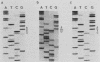Abstract
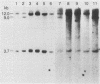
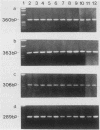
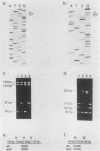
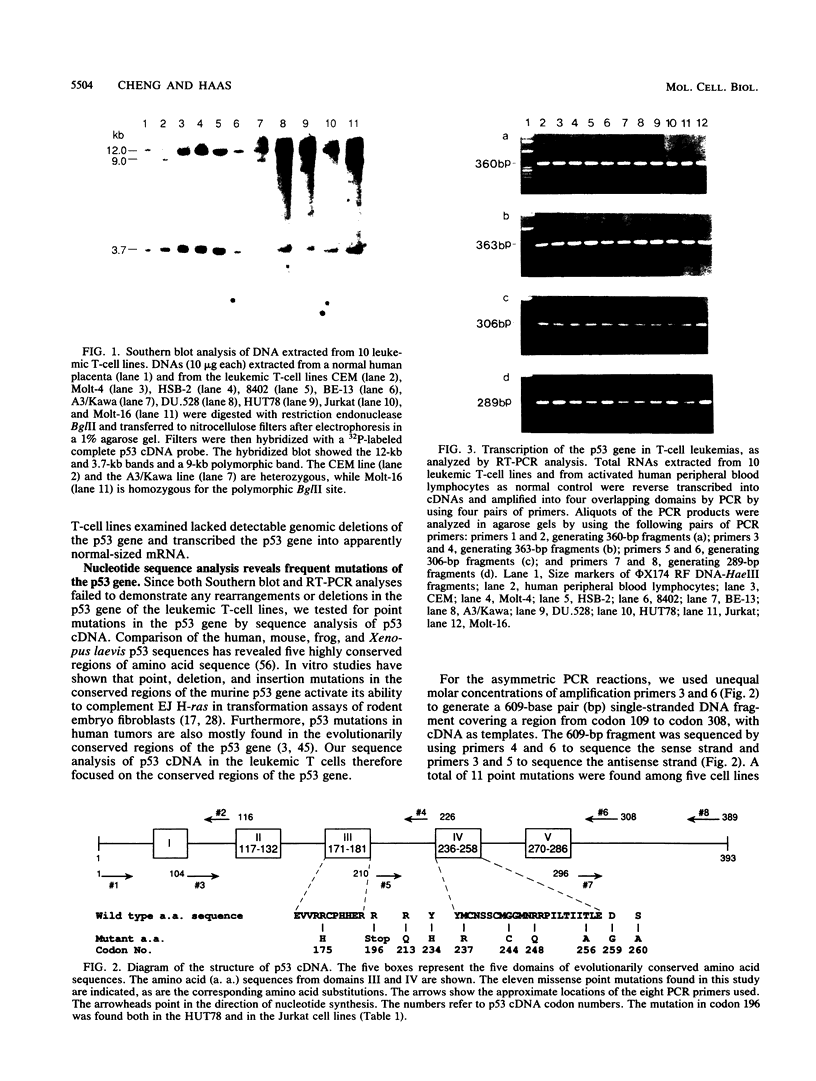
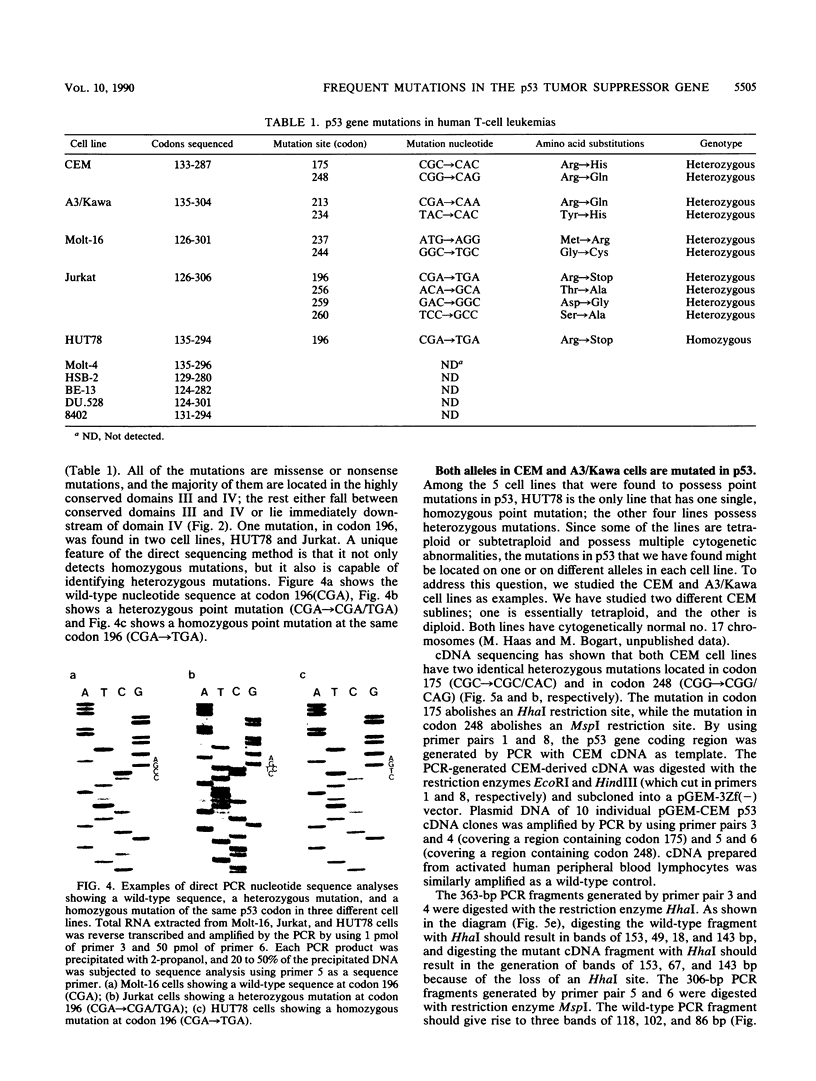
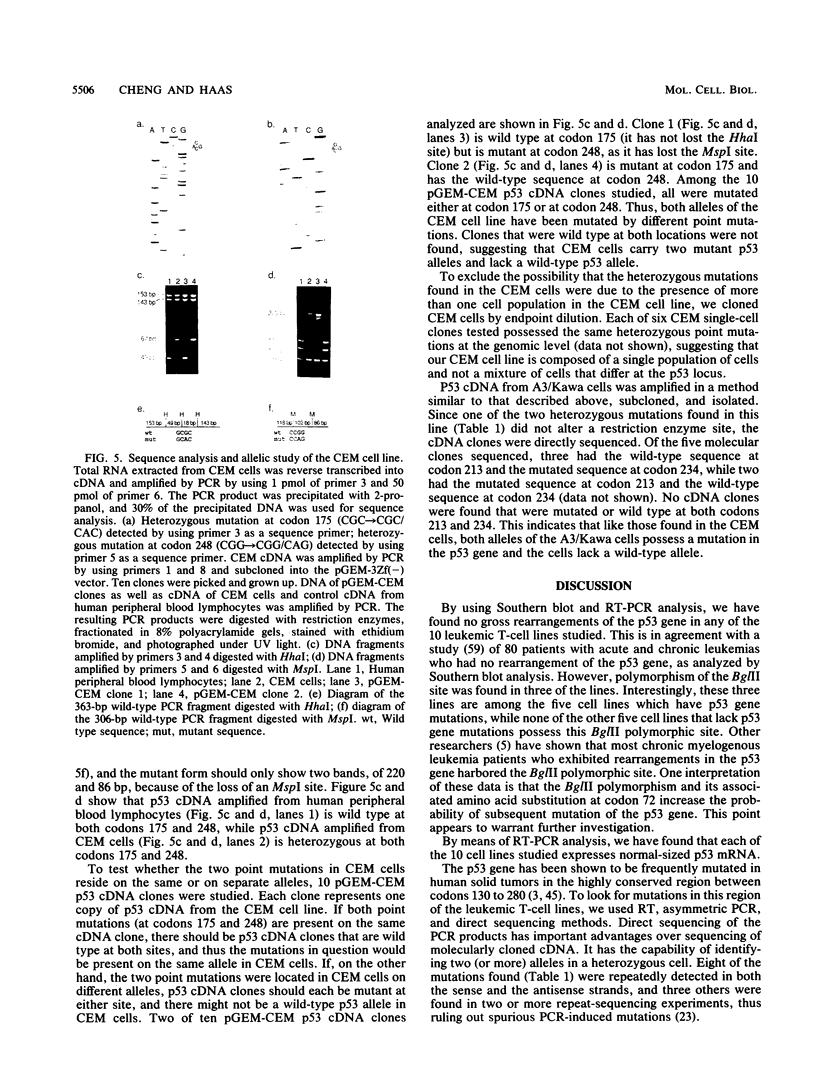
Human T-cell leukemia and T-cell acute lymphoblastic leukemia cell lines were studied for alterations in the p53 tumor suppressor gene. Southern blot analysis of 10 leukemic T-cell lines revealed no gross genomic deletions or rearrangements. Reverse transcription-polymerase chain reaction analysis of p53 mRNA indicated that all 10 lines produced p53 mRNA of normal size. By direct sequencing of polymerase chain reaction-amplified cDNA, we detected 11 missense and nonsense point mutations in 5 of the 10 leukemic T-cell lines studied. The mutations are primarily located in the evolutionarily highly conserved regions of the p53 gene. One of the five cell lines in which a mutation was detected possesses a homozygous point mutation in both p53 alleles, while the other four cell lines harbor from two to four different point mutations. An allelic study of two of the lines (CEM, A3/Kawa) shows that the two missense mutations found in each line are located on separate alleles, thus both alleles of the p53 gene may have been functionally inactivated by two different point mutations. Since cultured leukemic T-cell lines represent a late, fully tumorigenic stage of leukemic T cells, mutation of both (or more) alleles of the p53 gene may reflect the selection of cells possessing an increasingly tumorigenic phenotype, whether the selection took place in vivo or in vitro. Previously, we have shown that the HSB-2 T-cell acute lymphoblastic leukemia cell line had lost both alleles of the retinoblastoma tumor suppressor gene. Taken together, our data show that at least 6 of 10 leukemic T-cell lines examined may have lost the normal function of a known tumor suppressor gene, suggesting that this class of genes serves a critical role in the generation of fully tumorigenic leukemic T cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. A., Flowers A., Davis B. J. Direct implantation and serial transplantation of human acute lymphoblastic leukemia in hamsters, SB-2. Cancer Res. 1968 Jun;28(6):1121–1125. [PubMed] [Google Scholar]
- Ahuja H., Bar-Eli M., Advani S. H., Benchimol S., Cline M. J. Alterations in the p53 gene and the clonal evolution of the blast crisis of chronic myelocytic leukemia. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6783–6787. doi: 10.1073/pnas.86.17.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Ben David Y., Prideaux V. R., Chow V., Benchimol S., Bernstein A. Inactivation of the p53 oncogene by internal deletion or retroviral integration in erythroleukemic cell lines induced by Friend leukemia virus. Oncogene. 1988 Aug;3(2):179–185. [PubMed] [Google Scholar]
- Buchman V. L., Chumakov P. M., Ninkina N. N., Samarina O. P., Georgiev G. P. A variation in the structure of the protein-coding region of the human p53 gene. Gene. 1988 Oct 30;70(2):245–252. doi: 10.1016/0378-1119(88)90196-5. [DOI] [PubMed] [Google Scholar]
- Cattoretti G., Rilke F., Andreola S., D'Amato L., Delia D. P53 expression in breast cancer. Int J Cancer. 1988 Feb 15;41(2):178–183. doi: 10.1002/ijc.2910410204. [DOI] [PubMed] [Google Scholar]
- Cheng J., Scully P., Shew J. Y., Lee W. H., Vila V., Haas M. Homozygous deletion of the retinoblastoma gene in an acute lymphoblastic leukemia (T) cell line. Blood. 1990 Feb 1;75(3):730–735. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Pim D. C., Lamb P. The cellular protein p53 in human tumours. Mol Biol Med. 1984 Aug;2(4):261–272. [PubMed] [Google Scholar]
- DeLeo A. B., Jay G., Appella E., Dubois G. C., Law L. W., Old L. J. Detection of a transformation-related antigen in chemically induced sarcomas and other transformed cells of the mouse. Proc Natl Acad Sci U S A. 1979 May;76(5):2420–2424. doi: 10.1073/pnas.76.5.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler H. G., Minowada J. Morphological, immunophenotypical and isoenzymatic profiles of human leukemia cells and derived T-cell lines. Hematol Oncol. 1989 Mar-Apr;7(2):115–125. doi: 10.1002/hon.2900070203. [DOI] [PubMed] [Google Scholar]
- Eliyahu D., Goldfinger N., Pinhasi-Kimhi O., Shaulsky G., Skurnik Y., Arai N., Rotter V., Oren M. Meth A fibrosarcoma cells express two transforming mutant p53 species. Oncogene. 1988 Sep;3(3):313–321. [PubMed] [Google Scholar]
- Eliyahu D., Michalovitz D., Eliyahu S., Pinhasi-Kimhi O., Oren M. Wild-type p53 can inhibit oncogene-mediated focus formation. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8763–8767. doi: 10.1073/pnas.86.22.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliyahu D., Raz A., Gruss P., Givol D., Oren M. Participation of p53 cellular tumour antigen in transformation of normal embryonic cells. Nature. 1984 Dec 13;312(5995):646–649. doi: 10.1038/312646a0. [DOI] [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Tan T. H., Eliyahu D., Oren M., Levine A. J. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988 Feb;8(2):531–539. doi: 10.1128/mcb.8.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U., Peleg A., Milner Y., Galili N. Be13, a human T-leukemia cell line highly sensitive to dexamethasone-induced cytolysis. Cancer Res. 1984 Oct;44(10):4594–4601. [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Bunn P. A., Russell E. K., Jaffe E. S., Schechter G. P., Guccion J. G. Mitogen requirements for the in vitro propagation of cutaneous T-cell lymphomas. Blood. 1980 Mar;55(3):409–417. [PubMed] [Google Scholar]
- Green M. R. When the products of oncogenes and anti-oncogenes meet. Cell. 1989 Jan 13;56(1):1–3. doi: 10.1016/0092-8674(89)90975-6. [DOI] [PubMed] [Google Scholar]
- Gronostajski R. M., Goldberg A. L., Pardee A. B. Energy requirement for degradation of tumor-associated protein p53. Mol Cell Biol. 1984 Mar;4(3):442–448. doi: 10.1128/mcb.4.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M., Yu A., Gjerset R. Characteristics of the leukemic cell in childhood acute lymphoblastic T cell leukemia at diagnosis. Leukemia. 1990 Mar;4(3):230–234. [PubMed] [Google Scholar]
- Halevy O., Hall A., Oren M. Stabilization of the p53 transformation-related protein in mouse fibrosarcoma cell lines: effects of protein sequence and intracellular environment. Mol Cell Biol. 1989 Aug;9(8):3385–3392. doi: 10.1128/mcb.9.8.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I. A regulatory hierarchy for cell specialization in yeast. Nature. 1989 Dec 14;342(6251):749–757. doi: 10.1038/342749a0. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. Functional inactivation of genes by dominant negative mutations. Nature. 1987 Sep 17;329(6136):219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- Hinds P., Finlay C., Levine A. J. Mutation is required to activate the p53 gene for cooperation with the ras oncogene and transformation. J Virol. 1989 Feb;63(2):739–746. doi: 10.1128/jvi.63.2.739-746.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose M., Minato K., Tobinai K., Shimoyama M., Watanabe S., Abe T., Deura K. Two novel cultured cell lines, A3/Kawakami and A4/Fukuda, derived from malignant lymphoma of B(non-T)-cell nature of the gastrointestinal tract. Gan. 1983 Feb;74(1):106–115. [PubMed] [Google Scholar]
- Huang C. C., Hou Y., Woods L. K., Moore G. E., Minowada J. Cytogenetic study of human lymphoid T-cell lines derived from lymphocytic leukemia. J Natl Cancer Inst. 1974 Sep;53(3):655–660. doi: 10.1093/jnci/53.3.655. [DOI] [PubMed] [Google Scholar]
- James C. D., Carlbom E., Nordenskjold M., Collins V. P., Cavenee W. K. Mitotic recombination of chromosome 17 in astrocytomas. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2858–2862. doi: 10.1073/pnas.86.8.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins J. R., Rudge K., Chumakov P., Currie G. A. The cellular oncogene p53 can be activated by mutagenesis. 1985 Oct 31-Nov 6Nature. 317(6040):816–818. doi: 10.1038/317816a0. [DOI] [PubMed] [Google Scholar]
- Jenkins J. R., Rudge K., Currie G. A. Cellular immortalization by a cDNA clone encoding the transformation-associated phosphoprotein p53. Nature. 1984 Dec 13;312(5995):651–654. doi: 10.1038/312651a0. [DOI] [PubMed] [Google Scholar]
- Kelman Z., Prokocimer M., Peller S., Kahn Y., Rechavi G., Manor Y., Cohen A., Rotter V. Rearrangements in the p53 gene in Philadelphia chromosome positive chronic myelogenous leukemia. Blood. 1989 Nov 15;74(7):2318–2324. [PubMed] [Google Scholar]
- Koeffler H. P., Miller C., Nicolson M. A., Ranyard J., Bosselman R. A. Increased expression of p53 protein in human leukemia cells. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4035–4039. doi: 10.1073/pnas.83.11.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzberg J., Bigner S. H., Hershfield M. S. Establishment of the DU.528 human lymphohemopoietic stem cell line. J Exp Med. 1985 Nov 1;162(5):1561–1578. doi: 10.1084/jem.162.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Levine A. J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979 May;17(1):43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- Lübbert M., Miller C. W., Crawford L., Koeffler H. P. p53 in chronic myelogenous leukemia. Study of mechanisms of differential expression. J Exp Med. 1988 Mar 1;167(3):873–886. doi: 10.1084/jem.167.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay J., Steel C. M., Elder P. A., Forrest A. P., Evans H. J. Allele loss on short arm of chromosome 17 in breast cancers. Lancet. 1988 Dec 17;2(8625):1384–1385. doi: 10.1016/s0140-6736(88)90584-3. [DOI] [PubMed] [Google Scholar]
- Mashal R., Shtalrid M., Talpaz M., Kantarjian H., Smith L., Beran M., Cork A., Trujillo J., Gutterman J., Deisseroth A. Rearrangement and expression of p53 in the chronic phase and blast crisis of chronic myelogenous leukemia. Blood. 1990 Jan 1;75(1):180–189. [PubMed] [Google Scholar]
- Masuda H., Miller C., Koeffler H. P., Battifora H., Cline M. J. Rearrangement of the p53 gene in human osteogenic sarcomas. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7716–7719. doi: 10.1073/pnas.84.21.7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer W. E., Baserga R. Expression of the p53 protein during the cell cycle of human peripheral blood lymphocytes. Exp Cell Res. 1985 Sep;160(1):31–46. doi: 10.1016/0014-4827(85)90233-2. [DOI] [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Oren M., Maltzman W., Levine A. J. Post-translational regulation of the 54K cellular tumor antigen in normal and transformed cells. Mol Cell Biol. 1981 Feb;1(2):101–110. doi: 10.1128/mcb.1.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokocimer M., Shaklai M., Bassat H. B., Wolf D., Goldfinger N., Rotter V. Expression of p53 in human leukemia and lymphoma. Blood. 1986 Jul;68(1):113–118. [PubMed] [Google Scholar]
- Reich N. C., Oren M., Levine A. J. Two distinct mechanisms regulate the levels of a cellular tumor antigen, p53. Mol Cell Biol. 1983 Dec;3(12):2143–2150. doi: 10.1128/mcb.3.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogel A., Popliker M., Webb C. G., Oren M. p53 cellular tumor antigen: analysis of mRNA levels in normal adult tissues, embryos, and tumors. Mol Cell Biol. 1985 Oct;5(10):2851–2855. doi: 10.1128/mcb.5.10.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter V. p53, a transformation-related cellular-encoded protein, can be used as a biochemical marker for the detection of primary mouse tumor cells. Proc Natl Acad Sci U S A. 1983 May;80(9):2613–2617. doi: 10.1073/pnas.80.9.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovinski B., Benchimol S. Immortalization of rat embryo fibroblasts by the cellular p53 oncogene. Oncogene. 1988 May;2(5):445–452. [PubMed] [Google Scholar]
- Sarnow P., Ho Y. S., Williams J., Levine A. J. Adenovirus E1b-58kd tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kd cellular protein in transformed cells. Cell. 1982 Feb;28(2):387–394. doi: 10.1016/0092-8674(82)90356-7. [DOI] [PubMed] [Google Scholar]
- Smith L. J., McCulloch E. A., Benchimol S. Expression of the p53 oncogene in acute myeloblastic leukemia. J Exp Med. 1986 Sep 1;164(3):751–761. doi: 10.1084/jem.164.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow K., Judd W. Heterogeneity of a human T-lymphoblastoid cell line. Exp Cell Res. 1987 Aug;171(2):389–403. doi: 10.1016/0014-4827(87)90171-6. [DOI] [PubMed] [Google Scholar]
- Soussi T., Caron de Fromentel C., Stürzbecher H. W., Ullrich S., Jenkins J., May P. Evolutionary conservation of the biochemical properties of p53: specific interaction of Xenopus laevis p53 with simian virus 40 large T antigen and mammalian heat shock proteins 70. J Virol. 1989 Sep;63(9):3894–3901. doi: 10.1128/jvi.63.9.3894-3901.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Nau M. M., Chiba I., Birrer M. J., Rosenberg R. K., Vinocour M., Levitt M., Pass H., Gazdar A. F., Minna J. D. p53: a frequent target for genetic abnormalities in lung cancer. Science. 1989 Oct 27;246(4929):491–494. doi: 10.1126/science.2554494. [DOI] [PubMed] [Google Scholar]
- Venturelli D., Ku D. H., Narni F., Gatti C., Calabretta B. Lack of rearrangements of p53 tumor antigen gene locus in human hematological malignancies. Haematologica. 1988 Jul-Aug;73(4):259–264. [PubMed] [Google Scholar]
- Werness B. A., Levine A. J., Howley P. M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990 Apr 6;248(4951):76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- Wolf D., Rotter V. Major deletions in the gene encoding the p53 tumor antigen cause lack of p53 expression in HL-60 cells. Proc Natl Acad Sci U S A. 1985 Feb;82(3):790–794. doi: 10.1073/pnas.82.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota J., Wada M., Shimosato Y., Terada M., Sugimura T. Loss of heterozygosity on chromosomes 3, 13, and 17 in small-cell carcinoma and on chromosome 3 in adenocarcinoma of the lung. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9252–9256. doi: 10.1073/pnas.84.24.9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakut-Houri R., Bienz-Tadmor B., Givol D., Oren M. Human p53 cellular tumor antigen: cDNA sequence and expression in COS cells. EMBO J. 1985 May;4(5):1251–1255. doi: 10.1002/j.1460-2075.1985.tb03768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg F. M., Tigges A. J., Schipper M. E., den Hartog-Jager F. C., Kroes W. G., Walboomers J. M. Expression of the nuclear oncogene p53 in colon tumours. J Pathol. 1989 Mar;157(3):193–199. doi: 10.1002/path.1711570304. [DOI] [PubMed] [Google Scholar]