Abstract
Cytomegalovirus (CMV) infection exerts an enormous effect on human immunity, as it is associated with an immune-impaired response, a variety of chronic diseases, and overall survival in elderly individuals. Levels of anti-CMV antibodies may be associated with the differentiation degree of T cell subsets. Titers are significantly higher in the elderly and positively correlated with specific CD4+ T cell responses to CMV. In the elderly, antibody titers are associated with the degree of differentiation and the T cell receptor excision circle (TREC) content in CD4+ T cells, with other features of the immune risk profile, and with a reduced ability to respond to immunization in vivo. Associations may be absent in young subjects because their anti-CMV antibody titers are lower than those of the elderly. However, comparing young and elderly individuals with similar antibody levels reveals differences in their highly differentiated and naïve T cells. These are more marked in individuals with high titers. In parallel with the increase in anti-CMV antibodies, the elderly experience a significant reduction in absolute counts of naïve CD4+ T cells, which may be a strategy to compensate for the expansion of differentiated cells and to avoid an increase in total T cells. In summary, our results show that titers of anti-CMV antibodies, and not only CMV seropositivity, are related to differentiation status and immunocompetence in the elderly, making this as an important prognostic marker of the status of immune system function.
INTRODUCTION
The betaherpesvirus cytomegalovirus (CMV) is a persistent activating virus that primarily resides in the myeloid cell compartment but also spreads to other cell types. Clinically, infection is considered essentially asymptomatic in immunocompetent hosts. However, immunosuppressed individuals may suffer serious consequences as a result of viral reemergence. After infection, the virus establishes lifelong latency within the host and periodically reactivates. Reactivation from latency is a key step in the pathogenesis of the infection and can be detected in response to inflammation, infection, stress, or immunosuppression (1, 2). Activation of protein kinase C and NF-κB by tumor necrosis factor alpha (TNF-α) and increasing concentrations of cyclic AMP by stress hormones and prostaglandins promote viral reactivation and replication. Reactivation of CMV is more frequent in the elderly, as demonstrated by the direct detection of CMV DNA in the urine. Although reactivation is frequent in the elderly, it is subclinical in nature (3).
Recently, CMV has been linked to a variety of chronic diseases with an inflammatory component, including cardiovascular diseases, cancer, and cognitive and functional impairment (4–7). The specific mechanisms responsible for these associations have not been fully determined but are likely to have an inflammatory and immune component. Reactivation may result in increased levels of proinflammatory molecules such as interleukin 6 (IL-6), TNF-α, and C-reactive protein (CRP). In fact, CMV has been associated with a significantly greater risk of all-cause mortality and cardiovascular disease-related mortality in subjects with high CRP levels (8). Longitudinal aging studies suggest that CMV seropositivity is associated with a cluster of immune parameters, the “immune risk profile” (IRP), which predicts 2-year mortality in a population-based sample of Swedish octogenarians (9). Moreover, CMV seropositivity is also associated with the phenomenon of immunosenescence, which is characterized by clonal expansion of T cells, mainly CD8+ T cells, with a highly differentiated cell surface phenotype and with the acquisition of new functional features. Moreover, the hallmark of human immunosenescence, irrespective of its association with CMV, is low numbers of naïve T cells, particularly CD8+ T cells, and a corresponding lower diversity of the T cell receptor (TCR) repertoire (10, 11). This has been proposed as an explanation for much of the decreased ability of the elderly to resist new infections and to respond effectively to reinfection and persistent infections (12). Indeed, despite the evidence suggesting that CMV induces aging of T lymphocytes, more frequent and/or intensive reactivations in the elderly may be a consequence rather than the cause of immunosenescence. A general result of these alterations is that adaptive immunity seems to weaken with age, whereas innate immunity is maintained or even enhanced. This may be one of several explanations for the commonly reported increase in proinflammatory status in the elderly, which itself is associated with frailty and mortality (13).
The frequency of CMV seropositivity in the elderly in developed countries is above 80%. In spite of the enormous impact of CMV infection on human immunity, the degree of immunosenescence varies greatly, as is apparent even when only CMV-infected individuals are studied (14). We postulate that the humoral response to CMV may be a consequence of deleterious changes in the immune system and may be related to other changes induced in the immune response, rather than merely infection per se. To investigate this, we studied the association between anti-CMV antibody titers and various characteristics that define the degree of differentiation and the functional abilities of the T cell fraction.
MATERIALS AND METHODS
Study population.
Blood samples for hematological and immunological analyses were obtained from 92 healthy elderly donors (70 females and 22 males) living in the Santa Teresa nursing home (Oviedo, Spain). To ensure that a representative sample of the population was studied, volunteers were not rigorously selected with respect to their health status. Exclusion criteria were conditions with a possible influence on the immune system, such as recent or current infection, inflammation, autoimmune or malignant disease, malnutrition, abnormal laboratory data, and pharmacological interference (steroids, nonsteroidal anti-inflammatory agents, and immunosuppressive drugs). Samples from 70 young healthy controls (24 females and 46 males) were obtained from the Centro de Transfusiones del Principado de Asturias (Oviedo, Spain). Inclusion criteria were a minimum age of 65 years for the elderly group and a maximum age of 50 for the young group. The mean age of the elderly individuals was 85.1 ± 6.3 years (range, 68 to 97 years), and that of the young controls was 38.7 ± 8.4 years (range, 20 to 50 years).
Informed consent was obtained from patients and controls before they participated in the study. The Hospital Universitario Central de Asturias Ethics Committee (Oviedo, Spain) gave ethical approval for the study.
CMV and influenza virus serology.
Levels of CMV-specific immunoglobulin G (IgG) antibodies were determined by the enzyme-linked immunosorbent Vir-ELISA anti-CMV IgG assay (Viro-Immun Labor-Diagnostika GmbH, Oberursel, Germany), carried out in accordance with the manufacturer's specifications.
Anti-influenza virus antibodies in serum obtained from elderly individuals were measured by enzyme-linked immunosorbent assay (ELISA) as previously described (15). The optical density (OD) values of individual samples were compared against a calibration curve comprising the OD values of serial dilutions of the same internal control serum from a healthy vaccinated young person throughout the experiments. Results are expressed as arbitrary units. The antibody titer decreased significantly with time since vaccination, so these values were normalized by adjusting the titer by the time elapsed since immunization.
Hematological analysis and immunological phenotyping.
Hematological parameters were determined with a Sysmex XT-2000i analyzer (Sysmex, Hamburg-Norderstedt, Germany). The results of the cytometric studies were analyzed using a FACSCalibur cytometer with CellQuest software (BD Biosciences, San Jose, CA). EDTA-treated peripheral blood was surface stained with the following antibodies (antibody labels are in parentheses): anti-CD8 (phycoerythrin [PE]), anti-CD45RA (fluorescein isothiocyanate [FITC]) (Immunostep, Salamanca, Spain), anti-CD4 (peridinin chlorophyll protein [PerCP]), anti-CCR7 (Alexa Fluor 647), anti-CD3 (FITC) (BD Biosciences), and anti-CD28 (PE) (eBioscience, San Diego, CA). One hundred microliters of whole blood from elderly individuals was stained with different combinations of labeled monoclonal antibodies (MAbs) for 20 min at room temperature. Samples were red-blood lysed with FACS lysing solution (BD Biosciences), washed in phosphate-buffered saline (PBS), and analyzed with CellQuest software in the FACSCalibur cytometer. Appropriate isotype control MAbs were used for marker settings. In the study of the parameters associated with immunosenescence, staining was performed with anti-CD3 (FITC), anti-CD28 (PE), anti-CD4 (allophycocyanin [APC]) (eBioscience), and anti-CD8 (PerCP). Frequencies of CD4+ and CD8+ T cells were quantified into gated CD3+ T cells, and those of CD8+ CD28null and CD4+ CD28null cells were gated into CD3+ CD8+ and CD3+ CD4+ T cells, respectively.
Activation studies.
CMV-infected cell lysate was prepared by infecting human embryonic lung fibroblasts with the AD169 strain of CMV, and viral titers in the supernatant were determined by standard plaque assays (viral stock, 105 PFU/ml). The virus was inactivated by five freeze-thaw cycles.
Activation of T lymphocytes from heparinized whole blood by anti-CD3 or CMV extract was assessed by surface staining with anti-CD69 (eBioscience). Briefly, heparinized whole blood (250 μl) was stimulated with soluble anti-CD3 (10 ng/ml) (eBioscience) or with the CMV supernatant (dilution, 104 PFU/ml) in 15-ml conical polypropylene tubes for 18 h at 37°C in an atmosphere of 5% carbon dioxide. After stimulation, cells were stained with anti-CD69 and anti-CD4 monoclonal antibodies.
Proliferation cultures.
To determine the proliferation status of CD4+ T cells, peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation on Ficoll-Hypaque gradients (Lymphoprep; Nycomed, Oslo, Norway). PBMCs were resuspended in PBS at a final concentration of 5 × 106 to 10 × 106 cells/ml and incubated with 1.5 μM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen, Paisley, United Kingdom) for 10 min at 37°C, washed with RPMI 1640 medium containing 2 × 103 M l-glutamine and HEPES (BioWhittaker, Verviers, Belgium) twice, and cultured at 1 × 106 cells/ml in the presence of soluble anti-CD3 (10 ng/ml) and CMV extract (104 PFU/ml). The proliferative responses of CD4+ T cells were analyzed on day 7 using a FACSCalibur after staining with anti-CD4.
TREC quantification.
DNA was extracted from isolated CD4+ T cells (>90% purity) from PBMCs using a QIAamp DNA minikit (Qiagen GmbH, Hilden, Germany), according to the manufacturer's instructions. To isolate CD4+ T cells, PBMCs were isolated by centrifugation on Ficoll-Hypaque gradients (Lymphoprep) after 20 min of incubation with RosetteSep human CD4+ T cell enrichment cocktail (StemCell Technologies, Grenoble, France). Signal-joint TREC was quantified by SYBR green real-time quantitative PCR and an iCycler thermocycler (Bio-Rad; Life Science Research Group, Hercules, CA), as previously described (16). Experimental samples were run in duplicate, and the replicate average was used as the sample result.
HLA-DRB1 typing.
HLA-DRB1 alleles were typed, in accordance with the manufacturer's protocols, using Lifecodes HLA-SSO typing kits (Tepnel Lifecodes Corporation, Stamford, CT) based on the Luminex Corporation's X-Map technology.
TNF-α promoter polymorphism genotyping.
Single nucleotide polymorphisms (SNPs) at position −308 of the TNF-α gene were determined by analyzing the melting temperature (Tm) of the probe/target duplex after PCR amplification and hybridization with fluorescently labeled probes matched with one sequence variant (LightCycler; Roche Diagnostics, Mannheim, Germany). The primers used were CCTGCATCCTGTCTGGAAGTTA and CTGCACCTTCTGTCTCGGTTT. The hybridization probes (designed by TIB Molbiol, Berlin, Germany) used were AACCCCGTCCCCATGCCCC-F and LC Red 640-CCAAACCTATTGCCTCCATTTCTTTTGGGGAC.
Cytokine quantification.
Cytokine levels were measured in the sera of elderly subjects using X-Map technology with a Milliplex map kit (Millipore, Billerica, MA), according to the manufacturer's specifications. This bead-based analyte detection system was used to quantify human TNF-α and IL-15 using the Luminex Corporation's X-Map technology.
Statistical analysis.
Groups were compared by the nonparametric Mann-Whitney U and Kruskal-Wallis tests (for non-normally distributed data) or by Student's independent-samples t test (for normally distributed data). Correlations between variables were assessed by the nonparametric Spearman rho test. The χ2 test was used to compare dichotomous variables, and multiple linear regression was used to consider variables simultaneously. Analyses were performed using SPSS 15.0 (SPSS Inc., Chicago, IL). Significance was concluded for P values of <0.05.
RESULTS
CMV seropositivity and antibody titers in young and elderly people.
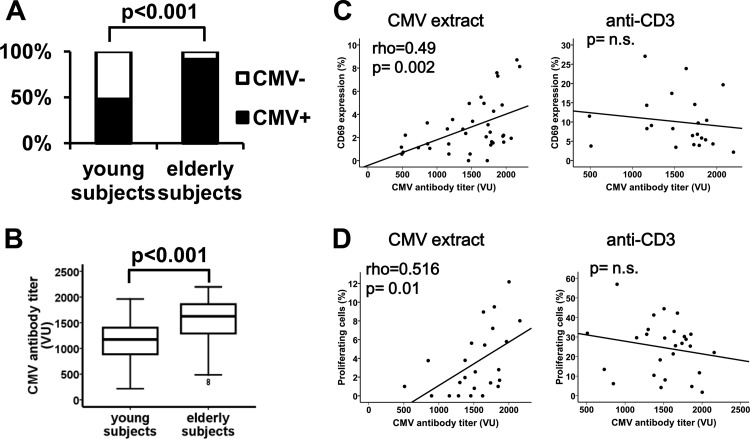
The characteristics of the individuals enrolled in the study are shown in Table 1. We quantified levels of anti-CMV antibodies in the sera of the 70 young and 92 elderly donors. The frequencies of seropositivity were 52% and 91%, respectively (Fig. 1A) (χ2 test; odds ratio [OR], 9.64 to 22.8; P < 0.001). Levels of anti-CMV antibodies in seropositive individuals were significantly higher in elderly than in young individuals, with medians of 1,625 VIRO units (VU)/ml (interquartile range [IR], 586 VU/ml) and 1,150 VU/ml (IR, 535.5 VU/ml), respectively (Mann-Whitney U test; P < 0.001) (Fig. 1B).
Table 1.
Characteristics of the study subjects
| Parametera | Elderly donors (n = 92) | Young donors (n = 70) |
|---|---|---|
| Demographic data | ||
| Age (yrs) | ||
| Mean ± SE | 85.1 ± 6.3 | 38.7 ± 8.4 |
| Range | 68–97 | 20–50 |
| No. of subjects investigated | ||
| Women | 70 | 24 |
| Men | 22 | 46 |
| Hematology values (means ± SDs) | ||
| RBCs (106/μl) | 4.28 ± 0.63 | 5.19 ± 0.71 |
| Hemoglobin (g/dl) | 13.0 ± 1.89 | 14.76 ± 1.17 |
| Hematocrit (%) | 39.19 ± 5.6 | 44.06 ± 2.87 |
| MCV (fl) | 91.78 ± 6.47 | 90.6 ± 3.7 |
| Platelets (103/μl) | 221.65 ± 71.08 | 312.56 ± 62.44 |
| WBCs (103/μl) | 6.30 ± 1.80 | 7.54 ± 1.53 |
| Neutrophils (103/μl) | 3.67 ± 1.32 | 4.18 ± 0.98 |
| Monocytes (103/μl) | 0.53 ± 0.36 | 0.46 ± 0.25 |
| Lymphocytes (103/μl) | 1.84 ± 0.68 | 3.0 ± 0.76 |
RBCs, red blood cells; MCV, mean corpuscular volume; WBCs, white blood cells.
Fig 1.
Frequencies of CMV infection and titers of anti-CMV antibodies in young and elderly subjects and response of CD4+ T cells from elderly subjects to CMV and anti-CD3. Immunoglobulin G levels of CMV-specific antibodies were determined by ELISA and a semiquantitative diagram was used to calculate the CMV titer. (A) Histograms show the percentages of CMV-seropositive (black bars) and CMV-seronegative (white bars) young (n = 70) and elderly (n = 92) subjects. The calculated titers of the patient samples are indicated as VIRO units (VU). Percentages of CMV-infected individuals were compared using the χ2 test. (B) Titers of anti-CMV antibodies in infected young (n = 37) and elderly (n = 82) subjects are illustrated in the box plots. Significant group differences, assessed with the Mann-Whitney U test, are indicated. (C and D) CD69 expression and proliferative capacity of CD4+ T cells from elderly subjects in response to CMV in vitro stimulation were analyzed. (C) Correlation between anti-CMV antibody titers and the frequency of CD69 expression in CD4+ subsets from elderly individuals in response to a CMV supernatant (104 PFU/ml) (n = 37) and to anti-CD3 (10 ng/ml) (n = 22). Whole blood from CMV-seropositive elderly people was stimulated for 18 h. (D) Proliferative capacity of CD4+ T cell subsets in response to CMV stimulation (n = 28) and to anti-CD3 (n = 27). PBMCs were labeled with CFSE (1.5 μM) and cultured in the presence of CMV supernatant or anti-CD3 for 5 days. The percentage of dividing CD4+ T cells is represented. Spearman correlation coefficients and corresponding P values are presented in the upper left-hand corner. n.s., not significant.
Aging was associated not only with the percentage of CMV seropositivity but also with the levels of anti-CMV antibodies.
Correlation between anti-CMV-specific T cells and antibody titer.
To analyze whether individuals with higher anti-CMV antibody titers also have stronger CMV-specific T cell responses, the CD4+ T cell response was measured by stimulating whole-blood cultures with CMV antigens and with anti-CD3. CD69 expression in response to CMV extracts and to anti-CD3 was analyzed in CD4+ T cells. The magnitude of the CD4+ T cell immune responses to CMV was positively correlated with anti-CMV antibody titers in the elderly (Spearman test; rho = 0.490 and P = 0.002) (Fig. 1C) but not in young donors (data not shown). No correlations were found between antibody titers and activation in response to anti-CD3 in CD4+ T cells in elderly subjects (Fig. 1C). Similarly, when proliferative responses were quantified in PBMC cultures, there was a significant correlation with CD4+ T cell proliferation only in the elderly group in response to CMV (Spearman test; rho = 0.516 and P = 0.01) but not in response to anti-CD3 (Fig. 1D). No correlations were found between activation or proliferation in CD4+ T cells with anti-CMV antibody titers in young donors (data not shown).
Levels of anti-CMV antibodies and CMV-specific CD4+ T cells were clearly related in elderly individuals.
T cell differentiation subsets and anti-CMV antibody titer.
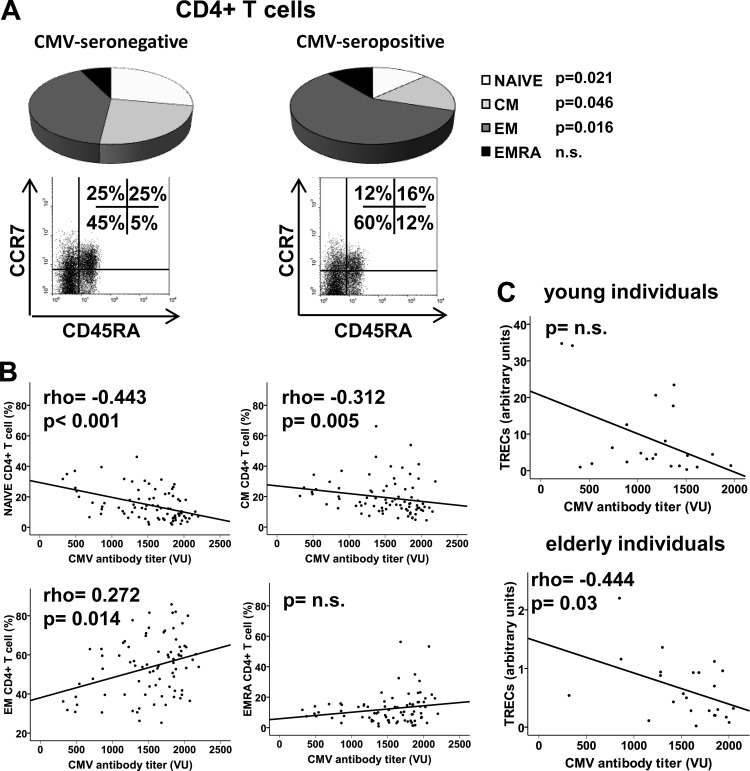
It is widely accepted that the progressive deterioration of the T cell compartment with advancing age is related to CMV seropositivity. T cells can be separated into functionally distinct populations using combinations of cell surface markers such as CD45RA and CCR7. We used these markers to classify the T cells into naïve (CD45RA+ CCR7+), central memory (CM; CD45RA− CCR7+), effector memory (EM; CD45RA− CCR7−), and effector memory RA (EMRA; CD45RA+ CCR7−) groups (17). We wanted to verify the association between CMV seropositivity and the degree of differentiation of T cell subsets in young and elderly individuals. First, we compared the distributions of the T cell subpopulations in seropositive and seronegative individuals and found that CMV seropositivity was related to the reduced frequency of undifferentiated subsets, naïve and CM, only in the CD4+ T cells of elderly individuals (Fig. 2A). No differences were found in the CD8+ T cells from elderly people. Most CD8+ T cells belonged to the EM and EMRA subsets, which are the final stages of differentiation (data not shown). Moreover, the frequencies of the four populations were similar in young seropositive and seronegative subjects in CD4+ and CD8+ T cells (data not shown).
Fig 2.
Distribution of CD4+ T cells into naïve (CD45RA+ CCR7+), central memory (CD45RA− CCR7+), effector memory (CD45RA− CCR7−), and effector memory RA (CD45RA+ CCR7−) related to CMV seropositivity and anti-CMV antibody titers in elderly subjects. CD45RA and CCR7 expression was analyzed by flow cytometry in gated CD3+ CD4+ T cells. (A) Individual segments of the pie charts show the proportions of each subset in CMV-seronegative and CMV-seropositive elderly subjects. Representative dot plots of the subsets defined by CD45RA and CCR7 expression for individuals in each group show the percentages of positive cells. Student's t test or the Mann-Whitney U test was used to examine differences between the groups, and P values are represented. (B) Relationship between anti-CMV antibody titers and frequency of the CD4+ T cell populations defined by CD45RA and CCR7 expression. (C) TREC content was measured in CD4+ T cells from young (n = 25) and elderly (n = 30) CMV-seropositive subjects. The CD4+ population was isolated by magnetic bead separation and the TREC copy number was determined by real-time PCR. Experiments were conducted in duplicate, and the results are presented relative to anti-CMV antibody titers in each subject. Spearman correlation coefficients and corresponding P values are shown in the upper left-hand corner.
We then calculated the correlation between the anti-CMV antibody titer and the frequency of these T cell subsets. We were unable to demonstrate any associations in young people, but the effect was evident in CD4+ T cells in elderly individuals. In fact, the frequency of naïve and CM CD4+ T cells showed a significant negative correlation with antibody titers (Spearman test; rho = −0.443 and P < 0.001 and rho = −0.312 and P = 0.005, respectively) (Fig. 2B). On the other hand, the more differentiated EM subset showed a significant and progressive increase (Spearman test; rho = 0.272 and P = 0.014), and no effect on the proportions of EMRA was found (Fig. 2B). Again, frequencies of CD8+ T cell subsets were independent of the antibody titers (data not shown).
To examine the differences in the differentiated status of CD4+ subsets in CMV-seropositive individuals further, we assessed the replicative history of these cells by quantifying the content of T cell receptor excision circles (TREC) in CD4+ T cells. TREC constitute a traceable molecular marker produced in newly naïve T cells; the content of TREC in peripheral T cells is an indicator of the number of divisions that the cell has undergone (18). The analysis revealed significant differences in TREC content depending on the levels of anti-CMV antibodies in elderly patients (Spearman test; rho = −0.444 and P = 0.03) and a nonsignificant trend in young individuals (Fig. 2C).
In conclusion, both CMV seropositivity and antibody titers influenced the degree of CD4+ T cell differentiation in the elderly, without affecting their CD8+ T cells, and the degree of differentiation in CD4+ and CD8+ T cells of young people.
IRP parameters.
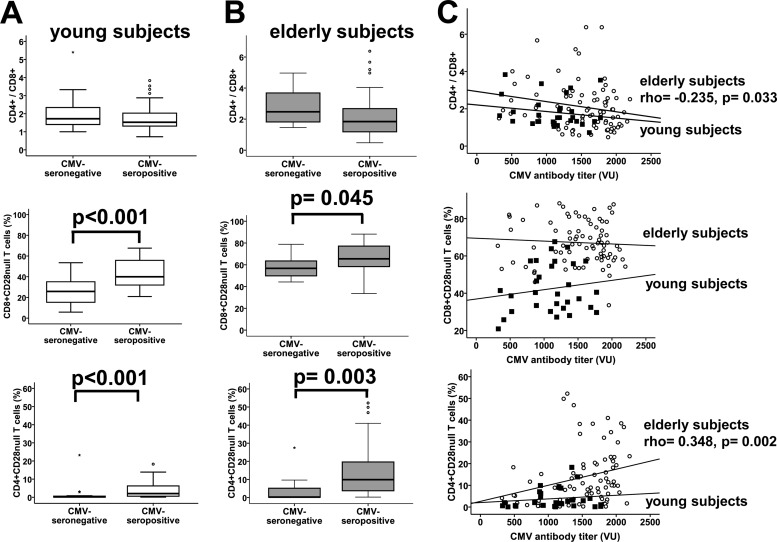
CMV infection is considered a predictor of survival in very elderly people, but we wanted to evaluate the association of CMV serological status with the other parameters that define the IRP, the CD4/CD8 ratio and the frequency of CD8+ CD28null T cells (9, 19). The CD4/CD8 ratio was slightly lower in seropositive than in seronegative young (Fig. 3A) and elderly (Fig. 3B) individuals, although neither was statistically significant. However, a significant negative association between anti-CMV antibody titers and the CD4/CD8 ratio was found in elderly people (Spearman test; rho = −0.235 and P = 0.033) (Fig. 3C). Conversely, the proportion of CD8+ CD28null T cells was higher in CMV-seropositive than CMV-seronegative individuals among both the young and elderly subjects (Fig. 3A and B) (Student's t test; P < 0.001 and P = 0.045, respectively) but was not correlated with the levels of antibodies (Fig. 3C).
Fig 3.
Relationship between CMV seropositivity and anti-CMV antibody titer with CD4/CD8 ratio and percentages of CD8+ CD28null and CD4+ CD28null T cells in young and elderly subjects. CD4/CD8 ratio and percentages of CD8+ CD28null and CD4+ CD28null T cells were compared between CMV-seropositive and CMV-seronegative young (A) and elderly (B) subjects. Significant differences are indicated (Student's t test or Mann-Whitney U test). (C) Correlation of anti-CMV antibody titers and CD4/CD8 ratio and percentages of CD8+ CD28null and CD4+ CD28null T cells in young (black squares) and elderly (white circles) subjects are represented in the dot plots. Spearman correlation coefficients and corresponding P values are listed on the right-hand side.
Since we had demonstrated that differences in the differentiation status of CD4+ T cells are related to CMV infection, we proceeded to analyze the relationship between the virus and the abundance of CD4+ CD28null T cells. As expected, young and elderly CMV-seropositive people had the highest percentages of CD4+ CD28null T cells (Fig. 3A and B) (Mann-Whitney U test; P < 0.001 and P = 0.003, respectively). Furthermore, this subset was significantly increased with higher levels of anti-CMV antibodies in the elderly (Spearman test; rho = 0.348 and P = 0.002), but not in the young (Fig. 3C).
Anti-CMV antibody titers were related to other parameters of IRP and to CD4+ CD28null T cells in the elderly but not in young individuals.
Comparisons between young and elderly individuals with similar anti-CMV antibody titers.
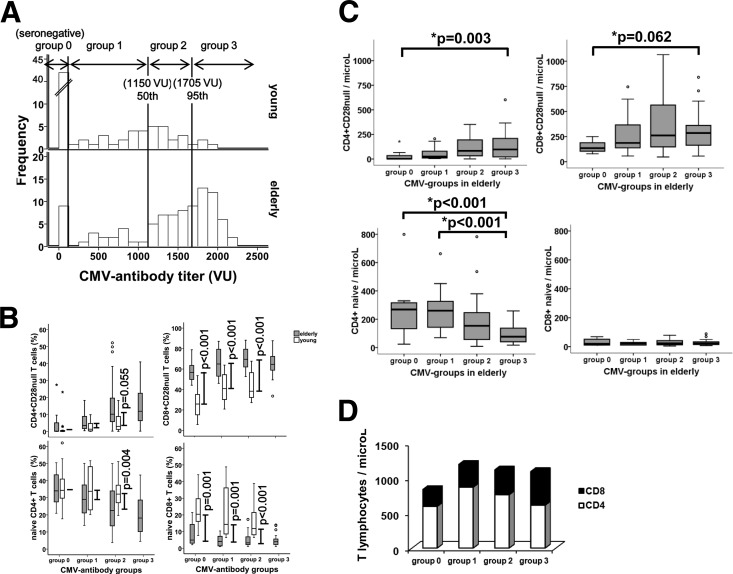
The lack of correlation between anti-CMV antibodies and other aspects of immunosenescence in the young but not in elderly people may be because the antibody levels in the elderly are much higher than in young individuals. To check whether the profile of cell differentiation found in these groups was due solely to these differences in the antibody titer, we compared the degrees of cell differentiation between young and elderly individuals with similar anti-CMV antibody titers. Individuals were classified into groups defined according to their CMV serological status and the antibody levels in young people, as shown in Fig. 4A (group 0, seronegative; group 1, ≤50th percentile in young individuals; group 2, between 50th and 95th percentiles in young people; group 3, >95th percentile in young people).
Fig 4.
Grouping of young and elderly subjects by anti-CMV antibody titer. (A) Distribution of frequencies of anti-CMV antibody titers in young and elderly subjects and classification into groups defined according to the antibody levels in young people (50th and 95th percentiles). (B) Comparisons of naïve and CD28null T cells (CD4+ and CD8+) between young and elderly individuals with similar anti-CMV antibody titers. Differences in the median frequencies between the young and the elderly in each group are represented by lines. Significant differences between subsets, assessed by Student's t test or the Mann-Whitney U test, are indicated. (C) Absolute counts of naïve and CD28null T cells (CD4+ and CD8+) in elderly subjects. The Kruskal-Wallis test was used to identify significant differences in frequencies between groups. “*p” indicates a significant difference in comparisons of all groups; “**p” indicates a significant difference when only groups of CMV-seropositive subjects were considered. (D) Absolute counts of CD3+ T cells, distributed into CD4+ (white bars) and CD8+ (black bars), belonging to each group in elderly subjects.
As there were too few young individuals (only three) in group 3 to carry out any meaningful statistical analysis, they were excluded, so only the elderly individuals in the group were considered. The median anti-CMV antibody titer in each group did not differ between elderly and young people. The mean ages of the elderly individuals were 82.5 ± 8.5 years in group 0, 86 ± 5.5 years in group 1, 85.5 ± 6.3 years in group 2, and 87 ± 5.6 years in group 3. For the young individuals, the mean ages were 38 ± 7.2 years in group 0, 41 ± 10 years in group 1, and 42 ± 9 years in group 2.
Frequencies of CD4+ CD28null and naïve CD4+ T cells in elderly and young CMV-seronegative individuals (group 0) were very similar, but the differences were progressively greater in the groups with higher antibody levels. This pattern arose mainly because of the variations experienced by these cellular subsets in the elderly groups (Fig. 4B). On the other hand, in the case of CD8+ T cells, differences were observed between young and elderly individuals in all groups (Fig. 4B). There were no changes in the differences in the frequency of CD28null subsets, while differences in naïve subsets diminished in the groups with higher anti-CMV antibody titers. The reason for this in the latter case was the reduction in the number of naïve cells in young people, since their frequency in the elderly was very low in all groups.
The frequency of naïve CD4+ T cells was related to CMV serological status and to anti-CMV antibody titers in elderly people but showed no association in young individuals. We checked whether this effect merely reflects the increase in the number of more differentiated cells, a consequence of which is a reduction in the frequency of naïve cells in the CD4+ T cell compartment. We compared the absolute counts of CD28null and naïve subsets in CD4+ and CD8+ T cells in the elderly groups (Fig. 4C). As expected, levels of highly differentiated cells increased significantly in the groups with higher antibody titers, but the differences were not statistically significant when only CMV-seropositive individuals were compared. Surprisingly, a significant reduction in the absolute numbers of CD4+ naïve T cells and an almost complete absence of CD8+ naïve T cells were found in comparisons of all elderly individuals and those who were CMV seropositive. This may be due to some homeostatic mechanisms that limit the generation of new naïve T cells in order to maintain the levels of total T lymphocytes. In fact, in a comparison of the counts of T lymphocytes, those of the three groups of CMV-infected individuals were very similar and higher than those of CMV-seronegative individuals (Fig. 4D), despite the notable increase in CD28null cells. We also found a gradual reduction in CD4+ and an increase in CD8+ T cells as the antibody titers rose.
Overall, differences between elderly and young individuals in highly differentiated and naïve CD4+ T cells increased with their anti-CMV antibody titers. The reduction in naïve cells may be a strategy to compensate for the expansion of differentiated cells and to avoid an increase in the total number of T cells.
Genetic features of CMV-infected elderly people.
To identify factors favoring CMV reactivation or the poor control of the infection, which may be responsible for the higher antibody titers, we analyzed some genetic characteristics of elderly individuals. Although HLA-DRB1 alleles are associated with increased susceptibility to CMV pathologies in HIV-infected and transplant patients, we were unable to find any associations between levels of anti-CMV antibody and the HLA-DRB1 profile of the elderly donors (data not shown).
Conversely, TNF-α is a key mediator in the reactivation of CMV, as well as a differentiation factor associated with the loss of expression of CD28 in T lymphocytes (20). Mutations in this gene leading to over- or underexpression of the cytokine may be significant in reactivation and the immune response against the virus. We studied the SNP at position −308 (G/A), which has functional effects on gene transcriptional activity, whereby ∼308A* is a stronger transcriptional activator than ∼308G* after in vitro lymphocyte stimulation (21). Sixty (65.2%) elderly individuals had the GG, 27 (30%) the GA, and 5 (5.5%) the AA genotype. These are frequencies similar to those described for our population. There were no overall differences in the frequency of CMV seropositivity or the anti-CMV antibody titers between the TNF-α genotype groups.
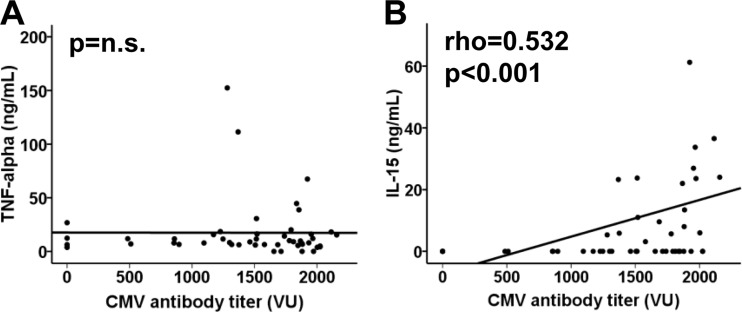
We nevertheless tested the levels of TNF-α and IL-15, a cytokine that could have a significant role in the proliferation of CD28null T cells, in the sera of the CMV-seropositive elderly individuals. Again, no association was found between CMV antibody titers and TNF-α levels (Fig. 5A). However, IL-15 levels were positively correlated with antibody titers (Spearman test; rho = 0.532 and P < 0.001), although they were undetectable in 60.4% of the elderly individuals analyzed (Fig. 5B).
Fig 5.
Levels of TNF-α and IL-15 related to anti-CMV antibody titers. Spearman correlation coefficients and corresponding P values are shown in the upper left-hand corner.
Our results suggest that anti-CMV antibody titers are not related to either HLA-DRB1 alleles or the −308 TNF-α polymorphism in elderly individuals but are associated with serum IL-15 levels.
Response to vaccination and CMV infection.
To determine whether CMV serological status influences the ability to respond to immunization in vivo, we measured the specific antibodies produced against influenza virus vaccination in the four groups of elderly people. The production of specific antibodies to the vaccine diminished gradually and significantly in the groups of CMV-seropositive individuals and was related to levels of anti-CMV antibodies (comparisons within all groups, Kruskal-Wallis test; P < 0.001; within CMV-seropositive groups, P = 0.002) (Fig. 6). The association between CD8+ CD28null T cells and the response to vaccination has been demonstrated previously (22). To verify the influence of CMV seropositivity or the titer of anti-CMV antibodies on the response to vaccination, we performed a multivariate linear regression, including age and levels of CD8+ CD28null T cells in elderly individuals as predictor variables (Table 2). It should be noted that in both models, including CMV seropositivity and anti-CMV antibody titer, only CMV status and age emerged as independent predictors of the magnitude of the humoral response to influenza virus vaccination. The influence of levels of CD8+ CD28null T cells could not be determined in the individuals studied. However, when the titer of anti-CMV antibodies in all elderly individuals (CMV seronegative and CMV seropositive) was included in the model, CD8+ CD28null T cells were also significantly associated (data not shown).
Fig 6.
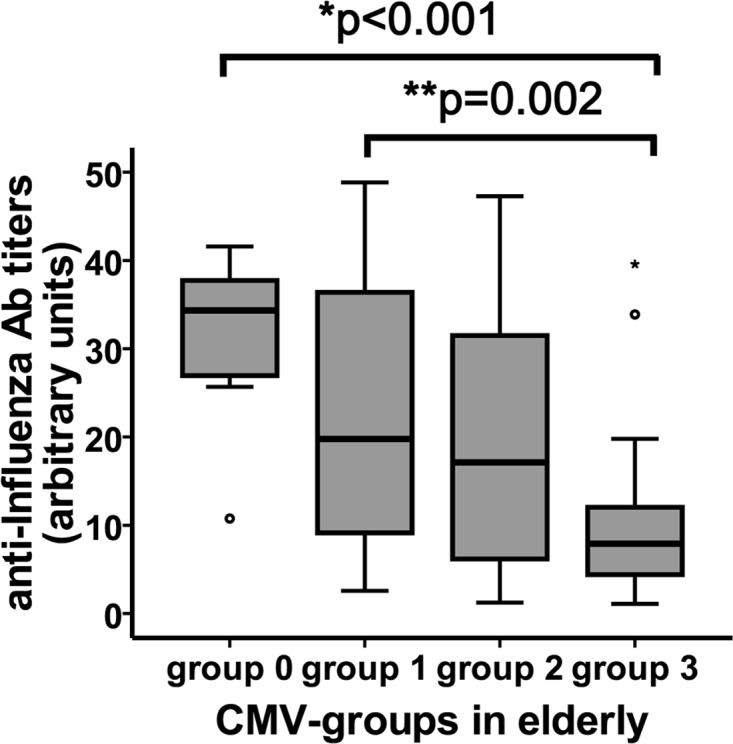
Response to influenza virus vaccination and its correlation with anti-CMV antibody (Ab) titer. Anti-influenza virus antibody titers were quantified by ELISA in the sera of elderly individuals after vaccination. The Kruskal-Wallis test was used to compare the influenza antibody titer between CMV groups. “*p” indicates a significant difference in comparisons between all groups; “**p” indicates a significant difference when only groups of CMV-seropositive subjects were considered.
Table 2.
Effect of CMV infection on response to influenza virus vaccination in elderly individuals, corrected by age and absolute counts of CD8+ CD28null cellsa
| Variable | Regression coefficient | SE | 95% confidence interval | P value |
|---|---|---|---|---|
| CMV seropositivity | −15.24 | 5.083 | −25.3 to −5.14 | 0.004 |
| Age | −0.437 | 0.233 | −0.9 to 0.027 | 0.064 |
| CD8+ CD28null (cells/μl) | 0.006 | 0.005 | −0.003 to 0.16 | 0.191 |
| Anti-CMV antibody titer | −0.011 | 0.003 | −0.017 to −0.005 | <0.001 |
| Age | −0.485 | 0.242 | −0.967 to −0.002 | 0.049 |
| CD8+ CD28null (cells/μl) | 0.016 | 0.012 | −0.007 to 0.04 | 0.178 |
Data from two regression models are given.
The in vivo ability to respond to new antigens is influenced by both CMV infection and antibody titers in elderly individuals.
DISCUSSION
This study examined whether anti-CMV antibody titers in the serum of young and elderly individuals are related to the phenotypic and functional status of their immune system. Our results suggest a relationship between aging and CMV seropositivity frequency and the levels of anti-CMV antibodies, which are clearly associated with CMV-specific CD4+ T cells in elderly individuals. Moreover, both CMV seropositivity and antibody titers are related to the degree of differentiation of CD4+ T cells and to the IRP parameters of elderly people. Differences between elderly and young individuals in highly differentiated and naïve CD4+ T cells increase depending on their anti-CMV antibody titers, and the reduction in the frequency of naïve cells may be a strategy to compensate for the expansion of differentiated cells and to avoid an increase in the total number of T cells. Our results also suggest that anti-CMV antibody titers are not related to HLA-DRB1 alleles or to the TNF-α polymorphism −308 in elderly individuals but are correlated with serum IL-15 levels. The in vivo ability of elderly individuals to respond to new antigens is also influenced by CMV infection.
The wide-ranging means of transmission of CMV lead to very high seroprevalence, estimated to be between 30% and 90% in developed countries, where it also increases with age (23). After primary infection, the virus persists for the rest of an individual's life, most commonly in a latent form in a variety of tissues but particularly in precursor cells of the monocytic lineage (24). Reactivations from latency are likely to occur routinely in healthy virus carriers and chronically in elderly individuals (3). The relationship between anti-CMV antibodies and the evolution of the infection has been poorly understood. We found that elderly people have higher levels of antibodies than young individuals. One possible explanation for this is that titers of anti-CMV antibodies are indicative of the history of infection, but this hypothesis could be tested by analyzing other characteristics, such as time since infection and the severity or frequency of reactivations. In this way, while individuals with detectable CMV DNA in monocytes have significantly higher percentages of anti-CMV-specific CD8+ T cells, it has not been possible to determine the association with anti-CMV antibody titers. However, the detection of CMV DNA in monocytes could be a marker of current CMV reactivation, and the associated cellular response may reflect the expansion of memory T cells to control the infection (25). In the present study, we found that activation and proliferation of anti-CMV CD4+ T cells in the elderly was associated with levels of specific antibodies. These results are consistent with a recent paper (26) reporting a correlated increase in humoral and CD4+ T cell responses to CMV antigens of extracellular origin in very old individuals. Furthermore, we demonstrated a clear association between higher levels of antibodies and a greater degree of CD4+ T cell differentiation in the elderly. The process of human immunosenescence, irrespective of its association with CMV, induces important changes in the T cell compartment. Although the two populations of CD8+ and CD4+ T cells undergo the same principal phenotypic shifts, the rates at which they occur or accumulate with age are very different. CD4+ T cells are more resistant than CD8+ T cells to phenotypic and functional changes with aging, probably because they have important regulatory roles and need to be subjected to strict control mechanisms (27). In this way, there was an association between titers of anti-CMV antibodies and the CD4+ T cell phenotype in elderly but not in young individuals. This might be explained by the different levels of antibodies in the two age groups, but when we compared elderly and young individuals with equal levels of anti-CMV antibodies, we found that, as expected, the differences were not explained solely by anti-CMV antibody titers. Nevertheless, the changes were more pronounced in CD4+ than in CD8+ T cells in the elderly, and there were substantial differences with young individuals with increasing antibody titers. The accumulation of highly differentiated cells may not be the only reason for the changes in T cell phenotype, and the impaired ability to replenish the pool of naïve T cells in elderly individuals also contributes to this. In humans, a dramatic drop in the absolute cell count of recent thymic emigrants with age is evident (28). Despite this thymic degeneration, clinical lymphopenia is rare in elderly people and the number of circulating T cells is maintained over the life span, probably due to the increase in highly differentiated memory cells. Accordingly, we found that higher anti-CMV antibody titers were correlated with lower absolute counts of naïve and more highly differentiated T cells. However, there was no correlation with the total T lymphocyte count. Experienced T lymphocytes may fill the immunological space and homeostatic mechanisms block the generation of new naïve cells to maintain the numbers of peripheral T lymphocytes. These mechanisms make it difficult to preserve the T cell repertoire diversity that combats new pathogens as well as the host's ability to mount vigorous recall responses to recurrent infections (11). Indeed, the loss of functionality with increasing differentiation and the reduction in naïve cells may be responsible for the suppression of immunity to other viruses and the impaired response to influenza vaccination reported for elderly donors with CMV infection (29). In this way, an insufficient antibody response following influenza vaccination in elderly individuals has also been linked to a high frequency of CD8+ CD28null T cells (22). In our study, both CMV seropositivity and the titer of anti-CMV antibodies corrected by age and CD8+ CD28null T cells were independently associated with the antibody response to influenza virus vaccination. The impaired response to new antigens may be considered a marker of CMV infection-related immunosuppression, which has other possible consequences. CMV seropositivity is strongly associated with an IRP that is known to predict 2-year mortality in a population-based sample of Swedish octogenarians (9). The IRP may be summarized by the presence of expanded populations of CD8+ CD28null memory T cells and an inverted CD4/CD8 ratio (30). Recently, two studies have reported that not only the CMV infection but also the titer of CMV-specific antibodies is crucial for determining the risk of increased mortality (31, 32). Differences related to CMV seropositivity in our study were more striking in CD28null T lymphocytes from elderly and young individuals, but only the CD4/CD8 ratio and the frequency of CD4+ CD28null T cells were correlated with antibody titers, and again not in the case of CD8+ CD28null T cells of elderly individuals.
The magnitude of CMV infection may be influenced by genetic factors in the host. In HIV infection and renal transplantation, the presence of HLA-DR7 has been correlated with an increased risk of infection and CMV disease and with poor immune responses to CMV (33, 34). It has been postulated that defective control of infection in HLA-DR7 patients could lead to frequent virus reactivations, contributing to the clonal expansion of specific subsets of CD4+ T cells restricted against a limited number of CMV antigens (35). In our study, we found no association between titers of anti-CMV antibodies and the HLA-DR alleles of the elderly individuals. Moreover, we found no association with their TNF-α polymorphisms, even though TNF-α is considered to be a key mediator of CMV reactivation. In turn, TNF-α, which is augmented by aging and CMV infection, induces CD28 expression loss and T cell differentiation (36). Despite the high levels of anti-CMV antibodies and the high frequencies of CD28null T cells, we found no association between CMV infection and TNF-α levels in the sera of elderly individuals. There was a significant correlation with the level of IL-15, another cytokine implicated in the loss of CD28 expression in CD8+ T cells and an inducer of preferential proliferation and functional capabilities of CD4+ CD28null T cells (20, 37).
In summary, our data show that levels of anti-CMV antibodies and CMV seropositivity are related to differentiation status and immunocompetence in the elderly. Elevated anti-CMV antibody titers could be a consequence rather than the cause of immunosenescence, but they may have value as a prognostic marker of the deterioration of immunological status and the risk of age-related diseases.
ACKNOWLEDGMENTS
This work was supported by the Red de Investigación Renal (REDinREN) and FIS-Fondos FEDER European Union PI080566 and PI1202527 from the Instituto Carlos III.
We thank Isabel Cuevas Pérez and José María Díaz Pérez for their excellent technical assistance.
Footnotes
Published ahead of print 6 February 2013
REFERENCES
- 1. Kutza AS, Muhl E, Hackstein H, Kirchner H, Bein G. 1998. High incidence of active cytomegalovirus infection among septic patients. Clin. Infect. Dis. 26:1076–1082 [DOI] [PubMed] [Google Scholar]
- 2. Prösch S, Wendt CE, Reinke P, Priemer C, Oppert M, Kruger DH, Volk HD, Docke WD. 2000. A novel link between stress and human cytomegalovirus (HCMV) infection: sympathetic hyperactivity stimulates HCMV activation. Virology 272:357–365 [DOI] [PubMed] [Google Scholar]
- 3. Stowe RP, Kozlova EV, Yetman DL, Walling DM, Goodwin JS, Glaser R. 2007. Chronic herpesvirus reactivation occurs in aging. Exp. Gerontol. 42:563–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aiello AE, Haan M, Blythe L, Moore K, Gonzalez JM, Jagust W. 2006. The influence of latent viral infection on rate of cognitive decline over 4 years. J. Am. Geriatr. Soc. 54:1046–1054 [DOI] [PubMed] [Google Scholar]
- 5. Harkins L, Volk AL, Samanta M, Mikolaenko I, Britt WJ, Bland KI, Cobbs CS. 2002. Specific localisation of human cytomegalovirus nucleic acids and proteins in human colorectal cancer. Lancet 360:1557–1563 [DOI] [PubMed] [Google Scholar]
- 6. Moro-Garcia MA, Alonso-Arias R, Lopez-Vazquez A, Suarez-Garcia FM, Solano-Jaurrieta JJ, Baltar J, Lopez-Larrea C. 2012. Relationship between functional ability in older people, immune system status, and intensity of response to CMV. Age (Dordr.) 34:479–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simanek AM, Dowd JB, Aiello AE. 2009. Persistent pathogens linking socioeconomic position and cardiovascular disease in the US. Int. J. Epidemiol. 38:775–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simanek AM, Dowd JB, Pawelec G, Melzer D, Dutta A, Aiello AE. 2011. Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS One 6:e16103 doi:10.1371/journal.pone.0016103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Olsson J, Wikby A, Johansson B, Lofgren S, Nilsson BO, Ferguson FG. 2000. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech. Ageing Dev. 121:187–201 [DOI] [PubMed] [Google Scholar]
- 10. Goronzy JJ, Lee WW, Weyand CM. 2007. Aging and T-cell diversity. Exp. Gerontol. 42:400–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nikolich-Zugich J. 2008. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat. Rev. Immunol. 8:512–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. 2009. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 10:29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giunta S. 2008. Exploring the complex relations between inflammation and aging (inflamm-aging): anti-inflamm-aging remodelling of inflamm-aging, from robustness to frailty. Inflamm. Res. 57:558–563 [DOI] [PubMed] [Google Scholar]
- 14. Alonso-Arias R, Moro-Garcia MA, Lopez-Vazquez A, Rodrigo L, Baltar J, Garcia FM, Jaurrieta JJ, Lopez-Larrea C. 2011. NKG2D expression in CD4+ T lymphocytes as a marker of senescence in the aged immune system. Age (Dordr.) 33:591–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kang I, Hong MS, Nolasco H, Park SH, Dan JM, Choi JY, Craft J. 2004. Age-associated change in the frequency of memory CD4+ T cells impairs long term CD4+ T cell responses to influenza vaccine. J. Immunol. 173:673–681 [DOI] [PubMed] [Google Scholar]
- 16. Hazenberg MD, Verschuren MC, Hamann D, Miedema F, van Dongen JJ. 2001. T cell receptor excision circles as markers for recent thymic emigrants: basic aspects, technical approach, and guidelines for interpretation. J. Mol. Med. (Berl.) 79:631–640 [DOI] [PubMed] [Google Scholar]
- 17. Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708–712 [DOI] [PubMed] [Google Scholar]
- 18. Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, Polis MA, Haase AT, Feinberg MB, Sullivan JL, Jamieson BD, Zack JA, Picker LJ, Koup RA. 1998. Changes in thymic function with age and during the treatment of HIV infection. Nature 396:690–695 [DOI] [PubMed] [Google Scholar]
- 19. Hadrup SR, Strindhall J, Kollgaard T, Seremet T, Johansson B, Pawelec G, thor Straten P, Wikby A. 2006. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J. Immunol. 176:2645–2653 [DOI] [PubMed] [Google Scholar]
- 20. Chiu WK, Fann M, Weng NP. 2006. Generation and growth of CD28nullCD8+ memory T cells mediated by IL-15 and its induced cytokines. J. Immunol. 177:7802–7810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW. 1997. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc. Natl. Acad. Sci. U. S. A. 94:3195–3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saurwein-Teissl M, Lung TL, Marx F, Gschosser C, Asch E, Blasko I, Parson W, Bock G, Schonitzer D, Trannoy E, Grubeck-Loebenstein B. 2002. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J. Immunol. 168:5893–5899 [DOI] [PubMed] [Google Scholar]
- 23. Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. 2006. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin. Infect. Dis. 43:1143–1151 [DOI] [PubMed] [Google Scholar]
- 24. Reeves M, Sinclair J. 2008. Aspects of human cytomegalovirus latency and reactivation. Curr. Top. Microbiol. Immunol. 325:297–313 [DOI] [PubMed] [Google Scholar]
- 25. Leng SX, Qu T, Semba RD, Li H, Yao X, Nilles T, Yang X, Manwani B, Walston JD, Ferrucci L, Fried LP, Margolick JB, Bream JH. 2011. Relationship between cytomegalovirus (CMV) IgG serology, detectable CMV DNA in peripheral monocytes, and CMV pp65(495–503)-specific CD8+ T cells in older adults. Age (Dordr.) 33:607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vescovini R, Biasini C, Telera AR, Basaglia M, Stella A, Magalini F, Bucci L, Monti D, Lazzarotto T, Dal Monte P, Pedrazzoni M, Medici MC, Chezzi C, Franceschi C, Fagnoni FF, Sansoni P. 2010. Intense antiextracellular adaptive immune response to human cytomegalovirus in very old subjects with impaired health and cognitive and functional status. J. Immunol. 184:3242–3249 [DOI] [PubMed] [Google Scholar]
- 27. Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, Ouslander JG, Weyand CM, Goronzy JJ. 2008. T cell subset-specific susceptibility to aging. Clin. Immunol. 127:107–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McFarland RD, Douek DC, Koup RA, Picker LJ. 2000. Identification of a human recent thymic emigrant phenotype. Proc. Natl. Acad. Sci. U. S. A. 97:4215–4220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trzonkowski P, Mysliwska J, Pawelec G, Mysliwski A. 2009. From bench to bedside and back: the SENIEUR Protocol and the efficacy of influenza vaccination in the elderly. Biogerontology 10:83–94 [DOI] [PubMed] [Google Scholar]
- 30. Wikby A, Johansson B, Olsson J, Lofgren S, Nilsson BO, Ferguson F. 2002. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp. Gerontol. 37:445–453 [DOI] [PubMed] [Google Scholar]
- 31. Strandberg TE, Pitkala KH, Tilvis RS. 2009. Cytomegalovirus antibody level and mortality among community-dwelling older adults with stable cardiovascular disease. JAMA 301:380–382 [DOI] [PubMed] [Google Scholar]
- 32. Wang GC, Kao WH, Murakami P, Xue QL, Chiou RB, Detrick B, McDyer JF, Semba RD, Casolaro V, Walston JD, Fried LP. 2010. Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. Am. J. Epidemiol. 171:1144–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kraat YJ, Christiaans MH, Nieman FH, van den Berg-Loonen PM, van Hooff JP, Bruggeman CA. 1993. Increased frequency of CMV infection in HLA-DR7 matched renal allograft recipients. Lancet 341:494–495 [DOI] [PubMed] [Google Scholar]
- 34. Schrier RD, Freeman WR, Wiley CA, McCutchan JA. 1995. Immune predispositions for cytomegalovirus retinitis in AIDS. The HNRC Group. J. Clin. Invest. 95:1741–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alonso-Arias R, Lopez-Vazquez A, Diaz-Pena R, Sampere A, Tricas L, Asensi V, Rodrigo L, Lopez-Larrea C. 2009. CD8dim and NKG2D expression defines related subsets of CD4+ T cells in HIV-infected patients with worse prognostic factors. J. Acquir. Immune Defic. Syndr. 51:390–398 [DOI] [PubMed] [Google Scholar]
- 36. Bryl E, Vallejo AN, Matteson EL, Witkowski JM, Weyand CM, Goronzy JJ. 2005. Modulation of CD28 expression with anti-tumor necrosis factor alpha therapy in rheumatoid arthritis. Arthritis Rheum. 52:2996–3003 [DOI] [PubMed] [Google Scholar]
- 37. Alonso-Arias R, Moro-Garcia MA, Vidal-Castineira JR, Solano-Jaurrieta JJ, Suarez-Garcia FM, Coto E, Lopez-Larrea C. 2011. IL-15 preferentially enhances functional properties and antigen-specific responses of CD4+CD28(null) compared to CD4+CD28+ T cells. Aging Cell 10:844–852 [DOI] [PubMed] [Google Scholar]