Abstract
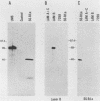
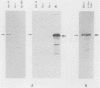
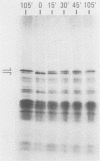
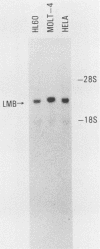
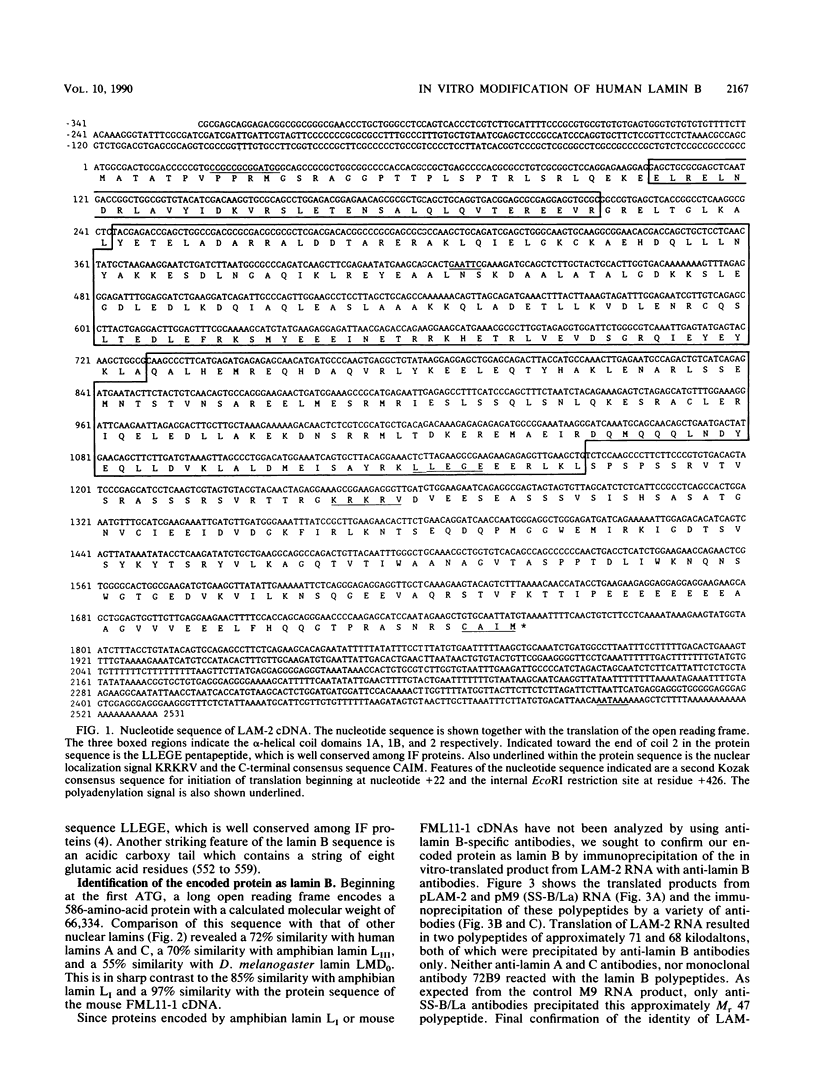
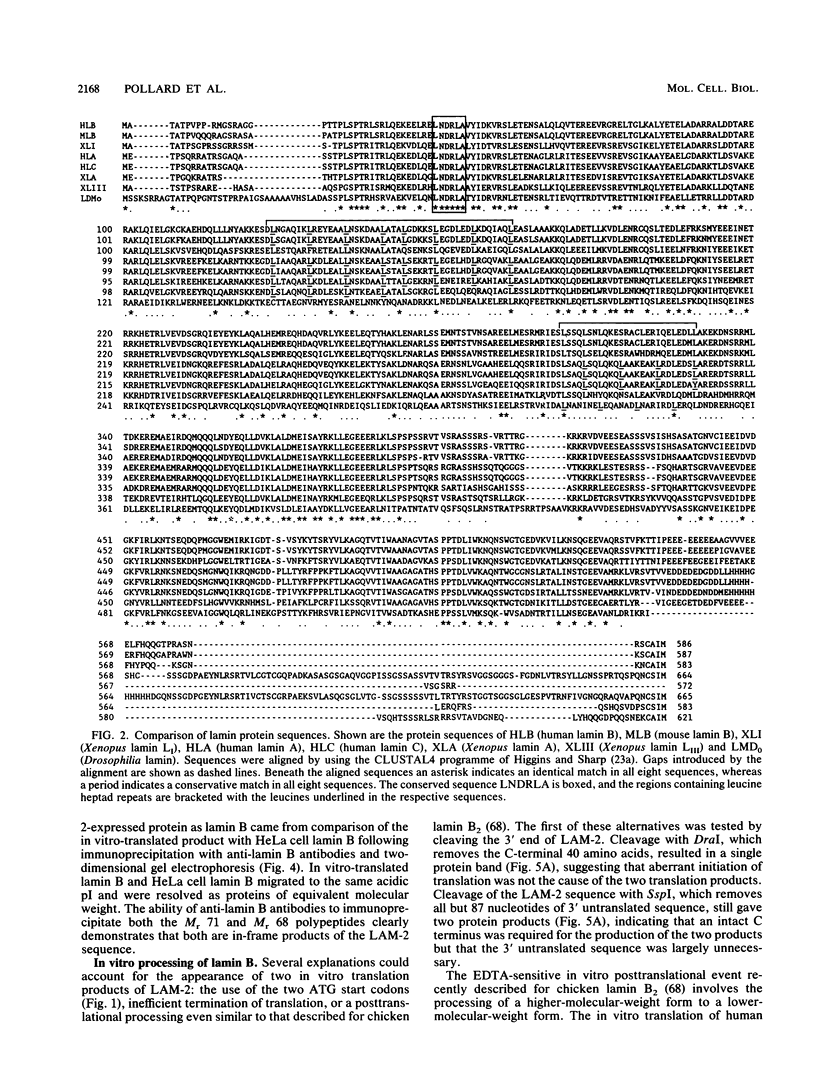
Autoimmune diseases are characterized by spontaneously occurring autoantibodies which have proven to be useful reagents for the characterization of specific nuclear proteins. Using a monoclonal autoantibody (72B9) derived from a murine lupus strain, we have cloned a cDNA from the human T-cell line MOLT-4, which encodes nuclear lamin B. The identity of the encoded protein as lamin B was established by both biochemical and immunological criteria. Inspection of the deduced amino acid sequence of lamin B revealed the presence in coil 1B of the alpha-helical domain of a leucine heptad repeat region. Analysis of mRNA in HL60 and MOLT-4 cells, which express only lamin B, or HeLa cells, which express all three major lamins (A, B, and C), together with the comigration of in vitro-translated product with isolated HeLa cell lamin B by two-dimensional gel electrophoresis, suggests that a single lamin B is expressed in mammalian somatic cells. In vitro translation with the cDNA clone revealed an EDTA-sensitive posttranslational modification which resulted in an increase in the apparent molecular weight to that equivalent to the native in vivo-synthesized lamin B protein. This in vitro modification included incorporation of a product of mevalonolactone and required an intact carboxy terminus.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck L. A., Hosick T. J., Sinensky M. Incorporation of a product of mevalonic acid metabolism into proteins of Chinese hamster ovary cell nuclei. J Cell Biol. 1988 Oct;107(4):1307–1316. doi: 10.1083/jcb.107.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bloemendal H., Pieper F. R. Intermediate filaments: known structure, unknown function. Biochim Biophys Acta. 1989 Apr 12;1007(3):245–253. doi: 10.1016/0167-4781(89)90144-9. [DOI] [PubMed] [Google Scholar]
- Blumenberg M. Evolution of homologous domains of cytoplasmic intermediate filament proteins and lamins. Mol Biol Evol. 1989 Jan;6(1):53–65. doi: 10.1093/oxfordjournals.molbev.a040533. [DOI] [PubMed] [Google Scholar]
- Chan E. K., Sullivan K. F., Tan E. M. Ribonucleoprotein SS-B/La belongs to a protein family with consensus sequences for RNA-binding. Nucleic Acids Res. 1989 Mar 25;17(6):2233–2244. doi: 10.1093/nar/17.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelsky D., Sobotka C., O'Neill C. L. Lamin B methylation and assembly into the nuclear envelope. J Biol Chem. 1989 May 5;264(13):7637–7643. [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffley J. F., Stillman B. Transcriptional silencing and lamins. Nature. 1989 Nov 2;342(6245):24–24. doi: 10.1038/342024a0. [DOI] [PubMed] [Google Scholar]
- Fisher D. Z., Chaudhary N., Blobel G. cDNA sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6450–6454. doi: 10.1073/pnas.83.17.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W. Nuclear lamins and cytoplasmic intermediate filament proteins: a growing multigene family. Cell. 1987 Jan 16;48(1):3–4. doi: 10.1016/0092-8674(87)90345-x. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Tyner A. L., Giudice G. J., Marchuk D., RayChaudhury A., Rosenberg M. The human keratin genes and their differential expression. Curr Top Dev Biol. 1987;22:5–34. doi: 10.1016/s0070-2153(08)60097-6. [DOI] [PubMed] [Google Scholar]
- Gerace L., Burke B. Functional organization of the nuclear envelope. Annu Rev Cell Biol. 1988;4:335–374. doi: 10.1146/annurev.cb.04.110188.002003. [DOI] [PubMed] [Google Scholar]
- Gerace L., Comeau C., Benson M. Organization and modulation of nuclear lamina structure. J Cell Sci Suppl. 1984;1:137–160. doi: 10.1242/jcs.1984.supplement_1.10. [DOI] [PubMed] [Google Scholar]
- Glass C., Fuchs E. Isolation, sequence, and differential expression of a human K7 gene in simple epithelial cells. J Cell Biol. 1988 Oct;107(4):1337–1350. doi: 10.1083/jcb.107.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenbaum Y., Landesman Y., Drees B., Bare J. W., Saumweber H., Paddy M. R., Sedat J. W., Smith D. E., Benton B. M., Fisher P. A. Drosophila nuclear lamin precursor Dm0 is translated from either of two developmentally regulated mRNA species apparently encoded by a single gene. J Cell Biol. 1988 Mar;106(3):585–596. doi: 10.1083/jcb.106.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilly M. N., Bensussan A., Bourge J. F., Bornens M., Courvalin J. C. A human T lymphoblastic cell line lacks lamins A and C. EMBO J. 1987 Dec 1;6(12):3795–3799. doi: 10.1002/j.1460-2075.1987.tb02715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989 Jun 30;57(7):1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Hedberg K. K., Chen L. B. Absence of intermediate filaments in a human adrenal cortex carcinoma-derived cell line. Exp Cell Res. 1986 Apr;163(2):509–517. doi: 10.1016/0014-4827(86)90081-9. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biosci. 1989 Apr;5(2):151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- Höger T. H., Krohne G., Franke W. W. Amino acid sequence and molecular characterization of murine lamin B as deduced from cDNA clones. Eur J Cell Biol. 1988 Dec;47(2):283–290. [PubMed] [Google Scholar]
- Högner D., Telling A., Lepper K., Jost E. Patterns of nuclear lamins in diverse animal and plant cells and in germ cells as revealed by immunofluorescence microscopy with polyclonal and monoclonal antibodies. Tissue Cell. 1984;16(5):693–703. doi: 10.1016/0040-8166(84)90003-x. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. Additional members of the rat liver lamin polypeptide family. Structural and immunological characterization. J Biol Chem. 1989 Aug 15;264(23):13946–13955. [PubMed] [Google Scholar]
- Kouzarides T., Ziff E. The role of the leucine zipper in the fos-jun interaction. Nature. 1988 Dec 15;336(6200):646–651. doi: 10.1038/336646a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohne G., Waizenegger I., Höger T. H. The conserved carboxy-terminal cysteine of nuclear lamins is essential for lamin association with the nuclear envelope. J Cell Biol. 1989 Nov;109(5):2003–2011. doi: 10.1083/jcb.109.5.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohne G., Wolin S. L., McKeon F. D., Franke W. W., Kirschner M. W. Nuclear lamin LI of Xenopus laevis: cDNA cloning, amino acid sequence and binding specificity of a member of the lamin B subfamily. EMBO J. 1987 Dec 1;6(12):3801–3808. doi: 10.1002/j.1460-2075.1987.tb02716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The DNA binding domain of the rat liver nuclear protein C/EBP is bipartite. Science. 1989 Mar 31;243(4899):1681–1688. doi: 10.1126/science.2494700. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Lassoued K., Guilly M. N., Danon F., Andre C., Dhumeaux D., Clauvel J. P., Brouet J. C., Seligmann M., Courvalin J. C. Antinuclear autoantibodies specific for lamins. Characterization and clinical significance. Ann Intern Med. 1988 Jun;108(6):829–833. doi: 10.7326/0003-4819-108-6-829. [DOI] [PubMed] [Google Scholar]
- Lebel S., Lampron C., Royal A., Raymond Y. Lamins A and C appear during retinoic acid-induced differentiation of mouse embryonal carcinoma cells. J Cell Biol. 1987 Sep;105(3):1099–1104. doi: 10.1083/jcb.105.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel S., Raymond Y. Lamins A, B and C share an epitope with the common domain of intermediate filament proteins. Exp Cell Res. 1987 Apr;169(2):560–565. doi: 10.1016/0014-4827(87)90216-3. [DOI] [PubMed] [Google Scholar]
- Lehner C. F., Kurer V., Eppenberger H. M., Nigg E. A. The nuclear lamin protein family in higher vertebrates. Identification of quantitatively minor lamin proteins by monoclonal antibodies. J Biol Chem. 1986 Oct 5;261(28):13293–13301. [PubMed] [Google Scholar]
- Lowy D. R., Willumsen B. M. Protein modification: new clue to Ras lipid glue. Nature. 1989 Oct 5;341(6241):384–385. doi: 10.1038/341384a0. [DOI] [PubMed] [Google Scholar]
- Magee T., Hanley M. Protein modification. Sticky fingers and CAAX boxes. Nature. 1988 Sep 8;335(6186):114–115. doi: 10.1038/335114a0. [DOI] [PubMed] [Google Scholar]
- Maltese W. A., Erdman R. A. Characterization of isoprenoid involved in the post-translational modification of mammalian cell proteins. J Biol Chem. 1989 Oct 25;264(30):18168–18172. [PubMed] [Google Scholar]
- Maul G. G., Schatten G., Jimenez S. A., Carrera A. E. Detection of nuclear lamin B epitopes in oocyte nuclei from mice, sea urchins, and clams using a human autoimmune serum. Dev Biol. 1987 Jun;121(2):368–375. doi: 10.1016/0012-1606(87)90173-4. [DOI] [PubMed] [Google Scholar]
- McKeon F. D., Kirschner M. W., Caput D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature. 1986 Feb 6;319(6053):463–468. doi: 10.1038/319463a0. [DOI] [PubMed] [Google Scholar]
- McKeon F. D., Tuffanelli D. L., Fukuyama K., Kirschner M. W. Autoimmune response directed against conserved determinants of nuclear envelope proteins in a patient with linear scleroderma. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4374–4378. doi: 10.1073/pnas.80.14.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviano Y., Gerace L. Phosphorylation of the nuclear lamins during interphase and mitosis. J Biol Chem. 1985 Jan 10;260(1):624–632. [PubMed] [Google Scholar]
- Parker K. A., Steitz J. A. Structural analysis of the human U3 ribonucleoprotein particle reveal a conserved sequence available for base pairing with pre-rRNA. Mol Cell Biol. 1987 Aug;7(8):2899–2913. doi: 10.1128/mcb.7.8.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry D. A., Conway J. F., Steinert P. M. Structural studies on lamin. Similarities and differences between lamin and intermediate-filament proteins. Biochem J. 1986 Aug 15;238(1):305–308. doi: 10.1042/bj2380305. [DOI] [PMC free article] [PubMed] [Google Scholar]
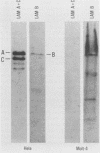
- Paulin-Levasseur M., Scherbarth A., Giese G., Röser K., Bohn W., Traub P. Expression of nuclear lamins in mammalian somatic cells lacking cytoplasmic intermediate filament proteins. J Cell Sci. 1989 Mar;92(Pt 3):361–370. doi: 10.1242/jcs.92.3.361. [DOI] [PubMed] [Google Scholar]
- Paulin-Levasseur M., Scherbarth A., Traub U., Traub P. Lack of lamins A and C in mammalian hemopoietic cell lines devoid of intermediate filament proteins. Eur J Cell Biol. 1988 Oct;47(1):121–131. [PubMed] [Google Scholar]
- Raymond Y., Chauvette M. Minor lamin polypeptides from rat liver nuclei can be cross-linked into heteropolymers by disulfide bridges. Biochem Cell Biol. 1988 Dec;66(12):1295–1302. doi: 10.1139/o88-150. [DOI] [PubMed] [Google Scholar]
- Raymond Y., Gagnon G. Lamin B shares a number of distinct epitopes with lamins A and C and with intermediate filament proteins. Biochemistry. 1988 Apr 5;27(7):2590–2597. doi: 10.1021/bi00407a048. [DOI] [PubMed] [Google Scholar]
- Reeves W. H., Chaudhary N., Salerno A., Blobel G. Lamin B autoantibodies in sera of certain patients with systemic lupus erythematosus. J Exp Med. 1987 Mar 1;165(3):750–762. doi: 10.1084/jem.165.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer G., Pollard K. M., Penning C. A., Ochs R. L., Lischwe M. A., Busch H., Tan E. M. Monoclonal autoantibody from a (New Zealand black x New Zealand white)F1 mouse and some human scleroderma sera target an Mr 34,000 nucleolar protein of the U3 RNP particle. Arthritis Rheum. 1987 Jul;30(7):793–800. doi: 10.1002/art.1780300709. [DOI] [PubMed] [Google Scholar]
- Riedel W., Werner D. Nucleotide sequence of the full-length mouse lamin C cDNA and its deduced amino-acid sequence. Biochim Biophys Acta. 1989 Jun 1;1008(1):119–122. doi: 10.1016/0167-4781(89)90179-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P., Ransone L. J., Lamph W. W., Verma I. M. Direct interaction between fos and jun nuclear oncoproteins: role of the 'leucine zipper' domain. Nature. 1988 Dec 15;336(6200):692–695. doi: 10.1038/336692a0. [DOI] [PubMed] [Google Scholar]
- Schuermann M., Neuberg M., Hunter J. B., Jenuwein T., Ryseck R. P., Bravo R., Müller R. The leucine repeat motif in Fos protein mediates complex formation with Jun/AP-1 and is required for transformation. Cell. 1989 Feb 10;56(3):507–516. doi: 10.1016/0092-8674(89)90253-5. [DOI] [PubMed] [Google Scholar]
- Song M. K., Adolph K. W. ADP-ribosylation of nonhistone proteins during the HeLa cell cycle. Biochem Biophys Res Commun. 1983 Sep 30;115(3):938–945. doi: 10.1016/s0006-291x(83)80025-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Stewart C., Burke B. Teratocarcinoma stem cells and early mouse embryos contain only a single major lamin polypeptide closely resembling lamin B. Cell. 1987 Nov 6;51(3):383–392. doi: 10.1016/0092-8674(87)90634-9. [DOI] [PubMed] [Google Scholar]
- Stick R., Angres B., Lehner C. F., Nigg E. A. The fates of chicken nuclear lamin proteins during mitosis: evidence for a reversible redistribution of lamin B2 between inner nuclear membrane and elements of the endoplasmic reticulum. J Cell Biol. 1988 Aug;107(2):397–406. doi: 10.1083/jcb.107.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stick R. cDNA cloning of the developmentally regulated lamin LIII of Xenopus laevis. EMBO J. 1988 Oct;7(10):3189–3197. doi: 10.1002/j.1460-2075.1988.tb03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear lamins. Ann Intern Med. 1988 Jun;108(6):897–898. doi: 10.7326/0003-4819-108-6-897. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R., Tjian R. Leucine repeats and an adjacent DNA binding domain mediate the formation of functional cFos-cJun heterodimers. Science. 1989 Mar 31;243(4899):1689–1694. doi: 10.1126/science.2494701. [DOI] [PubMed] [Google Scholar]
- Vorburger K., Lehner C. F., Kitten G. T., Eppenberger H. M., Nigg E. A. A second higher vertebrate B-type lamin. cDNA sequence determination and in vitro processing of chicken lamin B2. J Mol Biol. 1989 Aug 5;208(3):405–415. doi: 10.1016/0022-2836(89)90505-6. [DOI] [PubMed] [Google Scholar]
- Weber K., Plessmann U., Ulrich W. Cytoplasmic intermediate filament proteins of invertebrates are closer to nuclear lamins than are vertebrate intermediate filament proteins; sequence characterization of two muscle proteins of a nematode. EMBO J. 1989 Nov;8(11):3221–3227. doi: 10.1002/j.1460-2075.1989.tb08481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesierska-Gadek J., Penner E., Hitchman E., Sauermann G. Antibodies to nuclear lamins in autoimmune liver disease. Clin Immunol Immunopathol. 1988 Oct;49(1):107–115. doi: 10.1016/0090-1229(88)90100-6. [DOI] [PubMed] [Google Scholar]
- Wolda S. L., Glomset J. A. Evidence for modification of lamin B by a product of mevalonic acid. J Biol Chem. 1988 May 5;263(13):5997–6000. [PubMed] [Google Scholar]
- Wolin S. L., Krohne G., Kirschner M. W. A new lamin in Xenopus somatic tissues displays strong homology to human lamin A. EMBO J. 1987 Dec 1;6(12):3809–3818. doi: 10.1002/j.1460-2075.1987.tb02717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worman H. J., Lazaridis I., Georgatos S. D. Nuclear lamina heterogeneity in mammalian cells. Differential expression of the major lamins and variations in lamin B phosphorylation. J Biol Chem. 1988 Aug 25;263(24):12135–12141. [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]