Abstract
Background and Purpose
Hippocampus-dependent memory involves the activity of sharp wave ripples (SWRs), which are thought to participate in the process of memory consolidation. The hippocampus contains high levels of endogenous opioids and of μ-opioid receptors (MORs). Here, we have assessed the role of MOR agonists in the modulation of SWRs.
Experimental Approach
Using recordings of extracellular potentials from the CA1 field of rat hippocampal slices, we examined the pharmacological actions of morphine, DAMGO and fentanyl on SWRs and on network excitability and paired-pulse inhibition.
Key Results
All three MOR agonists (1 nM–10 μM) significantly increased the amplitude of sharp waves and the occurrence of SWR sequences, but reduced the initiation of episodes of SWRs. Fentanyl was most potent in producing these effects and morphine the least. Interestingly, although SWRs were reduced by relatively high concentrations (≥100 nM) of all agonists, they were significantly enhanced by very low concentrations of morphine (5–10 nM). Morphine and DAMGO at moderate-to-high concentrations increased network excitability and reduced inhibition. Furthermore, DAMGO suppressed inhibition more readily than it increased excitation, whereas morphine suppressed inhibition only at high concentrations. These drug effects were reversed by the MOR antagonists naloxone and CTOP.
Conclusions and Implications
We found that the SWRs were significantly modulated by three MOR agonists and that the SWRs were very sensitive to subtle changes in the excitation/inhibition balance induced by MOR agonists. Such modulation might underlie the effects of these agonists on hippocampus-dependent memory.
Keywords: hippocampus, sharp wave, ripple oscillation, μ-opioid receptor, opiate, memory, recurrent inhibition, network excitability, CA1, slices
Introduction
Endogenous and clinically administered opioids act on many receptors (Waldhoer et al., 2004) and are involved in a wide variety of functions (Drolet et al., 2001) including hippocampus-dependent learning and memory (Wise, 1989; Meilandt et al., 2004; Bodnar, 2010; Kesner and Warthen, 2010; Dacher and Nugent, 2011). Nevertheless, the mechanisms by which opioids affect hippocampus-dependent memory appear to be highly complex and poorly understood.
The critical involvement of the hippocampus in learning and memory (Eichenbaum, 2004; Squire et al., 2004) is underpinned by a number of different types of neuronal activity supporting distinct processes (Battaglia et al., 2011). For example, it is assumed that the encoding of new information that occurs during active behaviour is associated with θ oscillation (Hasselmo, 2005) whereas memory consolidation takes place during inactivity (Alvarez and Squire, 1994; McGaugh, 2000). It is generally thought that memory consolidation entails the generation of irregular activity of sharp wave ripples (SWRs) (O'Neill et al., 2010), which is an endogenous hippocampal activity that occurs during awake quietness and slow wave sleep. This activity was first recorded in rodents (O'Keefe and Nadel, 1978; Buzsaki, 1986) and more recently in primates and humans (Bragin et al., 1999; Skaggs et al., 2007). SWR activity is thought to represent a ‘carrier’ of information from the hippocampus to the neocortex (Wierzynski et al., 2009) in a process that is repeated off-line; this leads to long-term establishment of the memory in the neocortex, that is, consolidation (McGaugh, 2000). Accordingly, changes in the activity of SWRs have recently been correlated with performance in hippocampus-dependent learning and memory (Girardeau et al., 2009; Ramadan et al., 2009; Singer and Frank, 2009; Ego-Stengel and Wilson, 2010). SWRs are a complex field activity generated by the CA3 and CA1 hippocampal circuits and consist of a slow potential that lasts for 50–100 ms (sharp wave), which is generated by synaptic activity on pyramidal cells (Ylinen et al., 1995; Papatheodoropoulos and Kostopoulos, 2002; Maier et al., 2003) and a high-frequency (100–200 Hz) network oscillation (ripples) that occurs during the sharp wave and is the result of a rather complicated and spatio-temporally coordinated interaction between principal cells and circuits of specific classes of GABAergic interneurons (Chrobak and Buzsaki, 1994; Klausberger et al., 2004). Specific classes of GABAergic interneurons that innervate other types of interneurons are among the main sources of endogenous opioids in the hippocampus (Gall et al., 1981; Chavkin et al., 1985; Blasco-Ibanez et al., 1998). The hippocampus contains a relatively high level of opioid receptors (Zastawny et al., 1994), and the most abundant opioid receptors in the rat hippocampus belong to the class of μ-opioid receptors (MORs) (Mansour et al., 1987; receptor nomenclature follows Alexander et al., 2011). MORs are found in the dentate gyrus and CA3 and CA1 fields (Schnell and Wessendorf, 2008), and they are strongly expressed by and strategically located on those GABAergic interneurons that target the somatic domain of principal cells (Drake and Milner, 2002). In addition, within the perisomatically projecting GABAergic cells, MORs are selectively located on parvalbumin (PV)-expressing cells (Drake and Milner, 2002). Interestingly, PV cells in CA1 field belong to those GABAergic neurons that intensively increase their firing rate during SWRs (Klausberger et al., 2004), and they are the preferred targets of the recently discovered enkephalin-expressing GABAergic cells that are silent during SWRs but strongly activated after episodes of SWRs (Fuentealba et al., 2008b).
In order to better understand how the various neurotransmission and neuromodulation systems are involved in learning and memory, it is necessary to understand their interactions with the functioning of local brain circuits. The results from earlier studies suggest that opioid agonists acting at MORs might significantly affect information processing in the hippocampus. For example, MORs modulate temporal integration of synaptic activity in the hippocampus (McQuiston, 2011) and disrupt γ (Whittington et al., 1998) and β oscillation (Faulkner et al., 1999), yet the role of MOR in the modulation of the complex activity of SWRs remains unknown. In the present study using a recently developed in vitro model of SWRs in slices from the rat ventral hippocampus (Papatheodoropoulos and Kostopoulos, 2002), we aimed to determine the modulatory effects of opioid agonists that act primarily at MORs on the activity of SWRs. Using morphine, DAMGO and fentanyl over a wide range of concentrations that included clinically relevant concentrations and concentrations that were similar to extracellular concentrations of endogenous opioids, we found that all three opioid agonists significantly modulated SWRs. However, their effects were not identical but differed between the various characteristics of SWRs. The present findings demonstrate that MOR agonists significantly modulate SWRs and also suggest that this action may be mediated by modifications of the balance between excitation and inhibition of the local neuronal circuit.
Methods
All animal care and experimental procedures complied with the European Communities Council Directive Guidelines (86/609/EEC) for the care and use of laboratory animals and were approved by the local Ethical Committee of the University of Patras. Male Wistar rats (200–400 g; Hellenic Pasteur Institute, Athens, Greece) were housed under controlled conditions of temperature (20–22°C), 12/12 h light-dark cycle, and free access to food and water. All measures were taken to minimize animal suffering and to reduce the number of the animals used. Studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). In the present study 71 Wistar rats were used.
Slice preparation
Animals were deeply anaesthetized with diethyl ether and decapitated immediately after they stopped breathing. The brain was removed from the skull, placed in a chilled (2–4°C) artificial cerebrospinal fluid containing (in mM): 124 NaCl, 4 KCl, 2 MgSO4, 2 CaCl2, 1.25 NaH2PO4, 26 NaHCO3 and 10 glucose. The solution was equilibrated with 95% O2 and 5% CO2 gas mixture to achieve pH 7.4. The two hippocampi were excised free inside the chilled medium, then transverse slices of 500–550 μm thick were prepared from the ventral hippocampus, extending between 1 and 4 mm from the end of the structure using a McIlwain tissue chopper (Mickle Laboratory Engineering Co. Ltd, Gomshall, Surrey, UK). The slices were immediately transferred to an interface type recording chamber where they were maintained at a constant temperature of 31 ± 0.5°C. Slices were continuously supplied with a humidified mixed gas containing 95% O2 and 5% CO2 and they were perfused with standard artificial cerebrospinal fluid containing (in mM): 124 NaCl, 4 KCl, 2 MgSO4, 2 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 glucose, pH 7.4, equilibrated with 95% O2 and 5% CO2 gas mixture.
Recordings and data processing
Recordings started approximately 3 h after the slices were placed in the recording chamber to allow them to recover and organize spontaneous activity. Recordings of spontaneous and evoked extracellular potentials were made from the CA1 stratum pyramidale using carbon fibre electrodes (diameter 7 μm; Kation Scientific, Minneapolis, MN, USA). Evoked synaptic responses consisting of field EPSPs and population spikes (PSs) were recorded by delivering electrical pulses (intensity 0.1–0.6 mA, duration 0.1 ms) at the Schaffer collaterals using a bipolar platinum/iridium wire electrode (wire diameter of 25 μm; World Precision Instruments, Sarasota, FL, USA). PS with amplitude of about 2 mV was evoked every 30 s and continuously monitored in order to determine the stability of the response. Only slices with a stable response for at least 10 min were selected for further experimentation. Signals were recorded with a Neurolog amplifier (Digitimer Limited, Welwyn Garden City, Hertforshire, UK), band-pass filtered at 0.5 Hz–2 kHz, digitized at 5 kHz, and stored in a computer disc using the CED 1401-plus interface and the Spike5 and Signal software (Cambridge Electronic Design, Cambridge, UK).
As described in Results section, episodes of spontaneous network potentials identified as SWR activity consisted of either isolated single events or sequences of multiple consecutive events termed clusters (see Figure 1A). We call the first event in a sequence the primary event, while the subsequent events are called secondary events. Events were further analysed into slow and fast components, the slow sharp wave and the high-frequency ripple oscillation (which, in rats, peaks at 110–200 Hz). The following parameters were quantified: the inter-episode interval (IEpI) determined as the time interval between episodes, that is, the interval between the last event of an episode and the first event of the following episode; the inter-event interval (IEI) determined as the time interval between successive individual SPWs regardless of whether they appeared as isolated events or clusters; the intra-cluster interval (ICI, the mean time interval between consecutive events inside a cluster); the probability of occurrence of clusters of SWRs (probability of clusters); the number of events per minute; and the number of episodes per minute. Additionally, the amplitude, the duration at half amplitude and the maximum duration of the primary event were also measured. IEI, IEpI, ICI, amplitude and duration were measured after down sampling (at 1–2 kHz) and then low-pass filtering (at 30 Hz) original data. Individual sharp waves were detected by setting a threshold at a level where all putative events were marked as verified by visual inspection. Measurements of the above parameters were made during the last 2 min before applying the drug and during the last 2 min of drug application. High-frequency ripple oscillation was revealed by applying fast Fourier transform or filtering original records at 90–300 Hz. We used power spectra to measure the power of the peak of the ripple oscillation. In filtered recordings, ripples were detected by setting a threshold at four times the SD of event-free baseline noise; we measured (i) the number of troughs of a ripple event; (ii) the duration of the ripple event; (iii) the amplitude of the ripple event; and (iv) the mean intra-ripple frequency during each event. In episodes consisting of multiple events (i.e. clusters), measurements of ripple parameters were obtained from the primary event only. For accuracy reasons, all measurements were made manually, with the exception of ripple power that was measured using plots of fast Fourier transforms. In a given slice, the values obtained from the measurement of all sharp waves contained in the 2 min periods of control/drug conditions were averaged and the resulting mean value was the representative value from this particular slice. Manual measures of ripple characteristics from each slice were made from an epoch of 30 consecutive events inside the 2 min periods. Mean values of each ripple parameter were calculated by averaging the values of all 30 ripple episodes in a particular slice.
Figure 1.
The characteristics of SWR activity. (A) In the left panel recording from the CA1 pyramidal layer is shown with SWR complexes (upper trace) and the corresponding band-pass (90–300 Hz) filtered waveform revealing the ripple oscillation (lower trace). In this example are shown four episodes of activity, each of them consisting of a sequence of SWR events. The framed event is shown at fast sweep speed in the right upper panel. The power spectra in the bottom of the right panel illustrate the three peaks around 2, 10 and 160 Hz produced by the occurrence of SWR episodes (every ∼500 ms), the occurrence of SWR events in sequences (every ∼100 ms), and the ripple oscillation. (B) Distribution histograms of the amplitude of sharp waves, the inter-event interval, the intra-cluster interval, and the frequency of ripple oscillation; ‘n’ indicates the number of slices used.
In order to examine the effects of opioid agonists on neuronal excitability and on GABAA receptor-mediated synaptic inhibition, we constructed input/output curves of the stimulation strength and the amplitude of PS, as well as of EPSP and PS before and during drug application. Neuronal excitability was quantified by measuring the strength of the stimulation required to produce half-maximal PS (I-50), the postsynaptic excitation required to produce half-maximal PS (EPSP50), and the ratio between PS and the corresponding EPSP50 (PS/EPSP). We quantified changes in excitability by measuring the changes of I-50, EPSP50 and PS/EPSP. PS was quantified from its amplitude, measured as the length of the projection of the negative peak on the line connecting the two positive peaks of the PS waveform. EPSP was quantified from the maximal slope of its rising phase.
Synaptic GABAA receptor-mediated recurrent inhibition was evoked using a protocol of paired-pulse stimulation consisting of two consecutive stimuli of identical intensity delivered at Shaffer collaterals with an inter-pulse interval of 10 ms. The strength of so-produced paired-pulse inhibition (PPI) was quantified using the ratio response evoked by the second (test) stimulus (PS2)/response evoked by the first (conditioning) stimulus (PS1). Based on the constructed input/output curves and previous observations (Petrides et al., 2007), the strength of PPI and its changes were measured at two specific points of the input/output curves. More specifically, the stimulation strengths causing 50% of the maximum PS were chosen to calculate the PS2/PS1 ratio. The effect of opioids on PPI was quantified from the change produced in the I50 (shift of I-50) as well as the change of the PS2/PS1 ratio.
Data analysis
Values are expressed as mean ± SEM, and ‘n’ indicates the number of slices included in the analysis. In several instances, the number of animals is also given. For statistical comparisons (using paired and independent t-tests) of the drug effects on SWRs, measurements were made immediately before drug application and during the last 2–5 min of drug application. The EC50 were calculated from log concentration–response curves after sigmoidal or non-linear (dose–response) fitting to experimental data. In case of lack of fit with the previous methods, polynomial fit was applied. For data analysis and preparation of figures we used the software SPSS 14 (SPSS Inc., Chicago, IL, USA) and Origin 7.5 (OriginLab Corporation, Northampton, MA, USA).
Materials
We used three opioid receptor agonists – morphine, the synthetic analogue of endogenous enkephalin [D-Ala2,N-Me-Phe4,Gly-ol5]enkephalin (DAMGO), and fentanyl. All three agonists exhibit selectivity for MORs (Waldhoer et al., 2004; Crooks et al., 2006). We also used the opioid receptor antagonist naloxone and the highly selective MOR antagonist, D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP). Morphine, fentanyl and naloxone were obtained from Sigma-Aldrich, Taufkirchen, Germany. DAMGO and CTOP were purchased from Tocris Cookson Ltd, Bristol, UK. All drugs were first prepared as stock solutions and then were dissolved in the artificial cerebrospinal fluid and bath applied. Drug effects on the parameters of the spontaneous and evoked activity were measured 30 min after adding them to the perfusion medium. However, when low drug concentrations were used, measurements were also obtained at 60 min of application.
Results
Recordings of field potentials were made from the CA1 pyramidal cell layer of transverse ventral hippocampal slices maintained in standard conditions. Spontaneous activity was recorded from the majority of hippocampal slices (>80%) approximately 3 h after placing in the recording chamber. As shown in Figure 1A,B, spontaneous activity consisted of relatively slow deflections with a main positive phase associated with multiunit activity. The amplitude of slow potentials ranged from 20 μV to 1 mV among slices. Band-pass filtering of original waveforms at 90–300 Hz disclosed a transient oscillation associated with the slow potentials. The mean frequency of this oscillation was ∼160 Hz forming a characteristic peak in power spectra made from the original records (Figure 1A, right panel). The slow potentials in combination with the fast oscillation formed a complex activity that corresponds to SWRs recorded from the intact hippocampus (O'Keefe and Nadel, 1978). Episodes of the compound activity of SWRs consisted of either single events or multiple events characteristically clustered in sequences of two to five events (Figure 1A). The generation of these sequences represents a regular mode of SWR occurrence in the intact hippocampus and the consecutive events within the sequences occurred at the particularly consistent interval of 107 ± 2.1 ms (range 62–162 ms, n = 66 slices) (Figure 1B). In the present study, SWRs were recorded from more than 200 slices and drug effects were examined in 165 slices obtained from 71 animals. Figure 1B shows the distribution histograms for the amplitude of sharp waves, the IEI, the ICI as well as the ripple frequency. Primary sharp waves had mean amplitude of 232 ± 17 μV (range 100–1000 μV, n = 133), duration of 51 ± 1.7 ms (range 26–115 ms, n = 127) and episodes that occurred every 532 ± 18 ms (range of IEpI 206–922 ms, n = 72). The sharp wave-associated ripple oscillation displayed a frequency of 163 ± 1.6 Hz (range 121–195 Hz, n = 101). Furthermore, ripple events had an amplitude of 111 ± 8.4 μV (range 26–254, n = 45) and duration of 35 ± 1.7 ms (range 17.5–68.7, n = 45), and they consisted of 6.4 ± 0.3 ripple cycles (range 3.5–12, n = 45). We observed that episodes of clustered SWR events (i.e. sequences) occurred in about 70% of the slices. Single events and sequences occurred with similar (∼0.5) probability in the population of slices that displayed both types of episodes.
In order to examine whether tonic activity of MORs is implicated in the generation of SWRs, we perfused slices displaying ongoing SWRs with the wide-spectrum opioid antagonist naloxone or the selective MOR antagonist CTOP. None of the two drugs produced consistent effects on any of the parameters of SWRs. We then studied the effects of the three MOR agonists, morphine, DAMGO and fentanyl, used in a wide range of concentrations (1 nM–10 μM ), which includes concentrations similar to brain extracellular levels of endogenous opioids and clinically relevant concentrations of exogenous opioid agonists. Thus morphine, fentanyl and DAMGO were applied at concentrations (10−9 to 10−5 M) that included the nanomole regime covering the range of clinically relevant brain concentrations (Bouw et al., 2001), the opioid concentrations found in the cerebrospinal fluid (Bernards et al., 2003) or the plasma/serum (Veselis et al., 1994; Klepstad et al., 2003; Gunnar et al., 2004; Solassol et al., 2005) as well as the extracellular levels of endogenous brain opioids (∼1 to 10 nM) (Lam et al., 2008).
In general, all three drugs displayed pronounced effects on SWRs. Most of the drug actions were concentration-dependent and qualitatively similar although they differed in magnitude. More specifically, all drugs significantly increased the amplitude of the primary sharp waves, the incidence of occurrence of sequences of SWRs, and the interval between episodes of SWRs (IEpI). It is of note that these effects were significant even at very low concentrations of 1–10 nM (Figures 2A, 3A & 4A). As shown in Figures 2B, 3B and 4B, all MOR agonists increased the amplitude of sharp waves by 15–60%, and the interval between episodes (IEpI) by 6–280% in a concentration-dependent manner. The apparent consequence of the increased IEpI was the reduction in the rate of occurrence of episodes by 10–70%. Another effect of MOR agonists was the increase in the probability of occurrence of SWR sequences. The greatest effect was observed with a high concentration of DAMGO (1 μM) which elicited a 660% increase in this parameter. Because of the increased probability of occurrence of sequences, the total number of events was not changed at low concentrations of MOR agonists. However, when high concentrations were used, the reduction of the rate of episodes was high enough to surpass the increase in events due to an increase in clusters; this resulted in a significant reduction of the total number of events by 24–65%. In order to compare the potencies of the three drugs, we calculated the values of the EC50 (in nM) for a given effect using concentration–response curves. As shown in Table 1, the lowest EC50 values for all effects on sharp waves were observed with fentanyl, while morphine displayed the highest. DAMGO displayed rather intermediate EC50 values. We also estimated the relative potencies by pooling the EC50 values for all parameters together and calculating the mean EC50 value for the effect of each drug on sharp waves. The series of increasing potency was again morphine (269 ± 59), DAMGO (182 ± 66) and fentanyl (33.8 ± 5.3).
Figure 2.
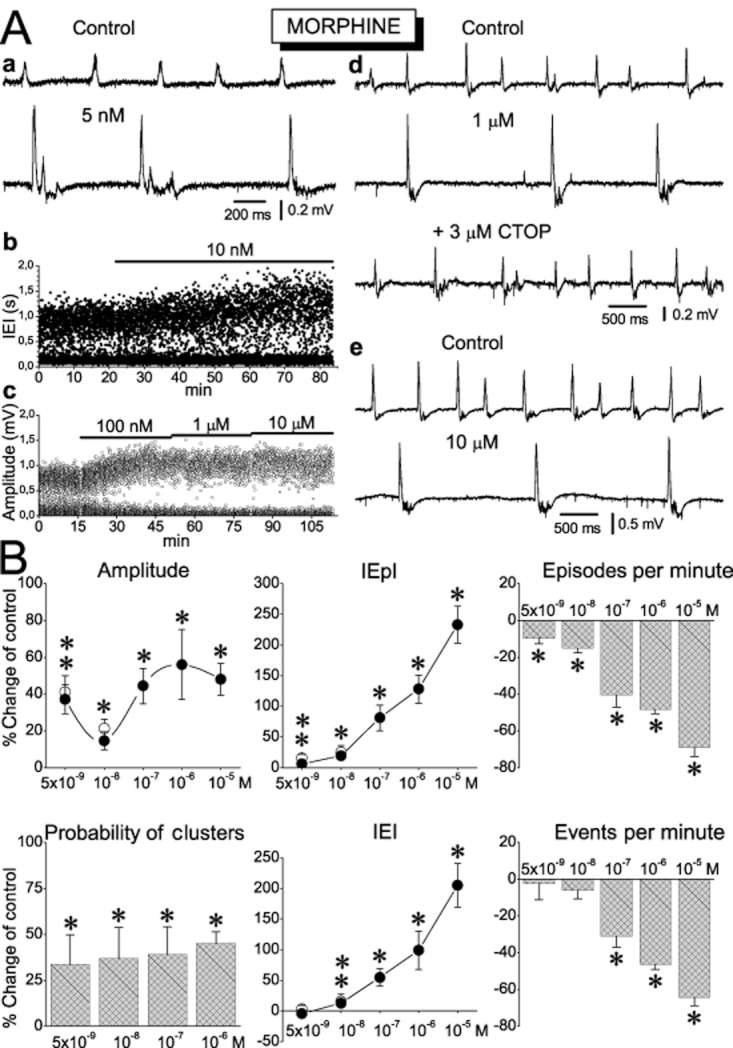
The effects of morphine on sharp waves. (A) Examples of CA1 recordings (a, d, e), scatter plot of instantaneous interval between events (b) and instantaneous amplitude of sharp waves (c) illustrating that morphine used at a wide range of concentrations promoted the appearance of sequences, increased the amplitude of sharp waves, and increased the interval between SWR events. Note in ‘d’ that CTOP fully reversed the effect of morphine. (B) Concentration–response collective plots of the effects of the various parameters of sharp waves. Note that at the low concentrations of 5 and 10 nM, morphine reduced the rate of episodes without changing the total number of events. The number of slices/animals used at the concentrations of 5 × 10−9, 10−8, 10−7, 10−6 and 10−5 M was respectively 10/4, 7/4, 7/4, 6/3, 8/4 for the amplitude, the IEpI, and the IEI, and 7/4, 6/4, 6/4, 4/3, 7/4 for the probability of clusters, the episodes per minute and the events per minute. *P < 0.05. Open circles at 5 and 10 nM represent measurements at 60 min of drug application and for these data, **P < 0.05.
Figure 3.

The effects of DAMGO on sharp waves. (A) Examples of CA1 recordings (a, b, d) and scatter plot of instantaneous interval (c) are shown. In the experiment shown in ‘c’, representative traces of sharp waves before and during 0.5 μM DAMGO are shown in insets. The start of the drug application is indicated by the arrow. Note that DAMGO induced the appearance of a new band of values at around 0.1 s which corresponds to short intervals between the consecutive events in the newly produced sequences. Calibration bars in insets 0.2 mV, 50 ms. (B) Concentration–response plots of the effects of DAMGO on the various parameters of sharp waves. The number of slices/animals used at the concentrations of 10−9, 10−8, 10−7, 5 × 10−7 and 10−6 M is respectively 12/5, 9/3, 8/3, 8/4, 9/8 for the amplitude, the IEpI, and the IEI, and 4/3, 3/3, 3/3, 4/3, 7/4 for the probability of clusters, the episodes per minute and the events per minute. *P < 0.05. Open circles at 5 and 10 nM represent measurements at 60 min of drug application and for these data, **P < 0.05.
Figure 4.
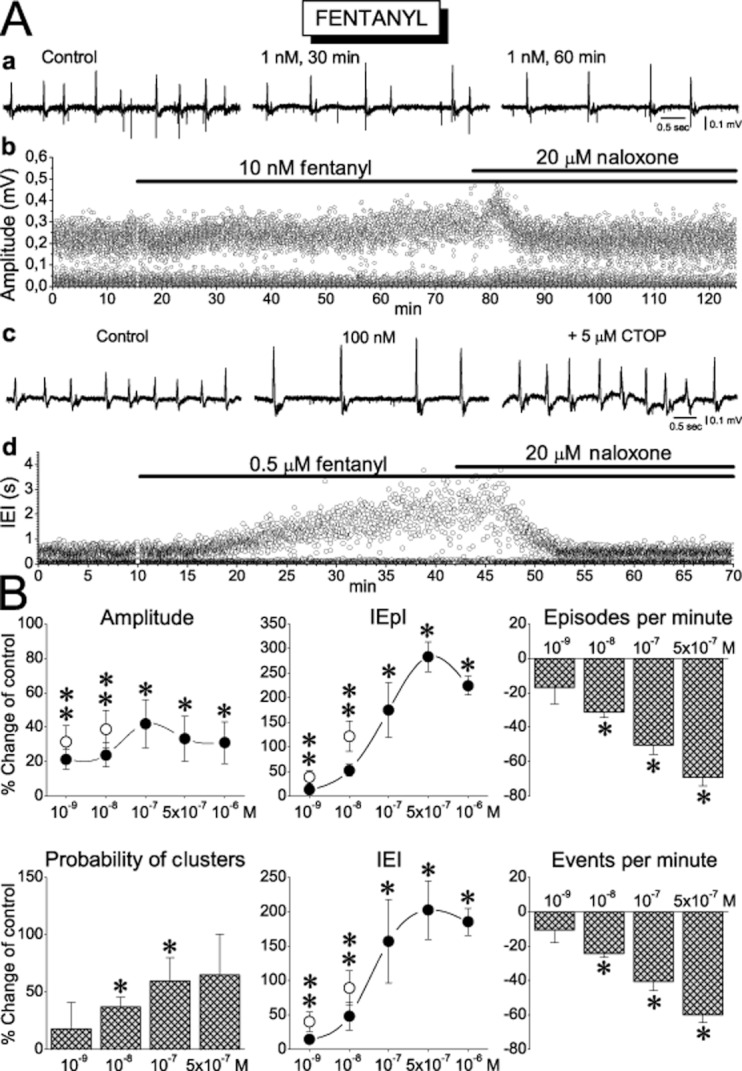
The effects of fentanyl on sharp waves. (A) Examples of recordings (a, c) and scatter plot of instantaneous amplitude (b) and interval (d) are shown. In the experiment shown in ‘d’, note that naloxone fully reversed the effect of high concentration of fentanyl. (B) Concentration–response plots of the effects of fentanyl on the characteristics of sharp waves. The number of slices/animals used at the concentrations of 10−9, 10−8, 10−7, 5 × 10−7 and 10−6 M was respectively 10/4, 12/6, 12/7, 6/3, 6/3 for the amplitude, the IEpI, and the IEI, and 4/3, 11/6, 9/5, 4/3, 7/4 for the probability of clusters, the episodes per minute and the events per minute. *P < 0.05. Open circles at 5 and 10 nM represent measurements at 60 min of drug application and for these data, **P < 0.05.
Table 1.
EC50 values (nM) for the three MOR agonists and for the parameters of spontaneous and evoked potentials
| Morphine | DAMGO | Fentanyl | |
|---|---|---|---|
| Spontaneous activity (SWRs) | |||
| Amplitude of sharp waves | 167 | a | 10.3 |
| IEpI | 440 | 366.2 | 30.8 |
| Episodes per minute | 278.8 | 49.2 | 47.9 |
| IEI | 433.2 | 275.3 | 37.5 |
| Events per minute | 233.4 | 22.4 | 41.7 |
| Probability of clusters | 72.1 | 198.3 | 34.4 |
| Ripple power | 23.3 | 167.5 | 132.5 |
| Evoked responses | |||
| I50 | 885.5 | 85.8 | – |
| EPSP50 | 316.8 | 223 | – |
| PS/EPSP50 | 347.8 | 319.5 | – |
| Second spike | 1578 | 372.5 | – |
| Shift of I50 | 1477 | 22.5 | – |
| PS2/PS1 | 2590 | 98.4 | – |
Fitting to the data not possible.
Based on our observation that the effects of the drugs at high concentrations (>100 nM) reached a plateau after 15–25 min application (see e.g. Figures 3A-c and 4A-d), we decided to make our measurements at 30 min. However, taking into account that equilibration between drug concentrations in the perfusion medium and the tissue might take longer at low than high concentrations, we examined the effects of the two lower concentrations (≤10 nM) of all three MOR agonists at both 30 and 60 min after application. The rationale behind this was that if the action of a given MOR agonist used at a low concentration continued to increase beyond 30 min, it would be difficult to reach a safe conclusion about the action of low opioid concentrations. After we applied drugs for 30 min of additional time, we observed either no change or a small but not significant further effect of the drug. The effects of MOR agonists reversed upon application of naloxone (20 μM) or CTOP (3–5 μM) or by washing out the agonist (see examples in Figures 2A-d, 3A-b,d and 4A-b,c,d). MOR agonists did not produce any consistent effect on the interval between consecutive events in the sequences (ICI) or the half-maximal and the maximal duration of the primary sharp waves.
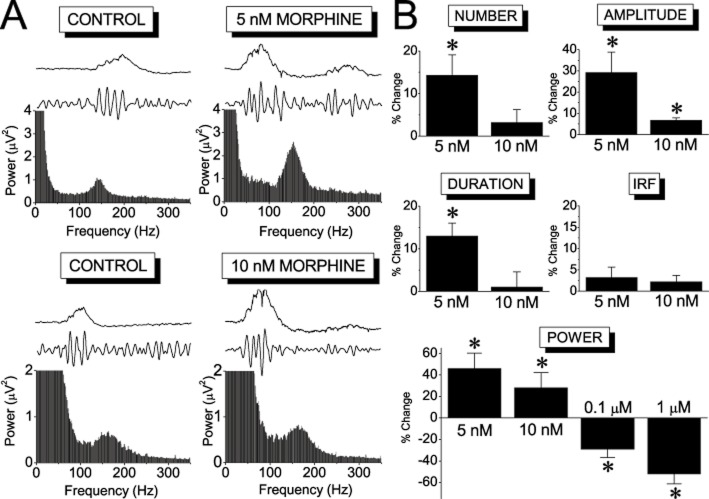
Although the effects of the three MOR agonists on sharp waves were qualitatively similar, their effects on the ripple oscillation differed (Figures 5 & 6). All three drugs significantly suppressed ripples at moderate-to-high concentrations (≥100 nM), with the effects being quantitatively similar between the three drugs. However, at lower concentrations (≤10 nM) morphine, but not the other agonists, enhanced the ripple oscillation (Figure 5). This enhancing effect was observed in all parameters of the oscillation and it was maximal at the lowest concentration of 5 nM, at which the observed increase in the amplitude, the number of ripples, the duration of ripple events, and the ripple power were 29%, 14%, 13% and 52% respectively. None of the MOR agonists significantly affected the frequency of the oscillation.
Figure 5.
Morphine enhanced ripple oscillation at low concentrations. Examples (A) and collective results (B) of the effects of morphine on the ripple oscillation. In each plot in (A), the power spectrum and the ripple oscillation are shown. The corresponding sharp waves are also indicated on the top of each plot. The number of slices/animals used at the concentrations of 5 nM, 10 nM, 0.1 μM and 1 μM was 16/8, 6/4, 13/7 and 13/7 respectively. *P < 0.05.
Figure 6.
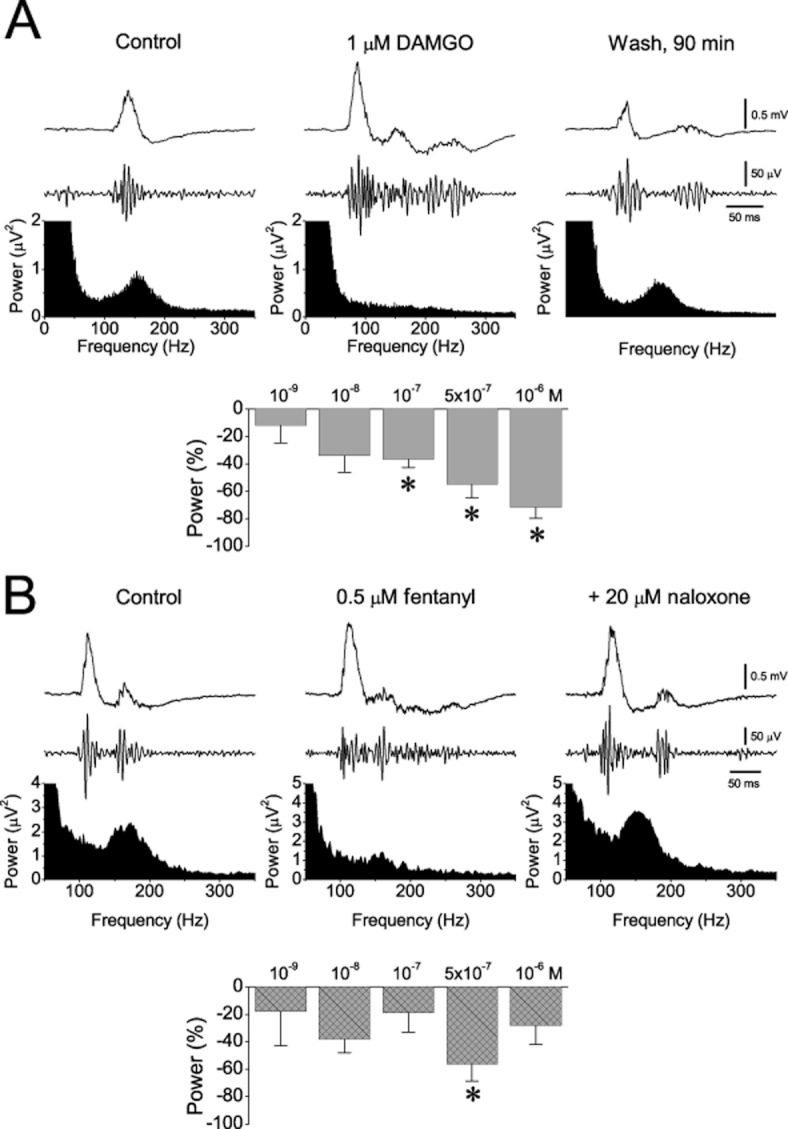
The effects of DAMGO (A) and fentanyl (B) on the ripple oscillation. Examples (power spectra and traces of sharp wave and ripples) and collective results for the power of the oscillation are shown. The number of slices/animals used at the concentrations of 10−9, 10−8, 10−7, 5 × 10−7 and 10−6 M was 9/4, 3/2, 8/3, 8/4, 6/6 for DAMGO and 4/2, 11/6, 12/7, 4/2, 3/2 for fentanyl respectively. *P < 0.05.
Effects of μ-opioids on network excitability and recurrent inhibition
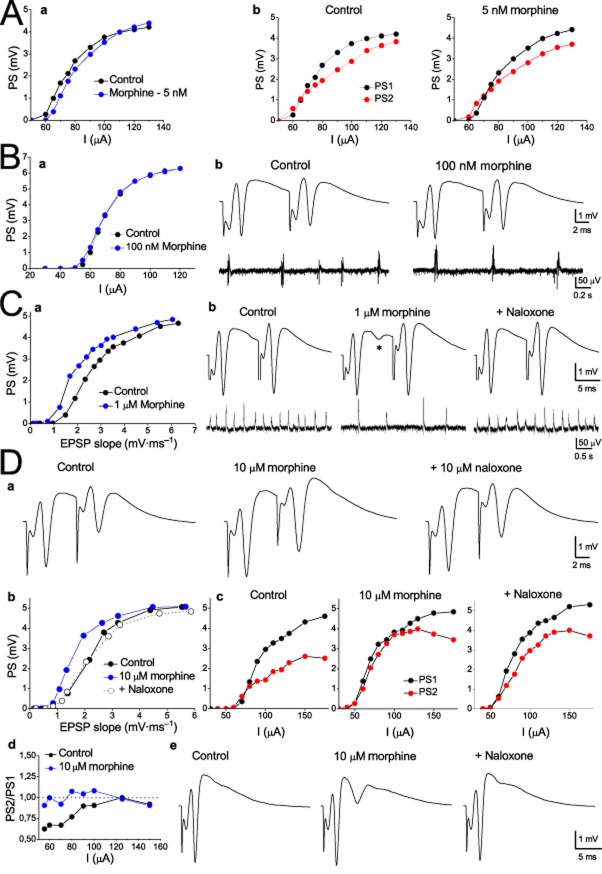
It is thought that the action of opioids in the hippocampus is produced mainly by a disinhibitory effect on the principal cells that results in an enhanced neuronal excitability of the network (Zieglgansberger et al., 1979; Nicoll et al., 1980; Masukawa and Prince, 1982; Swearengen and Chavkin, 1987). We thus examined the effects of morphine (5 nM, 10 μM) and DAMGO (10 nM, 1 μM) on the neuronal network excitability and paired-pulse recurrent inhibition in 41 slices obtained from 27 animals. In general, both drugs affected network excitability and inhibition, yet some differences in their action were revealed. Figures 7 and 8 show examples of the effects of morphine and DAMGO respectively. Summary results are shown in Figure 9. As shown by the excitability curves for morphine (Figure 7A-a, B-a) and DAMGO (Figure 8A-a, B-a) applied at relatively low concentrations (5–100 nM), neither of the two drugs significantly altered network excitability (see also Figure 9). However, at higher concentrations both morphine (1 and 10 μM, Figure 7C-a, D-b) and DAMGO (10 μM, Figure 8C-a) enhanced network excitability as shown by the leftward shift in the input/output curves, the corresponding decrease in I50 and EPSP50, and the increase in PS/EPSP ratio (Figure 9). The opioid-induced shift to the left of the stimulus-to-PS and EPSP-to-PS curves is consistent with previous reports (Dingledine, 1981; Watson and Lanthorn, 1993). Additionally, DAMGO was more potent than morphine in increasing network excitability, as shown by the lower mean EC50 value (i.e. from all three excitability indexes) of DAMGO (209) compared with morphine (517). Neither of the two drugs produced a consistent effect on the amplitude of maximal PS and maximal EPSP.
Figure 7.
Low concentrations of morphine enhance network excitability more than they affect recurrent inhibition (A) Examples of excitability curves obtained from one slice illustrating the effects of 5 nM morphine on the network excitability (a) and PPI (b) are shown. Input/output curves were made by plotting the PS amplitude against the stimulus strength before and during the application of the drug. Note that in (a), input/output curves were made for the first PS produced with the paired-pulse stimulation protocol, while in (b) curves were constructed for both the first and the second PS (PS1, black symbol and PS2, red symbol respectively) evoked by the pair of stimuli in order to illustrate the suppressive effect of the first stimulus to the second response attributed to the recurrent activation of GABAergic inhibition. At 5 nM, morphine did not produce any significant change in either the excitability curve (a) or the suppressing effect of the inhibition on the PS2. (B) Examples of excitability curve (a) with recordings of paired-pulse evoked potentials and concomitant spontaneous SWRs (upper and bottom traces respectively) obtained from another slice illustrating that although morphine reduced the rate of occurrence of SWRs, it did not change network excitability or PPI. (C) Examples of input/output curve (a) and recordings of evoked (upper traces) and simultaneous spontaneous SWRs (bottom traces) (b) showing that morphine at the concentration of 1 μM enhanced excitability, as demonstrated by the drug-induced shift to the left in the curve, but it did not affect recurrent inhibition because it did not change the relationship between PS1 and PS2. The asterisk indicates the appearance of a secondary PS. Note the concomitant strong drug effect on the spontaneous activity. Data were obtained from three different slices. Stimulation artefacts in (b) were truncated. (D) Examples of the effects of 10 μM morphine. Morphine reversibly enhanced network excitability (b) and reduced PPI (a) and (c). In (a), note that because of the short inter-pulse interval in this experiment (8 ms) the secondary PS was masked by the second stimulus artefact. The reduction in inhibition is also shown in the plot made between the PS2/PS1 ratio and the stimulation strength shown in (d). PS2/PS1 ratio was increased with 10 μM morphine at most stimulation strengths. Example of evoked maximal PSs from another slice (e) shows the generation of a secondary PS with 10 μM of morphine. Note that the secondary spikes were abolished by naloxone.
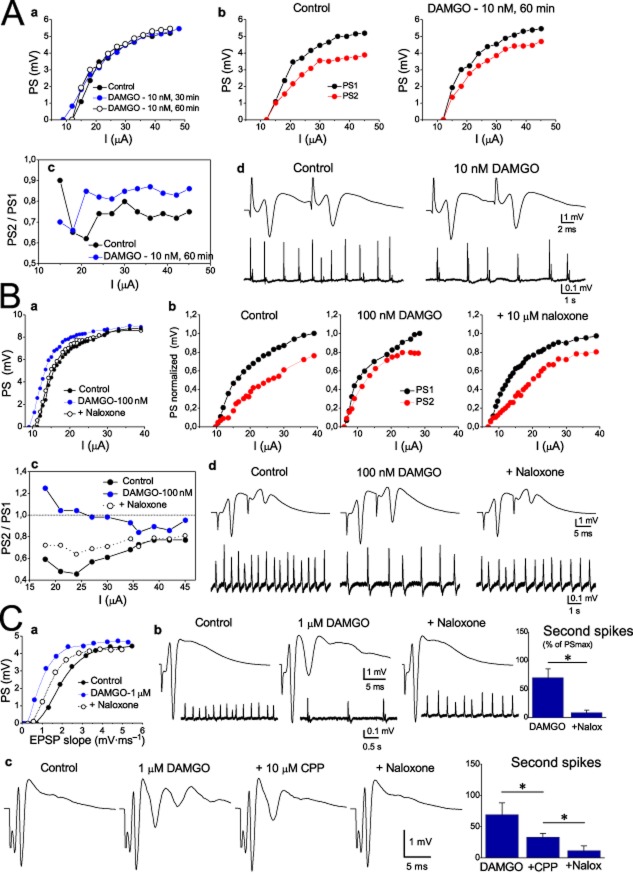
Figure 8.
DAMGO reduces the strength of recurrent inhibition at a lower concentration than that enhancing network excitability. (A) Examples of the effects of 10 nM DAMGO on the neuronal excitability (a) and PPI (b) are shown. A plot of the PS2/PS1 ratio against the stimulation strength, before and during 10 nM DAMGO, is shown in (c) and examples of recording of PSs evoked by the paired-pulse stimulation (upper traces) and of the simultaneously recorded spontaneous SWRs (lower traces) are shown in (d). (B) The effects of 100 nM DAMGO on the neuronal excitability and PPI are shown in (a) and (b) respectively. Input/output curves between PS amplitude and the stimulus strength show that DAMGO reversibly eliminated the suppressive effect of recurrent inhibition at 100 nM; this effect was reversed by naloxone. The plot in (c) illustrates the significant and reversible effect of 100 nM DAMGO on the PS2/PS1 ratio at a wide range of stimulus intensity. The example in (d) illustrates that 100 nM DAMGO reversibly reduced PPI (upper traces) while at the same time increased the amplitude and reduced the rate of occurrence of spontaneous activity (lower traces). (C) Effects of 1 μM DAMGO. Example of PS/EPSP curves that illustrate the strong leftward shift in the curve induced by 1 μM DAMGO is presented in (a). The example in (b) shows the generation of a large secondary PS under 1 μM DAMGO and its complete block by naloxone (Nalox). The plot on the right shows the collective results of the effect of naloxone (data collected from 5 slices/3 animals). In the example shown in (c), secondary spikes were partly blocked by the antagonist of NMDA receptor CPP, while the remaining component of secondary spikes was blocked by naloxone. The collective results of the consecutive effects of CPP and naloxone on secondary spikes are presented in the right plot (data from 4 slices/2 animals). *P < 0.05, significant effects of CPP or naloxone. Stimulation artefacts in (c) were truncated.
Figure 9.
Collective results of the effects of morphine and DAMGO on the indexes of network excitability (I50, EPSP50, PS/EPSP, second spikes) and paired-pulse inhibition (Shift-I50, PS2/PS1). The numbers in the I50 plot indicate the number of slices/animals used for each drug concentration. *P < 0.05, **P < 0.01, ***P < 0.005. Note that compared with morphine, DAMGO affected the indexes of inhibition at much lower concentrations.
Striking results were obtained from the drug effects on inhibition. Their effects on PPI were measured using the decrease in the rightward displacement of the input/output curve of the second PS (PS2) in relation to the first PS (PS1) evoked by the paired-pulse stimulation. This effect was reflected in the lower shift of I50 and in the higher PS2/PS1 ratio. Although both drugs reduced PPI they displayed different profiles of action. As shown in Figures 7 and 9, morphine reduced PPI only at 10 μM, whereas DAMGO reduced PPI at a much lower concentration (100 nM) (Figures 8B and 9) and yielded a lower EC50 value for inhibition (60.4), compared with that for morphine (2033) (see Table 1). Thus, DAMGO was more potent than morphine in reducing inhibition. Furthermore, the mean EC50 value of DAMGO for inhibition was more than threefold lower than that for excitability (60.4 vs. 209), whereas morphine was less potent in reducing inhibition than in increasing excitability (mean EC50 values, 2033 and 517 respectively).
In addition to the changes in the excitability indexes derived from the input/output curve, both morphine and DAMGO at relatively high concentrations induced secondary PSs following a primary PS (Figure 7C, D-d and Figure 8C). This is in line with previous observations that opioid agonists induce secondary PSs in hippocampal slices (Dingledine, 1981; Swearengen and Chavkin, 1987; Watson and Lanthorn, 1993). DAMGO was more effective than morphine in generating secondary spikes as its maximal effect was more than threefold greater than that of morphine (Figure 9); this effect was produced at a lower concentration (1 μM vs. 10 μM). In addition to the above, DAMGO began inducing secondary spikes at a lower concentration than morphine (100 nM vs. 1 μM). This difference in potency was also expressed in the considerably lower EC50 value for DAMGO (372.5) when compared with morphine (1578). In keeping with previous reports (Dingledine, 1981), we found that the secondary spikes were completely blocked by naloxone (Figures 7D-e and 8C-b). We also found that the secondary spikes induced by the MOR agonists were partly blocked by an antagonist of NMDA receptor CPP (Figure 8C-c), as previously reported (Swearengen and Chavkin, 1987). The remaining component of secondary spikes that was not blocked by CPP was abolished by naloxone (Figure 8C-d). It has been previously shown that morphine reduces the post-spike afterhyperpolarization (Faulkner et al., 1999). This reduction may lead to increased excitability in pyramidal cells and the appearance of multiple spikes (Azouz et al., 1996). Consequently, the greater ability of DAMGO to induce secondary spikes may reflect a greater effect of this drug in enhancing NMDA receptor activity and/or reducing post-spike afterhyperpolarization when compared with morphine.
The difference in the effects of morphine and DAMGO on excitability and inhibition suggests that their mechanisms of action on the local neuronal circuit are not identical. Morphine and DAMGO have high affinity for MORs but they also bind to δ, and κ opioid receptors (Mansour et al., 1987; Waldhoer et al., 2004; Crooks et al., 2006). M, δ and κ opioid receptors can be found in the CA1 field of the ventral hippocampus (Mansour et al., 1987), the part of the hippocampus that has been used in the current study. Because these opioid receptors elicit similar cellular events (Pan, 1998), the differences of the drug potencies observed here could be interpreted as differences in the affinity/efficacy of the drugs for the various opioid receptors (Zernig et al., 1995; Crooks et al., 2006), as well as different expression of the receptors from physiologically different cell types (Pan, 1998). DAMGO's higher potency to suppress inhibition, compared with morphine, could be accounted for by its higher affinity and efficacy for MORs (Chaturvedi et al., 2000; Crooks et al., 2006) and possibly for the various MOR subtypes (Pasternak, 2001).
Discussion
Our study demonstrates that the MORs play an important modulatory role on SWRs. All three MOR agonists used displayed prominent, concentration-dependent effects on SWRs. The effects were observed even at very low drug concentrations (1–10 nM). Moreover, MOR agonists affected the various characteristics of SWRs differently. All three agonists significantly enhanced the amplitude of sharp waves and increased the probability of occurrence of sequences of SWRs. They also reduced the mean rate of episodes of SWRs (i.e. they dampened the likelihood of initiation of new episodes). Ripple oscillation was suppressed by relatively high concentrations (≥100 nM) of opioid agonists; however, low concentrations of morphine (5–10 nM) significantly enhanced ripples. Morphine and DAMGO altered network excitability and recurrent inhibition, displaying different potencies. These effects were reversed by antagonists of MORs.
Interpretation of drug effects – implications for the mechanisms underlying SWRs
The similar effects of MOR agonists on the amplitude of sharp waves, the initiation of episodes and the incidence of sequences suggested a common mechanism of action. Because changes were observed at concentrations as low as those of endogenous μ-opioids in the rat brain (Lam et al., 2008), these changes might reflect the modulation of SWRs by endogenous opioids. In the hippocampus, MORs are almost exclusively located on GABAergic inhibitory interneurons (Drake and Milner, 2002) and opioid agonists modulate inhibition (Nicoll et al., 1980; Lupica, 1995) and increase the excitability of the pyramidal cells (Zieglgansberger et al., 1979; Masukawa and Prince, 1982; Swearengen and Chavkin, 1987). In CA1, opioids suppress synaptic inhibition by hyperpolarizing GABAergic interneurons especially those that project to the perisomatic domain of principal neurons (Madison and Nicoll, 1988; McQuiston, 2008). In keeping with these observations, we found that MOR agonists enhanced network excitability and decreased inhibition. However, as those effects on SWRs produced by low opioid concentrations (<100 nM) were observed in the absence of change on the overall neuronal network excitability and inhibition, we would propose that the modulation of SWRs requires relatively small and specific alterations in the GABAergic transmission which cannot be easily detected by stimulation that engages a rather large part of the local network. The different potencies observed between the three MOR agonists might result from the different affinities/efficacies of the three compounds for the MORs (Zernig et al., 1995; Crooks et al., 2006).
The reduction in the occurrence of SWR episodes is consistent with the suppressive effect of MOR agonists on the activity of GABAergic interneurons, and recent findings suggest that the initiation of episodes of SWRs is favoured by slight enhancement of GABAA receptor-mediated transmission (Ellender et al., 2010; Koniaris et al., 2011).
The generation of the ripple oscillation requires an accurate balance between excitation and inhibition in the local network. Interestingly, in the present study MOR agonists suppressed ripple oscillation at the same concentration (100 nM) that they disturbed the excitation/inhibition balance. This suppressive effect is consistent with the selective localization of MORs on PV cells (Drake and Milner, 2002) and the critical role these cells play in the generation of ripples (Klausberger et al., 2004). In addition to the strong activation of PV cells, recent observations suggest that ripples also require a rather mild increase in principal cell excitation (Racz et al., 2009; Koniaris et al., 2011; Maier et al., 2011). This may be related to our finding that low concentrations of morphine enhanced ripples. We found that DAMGO suppressed inhibition more readily than it increased excitability. Morphine displayed a much lower potency in reducing inhibition than in enhancing excitability. That is, at relatively low concentrations morphine (but not DAMGO) changed network excitability inducing a shift of the excitation/inhibition balance towards excitation. Although the effect of 5 nM morphine on the evoked responses was not statistically significant, it is plausible that small changes in the excitation/inhibition balance may also occur in the nanomolar range leading to moderate increase of the excitation in the local network and consequently to augmentation of ripples.
The enhancement of clusters by MOR agonists is consistent with their effect on excitation/inhibition balance and agrees with previous observations showing that even small enhancements in GABAA receptor-mediated transmission suppress clusters (Papatheodoropoulos and Koniaris, 2011). The generation of sequences requires activation of NMDA receptors (Papatheodoropoulos, 2010) whose activity can be uncovered by MORs (Swearengen and Chavkin, 1987; McQuiston, 2008). In the present study, DAMGO produced a marked increase in the probability of cluster occurrence at the same concentration (1 μM) that elicited NMDA-dependent secondary spikes. Thus, network disinhibition and NMDA receptor activation resulting from the increased activation of MORs is a condition that favours the generation of clusters.
We found that all three MOR agonists increased the amplitude of sharp waves. In the CA1 area, sharp waves correspond to GABAA receptor-mediated IPSPs (Papatheodoropoulos and Kostopoulos, 2002; Wu et al., 2002; Maier et al., 2003) and their amplitude is correlated with the amplitude of IPSPs on pyramidal neurons (Papatheodoropoulos, 2010). In view of the suppressive effect of MOR agonists on IPSPs (Nicoll et al., 1980; Masukawa and Prince, 1982; Capogna et al., 1993; McQuiston, 2008), these agonists should suppress rather than increase the amplitude of sharp waves in CA1. However, although MOR agonists act mainly on PV cells (Drake and Milner, 2002), the latter are not the only contributors to the generation of SWRs. Bistratified cells are also involved in SWRs (Klausberger et al., 2004). Additionally, SWRs in CA1 are triggered by excitatory input from CA3 (Csicsvari et al., 2000) and sharp waves in CA3 pyramidal cells correspond to excitatory rather than inhibitory synaptic activity (Wu et al., 2002; Colgin et al., 2004; Behrens et al., 2005). Thus, disinhibition of CA3 pyramidal cells by MOR agonists could lead to higher excitation of their targets in CA1 including pyramidal cells (Dingledine, 1981; McQuiston, 2008) and inhibitory bistratified cells (Freund and Buzsaki, 1996). Consequently, the enhancing effect of MOR agonists on the amplitude of sharp waves in CA1 could also be explained by a higher activity of bistratified inhibitory cells in this field, following their higher activation from disinhibited CA3 principal cells.
Recent findings show that MORs readily control the activity of ivy and neurogliaform GABAergic cells in the CA1 area (Krook-Magnuson et al., 2011). However, their contribution to the modulation of SWRs is uncertain because ivy and neurogliaform cells do not change their firing activity during SWRs (Fuentealba et al., 2008a).
Implications for the drug effects on memory
The effects of opioids on learning and memory appear diverse because of the variety of opioid receptors (Pan, 1998; Jamot et al., 2003), the learning task and animal species used (Morris and Johnston, 1995), as well as the interaction of opioids with other neurotransmitter/neuromodulator systems (McGaugh, 2002; Hadjiconstantinou and Neff, 2011). A consistent pattern of effects is disclosed when the time of drug administration is taken into account; morphine impairs memory when present during encoding (Castellano, 1975) but facilitates memory when administered after the training/learning sessions (White et al., 1978; Mondadori and Waser, 1979; Staubli and Huston, 1980). Furthermore, memory is more consistently facilitated by intrahippocampal/intraventricular (Meilandt et al., 2004; Hunsaker et al., 2007) than peripheral drug injections (Izquierdo, 1979; Veselis et al., 1994). The facilitatory effect may represent drug effect on the memory consolidation, a process thought to encompass hippocampo-neocortical information transfer through the activity of SWRs (Chrobak and Buzsaki, 1994; Wierzynski et al., 2009). SWRs might arise from an assembly of hippocampal cells interconnected by synapses that have been strengthened during encoding of new information, and such an assembly may underlie a particular memory trace in the hippocampus (Buzsaki, 1989). The SWR-associated, offline reactivation of this assembly may then contribute to further strengthening of hippocampal and neocortical synapses, assisting memory consolidation (Buzsaki, 1989). Recent evidence showing that synaptic strengthening accompanies the emergence (Behrens et al., 2005) and potentiation (Papatheodoropoulos, 2010) of SWRs supports this hypothesis. Similarly, the observed increase in amplitude of sharp waves induced by MOR agonists may contribute to synaptic strengthening inside a sharp wave-associated cell assembly assisting the establishment/maintenance of memory trace. Hence, the facilitatory effect of activating MORs on hippocampus-dependent memory may involve long-term synaptic modification. Indeed, activation of MORs facilitates the induction of long-term potentiation in hippocampal synapses (Martinez et al., 2011).
The effect of MOR agonists on the sequences of SWRs might have important consequences on memory function as the former appear to reflect specific aspects of information processing by the hippocampal circuit. Recent observations suggest that sequences may represent compressed activity that corresponds to long paths the animal traverses in space, and thus they may be involved in the memorization of prolonged experiences (Davidson et al., 2009).
It is of note that, although moderate to relatively high concentrations (≥100 nM) of all the MOR agonists tested reduced ripples, low concentrations of morphine (5–10 nM) produced a considerable increase in the ripple oscillation supporting the idea that endogenous opioid levels may positively modulate memory. An increase in ripple quantity and power has been found after learning, and has been correlated with increased hippocampus-dependent memory performance (Ramadan et al., 2009). Additionally, reduced performance was observed during pharmacologically induced reduction of the ripple power (Robbe et al., 2006). Interestingly, low concentrations of the MOR agonists did not alter the total number of SWR events. Stability in the quantity of SWRs has been observed in parallel with long-term increase in the amplitude of sharp waves and frequency of clusters, following θ-burst stimulation (Papatheodoropoulos, 2010). Therefore, the enhancing effects of MOR agonists on SWRs are consistent with the behavioural data that show MOR-induced facilitation of memory consolidation, and they suggest that this effect may entail the modulation of opioids on SWRs. Moreover, the fact that MOR-mediated modulation of SWRs apparently involves only subtle changes in the excitation/inhibition balance is consistent with the hypothesized role of SWRs in the processes of transfer and consolidation of discrete memory traces given that these processes have to be selective and accurate employing small neuronal circuits, and thus keeping fidelity (Sutherland and McNaughton, 2000).
In this study, we have determined the roles played by MOR agonists in the hippocampal activity of SWRs. Morphine, DAMGO and fentanyl significantly modulated the activity enhancing the amplitude and the sequencing of sharp waves and dampening the initiation of distinct episodes. Low-level activation of MORs, not affecting network excitability and recurrent inhibition, produced an augmentation of the ripple oscillation. These results show for the first time that MOR agonists at concentrations comparable to endogenous levels of opioids exerted a dynamic regulation on SWRs, presumably by finely tuning the balance between excitation and inhibition. To some extent, such modulation may underlie the actions of MOR agonists on hippocampus-dependent memory.
Acknowledgments
This work was supported by a ‘Karathodori’ grant (No. C176) from the Research Committee of the University of Patras. We would like to thank the reviewers for their constructive suggestions.
Glossary
- ICI
intra-cluster interval
- IEI
inter-event interval; IEpI, inter-episode interval
- MOR
μ-opioid receptor; PPI, paired-pulse inhibition
- PS
population spike; SWR, sharp wave ripple
Conflicts of interest
The authors have no actual or potential conflicts of interest to disclose.
References
- Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th edn. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez P, Squire LR. Memory consolidation and the medial temporal lobe: a simple network model. Proc Natl Acad Sci U S A. 1994;91:7041–7045. doi: 10.1073/pnas.91.15.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azouz R, Jensen MS, Yaari Y. Ionic basis of spike after-depolarization and burst generation in adult rat hippocampal CA1 pyramidal cells. J Physiol. 1996;492(Pt 1):211–223. doi: 10.1113/jphysiol.1996.sp021302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia FP, Benchenane K, Sirota A, Pennartz CM, Wiener SI. The hippocampus: hub of brain network communication for memory. Trends Cogn Sci. 2011;15:310–318. doi: 10.1016/j.tics.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Behrens CJ, van den Boom LP, de Hoz L, Friedman A, Heinemann U. Induction of sharp wave-ripple complexes in vitro and reorganization of hippocampal networks. Nat Neurosci. 2005;8:1560–1567. doi: 10.1038/nn1571. [DOI] [PubMed] [Google Scholar]
- Bernards CM, Shen DD, Sterling ES, Adkins JE, Risler L, Phillips B, et al. Epidural, cerebrospinal fluid, and plasma pharmacokinetics of epidural opioids (part 2): effect of epinephrine. Anesthesiology. 2003;99:466–475. doi: 10.1097/00000542-200308000-00030. [DOI] [PubMed] [Google Scholar]
- Blasco-Ibanez JM, Martinez-Guijarro FJ, Freund TF. Enkephalin-containing interneurons are specialized to innervate other interneurons in the hippocampal CA1 region of the rat and guinea-pig. Eur J Neurosci. 1998;10:1784–1795. doi: 10.1046/j.1460-9568.1998.00190.x. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ. Endogenous opiates and behavior: 2009. Peptides. 2010;31:2325–2359. doi: 10.1016/j.peptides.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Bouw R, Ederoth P, Lundberg J, Ungerstedt U, Nordstrom CH, Hammarlund-Udenaes M. Increased blood-brain barrier permeability of morphine in a patient with severe brain lesions as determined by microdialysis. Acta Anaesthesiol Scand. 2001;45:390–392. doi: 10.1034/j.1399-6576.2001.045003390.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Buzsaki G. High-frequency oscillations in human brain. Hippocampus. 1999;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Hippocampal sharp waves: their origin and significance. Brain Res. 1986;398:242–252. doi: 10.1016/0006-8993(86)91483-6. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Two-stage model of memory trace formation: a role for ‘noisy’ brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- Capogna M, Gahwiler BH, Thompson SM. Mechanism of mu-opioid receptor-mediated presynaptic inhibition in the rat hippocampus in vitro. J Physiol. 1993;470:539–558. doi: 10.1113/jphysiol.1993.sp019874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano C. Effects of morphine and heroin on discrimination learning and consolidation in mice. Psychopharmacologia. 1975;42:235–242. doi: 10.1007/BF00421262. [DOI] [PubMed] [Google Scholar]
- Chaturvedi K, Shahrestanifar M, Howells RD. Mu opioid receptor: role for the amino terminus as a determinant of ligand binding affinity. Brain Res Mol Brain Res. 2000;76:64–72. doi: 10.1016/s0169-328x(99)00332-0. [DOI] [PubMed] [Google Scholar]
- Chavkin C, Shoemaker WJ, McGinty JF, Bayon A, Bloom FE. Characterization of the prodynorphin and proenkephalin neuropeptide systems in rat hippocampus. J Neurosci. 1985;5:808–816. doi: 10.1523/JNEUROSCI.05-03-00808.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsaki G. Selective activation of deep layer (V-VI) retrohippocampal cortical neurons during hippocampal sharp waves in the behaving rat. J Neurosci. 1994;14:6160–6170. doi: 10.1523/JNEUROSCI.14-10-06160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, Kubota D, Jia Y, Rex CS, Lynch G. Long-term potentiation is impaired in rat hippocampal slices that produce spontaneous sharp waves. J Physiol. 2004;558:953–961. doi: 10.1113/jphysiol.2004.068080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks PA, Kottayil SG, Al-Ghananeem AM, Byrn SR, Butterfield DA. Opiate receptor binding properties of morphine-, dihydromorphine-, and codeine 6-O-sulfate ester congeners. Bioorg Med Chem Lett. 2006;16:4291–4295. doi: 10.1016/j.bmcl.2006.05.060. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Mamiya A, Buzsaki G. Ensemble patterns of hippocampal CA3-CA1 neurons during sharp wave-associated population events. Neuron. 2000;28:585–594. doi: 10.1016/s0896-6273(00)00135-5. [DOI] [PubMed] [Google Scholar]
- Dacher M, Nugent FS. Opiates and plasticity. Neuropharmacology. 2011;61:1088–1096. doi: 10.1016/j.neuropharm.2011.01.028. [DOI] [PubMed] [Google Scholar]
- Davidson TJ, Kloosterman F, Wilson MA. Hippocampal replay of extended experience. Neuron. 2009;63:497–507. doi: 10.1016/j.neuron.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R. Possible mechanisms of enkephalin action on hippocampal CA1 pyramidal neurons. J Neurosci. 1981;1:1022–1035. doi: 10.1523/JNEUROSCI.01-09-01022.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CT, Milner TA. Mu opioid receptors are in discrete hippocampal interneuron subpopulations. Hippocampus. 2002;12:119–136. doi: 10.1002/hipo.1107. [DOI] [PubMed] [Google Scholar]
- Drolet G, Dumont EC, Gosselin I, Kinkead R, Laforest S, Trottier JF. Role of endogenous opioid system in the regulation of the stress response. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:729–741. doi: 10.1016/s0278-5846(01)00161-0. [DOI] [PubMed] [Google Scholar]
- Ego-Stengel V, Wilson MA. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus. 2010;20:1–10. doi: 10.1002/hipo.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Ellender TJ, Nissen W, Colgin LL, Mann EO, Paulsen O. Priming of hippocampal population bursts by individual perisomatic-targeting interneurons. J Neurosci. 2010;30:5979–5991. doi: 10.1523/JNEUROSCI.3962-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner HJ, Traub RD, Whittington MA. Anaesthetic/amnesic agents disrupt beta frequency oscillations associated with potentiation of excitatory synaptic potentials in the rat hippocampal slice. Br J Pharmacol. 1999;128:1813–1825. doi: 10.1038/sj.bjp.0702948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Fuentealba P, Begum R, Capogna M, Jinno S, Marton LF, Csicsvari J, et al. Ivy cells: a population of nitric-oxide-producing, slow-spiking GABAergic neurons and their involvement in hippocampal network activity. Neuron. 2008a;57:917–929. doi: 10.1016/j.neuron.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba P, Tomioka R, Dalezios Y, Marton LF, Studer M, Rockland K, et al. Rhythmically active enkephalin-expressing GABAergic cells in the CA1 area of the hippocampus project to the subiculum and preferentially innervate interneurons. J Neurosci. 2008b;28:10017–10022. doi: 10.1523/JNEUROSCI.2052-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall C, Brecha N, Karten HJ, Chang KJ. Localization of enkephalin-like immunoreactivity to identified axonal and neuronal populations of the rat hippocampus. J Comp Neurol. 1981;198:335–350. doi: 10.1002/cne.901980211. [DOI] [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, Buzsaki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12:1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- Gunnar T, Mykkanen S, Ariniemi K, Lillsunde P. Validated semiquantitative/quantitative screening of 51 drugs in whole blood as silylated derivatives by gas chromatography-selected ion monitoring mass spectrometry and gas chromatography electron capture detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;806:205–219. doi: 10.1016/j.jchromb.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Hadjiconstantinou M, Neff NH. Nicotine and endogenous opioids: neurochemical and pharmacological evidence. Neuropharmacology. 2011;60:1209–1220. doi: 10.1016/j.neuropharm.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. What is the function of hippocampal theta rhythm? – Linking behavioral data to phasic properties of field potential and unit recording data. Hippocampus. 2005;15:936–949. doi: 10.1002/hipo.20116. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Mooy GG, Swift JS, Kesner RP. Dissociations of the medial and lateral perforant path projections into dorsal DG, CA3, and CA1 for spatial and nonspatial (visual object) information processing. Behav Neurosci. 2007;121:742–750. doi: 10.1037/0735-7044.121.4.742. [DOI] [PubMed] [Google Scholar]
- Izquierdo I. Effect of naloxone and morphine on various forms of memory in the rat: possible role of endogenous opiate mechanisms in memory consolidation. Psychopharmacology (Berl) 1979;66:199–203. doi: 10.1007/BF00427631. [DOI] [PubMed] [Google Scholar]
- Jamot L, Matthes HW, Simonin F, Kieffer BL, Roder JC. Differential involvement of the mu and kappa opioid receptors in spatial learning. Genes Brain Behav. 2003;2:80–92. doi: 10.1034/j.1601-183x.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Warthen DK. Implications of CA3 NMDA and opiate receptors for spatial pattern completion in rats. Hippocampus. 2010;20:550–557. doi: 10.1002/hipo.20676. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, Baude A, Roberts JD, Magill PJ, Somogyi P. Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nat Neurosci. 2004;7:41–47. doi: 10.1038/nn1159. [DOI] [PubMed] [Google Scholar]
- Klepstad P, Dale O, Kaasa S, Zahlsen K, Aamo T, Fayers P, et al. Influences on serum concentrations of morphine, M6G and M3G during routine clinical drug monitoring: a prospective survey in 300 adult cancer patients. Acta Anaesthesiol Scand. 2003;47:725–731. doi: 10.1034/j.1399-6576.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Koniaris E, Drimala P, Sotiriou E, Papatheodoropoulos C. Different effects of zolpidem and diazepam on hippocampal sharp wave-ripple activity in vitro. Neuroscience. 2011;175:224–234. doi: 10.1016/j.neuroscience.2010.11.027. [DOI] [PubMed] [Google Scholar]
- Krook-Magnuson E, Luu L, Lee SH, Varga C, Soltesz I. Ivy and neurogliaform interneurons are a major target of mu-opioid receptor modulation. J Neurosci. 2011;31:14861–14870. doi: 10.1523/JNEUROSCI.2269-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam MP, Marinelli PW, Bai L, Gianoulakis C. Effects of acute ethanol on opioid peptide release in the central amygdala: an in vivo microdialysis study. Psychopharmacology (Berl) 2008;201:261–271. doi: 10.1007/s00213-008-1267-8. [DOI] [PubMed] [Google Scholar]
- Lupica CR. Delta and mu enkephalins inhibit spontaneous GABA-mediated IPSCs via a cyclic AMP-independent mechanism in the rat hippocampus. J Neurosci. 1995;15:737–749. doi: 10.1523/JNEUROSCI.15-01-00737.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. Memory – a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory consolidation and the amygdala: a systems perspective. Trends Neurosci. 2002;25:456. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuiston AR. Layer selective presynaptic modulation of excitatory inputs to hippocampal cornu Ammon 1 by mu-opioid receptor activation. Neuroscience. 2008;151:209–221. doi: 10.1016/j.neuroscience.2007.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuiston AR. Mu opioid receptor activation normalizes temporo-ammonic pathway driven inhibition in hippocampal CA1. Neuropharmacology. 2011;60:472–479. doi: 10.1016/j.neuropharm.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Enkephalin hyperpolarizes interneurones in the rat hippocampus. J Physiol. 1988;398:123–130. doi: 10.1113/jphysiol.1988.sp017033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier N, Nimmrich V, Draguhn A. Cellular and network mechanisms underlying spontaneous sharp wave-ripple complexes in mouse hippocampal slices. J Physiol. 2003;550:873–887. doi: 10.1113/jphysiol.2003.044602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier N, Tejero-Cantero A, Dorrn AL, Winterer J, Beed PS, Morris G, et al. Coherent phasic excitation during hippocampal ripples. Neuron. 2011;72:137–152. doi: 10.1016/j.neuron.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- Martinez CO, Do VH, Derrick BE. Endogenous opioid peptides contribute to associative LTP in the hippocampal CA3 region. Neurobiol Learn Mem. 2011;96:207–217. doi: 10.1016/j.nlm.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masukawa LM, Prince DA. Enkephalin inhibition of inhibitory input to CA1 and CA3 pyramidal neurons in the hippocampus. Brain Res. 1982;249:271–280. doi: 10.1016/0006-8993(82)90061-0. [DOI] [PubMed] [Google Scholar]
- Meilandt WJ, Barea-Rodriguez E, Harvey SA, Martinez JL., Jr Role of hippocampal CA3 mu-opioid receptors in spatial learning and memory. J Neurosci. 2004;24:2953–2962. doi: 10.1523/JNEUROSCI.5569-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondadori C, Waser PG. Facilitation of memory processing by posttrial morphine: possible involvement of reinforcement mechanisms? Psychopharmacology (Berl) 1979;63:297–300. doi: 10.1007/BF00433566. [DOI] [PubMed] [Google Scholar]
- Morris BJ, Johnston HM. A role for hippocampal opioids in long-term functional plasticity. Trends Neurosci. 1995;18:350–355. doi: 10.1016/0166-2236(95)93927-p. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Alger BE, Jahr CE. Enkephalin blocks inhibitory pathways in the vertebrate CNS. Nature. 1980;287:22–25. doi: 10.1038/287022a0. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon Press; 1978. [Google Scholar]
- O'Neill J, Pleydell-Bouverie B, Dupret D, Csicsvari J. Play it again: reactivation of waking experience and memory. Trends Neurosci. 2010;33:220–229. doi: 10.1016/j.tins.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Pan ZZ. Mu-opposing actions of the kappa-opioid receptor. Trends Pharmacol Sci. 1998;19:94–98. doi: 10.1016/s0165-6147(98)01169-9. [DOI] [PubMed] [Google Scholar]
- Papatheodoropoulos C. Patterned activation of hippocampal network (approximately 10 Hz) during in vitro sharp wave-ripples. Neuroscience. 2010;168:429–442. doi: 10.1016/j.neuroscience.2010.03.058. [DOI] [PubMed] [Google Scholar]
- Papatheodoropoulos C, Koniaris E. α5GABAA receptors regulate hippocampal sharp wave-ripple activity in vitro. Neuropharmacology. 2011;60:662–673. doi: 10.1016/j.neuropharm.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Papatheodoropoulos C, Kostopoulos G. Spontaneous GABA(A)-dependent synchronous periodic activity in adult rat ventral hippocampal slices. Neurosci Lett. 2002;319:17–20. doi: 10.1016/s0304-3940(01)02505-8. [DOI] [PubMed] [Google Scholar]
- Pasternak GW. Insights into mu opioid pharmacology the role of mu opioid receptor subtypes. Life Sci. 2001;68:2213–2219. doi: 10.1016/s0024-3205(01)01008-6. [DOI] [PubMed] [Google Scholar]
- Petrides T, Georgopoulos P, Kostopoulos G, Papatheodoropoulos C. The GABAA receptor-mediated recurrent inhibition in ventral compared with dorsal CA1 hippocampal region is weaker, decays faster and lasts less. Exp Brain Res. 2007;177:370–383. doi: 10.1007/s00221-006-0681-6. [DOI] [PubMed] [Google Scholar]
- Racz A, Ponomarenko AA, Fuchs EC, Monyer H. Augmented hippocampal ripple oscillations in mice with reduced fast excitation onto parvalbumin-positive cells. J Neurosci. 2009;29:2563–2568. doi: 10.1523/JNEUROSCI.5036-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan W, Eschenko O, Sara SJ. Hippocampal sharp wave/ripples during sleep for consolidation of associative memory. PLoS ONE. 2009;4:e6697. doi: 10.1371/journal.pone.0006697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Montgomery SM, Thome A, Rueda-Orozco PE, McNaughton BL, Buzsaki G. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nat Neurosci. 2006;9:1526–1533. doi: 10.1038/nn1801. [DOI] [PubMed] [Google Scholar]
- Schnell SA, Wessendorf MW. Coexpression of the mu-opioid receptor splice variant MOR1C and the vesicular glutamate transporter 2 (VGLUT2) in rat central nervous system. J Comp Neurol. 2008;508:542–564. doi: 10.1002/cne.21712. [DOI] [PubMed] [Google Scholar]
- Singer AC, Frank LM. Rewarded outcomes enhance reactivation of experience in the hippocampus. Neuron. 2009;64:910–921. doi: 10.1016/j.neuron.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Permenter M, Archibeque M, Vogt J, Amaral DG, et al. EEG sharp waves and sparse ensemble unit activity in the macaque hippocampus. J Neurophysiol. 2007;98:898–910. doi: 10.1152/jn.00401.2007. [DOI] [PubMed] [Google Scholar]
- Solassol I, Bressolle F, Caumette L, Garcia F, Poujol S, Culine S, et al. Inter- and intraindividual variabilities in pharmacokinetics of fentanyl after repeated 72-hour transdermal applications in cancer pain patients. Ther Drug Monit. 2005;27:491–498. doi: 10.1097/01.ftd.0000160717.50704.42. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Staubli U, Huston JP. Avoidance learning enhanced by post-trial morphine injection. Behav Neural Biol. 1980;28:487–490. doi: 10.1016/s0163-1047(80)91860-9. [DOI] [PubMed] [Google Scholar]
- Sutherland GR, McNaughton B. Memory trace reactivation in hippocampal and neocortical neuronal ensembles. Curr Opin Neurobiol. 2000;10:180–186. doi: 10.1016/s0959-4388(00)00079-9. [DOI] [PubMed] [Google Scholar]
- Swearengen E, Chavkin C. NMDA receptor antagonist D-APV depresses excitatory activity produced by normorphine in rat hippocampal slices. Neurosci Lett. 1987;78:80–84. doi: 10.1016/0304-3940(87)90565-9. [DOI] [PubMed] [Google Scholar]
- Veselis RA, Reinsel RA, Feshchenko VA, Wronski M, Dnistrian A, Dutchers S, et al. Impaired memory and behavioral performance with fentanyl at low plasma concentrations. Anesth Analg. 1994;79:952–960. doi: 10.1213/00000539-199411000-00023. [DOI] [PubMed] [Google Scholar]
- Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu Rev Biochem. 2004;73:953–990. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- Watson GB, Lanthorn TH. Electrophysiological actions of delta opioids in CA1 of the rat hippocampal slice are mediated by one delta receptor subtype. Brain Res. 1993;601:129–135. doi: 10.1016/0006-8993(93)91703-u. [DOI] [PubMed] [Google Scholar]
- White N, Major R, Siegel J. Effects of morphine on one-trial appetitive learning. Life Sci. 1978;23:1967–1971. doi: 10.1016/0024-3205(78)90564-7. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Faulkner HJ, Jefferys JG, Chettiar K. Morphine disrupts long-range synchrony of gamma oscillations in hippocampal slices. Proc Natl Acad Sci U S A. 1998;95:5807–5811. doi: 10.1073/pnas.95.10.5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzynski CM, Lubenov EV, Gu M, Siapas AG. State-dependent spike-timing relationships between hippocampal and prefrontal circuits during sleep. Neuron. 2009;61:587–596. doi: 10.1016/j.neuron.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Opiate reward: sites and substrates. Neurosci Biobehav Rev. 1989;13:129–133. doi: 10.1016/s0149-7634(89)80021-1. [DOI] [PubMed] [Google Scholar]
- Wu C, Shen H, Luk WP, Zhang L. A fundamental oscillatory state of isolated rodent hippocampus. J Physiol. 2002;540:509–527. doi: 10.1113/jphysiol.2001.013441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sik A, et al. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zastawny RL, George SR, Nguyen T, Cheng R, Tsatsos J, Briones-Urbina R, et al. Cloning, characterization, and distribution of a mu-opioid receptor in rat brain. J Neurochem. 1994;62:2099–2105. doi: 10.1046/j.1471-4159.1994.62062099.x. [DOI] [PubMed] [Google Scholar]
- Zernig G, Issaevitch T, Broadbear JH, Burke TF, Lewis JW, Brine GA, et al. Receptor reserve and affinity of mu opioid agonists in mouse antinociception: correlation with receptor binding. Life Sci. 1995;57:2113–2125. doi: 10.1016/0024-3205(95)02204-v. [DOI] [PubMed] [Google Scholar]
- Zieglgansberger W, French ED, Siggins GR, Bloom FE. Opioid peptides may excite hippocampal pyramidal neurons by inhibiting adjacent inhibitory interneurons. Science. 1979;205:415–417. doi: 10.1126/science.451610. [DOI] [PubMed] [Google Scholar]