Abstract
The endogenous hippocampal opioid systems are implicated in learning associated with drug use. Recently, we showed that ovarian hormones regulate enkephalin levels in the mossy fiber pathway. This pathway overlaps with parvalbumin (PARV)-basket interneurons that contain the enkephalin-activated mu opioid receptors (MORs) and are important for controlling the “temporal timing” of granule cells. Here, we evaluated the influence of ovarian steroid on the trafficking of MORs in PARV interneurons. Two groups of female rats were analyzed: cycling rats in proestrus (relatively high estrogens) or diestrus; and ovariectomized rats euthanized 6, 24 or 72 hr after estradiol benzoate (10μg, s.c.) administration. Dorsal hippocampal sections were dually immunolabeled for MORs and PARV and examined by light and electron microscopy. As in males, in females MOR-immunoreactivity (-ir) was in numerous PARV-labeled perikarya, dendrites and terminals in the dentate hilar region. Variation in ovarian steroid levels altered the subcellular distribution of MORs in PARV-labeled dendrites but not terminals. In normal cycling rats, MOR-gold particles on the plasma membrane of small PARV-labeled dendrites (area < 1μm2) had higher density in proestrus rats than in diestrus rats. Likewise, in ovariectomized rats MORs showed higher density on the plasma membrane of small PARV-labeled dendrites 72 hrs after estradiol exposure. The number of PARV-labeled cells was not affected by estrous cycle phase or estrogen levels. These results demonstrate that estrogen levels positively regulate the availability of MORs on GABAergic interneurons in the dentate gyrus, suggesting cooperative interaction between opioids and estrogens in modulating principal cell excitability.
Keywords: estrogen, estrous cycle, ovariectomy, hippocampus, endogenous opioids
INTRODUCTION
Multiple reports have shown that ovarian steroids can alter hippocampal activity such as increased long-term potentiation (LTP) during periods of high estogen activity (Warren et al., 1995; Kim et al., 2002; Smith and McMahon, 2005), decreased excitatory transmission during periods of high progestins (Smith et al., 2002) and can thus affect associative learning processes (Zurkovsky et al., 2007). Recently, we reported that ovarian steroids regulate the levels of enkephalins and dynorphins in the hippocampal granule cell mossy fiber pathway (Torres-Reveron et al., 2008). Hippocampal output is regulated through a series of principal cell and inhibitory interneuron connections that can be modulated at several points by either endogenous opioids or exogenous opiates. Activation of the endogenous opioid system has been implicated in learning associated with drug use (Nestler et al, 2001) and in addiction to opiates such as morphine and heroin (Gerrits et al., 2003). Since enkephalin opioids preferentially activate mu opioid receptors (MORs) (Janecka et al., 2004), it is important to better understand any possible effects of ovarian steroids on MOR trafficking.
In the dentate gyrus, MORs are found primarily in parvalbumin (PARV)-containing GABAergic basket interneurons (Drake et al., 2002); (Mansour et al., 1988; Drake and Milner, 1999; Drake and Milner, 2006). MOR activation by enkephalin or exogenous agonists produces excitation and facilitates LTP (Bramham, 1992; Morris and Johnston, 1995; Xie and Lewis, 1995; Drake et al., 2007) by inhibiting GABAergic transmission, producing a net facilitation of glutamatergic neurotransmission (Chavkin et al., 1988; Xie et al., 1992; Morris and Johnston, 1995; Bramham and Sarvey, 1996; Drake et al., 2007). A major role of hippocampal GABAergic basket interneurons is to control the “temporal timing” of granule cell output during hippocampal-dependent memory processes (Paulsen and Moser, 1998). Both the somatodendritic and axonal compartments of MOR-containing interneurons are located within a functionally relevant distance of enkephalin-containing terminals (Drake et al., 2002). Consequently, MORs are situated to play a key role in hippocampal plasticity.
Several lines of evidence suggest that ovarian steroids, specifically estrogen, can modulate MORs, but contrasting results have been observed. In hypothalamic neurons, MORs are internalized one day after estradiol administration to ovariectomized (OVXed) rats (Sinchak and Micevych, 2001). In the striatum and hippocampus, administration of estradiol alone or in combination with progesterone to OVXed rats decreases the density of MORs as measured by autoradiography (Slamberova et al., 2003). Estradiol administration to OVXed rats increases MOR binding hippocampal homogenates (Piva et al., 1995) but does not alter MOR mRNA expression in the hippocampus (Quinones-Jenab et al., 1997). The apparent inconsistencies observed with different techniques suggest a complex mechanism, for example that estrogens may alter the availability or trafficking of MORs rather than altering MOR translation.
In the present study quantitative dual-labeling electron microscopic immunocytochemistry was used to determine if ovarian steroids alter the trafficking of MORs within hippocampal PARV interneurons. This study was limited to the dorsal hippocampus, where estrogen-induced morphological changes are consistently reported (Cooke and Woolley, 2005). Some of the data presented here were previously published in abstract form (Torres-Reveron et al., 2007).
EXPERIMENTAL PROCEDURES
Animals and estrous cycle determination
Adult female Sprague Dawley rats (225-250 g; approximately 60 days old) from Charles River Laboratories (Wilmington, MA) were pair-housed with ad libitum access to food and water and with 12:12 light/dark cycles (lights on 0600 - 1800). All procedures were approved by the Weill Cornell Medical College and Rockefeller Institutional Animal Care and Use Committees and were in accordance with the National Institutes of Health guidelines. Rats were allowed to acclimate for one week after which estrous cycle stage was determined using vaginal smear cytology (Turner and Bagnara, 1971). Only rats that showed two consecutive, regular, 4-5 day estrous cycles were included in the study. Animals in proestrus and diestrus 2 stages of the estrous cycle were analyzed. Diestrus 2 rather than metestrus (diestrus 1) was chosen to be certain that the animal was completely out of the estrus phase. For simplicity, we will use the term “diestrus” to refer specifically to diestrus 2 in this report. While vaginal smear cytology was the main method used to determine estrous stages, stages were further verified by measuring uterine weights and levels of estrogen and progestin from blood samples collected from the heart immediately prior to insertion of the needle during the perfusion procedure. Rats destined for ovariectomy were not cycled. Plasma serum levels for progesterone and estradiol were determined by radioimmunoassay using Coat-A-Count kits from Diagnostics Products Corporation (Los Angeles, CA). The rats used in the present study were the same rats used in our previous study, and the hormone assay data and uterine weights have been reported previously (Torres-Reveron et al., 2008).
Ovariectomy and steroid replacement models
Ovariectomy was performed either at Weill Cornell Medical College or Rockefeller University following modified surgical guidelines previously published for ovariectomy surgery (Eddy, 1986). Rats were anesthetized with isoflurane (2-3% in oxygen) and body temperature was monitored and maintained at 37°C using a heating pad during surgical procedures. The lumbar dorsum was shaved on both sides and cleaned with betadine scrub then 70% ethanol in accordance with surgical guidelines. Each ovary as well as associated fat was identified, exposed, severed and removed. The muscle wall was closed with absorbable suture and the skin was closed using stainless steel wound clips.
The ovariectomy model tested the effects of estradiol (E) at different time points (6, 24, and 72 hours). In the 6 hr and 24 hr groups, OVXed rats received a single subcutaneous injection of 10μg/0.2 mL of estradiol benzoate (EB; Sigma, St. Louis, MO) in sesame oil 6 or 24 hrs prior to perfusion. Rats in the 72 hr group received 2 injections of EB 24 hrs apart and then were perfused 2 days after the last injection; this protocol has been previously shown to induce dendritic spine changes in the hippocampal CA1 region (Woolley, 1998). Rats in the control or oil (O) groups received an injection of sesame oil 24 hrs before perfusion. All injections started 2 weeks after ovariectomy surgery.
Antisera
A rabbit polyclonal against MOR antibody was purchased from Neuromics (Minneapolis, MN). This antibody recognizes residues 384-398 from the carboxy terminus or MOR1 and does not recognize the splice variant MOR-1A-E or the cloned delta opioid receptor (Arvidsson et al., 1995; Abbadie et al., 2000). Specificity of the antibody has been demonstrated previously by western blotting, adsorption and omission controls and under the same labeling conditions used in the present study (Arvidsson et al., 1995; Drake and Milner, 1999). A mouse monoclonal antibody against PARV was purchased from Sigma (St. Louis, MO). This antibody has been previously characterized by radioimmunoassay, immunoblots and the ability to recognize PARV in brain tissue (Celio et al., 1988).
Section preparation
Rats were deeply anesthetized with pentobarbital (150 mg/kg) on the morning of proestrus or diestrus or following EB administration and their brains fixed by aortic arch perfusion with 3.75% acrolein and 2% paraformaldehyde in 0.1M phosphate buffer (pH 7.6) (Milner et al., 2001). The brains were removed from the skull and cut into 5 mm coronal blocks using a brain mold (Activational Systems, Inc.), and postfixed for 30 minutes in 2% paraformaldehyde in 0.1M phosphate buffer. The brains were sectioned on a Leica Vibratome (40 μm thick) and stored in cryoprotectant (30% sucrose and 30% ethylene glycol in 0.1M phosphate buffer) until immunocytochemical processing. Prior to immunocytochemistry, coronal sections of all treatment groups were rinsed in PB, coded with hole-punches and pooled into single crucibles. Sections then were treated with 1% sodium borohydride in PB for 30 minutes to neutralize free aldehydes. Tissue sections were rinsed in PB followed by Tris-buffered saline (TS; pH 7.6) and incubated in 0.5% bovine serum albumin (BSA) in TS for 30 min.
Light microscopic immunocytochemistry
To examine changes in numbers of PARV neurons between proestrus and diestrus rats or between OVXed and OVXed- estradiol administered rats, sections from each experimental group were marked and pooled into single containers. Sections then were processed for PARV immunocytochemistry using the avidin-biotin complex (ABC)- peroxidase technique (Hsu et al. 1981). For this, sections were incubated in PARV antibody 2 days at 4°C and then processed through 1) a 1:400 dilution of biotinylated horse anti-mouse immunoglobulin (IgG) (Vector Labs, Burlingame, CA), 30 min; 2) ABC (at twice the recommended dilution; Vector), 30 min and 3) 3,3'-diaminobenzidine (DAB; Sigma, St. Louis, MO) and H2O2 in TS for 6 minutes. All incubations were separated by washes in TS. Sections were mounted on gelatin-coated slides, dehydrated, and coverslipped from xylene with DPX mounting media (Aldrich). A person unaware of the experimental condition counted the number of PARV-labeled neurons in the hilus. Using the granule cell layer as a border, all PV cells were counted in the dorsal hilus (between AP -3.6 - -4.0 from bregma) (Swanson, 1992) from 1 section per rat. Differences between groups were analyzed using a Student's t-test.
For light microscopic immunofluorescent localization of MOR and PARV, single sections were incubated in MOR antibody (1:1000 dilution) for 48 hrs at 4 °C and then the PARV antibody (1:1500 dilution) was added to the diluent for an additional 24 hrs. Sections then were rinsed in TS and incubated sequentially for 1 hour each in Alexa Fluor 488 goat anti-rabbit IgG (1:400; Invitrogen-Molecular Probes, Carlsbad, CA) and Cy5 goat anti-mouse IgG (1:400; Invitrogen-Molecular Probes). Sections were mounted on gelatin-coated slides, air-dried and coverslipped with slowFade Gold antifade reagent (Invitrogen-Molecular Probes). Immunofluorescence images were acquired sequentially using a confocal laser-scanning microscope (Leica, Nussloch, Germany). Z-stack analysis was used to verify if neurons were dually labeled for MOR and PARV. Alexa Fluor 488 (MOR) was pseudocolored green while Cy5 (PARV) was pseudocolored blue.
Electron microscopic immunocytochemistry
For electron microscopic localization of MOR and PARV, single sections were labeled for PARV using immunoperoxidase and MOR using immunogold methods described previously (Towart et al., 2002). All sections to be compared in a given experiment (e.g., proestrus and diestrus) were processed together in the same containers to eliminate variables that could affect between-groups comparisons. Sections were incubated first in MOR antibody (diluted 1:1000) for 48 hrs at 4 °C. Following this, PARV antibody (final dilution 1:3000) was added and the incubation continued for an additional 72 hrs. PARV was visualized using the ABC method (Hsu et al., 1981) described above. To visualize MOR, sections next were processed with the silver-enhanced immunogold technique (Chan et al., 1990). For this, sections were rinsed in TS and incubated in a 1:50 dilution of goat anti-rabbit IgG conjugated to 1-nm gold particles (Electron Microscopy Sciences, EMS, Washington, PA) in 0.001% gelatin and 0.08% BSA in PBS overnight at 4°C. Sections were rinsed in PBS, postfixed in 1.25% glutaraldehyde in PBS for 10 min, rinsed again in PBS followed by 1.2% sodium citrate buffer, pH 7.4. The conjugated gold particles were enhanced by incubation in silver solution (IntenSE; Amersham) for 5-7 min. Sections were fixed 1 hr in 2% osmium tetroxide, dehydrated in ascending concentrations of ethanols and propylene oxide, and embedded in EMBed 812 (EMS) between two sheets of Aclar plastic (Honeywell, Pottsville, PA). Ultrathin sections (70-72 nm thick) through the dorsal dentate gyrus (AP -3.6 to -4.0 from Bregma; Swanson, 1992) were cut on a Leica UCT ultratome. Sections were counterstained with Reynold's lead citrate and uranyl acetate and examined with a FEI Tecnai Biotwin transmission electron microscope equipped with an Advanced Microscopy Techniques digital camera (software version 3.2; Danvers, MA).
Electron microscopic data analysis
For the normal cycling animals, tissue from 5 rats in proestrus and 5 rats in diestrus was analyzed. For the timed estrogen-replaced OVXed rats, tissue from 3 animals at each timepoint was analyzed. One or two sections of the DG per animal was thin sectioned and examined under the electron microscope. Immunoperoxidase labeling for PARV was distinguished as an electron-dense reaction product precipitate. Silver-intensified immunogold (SIG) labeling for MOR appeared as intense black electron-dense particles located within cytoplasmic compartments or plasma membrane. To avoid false negative labeling of smaller profiles, profiles were considered as dual-labeled if they contained electron-dense reaction product and at least one gold particle. To circumvent problems due to differences in antibody penetration, images were taken from the tissue-plastic interface (i.e., the surface of the tissue). As in the light microscopic study, the granule cell layer was used to define the border of the hilus. All profiles containing both PARV and MOR immunoreactivities were photographed and classified as dendrites or terminals based on the descriptions from Peters et al. (1991). On average, 76 profiles were collected per block. The area perimeter and Feret's diameter of the profiles was collected using Image J software (NIH).
MOR-SIG particle localization was recorded as either cytoplasmic, plasmalemmal or “near plasma membrane” (i.e., particles within 30 nM, but not touching, the plasma membrane). Profiles were divided into different size categories based on their area or perimeter, to analyze the density in cytoplasm or along the plasma membrane, respectively. Profiles with a perimeter smaller than 5 μm were categorized as “small”, and all other profiles as “large”. Similarly, profiles with an area ≤ 1 μm2 were categorized as “small” and all other profiles as “large”. The density of MOR SIG for each animal was calculated as follows: the total number of MOR-SIG particles for all the profiles sampled within a given size category was divided by the total sum of area or perimeter of the profiles. Several ratios were also calculated: 1) cytoplasmic SIG particles to total number of SIG particles in the profile (CY:total); 2) plasma membrane SIG particles to total number of gold particles (PL:total); and 3) near plasma membrane SIG particles per total number of SIG particles (CY:near). All values were calculated per animal. Thus, for statistical analyses N equals number of animals within a group, not numbers of profiles analyzed.
Data was analyzed by Student's t-test for the cycling groups or by ANOVA for the OVXed groups using SPSS for Windows V. 11.0 followed by protected Fisher Least Significant Difference (LSD) post hoc test. Pictures were corrected for brightness and contrast using Adobe Photoshop 7.01 (Adobe Systems, Inc., San Jose CA). Graphs were prepared with Graph Pad Prism 4.01 (Graph Pad Software, Inc., San Diego CA).
RESULTS
MORs are frequently colocalized with PARV in the female dentate gyrus
In agreement with previous studies in male rats (Drake and Milner, 1999; Drake and Milner, 2006), MOR-ir was present in numerous PARV-labeled interneurons in the female dorsal dentate gyrus (Fig. 1). The majority of dual-labeled neurons were in the subgranular zone of the hilus. MOR-labeled perikarya were small (8-15 μm in minimum diameter) and were usually fusiform or bipolar. PARV-labeled dendritic processes often extended into the molecular layer perpendicular to the laminae, and also extended into the hilus.
Figure 1.
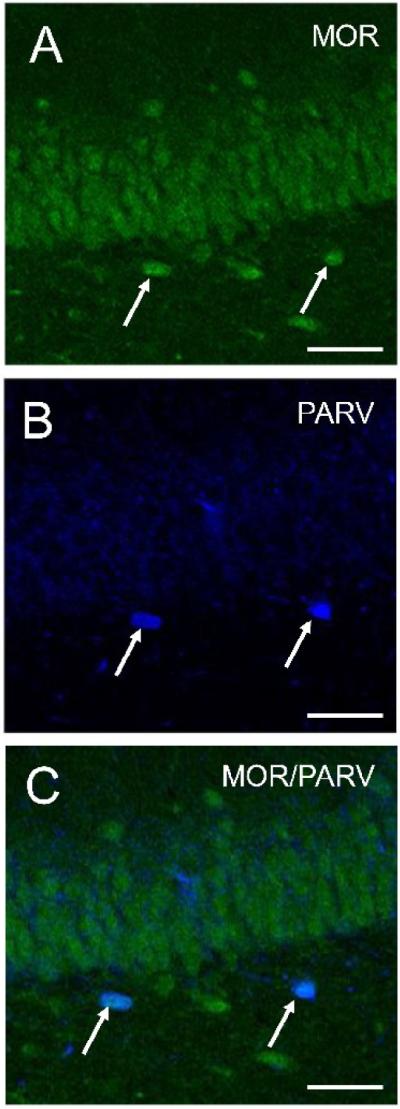
By light microscopy, MOR-ir is contained in numerous PARV-labeled neurons in the hilus of the dentate gyrus. Confocal micrographs show MOR alone (A), PARV alone (B) and a merged Z-stack reconstruction image (C). Arrows indicate dual labeled cells. Bars, 50 μm.
At the electron microscopic level, MOR-ir was prominent in perikarya, dendrites and terminals. Most, but not all, MOR labeled profiles contained PARV immunoreactivity (Fig. 2). Perikarya containing MOR and PARV immunoreactivities exhibited infolded nuclei and abundant organelles. Consistent with previous studies in males (Drake et al., 1999), perikarya contained few MOR SIG particles. These particles were usually affiliated with the endoplasmic reticulum (Fig. 3). Few MOR SIG particles were found on the plasma membrane of PARV-labeled perikarya, regardless of estrogen state. Dual labeled perikarya received few synaptic contacts and were surrounded by glial processes.
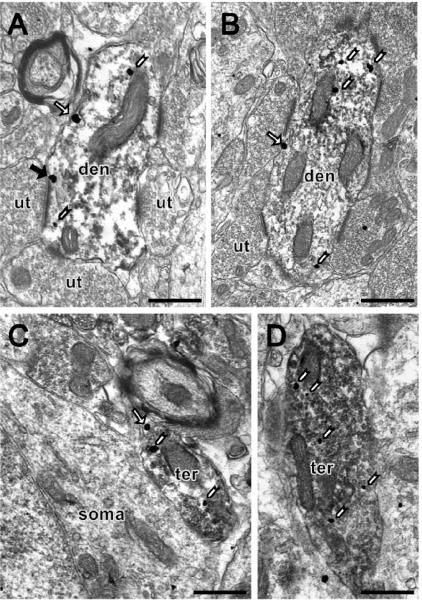
Figure 2.
By electron microscopy, MOR SIG particles are found in PARV-labeled dendrites and terminals in the female rat dentate gyrus. (A, B) PARV peroxidase labeled dendrites (den) contain MOR SIG particles in the cytoplasm (white arrowheads), and at the plasma membrane (regular arrow) from a proestrus rat (A) and a diestrus rat (B). In A, the PARV-labeled dendrite contains two MOR SIG particles at the plasma membrane and one of the particles is in close apposition to an asymmetric synapse (solid black arrow) with an unlabeled terminal (ut). (C, D) PARV peroxidase labeled small terminals (ter) containing MOR-SIG particles in the cytoplasm or in the plasma membrane from a proestrus rat (C) and a diestrus rat (D). In C, the small terminal has one SIG particle in the plasma membrane (regular arrowhead); the terminal is next to a MOR SIG labeled soma. Both terminals contain numerous small synaptic vesicles. Bars, 500 nm.
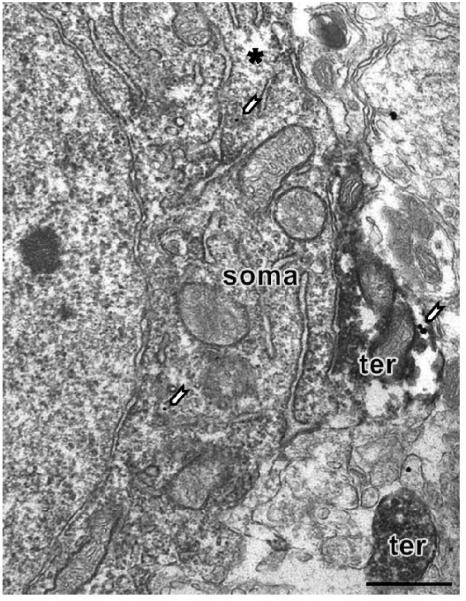
Figure 3.
By electron microscopy, few MOR SIG particles are found in PARV-labeled perikarya in the female rat dentate gyrus. Only two SIG particles (white arrows) are observed. Note that the lack of MOR-SIG labeling is not due to antibody penetration since this picture was taken from a superficial sample as noted by surface-tissue interface (asterisk). This dual labeled soma is in close apposition to a PARV-SIG MOR dual labeled terminal (ter). Another single labeled PARV terminal is observed on the field.
MOR SIG particles were more prominent in PARV-labeled dendrites and terminals. Dual-labeled dendrites were large (average Feret's diameter= 3.07 ± 0.19 μm) or small (average Feret's diameter= 1.25 ± 0.05 μm) and often contained mitochondria and smooth endoplasmic reticula. Dual-labeled dendrites frequently formed synapses, primarily of the asymmetric or excitatory type, with numerous unlabeled terminals (Fig. 2A and B). Within dendrites, MORSIG particles were localized on or near the plasma membrane and in the cytoplasm. Dual labeled terminals ranged in size from 0.26 to 5.84 μm in Feret's diameter and contained numerous small synaptic vesicles and few (1-3) large dense-core vesicles (Fig. 2C and D). Like dendrites, MOR SIG particles were on or near the plasma membrane and in the cytoplasm of PARV-labeled terminals. Since the majority of MOR SIG particles were in dendrites and terminals, these profiles were selected for the quantitative analysis.
Plasma membrane MORs in small PARV dendrites are more abundant during proestrus
The greatest changes in the subcellular distribution of MOR-SIG particles over the estrous cycle or following estradiol administration were detected in small PARV-labeled dendrites. Specifically, proestrus rats had a greater number of MOR-SIGs on the plasma membrane per μm perimeter of small PARV-labeled dendrites compared to diestrus rats (t= 2.45, d.f.= 8, p< 0.05; Fig. 4A). This increase in plasma membrane MOR SIG particles was accompanied by a decrease in cytoplasmic MOR-SIG particles per area unit of PARV-labeled dendrites in proestrus rats (t= 3.03, d.f.= 8, p< 0.05). Calculations of the ratio of plasmalemmal MOR-SIG particles to total MOR-SIG particles within PARV dendritic profiles showed a similar pattern. Proestrus rats had a larger ratio of MOR-SIG particles on the plasma membrane of small PARV-labeled dendrites compared to rats in diestrus (t= 4.25, d.f.= 8, p< 0.01), and a decreased ratio of MOR-SIG particles in the cytoplasm of small PARV-labeled dendrites (t= 2.43, d.f.= 8, p< 0.05; Fig. 4B). The total number of MOR-SIG particles within PARV dendritic profiles was not significantly different between the two groups (p > 0.05). No significant cycle-linked changes were observed in the number of MOR-SIG particles near the plasma membrane, or in the number or distribution of MOR SIG particles in large PARV-labeled dendrites.
Figure 4.
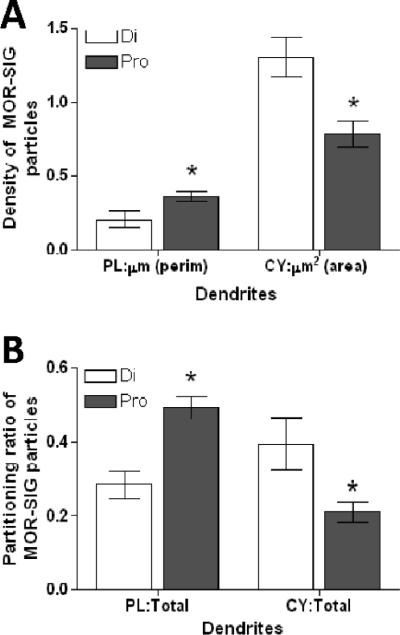
The distribution of MOR-SIG particles in small PARV-labeled dendrites changes in normal cycling female rats. (A) The number of MOR-SIG particles in small PARV dendrites (i.e., area < 1 μm2 and perimeter < 5 μm) is increased on the perimeter and decreased in the cytoplasm of proestrus compared to diestrus rats. (B) In proestrus compared to diestrus rats, small PARV dendrites show a higher ratio of plasma membrane:total MOR-SIG particles and lower ratio of cytoplasmic:total MOR-SIG particles. * represents significantly different from diestrus group (p< 0.05) on a t-test.
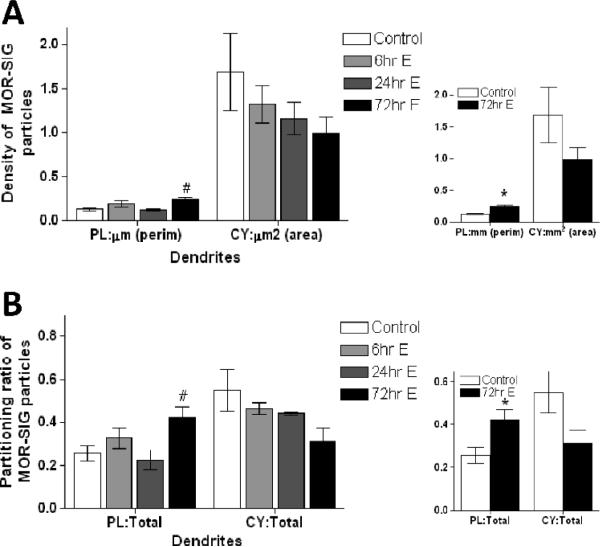
MORs increase on the plasma membrane of small PARV dendrites in OVXed rats 72 hrs after pulsatile estradiol
To confirm the role of estradiol in the changes seen on proestrus rats, OVXed rats were administered two pulsatile doses of EB and analyzed 48 hrs after the last injection (i.e., 72 hr EB group). The 72 hr EB treated group displayed an increase in plasma membrane MOR-SIG particles in small PARV-labeled dendrites. ANOVA revealed a main effect of group treatment (F(3,11)= 4.77, p<0.05). Post-hoc analysis showed that the 72 hr EB group had a significant increase in plasma membrane MOR-SIG particles per μm perimeter compared to control and 24 hrs EB groups (p< 0.05 both comparisons; Fig. 5A). While there was a strong tendency for decreased cytoplasmic MOR-SIG particles per unit area of PARV-labeled dendrites in the 72 hr group as compared to controls, this decrease was not significant (p = 0.1; Fig. 5A, right inset). The increase in the number of plasma membrane MOR-SIG particles was mirrored by an increase in the ratio of plasma membrane MOR-SIG to total MOR-SIG particles within PARV-labeled dendrites (F(3,11)= 3.78, p=0.05). Post hoc analyses revealed a significant increase in plasma membrane MOR-SIG particles to total MOR-SIG particles in PARV-labeled dendrites in the 72 hr EB group compared to control and 24 hr EB groups (p< 0.05 both comparisons; Fig. 5B). While the ratio of cytoplasmic to total MOR-SIG particles in PARV-labeled dendrites tended to decrease in the 72 hr EB group compared to the control group, it did not reach statistical significance (p = 0.10; Fig. 5B, right inset). The total number of MOR-SIG particles within small dendritic profiles was not significantly different between groups. The number of MOR-SIG particles near the plasma membrane of small PARV-labeled dendrites was not significantly different between groups (p > 0.05).
Figure 5.
The distribution of MOR-SIG particles in small PARV dendrites is altered in OVXed rats administered pulsatile EB over a 72 hr period (72 hr EB group). (A) The number of MOR-SIG particles is increased on the perimeter and tends to decrease in the cytoplasm of small PARV dendrites from the 72 hr EB group. (B) In the 72 hr EB group, the ratio of plasma membrane:total MOR-SIG particles is increased in small PARV dendrites. # represents significantly different from control and 24 hrs group. Smaller inserts on the right show a comparison of control and 72 hrs EB group only compared using Student's t-test. * represents significantly different from control group (p< 0.05).
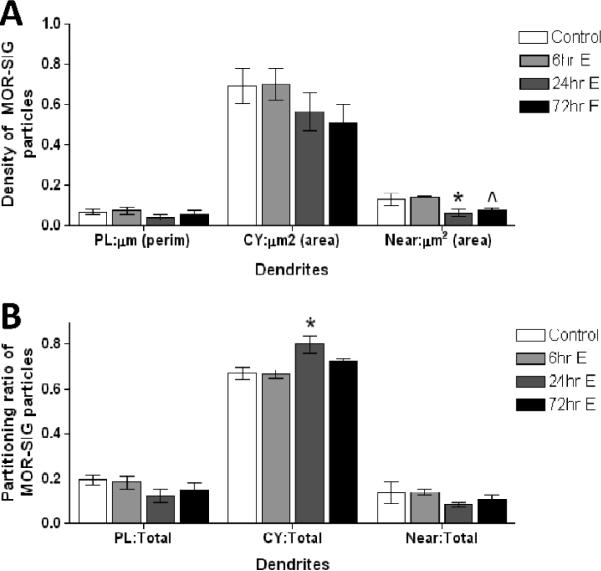
MOR distribution is altered in large PARV dendrites of OVXed rats administered EB
Large dual-labeled dendrites, unlike smaller dendrites, did not show group-linked differences in the density of MOR-SIG on the plasma membrane or in the cytoplasm. However, PARV-labeled large dendrites did show a significant main effect of group for the MOR-SIG particles near the plasma membrane (F(3,11)= 4.75, p=0.05) (Fig. 6). Post hoc analysis revealed that the 24 hr EB group was significantly lower than the control and 6 hr EB group (p< 0.05 both comparisons), and that the 72 hr EB group was different from the 6 hr EB group (p< 0.05). The partitioning ratio of total MOR-SIG particles within large PARV-containing dendritic profiles also was significantly different between EB administered groups. Specifically, there was a significant main effect of group in the ratio of cytoplasmic:total MOR-SIG particles (F(3,11)= 5.68, p=0.05). There were no significant differences detected for the plasma membrane:total and the near-plasma membrane: total MOR-SIG particles in large PARV-labeled dendrites. Subsequent post-hoc analysis revealed that a significantly higher ratio of MOR-SIG particles in the cytoplasm of large PARV-labeled dendrites from the 24 hr EB group compared to the control and 6 hr EB groups (p< 0.01, both comparisons).
Figure 6.
The distribution of MOR-SIG particles near the plasma membrane of large PARV-labeled dendrites is decreased in OVXed rats in the 24 and 72 hr EB groups. (A) The number of MOR-SIG particles that lie near (i.e., within 0.1 μm but not touching) the plasma membrane of large (area bigger than 1 μm2 and a perimeter bigger than 5 μm) PARV-labeled dendrites are decreased in the 24 and 72 hr EB groups. (B) The ratio of cytoplasmic:total MOR-SIG particles is increased in large PARV-labeled dendrites in the 24 hr EB group. * represents significantly different from control and 6 hr EB group and ^ represents significantly different from 6 hr EB group only.
MOR distribution was not changed in PARV immunoreactive terminals
PARV immunoreactive terminals were classified and analyzed in the same manner as dendritic profiles. However, there were no between group differences in the cytoplasmic or plasmalemmal distribution of MOR SIG particles for either the normal cycling animals or the ovariectomized animals (not shown). Thus the distribution of MOR in terminals from PARV immunoreactive neurons is not affected by changes in ovarian steroids.
The number of PARV-immunoreactive cells is not altered by estrous cycle or by estradiol administration
One possible basis for the hormone- or cycle-linked changes observed in dual labeled profiles is a change in the detectable number of profiles containing PARV. To address this possibility, in a separate set of experiments we quantified the number of PARV positive neurons in cycling animals and OVXed animals from the 72 hr EB group. No significant differences in the average number of PARV-immunoreactive neurons were found within the hilus of proestrus or diestrus rats (Proestrus: 8.4 ± 1.02; Diestrus: 6.8 ± 1.15; p> 0.05 on a Student's t-test; N = 4 each condition). Similarly, the number of PARV-labeled neurons in 72 hr EB OVXed rats was not significantly different from OVXed control rats (OVX-EB: 5.4 ± 1.5; OVX-Control: 7.4 ± 1.98; p> 0.05 on a t-test; N=5 each condition). This finding supports the idea that number of PARV-labeled profiles is not affected by cyclic changes in ovarian hormones, ovariectomy or estradiol administration after ovariectomy.
DISCUSSION
This study is the first to demonstrate that ovarian steroids alter the trafficking of MORs within subpopulations of hippocampal GABAergic interneurons. In particular, this study shows that elevated levels of estrogens, either at proestrus or after 72 hrs of pulsatile EB in OVXed rats, can increase the availability of MORs on PARV-containing GABAergic basket cells, potentially altering the disinhibitory effects of endogenous or exogenous opiates (Fig. 7).
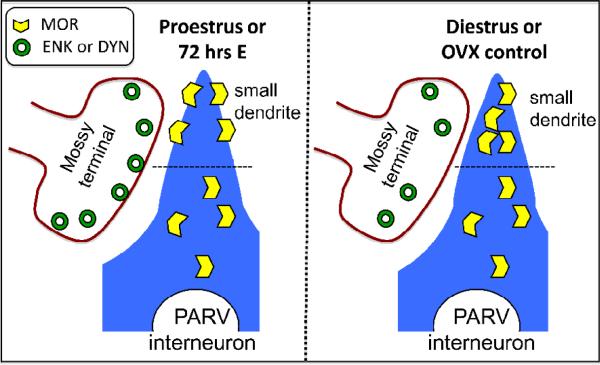
Figure 7.
Schematic diagram summarizing the observed effect on the redistribution of MORs in PARV interneurons in the female rat dentate gyrus. When estrogen levels are high (either at proestrus or 72 hr after EB administration) enkephalin/dynorphin levels are elevated in the mossy fibers and more MORs are on the small dendrites plasma membrane of PARV GABAergic interneurons. However, when estrogen levels are low (either at diestrus or in OVX control rats), less MORs are on the small dendrites plasma membrane and more on the cytoplasm. Together, these results suggest that during periods of high estrogen levels, more MORs are available to be activated and this can lead to greater disinhibition (e.g., more excitation) of pyramidal cells.
Methodological Considerations
The pre-embedding immunogold method was chosen to localize MOR immunoreactivity because it maintains morphological preservation while providing discrete subcellular localization of the antigen of interest (Leranth and Pickel, 1989). This method is more appropriate than post-embedding methods for localization of immunoreactivity at extrasynaptic sites, and thus is suitable for quantifying the regional distribution of MORs (Lujan et al., 1996; Drake et al., 2005). Although pre-embedding immunogold labeling can produce lower estimates of receptor number than immunoperoxidase labeling, due to reduced reagent penetration (Leranth and Pickel, 1989), this limitation was not likely to affect comparisons of MOR density between groups since (a) sections were pooled and processed together to facilitate relative comparisons (Pierce et al., 1999), and (b) ultrathin sections were collected from the plastic-tissue interface where immunoreagent access is maximal. For each animal, we analyzed a similar number of dual labeled processes from the hilus to insure that between-group comparisons were not affected by pre-embedding immunogold limitations.
Our experiments were conducted both in normal cycling animals and in ovariectomized animals. To provide the best correlation of hormonal levels between the two sets of experiments the cycling animals were studied at proestrus and diestrus; in proestrus circulating estrogen levels are highest and in diestrus circulating ovarian hormone levels are lowest (Belanger et al., 1981). The roles of ovarian hormones have been studied using many different OVXed models (for example: (McEwen, 2001; Cyr et al., 2001). Each model has different strengths and weaknesses since estradiol effects depend strongly on hormone dose, time elapsed after steroid administration, and interval following ovariectomy (Tanapat et al., 2005). The OVXed model used here was used previously in our laboratory to study the effects of estrogen levels on enkephalin and dynorphin (Torres-Reveron et al., 2008; Torres-Reveron et al., 2009). The OVXed model with 72 hr estrogen-replacement regimen used in this study has been shown to produce increases in dendritic spines in stratum radiatum of CA1 (Woolley and McEwen, 1994), as is seen at proestrus in cycling animals (Woolley et al., 1990).
Ovarian steroids alter trafficking of MORs
The number of MORs on the plasma membrane of small PARV labeled dendrites was higher in proestrus rats and OVXed rats 72 hrs after the onset of EB administration. These results indicate that increases in estrogen levels result in the mobilization of MORs from cytoplasm to plasma membrane. A previous study has shown that neurotensin receptors identified by SIG particles bound to plasma membrane show a similar distribution to radioactive-ligand binding sites, indicating that plasmalemmal associated SIG particles reflects functional receptors (Boudin et al., 1998). Interestingly we observed movement of MOR towards the plasma membrane only in smaller dendrites and not in larger dendrites. Becker and Hu (2008) suggested that the movement of NMDA-NR1 receptors in nucleus accumbens dopaminergic neurons from larger to smaller dendrites reflects the trafficking of receptors to an area where the likelihood of functional synaptic sites is higher. Therefore, a similar phenomenon might be occurring in PARV interneurons where MOR receptors are relocalizing from larger dendrites to smaller ones in response to increased levels of estradiol. Functionally, a larger number of MORs at the plasma membrane will results in more receptors available for activation by endogenous or exogenous ligands. More MORs activated can lead to augmented excitability at the mossy fiber-CA3 synapse (Derrick et al., 1992; Akaishi et al., 2000).
Consistent with an increased presence of MORs on the plasma membrane of PARV interneurons, we recently reported that leu-enkephalin levels are elevated in specific subregions of the mossy fiber pathway during proestrus and estrus (one day after the peak in ovarian steroids) or 24 hrs after estradiol administration to OVXed rats (Torres-Reveron et al., 2008). Our previous studies of the male dentate gyrus also localized enkephalin immunoreactivity to large dense-core vesicles in terminals that are situated within a functionally relevant distance of MOR-labeled dendrites (Commons and Milner, 1996; Drake et al., 2002). Together, these findings suggest that during the high frequency hippocampal activity that stimulates release of enkephalins (e.g., stress: (McLaughlin et al., 2003), activation of MORs on PARV-containing dendrites would be greater in proestrus rats than in diestrus rats. Additionally, they suggest that exposure to exogenous opioids (e.g., morphine) during proestrus would activate more MORs on hippocampal interneurons.
As with leu-enkephalin, we have observed that ovarian steroids increase dynorphin immunoreactivity in the mossy fiber pathway, with higher dynorphin observed at estrus, following 24 hrs of estradiol administration or following chronic medroxyprogesterone (Torres-Reveron et al., 2009). While dynorphin preferentially binds to kappa opioid receptors (KORs), it has only a modest selectivity for KORs over MORs (Hruby and Agnes, 1999). Moreover, relatively few KORs are detected in the rat dentate gyrus (Drake et al., 2007). Therefore, in addition to leu-enkephalin, released dynorphin could potentially activate MORs on the plasma membrane and work synergistically with leu-enkephalin to increase the disinhibition in principal cells (Plager and Vogt, 1988). However, whether MORs are internalized after activation by either dynorphin or enkephalin remains to be determined since MORs show ligand-dependent trafficking (Arttamangkul et al., 2008).
Possible mechanisms of steroid effects on MORs in the dentate gyrus
The estrogen receptor (ER) subtype ERα has been localized to both nuclear and extranuclear sites of GABAergic interneurons (Milner et al., 2001). Direct genomic effects of estradiol on MOR expression are unlikely since few PARV-containing interneurons in the hippocampus express nuclear ERα (Nakamura and McEwen, 2005). However, non-genomic effects of estradiol are supported by our previous studies demonstrating that some interneuron dendrites express non-nuclear ERα (Milner et al., 2001). Moreover, in the hypothalamus MOR internalization is through an ERα dependent mechanism (Micevych et al., 2003), suggesting that non-nuclear ERs might be affecting the trafficking of MORs in hippocampal dendrites. In addition, extranuclear ERβ-ir is prominent on interneuron perikarya and dendrites in the hilus (Milner et al., 2005) many of which colocalize PARV (Blurton-Jones and Tuszynski, 2002); Milner et al., 2005). Thus by activating non-nuclear ERs, increased estrogen levels may influence the mobilization of MORs from cytoplasmic to plasma membrane locations. The possibility of specific interactions between non- nuclear ERs and MORs merits further study.
Clinical considerations
The influence of estradiol on the trafficking of MORs in hippocampal interneurons has several important functional implications. Clinical reports have shown that seizure frequency changes during the menstrual cycle, a phenomenon known as catamenial epilepsy (Backstrom, 1976; Herzog et al., 2004). Seizure exacerbation appears to be related to estrogen surge during pre-ovulatory days which produces an increased ratio of serum estrogen over progesterone (Penovich and Helmers, 2008). The increase in estrogen favors hippocampal gutamatergic activity and reduces progesterone inhibitory effects mediated by GABAergic stimulation (Majewska et al., 1986; Wong and Moss, 1992). MOR activation on PARV- GABAergic interneurons results in more disinhibition and increased excitation susceptibility. We have shown that in rats, both leu-enkephalin and dynorphin are elevated during proestrus compared to diestrus (Torres-Reveron et al., 2008; Torres-Reveron et al., 2009). The present demonstration of an increased density of plasma membrane MORs in dendrites suggests that estradiol may increase both, endogenous ligand levels (leu-enkephalin and dynorphin) and mobilization of MORs to plasma membrane, contributing to increased seizure susceptibility during periods when the ratio of estrogen over progesterone is augmented.
Our results show evidence for the interaction of estrogen and opioid systems, which can provide a cellular substrate for functional differences in hippocampal activity related to drug-associated learning. Release of endogenous opioids and the induction of mossy fiber LTP both require high frequency stimulation (Derrick et al., 1992). Mossy fiber LTP is critical for hippocampal dependent contextual learning (Ishihara et al., 1997; Dumas et al., 2004). Contextual associative learning with a particular drug-abuse experience is thought to contribute to the maintenance of addictive processes (Berke and Hyman, 2000). In addition, endogenous peptides such as dynorphin or dynorphin-like compounds capable of reaching the mesolimbic system have been proposed to be potentially effective in managing drug addictions with a possible higher effect in female subjects (Kreek et al., 1999). Thus, our results demonstrate one additional interaction between estrogen and mu opioid systems that shed light on the mechanisms involving sex-linked differences in hippocampal activity related to drug-associated learning.
In conclusion, this study provides ultrastuctural evidence for increased MOR availability in response to cyclic ovarian hormones and estradiol administration. These findings, and our previous reports that endogenous opioid peptides increase in the mossy fiber pathway in response to ovarian steroids, contribute to a developing picture of interactions between estradiol and the hippocampal opioid system that potentially have powerful effects on excitability, plasticity and hippocampal-dependent learning processes.
ACKNOWLEDGEMENTS
This work was supported by the following grants: DA08259 (TAM; CTD); Minority supplement to DA08259 (ATR); HL18974 (TAM); NS07080 (BSM). We thank Ms. Priya Prasad for her assistance in data collection, Ms. Louisa Thompson for technical assistance and Dr. Joseph P. Pierce for his helpful advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Abbadie C, Pan YX, Drake CT, Pasternak GW. Comparative immunohistochemical distributions of carboxy terminus epitopes from the mu-opioid receptor splice variants MOR-1D, MOR-1 and MOR-1C in the mouse and rat CNS. Neuroscience. 2000;100(1):141–153. doi: 10.1016/s0306-4522(00)00248-7. [DOI] [PubMed] [Google Scholar]
- Akaishi T, Saito H, Ito Y, Ishige K, Ikegaya Y. Morphine augments excitatory synaptic transmission in the dentate gyrus through GABAergic disinhibition. Neuroscience Research. 2000;38(4):357–363. doi: 10.1016/s0168-0102(00)00177-2. [DOI] [PubMed] [Google Scholar]
- Arttamangkul S, Quillinan N, Low MJ, von ZM, Pintar J, Williams JT. Differential activation and trafficking of micro-opioid receptors in brain slices. Molecular Pharmacology. 2008;74(4):972–979. doi: 10.1124/mol.108.048512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law PY, Wessendorf MW, Elde R. Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. Journal of Neuroscience. 1995;15(5 Pt 1):3328–3341. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom T. Epileptic seizures in women related to plasma estrogen and progesterone during the menstrual cycle. Acta Neurol Scand. 1976;54(4):321–347. doi: 10.1111/j.1600-0404.1976.tb04363.x. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29(1):36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger A, Cusan L, Caron S, Barden N, Dupont A. Ovarian progestins, androgens and estrogen throughout the 4-day estrous cycle in the rat. Biology of Reproduction. 1981;24(3):591–596. doi: 10.1095/biolreprod24.3.591. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25(3):515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M, Tuszynski MH. Estrogen receptor-beta colocalizes extensively with parvalbumin-labeled inhibitory neurons in the cortex, amygdala, basal forebrain, and hippocampal formation of intact and ovariectomized adult rats. J.Comp Neurol. 2002;452(3):276–287. doi: 10.1002/cne.10393. [DOI] [PubMed] [Google Scholar]
- Boudin H, Pélaprat D, Rostène W, Pickel VM, Beaudet A. Correlative ultrastructural distribution of neurotensin receptor proteins and binding sites in the rat substantia nigra. Journal of Neuroscience. 1998;18(20):8473–8484. doi: 10.1523/JNEUROSCI.18-20-08473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR. Opioid receptor dependent long-term potentiation: Peptidergic regulation of synaptic plasticity in the hippocampus. Neurochem.Int. 1992;20:441–455. doi: 10.1016/0197-0186(92)90021-i. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Sarvey JM. Endogenous activation of μ and δ-1 opioid receptors is required for long-term potentiation induction in the lateral perforant path: Dependence on GABAergic inhibition. Journal of Neuroscience. 1996;16:8123–8131. doi: 10.1523/JNEUROSCI.16-24-08123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celio MR, Baier W, Scharer L, de Viragh PA, Gerday C. Monoclonal antibodies directed against the calcium binding protein parvalbumin. Cell Calcium. 1988;9(2):81–86. doi: 10.1016/0143-4160(88)90027-9. [DOI] [PubMed] [Google Scholar]
- Chan J, Aoki C, Pickel VM. Optimization of differential immunogold-silver and peroxidase labeling with maintenance of ultrastructure in brain sections before plastic embedding. Journal of Neuroscience Methods. 1990;33:113–127. doi: 10.1016/0165-0270(90)90015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, Neumaier JF, Swearengen E. Opioid receptor mechanisms in the rat hippocampus. Natl.Inst.Drug Abuse Res.Monogr.Ser. 1988;82:94–117. [PubMed] [Google Scholar]
- Commons KG, Milner TA. The ultrastructural relationships between leu-enkephalin and GABA containing neurons differ within the hippocampal formation. Brain Research. 1996;724:1–15. doi: 10.1016/0006-8993(96)00236-3. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol. 2005;64(1):34–46. doi: 10.1002/neu.20143. [DOI] [PubMed] [Google Scholar]
- Cyr M, Ghribi O, Thibault C, Morissette M, Landry M, Di Paolo T. Ovarian steroids and selective estrogen receptor modulators activity on rat brain NMDA and AMPA receptors. Brain Res.Brain Res.Rev. 2001;37(1-3):153–161. doi: 10.1016/s0165-0173(01)00115-1. [DOI] [PubMed] [Google Scholar]
- Derrick BE, Rodriguez SB, Lieberman DN, Martinez JL., Jr. Mu opioid receptors are associated with the induction of hippocampal mossy fiber long-term potentiation. Journal of Pharmacology and Experimental Therapeutics. 1992;263:725–733. [PubMed] [Google Scholar]
- Drake CT, Aicher SA, Montalmant FL, Milner TA. Redistribution of mu-opioid receptors in C1 adrenergic neurons following chronic administration of morphine. Experimental Neurology. 2005;196:365–372. doi: 10.1016/j.expneurol.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Drake CT, Chang PC, Harris JA, Milner TA. Neurons with mu opioid receptors interact indirectly with enkephalin-containing neurons in the rat dentate gyrus. Experimental Neurology. 2002;176(1):254–261. doi: 10.1006/exnr.2002.7948. [DOI] [PubMed] [Google Scholar]
- Drake CT, Chavkin C, Milner TA. Opioid systems in the dentate gyrus. Progress in Brain Research. 2007;163C:245–814. doi: 10.1016/S0079-6123(07)63015-5. [DOI] [PubMed] [Google Scholar]
- Drake CT, Milner TA. Mu opioid receptors are in somatodendritic and axonal compartments of GABAergic neurons in rat hippocampal formation. Brain Research. 1999;849(1-2):203–215. doi: 10.1016/s0006-8993(99)01910-1. [DOI] [PubMed] [Google Scholar]
- Drake CT, Milner TA. Mu opioid receptors are extensively co-localized with parvalbumin, but not somatostatin, in the dentate gyrus. Neuroscience Letters. 2006;403(1-2):176–180. doi: 10.1016/j.neulet.2006.04.047. [DOI] [PubMed] [Google Scholar]
- Dumas TC, Powers EC, Tarapore PE, Sapolsky RM. Overexpression of calbindin D(28k) in dentate gyrus granule cells alters mossy fiber presynaptic function and impairs hippocampal-dependent memory. Hippocampus. 2004;14(6):701–709. doi: 10.1002/hipo.10210. [DOI] [PubMed] [Google Scholar]
- Eddy CA. In: Experimental Surgery of the Genitalia System. Gay WI, Heavner JE, editors. Methods of Animal Experimentation Academic Press; Orlando, FL: 1986. p. 191. [Google Scholar]
- Gerrits MA, Lesscher HB, van Ree JM. Drug dependence and the endogenous opioid system. Eur.Neuropsychopharmacol. 2003;13(6):424–434. doi: 10.1016/j.euroneuro.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Harden CL, Liporace J, Pennell P, Schomer DL, Sperling M, Fowler K, Nikolov B, Shuman S, Newman M. Frequency of catamenial seizure exacerbation in women with localization-related epilepsy. Ann.Neurol. 2004;56(3):431–434. doi: 10.1002/ana.20214. [DOI] [PubMed] [Google Scholar]
- Hruby VJ, Agnes RS. Conformation-activity relationships of opioid peptides with selective activities at opioid receptors. Biopolymers. 1999;51(6):391–410. doi: 10.1002/(SICI)1097-0282(1999)51:6<391::AID-BIP3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. Journal of Histochemistry and Cytochemistry. 1981;29:557–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Mitsuno K, Ishikawa M, Sasa M. Behavioral LTP during learning in rat hippocampal CA3. Behavioral Brain Research. 1997;83:235–238. doi: 10.1016/s0166-4328(97)86077-9. [DOI] [PubMed] [Google Scholar]
- Janecka A, Fichna J, Janecki T. Opioid receptors and their ligands. Curr.Top.Med.Chem. 2004;4(1):1–17. doi: 10.2174/1568026043451618. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kim HY, Kim JH, Shin HK, Lee SH, Lee YS, Son H. Enhancement of rat hippocampal long-term potentiation by 17 beta-estradiol involves mitogen-activated protein kinase-dependent and -independent components. Neuroscience Letters. 2002;332(1):65–69. doi: 10.1016/s0304-3940(02)00902-3. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Schluger J, Borg L, Gunduz M, Ho A. Dynorphin A1-13 causes elevation of serum levels of prolactin through an opioid receptor mechanism in humans: gender differences and implications for modulation of dopaminergic tone in the treatment of addictions. J Pharmacol.Exp.Ther. 1999;288(1):260–269. [PubMed] [Google Scholar]
- Leranth C, Pickel VM. In: Electron microscopic preembedding double-labeling methods. Heimer L, Zaborszky L, editors. Neuroanatomical Tract-Tracing Methods 2 Plenum Press; New York: 1989. pp. 129–172. [Google Scholar]
- Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur.J.Neurosci. 1996;8(7):1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232(4753):1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends in Neurosciences. 1988;11(7):308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Invited review: Estrogens effects on the brain: multiple sites and molecular mechanisms. J.Appl.Physiol. 2001;91(6):2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. Journal of Neuroscience. 2003;23(13):5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Rissman EF, Gustafsson JA, Sinchak K. Estrogen receptor-alpha is required for estrogen-induced mu-opioid receptor internalization. Journal of Neuroscience Research. 2003;71(6):802–810. doi: 10.1002/jnr.10526. [DOI] [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J.Comp Neurol. 2005;491(2):81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. Journal of Comparative Neurology. 2001;429(3):355–371. [PubMed] [Google Scholar]
- Morris BJ, Johnston HM. A role for hippocampal opioids in long-term functional plasticity. Trends in Neurosciences. 1995;18:350–355. doi: 10.1016/0166-2236(95)93927-p. [DOI] [PubMed] [Google Scholar]
- Nakamura NH, McEwen BS. Changes in interneuronal phenotypes regulated by estradiol in the adult rat hippocampus: a potential role for neuropeptide Y. Neuroscience. 2005;136(1):357–369. doi: 10.1016/j.neuroscience.2005.07.056. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE, Malenka RC. Neural substrates of drug action. McGraw-Hill Companies, Inc,; New Yok: 2001. p. 224. [Google Scholar]
- Paulsen O, Moser EI. A model of hippocampal memory encoding and retrieval: GABAergic control of synaptic plasticity. Trends in Neurosciences. 1998;21(7):273–278. doi: 10.1016/s0166-2236(97)01205-8. [DOI] [PubMed] [Google Scholar]
- Penovich PE, Helmers S. Catamenial epilepsy. Int.Rev.Neurobiol. 2008;83:79–90. doi: 10.1016/S0074-7742(08)00004-4. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster H. d. The fine structure of the nervous system. 3rd ed. Oxford University Press; New York: 1991. [Google Scholar]
- Piva F, Limonta P, Dondi D, Pimpinelli F, Martini L, Maggi R. Effects of steroids on the brain opioid system. Journal of Steroid Biochemistry and Molecular Biology. 1995;53(1-6):343–348. doi: 10.1016/0960-0760(95)00072-8. [DOI] [PubMed] [Google Scholar]
- Plager MD, Vogt BA. Mu- and delta-opioid receptor binding peaks and kappa-homogeneity in the molecular layers of rat hippocampal formation. Brain Research. 1988;460:150–154. doi: 10.1016/0006-8993(88)91215-2. [DOI] [PubMed] [Google Scholar]
- Quinones-Jenab V, Jenab S, Ogawa S, Inturrisi C, Pfaff DW. Estrogen regulation of mu-opioid receptor mRNA in the forebrain of female rats. Brain Research. 1997;47:1. doi: 10.1016/s0169-328x(97)00041-7. [DOI] [PubMed] [Google Scholar]
- Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of mu-opioid receptors regulates reproductive behavior. Journal of Neuroscience. 2001;21(15):5723–5729. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamberova R, Rimanoczy A, Schindler CJ, Vathy I. Cortical and striatal mu-opioid receptors are altered by gonadal hormone treatment but not by prenatal morphine exposure in adult male and female rats. Brain Research Bulletin. 2003;62(1):47–53. doi: 10.1016/j.brainresbull.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Smith CC, McMahon LL. Estrogen-induced increase in the magnitude of long-term potentiation occurs only when the ratio of NMDA transmission to AMPA transmission is increased. J Neurosci. 2005;25(34):7780–7791. doi: 10.1523/JNEUROSCI.0762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MD, Jones LS, Wilson MA. Sex differences in hippocampal slice excitability: role of testosterone. Neuroscience. 2002;109(3):517–530. doi: 10.1016/s0306-4522(01)00490-0. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain Elsevier. Amsterdam: 1992. [Google Scholar]
- Tanapat P, Hastings NB, Gould E. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J.Comp Neurol. 2005;481(3):252–265. doi: 10.1002/cne.20385. [DOI] [PubMed] [Google Scholar]
- Torres-Reveron A, Khalid S, Williams TJ, Waters EM, Drake CT, McEwen B, Milner TA. Ovarian steroids modulate leu-enkephalin levels and target leuenkephalinergic profiles in the female hippocampal mossy fiber pathway. Brain Research. 2008;1232:70–84. doi: 10.1016/j.brainres.2008.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Reveron A, Khalid S, Williams TJ, Waters EM, Jacome L, Luine VN, Drake CT, McEwen BS, Milner TA. Hippocampal dynorphin immunoreactivity increases in response to gonadal steroids and is positioned for direct modulation by ovarian steroid receptors. Neuroscience. 2009;159(1):204–216. doi: 10.1016/j.neuroscience.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Reveron A, Williams TJ, Prasad P, Drake CT, Milner TA. Neuroscience Meeting Planner. Society for Neuroscience; San Diego, CA: 2007. Ovarian steroids affect the subcellular distribution of mu opioid receptors in parvalbumin interneurons of the dentate gyrus. 2007.Online 729.6. [Google Scholar]
- Towart LA, Alves SE, Znamensky V, Hayashi S. Cholinergic terminals in rat hippocampal formation contain estrogen receptor a. Society for Neuroscience Abstracts 28.2002. [Google Scholar]
- Turner CD, Bagnara JT. General Endocrinology W.B. Saunders. Philadelphia: 1971. [Google Scholar]
- Warren SG, Humphreys AG, Juraska JM, Greenough WT. LTP varies across the estrous cycle: Enhanced synaptic plasticity in proestrus rats. Brain Research. 1995;703:26–30. doi: 10.1016/0006-8993(95)01059-9. [DOI] [PubMed] [Google Scholar]
- Wong M, Moss RL. Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. Journal of Neuroscience. 1992;12:3217–3225. doi: 10.1523/JNEUROSCI.12-08-03217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Hormones and Behavior. 1998;34(2):140–148. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. Journal of Neuroscience. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. Journal of Neuroscience. 1994;14:7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie CW, Lewis DV. Depression of LTP in rat dentate gyrus by naloxone is reversed by GABAA blockade. Brain Research. 1995;688:56–60. doi: 10.1016/0006-8993(95)00510-w. [DOI] [PubMed] [Google Scholar]
- Xie CW, Morrisett RA, Lewis DV. Mu opioid receptor-mediated modulation of synaptic currents in dentate granule cells of rat hippocampus. Journal of Neurophysiology. 1992;68:1113–1120. doi: 10.1152/jn.1992.68.4.1113. [DOI] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience. 2007;144(1):26–37. doi: 10.1016/j.neuroscience.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]