Abstract
Mesenchymal stem cells (MSCs) are a prototypic adult stem cell with capacity for self-renewal and differentiation with a broad tissue distribution. Initially described in bone marrow, MSCs have the capacity to differentiate into mesodermal and non-mesodermal derived tissues. The endogenous role for MSCs is maintenance of stem cell niches (classically the hematopoietic), and as such MSCs participate in organ homeostasis, wound healing, and successful aging. From a therapeutic perspective, and facilitated by the ease of preparation and immunologic privilege, MSCs are emerging as an extremely promising therapeutic agent for tissue regeneration. Studies in animal models of myocardial infarction (MI) demonstrate the ability of transplanted MSCs to engraft and differentiate into cardiomyocytes and vasculature cells, recruit endogenous cardiac stem cells, and secrete a wide array of paracrine factors. Together these properties can be harnessed to both prevent and reverse remodeling in the ischemically injured ventricle. In proof-of-concept and phase I clinical trials, MSC therapy improve LV function, induces reverse remodeling, and decreases scar size. This article reviews the current understanding of MSC biology, mechanism of action in cardiac repair, translational findings, and early clinical trial data of MSC therapy for cardiac disease.
Keywords: Stem Cells, Regeneration, Differentiation, Niches
Introduction
Ischemic heart disease is the leading cause of death in developed countries and carries significant morbidity.1 After an acute myocardial infarction (MI), the heart has limited capacity for self-renewal and undergoes remodeling with resulting depressed left ventricular (LV) function.2 Over the past decade, there has been tremendous enthusiasm in the quest to find a stem cell capable of regenerating lost myocardium and restoring cardiac function.
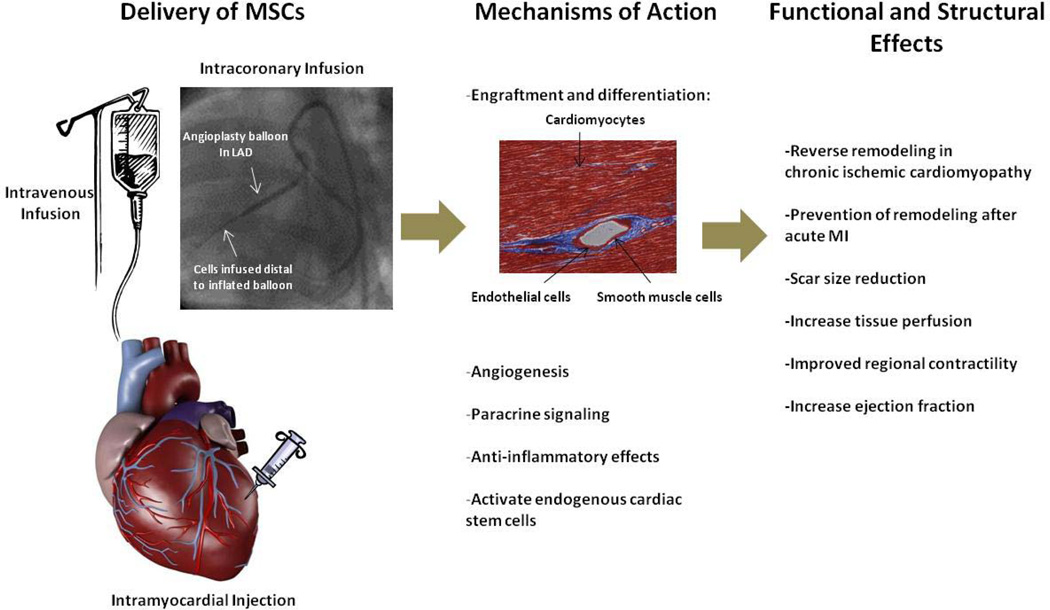
Mesenchymal stem cells (MSCs) were first identified and isolated from the bone marrow (BM) more than 40 years ago3, and have emerged as one of the leading candidates in cellular cardiomyoplasty (Figure 1). The unique properties of MSCs—easily isolated and amplified from the BM,4 immunologically tolerated as an allogeneic transplant5, and multilineage potential6—have lead to intense investigation as a cell-based therapeutic for cardiac repair. In this review, we describe the biology of MSCs, and discuss the data supporting the translation of MSC therapy to clinical trials for cardiac disease.
Figure 1. Delivery and potential effects of MSC therapy in cardiac disease.
Historical Overview
In 1970, Friedenstein and colleagues3 demonstrated that bone marrow (BM) contains a population of hematopoietic stem cells (HSCs) and a rare population of plastic-adherent stromal cells (1 in 10,000 nucleated cells in BM). These plastic adherent cells, initially referred to as stromal cells and now commonly called MSCs, were capable of forming single-cell colonies. As the plastic-adherent BM cells were expanded in culture, round-shaped colonies resembling fibroblastoid cells formed and were given the name Colony Forming Unit – fibroblasts (CFU-f). Friedenstein was the first investigator to demonstrate the ability of MSCs to differentiate into mesodermal derived tissue as well as identify their importance in controlling the hematopoietic niche7. Control of stem cell niches – functional and structural units that spatiotemporally regulate stem cell division and differentiation8, 9 – is emerging as a key role played by MSCs in a broad array of tissues, including hair follicles and the gut, and recently MSC ablation was shown to disrupt hematopoiesis.10
During the 1980s, MSCs were shown to differentiate into osteoblasts, chondrocytes, and adipocytes.11, 12 Caplan demonstrated that bone and cartilage turnover was mediated by MSCs, and the surrounding conditions were critical to inducing MSC differentiation.13 In the 1990s, MSCs were shown to differentiate into a myogenic phenotype,14 and Pittenger and colleagues demonstrated that individual adult human MSCs were capable of being expanded to colonies while still retaining their multilineage potential.6 Also during the late 1990s, Kopen et al. described the capacity of MSCs to transdifferentiate into ectodermal derived tissue.15
During the early 21st century, in-vivo studies demonstrated that human MSCs transdifferentiate into endodermal derived cells and cardiomyocytes;16, 17 and in-vitro co-culturing of ventricular myocytes with MSCs induced transdifferentiation into a cardiomyocyte phenotype.18 It was also during this time that MSCs were demonstrated to suppress T-lymphocyte proliferation, paving the way for the application of MSC therapy for allogeneic transplantation and as a potential immunomodulatory therapy.19 Large animal preclinical studies of MSC administration in post-MI hearts demonstrated the ability of MSCs to engraft, differentiate, and produce substantial functional recovery.20–22 Recently, MSC therapy has been translated to clinical trials for ischemic heart disease.23–25
Definition of an MSC
No unique cell surface marker unequivocally distinguishes MSCs from other HSCs, making a uniform definition difficult. The International Society for Cell Therapy (ISCT) proposed a criteria26 that comprises: (1) adherence to plastic in standard culture conditions; (2) expression of the surface molecules CD73, CD90, CD105 in the absence of CD34, CD45, HLA-DR, CD14 or CD11b, CD79a or CD19 surface molecules as assessed by FACS analysis; (3) capacity for differentiation to osteoblasts, adipocytes, and chondroblasts in vitro. These criteria were established to standardize human (h)MSC isolation but may not uniformly apply to other species. For example, murine MSCs have been shown to differ in marker expression and behavior compared to hMSCs.27 As MSCs are isolated and expanded in culture it has also been proposed that certain in-vivo surface markers may no longer be expressed after explantation, while new markers are acquired during expansion. For example, an MSC line was isolated that uniformly expressed HLA-DR (a marker that should not be expressed on MSCs by the above definition) while also expressing CD90 and CD105, adhering to plastic in culture, and were capable of differentiating into osteoblasts, adipocytes, and chondroblasts.28 Indeed, MSCs possess species specific characteristics, which make an unequivocal definition difficult to apply to both human and animal models.
Sources, Isolation, and Types of MSCs
MSCs isolated from bone marrow,6 adipose tissue,29 synovial tissue,30 lung tissue,31 umbilical cord blood,32 and peripheral blood33 are heterogeneous with variable growth potential, but all have similar surface markers and mesodermal differentiation potential.34 Furthermore, MSCs have been isolated from nearly every tissue type (brain, spleen, liver, kidney, lung, bone marrow, muscle, thymus, aorta, vena cava, pancreas) of adult mice, suggesting MSCs may reside in all post-natal organs.35 MSCs can be derived from many tissue sources, consistent with their broad, possibly ubiquitous distribution. From a translational perspective, as most studies of MSC therapy for cardiac repair have utilized BM derived MSCs, we will focus this review on BM-MSCs.
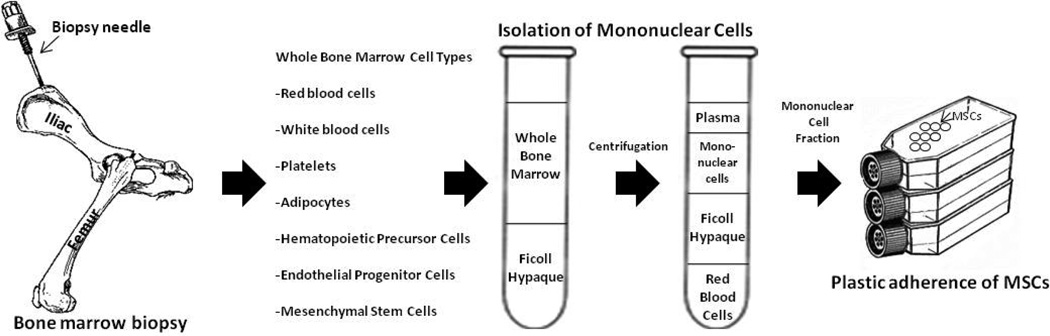
The BM is the major source of HSCs that continually renew red blood cells (RBCs), platelets, monocytes, and granulocytes. The HSCs are housed within the BM by non-HSCs that support the microenvironment necessary for development and differentiation of HSCs,36 and the MSC is one of the most important cells that supports the BM microenvironment, termed the hematopoietic niche.37 MSCs have been isolated from numerous species,6, 38, 39 and the three critical steps that allow MSCs to be isolated from other BM cells are: (1) the use of density gradient centrifugation (Ficoll or Percoll) to separate non-nucleated RBCs from nucleated cells; (2) the ability of MSCs to adhere to plastic; and (3) the ability of monocytes to be separated from MSCs by trypsinization.4 Protocols for isolation and expansion are similar among various species, as depicted in Figure 2.
Figure 2. Isolation of mesenchymal stem cells from a bone marrow biopsy.
Many stem cells and maturing cells exist in the bone marrow cavity. The mononuclear cells are isolated from red blood cells using Ficoll density centrifugation and the MSCs are separated from the MNCs by plastic adherence in culture.
Expansion of plastic adherence MSCs is the most widely employed method of obtaining MSCs and represents the most commonly used source of MSCs for cardiac repair. As knowledge evolves regarding MSC biology, attempts have been made to identify the endogenous MSC precursor based upon cell surface antigen expression. A subset of MSCs expressing the marker neurotrophic growth factor (CD271) has been shown to have similar differentiation capacity as traditional MSCs, but secrete higher levels of cytokines and have greater immunosuppressive properties.40 Another MSC subpopulation is the Stro-3-positive mesenchymal precursor cell (MPC), which has extensive capacity for proliferation and differentiation.41
Immunology of MSCs
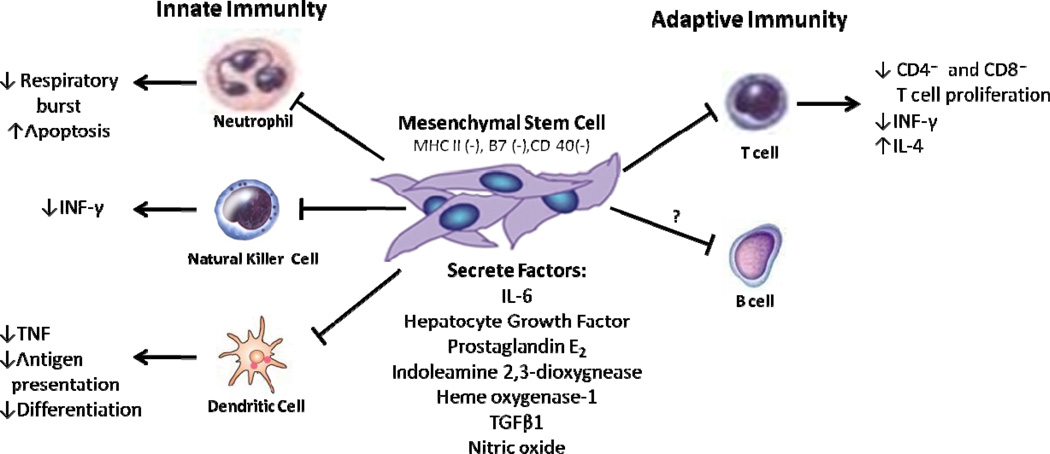
Human MSCs express moderate levels of human leukocyte antigen (HLA) major histocompatibility complex (MHC) class I, lack MHC class II expression, and do not express costimulatory molecules B7 and CD40 ligand.42–44 Tolerance of MSCs as an allogeneic transplant is due to this unique immunophenotype coupled with powerful immunosuppressive activity via cell-cell contact with target immune cells and secretion of soluble factors, such as nitric oxide, indoleamine 2,3-dioxygnease, and heme oxygenase-1.45–48 MSCs produce an immunomodulatory effect by interacting with both innate and adaptive immune cells (Figure 3).
Figure 3. MSC interactions with immune cells.
MSCs are immunoprivileged cells inhibit both innate (neutrophils, dendritic cells, natural killer cells) and adaptive (T cells and B cells) immune cells.
The innate immune cells—neutrophils, dendritic cells (DCs), natural killer (NK) cells, eosinophils, mast cells, and macrophages—are responsible for non-specific defense to infection, and MSCs have been shown to suppress most of these inflammatory cells. Neutrophils are one of the first cells to respond to an infection and an important process in their response to inflammatory mediators is the respiratory burst, characterized by large oxygen consumption and production of reactive oxygen species.5 MSCs have shown to dampen the respiratory burst process by releasing interleukin (IL)-6.49 DCs play an important role in antigen presentation to naïve T cells, and MSCs have been shown to inhibit the differentiation of immature monocytes into DCs.50 Additionally, co-cultures of MSCs and DCs inhibit the production of tumor necrosis factor (TNF-α), a potent inflammatory molecule.51 NK cells are important innate cells in defending against viral organisms and in tumor defense by secreting cytokines and cytolysis.5 MSCs cultured with freshly isolated resting NK cells have been shown to inhibit IL-2 induced proliferation and decrease secretion of interferon (IFN-γ) by 80%; however, IL-2 activated NK cells can lyse autologous and allogeneic MSCs.51, 52
The adaptive immune system, composed of T and B lymphocytes, is capable of generating specific immune responses to pathogens with the production of memory cells. Once a T-cell is activated by a foreign antigen binding to a specific T-cell receptor, the T-cell proliferates and releases cytokines.5 T-cells exist as CD8+ cytotoxic T-cells that induce death of target cells, or CD4+ helper T-cells which modulate the other immune cells, and MSCs have shown to suppress T-cell proliferation in a mixed lymphocyte culture.19, 51 Suppression of lymphocyte proliferation is mediated through cytokines released by MSCs, such as rhHGF and rhTGF-β1, that equally suppress the proliferation of cytotoxic and helper T cells.19 When MSCs are present during naïve T cell differentiation to CD4+ T helper cells, there is marked decrease in the production of IFN-γ and increase in the production of IL-4,51 suggesting that MSCs alter naïve T cells from a pro-inflammatory state (heavy production of IFN- γ) to an anti-inflammatory state (more production of IL-4). The B cell, which produce antibodies, are highly dependent on T-cells and MSC inhibition of T cells likely contributes to the interactions of MSCs with B cells.5 Conflicting data has shown that MSCs can inhibit and promote proliferation of B cells.53, 54
In-vivo studies have demonstrated that MSCs may play an important role in immune-mediated diseases and rejection of transplanted tissue. Skin allograft transplants survive longer with concomitant intravenous administration of MSCs in a baboon model,55 and systemic infusion of MSCs preferentially home to areas of injury.56 Infusion of MSCs in graft-versus-host disease (GVHD), a life threatening complication from allogeneic HSC transplant, has demonstrated encouraging results in steroid resistant GVHD.57
While MSCs have been shown to suppress innate and adaptive immune cells, in-vitro experiments with MSCs induced to acquire a cardiac or vascular phenotype have increased MHC Ia and II (immunogenic) expression and decreased MHC Ib (immunotolerant) expression.58 Furthermore, when allogeneic MSCs were transplanted into post-MI rats, the cells were eliminated from the heart and did not improve cardiac function.58 This data from rats suggests that MSCs may lose their immunoprivileged properties as they differentiate; however, allogeneic MSCs transplanted into pigs were shown to engraft to a large extent as undifferentiated MSCs (~75% of cell identified) with few MSCs differentiated (~25%), and did not induce an immune response.21 In addition, engraftment of these undifferentiated MSCs in post-MI hearts can stimulate endogenous repair mechanisms.39
Multilineage Potential of MSCs
Stem cells are characterized by their ability to self renew, clone, and differentiate into multiple tissues.59 The first MSCs isolated more than 40 years ago were observed in culture to form bone and cartilage deposits;3, 60 and this multilineage capability has become a defining feature of MSCs.61 Subsequent experiments in the 1970s using autologous transplantation of pellets of BM-MSCs to the sub-capsular region of a rabbit kidney demonstrated the in-vivo differentiation capabilities of MSCs to osteocytes7. Caplan expanded on this pioneering work by demonstrating that MSC differentiation into a distinct phenotype (e.g. chondrocytes or osteoblasts) is dependent on surrounding conditions.13 For example, MSC differentiation into osteoblasts is dependent on close proximity to vasculature; however, differentiation to the chondrocyte requires no vasculature.62 Ultimately, these early observations paved the way for experiments that have defined specific conditions to promote MSC differentiation.6
In-vitro Studies
MSCs in the presence of dexamethasone, β-glycerol phosphate, and ascorbic acid express alkaline phosphatase and calcium accumulation, a morphology consistent with osteogenic differentiation.6, 63–65 These osteogenic cells will react with antiosteogenic antibodies and form a mineralized extracellular matrix. The osteogenic potential of MSCs is conserved through numerous passages by their increased alkaline phosphatase activity.64 To induce chondrocyte differentiation, MSCs are cultured in a medium containing dexamethasone and transforming growth factor-beta3 (TGF-β3).66 Subsequently, the cells begin secreting an extracellular matrix, including type II collagen, aggrecan, anionic proteoglycans, consistent with articular cartilage formation.6 Glucocorticoids play an important role in the differentiation of MSCs to the chondrocyte lineage by promoting TGF-β mediated upregulation of collagen type II and inducing matrix components aggrecan, dermatopontin, and collagen type XI.67 MSC differentiation into an adipogenic lineage can be induced by culturing cells in the presence of dexamethasone, insulin, indomethacin, and 1-methyl-3-isobutylxanthine.6 Cells express markers consistent with adipocytes, such as peroxisome proliferation-activated receptor γ2 (PPAR γ2), lipoprotein lipase (LPL), and fatty acid binding protein aP2.6 These adipocytes accumulate lipid rich vacuoles and eventually coalesce.
BM-MSCs also have capacity for differentiation into other mesodermal derived tissue, notably myocytes, as shown in some but not all studies.14, 68 Rat and human BM-MSCs cultured with 5-azacytidine differentiate into multinucleated myotubes, consistent with a myocyte lineage.14, 18 These induced myocytes express β-myosin heavy chain (β-MHC), desmin, and α-cardiac actin and display spontaneous rhythmic calcium fluxes and potassium induced calcium fluxes.18 Further experiments have also shown that both human and porcine MSCs can be induced to skeletal myogenic differentiation.17, 69 Murine BM-MSCs exposed to 5-azacytidine have been shown to spontaneously beat alone, connect to adjoining cells, and at after 2–3 weeks beat in synchrony.70 Interestingly, electrophysiological evaluation of these cells showed two types of morphological action potentials, sinus node like potentials and ventricular myocyte like potentials. Reverse- transcription-polymerase chain reaction (RT-PCR) demonstrated atrial natriuretic, brain natriuretic peptide, α and β myosin heavy chain, Nkx2.5 and GATA4 genes. The authors concluded that MSCs are capable of adopting a cardiomyocyte phenotype in-vitro.70
Several co-culture experiments with cardiac myocytes have shown MSCs’ ability to transdifferentiate into a cardiac phenotype.68, 71 When mouse MSCs and rat ventricular myocytes were co-cultured, MSCs became α-actin positive, formed gap junctions with native myocytes, and these differentiating MSCs exhibited synchronous contractions with native myocytes. However, MSCs did not become α-actin positive when separated by a semi-permeable membrane from myocytes, suggesting that transdifferentiation requires cell-cell contact.71 Contrary to these findings, rat BM-MSCs co-cultured with neonatal rat ventricular myocytes separated by semi-permeable membrane were shown to transdifferentiate into cardiomyocytes.68 After one week, these MSCs were observed to be contracting; expressed SERCA2 and RyR2 by RT-PCR; and were positive for cardiac troponin T, sarcomeric α-actinin, and desmin by immunohistochemistry. These studies suggest that physical cell-cell contact as well as the local cardiac milieu and the factors secreted by myocytes play important roles in MSC transdifferentiation to cardiomyocytes. Future studies will be required to establish the relative roles of these two factors in MSC differentiation.
MSCs transdifferentiate into non-mesodermal derived tissue, such as neurons, when exposed to β-mercaptoethanol (BME).72, 73 As early as thirty-minutes in culture MSCs express the neuronal markers NSE and neurofilament–M (NF-M).72 Importantly, these MSCs which appeared to adopt a neuronal phenotype were not shown to produce an action potential.
Regulation of Differentiation
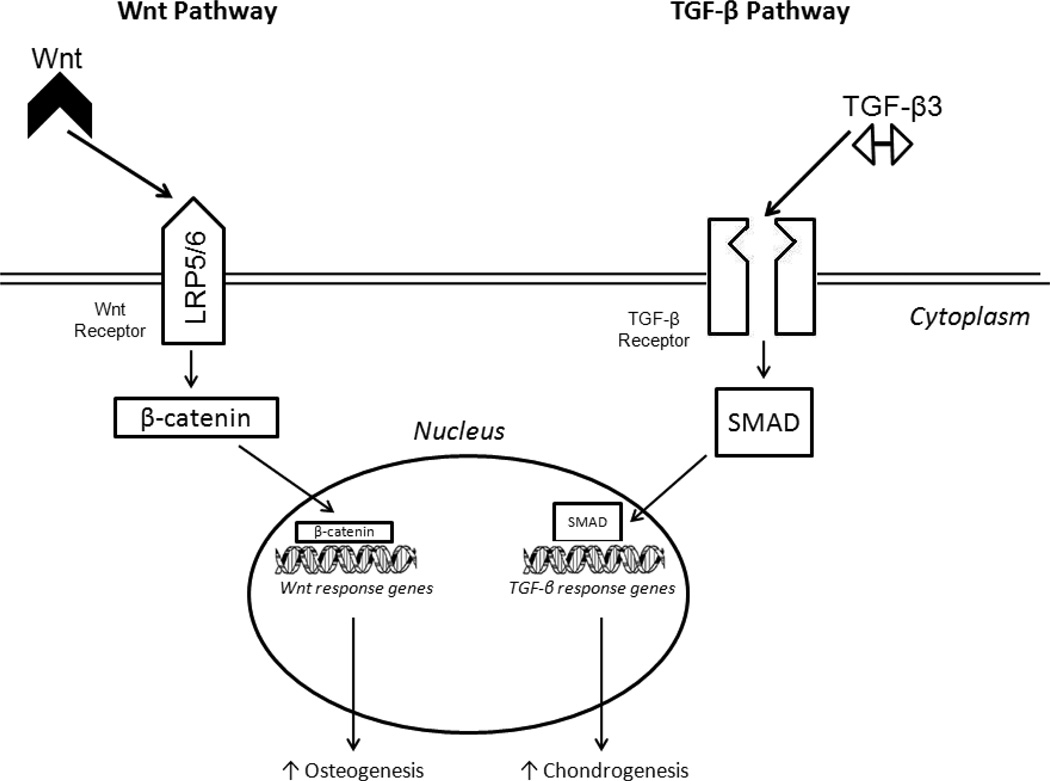
The molecular regulation of MSC differentiation has mainly focused on two pathways, the Wnt canonical pathway and the TGF-β superfamily pathway.61 Wnt are soluble glycoproteins which engage receptor complexes composed of Lrp5/6 proteins to induce a cascade of intracellular events that regulate cell proliferation and differentiation (Figure 4).74 The Wnt pathway has been shown to be critical in skeletogenesis by promoting osteoblast proliferation74 as well as suppression of chondrocyte formation via beta-catenin, an essential component in transducing Wnt signaling to the nucleus.75 Numerous Wnt ligands and receptors are expressed on MSCs, and regulation of Wnt proteins controls MSC differentiation.76 For example, exposure to Wnt3a inhibited MSC differentiation into osteoblasts while promoting undifferentiated MSC proliferation.77
Figure 4. Molecular regulation of MSC differentiation.
Wnt signaling and TGF-β induce intracellular signaling regulate differentiation of MSCs.
The other molecular pathway regulating MSC differentiation is the TGF-β pathway, a family of proteins involved skeletal tissue growth and regulation of MSC differentiation into chondrocytes.6, 61, 66 TGF-β3 upregulates gene expression in MSCs to promote chondrogenic differentiation via several intracellular cascades, including extracellular-signal regulated kinase (ERK1/2), SMAD proteins, mitogen-activated protein (MAP) kinases, p38, and JNK (Figure 4).61, 78 Several other molecules are have been shown to regulate MSC differentiation, such as epidermal growth factor (EGF), platelet derived growth factor (PDGF), and fibroblast growth factor (FGF).79, 80 Indeed, many growth factors likely interact with the Wnt and TGF-β pathways to control MSC differentiation.61
In-Vivo Mechanism of Action in Cardiac Repair
Engraftment and Differentiation: Cardiomyocytes and Vasculature
Numerous in-vivo rodent and swine studies have demonstrated the ability of MSCs to engraft and differentiate within the heart.17, 20, 21, 81–84 In 2001, Toma and colleagues reported the results of human BM-MSCs transfected with β-galactosidase reporter gene injected into immunodeficient adult mouse hearts.17 The majority hMSCs were found in the spleen, lung, and liver at 4 days post-injection. Only 0.44% of injected hMSCs were identified in the myocardium, and over time adopted a morphology indistinguishable from host cardiomyocytes. Immunofluorescence staining with anti-β-galactosidase identified engrafted MSCs in the myocardium, which co-localized with markers of cardiomyocyte lineage (desmin, α-actin, and cardiac troponin T) as early as 14 days post-injection. Shake and colleagues expanded on these early murine studies by surgically injecting autologous porcine Di-I-labeled MSCs directly into post-MI swine myocardium.20 Successful engraftment was demonstrated by observing the labeled MSC engrafting into scarred myocardium, and also expressing the cardiomyocyte markers α-actin, tropomyosin, troponin-T, myosin heavy chain, and phospholamban within 2-weeks post-injection.
In a swine model of chronic ischemic cardiomyopathy, our group reported the capacity of allogeneic MSCs to engraft and differentiate into cardiomyocytes, smooth muscle cells, and endothelium.21 Male BM-MSCs were injected into female swine, and identified by co-localization using Y-chromosome FISH. Cardiomyocyte differentiation was present in approximately 14% of Y-positive MSCs as identified by co-staining for the cardiac structural proteins α-sarcomeric actinin and tropomyosin, and transcriptional factors GATA-4 and Nkx2.5. Additionally, differentiated myocytes exhibited the capacity for coupling with host myocytes via connexin-43. MSCs also were shown to participate in coronary angiogenesis (actinin, calponin, smooth muscle protein 22-α expression) and accounted for approximately 10% of Y-positive cells identified, with the remaining 76% of engrafted cells identified as clusters of immature cells in the interstitial compartment. In another swine study, Makkar and colleagues also demonstrated the ability of allogeneic MSCs to engraft and differentiate into vascular cells and cardiomyocytes that formed connexin-43 gap junctions with adjacent host cardiomyocytes.84 Yang and colleagues found that combining a statin with intramyocardial injections of BM-MSCs improves the efficiency of cardiomyocyte differentiation by approximately 4-fold in a swine model of acute myocardial infarction.81 In a dog model of chronic ischemic cardiomyopathy, Silva and colleagues showed that canine MSCs engraft and differentiate into cells with a vascular phenotype, but were unable to show MSC differentiation into cardiomyocytes using troponin I co-localization techniques, suggesting important species differences.82
In contrast to numerous reports of engraftment, Dixon and colleagues transplanted male mesenchymal precursor cells (a subpopulation of MSCs expressing STRO-3) into post-MI female sheep and were unable to demonstrate engraftment.85 At one-hour post injection successful implantation was confirmed by immunolocalization for the Y-chromosome, but at 8 weeks post-transplantation staining for the Y-chromosome was not detected, suggesting failure of prolonged engraftment. Furthermore, PCR confirmed the lack of engraftment by the inability to detect sex determining region genes in any female swine hearts at 8 weeks post-injection.
Paracrine Signaling
The frequency of MSC engraftment and differentiation in the heart is low compared to the robust functional recovery observed after cell transplantation, which has raised questions as to whether MSC engraftment and differentiation is the predominant mechanism of action. MSCs are known to secrete soluble paracrine factors that have been postulated to contribute to endogenous cardiomyogenesis and angiogenesis.86–91 MSCs secrete a wide array of cytokines and growth factors, which can suppress the immune system, inhibit fibrosis and apoptosis, enhance angiogenesis, and stimulate differentiation of tissue specific stem cells (Table 1).89, 92–94
Table 1.
Paracrine factors secreted by MSCs
| Secreted Factor | Function |
|---|---|
| Pro-angiogenesis: | |
| Fibroblast growth factor-2 (FGF-2)87, 93 | Induces endothelial and smooth muscle cell proliferation |
| Fibroblast growth factor-7 (FGF-7)93 | Induces endothelial cell proliferation |
| Monocyte chemoattractanat protein-1 (MCP-1)89 | Induce angiogenesis; recruit monocytes |
| Platelet derived growth factor (PDGF)93 | Smooth muscle cell proliferation |
| Placental growth factor (PlGF)89, 93 | Promotes angiogenesis |
| Transforming growth factor-β (TGF-β)93 | Vessel maturation |
| Vascular endothelial growth factor (VEGF)87, 89 | Endothelial cell proliferation, migration, tube formation |
| Remodeling of extracellular matrix:: | |
| Metalloproteinase-1 (MMP1)93 | Loosens matrix; tubule formation |
| Metalloproteinase-2 (MMP2)93 | Loosens matrix; tubule formation |
| Metalloproteinase-9 (MMP9)93 | Loosens matrix |
| Plasminogen activator (PA)93 | Degrades matrix molecules |
| Tumor necrosis factor -α (TNF-α)93 | Degrades matrix molecules; cell proliferation |
| Stem cell proliferation, recruitment, & survival: | |
| Basic fibroblast growth factor (bFGF)89 | Enhance proliferation of endothelial and smooth muscle cells |
| Granulocyte colony stimulating factor (G-CSF)94 | Increases proliferation and differentiation of neutrophils |
| Insulin –like growth factor-1 (IGF-1)87 | Regulates cell growth and proliferation; inhibits apoptosis |
| Macrophage colony stimulating factor (M-CSF)94 | Increases proliferation and differentiation of monocytes |
| Thymosin-β4 (Tβ4)87 | Promotes cell migration |
| Stem cell-derived factor (SDF)93 | Progenitor cell homing |
| Secreted frizzled-related protein-1 (SFRP1)130 | Enhance cell development |
| Secreted frizzled-related protein-2 (SFRP2)90 | Inhibit apoptosis; enhance cell development |
| Immunomodulatory: | |
| Heme oxygenase-1(HO1)47 | Inhibits T cell proliferation |
| Hepatocyte Growth Factor (HGF)87 | Inhibit CD4+ T cell proliferation |
| Indoleamine 2,3-dioxygenase (IDO)45 | Inhibits innate and adaptive immune cell proliferation |
| Inducible nitric-oxide synthase (iNOS)48 | Inhibits inflammation |
| Interleukin-6 (IL-6)94 | Regulates inflammation; VEGF induction |
| Prostaglandin E2 (PGE2)52 | Inhibits inflammation |
In-vitro studies have shown that conditioned medium from hypoxic akt-MSCs inhibits apoptosis and can trigger spontaneous contractions of rat cardiomyocytes.87 When this conditioned medium from akt-MSCs was injected into post-MI rat hearts, infarct size reduced and LV function improved.87 Several genes coding for VEGF, FGF-2, HGF, IGF-I are upregulated in hypoxic akt-MSCs, and these factors could mediate the functional improvements observed.87 Furthermore, these investigators suggest that these improvements are seen within 72-hours and meaningful engraftment is unlikely to provide such rapid improvements, but secreted paracrine mediators are able to immediately affect the milieu.88 Additionally, Akt-MSCs secrete frizzled related protein (Sfrp2), a paracrine factor that exerts a pro-survival effect on ischemic myocardium through modulating Wnt signaling.90
Murine BM-MSC concentrated conditioned media (CCM) has been shown to consist of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), placental growth factor (PlGF), and monocyte chemoattractat protein-1 (MCP-1), which enhance proliferation of endothelial and smooth muscle cells.89 Increased local levels of VEGF and FGF proteins were detected when β-galactosidase labeled MSCs were injected into ischemic murine peripheral muscles compared to controls. These MSCs were detected in decreasing quantities from days 7 to 28 post-transplantation and failed to incorporate into vessels. Administering the factors secreted by MSCs alone may not have the same impact as administering the cells. In a swine model of MI, intramyocardial injection of MSCs reduced infarct size by recruiting endogenous c-kit+ cardiac stem cells (CSCs), whereas a single application of CCM did not.39
Cell-Cell Interactions and Niche Reconstitution
Our group has shown the ability of MSCs to stimulate proliferation of endogenous c-kit+ CSCs and enhance cardiomyocyte cell cycling.39 Post-MI female swine were injected with GFPlabeled allogeneic MSCs to the infarct borderzone, and on histological examination were shown to exhibit chimeric clusters containing immature MSCs and c-kit+ CSCs. These niches contained adult cardiomyocytes, GFP+ MSCs, and c-kit+ CSCs expressing connexin-43 mediated gap junctions and N-cadherin mechanical connections between cells. A 20–fold increase in the endogenous c-kit+ CSCs was observed in MSC treated animals compared to placebo. Additional co-culture experiments with c-kit+ CSCs and MSCs demonstrated a 10-fold increase in c-kit+ CSC proliferation compared to c-kit+ CSC cultures without MSCs. These data suggest that MSCs induce endogenous c-kit+ CSC proliferation, and cell-cell interactions play an important role in MSC based cardiac repair mechanisms.
Using genetically engineered mice that permanently express GFP in all cardiomyocytes after tamoxifen therapy, Loffredo and colleagues compared the ability of BM derived c-kit+ stem cells and BM-MSCs to induce proliferation of endogenous CSCs.95 Mice were treated with tomoxifen to induce GFP expression in cardiomyocytes, underwent MI, and administered intramyocardial injections of stem cells to the infarct borderzone. At 8 weeks, BM c-kit+ stem cells led to a significant reduction in the GFP+ cardiomyocyte pool and parallel increases in β-galactosidase+ cardiomyocytes compared to control, a finding consistent with increased progenitor activity induced by BM c-kit+ stem cells. However, BM-MSCs did not lead to reduction in the GFP+ cardiomyocyte pool or increase β-galactosidase+ cardiomyocytes, suggesting that MSCs may not stimulate endogenous progenitors. These findings in a murine model are clearly different from the body of work conducted using porcine models, and additionally reflect species specific functions of MSCs.
MSC and Cardiomyocyte Fusion
Fusion of BM stem cells with adult cells, including cardiomyocytes, has been proposed as a potential mechanism of action.96, 97 BM-MSCs expressing Cre recombinase were injected into Cre reporter gene mice hearts, which can detect fusion of MSCs with mouse cells by LacZ gene expression.98 As early as three days after transplant, rare cellular fusion was detected at injection sites by H&E staining with LacZ gene expressing distinct blue cells, which persisted at 28 days post-transplant. These findings lend support to the potential for MSC fusion with cardiomyocytes, but the paucity of fused cells detected makes it unlikely to be a predominant mechanism of action considering the degree of functional recovery.
Preclinical Trials of MSC Therapy: Effects on Cardiac Structure and Function
Several large animal species, including swine, sheep, and dogs, have been used to investigate the effects of MSC therapy in models of chronic and acute post-MI left ventricular dysfunction.20, 21, 41, 82–85, 99–108 Stem cells can be delivered to the heart via peripheral intravenous infusion; direct surgical injection during open heart surgery; or via catheter-based intracoronary infusion, retrograde coronary venous infusion, or transendocardial injection.109, 110 Using radiolabeled BM stem cells, γ-emission counting of harvested organs 1-hour after stem cell delivery demonstrated that intramyocardial injection has the highest retention rate of cells.110 Interestingly, most cells delivered to the heart by any method are not retained in the myocardium and found commonly in the lungs and spleen.110 Positron emission tomographic (PET) tracking of MSCs delivered by catheter-based transendocardial injection showed retention of approximately 6% of injected cells in the myocardium at 10 days after injection with increased uptake in the pericardium and pluera.111 While stem cell retention in the myocardium appears to be low by any delivery route, preclinical data of MSC therapy for cardiac disease has shown highly promising results. Here, we will review the preclinical trials using allogeneic and autologous MSCs delivered to the heart via the four most common techniques: peripheral intravenous infusion, intracoronary infusion, catheter-based transendocardial injection, and direct surgical injection.
Intravenous MSC therapy
Intravenous infusion of MSCs is the easiest and most practical method for delivery as it only requires peripheral venous access; however, for the cells to reach the myocardium they have to transit through the pulmonary circulation where entrapment of cells is a concern.112 Intravenous infusion of MSCs in a swine model of acute MI was conducted at fifteen minutes following left anterior descending (LAD) coronary artery occlusion with swine randomized to vehicle or various doses (1, 3, or 10 million cells/kg) of allogeneic BM-MSCs.101 At 12 weeks post-MI, LV ventriculography showed no difference in EF between MSC treated animals versus placebo. Pressure-volume (PV) loop analysis demonstrated a significant improvement in the end systolic pressure volume relationship (ESPVR) and preload recruitable stroke work (PRSW) in MSC treated animals compared to placebo. Histological analysis of post-mortem hearts showed significantly greater density of von Willebrand Factor (vWF) positive blood vessels and vascular endothelial growth factor (VEGF) expression in MSC treated animals. Steady state coronary blood flow reserve was similar among groups, but adenosine recruited coronary blood reserve was improved in MSC treated animals.
In another acute MI swine model, hemodynamic and electrophysiological effects of intravenous infusion of allogeneic MSCs were studied.100 Using transthoracic echocardiography, EF improved and less eccentric hypertrophy in MSC treated animals was detected at 3 months follow-up compared to placebo. Confocal spectral imaging of explanted organs confirmed DiI fluorescence in the lungs and rarely in the hearts of MSC treated animals. Electrophysiological studies at 3 months post-MI showed shortened epicardial effective refractory periods (ERP) in MSC treated animals compared to placebo. Shortened ERP may induce ventricular tachycardia,113 raising the possibility that MSCs may induce pro-arrhythmic remodeling.
Intracoronary infusion of MSCs
Intracoronary infusion of stem cells is delivered using a standard over-the-wire balloon angioplasty catheter placed into the target coronary artery.114 After positioning the angioplasty catheter, the balloon is inflated at a low pressure to block blood flow (allowing adhesion and potential transmigration), while the cells are infused through the distal lumen.114, 115 Concern for inducing ischemia during coronary artery occlusion and the lack of vessels in scar tissue may not allow effective cell delivery. Advantages of this technique are the familiarity of angioplasty techniques to interventional cardiologists and the ability to deliver cells during percutaneous intervention for acute MI.114
Most preclinical studies of intracoronary infusion of MSCs have focused on models of acute MI.99, 102, 103, 115 Cardiac MRI was used to investigate the effects of intracoronary infusion of iron oxide labeled MSCs or placebo in swine at approximately 5 days post LAD MI.115 Delayed enhancement (DE) cardiac MRI showed an 8% reduction in scar size in MSC treated swine compared to increased scar size in placebo infused animals. EF was unchanged in placebo infused animals, while a 15-point improvement in EF was observed in MSC treated swine. At 8 weeks post-infusion, prussian blue staining identified engraftment of iron-oxide labeled MSCs in the peri-infarct zone.
In an ischemia-reperfusion canine model of acute MI, intracoronary infusion was compared to electroanatomical guided transendocardial injection of allogeneic MSCs (100 million cells) at 7 days post-MI with a no-infusion placebo control arm.99 Two animals died in the intracoronary infusion arm, one due to microvascular plugging associated with MSC infusion and another due to intestinal ischemia, with no deaths in the other groups. Echocardiography at 21-days post transplant showed no difference in EF or LV chamber sizes in the MSC intracoronary infusion group compared to placebo, while EF, EDV, and ESV improved in the transendocardial MSC injection group. Histological analysis showed that intracoronary infusion produces a more uniformly distributed pattern of MSCs, concentrated in the borderzone and normal myocardium. Transendocardial injection was also used to deliver MSCs to the borderzone and normal myocardium, and achieved higher MSC concentration per square micron compared to intracoronary delivery.
Catheter-based intramyocardial injection of MSCs
Transendocardial stem cell injection (TESI) delivers MSCs directly into the myocardium using a catheter navigated in the LV by fluoroscopic guidance or electroanatomical mapping.114 TESI uses a needle deployed from the tip of a catheter that engages and infuses cells into the myocardium. Perforation of myocardium with the potential for cardiac tamponade and inducing arrhythmias need to be monitored with TESI, but the minimally invasive ability to directly inject cells into scar and borderzones make it an attractive delivery technique.110, 111
In a series of acute MI studies in swine, our group demonstrated the ability of transendocardial MSC injection to improve cardiac function and reduce scar size (Figure 5).39, 83, 107 Allogeneic MSCs were administered to the border and infarct zones at 3 days post-MI, and DE cardiac MRI showed approximately 50% reduction in scar size at 8 weeks post-transplant while control animals had no change in scar size.83 On gross pathological examination, MSC treated animals had infarct confined to the mid-myocardium with visible myocardium present at both the subendocardial and subepicardial zones. The subendocardial tissue, also present on DEMRI was consistent with new tissue growth and was not evident on control animals. Cardiac MRI demonstrated an increase in EF from 25% to 42% at 8 weeks post injection, while the non-treated animals had minimal change in EF. Pressure volume loops showed improved LV relaxation (LV end diastolic pressure and relaxation time) and systolic compliance (end systolic pressure volume relationship) in MSC treated animals compared to placebo. Additional work by our group has also showed that transendocardial MSC injection improves resting myocardial blood flow by first-pass gadolinium perfusion MRI at 8 weeks post-injection compared to placebo.107 Vessel density assessed by vWF expression was similar in MSC and placebo treated swine, but MSC injected animals had significantly larger vessels that likely contributed to improved tissue perfusion.
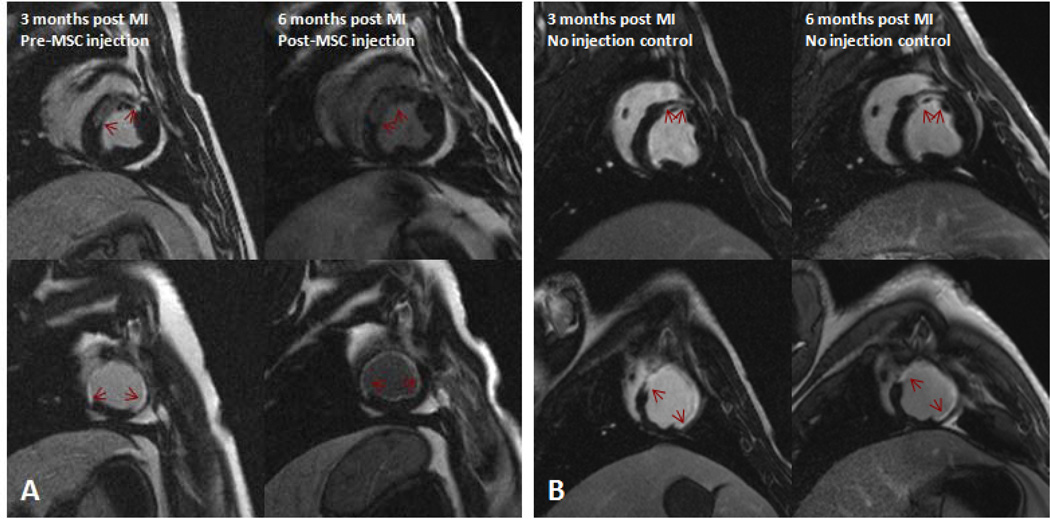
Figure 5. Impact of allogeneic MSC therapy on infarct size.
DE-MRI images of swine with chronic ischemic cardiomyopathy (A) injected with 200M allogeneic MSCs before and 3 months after therapy showing a 17% reduction in scar size; and (B) a control animal showing no change in scar size (red arrows indicate gadolinium enhanced scar).
In another study, three allogeneic MSC doses (2.4 × 107, 2.4 × 108, or 4.4 × 108) versus placebo delivered by electroanatomical mapping guided TESI were compared at three days post-MI.104 DE-MRI at 12-weeks post-injection showed reduced infarct size in all MSC dosage groups, while placebo injected animals had increased infarct size. Interestingly, there was no dose dependent effect on scar size and EF at 12 weeks was similar in all groups. In another study, transendocardial injection of autologous MSCs also produced a significant reduction in DE-MRI derived scar size at 10 days post-injection compared to placebo injection.111
To investigate the impact of MSCs on healed scars, our group studied transendocardial allogeneic MSC injection in swine with three-month old anterior wall infarcts.21 At three months post MI, remodeling in swine is nearly complete and results in depressed LV function.116 DE-MRI at 12-weeks post-injection showed that MSC therapy resulted in a 30% reduction in scar size, while the placebo injected animals had unchanged scars. Furthermore, the circumferential extent of scar decreased approximately 14% in MSC treated swine and slightly increased in placebo injected animals. Using tagged MRI to assess regional LV function, peak Eulerian circumferential shortening (Ecc) demonstrated improved contractility of the infarct and border zones in MSC treated animals while further declines in function were evident in the placebo group. Scar size reduction and increased regional function resulted led to improved global LV function in MSC treated animals, whereas EF remained depressed in placebo injected swine. These findings are consistent with the ability of intramyocardial injection of MSCs to lead to reverse remodeling in the chronically injured heart.
Direct surgical intramyocardial injection of MSCs
The largest preclinical experience with allogeneic and autologous MSC therapy for cardiac disease has utilized direct surgical intramyocardial injection.20, 22, 41, 82, 84, 85, 117 Surgical injection is the most invasive delivery technique as it requires either a thoracotomy or sternotomy for cell delivery, but allows direct visualization of scarred myocardium and needle engagement. If perforation occurs, it can be controlled at the time of injection with sutures. Direct surgical injection of MSCs has been studied in large animals with acute MI, sub-acute MI, and chronic ischemic cardiomyopathy.
In a sheep model of acute LAD coronary artery occlusion without reperfusion, BM derived STRO-3 positive mesenchymal precursor cells (MPC) were injected directly into the infarct borderzone one-hour following MI.41, 85 As discussed above, STRO-3 positive MPCs are a sub-set of BM-MSCs with extensive capacity for proliferation and differentiation.118 Animals were allocated to one of four different cell dosages (25 million; 75 million; 225 million; or 450 million cells) or placebo. Echocardiography at 8-weeks post-injection demonstrated improved EF in all MPC treated animals compared to placebo. Compared to placebo treated animals, EDV was significantly smaller in the two low dose groups (25 million and 75 million cells), but was unchanged in the two high dose groups (225 million and 450 million cells). EF significantly improved in all cell treated animals compared to placebo. Echocardiography derived MI length was significantly smaller in the three low dose groups (25 million, 75 million, and 225 million) compared to placebo, but was not significantly different in the high dose (450 million) treated group compared to placebo. This data suggests that there may be threshold effect with this particular subpopulation of MSCs.
In an ischemia-reperfusion swine model of sub-acute MI, autologous MSCs (60 million cells) were directly injected into two-week old infarcts using a midline sternotomy.20 Sonomicrometry crystals were placed in the infarct region during exposure of the heart to assess wall thickness and regional contractility. At 4-weeks post injection a trend towards improved end diastolic wall thickness and improved systolic wall thickening was seen in MSC treated animals compared to placebo.
Our group reported the results of direct surgical injections of autologous MSCs in a swine model of ischemic cardiomyopathy.22 Animals underwent LAD coronary artery occlusion followed by reperfusion, and at 12-weeks post-MI received either 20 million or 200 million autologous MSCs or placebo injections to the infarct and borderzone. Serial cardiac enzymes (troponin-I, creatine kinase, and creatine kinase MB) and white blood cell (WBC) obtained after injections showed no difference between groups. Cardiac MRI was used to assess LV function, scar size, and myocardial blood flow. DE-MRI conducted at 12-weeks post-injection demonstrated a trend towards decreased infarct size in low-dose MSC treated animals and a significant reduction in infarct size in high-dose MSC treated animals. Furthermore, decreased circumferential extent of scar was evident in MSC treated animals while there was a trend in scar expansion in placebo treated animals. Regional contractility as assessed by tagged MRI derived peak Ecc showed improved contractility of the infarct borderzones of low and high dose MSC treated animals. The high dose MSC treated animals also had significant improvements in regional contractility of the infarct zone. First-pass myocardial perfusion MRI showed improved myocardial blood flow in both high and low dose MSC treated animals compared to no change in placebo. The reduction in scar size, improved regional contractility, and increased myocardial blood flow resulted in increased EF of MSC treated animals compared to unchanged EF in placebo treated animals.
In a swine model of LAD coronary artery occlusion without reperfusion, allogeneic bone marrow MSCs (200 million cells) or placebo were surgically injected during thoracotomy at 30 days post-MI.84 Sixty days after injection, global cardiac function assessed by contrast LV angiography showed deterioration in EF of placebo treated animals, while MSC treated animals had preserved EF. Echocardiography derived LV chamber dimensions were not different between MSC or placebo treated animals. Infarct size determined by planimetry imaging showed no difference between treated and placebo groups. This is a particularly important study as it stands in contrast to the totality of large animal experience with reperfusion models, in which MI size is reduced by MSC injection.
MSC therapy has also been studied in dogs with chronic ischemic hibernating myocardium.82 Dogs underwent ameroid constrictor placement around the LAD coronary artery to induce chronic ischemia, and thirty days later direct surgical injections of allogeneic BMMSCs (100 million cells) or placebo were administered to the ischemic territory during thoracotomy. Serial cardiac enzymes (creatine kinase – MB and troponin I), c-reactive protein (CRP), and WBC obtained after injections showed no comparable levels between groups. Cardiac function assessed by echocardiography showed significant attenuation in EF in MSC treated animals compared to placebo at 30 days post-injection. Quantitative morphometry assessment of scar size showed a trend towards reduced fibrosis in the anterolateral wall of MSC treated animals compared to controls.
In summary, MSC therapy improves LV function, reduces scar size and increases myocardial tissue perfusion in post-MI large animal models regardless of delivery method or species. Intramyocardial injection appears to have the highest retention rate, and when compared to intracoronary infusion accordingly has a greater impact on LV function.99, 110 The optimal time to deliver MSCs in the post-MI setting, whether acutely during the remodeling phase or in the chronic setting after a healed scar is developed, needs better and more rigorous definition in future studies. Other important lessons from cell injection trials include the importance of reperfusion.
Genetically and Pharmacologically Modified MSCs
Several preclinical studies have investigated the effects of genetically modified MSCs and concomitant pharmacological therapy on cardiac repair.81, 119, 120 In an acute MI swine model of ischemia-reperfusion, direct surgical injections of autologous MSCs (30 million cells) alone or in combination with oral simvastatin were compared to placebo injected swine with and without oral simvastatin therapy. At 6 weeks post-injection, myocardial single photon emission tomography (SPECT) assessment of myocardial perfusion showed a reduction in the perfusion defect in both the simvastatin alone and combination (simvastatin + MSC) groups compared to control; however, there was no added benefit to improving myocardial perfusion by adding MSC therapy to simvastatin alone. Cardiac MRI showed improvement in EF in the combination (simvastatin + MSC) group compared to control. There was no difference in EDV between groups, and ESV was significantly less in the combination (simvastatin + MSC) group compared to control. DE-MRI showed reduced scar size in both simvastatin alone and combination (simvastatin and MSC) groups compared to placebo, but not between groups. Systolic wall thickening improved only in the combination (simvastatin + MSC) group compared to control. Histology showed increased engraftment of MSCs with concomitant treatment with simvastatin compared to MSC therapy alone, suggesting that a statin may improve retention and survival of MSCs in post-MI hearts.
In a swine model of chronic ischemic cardiomyopathy, the effect of adding hepatocyte growth factor (HGF) to BM-MSC therapy was investigated.119 Four weeks after permanent LAD occlusion, swine were administered autologous MSCs (5 million cells) alone, combination HGF/MSCs (5 million cells), or placebo via intracoronary infusion to the non-infarct related artery. Gated myocardial SPECT showed no change in myocardial perfusion of placebo treated swine; however, both the MSC alone and MSC/HGF groups had significant improvements at 4 weeks with no difference in myocardial perfusion between MSC alone and MSC/HGF groups. SPECT derived EF should significant improvement in MSC alone and MSC/HGF treated animals compared to no change in controls. Histological analysis showed increased vessel density in MSC alone and MSC/HGF treated animals compared to placebo, but no difference between treated groups. Taken together this study suggests that adding HGF to MSC therapy does not provide additional benefit over MSC therapy alone.
Heme oxygenase-1 (HO-1) is an inducible enzyme that rapidly degrades heme resulting in anti-apoptotic and anti-inflammatory activity, and in-vitro studies have been shown HO-1 to enhance the tolerance of MSCs to hypoxia-reoxygenation injury.121 BM-MSCs transfected to overexpress heme oxygenase-1 (HO-1) were administered to cells or placebo via intracoronary infusion following acute MI in a swine model of ischemia-reperfusion.120 Cardiac MRI at 3 months post-injection demonstrated improved EF in HO-1 transfected MSCs compared to placebo and plasmid transfected MSCs. EDV was similar among groups, while ESV was significantly lower in the HO-1 transfected MSC group. Western blot demonstrated significantly enhanced myocardial expression of VEGF and reduced expression of TNF-α and IL-6 in Ho-1 transfected MSC treated hearts compared to control. Histological analysis demonstrated increased capillary and arteriolar density in HO-1 MSC treated hearts. Taken together, HO-1 overexpression in MSCs appears to be associated with decreased inflammatory cytokine levels and improved angiogenesis when transplanted to post-MI swine hearts.
In another genetically modified MSC study, BM-MSCs were transfected with Akt, an enzyme that has been shown to protect cardiomyocytes against apoptosis after ischemia-reperfusion injury.122, 123 Transfected Akt-MSCs, MSCs alone, or placebo were administered via intracoronary infusion three days post-MI. Coronary angiograms following intracoronary MSC infusion showed several cases of slow coronary flow requiring administration of intracoronary adenosine, nitroglycerin, or nicorandil. At 4 weeks post-transplant, SPECT showed significantly improved EF in akt-MSC treated animals compared to MSC alone, while both showed LV function compared to placebo. Infarct size by SPECT was significantly less in both MSC alone and akt-MSC treated animals compared to placebo. Furthermore, the infarct size was significantly smaller in akt-MSC treated animals compared to MSC alone, suggesting that akt-MSCs may provide additional scar size reduction.
Safety of MSC Therapy
Whereas, evidence continues to accrue that MSCs have great potential as a new therapy to treat damaged myocardium, it is crucial to keep safety concerns in mind. Two key concerns that warrant mention include pro-arrhythmia and tumor formation.124 Intravenous infusion of MSCs in swine was shown to alter the electrophysiological properties of the myocardium;100 but contrary to these findings in swine, intravenous infusion of allogeneic MSCs in humans with acute MI demonstrated less ventricular arrhythmias compared to placebo infusion.125 Furthermore, other clinical trials have not shown increased arrhythmias after MSC therapy.126, 127 In the setting of ischemic cardiomyopathy, programmed electrical stimulation did not detect a higher level of inducibility in MSC treated pigs.
Several reports have raised concern for tumor formation using BM cultured MSCs.124, 128 Murine derived BM-MSCs were used in these studies and shown to undergo chromosomal abnormalities that result in tumor formation in numerous organs. As previously discussed, in a series of large animal preclinical studies by our group and others, MSCs were safe in large animals with no evidence of tumor formation or ectopic tissue growth.22, 39, 41, 82–85, 99–104, 106, 107 Furthermore, there have been no reports of ectopic tissue growth in the early phase human studies using MSCs.23, 126, 127 Nonetheless, these reports of tumorgenesis in murine models highlights the importance of continued long-term surveillance of patients treated with MSCs.
Clinical Trials of MSC Therapy for Cardiac Repair
Acute MI
Based upon rigorous preclinical testing, highlighted above that demonstrated the safety of MSC delivery to patients with cardiac disease, clinical trials have been initiated for both acute MI as well as ischemic cardiomyopathy. Phase I/II clinical data has been reported using intravenous therapy, intracoronary infusion, and intramyocardial injection.23, 126, 127 Chen and colleagues investigated the effects of intracoronary infusion of autologous BM-MSCs (8 to 10 × 109, n=34) or saline (n=35) in patients with sub-acute MI.126 PET showed improvement in perfusion defects at 3 months after BM-MSC therapy, and left ventriculography demonstrated improved EF and LV chamber sizes in MSC treated patients compared to placebo. Importantly, this study showed that MSC infusion was safe with no deaths reported during follow-up and electrocardiographic monitoring showed no arrhythmias.
Our group was part of a randomized study investigating allogeneic MSCs (n=39) versus placebo (n=21) administered intravenously following acute MI.23 This dose ranging study showed that IV allogeneic MSCs are safe in post-MI patients with no difference in adverse events between groups. Interestingly, follow-up electrocardiograms demonstrated a reduction in ventricular arrhythmias and improved pulmonary function in MSC treated patients. Additionally, echocardiography showed MSC treated patients experienced a 6% increase in EF at 3 months.
Ischemic Cardiomyopathy
Based upon the large amount of mechanistic data available and the extensive translational findings that MSCs can stimulate reverse remodeling in porcine models of ischemic cardiomyopathy, clinical trial programs are underway testing the safety and efficacy of MSCs and MSC precursor cells for patients with cardiac injury due to previous MI. Our group directly tested the hypothesis that transendocardial, intramyocardial injection of MSCs (and/or whole bone marrow) produced a cardiac phenotype of reverse remodeling by employing cardiac MRI imaging. In this study, 8 patients with chronic ischemic cardiomyopathy were administered bone marrow MSCs (n=4) or mononuclear cells (MNCs, n=4) to the scar and border zone.127 We demonstrated reverse remodeling and improved regional contractility of the treated scar, which was evident as early as 3 months post-injection and persisted at 12 months (Figure 6). The improved regional contractility strongly correlated with the reduction in both EDV (r2=0.69, p=0.04) and ESV (r2=0.83, p=0.01). Importantly, we used serial whole body computed tomography (CT) scans that showed no evidence of ectopic tissue growth at one year after transplantation, and serial Holter ECG recordings showed no sustained arrhythmias. Taken together, the early phase clinical trial data demonstrates that MSC therapy for post-MI is safe and has favorable effects on cardiac structure and function.
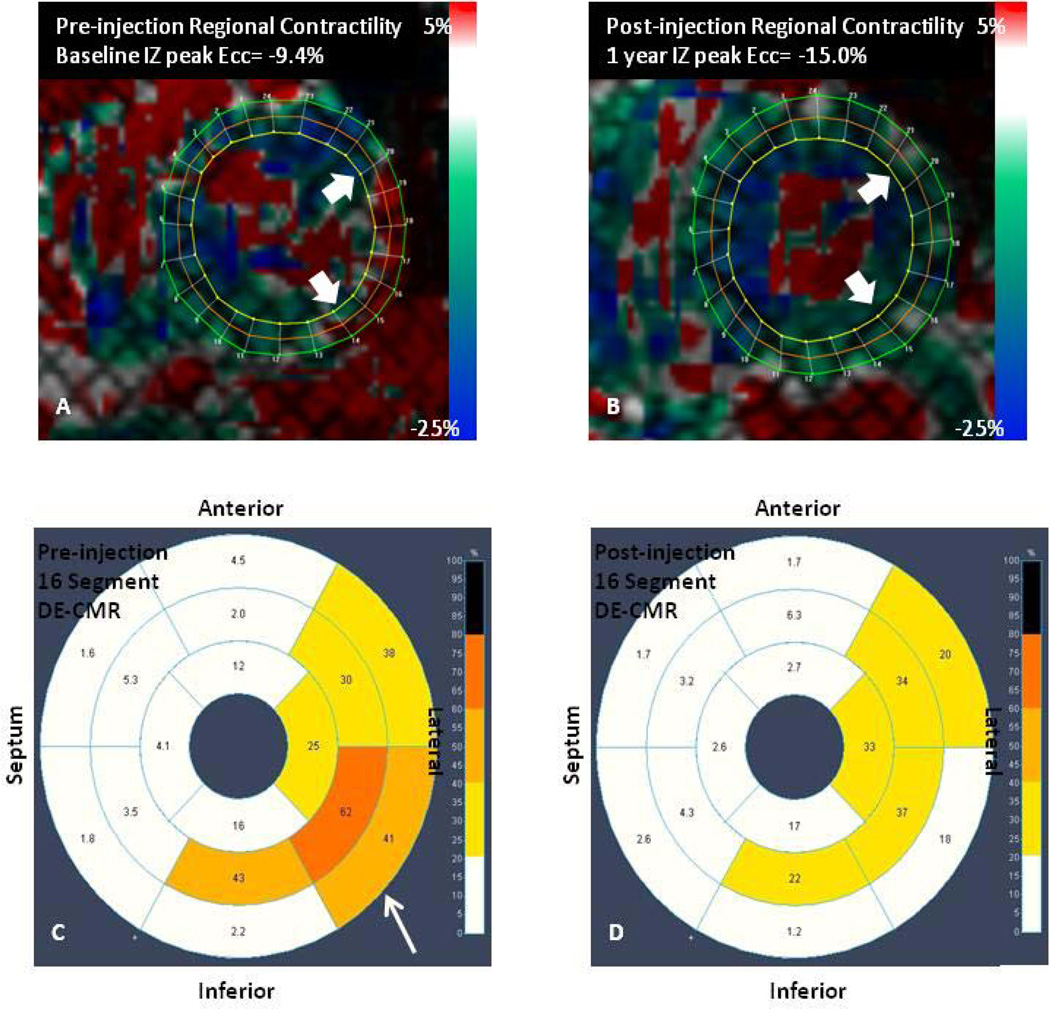
Figure 6. Intramyocardial injection of autologous MSCs improves regional contractility.
Tagged CMR strain maps (A) before MSC injection showing a lateral infarct (white arrows) with decreased contractility (red) and (B) post-injection showing improved contractility (green/blue). Corresponding DE-CMR 16 segment model of infarct size (A) before MSC injection and (D) 1 year after injection (less transmularity depicted by decreased orange/yellow). (Panels A and B are reproduced from Williams et al. Circ Res. 2011;108:792–796, courtesy of the American Heart Association)
To follow-up on this finding, two larger studies are underway: the Transendocardial Autologous Cells in Ischemic Heart Failure Trial (TAC-HFT) and Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis (POSEIDON). TAC-HFT is a randomized, double-blinded, placebo controlled trial comparing bone marrow MSCs versus MNCs, and POSEIDON is comparing the effects of allogeneic versus autologous MSC therapy in patients with ischemic cardiomyopathy.24 Additionally, we have completed enrollment in the Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS), a trial investigating MSC therapy delivered during coronary artery bypass surgery; and recently we initiated POSEIDON-DCM studying MSC therapy for the treatment of idiopathic dilated cardiomyopathy.
Several other clinical trial efforts are also underway including the use of MPCs and MSCs treated ex-vivo with cytokines to enhance cardiopoiesis. The next few years will witness the results of these early clinical trial efforts.
Strategies and Future Directions
Retention of stem cells in the heart is low by any method of delivery,110 and strategies to enhance engraftment and differentiation are needed. Guided cardiopoiesis has been a proposed technique to enhance the cardiac phenotype of MSCs and was shown to increase engraftment in murine hearts.129 The exact mechanism of action of MSCs—whether via paracrine signaling, cell fusion, cell-cell interaction, or differentiation to cardiomyocytes and vascular cells—is still highly debated and unresolved.21, 39, 86 The mechanism of action of MSCs in cardiac repair are likely multifaceted and the data accumulated to date in large animal models and humans has shown that MSC therapy for cardiac disease is safe and provides substantial improvements in cardiac structure and function.
In summary, understanding of endogenous roles of MSCs and their therapeutic potential has substantially evolved over the past few decades. Initially considered bystanders of uncertain significance, MSCs are now appreciated as essential cells governing tissue homeostasis by regulating niches. This property, along with a capacity for multipotential differentiation, has greatly facilitated a therapeutic role for these cells as a cell-based therapeutic. Enhanced by ease of preparation and immunoprivilege, MSCs have been tested in preclinical models and proof-ofconcept clinical trials for ability to both prevent and reverse ventricular remodeling. The early demonstrations of these effects, coupled with a remarkable safety profile, have set the stage for pivotal testing of these cells as a therapeutic. Should these trials be positive, and start to reveal clinical benefits for MSC-based therapy, a truly transformative approach to cardiac therapeutics will have been achieved.
Acknowledgments
Funding Sources: Dr. Hare is funded by National Institutes Health grants U54-HL081028 (Specialized Center for Cell Based Therapy), P20-HL101443, and R01- grants HL084275, HL110737-01, HL107110, and HL094849.
Non-standard abbreviations and acronyms
- MSC
mesenchymal stem cell
- HSCs
hematopoietic stem cells
- BM
bone marrow
- MPC
mesenchymal precursor cell
- CFU-f
Colony Forming Unit – fibroblasts
- SPECT
single photon emission tomography
- MI
myocardial infarction
- LV
left ventricle
- EDV
end-diastolic volume
- ESV
end-systolic volume
Footnotes
Disclosures: None.
Reference List
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, de SG, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 2.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 3.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 4.Lennon DP, Caplan AI. Isolation of human marrow-derived mesenchymal stem cells. Exp Hematol. 2006;34:1604–1605. doi: 10.1016/j.exphem.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008 doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 6.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 7.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 10.Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian RP, Scadden EO, Aung Z, Matza M, Merkenschlager M, Lin C, Rommens JM, Scadden DT. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464:852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piersma AH, Brockbank KG, Ploemacher RE, van VE, Brakel-van Peer KM, Visser PJ. Characterization of fibroblastic stromal cells from murine bone marrow. Exp Hematol. 1985;13:237–243. [PubMed] [Google Scholar]
- 12.Caplan AI. Molecular and cellular differentiation of muscle, cartilage, and bone in the developing limb. Prog Clin Biol Res. 1986;217B:307–318. [PubMed] [Google Scholar]
- 13.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 14.Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 15.Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M, Sato T, Miyanishi K, Takayama T, Takahashi M, Takimoto R, Iyama S, Matsunaga T, Ohtani S, Matsuura A, Hamada H, Niitsu Y. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106:756–763. doi: 10.1182/blood-2005-02-0572. [DOI] [PubMed] [Google Scholar]
- 17.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 18.Xu W, Zhang X, Qian H, Zhu W, Sun X, Hu J, Zhou H, Chen Y. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp Biol Med (Maywood) 2004;229:623–631. doi: 10.1177/153537020422900706. [DOI] [PubMed] [Google Scholar]
- 19.Di NM, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 20.Shake JG, Gruber PJ, Baumgartner WA, Senechal G, Meyers J, Redmond JM, Pittenger MF, Martin BJ. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg. 2002;73:1919–1925. doi: 10.1016/s0003-4975(02)03517-8. [DOI] [PubMed] [Google Scholar]
- 21.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, Kellner J, Zviman MM, Hatzistergos KE, Detrick B, Conte JV, McNiece I, Steenbergen C, Lardo AC, Hare JM. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009;30:2722–2732. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trachtenberg B, Velazquez DL, Williams AR, McNiece I, Fishman J, Nguyen K, Rouy D, Altman P, Schwarz R, Mendizabal A, Oskouei B, Byrnes J, Soto V, Tracy M, Zambrano JP, Heldman AW, Hare JM. Rationale and design of the Transendocardial Injection of Autologous Human Cells (bone marrow or mesenchymal) in Chronic Ischemic Left Ventricular Dysfunction and Heart Failure Secondary to Myocardial Infarction (TAC-HFT) trial: A randomized, double-blind, placebo-controlled study of safety and efficacy. Am Heart J. 2011;161:487–493. doi: 10.1016/j.ahj.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 25.Hare JM. Translational development of mesenchymal stem cell therapy for cardiovascular diseases. Tex Heart Inst J. 2009;36:145–147. [PMC free article] [PubMed] [Google Scholar]
- 26.Dominici M, Le BK, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 27.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 28.Jones EA, Kinsey SE, English A, Jones RA, Straszynski L, Meredith DM, Markham AF, Jack A, Emery P, McGonagle D. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46:3349–3360. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- 29.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 30.De BC, Dell'Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 31.Sabatini F, Petecchia L, Tavian M, Jodon dV V, Rossi GA, Brouty-Boye D. Human bronchial fibroblasts exhibit a mesenchymal stem cell phenotype and multilineage differentiating potentialities. Lab Invest. 2005;85:962–971. doi: 10.1038/labinvest.3700300. [DOI] [PubMed] [Google Scholar]
- 32.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 33.Zvaifler NJ, Marinova-Mutafchieva L, Adams G, Edwards CJ, Moss J, Burger JA, Maini RN. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2:477–488. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 35.da Silva ML, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 36.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 37.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 38.Nardi NB, Camassola M. Isolation and culture of rodent bone marrow-derived multipotent mesenchymal stromal cells. Methods Mol Biol. 2011;698:151–160. doi: 10.1007/978-1-60761-999-4_12. [DOI] [PubMed] [Google Scholar]
- 39.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuci S, Kuci Z, Kreyenberg H, Deak E, Putsch K, Huenecke S, Amara C, Koller S, Rettinger E, Grez M, Koehl U, Latifi-Pupovci H, Henschler R, Tonn T, von LD, Klingebiel T, Bader P. CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. Haematologica. 2010;95:651–659. doi: 10.3324/haematol.2009.015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamamoto H, Gorman JH, III, Ryan LP, Hinmon R, Martens TP, Schuster MD, Plappert T, Kiupel M, St John-Sutton MG, Itescu S, Gorman RC. Allogeneic mesenchymal precursor cell therapy to limit remodeling after myocardial infarction: the effect of cell dosage. Ann Thorac Surg. 2009;87:794–801. doi: 10.1016/j.athoracsur.2008.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devine SM, Hoffman R. Role of mesenchymal stem cells in hematopoietic stem cell transplantation. Curr Opin Hematol. 2000;7:358–363. doi: 10.1097/00062752-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, Mosca JD. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003;10:228–241. doi: 10.1007/BF02256058. [DOI] [PubMed] [Google Scholar]
- 44.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 45.Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, Romagnani P, Maggi E, Romagnani S, Annunziato F. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 46.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 47.Chabannes D, Hill M, Merieau E, Rossignol J, Brion R, Soulillou JP, Anegon I, Cuturi MC. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110:3691–3694. doi: 10.1182/blood-2007-02-075481. [DOI] [PubMed] [Google Scholar]
- 48.Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 49.Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri F, Ottonello L, Pistoia V. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells. 2008;26:151–162. doi: 10.1634/stemcells.2007-0416. [DOI] [PubMed] [Google Scholar]
- 50.Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 51.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 52.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 53.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 54.Traggiai E, Volpi S, Schena F, Gattorno M, Ferlito F, Moretta L, Martini A. Bone marrow-derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells. 2008;26:562–569. doi: 10.1634/stemcells.2007-0528. [DOI] [PubMed] [Google Scholar]
- 55.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 56.Chapel A, Bertho JM, Bensidhoum M, Fouillard L, Young RG, Frick J, Demarquay C, Cuvelier F, Mathieu E, Trompier F, Dudoignon N, Germain C, Mazurier C, Aigueperse J, Borneman J, Gorin NC, Gourmelon P, Thierry D. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gene Med. 2003;5:1028–1038. doi: 10.1002/jgm.452. [DOI] [PubMed] [Google Scholar]
- 57.Le BK, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringden O. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 58.Huang XP, Sun Z, Miyagi Y, McDonald KH, Zhang L, Weisel RD, Li RK. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation. 2010;122:2419–2429. doi: 10.1161/CIRCULATIONAHA.110.955971. [DOI] [PubMed] [Google Scholar]
- 59.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 60.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–274. [PubMed] [Google Scholar]
- 61.Augello A, De BC. The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther. 2010;21:1226–1238. doi: 10.1089/hum.2010.173. [DOI] [PubMed] [Google Scholar]
- 62.Caplan AI. Bone development and repair. BioAssays. 1987;6:171–175. doi: 10.1002/bies.950060406. [DOI] [PubMed] [Google Scholar]
- 63.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- 64.Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 65.Pereira RF, Halford KW, O'Hara MD, Leeper DB, Sokolov BP, Pollard MD, Bagasra O, Prockop DJ. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci U S A. 1995;92:4857–4861. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–428. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 67.Derfoul A, Perkins GL, Hall DJ, Tuan RS. Glucocorticoids promote chondrogenic differentiation of adult human mesenchymal stem cells by enhancing expression of cartilage extracellular matrix genes. Stem Cells. 2006;24:1487–1495. doi: 10.1634/stemcells.2005-0415. [DOI] [PubMed] [Google Scholar]
- 68.Li X, Yu X, Lin Q, Deng C, Shan Z, Yang M, Lin S. Bone marrow mesenchymal stem cells differentiate into functional cardiac phenotypes by cardiac microenvironment. J Mol Cell Cardiol. 2007;42:295–303. doi: 10.1016/j.yjmcc.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 69.Moscoso I, Centeno A, Lopez E, Rodriguez-Barbosa JI, Santamarina I, Filgueira P, Sanchez MJ, Dominguez-Perles R, Penuelas-Rivas G, Domenech N. Differentiation "in vitro" of primary and immortalized porcine mesenchymal stem cells into cardiomyocytes for cell transplantation. Transplant Proc. 2005;37:481–482. doi: 10.1016/j.transproceed.2004.12.247. [DOI] [PubMed] [Google Scholar]
- 70.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu M, Wani M, Dai YS, Wang J, Yan M, Ayub A, Ashraf M. Differentiation of bone marrow stromal cells into the cardiac phenotype requires intercellular communication with myocytes. Circulation. 2004;110:2658–2665. doi: 10.1161/01.CIR.0000145609.20435.36. [DOI] [PubMed] [Google Scholar]
- 72.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 73.Black IB, Woodbury D. Adult rat and human bone marrow stromal stem cells differentiate into neurons. Blood Cells Mol Dis. 2001;27:632–636. doi: 10.1006/bcmd.2001.0423. [DOI] [PubMed] [Google Scholar]
- 74.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 75.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 76.Etheridge SL, Spencer GJ, Heath DJ, Genever PG. Expression profiling and functional analysis of wnt signaling mechanisms in mesenchymal stem cells. Stem Cells. 2004;22:849–860. doi: 10.1634/stemcells.22-5-849. [DOI] [PubMed] [Google Scholar]
- 77.Boland GM, Perkins G, Hall DJ, Tuan RS. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem. 2004;93:1210–1230. doi: 10.1002/jcb.20284. [DOI] [PubMed] [Google Scholar]
- 78.Arita NA, Pelaez D, Cheung HS. Activation of the extracellular signal-regulated kinases 1 and 2 (ERK1/2) is needed for the TGFbeta-induced chondrogenic and osteogenic differentiation of mesenchymal stem cells. Biochem Biophys Res Commun. 2011;405:564–569. doi: 10.1016/j.bbrc.2011.01.068. [DOI] [PubMed] [Google Scholar]
- 79.Martin I, Muraglia A, Campanile G, Cancedda R, Quarto R. Fibroblast growth factor-2 supports ex vivo expansion and maintenance of osteogenic precursors from human bone marrow. Endocrinology. 1997;138:4456–4462. doi: 10.1210/endo.138.10.5425. [DOI] [PubMed] [Google Scholar]
- 80.Tokunaga A, Oya T, Ishii Y, Motomura H, Nakamura C, Ishizawa S, Fujimori T, Nabeshima Y, Umezawa A, Kanamori M, Kimura T, Sasahara M. PDGF receptor beta is a potent regulator of mesenchymal stromal cell function. J Bone Miner Res. 2008;23:1519–1528. doi: 10.1359/jbmr.080409. [DOI] [PubMed] [Google Scholar]
- 81.Yang YJ, Qian HY, Huang J, Li JJ, Gao RL, Dou KF, Yang GS, Willerson JT, Geng YJ. Combined therapy with simvastatin and bone marrow-derived mesenchymal stem cells increases benefits in infarcted swine hearts. Arterioscler Thromb Vasc Biol. 2009;29:2076–2082. doi: 10.1161/ATVBAHA.109.189662. [DOI] [PubMed] [Google Scholar]
- 82.Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RV, Oliveira EM, He R, Geng YJ, Willerson JT, Perin EC. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 83.Amado LC, Saliaris AP, Schuleri KH, St JM, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Makkar RR, Price MJ, Lill M, Frantzen M, Takizawa K, Kleisli T, Zheng J, Kar S, McClelan R, Miyamota T, Bick-Forrester J, Fishbein MC, Shah PK, Forrester JS, Sharifi B, Chen PS, Qayyum M. Intramyocardial injection of allogenic bone marrow-derived mesenchymal stem cells without immunosuppression preserves cardiac function in a porcine model of myocardial infarction. J Cardiovasc Pharmacol Ther. 2005;10:225–233. doi: 10.1177/107424840501000403. [DOI] [PubMed] [Google Scholar]
- 85.Dixon JA, Gorman RC, Stroud RE, Bouges S, Hirotsugu H, Gorman JH, III, Martens TP, Itescu S, Schuster MD, Plappert T, St John-Sutton MG, Spinale FG. Mesenchymal cell transplantation and myocardial remodeling after myocardial infarction. Circulation. 2009;120:S220–S229. doi: 10.1161/CIRCULATIONAHA.108.842302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 88.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 89.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 90.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li H, Zuo S, He Z, Yang Y, Pasha Z, Wang Y, Xu M. Paracrine factors released by GATA-4 overexpressed mesenchymal stem cells increase angiogenesis and cell survival. Am J Physiol Heart Circ Physiol. 2010;299:H1772–H1781. doi: 10.1152/ajpheart.00557.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 93.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 94.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996;166:585–592. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 95.Loffredo FS, Steinhauser ML, Gannon J, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389–398. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 97.Nygren JM, Jovinge S, Breitbach M, Sawen P, Roll W, Hescheler J, Taneera J, Fleischmann BK, Jacobsen SE. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 98.Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, Dzau VJ, Pratt RE. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006;14:840–850. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 99.Perin EC, Silva GV, Assad JA, Vela D, Buja LM, Sousa AL, Litovsky S, Lin J, Vaughn WK, Coulter S, Fernandes MR, Willerson JT. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J Mol Cell Cardiol. 2008;44:486–495. doi: 10.1016/j.yjmcc.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 100.Price MJ, Chou CC, Frantzen M, Miyamoto T, Kar S, Lee S, Shah PK, Martin BJ, Lill M, Forrester JS, Chen PS, Makkar RR. Intravenous mesenchymal stem cell therapy early after reperfused acute myocardial infarction improves left ventricular function and alters electrophysiologic properties. Int J Cardiol. 2006;111:231–239. doi: 10.1016/j.ijcard.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 101.Halkos ME, Zhao ZQ, Kerendi F, Wang NP, Jiang R, Schmarkey LS, Martin BJ, Quyyumi AA, Few WL, Kin H, Guyton RA, Vinten-Johansen J. Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic Res Cardiol. 2008;103:525–536. doi: 10.1007/s00395-008-0741-0. [DOI] [PubMed] [Google Scholar]
- 102.Wang X, Jameel MN, Li Q, Mansoor A, Qiang X, Swingen C, Panetta C, Zhang J. Stem cells for myocardial repair with use of a transarterial catheter. Circulation. 2009;120:S238–S246. doi: 10.1161/CIRCULATIONAHA.109.885236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vela DC, Silva GV, Assad JA, Sousa AL, Coulter S, Fernandes MR, Perin EC, Willerson JT, Buja LM. Histopathological study of healing after allogenic mesenchymal stem cell delivery in myocardial infarction in dogs. J Histochem Cytochem. 2009;57:167–176. doi: 10.1369/jhc.2008.952507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hashemi SM, Ghods S, Kolodgie FD, Parcham-Azad K, Keane M, Hamamdzic D, Young R, Rippy MK, Virmani R, Litt H, Wilensky RL. A placebo controlled, dose-ranging, safety study of allogenic mesenchymal stem cells injected by endomyocardial delivery after an acute myocardial infarction. Eur Heart J. 2008;29:251–259. doi: 10.1093/eurheartj/ehm559. [DOI] [PubMed] [Google Scholar]
- 105.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Amado LC, Schuleri KH, Saliaris AP, Boyle AJ, Helm R, Oskouei B, Centola M, Eneboe V, Young R, Lima JA, Lardo AC, Heldman AW, Hare JM. Multimodality noninvasive imaging demonstrates in vivo cardiac regeneration after mesenchymal stem cell therapy. J Am Coll Cardiol. 2006;48:2116–2124. doi: 10.1016/j.jacc.2006.06.073. [DOI] [PubMed] [Google Scholar]
- 107.Schuleri KH, Amado LC, Boyle AJ, Centola M, Saliaris AP, Gutman MR, Hatzistergos KE, Oskouei BN, Zimmet JM, Young RG, Heldman AW, Lardo AC, Hare JM. Early improvement in cardiac tissue perfusion due to mesenchymal stem cells. Am J Physiol Heart Circ Physiol. 2008;294:H2002–H2011. doi: 10.1152/ajpheart.00762.2007. [DOI] [PubMed] [Google Scholar]
- 108.Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, Kellner J, Zviman MM, Hatzistergos KE, Detrick B, Conte JV, McNiece I, Steenbergen C, Lardo AC, Hare JM. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009;30:2722–2732. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thompson CA, Nasseri BA, Makower J, Houser S, McGarry M, Lamson T, Pomerantseva I, Chang JY, Gold HK, Vacanti JP, Oesterle SN. Percutaneous transvenous cellular cardiomyoplasty. A Circulation Research Manuscript #CIRCRESAHA/2011/243147 Review novel nonsurgical approach for myocardial cell transplantation. J Am Coll Cardiol. 2003;41:1964–1971. doi: 10.1016/s0735-1097(03)00397-8. [DOI] [PubMed] [Google Scholar]
- 110.Hou D, Youssef EA, Brinton TJ, Zhang P, Rogers P, Price ET, Yeung AC, Johnstone BH, Yock PG, March KL. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112:I150–I156. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- 111.Gyongyosi M, Blanco J, Marian T, Tron L, Petnehazy O, Petrasi Z, Hemetsberger R, Rodriguez J, Font G, Pavo IJ, Kertesz I, Balkay L, Pavo N, Posa A, Emri M, Galuska L, Kraitchman DL, Wojta J, Huber K, Glogar D. Serial noninvasive in vivo positron emission tomographic tracking of percutaneously intramyocardially injected autologous porcine mesenchymal stem cells modified for transgene reporter gene expression. Circ Cardiovasc Imaging. 2008;1:94–103. doi: 10.1161/CIRCIMAGING.108.797449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, Kloner RA, Leor J. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 113.Kuo CS, Munakata K, Reddy CP, Surawicz B. Characteristics and possible mechanism of ventricular arrhythmia dependent on the dispersion of action potential durations. Circulation. 1983;67:1356–1367. doi: 10.1161/01.cir.67.6.1356. [DOI] [PubMed] [Google Scholar]
- 114.Sherman W, Martens TP, Viles-Gonzalez JF, Siminiak T. Catheter-based delivery of cells to the heart. Nat Clin Pract Cardiovasc Med. 2006;3(Suppl 1):S57–S64. doi: 10.1038/ncpcardio0446. [DOI] [PubMed] [Google Scholar]
- 115.Qi CM, Ma GS, Liu NF, Shen CX, Chen Z, Liu XJ, Hu YP, Zhang XL, Teng GJ, Ju SH, Ma M, Tang YL. Transplantation of magnetically labeled mesenchymal stem cells improves cardiac function in a swine myocardial infarction model. Chin Med J (Engl) 2008;121:544–550. [PubMed] [Google Scholar]
- 116.Schuleri KH, Boyle AJ, Centola M, Amado LC, Evers R, Zimmet JM, Evers KS, Ostbye KM, Scorpio DG, Hare JM, Lardo AC. The adult Gottingen minipig as a model for chronic heart failure after myocardial infarction: focus on cardiovascular imaging and regenerative therapies. Comp Med. 2008;58:568–579. [PMC free article] [PubMed] [Google Scholar]
- 117.Tomita S, Mickle DA, Weisel RD, Jia ZQ, Tumiati LC, Allidina Y, Liu P, Li RK. Improved heart function with myogenesis and angiogenesis after autologous porcine bone marrow stromal cell transplantation. J Thorac Cardiovasc Surg. 2002;123:1132–1140. doi: 10.1067/mtc.2002.120716. [DOI] [PubMed] [Google Scholar]
- 118.Gronthos S, Fitter S, Diamond P, Simmons PJ, Itescu S, Zannettino AC. A novel monoclonal antibody (STRO-3) identifies an isoform of tissue nonspecific alkaline phosphatase expressed by multipotent bone marrow stromal stem cells. Stem Cells Dev. 2007;16:953–963. doi: 10.1089/scd.2007.0069. [DOI] [PubMed] [Google Scholar]
- 119.Yang ZJ, Ma DC, Wang W, Xu SL, Zhang YQ, Chen B, Zhou F, Zhu TB, Wang LS, Xu ZQ, Zhang FM, Cao KJ, Ma WZ. Experimental study of bone marrow-derived mesenchymal stem cells combined with hepatocyte growth factor transplantation via noninfarct-relative artery in acute myocardial infarction. Gene Ther. 2006;13:1564–1568. doi: 10.1038/sj.gt.3302820. [DOI] [PubMed] [Google Scholar]
- 120.Jiang Y, Chen L, Tang Y, Ma G, Shen C, Qi C, Zhu Q, Yao Y, Liu N. HO-1 gene overexpression enhances the beneficial effects of superparamagnetic iron oxide labeled bone marrow stromal cells transplantation in swine hearts underwent ischemia/reperfusion: an MRI study. Basic Res Cardiol. 2010;105:431–442. doi: 10.1007/s00395-009-0079-2. [DOI] [PubMed] [Google Scholar]
- 121.Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339–1350. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 122.Lim SY, Kim YS, Ahn Y, Jeong MH, Hong MH, Joo SY, Nam KI, Cho JG, Kang PM, Park JC. The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Cardiovasc Res. 2006;70:530–542. doi: 10.1016/j.cardiores.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 123.Matsui T, Tao J, del MF, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 124.Jeong JO, Han JW, Kim JM, Cho HJ, Park C, Lee N, Kim DW, Yoon YS. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res. 2011;108:1340–1347. doi: 10.1161/CIRCRESAHA.110.239848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, Sun JP. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 127.Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, Mendizabal AM, Pattany PM, Lopera GA, Fishman J, Zambrano JP, Heldman AW, Hare JM. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res. 2011;108:792–796. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Miura M, Miura Y, Padilla-Nash HM, Molinolo AA, Fu B, Patel V, Seo BM, Sonoyama W, Zheng JJ, Baker CC, Chen W, Ried T, Shi S. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24:1095–1103. doi: 10.1634/stemcells.2005-0403. [DOI] [PubMed] [Google Scholar]
- 129.Behfar A, Yamada S, Crespo-Diaz R, Nesbitt JJ, Rowe LA, Perez-Terzic C, Gaussin V, Homsy C, Bartunek J, Terzic A. Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction. J Am Coll Cardiol. 2010;56:721–734. doi: 10.1016/j.jacc.2010.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dufourcq P, Descamps B, Tojais NF, Leroux L, Oses P, Daret D, Moreau C, Lamaziere JM, Couffinhal T, Duplaa C. Secreted frizzled-related protein-1 enhances mesenchymal stem cell function in angiogenesis and contributes to neovessel maturation. Stem Cells. 2008;26:2991–3001. doi: 10.1634/stemcells.2008-0372. [DOI] [PubMed] [Google Scholar]