Abstract
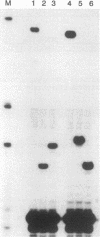
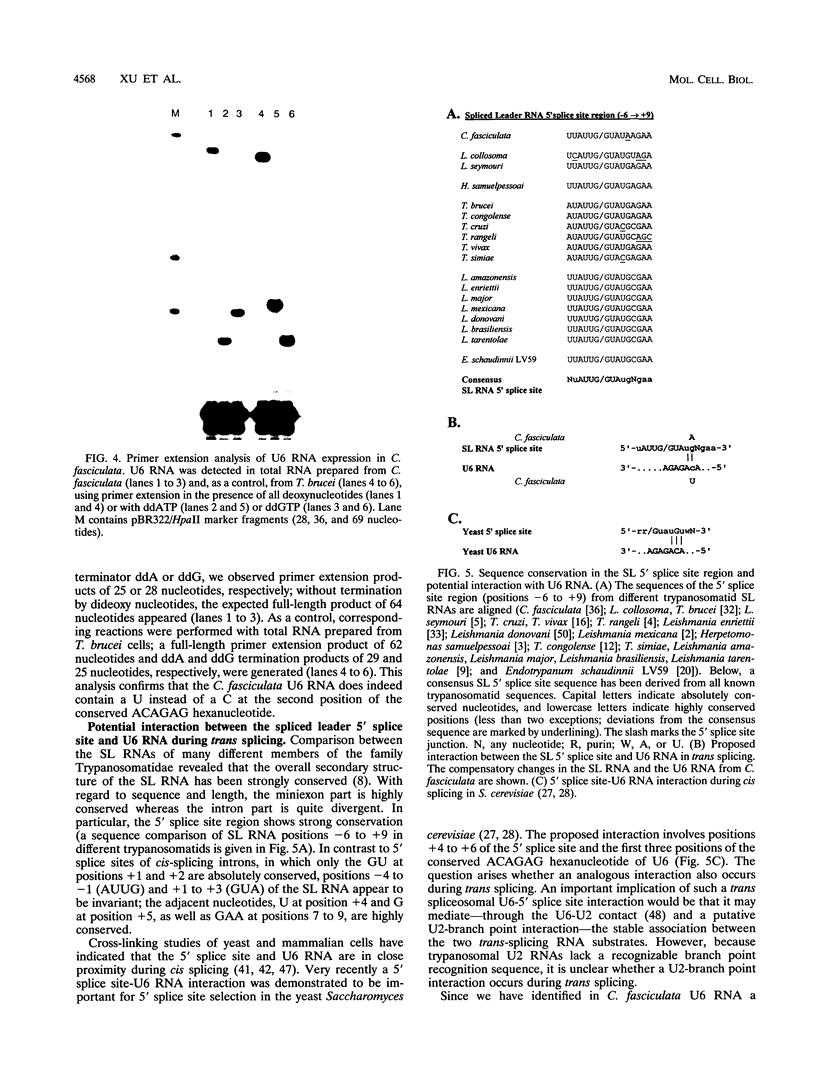
U6 RNA genes from the trypanosomatids Crithidia fasciculata and Leptomonas seymouri have been isolated and sequenced. As in Trypanosoma brucei, the U6 RNA genes in both C. fasciculata and L. seymouri are arranged in close linkage with upstream tRNA genes. The U6 RNA sequences from C. fasciculata and L. seymouri deviate in five and three positions, respectively, from the published T. brucei sequence. Interestingly, both C. fasciculata U6 RNA genes carry a C-->T change at the second position of the ACAGAG hexanucleotide sequence, which is important for splicing function and has been considered phylogenetically invariable. A compensatory base change of the C. fasciculata spliced leader RNA at the highly conserved 5' splice site position +5, G-->A, suggests that an interaction between the 5' splice site region and U6 RNA recently proposed for the yeast cis-splicing system may also occur in trans splicing.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agabian N. Trans splicing of nuclear pre-mRNAs. Cell. 1990 Jun 29;61(7):1157–1160. doi: 10.1016/0092-8674(90)90674-4. [DOI] [PubMed] [Google Scholar]
- Agami R., Shapira M. Nucleotide sequence of the spliced leader RNA gene from Leishmania mexicana amazonensis. Nucleic Acids Res. 1992 Apr 11;20(7):1804–1804. doi: 10.1093/nar/20.7.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy S., Shay G. L., Villanueva M. S., Beard C. B., Richards F. F. Spliced leader RNA sequences of Trypanosoma rangeli are organized within the 5S rRNA-encoding genes. Gene. 1992 Apr 15;113(2):239–243. doi: 10.1016/0378-1119(92)90401-a. [DOI] [PubMed] [Google Scholar]
- Aksoy S. Spliced leader RNA and 5S rRNA genes in Herpetomonas spp. are genetically linked. Nucleic Acids Res. 1992 Feb 25;20(4):913–913. doi: 10.1093/nar/20.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellofatto V., Cooper R., Cross G. A. Discontinuous transcription in Leptomonas seymouri: presence of intact and interrupted mini-exon gene families. Nucleic Acids Res. 1988 Aug 11;16(15):7437–7456. doi: 10.1093/nar/16.15.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellofatto V., Hartree D. E., Torres-Munoz J. Leptomonas seymouri as a model system for the analysis of gene expression in trypanosomatids. J Parasitol. 1993 Oct;79(5):637–644. [PubMed] [Google Scholar]
- Brow D. A., Guthrie C. Spliceosomal RNA U6 is remarkably conserved from yeast to mammals. Nature. 1988 Jul 21;334(6179):213–218. doi: 10.1038/334213a0. [DOI] [PubMed] [Google Scholar]
- Bruzik J. P., Van Doren K., Hirsh D., Steitz J. A. Trans splicing involves a novel form of small nuclear ribonucleoprotein particles. Nature. 1988 Oct 6;335(6190):559–562. doi: 10.1038/335559a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clayton C. E. The molecular biology of the Kinetoplastidae. Genet Eng. 1988;(7):1–56. [PubMed] [Google Scholar]
- Cook G. A., Donelson J. E. Mini-exon gene repeats of Trypanosoma (Nannomonas) congolense have internal repeats of 190 base pairs. Mol Biochem Parasitol. 1987 Aug;25(1):113–122. doi: 10.1016/0166-6851(87)90024-7. [DOI] [PubMed] [Google Scholar]
- Cross M., Günzl A., Palfi Z., Bindereif A. Analysis of small nuclear ribonucleoproteins (RNPs) in Trypanosoma brucei: structural organization and protein components of the spliced leader RNP. Mol Cell Biol. 1991 Nov;11(11):5516–5526. doi: 10.1128/mcb.11.11.5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross M., Wieland B., Palfi Z., Günzl A., Röthlisberger U., Lahm H. W., Bindereif A. The trans-spliceosomal U2 snRNP protein 40K of Trypanosoma brucei: cloning and analysis of functional domains reveals homology to a mammalian snRNP protein. EMBO J. 1993 Mar;12(3):1239–1248. doi: 10.1002/j.1460-2075.1993.tb05765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta B., Weiner A. M. The phylogenetically invariant ACAGAGA and AGC sequences of U6 small nuclear RNA are more tolerant of mutation in human cells than in Saccharomyces cerevisiae. Mol Cell Biol. 1993 Sep;13(9):5377–5382. doi: 10.1128/mcb.13.9.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lange T., Berkvens T. M., Veerman H. J., Frasch A. C., Barry J. D., Borst P. Comparison of the genes coding for the common 5' terminal sequence of messenger RNAs in three trypanosome species. Nucleic Acids Res. 1984 Jun 11;12(11):4431–4443. doi: 10.1093/nar/12.11.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund P. T. The replication of kinetoplast DNA networks in Crithidia fasciculata. Cell. 1978 May;14(1):157–168. doi: 10.1016/0092-8674(78)90310-0. [DOI] [PubMed] [Google Scholar]
- Fabrizio P., Abelson J. Two domains of yeast U6 small nuclear RNA required for both steps of nuclear precursor messenger RNA splicing. Science. 1990 Oct 19;250(4979):404–409. doi: 10.1126/science.2145630. [DOI] [PubMed] [Google Scholar]
- Fernandes O., Degrave W., Campbell D. A. The mini-exon gene: a molecular marker for Endotrypanum schaudinni. Parasitology. 1993 Sep;107(Pt 3):219–224. doi: 10.1017/s003118200007918x. [DOI] [PubMed] [Google Scholar]
- Fortner D. M., Troy R. G., Brow D. A. A stem/loop in U6 RNA defines a conformational switch required for pre-mRNA splicing. Genes Dev. 1994 Jan;8(2):221–233. doi: 10.1101/gad.8.2.221. [DOI] [PubMed] [Google Scholar]
- Geiduschek E. P., Tocchini-Valentini G. P. Transcription by RNA polymerase III. Annu Rev Biochem. 1988;57:873–914. doi: 10.1146/annurev.bi.57.070188.004301. [DOI] [PubMed] [Google Scholar]
- Guthrie C., Patterson B. Spliceosomal snRNAs. Annu Rev Genet. 1988;22:387–419. doi: 10.1146/annurev.ge.22.120188.002131. [DOI] [PubMed] [Google Scholar]
- Hannon G. J., Maroney P. A., Yu Y. T., Hannon G. E., Nilsen T. W. Interaction of U6 snRNA with a sequence required for function of the nematode SL RNA in trans-splicing. Science. 1992 Dec 11;258(5089):1775–1780. doi: 10.1126/science.1465612. [DOI] [PubMed] [Google Scholar]
- Hausner T. P., Giglio L. M., Weiner A. M. Evidence for base-pairing between mammalian U2 and U6 small nuclear ribonucleoprotein particles. Genes Dev. 1990 Dec;4(12A):2146–2156. doi: 10.1101/gad.4.12a.2146. [DOI] [PubMed] [Google Scholar]
- Kandels-Lewis S., Séraphin B. Involvement of U6 snRNA in 5' splice site selection. Science. 1993 Dec 24;262(5142):2035–2039. doi: 10.1126/science.8266100. [DOI] [PubMed] [Google Scholar]
- Lesser C. F., Guthrie C. Mutations in U6 snRNA that alter splice site specificity: implications for the active site. Science. 1993 Dec 24;262(5142):1982–1988. doi: 10.1126/science.8266093. [DOI] [PubMed] [Google Scholar]
- Madhani H. D., Bordonné R., Guthrie C. Multiple roles for U6 snRNA in the splicing pathway. Genes Dev. 1990 Dec;4(12B):2264–2277. doi: 10.1101/gad.4.12b.2264. [DOI] [PubMed] [Google Scholar]
- Michaeli S., Roberts T. G., Watkins K. P., Agabian N. Isolation of distinct small ribonucleoprotein particles containing the spliced leader and U2 RNAs of Trypanosoma brucei. J Biol Chem. 1990 Jun 25;265(18):10582–10588. [PubMed] [Google Scholar]
- Milhausen M., Nelson R. G., Sather S., Selkirk M., Agabian N. Identification of a small RNA containing the trypanosome spliced leader: a donor of shared 5' sequences of trypanosomatid mRNAs? Cell. 1984 Oct;38(3):721–729. doi: 10.1016/0092-8674(84)90267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. I., Landfear S. M., Wirth D. F. Cloning and characterization of a Leishmania gene encoding a RNA spliced leader sequence. Nucleic Acids Res. 1986 Sep 25;14(18):7341–7360. doi: 10.1093/nar/14.18.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottram J. C., Bell S. D., Nelson R. G., Barry J. D. tRNAs of Trypanosoma brucei. Unusual gene organization and mitochondrial importation. J Biol Chem. 1991 Sep 25;266(27):18313–18317. [PubMed] [Google Scholar]
- Mottram J., Perry K. L., Lizardi P. M., Lührmann R., Agabian N., Nelson R. G. Isolation and sequence of four small nuclear U RNA genes of Trypanosoma brucei subsp. brucei: identification of the U2, U4, and U6 RNA analogs. Mol Cell Biol. 1989 Mar;9(3):1212–1223. doi: 10.1128/mcb.9.3.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhich M. L., Hughes D. E., Simpson A. M., Simpson L. The monogenetic kinetoplastid protozoan, Crithidia fasciculata, contains a transcriptionally active, multicopy mini-exon sequence. Nucleic Acids Res. 1987 Apr 10;15(7):3141–3153. doi: 10.1093/nar/15.7.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfi Z., Bindereif A. Immunological characterization and intracellular localization of trans-spliceosomal small nuclear ribonucleoproteins in Trypanosoma brucei. J Biol Chem. 1992 Oct 5;267(28):20159–20163. [PubMed] [Google Scholar]
- Palfi Z., Günzl A., Cross M., Bindereif A. Affinity purification of Trypanosoma brucei small nuclear ribonucleoproteins reveals common and specific protein components. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9097–9101. doi: 10.1073/pnas.88.20.9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinke J., Appel B., Digweed M., Lührmann R. Localization of a base-paired interaction between small nuclear RNAs U4 and U6 in intact U4/U6 ribonucleoprotein particles by psoralen cross-linking. J Mol Biol. 1985 Oct 20;185(4):721–731. doi: 10.1016/0022-2836(85)90057-9. [DOI] [PubMed] [Google Scholar]
- Roiha H., Shuster E. O., Brow D. A., Guthrie C. Small nuclear RNAs from budding yeasts: phylogenetic comparisons reveal extensive size variation. Gene. 1989 Oct 15;82(1):137–144. doi: 10.1016/0378-1119(89)90038-3. [DOI] [PubMed] [Google Scholar]
- Sawa H., Abelson J. Evidence for a base-pairing interaction between U6 small nuclear RNA and 5' splice site during the splicing reaction in yeast. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11269–11273. doi: 10.1073/pnas.89.23.11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa H., Shimura Y. Association of U6 snRNA with the 5'-splice site region of pre-mRNA in the spliceosome. Genes Dev. 1992 Feb;6(2):244–254. doi: 10.1101/gad.6.2.244. [DOI] [PubMed] [Google Scholar]
- Swinkels B. W., Evers R., Borst P. The topogenic signal of the glycosomal (microbody) phosphoglycerate kinase of Crithidia fasciculata resides in a carboxy-terminal extension. EMBO J. 1988 Apr;7(4):1159–1165. doi: 10.1002/j.1460-2075.1988.tb02926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J., Lea K., Zucker-Aprison E., Blumenthal T. The spliceosomal snRNAs of Caenorhabditis elegans. Nucleic Acids Res. 1990 May 11;18(9):2633–2642. doi: 10.1093/nar/18.9.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschudi C., Krainer A. R., Ullu E. The U6 small nuclear RNA from Trypanosoma brucei. Nucleic Acids Res. 1988 Dec 9;16(23):11375–11375. doi: 10.1093/nar/16.23.11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman D. A., Steitz J. A. Interactions of small nuclear RNA's with precursor messenger RNA during in vitro splicing. Science. 1992 Sep 25;257(5078):1918–1925. doi: 10.1126/science.1411506. [DOI] [PubMed] [Google Scholar]
- Watkins K. P., Agabian N. In vivo UV cross-linking of U snRNAs that participate in trypanosome trans-splicing. Genes Dev. 1991 Oct;5(10):1859–1869. doi: 10.1101/gad.5.10.1859. [DOI] [PubMed] [Google Scholar]
- Weiner A. M. mRNA splicing and autocatalytic introns: distant cousins or the products of chemical determinism? Cell. 1993 Jan 29;72(2):161–164. doi: 10.1016/0092-8674(93)90654-9. [DOI] [PubMed] [Google Scholar]
- Wilson K., Hanson S., Landfear S., Ullman B. Nucleotide sequence of the Leishmania donovani medRNA gene. Nucleic Acids Res. 1991 Oct 25;19(20):5787–5787. doi: 10.1093/nar/19.20.5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff T., Bindereif A. Conformational changes of U6 RNA during the spliceosome cycle: an intramolecular helix is essential both for initiating the U4-U6 interaction and for the first step of slicing. Genes Dev. 1993 Jul;7(7B):1377–1389. doi: 10.1101/gad.7.7b.1377. [DOI] [PubMed] [Google Scholar]
- Wolff T., Menssen R., Hammel J., Bindereif A. Splicing function of mammalian U6 small nuclear RNA: conserved positions in central domain and helix I are essential during the first and second step of pre-mRNA splicing. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):903–907. doi: 10.1073/pnas.91.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. T., Maroney P. A., Nilsen T. W. Functional reconstitution of U6 snRNA in nematode cis- and trans-splicing: U6 can serve as both a branch acceptor and a 5' exon. Cell. 1993 Dec 17;75(6):1049–1059. doi: 10.1016/0092-8674(93)90315-h. [DOI] [PubMed] [Google Scholar]