3-min intervals. The contractions were recorded using the Myomed myograph system (MED Associates).
Solutions and drugs.
The Ca2+-free DS had the following composition (in mM): 80 monosodium glutamate, 55 NaCl, 6 KCl, 2 MgCl2, 10 HEPES, and 10 glucose, adjusted to pH 7.3 with NaOH. For DSM contraction studies, the PSS was prepared daily and had the following composition (mM): 119 NaCl, 4.7 KCl, 24 NaHCO3, 1.2 KH2PO4, 2.5 CaCl2, 1.2 MgSO4, and 11 glucose, aerated with 95% O2-5% CO2 (pH 7.4). The extracellular (bath) solution used in the patch-clamp and Ca2+ imaging experiments contained (in mM) 134 NaCl, 6 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES, pH adjusted to 7.4 with NaOH. The patch-clamp pipette solution contained (in mM) 110 potassium aspartate, 30 KCl, 10 NaCl, 1 MgCl2, 10 HEPES, and 0.05 EGTA, pH adjusted to 7.2 with NaOH and supplemented with freshly dissolved (every 1–2 h) 200 μg/ml amphotericin B in DMSO. BSA and amphotericin B were obtained from Thermo Fisher Scientific. All other drugs were obtained from Sigma-Aldrich. 9-Phenanthrol was dissolved daily in DMSO. Carbachol and atropine were dissolved in double distilled water, and TTX was dissolved in citrate buffer. The final concentration of DMSO in the bath solutions did not exceed 0.05%.
Statistical analysis.
MiniAnalysis software (Synaptosoft, Decatur, GA) was used to analyze the DSM contraction parameters. Specifically, the DSM contractile activity was quantified by measuring the average phasic contraction amplitude (the difference between the force-time baseline curve and the maximum peak of the contractions), the frequency (contractions/min), muscle force integral (calculated by integrating the area under the force-time baseline curve), the phasic contraction duration (defined as width of the individual phasic contraction at 50% of the amplitude), and the DSM tone (the difference between the zero line and the force-time baseline curve). Statistical analyses were performed with GraphPad Prism 4.03 software (GraphPad Software, La Jolla, CA) and CorelDraw Graphic Suite × 3 software (Corel) was used to illustrate the data. IC50 values were obtained using a sigmoidal fitting function in GraphPad Prism 4.03 software and are reported as means (95% confidence interval). To compare the phasic contraction parameters for spontaneous, 0.1 μM carbachol-induced, or 20 mM KCl-induced contractions, data were normalized to the last 5 min of the contractions before the addition of the first concentration of 9-phenanthrol (taken to be 100%) and expressed as percentages. For the EFS-induced contractions, the contraction amplitude at EFS frequency of 50 Hz under control conditions was taken to be 100%, and the data were normalized. To evaluate the effect of the cumulative concentrations of 9-phenanthrol (0.1–30 μM), the last 5 min before addition of each concentration to the bath was analyzed. In the electrophysiology experiments, the channel open probability (NPo) values were calculated in the absence and presence of 9-phenanthrol as described previously (9, 10). The spontaneous inward whole cell current activity at −70 mV was calculated as the sum of the NPo values of multiple open states using the build-in single-channel analysis feature of the Clampfit software. The baseline/channel closed state was obtained in the presence of 9-phenanthrol. The single-channel TRPM4 current of 1.75 pA was chosen in the data analysis based on the reported unitary conductance of the TRPM4 channel (25 pS) (21). Data are summarized as means ± SE for n (the number of DSM strips or cells) isolated from N (the number of rats). In Ca2+ imaging experiments, the effects of 9-phenanthrol were analyzed before and at least 10 min after application allowing the levels to reach steady state. Data were compared using a two-way ANOVA followed by a Bonferroni posttest or two-tailed paired Student's t-test. A P value <0.05 was considered statistically significant.
RESULTS
Rat DSM expresses mRNA for TRPM4 channels.
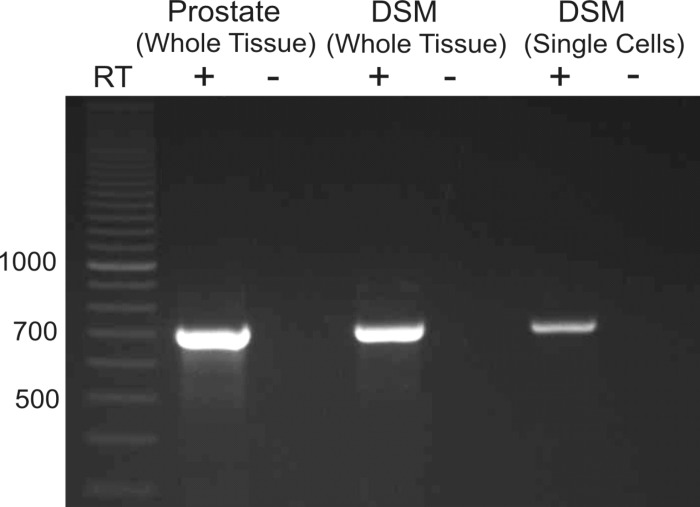
We detected expression of mRNA message for TRPM4 channels in whole DSM tissue (Fig. 1). In whole DSM tissue, detection of mRNA message could come directly from DSM cells or non-DSM cells such as fibroblasts, neuronal cells, and vascular smooth muscle. To investigate the expression of TRPM4 channels in DSM cells directly, RT-PCR experiments were performed in isolated, pooled single DSM cells. The single-cell RT-PCR results confirmed that mRNA message for TRPM4 channels was detected in the DSM cells (Fig. 1). A lack of genomic DNA contamination was confirmed using the negative control reactions lacking RT (Fig. 1).
Fig. 1.
RT-PCR detection of mRNA for the melastatin transient receptor potential channel (TRPM4) (707 bp) in rat detrusor smooth muscle (DSM) whole tissue and pooled single DSM cells. The illustrated gel image is a selected representation of 12 independent RT-PCR experiments based on RNA extracted from 12 rats. Rat prostate was used as a positive control (+). Negative control experiments carried out with omission of RT enzyme (−RT) in reaction mixtures showed no mRNA products.
Rat DSM expresses TRPM4 channel protein.
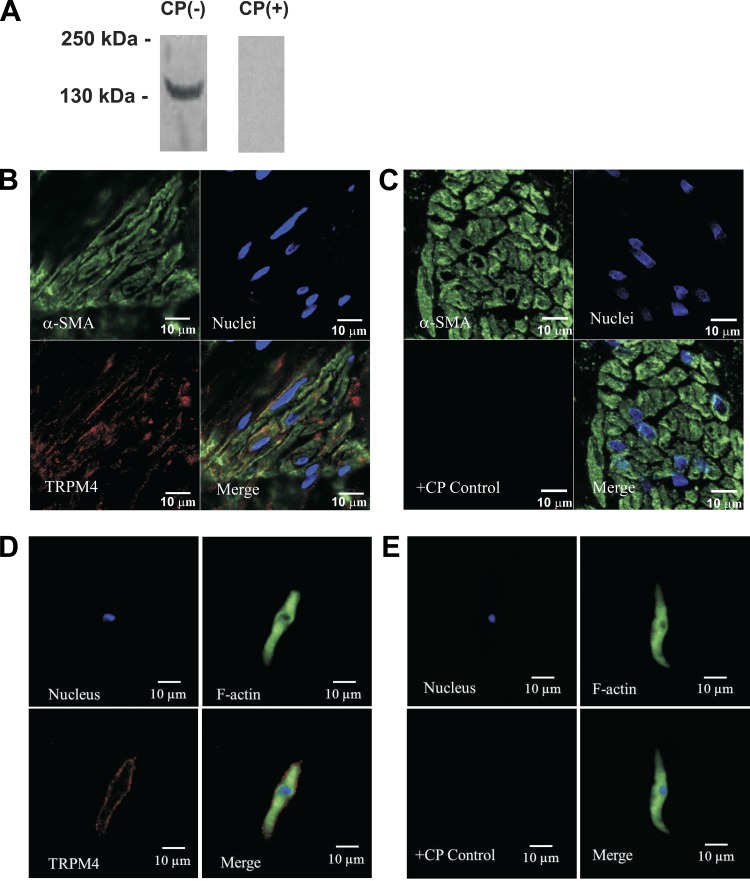
To confirm the presence of TRPM4 channel protein in mucosa-free DSM tissue, we used the Western blot technique and a TRPM4 channel-specific antibody (Fig. 2A). The expected molecular mass of TRPM4 channel protein was ∼134 kDa, consistent with the experimentally determined molecular mass. In control experiments, preabsorption of the primary antibody in the presence of its antigenic competing peptide resulted in the loss of the band, indicating the specificity of the antibody for its intended epitope (Fig. 2A). Thus the Western blot experiments confirmed the presence of TRPM4 channel protein in rat DSM whole tissue. Furthermore, immunohistochemical analysis also confirmed the expression and localization of TRPM4 channel protein in rat DSM whole tissue (Fig. 2B).
Fig. 2.
Western blot, immunohistochemical, and immunocytochemical detection of TRPM4 channel protein in rat DSM tissue and freshly isolated single DSM cells using a TRPM4-specific antibody. A: Western blot detection of TRPM4 channel protein expression in rat DSM tissue. The illustrated image is a representation of 8 independent Western blot experiments based on protein extracted from 8 rats. The lack of an immunoreactive band in the presence of competing peptide (CP) confirmed the specificity of the primary antibody. B: confocal images showing immunohistochemical detection of TRPM4 channel protein expression in rat DSM whole tissue. Red staining (bottom left) indicates the presence of TRPM4 channels. Cell nuclei are shown in blue (top right); α-smooth muscle actin (α-SMA) is shown in green (top left). The merged image (bottom right) illustrates the overlap of all 3 images. D: high-resolution confocal images showing immunocytochemical detection of TRPM4 channel protein expression in a single DSM cell. Red staining (bottom left) indicates detection of TRPM4 channels. Cell nucleus is shown in blue (top left); F-actin is shown in green (top right). The merged image (bottom right) illustrates the overlap of all 3 images. In control experiments, no staining was visible after absorption of the primary antibody with a competing peptide (C and E; +CP control). The merged images (bottom right) illustrate the overlap of all 3 images. The results were verified in 3 separate experiments using DSM whole tissue or multiple DSM cells isolated from 3 rats.
To determine whether isolated single DSM cells express TRPM4 channel protein, immunocytochemistry was carried out with a TRPM4 channel-specific antibody in isolated single DSM cells. As illustrated in Fig. 2D, immunolabeling confirmed the expression of TRPM4 channel protein in freshly isolated DSM cells and demonstrated that TRPM4 channel protein is localized to the plasma membrane. Control treatments were further conducted by preabsorption of the primary antibody with its antigenic competing peptide to verify the specificity of the antibody for its intended epitope and demonstrated no specific staining (Fig. 2, C and E).
Attenuation of spontaneous inward current activity by the selective TRPM4 channel inhibitor 9-phenanthrol in freshly isolated rat DSM cells.
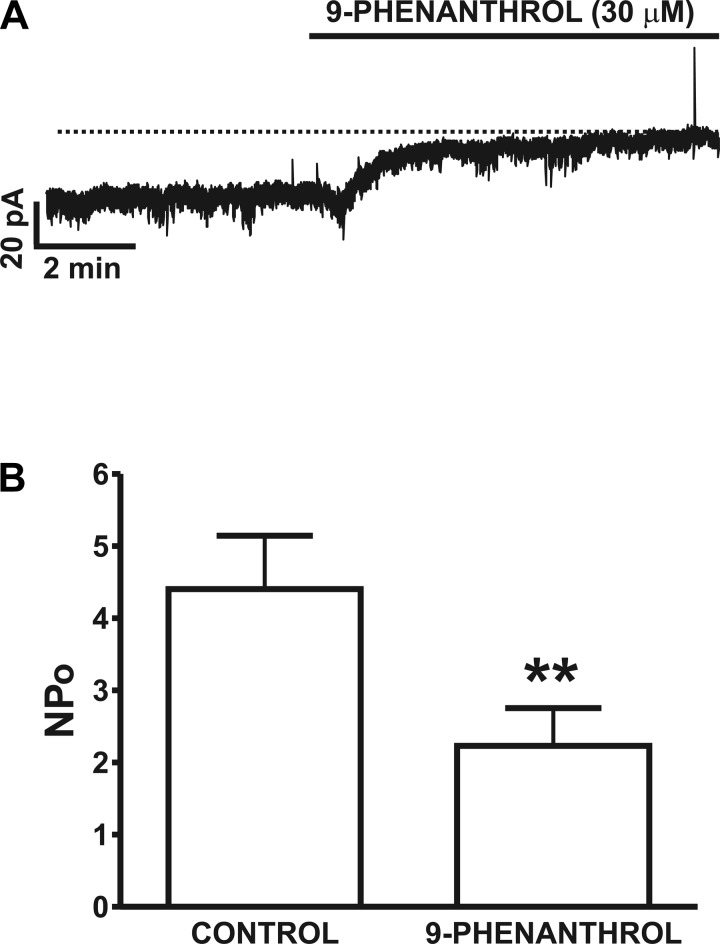
To examine the role of the TRPM4 channel in DSM cell excitability, we conducted electrophysiological studies using the perforated patch-clamp technique and the TRPM4 channel-selective inhibitor 9-phenanthrol. At a holding potential of −70 mV, we observed stable spontaneous basal activity along with numerous TICCs as illustrated in Fig. 3A. This activity is reminiscent of the TRPM4-mediated TICCs recently reported in rat cerebral arterial myocytes (10). We then examined the effect of 9-phenanthrol on the spontaneous inward current in DSM cells. We applied 9-phenanthrol at 30 μM, since this is a threefold higher concentration than its IC50 value identified in recombinant cells (11) or cerebral artery myocytes (10). The addition of 30 μM 9-phenanthrol attenuated the spontaneous inward current activity by ∼50% from an NPo of 4.41 ± 0.7 in the absence of the compound to 2.2 ± 0.5 in its presence (n = 10, N = 6; P < 0.01; Fig. 3B). In the majority of the cells examined including the representative one shown in Fig. 3A, 9-phenanthrol (30 μM) reduced the apparent inward current. This suggests that TRPM4 channels are tonically active under the experimental conditions of this study and are important regulators of rat DSM cell excitability.
Fig. 3.
Inhibition of spontaneous inward current activity by the TRPM4 channel inhibitor 9-phenanthrol in freshly isolated DSM cells. A: original recording of the inhibitory effect of 9-phenanthrol (30 μM) on spontaneous inward current activity recorded at −70 mV using the perforated whole cell patch-clamp technique in a single DSM cell. The dotted line indicates the closed state of the TRPM4 channels. B: summary data for the inhibitory effect of 9-phenanthrol on TRPM4 spontaneous inward current, presented as NPo activity before and after its application at 30 μM (n = 10, N = 6; **P < 0.01).
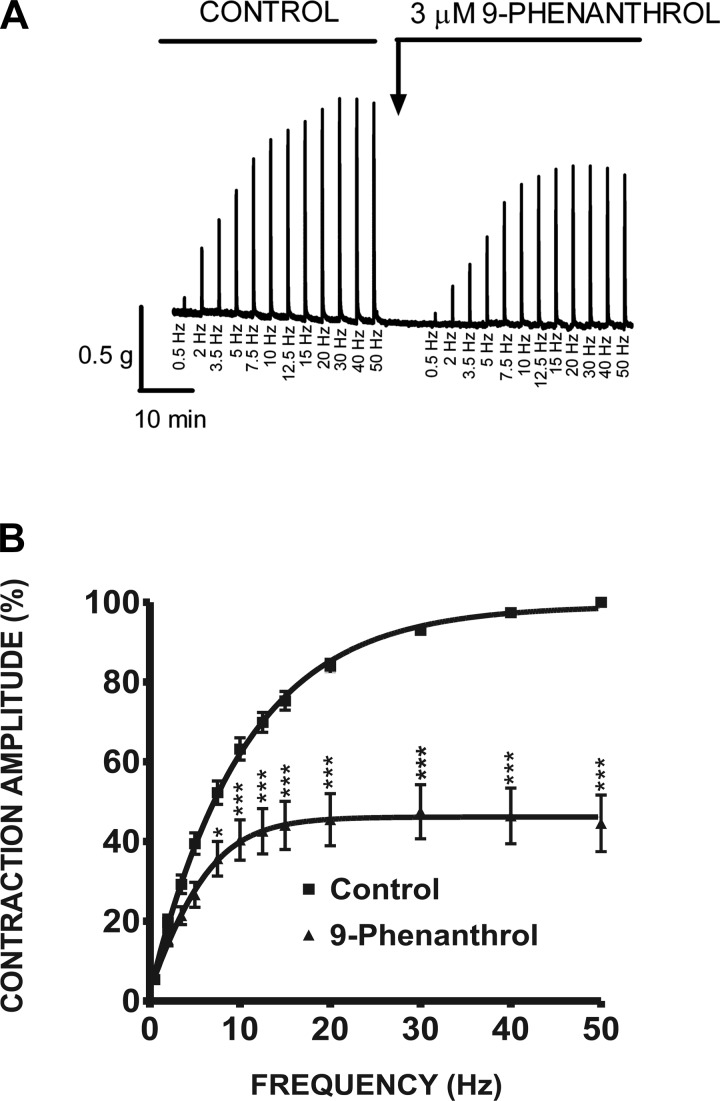
Fig. 9.
Selective TRPM4 inhibition with 9-phenanthrol decreases the amplitude of the 0.5- to 50-Hz EFS-induced contractions of rat DSM isolated strips. A: original myograph recording of EFS-induced contractions (stimulation frequency 0.5–50 Hz) in the absence (control) and 10 min after application of 3 μM 9-phenanthrol. B: frequency-response curves in the presence or absence of 3 μM 9-phenanthrol illustrating a decrease in the amplitude of EFS-induced contractions of rat DSM isolated strips (n = 11, N = 7; *P < 0.05, ***P < 0.005).
Selective inhibition of TRPM4 channels with 9-phenanthrol reduces intracellular Ca2+ levels in freshly isolated rat DSM cells.
To study the effects of TRPM4 channel inhibition with 9-phenanthrol on intracellular Ca2+ levels, real-time live-cell Ca2+ imaging was carried out using the ratiometric dye fura 2. The control resting Ca2+ level was measured for at least 10 min, and then 30 μM 9-phenanthrol was added into the bath solution and Ca2+ levels were recorded for at least 30 additional min until reaching a steady-state level. Under control conditions, the fluorescent ratio (340/380 nm) was 0.996 ± 0.049, and in the presence of 9-phenanthrol the fluorescent ratio (340/380 nm) decreased to 0.607 ± 0.004 (n = 19, N = 6; P < 0.005). The responses of the TRPM4 channel-selective inhibitor 9-phenanthrol on the intracellular Ca2+ levels are likely associated with diminished Ca2+ influx via VDCCs due to decreased DSM membrane excitability.
Inhibition of spontaneous phasic contractions by the TRPM4 channel-selective inhibitor 9-phenanthrol in rat DSM isolated strips.
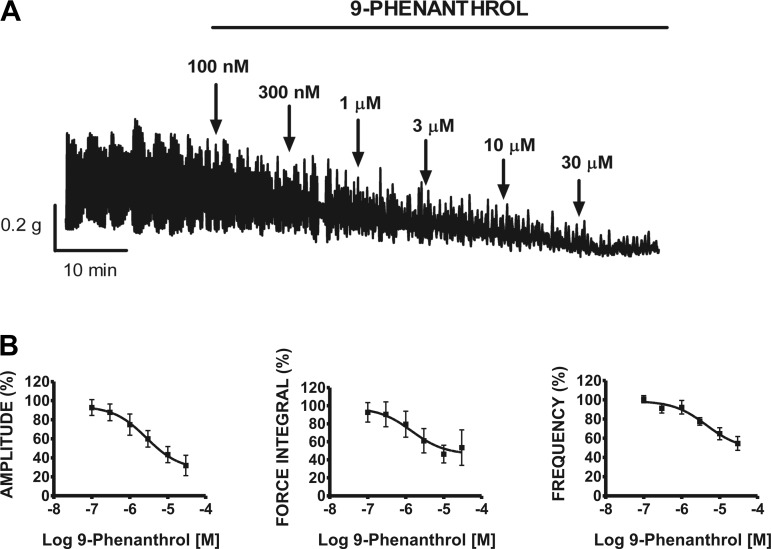
In this experimental series, functional studies were carried out to elucidate the inhibitory effect of the TRPM4 channel blocker 9-phenanthrol on the spontaneous contractile activity of rat DSM isolated strips. After initial spontaneous phasic contractions stabilized (30–60 min), cumulative addition of 9-phenanthrol (0.1–30 μM) to rat DSM isolated strips was carried out as exemplified in Fig. 4A. As illustrated, 9-phenanthrol dramatically decreased the DSM spontaneous contractile activity. The concentration-response curves for the effect of cumulative addition of 9-phenanthrol (0.1–30 μM) on the contractile parameters in rat DSM isolated strips are illustrated in Fig. 4B. 9-Phenanthrol caused a concentration-dependent inhibition of the spontaneous contraction amplitude, muscle force integral, and frequency with IC50 values ranging from 1.4 to 4.6 μM (n = 8, N = 7; Table 1). Control experiments showed that the vehicle (DMSO) had no effect on DSM spontaneous phasic contraction amplitude, muscle force integral, duration, frequency, and tone (Table 2).
Fig. 4.
Cumulative addition of 9-phenanthrol significantly inhibited the spontaneous phasic contraction amplitude, muscle force integral, and frequency of rat DSM isolated strips. A: representative myograph recording obtained from a rat DSM isolated strip showing the effects of 0.1–30 μM 9-phenanthrol on spontaneous phasic contractions. B: cumulative concentration-response curves for 9-phenanthrol (0.1–30 μM) on phasic contraction amplitude, muscle force integral, and frequency on rat DSM isolated strips. Spontaneous contractions were taken to be 100% before the addition of 100 nM 9-phenanthrol (n = 8, N = 7).
Table 1.
IC50 and maximum inhibition values of 9-phenanthrol on DSM phasic contraction parameters under various experimental conditions
| Condition | Spontaneous Phasic Contractions | Carbachol-Induced Phasic Contractions | 20 mM KCl-Induced Phasic Contractions | 10-Hz EFS-Induced Contractions | 10-Hz EFS-Induced Contractions+Atropine (1 μM) |
|---|---|---|---|---|---|
| Phasic contraction amplitude | 2.8 (1.8–4.2) μM 68.1 ± 10.6% | 1.9 (1.0–3.9) μM 86.9 ± 3.9% | 2.3 (0.8–6.7) μM 81.3 ± 2.4% | 2.3 (1.8–2.9) μM 79.97 ± 6.82% | 20.5 (10.1–41.7) μM 66.6 ± 4.0% |
| Muscle force integral | 1.4 (0.2–8.3) μM 45.5 ± 19.7% | 3.3 (1.9–5.8) μM 83.2 ± 4.0% | 3.8 (0.7–20.0) μM 69.0 ± 6.9% | 2.8 (1.5–5.2) μM 77.5 ± 7.9% | 20.8 (10.3–42.2) μM61.0 ± 4.1% |
| Phasic contraction frequency | 4.6 (1.2–17.5) μM 68.1 ± 10.6% | 1.7 (0.7–3.9) μM 63.2 ± 5.9% | 5.5 (1.4–22.0) μM 52.0 ± 7.2% | N/A | N/A |
| DSM tone | No effect | 10.5 ± 18.4% | 1.9 (0.9–3.9) μM 45.8 ± 7.5% | No effect | No effect |
IC50 values are reported as means (95% confidence interval); maximum inhibition values are reported as percentages of means ± SE. DSM, detrusor smooth muscle; EFS, electrical field stimulation; N/A, not applicable.
Table 2.
Lack of DMSO effect on DSM phasic contraction parameters under various experimental conditions
| Condition | Spontaneous Phasic Contractions | Carbachol-Induced Phasic Contractions | 20 mM KCl-Induced Phasic Contractions | 10-Hz EFS-Induced Contractions |
|---|---|---|---|---|
| Phasic contraction amplitude | 96.0 ± 7.4% | 85.0 ± 6.1% | 91.3 ± 5.5% | 102.1 ± 5.0% |
| n = 12, N = 7 | n = 6, N = 5 | n = 10, N = 6 | n = 7, N = 5 | |
| P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | |
| Muscle force integral | 103.3 ± 14.7% | 94.1 ± 7.3% | 89.4 ± 11.4% | 106.6 ± 6.5% |
| n = 12, N = 7 | n = 6, N = 5 | n = 10, N = 6 | n = 7, N = 5 | |
| P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | |
| Phasic contraction duration | 95.3 ± 5.8% | 98.8 ± 6.0% | 97.7 ± 5.5% | 103.1 ± 6.5% |
| n = 12, N = 7 | n = 6, N = 5 | n = 10, N = 6 | n = 7, N = 5 | |
| P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | |
| Phasic contraction frequency | 96.6 ± 3.7% | 96.1 ± 2.6% | 102.1 ± 3.6% | N/A |
| n = 12, N = 7 | n = 6, N = 5 | n = 10, N = 6 | ||
| P > 0.05 | P > 0.05 | P > 0.05 | ||
| DSM tone | 101.1 ± 4.8% | 106.9 ± 4.3% | 96.8 ± 2.6% | 101.4 ± 2.3% |
| n = 12, N =7 | n = 6, N = 5 | n = 10, N = 6 | n = 7, N = 5 | |
| P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 |
Values are means ± SE for n = number of isolated DSM strips and N = number of rats; normalized to the control taken as 100%.
Selective TRPM4 channel inhibition with 9-phenanthrol decreases pharmacologically induced contractions of rat DSM isolated strips.
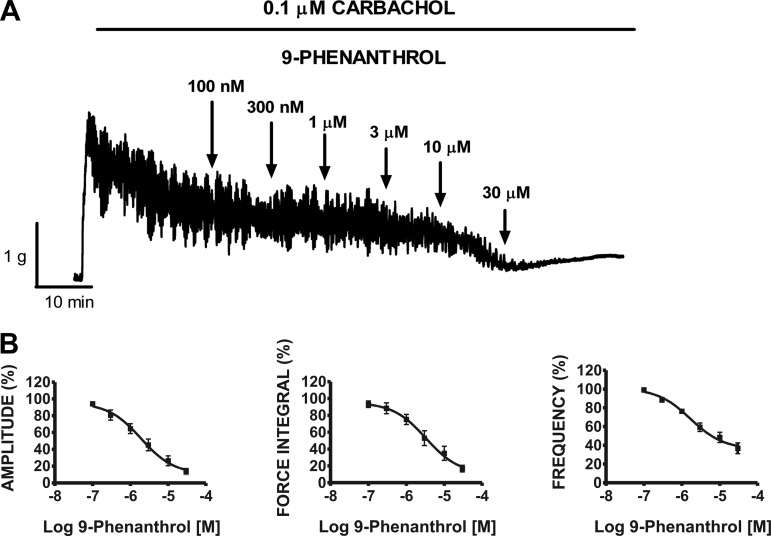
In the next experimental series, we sought to test the effect of cumulative addition of 9-phenanthrol (0.1–30 μM) under conditions of either muscarinic receptor stimulation or sustained membrane depolarization. Carbachol (0.1 μM) rapidly and robustly increased the basal tension and spontaneous contractions of DSM strips (Fig. 5A). Cumulative addition of 9-phenanthrol (0.1–30 μM) significantly decreased the amplitude, muscle force integral, and frequency of the carbachol-induced contractions of DSM isolated strips with IC50 values ranging from 1.7 to 3.3 μM (n = 11, N = 7; Fig. 5B; Table 1). In comparison, carbachol-induced DSM tone was reduced to a lesser extent with a maximum inhibition value of 10.5 ± 18.4% (Table 1). Control experiments showed that the vehicle (DMSO) had no effect on carbachol-induced phasic contraction amplitude, muscle force integral, duration, frequency, and tone (see Table 2).
Fig. 5.
Cumulative addition of 9-phenanthrol significantly inhibited the 0.1 μM carbachol-induced contraction amplitude, muscle force integral, and frequency of rat DSM isolated strips. A: representative myograph recording obtained from a DSM isolated strip showing the effects of 0.1–30 μM 9-phenanthrol on 0.1 μM carbachol-induced phasic and tonic contractions. B: cumulative concentration-response curves for 9-phenanthrol (0.1–30 μM) on 0.1 μM carbachol-induced phasic contraction amplitude, muscle force integral, and frequency on rat DSM strips. Phasic contractions during the 30-min equilibrium period after addition of 0.1 μM carbachol and before the addition of 100 nM 9-phenanthrol were taken to be 100% (n = 11, N = 7).
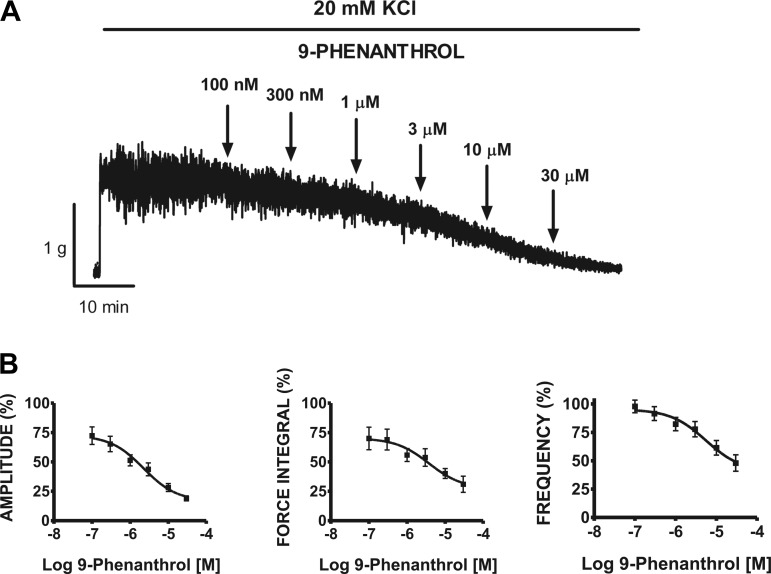
Additionally, we used 20 mM KCl to mildly depolarize rat DSM strips and induce phasic contractions. As illustrated in Fig. 6A, exposure of DSM strips to cumulative addition of 9-phenanthrol (0.1–30 μM) caused a significant inhibition of the 20 mM KCl-induced contractions. Cumulative addition of 9-phenanthrol significantly decreased the amplitude, muscle force integral, and frequency of the 20 mM KCl-induced DSM contractions with IC50 values ranging from 1.9 to 5.5 μM (n = 8, N = 7; Fig. 6B; Table 1). Control experiments showed that the DMSO vehicle at 0.05% had no effect on 20 mM KCl-induced contraction parameters (Table 2).
Fig. 6.
Cumulative addition of 9-phenanthrol significantly inhibited the 20 mM KCl-induced contraction amplitude, muscle force integral, and frequency of rat DSM isolated strips. A: representative myograph recording obtained from a rat DSM strip showing the effects of 0.1–30 μM 9-phenanthrol on 20 mM KCl-induced phasic and tonic contractions. B: cumulative concentration-response curves for 9-phenanthrol (0.1–30 μM) on 20 mM KCl-induced phasic contraction amplitude, muscle force integral, and frequency on rat DSM. Phasic contractions during the 30-min equilibrium period after addition of 20 mM KCl and before the addition of 100 nM 9-phenanthrol were taken to be 100% (n = 8, N = 7).
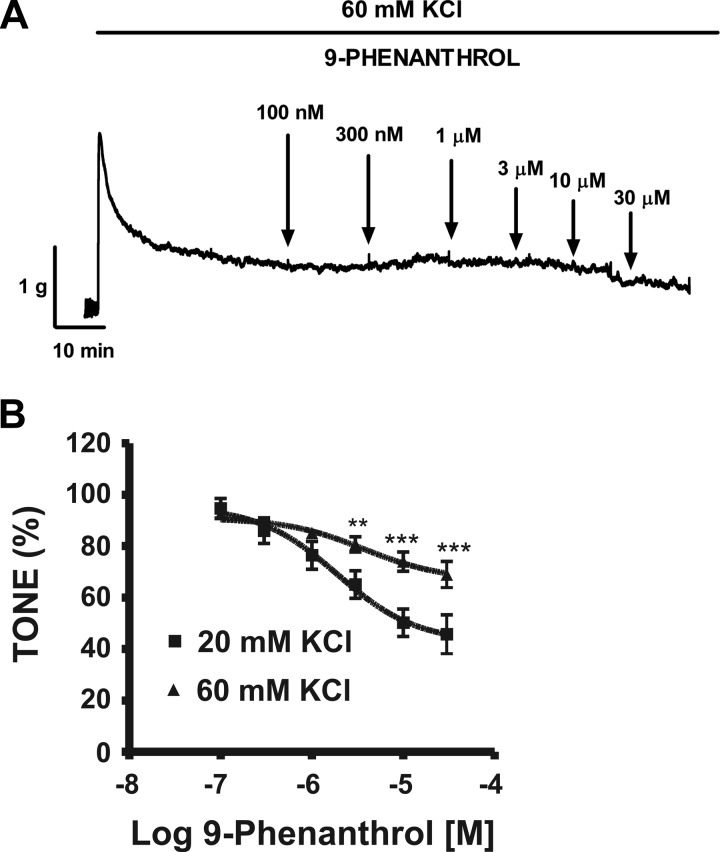
We also tested the effect of 9-phenanthrol on depolarization-mediated DSM contraction elicited by a higher K+ concentration (60 mM KCl). The 60 mM KCl-induced tonic contractions were manifested by an initial peak followed by recovery and stabilization at a steady-state level. The effect of 9-phenanthrol during the steady-state phase of the 60 mM KCl-induced tonic contraction is illustrated in Fig. 7A. In the 20 mM KCl solution, cumulative addition of 9-phenanthrol (0.1–30 μM) significantly inhibited DSM tone to 45.8 ± 7.5% at 30 μM (Fig. 7B; Table 1). However, cumulative addition of 9-phenanthrol (0.1–30 μM) had a substantially reduced inhibitory effect on DSM tone in the presence of the 60 mM KCl solution (n = 12, N = 8; Fig. 7B).
Fig. 7.
Cumulative addition of 9-phenanthrol significantly inhibited the tonic contractions in rat DSM isolated strips A: representative myograph recording obtained from a rat DSM strip showing the effects of 0.1–30 μM 9-phenanthrol on 60 mM KCl-induced tonic contractions. B: cumulative concentration-response curve for the effect of 9-phenanthrol (0.1–30 μM) on the muscle tone in the presence of extracellular 20 or 60 mM KCl in rat DSM. Tonic contractions during the 30-min equilibration period after addition of 20 or 60 mM KCl, respectively, were taken to be 100% (n = 8, N = 7 for the 20 mM KCl and n = 12, N = 8 for the 60 mM KCl responses. **P < 0.01; ***P < 0.005; 20 vs. 60 mM KCl).
Attenuation of nerve-evoked contractions by TRPM4 channel-selective inhibitor 9-phenanthrol in rat DSM isolated strips.
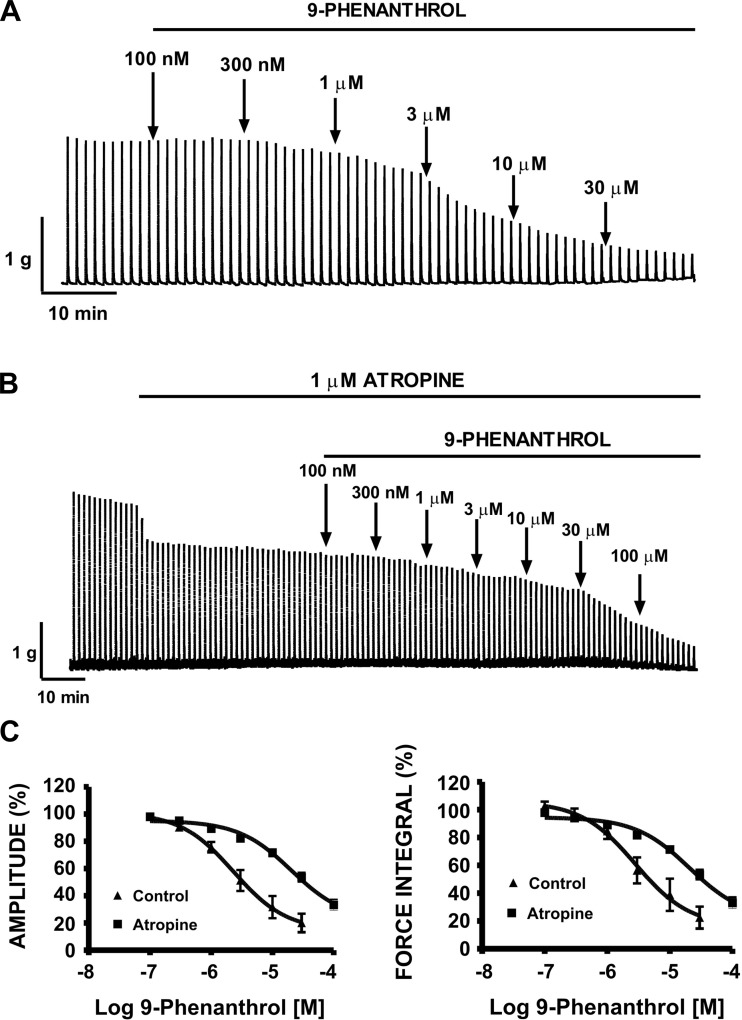
Activation of parasympathetic nerves causes DSM contractions during bladder voiding. In this experimental series, we sought to explore the ability of cumulative addition of 9-phenanthrol (0.1–30 μM) to reduce DSM neurogenic contractions evoked by repetitive EFS of 10-Hz frequency. These experiments were conducted in the absence of TTX. A representative myographic recording in Fig. 8A shows that cumulative addition of 9-phenanthrol (0.1–30 μM) reduced the EFS-induced contractions at a stimulation frequency of 10 Hz in DSM isolated strips. Figure 8C summarizes the mean-concentration responses and demonstrates significant inhibitory effects on nerve-evoked contraction amplitude and muscle force integral at a stimulation frequency of 10 Hz with IC50 values of 2.3 and 2.8 μM, respectively (n = 8, N = 6, Table 1). Control experiments showed that the DMSO vehicle had no effect on 10-Hz EFS-induced contraction amplitude, muscle force integral, duration, and tone (Table 2).
Fig. 8.
Inhibitory effect of cumulative addition of 9-phenanthrol on continuous 10-Hz electrical field stimulation (EFS)-induced contractions of rat DSM isolated strips. A: representative myograph recording obtained from a rat DSM strip showing the effects of 0.1–30 μM 9-phenanthrol on 10-Hz EFS-induced contraction in the absence of atropine. B: representative myograph recording obtained from a rat DSM strip showing the effects of 0.1–100 μM 9-phenanthrol on 10-Hz EFS-induced contractions in the presence of atropine (1 μM). Contractile responses were evoked by repetitive (1/min) EFS (0.75-ms pulse width, 3-s duration, 20-V pulse amplitude). C: cumulative concentration-response curves for the inhibitory effects of 9-phenanthrol on 10-Hz EFS-induced contraction amplitude and muscle force integral of DSM strips in the presence (n = 23, N = 5) or absence of 1 μM atropine (n = 8, N = 6).
In addition, we sought to test the effect of cumulative addition of 9-phenanthrol (0.1–100 μM) on purinergic-mediated DSM contractions. DSM purinergic contractions were evoked by repetitive EFS of 10-Hz frequency in the presence of atropine (1 μM) to block the muscarinic receptors. As illustrated in Fig. 8B, in the presence of atropine, cumulative addition of 9-phenanthrol (0.1–100 μM) had less of an inhibitory effect on purinergic-mediated, nerve-evoked contraction amplitude and muscle force integral at a stimulation frequency of 10 Hz with IC50 values of 20.5 and 20.8 μM, respectively (n = 23, N = 5; Fig. 8C; Table 1). Therefore, 9-phenanthrol is more effective at inhibiting cholinergic than purinergic DSM contractions.
In another group of experiments, DSM isolated strips were stimulated by increasing EFS frequencies (0.5–50 Hz). Following a frequency-response curve under control conditions, DSM strips were incubated for 10 min with 9-phenanthrol (3 μM) and a frequency-response curve in the presence of 9-phenanthrol was generated again. As illustrated in Fig. 9A, 9-phenanthrol significantly decreased the maximum amplitude generated in response to EFS frequencies of 7.5, 10, 12.5, 15, 20, 30, 40, and 50 Hz (n = 11, N = 7; P < 0.05; Fig. 9B). At the highest frequency tested of 50 Hz, addition of 3 μM 9-phenanthrol to the bath solution inhibited the EFS-induced contraction amplitude in rat DSM isolated strips to 45.2 ± 14.7% compared with the control responses (Fig. 9B). This experimental series further suggests that selective inhibition of the TRPM4 channel can reduce nerve-evoked contractions at various stimulation frequencies in rat DSM isolated strips.
DISCUSSION
In this study, we provide evidence that TRPM4 channels are physiologically relevant regulators of rat DSM excitability and contractility. To our knowledge, this is the first study to elucidate the expression and physiological role of TRPM4 channels in rat DSM isolated strips and freshly isolated cells. On the basis of our data obtained from molecular biology, Ca2+ imaging, patch-clamp, and functional studies on DSM contractility, we can draw several important conclusions. First, rat DSM whole tissue and single cells express TRPM4 channel mRNA. Second, TRPM4 channels were identified at the protein level in DSM whole tissue and DSM single cells by Western blotting, immunohistochemistry, and immunocytochemistry. Third, in single DSM cells 9-phenanthrol attenuated both spontaneous inward current activity and intracellular Ca2+ levels. Fourth, the selective TRPM4 channel blocker 9-phenanthrol decreased spontaneous, pharmacologically induced and nerve-evoked contractions in rat DSM isolated strips. Collectively, these observations support the roles of TRPM4 channels in regulating rat DSM excitability and contractility.
To identify the molecular fingerprints of the TRPM4 channel in rat DSM, we applied a combined molecular biology approach. Our RT-PCR experiments revealed mRNA expression of the TRPM4 channel in whole DSM tissue and freshly isolated DSM cells (Fig. 1). Furthermore, the Western blot experiments confirmed the expression of the TRPM4 channel at the protein level (Fig. 2A). Immunohistochemical and immunocytochemical analysis also confirmed the expression of the TRPM4 channel protein in rat DSM tissue and freshly isolated DSM single cells as well as its localization to the plasma membrane (Fig. 2, B–E). A recent study shows that TRPM4 channels are also expressed in the apical membrane of the umbrella cells in bladder urothelium as revealed by RT-PCR, Western blotting, and immunohistochemistry (34). However, the function of the TRPM4 channel in bladder urothelium has yet to be elucidated. In contrast, our study provides clear evidence for a physiological role of TRPM4 channels in the regulation of DSM excitability and contractility.
To address the cellular specificity of the effects of 9-phenanthrol, and thus to elucidate the potential roles of TRPM4 channels at the level of DSM cells directly, we conducted perforated whole cell patch-clamp and Ca2+ imaging experiments in freshly isolated single DSM cells. This series of experiments was important since other cell types within the DSM tissue layer including interstitial cells, neuronal cells, vascular smooth muscle, vascular endothelial, or other cell type may directly or indirectly modulate the DSM contractility (1). Our experiments revealed that 9-phenanthrol at 30 μM reduced the spontaneous inward current activity and intracellular Ca2+ levels in single DSM cells indicating the effects at the level of DSM cells directly. Although our study does not exclude the potential contribution of the TRPM4 channels expressed in non-myocytes within the DSM layer, the observation of modulation of DSM cell function by 9-phenanthrol strongly supports that the inhibition of rat DSM contractility was primarily due to reduced excitability and attenuation of basal intracellular Ca2+ levels in DSM cells. When the TRPM4 channel is blocked by 9-phenanthrol, this reduces the cationic inward current (Fig. 3), hyperpolarizing the membrane potential, thus reducing the entry of Ca2+ via the VDCC into the cell. Therefore, the reduction in intracellular Ca2+ levels in the DSM is thus consistent with the reduction in DSM contractility. The observations of our study are in line with the effects of 9-phenanthrol and a physiological role of the TRPM4 channels in cerebral artery smooth muscle cells (9, 10) and ventricular myocytes (29). In a recent study, 9-phenanthrol was shown to block the TRPM4 channels in rat vascular smooth muscle, resulting in substantial membrane potential hyperpolarization and dilation of arteries exhibiting myogenic tone (10). This report showed that TRPM4 channel currents play a major role in establishing the membrane potential of arterial myocytes and maintaining myogenic tone (10). Under physiological conditions of DSM cell membrane potential being in the range of −40 mV (13, 14, 27), open TRPM4 channels provide an excitatory influence. Thus selective TRPM4 channel inhibition with 9-phenanthrol attenuates this excitatory contribution (Fig. 3), leading to DSM relaxation (Fig. 4). Rat DSM cells, however, responded somewhat differently to 9-phenanthrol (Fig. 3) than did cerebral arterial cells. In the latter cell type, the addition of 9-phenanthrol at 30 μM almost completely inhibited transient inward current activity (10), similar to the observation in recombinant systems (11) and did not affect the basal minimum current indicative of the closed state. In DSM cells, 9-phenanthrol only partially inhibited the responses by ∼50% in NPo (Fig. 3), suggesting that the remaining activity is due to non-TRPM4 channel background current activity. However, in rat DSM cells we observed a robust tonically active 9-phenanthrol-sensitive basal current activity (Fig. 3) much stronger than in cerebral arterial cells. This suggests that DSM cells have a distinct phenotype of TRPM4 channel activity similar to the TRPM4 activity reported for human and monkey colonic myocytes (6).
In the present study, 9-phenanthrol reduced spontaneous phasic contraction amplitude, muscle force, and frequency in a concentration-dependent manner with IC50 values ranging from 1.4 to 4.6 μM, which is consistent with IC50 values recently reported in recombinant and vascular smooth muscle cells (10, 11). This provides strong evidence that the observed effects on rat DSM contractility are mediated by TRPM4 channels and therefore may represent an important pharmacological target for reducing DSM myogenic contractions. To reveal the effect of TRPM4 channel inhibition on cholinergic DSM contractions, we stimulated muscarinic receptors directly using carbachol, a chemically stable cholinergic agonist that is resistant to acetylcholinesterase activity. Our data demonstrate that 9-phenanthrol reduced carbachol-induced contraction amplitude, force integral, and frequency (Fig. 5), suggesting that the activity of Ca2+-activated TRPM4 channels works to facilitate cholinergic neurotransmission. In addition to cholinergic input, purinergic signaling pathways have also been reported to play an important role in DSM contractility. Our results show that in the presence of atropine, 9-phenanthrol inhibited the amplitude and muscle force integral of nerve-evoked contractions (EFS of 10-Hz frequency) with IC50 values of ∼20 μM (Fig. 8; Table 1). This demonstrates that 9-phenanthrol caused preferential (∼10-fold) inhibition of cholinergic- vs. purinergic-mediated DSM contractions. Since the selective inhibition of the TRPM4 channels with 9-phenanthrol produces an inhibitory effect on nerve-evoked contractions, we conclude that the TRPM4 channels could be novel pharmacological targets for neurogenic OAB.
Furthermore, 9-phenanthrol reduced 20 mM KCl-induced contraction amplitude, force integral, frequency, and tone, suggesting that TRPM4 channel inhibition can reduce contractility under conditions of sustained membrane depolarization (Fig. 6). Since OAB and/or DO are thought to be associated with altered myogenic contractility (1, 2), the observation of reduced contractility under this condition has a potential translational utility for idiopathic OAB.
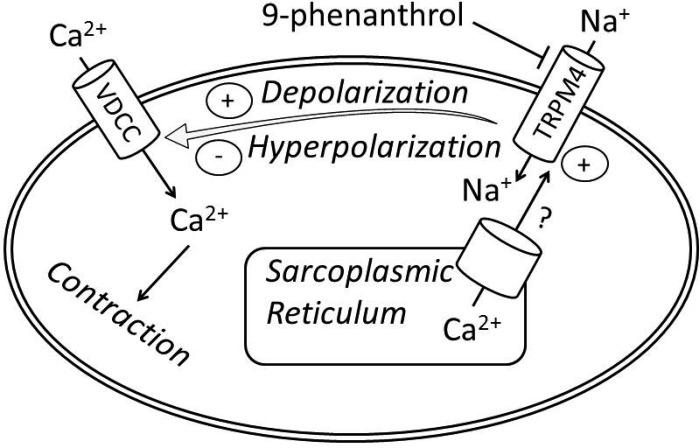
To further investigate the mechanism of TRPM4 channel inhibition in decreasing DSM contractility, DSM strips were precontracted under conditions of moderately (20 mM K+) or highly elevated extracellular K+ (60 mM K+; Figs. 6 and 7). The elevated extracellular K+ concentration raises the K+ equilibrium potential to a more positive value; thus the cell membrane becomes more depolarized. Our experiments revealed that increasing K+ concentration to 60 mM reduced 9-phenanthrol's inhibitory effect on DSM tone, indicating that the effect depends on the level of the resting membrane potential (Fig. 7B). These experiments further provide evidence that the effects of 9-phenanthrol on DSM contractility were not mediated by other mechanisms such as the VDCC and the myosin light chain kinase (MLCK) pathway on DSM contractility (32). Based on our DSM contraction and electrophysiology data, we postulate that upon activation of the TRPM4 channel by intracellular Ca2+, the TRPM4 channel induces the entry of Na+ into the cell, leading to membrane depolarization and subsequent activation of the VDCC, which causes an influx of Ca2+ into the cell, increasing the intracellular Ca2+ level and resulting in DSM contraction (Fig. 10). Previous reports have shown that Ca2+ release from the sarcoplasmic reticulum activates the TRPM4 channels in vascular smooth muscle; however, additional studies are needed to confirm this observation in DSM (8). It is possible that Ca2+ influx via VDCC also directly or indirectly activates TRPM4 channels in DSM cells.
Fig. 10.
Schematic illustration of the proposed physiological role for TRPM4 channels in rat DSM cells. According to the postulated mechanism, the TRPM4 channels via sarcoplasmic reticulum Ca2+-dependent activation participate in a positive feedback loop to maximize DSM contractility by providing Na+-depolarizing conductance. Selective pharmacological inhibition of the TRPM4 channel with 9-phenanthrol would cause membrane hyperpolarization in DSM cells, leading to blockade of VDCC and thus reduction in DSM contractility.
In conclusion, this is the first study to identify the expression and to address the functional role of TRPM4 channels in rat DSM. We identified TRPM4 channel expression in mucosa-free whole DSM tissue and isolated single DSM cells at mRNA and protein levels. We showed that the selective TRPM4 channel inhibitor 9-phenanthrol reduced the spontaneous TRPM4 current activity, decreased the level of global intracellular Ca2+, and inhibited myogenic, pharmacologically induced, and nerve-evoked contractions in rat DSM. Thus our data provide strong evidence that TRPM4 channels are fundamental regulators of DSM excitation-contraction coupling and may represent an attractive therapeutic target for the treatment of OAB.
GRANTS
This work was supported by National Institutes of Health Grant DK-084284 and University of South Carolina Research Foundation ASPIRE-I A033 grants to G. V. Petkov.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.C.S., S.P.P., K.L.H., Q.C., W.X., J.M., and G.V.P. performed experiments; A.C.S., S.P.P., K.L.H., Q.C., W.X., J.M., and G.V.P. analyzed data; A.C.S., S.P.P., K.L.H., Q.C., S.E., W.X., J.M., and G.V.P. interpreted results of experiments; A.C.S., S.P.P., K.L.H., and G.V.P. prepared figures; A.C.S., S.P.P., K.L.H., Q.C., R.P.S., S.A.A., S.E., W.X., J.M., and G.V.P. edited and revised manuscript; A.C.S., S.P.P., K.L.H., Q.C., R.P.S., S.A.A., S.E., W.X., J.M., and G.V.P. approved final version of manuscript; S.E., J.M., and G.V.P. provided conception and design of research; A.C.S., J.M. and G.V.P. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Robert Price for knowledge and guidance in obtaining the confocal images for the immunocytochemical data, Dr. Matthias Werner for conducting some of the initial feasibility experiments, and Ning Li for a critical evaluation of the manuscript.
REFERENCES
- 1. Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84: 935– 986, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev 56: 581– 631, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Chen M, Kellett WF, Petkov GV. Voltage-gated K+ channels sensitive to stromatoxin-1 regulate myogenic and neurogenic contractions of rat urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 299: R177– R184, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen M, Petkov GV. Identification of large conductance calcium activated potassium channel accessory beta4 subunit in rat and mouse bladder smooth muscle. J Urol 182: 374– 381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng H, Beck A, Launay P, Gross SA, Stokes AJ, Kinet JP, Fleig A, Penner R. TRPM4 controls insulin secretion in pancreatic beta-cells. Cell Calcium 41: 51– 61, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dwyer L, Rhee PL, Lowe V, Zheng H, Peri L, Ro S, Sanders KM, Koh SD. Basally activated nonselective cation currents regulate the resting membrane potential in human and monkey colonic smooth muscle. Am J Physiol Gastrointest Liver Physiol 301: G287– G296, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res 95: 922– 929, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Gonzales AL, Amberg GC, Earley S. Ca2+ release from the sarcoplasmic reticulum is required for sustained TRPM4 activity in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 299: C279– C288, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gonzales AL, Earley S. Endogenous cytosolic Ca2+ buffering is necessary for TRPM4 activity in cerebral artery smooth muscle cells. Cell Calcium 51: 82– 93, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gonzales AL, Garcia ZI, Amberg GC, Earley S. Pharmacological inhibition of TRPM4 hyperpolarizes vascular smooth muscle. Am J Physiol Cell Physiol 299: C1195– C1202, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grand T, Demion M, Norez C, Mettey Y, Launay P, Becq F, Bois P, Guinamard R. 9-Phenanthrol inhibits human TRPM4 but not TRPM5 cationic channels. Br J Pharmacol 153: 1697– 1705, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guinamard R, Demion M, Launay P. Physiological roles of the TRPM4 channel extracted from background currents. Physiology (Bethesda) 25: 155– 164, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Hashitani H, Brading AF. Electrical properties of detrusor smooth muscles from the pig and human urinary bladder. Br J Pharmacol 140: 146– 158, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heppner C, Kester MB, Agarwal SK, Debelenko LV, Emmert-Buck MR, Guru SC, Manickam P, Olufemi SE, Skarulis MC, Doppman JL, Alexander RH, Kim YS, Saggar SK, Lubensky IA, Zhuang Z, Liotta LA, Chandrasekharappa SC, Collins FS, Spiegel AM, Burns AL, Marx SJ. Somatic mutation of the MEN1 gene in parathyroid tumours. Nat Genet 16: 375– 378, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol Cell Physiol 273: C110– C117, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Hristov KL, Chen M, Kellett WF, Rovner ES, Petkov GV. Large-conductance voltage- and Ca2+-activated K+ channels regulate human detrusor smooth muscle function. Am J Physiol Cell Physiol 301: C903– C912, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hristov KL, Chen M, Soder RP, Parajuli SP, Cheng Q, Kellett WF, Petkov GV. KV2.1 and electrically silent KV channel subunits control excitability and contractility of guinea pig detrusor smooth muscle. Am J Physiol Cell Physiol 302: C360– C372, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hristov KL, Cui X, Brown SM, Liu L, Kellett WF, Petkov GV. Stimulation of β3-adrenoceptors relaxes rat urinary bladder smooth muscle via activation of the large-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 295: C1344– C1353, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hristov KL, Parajuli SP, Soder RP, Cheng Q, Rovner ES, Petkov GV. Suppression of human detrusor smooth muscle excitability and contractility via pharmacological activation of large conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 302: C1632– C1641, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kruse M, Schulze-Bahr E, Corfield V, Beckmann A, Stallmeyer B, Kurtbay G, Ohmert I, Brink P, Pongs O. Impaired endocytosis of the ion channel TRPM4 is associated with human progressive familial heart block type I. J Clin Invest 119: 2737– 2744, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 109: 397– 407, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Nilius B, Mahieu F, Prenen J, Janssens A, Owsianik G, Vennekens R, Voets T. The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J 25: 467– 478, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parajuli SP, Soder RP, Hristov KL, Petkov GV. Pharmacological activation of small conductance calcium-activated potassium channels with naphtho[1,2-d]thiazol-2-ylamine decreases guinea pig detrusor smooth muscle excitability and contractility. J Pharmacol Exp Ther 340: 114– 123, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Petkov GV. Ion channels. In: Pharmacology: Principles and Practice, edited by Hacker M, Messer W, Bachmann K. New York: Elsevier, 2009, chapt. 16, p. 382–425 [Google Scholar]
- 25. Petkov GV. Role of potassium ion channels in detrusor smooth muscle function and dysfunction. Nat Rev Urol 9: 30– 40, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petkov GV, Balemba OB, Nelson MT, Mawe GM. Identification of a spontaneously active, Na+-permeable channel in guinea pig gallbladder smooth muscle. Am J Physiol Gastrointest Liver Physiol 289: G501– G507, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol 537: 443– 452, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Petkov GV, Heppner TJ, Bonev AD, Herrera GM, Nelson MT. Low levels of KATP channel activation decrease excitability and contractility of urinary bladder. Am J Physiol Regul Integr Comp Physiol 280: R1427– R1433, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Simard C, Salle L, Rouet R, Guinamard R. Transient receptor potential melastatin 4 inhibitor 9-phenanthrol abolishes arrhythmias induced by hypoxia and re-oxygenation in mouse ventricle. Br J Pharmacol 165: 2354– 2364, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Soder RP, Petkov GV. Large conductance Ca2+-activated K+ channel activation with NS1619 decreases myogenic and neurogenic contractions of rat detrusor smooth muscle. Eur J Pharmacol 670: 252– 259, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thorneloe KS, Nelson MT. Properties of a tonically active, sodium-permeable current in mouse urinary bladder smooth muscle. Am J Physiol Cell Physiol 286: C1246– C1257, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Wang BH, Ternai B, Polya GM. Specific inhibition of cyclic AMP-dependent protein kinase by the antimalarial halofantrine and by related phenanthrenes. Biol Chem Hoppe Seyler 375: 527– 535, 1994 [DOI] [PubMed] [Google Scholar]
- 33. Wang HP, Pu XY, Wang XH. Distribution profiles of transient receptor potential melastatin-related and vanilloid-related channels in prostatic tissue in rat. Asian J Androl 9: 634– 640, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Yu W, Hill WG, Apodaca G, Zeidel ML. Expression and distribution of transient receptor potential (TRP) channels in bladder epithelium. Am J Physiol Renal Physiol 300: F49– F59, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang Z, Okawa H, Wang Y, Liman ER. Phosphatidylinositol 4,5-bisphosphate rescues TRPM4 channels from desensitization. J Biol Chem 280: 39185– 39192, 2005 [DOI] [PubMed] [Google Scholar]