Abstract
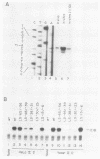
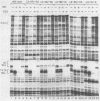
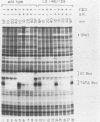
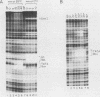
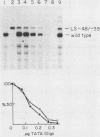
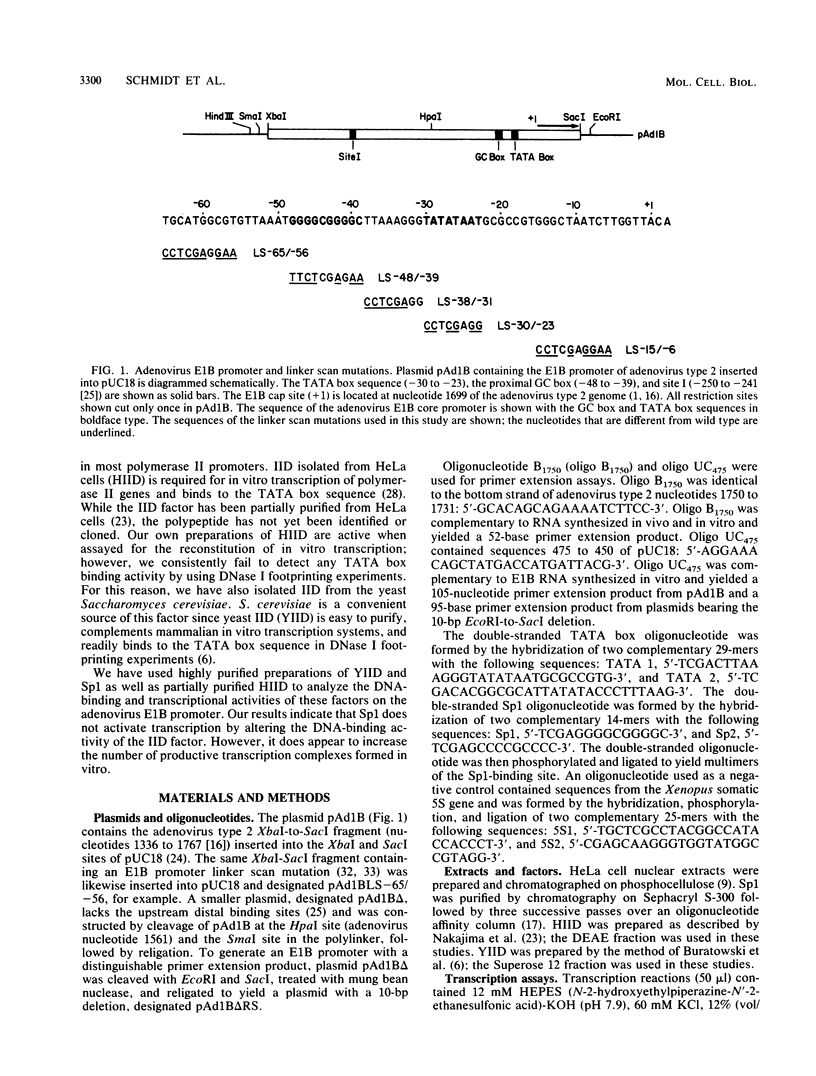
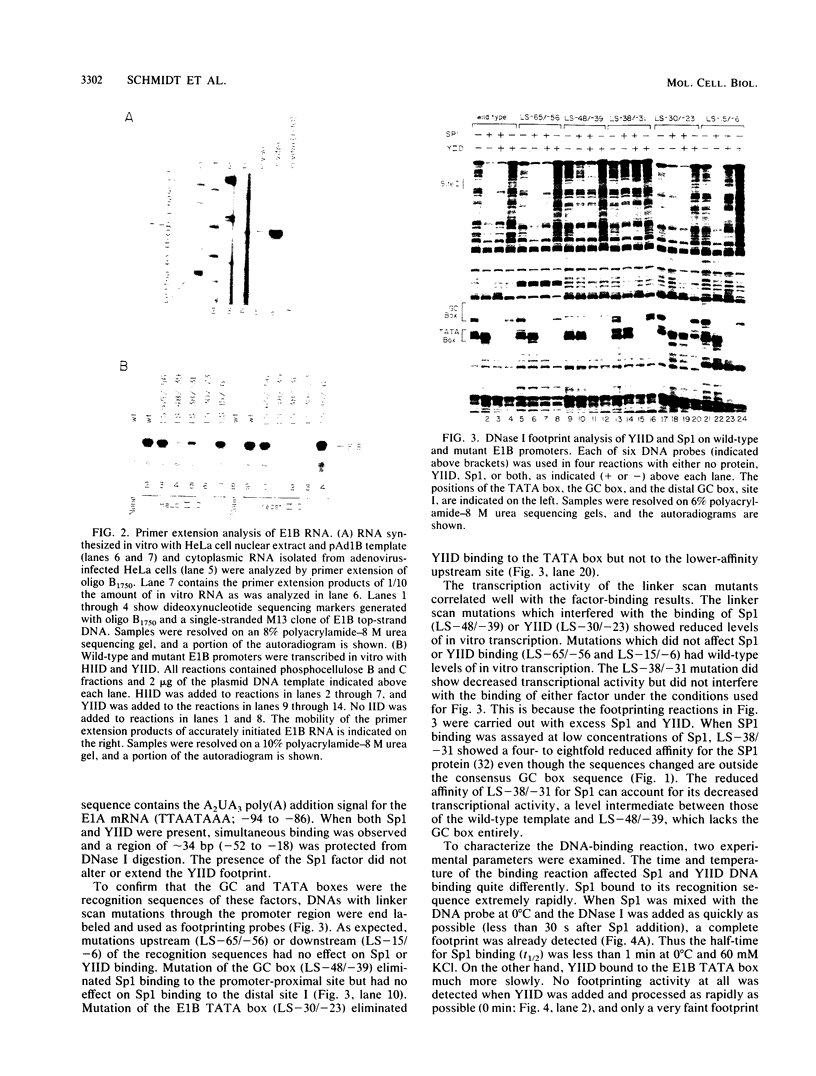
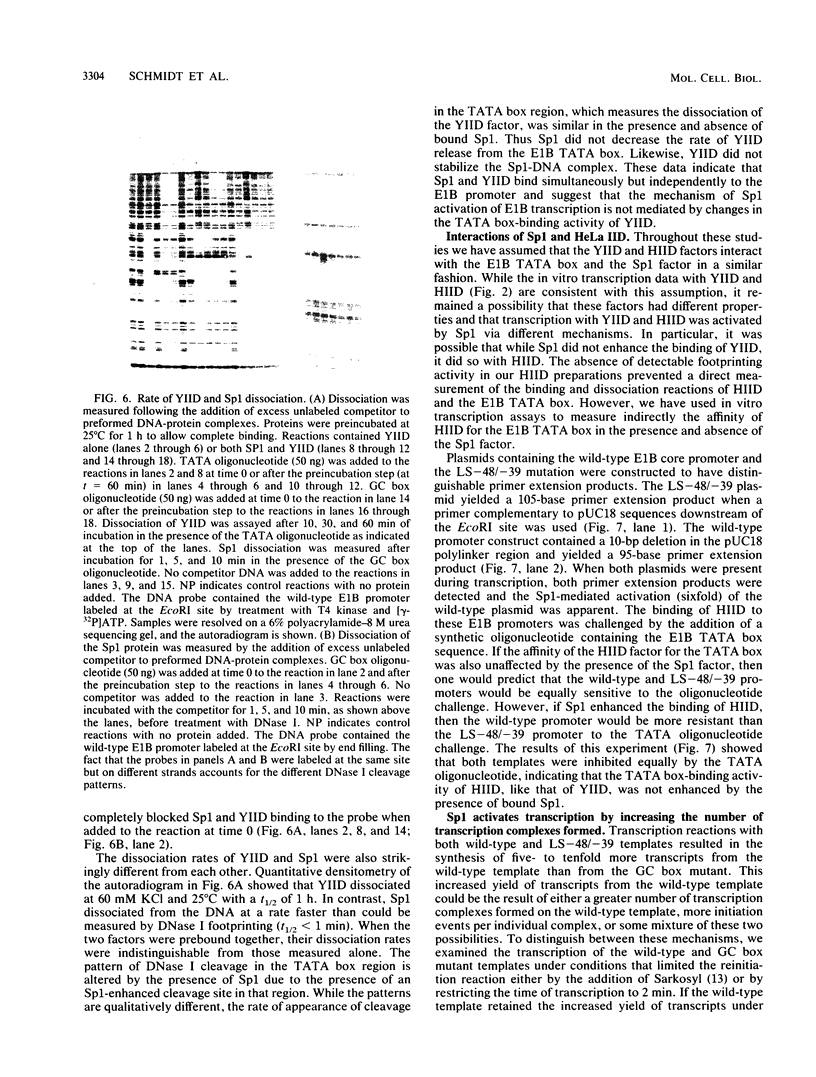
We have studied the interactions of the Sp1 and IID transcription factors with a simple RNA polymerase II promoter. The adenovirus E1B core promoter consists essentially of a GC box and a TATA box, binding sites for the Sp1 and IID transcription factors, respectively. The E1B promoter is accurately transcribed in vitro using a mammalian transcription system. Sp1 activates E1B transcription in vitro in reactions using IID factor isolated from either human or yeast cells. In DNase I footprinting studies, Sp1 bound rapidly to its recognition sequence even at 0 degrees C (t1/2 less than 1 min). In contrast, yeast IID bound more slowly (t1/2 approximately 6 min at 25 degrees C) and required thermal energy for stable binding to the TATA box sequence. Dissociation rates were measured by the addition of specific oligonucleotide competitors to preformed DNA-protein complexes. Sp1 dissociates rapidly (t1/2 less than 1 min) at 25 degrees C, while yeast IID dissociates with an estimated t1/2 of 1 h at 25 degrees C. Sp1 and yeast IID bound to the E1B promoter simultaneously but independently. The rates of binding and dissociation of these factors were not significantly affected by the presence of the other factor. Bound Sp1 factor did not alter or enhance the yeast IID footprint. Oligonucleotide challenge of in vitro transcription reactions indicated that Sp1 also did not enhance the binding of the human IID factor to the E1B promoter. Thus the Sp1 factor activates transcription of the E1B gene by a mechanism that does not enhance the DNA-binding activity of the IID factor. Sp1 factor activates E1B transcription by 5- to 10-fold in vitro. Under these in vitro transcription conditions, transcripts due to reinitiation from an individual promoter complex contribute only a small portion of the total yield of E1B transcripts. Thus Sp1 cannot activate transcription by increasing the rate of initiation events per complex. Instead it appears that Sp1 acts by increasing the number of productive transcription complexes formed in vitro.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. C., Ziff E. B. Promoters and heterogeneous 5' termini of the messenger RNAs of adenovirus serotype 2. J Mol Biol. 1981 Jun 25;149(2):189–221. doi: 10.1016/0022-2836(81)90298-9. [DOI] [PubMed] [Google Scholar]
- Berk A. J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- Brent R., Ptashne M. A eukaryotic transcriptional activator bearing the DNA specificity of a prokaryotic repressor. Cell. 1985 Dec;43(3 Pt 2):729–736. doi: 10.1016/0092-8674(85)90246-6. [DOI] [PubMed] [Google Scholar]
- Briggs M. R., Kadonaga J. T., Bell S. P., Tjian R. Purification and biochemical characterization of the promoter-specific transcription factor, Sp1. Science. 1986 Oct 3;234(4772):47–52. doi: 10.1126/science.3529394. [DOI] [PubMed] [Google Scholar]
- Bucher P., Trifonov E. N. Compilation and analysis of eukaryotic POL II promoter sequences. Nucleic Acids Res. 1986 Dec 22;14(24):10009–10026. doi: 10.1093/nar/14.24.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratowski S., Hahn S., Sharp P. A., Guarente L. Function of a yeast TATA element-binding protein in a mammalian transcription system. Nature. 1988 Jul 7;334(6177):37–42. doi: 10.1038/334037a0. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. J. The selectivity of transcription. Annu Rev Biochem. 1974;43(0):721–775. doi: 10.1146/annurev.bi.43.070174.003445. [DOI] [PubMed] [Google Scholar]
- Courey A. J., Tjian R. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell. 1988 Dec 2;55(5):887–898. doi: 10.1016/0092-8674(88)90144-4. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Martin P. L., Shastry B. S., Roeder R. G. Eukaryotic gene transcription with purified components. Methods Enzymol. 1983;101:582–598. doi: 10.1016/0076-6879(83)01039-3. [DOI] [PubMed] [Google Scholar]
- Fire A., Baker C. C., Manley J. L., Ziff E. B., Sharp P. A. In vitro transcription of adenovirus. J Virol. 1981 Dec;40(3):703–719. doi: 10.1128/jvi.40.3.703-719.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores O., Maldonado E., Burton Z., Greenblatt J., Reinberg D. Factors involved in specific transcription by mammalian RNA polymerase II. RNA polymerase II-associating protein 30 is an essential component of transcription factor IIF. J Biol Chem. 1988 Aug 5;263(22):10812–10816. [PubMed] [Google Scholar]
- Gingeras T. R., Sciaky D., Gelinas R. E., Bing-Dong J., Yen C. E., Kelly M. M., Bullock P. A., Parsons B. L., O'Neill K. E., Roberts R. J. Nucleotide sequences from the adenovirus-2 genome. J Biol Chem. 1982 Nov 25;257(22):13475–13491. [PubMed] [Google Scholar]
- Hai T. W., Horikoshi M., Roeder R. G., Green M. R. Analysis of the role of the transcription factor ATF in the assembly of a functional preinitiation complex. Cell. 1988 Sep 23;54(7):1043–1051. doi: 10.1016/0092-8674(88)90119-5. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., Roeder R. G. Functional steps in transcription initiation and reinitiation from the major late promoter in a HeLa nuclear extract. J Biol Chem. 1987 Mar 15;262(8):3452–3461. [PubMed] [Google Scholar]
- Hope I. A., Struhl K. Functional dissection of a eukaryotic transcriptional activator protein, GCN4 of yeast. Cell. 1986 Sep 12;46(6):885–894. doi: 10.1016/0092-8674(86)90070-x. [DOI] [PubMed] [Google Scholar]
- Horikoshi M., Hai T., Lin Y. S., Green M. R., Roeder R. G. Transcription factor ATF interacts with the TATA factor to facilitate establishment of a preinitiation complex. Cell. 1988 Sep 23;54(7):1033–1042. doi: 10.1016/0092-8674(88)90118-3. [DOI] [PubMed] [Google Scholar]
- Jackson S. P., Tjian R. O-glycosylation of eukaryotic transcription factors: implications for mechanisms of transcriptional regulation. Cell. 1988 Oct 7;55(1):125–133. doi: 10.1016/0092-8674(88)90015-3. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Carner K. R., Masiarz F. R., Tjian R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell. 1987 Dec 24;51(6):1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- Lewis E. D., Manley J. L. Control of adenovirus late promoter expression in two human cell lines. Mol Cell Biol. 1985 Sep;5(9):2433–2442. doi: 10.1128/mcb.5.9.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Nakajima N., Horikoshi M., Roeder R. G. Factors involved in specific transcription by mammalian RNA polymerase II: purification, genetic specificity, and TATA box-promoter interactions of TFIID. Mol Cell Biol. 1988 Oct;8(10):4028–4040. doi: 10.1128/mcb.8.10.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Parks C. L., Banerjee S., Spector D. J. Organization of the transcriptional control region of the E1b gene of adenovirus type 5. J Virol. 1988 Jan;62(1):54–67. doi: 10.1128/jvi.62.1.54-67.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Sawadogo M. Multiple forms of the human gene-specific transcription factor USF. II. DNA binding properties and transcriptional activity of the purified HeLa USF. J Biol Chem. 1988 Aug 25;263(24):11994–12001. [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985 Nov;43(1):165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- Wu L., Berk A. J. Transcriptional activation by the pseudorabies virus immediate early protein requires the TATA box element in the adenovirus 2 E1B promoter. Virology. 1988 Nov;167(1):318–322. doi: 10.1016/0042-6822(88)90089-x. [DOI] [PubMed] [Google Scholar]
- Wu L., Berk A. Constraints on spacing between transcription factor binding sites in a simple adenovirus promoter. Genes Dev. 1988 Apr;2(4):403–411. doi: 10.1101/gad.2.4.403. [DOI] [PubMed] [Google Scholar]
- Wu L., Rosser D. S., Schmidt M. C., Berk A. A TATA box implicated in E1A transcriptional activation of a simple adenovirus 2 promoter. Nature. 1987 Apr 2;326(6112):512–515. doi: 10.1038/326512a0. [DOI] [PubMed] [Google Scholar]
- von Hippel P. H., Bear D. G., Morgan W. D., McSwiggen J. A. Protein-nucleic acid interactions in transcription: a molecular analysis. Annu Rev Biochem. 1984;53:389–446. doi: 10.1146/annurev.bi.53.070184.002133. [DOI] [PubMed] [Google Scholar]
- von Hippel P. H., Berg O. G. Facilitated target location in biological systems. J Biol Chem. 1989 Jan 15;264(2):675–678. [PubMed] [Google Scholar]