Abstract
Background
Chronic hepatitis C (HCV) is a difficult to treat disease affecting over 3 million Americans. Protease inhibitors increase the effectiveness of standard therapy but are costly. A genetic assay may identify patients most likely to benefit from this treatment advance.
Objective
Cost-effectiveness assessment of new protease inhibitors and an Interleukin-28B (IL- 28B) genotyping assay for treating chronic HCV
Design
Decision-analytic Markov model
Data Sources
Published literature and expert opinion
Target Population
Treatment-naïve patients with chronic, genotype 1 HCV mono-infection
Time Horizon
Lifetime
Perspective
Societal
Interventions
Strategies are defined by the use of IL-28B genotyping and type of treatment (standard therapy: pegylated interferon with ribavirin; triple therapy: standard therapy and a protease inhibitor). IL-28B guided triple therapy stratifies CC genotype patients to standard therapy and non-CC types to triple therapy.
Outcome Measures
Discounted costs (2010 U.S. dollars) and quality-adjusted life years (QALYs); incremental cost effectiveness ratios
Results of Base-Case Analysis
For patients with mild and advanced fibrosis, universal triple therapy reduces life-time risk of hepatocellular-carcinoma by 39% and 29% and increases quality-adjusted life expectancy by 3% and 8% compared to standard therapy. Gains from IL- 28B guided triple therapy are smaller. If the protease inhibitor costs $1,100 per week, universal triple therapy costs $102,600 per QALY (mild fibrosis) or $51,500 per QALY (advanced fibrosis) compared to IL-28B guided triple therapy and $70,100 per QALY and $36,300 per QALY compared to standard therapy.
Results of Sensitivity Analysis
Results are sensitive to the cost of protease inhibitors and treatment adherence rates.
Limitations
Lack of long-term comparative effectiveness data on the new protease inhibitors
Conclusions
Both universal triple therapy and IL-28B guided triple therapy are cost-effective with the least expensive protease inhibitor for patients with advanced fibrosis.
Primary Funding Source
Stanford Graduate Fellowship
Introduction
Hepatitis C virus (HCV) infection is a serious liver disease affecting 180 million people worldwide (1). In the U.S., between 2.7 to 3.9 million people live with chronic HCV infection, approximately 75% of whom are infected with HCV genotype 1 (2, 3). Chronic HCV causes liver fibrosis, cirrhosis, and hepatocellular carcinoma, and is the most common cause of liver transplantation (1). Standard therapy for chronic HCV infection is pegylated interferon and ribavirin, which is effective in 40–60% of HCV genotype 1 patients (2, 4).
New viral protease inhibitors, boceprevir (VictrelisTM, Merck) and telaprevir (IncivekTM, Vertex), used in conjunction with standard therapy, have significantly increased treatment success in genotype 1 infected individuals and also shorten treatment duration (5, 6). These new treatment regimens are more expensive (boceprevir $1,100 per week; telaprevir $4,100 per week) and can cause more severe side effects than standard therapy (7). It is unclear whether they are best used as first-line therapy in all genotype 1 infected patients or for the subset of patients with the poorest expected outcomes on standard therapy.
Interleukin 28B (IL-28B) genotype (CC, CT, or TT type) predicts response to HCV therapy and may prove valuable in targeting protease inhibitors to those least likely to benefit from standard therapy (8–11). Patients with non-CC types have a 30% sustained viral response (SVR) on standard therapy and up to 70% SVR rate when treated with triple therapy (12–14). In contrast, CC types are more responsive to treatment: 70% achieve SVR on standard therapy and up to 90% achieve SVR on triple therapy.
We performed a model-based cost-effectiveness analysis of treatment strategies for eligible chronic HCV genotype 1 infected patients. We evaluated adding new protease inhibitors to standard therapy in the context of response guided therapy and the use of IL-28B genotyping to target triple therapy.
Methods
We used a decision-analytic Markov model of HCV natural history and progression towards advanced liver disease to assess the cost-effectiveness of alternative treatment strategies for treatment-naïve patients with genotype 1 chronic HCV mono-infection. Cohorts are defined by age (base case: 50 years), sex, race (white and black), IL-28B genotype (CC and non-CC types), and initial fibrosis stage (Metavir score: F0, F1, F2, F3, F4). Since patients’ fibrosis stage is not always known, we considered two groups of patients: those with ‘mild fibrosis’ (a mix of F0-F2) and ‘advanced fibrosis’ (a mix of F2-F4).
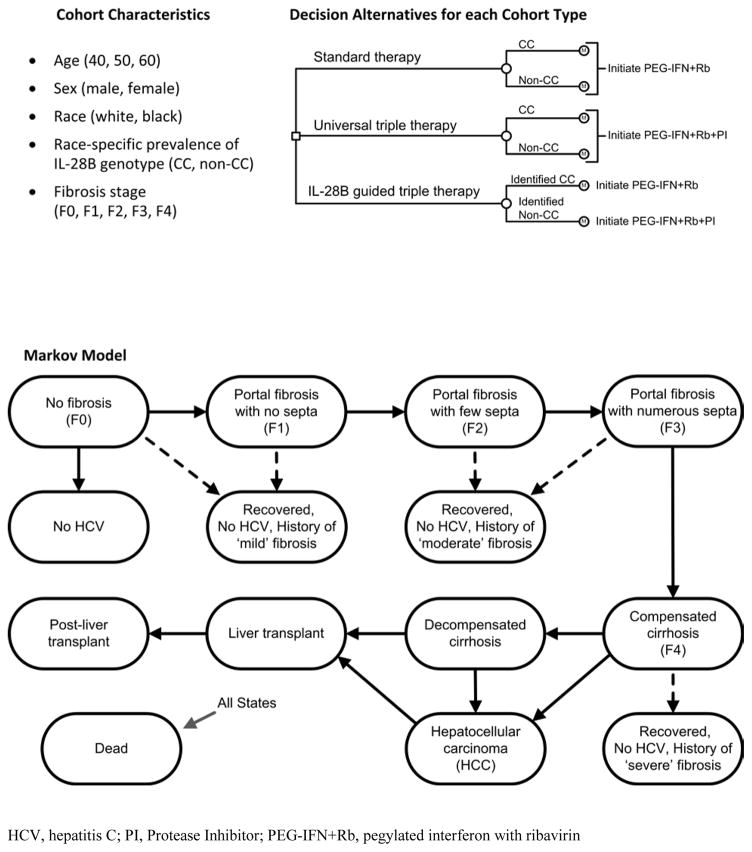
We considered three strategies (Figure 1). Patients can be treated without IL-28B genotyping with either standard therapy (pegylated interferon with ribavirin) or with triple therapy (pegylated interferon with ribavirin and a new protease inhibitor). The IL-28B guided triple therapy strategy stratifies non-CC type patients to triple therapy and CC type patients to standard therapy.
Figure 1. Model Schematics.
The small square represents the decision to implement a policy of standard therapy, universal triple therapy, or IL-28B guided triple therapy. The small circle with inset M indicates the Markov model. During each 12-week cycle of the model, all individuals face a risk of death depending on their age and health state. Individuals begin the model receiving treatment and if treatment is successful (the patient achieves sustained viral response) the patient may transition along one of the dashed arrows to a fibrosis-stage stratified recovered state. Treatment effectiveness is determined by type of treatment, race, fibrosis stage, and IL-28B genotype. If treatment is not successful the individual continues progressing through the natural history of HCV (indicated by solid arrows). Death can occur from any health state in the Markov model.
Natural History Model
The natural history model is similar to a previously published, empirically calibrated model (15, 16) (Appendix I). In brief, it simulates the lifetime disease progression of individuals with chronic HCV infections (Figure 1). Progression through fibrosis stages is characterized by Metavir Score, with possible transitions occurring every 12 weeks. Rates of disease progression depend on age and sex. Health states include healthy (No HCV), no fibrosis (F0), portal fibrosis with no septa (F1), portal fibrosis with few septa (F2), numerous septa without cirrhosis (F3), compensated cirrhosis (F4), decompensated cirrhosis, hepatocellular carcinoma, and liver transplantation. Without treatment, spontaneous clearance of the virus and return to the HCV negative state is only possible from F0. A proportion of patients who start at F0 are “nonprogressors” and do not progress to more severe fibrosis stages. Patients who achieve SVR transition to recovered health states stratified by fibrosis severity. A proportion of decompensated cirrhosis and hepatocellular carcinoma patients receive liver transplants. Death can occur from any state.
Treatment Model
Patients initiate treatment at the outset of the model. The goal of treatment is SVR – absence of HCV RNA from serum 24 weeks following discontinuation of treatment. Both standard therapy and triple therapy employ specific response guided protocols (1, 5, 6, 17, 18) (Appendix Figure 1 and 2). Null responders, partial responders, and relapsers resume fibrosis progression after treatment failure since no retreatment is offered. Retreatment scenarios are explored in Appendix I.
Since no randomized controlled trials directly compare the two protease inhibitors and their reported efficacies are similar, we considered a general protease inhibitor whose effectiveness was similar and then explicitly modeled costs, treatment durations, and response guided rules specific to each drug. In scenario analyses, we explicitly compared the two protease inhibitors under a range of effectiveness and cost scenarios.
Data and Sources
Tables 1 and 2 show the data and sources.
Table 1.
Model Parameter Values and Ranges
| Variable | Base Case | Range (Low – High) | Reference |
|---|---|---|---|
| Model Assumptions | |||
| Discount rate (annual) | 0.03 | 0 – 0.05 | (19) |
| Time horizon | Lifetime | ||
| Perspective | Societal | ||
| Cohort characteristics | |||
| Cohort age | 50 | 40 – 60 | |
| Mild/Advanced | (20) | ||
| Stage of Fibrosis Distribution* | |||
| No fibrosis (F0) | 0.30 / 0 | ||
| Portal fibrosis (F1) | 0.41 / 0 | ||
| Periportal fibrosis (F2) | 0.29 / 0.29 | ||
| Bridging fibrosis (F3) | 0 / 0.23 | ||
| Compensated fibrosis (F4) | 0 / 0.48 | ||
| IL-28B genotype, proportion CC-type polymorphism (vs. non-CC type) | |||
| White | 0.37 | 0.28 – 0.46 | (12) |
| Black | 0.14 | 0.11 – 0.18 | |
| Hepatitis C (HCV) natural history | |||
| Proportion of patients with no fibrosis (F0) who are non- progressors | 0.24 | 0.20 – 0.33 | (21) |
| Annual probability of spontaneous remission (F0 → Remission) | 0.012 | 0.007 – 0.017 | (15, 21) |
| Fibrosis progression (annual probability) | (15, 21) | ||
| Males, by age | |||
| 40–49 | 0.05 | 0.03 – 0.09 | |
| 50–59 | 0.12 | 0.07 – 0.14 | |
| 60–69 | 0.20 | 0.12 – 0.30 | |
| >=70 | 0.26 | 0.14 – 0.38 | |
| Females, by age | |||
| 40–49 | 0.03 | 0.01 – 0.06 | |
| 50–59 | 0.06 | 0.03 – 0.11 | |
| 60–69 | 0.11 | 0.04 – 0.21 | |
| 70–79 | 0.14 | 0.08 – 0.24 | |
| >=80 | 0.20 | 0.08 – 0.30 | |
| Cirrhosis → decompensated cirrhosis | 0.04 | 0.03 – 0.05 | |
| Cirrhosis (both F4 and decompensated cirrhosis) → hepatocellular carcinoma | 0.02 | 0.017 – 0.03 | |
| Liver transplant (annual probability) | |||
| Decompensated cirrhosis → liver transplant | 0.05 | 0 – 0.40 | (22) |
| Hepatocellular carcinoma → liver transplant | 0.15 | 0.05 – 0.40 | (22) |
| Background mortality | |||
| Sex-, Race-, Age-specific background mortality | (23) | ||
| Hazard ratio for non-liver causes of death in individuals with chronic HCV infection | |||
| White, male | 2.56 | 1.80 – 3.30 | NHANES III |
| White, female | 1.90 | 1.30 – 2.50 | NHANES III |
| Black, male | 2.75 | 1.90 – 3.60 | NHANES III |
| Black, female | 2.48 | 1.70 – 3.20 | NHANES III |
| Liver related mortality (annual probability) | |||
| Liver transplant | 0.14 | 0.134 – 0.15 | (24) |
| Post liver transplant | 0.05 | 0.049 – 0.051 | (24) |
| Decompensated cirrhosis | 0.26 | 0.12 – 0.33 | (21) |
| Hepatocellular carcinoma | (25) | ||
| First year | 0.72 | 0.58 – 0.80 | |
| Subsequent year | 0.25 | 0.16 – 0.30 | |
| Treatment related mortality | 0.005 | 0.0005 – 0.011 | (26) |
| Effectiveness of treatment in treatment naïve patients | |||
| Standard therapy (PEG-INF+Rb) | |||
| Mild fibrosis (F0/F1/F2), White race | CC / non-CC | (4, 12, 27) | |
| Probability of early viral response (assessed at 12 weeks) | 0.90/0.66 | ||
| Probability of viral response at 24 weeks, conditional on early viral response | 0.92/0.75 | ||
| Probability of sustained viral response, conditional on completed treatment (48 weeks) | 0.83/0.64 | ||
| Overall probability of sustained viral response@ | 0.46 | 0.42 – 0.49 | |
| Mild fibrosis (F0/F1/F2), Black race | CC/non-CC | ||
| Probability of early viral response (assessed at 12 weeks) | 0.76/0.45 | ||
| Probability of viral response at 24 weeks, conditional on early viral response | 0.95/0.78 | ||
| Probability of sustained viral response, conditional on completed treatment (48 weeks) | 0.67/0.40 | ||
| Overall probability of sustained viral response@ | 0.19 | 0.13 – 0.24 | |
| Treatment adherence on triple therapy | 0.70 | 0.50 – 0.70 | |
| Triple Therapy (PEG-INF+Rb+PI) § | 0 | (5, 13, 14, 28–30) | |
| Mild fibrosis (F0/F1/F2), White race | CC/non-CC | ||
| Probability of early viral response (assessed at 12 weeks) | 0.98/0.90 | ||
| Probability of treatment failure at 24 weeks | 0.10/0.15 | ||
| Probability of treatment completion at 24/28 weeks | 0.62/0.43 | ||
| Probability of continuing treatment until 48 weeks | 0.28/0.42 | ||
| Probability of sustained viral response, conditional on completed treatment (24/28 weeks) | 0.98/0.95 | ||
| Probability of sustained viral response, conditional on completed treatment (48 weeks) | 0.75/0.65 | ||
| Overall probability of sustained viral response@ | 0.68 | 0.60 – 0.72 | |
| Mild fibrosis (F0/F1/F2), Black race | CC/non-CC | ||
| Probability of early viral response (assessed at 12 weeks) | 0.80/0.60 | ||
| Probability of treatment failure at 24 weeks | 0.14/0.14 | ||
| Probability of treatment completion at 24/28 weeks | 0.48/0.48 | ||
| Probability of continuing treatment until 48 weeks | 0.38/0.38 | ||
| Probability of sustained viral response, conditional on completed treatment (24/28 weeks) | 0.95/0.89 | ||
| Probability of sustained viral response, conditional on completed treatment (48 weeks) | 0.70/0.60 | ||
| Overall probability of sustained viral response@ | 0.42 | 0.24 – 0.47 | |
| Reduction in sustained viral response for advanced (F3 and F4) fibrosis stage | 0.80 | 0.70 – 1.00 | |
| Effectiveness of retreatment | |||
| Proportion of patients who do not achieve sustained viral response who seek retreatment | 0.80 | 0.50 – 1.00 | Expert opinion |
| Overall sustained viral response after retreatment with triple therapy | white/black | (31) | |
| Null responders | 0.35/0.26 | 0.28 – 0.39/ 0.21 – 0.28 | |
| Partial responders | 0.64/0.47 | 0.51 – 0.70/ 0.37 – 0.52 | |
| Relapsers | 0.86/0.63 | 0.69 – 0.95/ 0.50 – 0.69 |
NHANES: National Health and Nutrition Examination Survey
PI, Protease Inhibitor
PEG-IFN+Rb, pegylated interferon with ribavirin
The fibrosis stage distribution from the Detroit study is 18% F0, 24% F1, 17% F2, 13% F3, and 28% F4. To obtain the distribution of F0-F2 in the mild fibrosis group, we re-normalized the denominator, F0 = 18/(18+24+17), F1= 24/(18+24+17), F2=17/(18+24+17). For the distribution of F2-F4 in the advanced fibrosis group, F2=17/(17+13+28), F3=13/(17+13+28), F4=28/(17+13+28).
Calculated final SVR for the full cohort stratified by race, but not by IL-28B genotypes.
The reported triple therapy effectiveness used in the base case analysis is similar to boceprevir. In the scenario analysis of telaprevir, we increased the overall probability of SVR to represent the effectiveness reported in the telaprevir ADVANCE trial, whites (SVR=75%) and black (SVR=61%).
Table 2.
Utilities and Cost
| Variable | Base Case | Range (Low – – High) | Reference |
|---|---|---|---|
| Quality of Life (Year)& | |||
| Age-specific quality of life weights | (32, 33) | ||
| HCV-specific weights | (15, 34–37) | ||
| HCV mild fibrosis (F0, F1) | 0.980 | 0.700 – 1.000 | |
| SVR following mild fibrosis | 1.000 | 0.740 – 1.000 | |
| HCV moderate fibrosis (F2, F3) | 0.850 | 0.660 – 1.000 | |
| SVR following moderate fibrosis | 0.933 | 0.710 – 1.000 | |
| Compensated cirrhosis (F4) | 0.790 | 0.460 – 1.000 | |
| SVR following cirrhosis | 0.933 | 0.600 – 1.000 | |
| Decompensated cirrhosis (DC) | 0.720 | 0.257 – 0.913 | |
| HCC | 0.720 | 0.150 – 0.950 | |
| Liver transplant (during/post) | 0.825 | 0.636 – 1.000 | |
| Standard therapy decrement # | −0.110 | −0.200 – 0.000 | |
| Triple therapy decrement # | −0.165 | −0.400 – 0.000 | |
| Liver transplant decrement # | −0.200 | −0.364 – 0.000 | |
| Cost (2010 U.S. dollar) | |||
| Age-specific baseline costs | (38) | ||
| IL-28B Testing | $371 | $186 – $557 | |
| Treatment (drug and medical care) | |||
| Peg Interferon and ribavirin (F0-F3, 48 weeks) | $32,692 | $12,002 – $49,460 | (39, 40) |
| Peg Interferon and ribavirin (F4, 48 weeks) | $35,814 | $15,123 – $52,582 | (39, 40) |
| PIs (per week)$ | $1,100 | $781 – $1,430 | (7, 41) |
| Adverse events, standard therapy | $1,920 | $1,344 – $2,496 | (42) |
| Adverse events, standard therapy, PI | $2,586 | $1,810 – $3,361 | (42) |
| Retreatment (48 weeks) ~ | $83,677 | $48,176 – $115,742 | |
| Cost of Annual Care^ | |||
| HCV mild fibrosis (F0, F1) | $1,404 | $152 – $4,194 | (15, 40, 43–45) |
| HCV portal fibrosis (F2) | $1,404 | $152 – $4,194 | |
| HCV bridging fibrosis (F3) | $1,404 | $152 – $4,194 | |
| Compensated cirrhosis (F4) | $4,194 | $152 – $4,194 | |
| Decompensated Cirrhosis (DC) | $11,109 | $5,560 – $16,669 | |
| HCC | $44,224 | $22,117 – $66,341 | |
| Liver transplant, first year | $145,640 | $72,825 – $218,455 | |
| Liver transplant, subsequent | $25,430 | $12,715 – $38,156 | |
| Recovered states from F0-F3 | $406 | $0 – $702 | Assumed** |
| Recovered states from F4 | $811 | $0 – $2,097 | |
The quality of life weight for a given age and HCV disease state is computed as the product of the utility associated with the HCV disease state and a mean age-specific quality weight obtained from published data (32, 33).
Unlike other utilities in Table 2, these utility decrements are for short-term states (i.e., being on HCV treatment or receiving a liver transplant). The total QALY decrement for being on HCV treatment involves multiplying the annual utility decrement by the time on treatment, which can vary given each strategy’s Response Guided Therapy rules. For example, the utility decrement of −0.11 is reported in terms of QALYs lost per year while on standard therapy. For an individual on standard therapy for 12 weeks, the loss of QALYs from treatment is −0.0254 (−0.11*12/52). Similarly, for the 12 weeks surrounding the liver transplant, the loss of QALYs from transplantation is -0.0462 (−0.20*12/52).
The PI cost is added to the standard therapy cost while on triple therapy.
Base case retreatment cost is the sum of 48 weeks of standard therapy cost and 44 weeks of PI cost ($1,100 per week), similar to boceprevir. In the telaprevir case, retreatment cost is the sum of 48 weeks of standard therapy cost and 12 weeks of PI cost ($4,100 per week).
Baseline healthcare cost by age is included in the model (38).
Assume costs in the recovered states are half of the hepatitis C related care costs in the year before diagnosis of the corresponding states (43).
Background Mortality
2006 U.S. life tables provide sex-, age-, and race-specific mortality rates from causes other than HCV (23). Patients with chronic HCV have higher risks of death from other causes. We increased the relevant non-liver related mortality rates using sex- and race-specific factors (1.9 to 2.75) estimated from the National Health and Nutrition Examination Survey III (Appendix I). Patients who achieved SVR are no longer at higher risk for liver-related mortality, but they continue to be at higher risk for non-liver related mortality.
Fibrosis
A patient’s fibrosis stage can be assessed using liver biopsy—the gold standard, though due to the risk of complication and potential sampling error, other non-invasive methods are also commonly used to coarsely stage patients as having mild or advanced fibrosis (46–48). We considered two representative patient cohorts (Table 1): one in which patients have mild fibrosis (a mix of F0–F2); and one in which they have advanced fibrosis (a mix of F2–F4) (20). The F2 stage appeared in both groups due to the high likelihood of misclassification from non-invasive staging methods (46–48). In scenario analyses, we considered clinical situations in which fibrosis stage is known and we evaluated cohorts of each fibrosis stage separately.
IL-28B Genotypes
Among study participants who were IL-28B genotyped (12), CT and TT types had similar treatment response rates; therefore, we combined them into a single category, ‘non-CC’. We used race-specific IL-28B genotype distributions: 37% CC-type in white and 14% CC-type in blacks. Additional studies have estimated IL-28B genotype distributions and provide similar estimates of CC prevalence (35–37%) (13, 14).
Treatment Effectiveness
Complete virologic response profiles for the duration of treatment stratified by race and IL-28B genotype are not available from clinical trials. For standard therapy effectiveness, we used data from the intention-to-treat IL-28B analysis of “The Individualized Dosing Efficacy vs. Flat Dosing to Assess Optimal Pegylated Interferon Therapy” (IDEAL) cohorts (4, 8, 12). For the effectiveness of treatments including the new protease inhibitors, we used data reported from the telaprevir “A New Direction in HCV Care: A Study of Treatment-Naive Hepatitis C Patients with Telaprevir” (ADVANCE) and boceprevir “Serine Protease Inhibitor Therapy 2” (SPRINT-2) Phase III clinical trials (5, 13, 14, 29). We inferred missing subgroup estimates stratified by race and IL-28B genotype, consistent with the SVRs reported (Appendix Table 1 and 2).
Treatment Adherence
In the base case, we assumed that treatment adherence was similar for standard therapy and triple therapy (i.e., approximately 70% patients were adherent, defined as taking ≥80% of both pegylated interferon and ribavirin (12)). Comparable adherence metrics were not reported in the protease inhibitor trials, though previous experience suggests that adherence in real-world settings can be lower than in clinical trials. Therefore, in sensitivity analyses, we reduced adherence for triple therapy from 70% to 50% for all patients on triple therapy.
Health Outcomes
Age-specific quality of life weights were derived from the Medical Expenditure Panel Survey (32, 33). Quality of life reductions associated with chronic HCV infection were estimated by combining several published studies (15, 34–37) (Appendix I). Utility decrements for standard therapy for one year were -0.110 (equivalent to a loss of 40 quality-adjusted days) (34) and were -0.165 for a year of triple therapy (equivalent to a loss of 60 quality-adjusted days). Decrements were scaled by the actual time on treatment, which can be shorter in response guided triple therapy (Table 2).
Costs
Age-specific baseline healthcare costs included patient out-of-pocket expenses (38). We also included additional fibrosis-stage-specific costs attributable to chronic HCV infection derived from HCV-related medical expenditures from the year following HCV diagnosis (43). Ongoing fibrosis-stage-specific patient costs were halved for those who achieved SVR post-treatment (40, 43), an assumption which we varied widely in sensitivity analyses (15, 40, 43–45) (Table 2).
Treatment costs include drugs and medical care (Table 2). We assumed patients received pegylated interferon alfa-2b 150 mcg once weekly ($584/week, PegIntronTM, Schering Corp.; and similarly $580/week, 180 mcg once weekly of pegylated interferon with ribavirin alfa 2a, Pegasys®, Roche), plus ribavirin 1,000 mg daily ($370.87/week, Rebetol®, Schering Corp.) (39, 49), converting average wholesale prices to best prices using a 0.64 conversion factor, consistent with the Congressional Budget Office estimates (50). The price for boceprevir (Victrelis, Merck) is $1,100 per week and for telaprevir (Incivek, Vertex) is $4,100 per week (7). Given the divergent treatment duration and costs of the two protease inhibitors, we assumed the cost for the short treatment course of a general protease inhibitor to be $26,400 applied during the first 28 weeks of treatment (24 weeks of boceprevir cost), and $35,200 (32 weeks of boceprevir cost) for the long treatment course. In scenario analyses, we increased the cost for a general protease inhibitor to be $49,200 during the first 12 weeks of treatment (12 weeks of telaprevir cost). Annual medical care costs related to HCV treatment were based on chronic HCV medical claims data, estimated to be $3,122 for F0-F3 patients, and $6,244 for F4 patients (40). We included additional costs from side-effects since triple therapy may be associated with differentially higher rates or severity of these events (42).
When necessary, costs were inflation adjusted to 2010 dollars using the U.S. Consumer Price Index (51).
Analysis
Outcomes included lifetime discounted costs, quality-adjusted life-years (QALYs) gained, and incremental cost-effectiveness ratios (ICERs) of the three treatment strategies. Results are presented as weighted averages over race and sex based on the distribution observed in patients with chronic HCV from National Health and Nutrition Examination Survey III data (51% white male, 23% white female, 17% black male, 9% black female).
We followed the recommendations of the U.S. Panel on Cost-Effectiveness, adopting a societal perspective, considering costs and benefits over a lifetime horizon, and discounting future costs and health benefits at 3% annually (19). Given the multiplicity of sources used, we performed deterministic sensitivity analyses for all variables and probabilistic sensitivity analyses to examine the impact of uncertainty on policy recommendations.
Role of the Funding Source
The funders of the project had no role in the design, conduct, or reporting of this analysis.
Results
Using new protease inhibitors as part of triple therapy for all patients chronically infected with genotype 1 HCV or targeted to those most likely to benefit via IL-28B genotyping improves health outcomes compared to current standard two-drug therapy (Table 3). For patients with advanced fibrosis, universal triple therapy increases the proportion achieving SVR to 51% compared to 31% SVR for standard therapy. This increase in SVR results in reductions in the lifetime risk of decompensated cirrhosis (from 23.2% to 16.5%), hepatocellular carcinoma (from 13.3% to 9.5%), and liver transplant (from 4.1% to 3.3%). For patients with mild fibrosis, universal triple therapy increases SVR from 37% to 60% and reduces lifetime risk of decompensated cirrhosis (from 8.5% to 5.2%), hepatocellular carcinoma (from 4.8% to 3.0%), and liver transplant (from 1.6% to 1.0%) compared to standard therapy. IL-28B guided triple therapy achieved SVR in 48% of patients with advanced fibrosis and 57% of patients with mild fibrosis. Reductions in lifetime decompensated cirrhosis and hepatocellular carcinoma obtained with IL-28B guided triple therapy were approximately 83% of those achieved with universal triple therapy.
Table 3.
Lifetimediscounted c ostsand health benefits of treatment strategies byseverity of fibrosis stage.* a. base case, b. telaprevir scenario
| a. Base case
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Strategy | Sustained Viral Response | Lifetime Risk
|
Cost ($) | QALYs | ICER ($ / QALY) | ICER& Excluding IL28B ($ / QALY) | ||
| Decompensated Cirrhosis | HCC | Liver Transplant | ||||||
| Mild Fibrosis** | ||||||||
| Standard therapy | 37% | 8.5% | 4.8% | 1.6% | 160,456 | 10.97 | - | - |
| IL-28B guided triple therapy | 57% | 5.8% | 3.3% | 1.1% | 177,152 | 11.24 | 62,900 | - |
| Universal triple therapy | 60% | 5.2% | 3.0% | 1.0% | 183,257 | 11.30 | 102,600 | 70,100 |
| Advanced Fibrosis** | ||||||||
| Standard therapy | 31% | 23.2% | 13.3% | 4.1% | 161,312 | 8.84 | - | - |
| IL-28B guided triple therapy | 48% | 17.7% | 10.1% | 3.6% | 179,090 | 9.38 | 32,800 | - |
| Universal triple therapy | 51% | 16.5% | 9.5% | 3.3% | 185,447 | 9.51 | 51,500 | 36,300 |
| b. Telaprevir scenario
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Mild Fibrosis** | Advanced Fibrosis** | |||||||
|
|
||||||||
| Strategy | Cost ($) | QALYs | ICER ($ / QALY) | ICER& Excluding IL28B ($ / QALY) | Cost ($) | QALYs | ICER ($ / QALY) | ICER& Excluding IL28B ($ / QALY) |
| Standard therapy | 160,456 | 10.97 | _ | 161,312 | 8.84 | _ | ||
| IL-28B guided triple therapy | 191,559 | 11.33 | 86,800 | _ | 193,805 | 9.56 | 45,300 | _ |
| Universal triple therapy | 203,285 | 11.44 | 102,400 | 91,000 | 206,010 | 9.78 | 54,100 | 47,400 |
HCC – hepatocellular carcinoma; QALY – Quality adjusted life year; ICER – Incremental cost effectiveness ratio
Results are weighted averages over race and sex based on relative prevalence of these groups for patients with chronic HCV from NHANES III data (51% white male, 23% white female, 17% black male, 9% black female).
Mild fibrosis describes a population of patients with 30% F0, 41% F1, and 29% F2; Advanced fibrosis describes a population of patients with 29% F2, 23% F3, and 48% F4.
If IL-28B genotyping is unavailable, ICER compares universal triple therapy to standard therapy.
For patients with advanced fibrosis, IL-28 guided triple therapy and universal triple therapy increase discounted quality-adjusted life expectancy by 0.54 and 0.67 years, respectively, compared to standard therapy. For patients with mild fibrosis, the increases are 0.27 and 0.33 years.
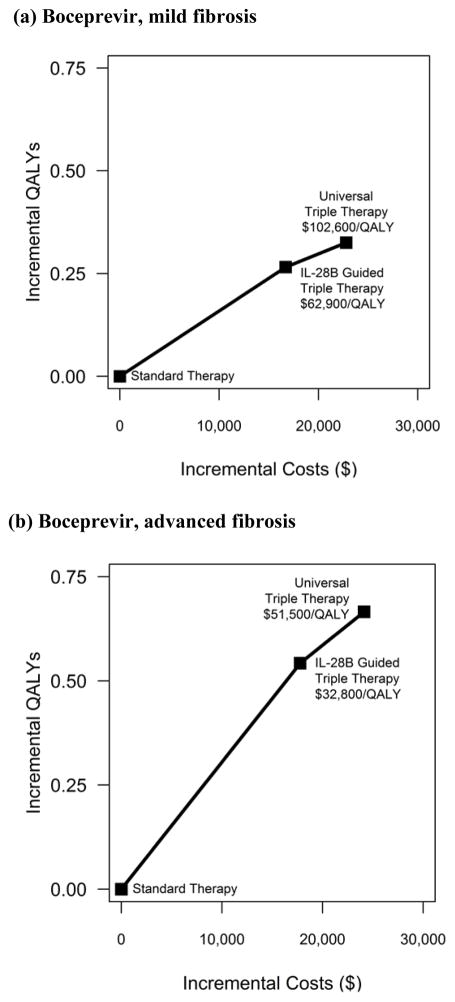
If the protease inhibitor costs $1,100 per week, universal triple therapy improves outcomes but also substantially increases total costs ($24,135 for advanced fibrosis and $22,801 for mild fibrosis compared to standard therapy). Compared to IL-28B guided triple therapy, universal triple therapy costs $51,500/QALY for patients with advanced fibrosis and $102,600/QALY for patients with mild fibrosis (Figure 2a, b). The more favorable costeffectiveness results for patients with advanced fibrosis are due largely to the greater health gains achieved by universal triple therapy for patients at roughly equivalent increases in costs.
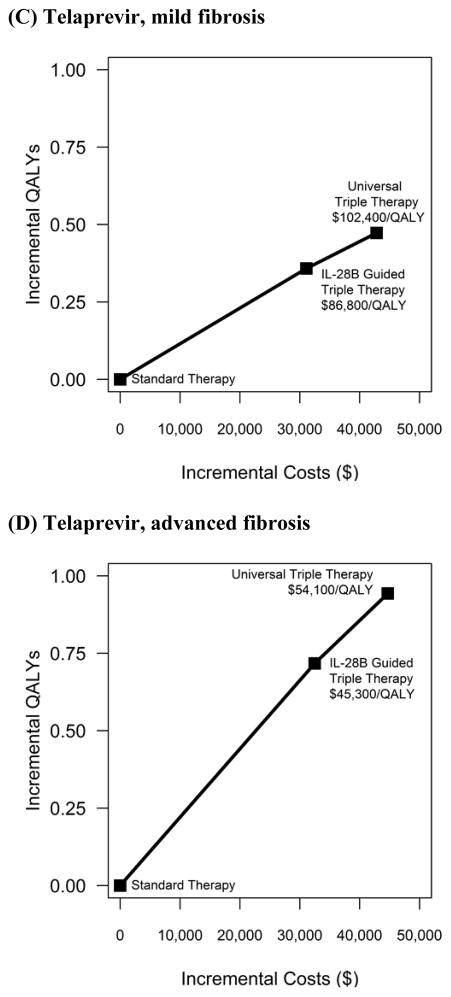
Figure 2. Cost-effectiveness Results: incremental costs incurred and quality-adjusted lifeyears (QALYs) experienced for each intervention (a) Boceprevir, mild fibrosis (b) Boceprevir, advanced fibrosis (c) Telaprevir, mild fibrosis (d) Telaprevir, advanced fibrosis.
The graph plots the incremental discounted quality adjusted life year (y-axis) and incremental discounted total expected lifetime costs (x-axis) for each treatment strategy separately for cohorts of patients with mild and advanced fibrosis. The solid lines represent the cost-effectiveness efficient frontier, those strategies that are potentially cost-effective depending on one’s willingness to pay per unit of health benefit gained, expressed as an incremental costeffectiveness ratio (ICER) (defined as the ratio of the additional costs of an intervention and its additional effects as compared to the next best alternative).
In situations where IL-28B guided therapy is unavailable, universal triple therapy costs $36,300/QALY for patients with advanced fibrosis and $70,100/QALY for patients with mild fibrosis compared to standard therapy (Table 3).
Chronic HCV is a slowly progressing disease that can take 30 years to cause end-stage liver disease. Meanwhile, patients may die from non-liver related causes. Therefore, initial fibrosis stage affects the cost-effectiveness of treatment strategies. We considered clinical scenarios in which a patient’s fibrosis stage is known (Appendix Figure 5). In general, universal triple therapy provides relatively more benefit per dollar spent for patients with more advanced liver fibrosis than those with less advanced fibrosis (ICERs below $50,000/QALY for those with F4 fibrosis rising to >$150,000/QALY for those with F0 fibrosis).
Treatment Costs
The treatment cost of the two protease inhibitors is very different, and in our base case analysis, the cost considered was that of boceprevir ($1,100 per week for 24–32 weeks). If the price of the protease inhibitor were higher, equivalent to telaprevir ($4,100 per week for 12 weeks), and the effectiveness was that of telaprevir reported in the ADVANCE trial, then the ICERs of universal triple therapy to IL-28B guided triple therapy are $54,100/QALY for patients with advanced fibrosis and $102,400/QALY for patients with mild fibrosis, very similar to the results for a protease inhibitor like boceprevir (Figure 2c, d). In situations where IL-28B guided therapy is unavailable, universal triple therapy costs $47,400/QALY for patients with advanced fibrosis and $91,000/QALY for patients with mild fibrosis compared to standard therapy (Table 3).
Comparing the two drugs to each other, telaprevir’s effectiveness needs to be substantially greater than that observed in the ADVANCE trial to yield an ICER below $50,000/QALY compared to universal triple therapy with boceprevir (Appendix Figure 6), though differences including patterns of side effects and uncertainties about adherence and effectiveness make a definitive comparison difficult.
Prices for brand-name drugs vary significantly between purchasing institutions. For example, the Veteran Affairs Health System may purchase drugs at the Federal Supply Schedule price. In their case, the ICERs for universal triple therapy compared to IL-28B guided triple therapy are $41,100/QALY gained for advanced fibrosis and $81,300/QALY gained for mild fibrosis, though specific estimates depend on the price levels and relative prices of standard therapy and new protease inhibitors (Appendix Table 5).
Adverse Events from Therapy
Side effects from triple therapy are more frequent and potentially more severe than those of standard therapy which include anemia, depression, rash and flu-like symptoms. Higher rates and severity of side effects may undermine the cost-effectiveness of universal triple therapy. We performed a threshold analysis to determine how severe the side effect profile would need to be for the incremental cost effectiveness ratio to exceed $100,000/QALY for those with advanced fibrosis (Appendix Table 6), finding that it did not exceed this threshold even when the costs of side effects were tripled ($7,500) and the disutility of side effects were doubled (equivalent to a disutility of −0.36 QALYs/year on triple therapy).
Adherence
In our base case analysis we assumed equal adherence for standard therapy and triple therapy (70% of patients taking ≥80% of their HCV medications). In a threshold analysis, we identified that if adherence for standard therapy remained 70% but for triple therapy was as low as 50% of patients taking ≥80% of their HCV medications, then universal triple therapy is more costly but achieves no additional benefit compared to IL-28B guided triple therapy and was consequently not cost-effective for those with either mild or advanced fibrosis (Appendix Table 7). However, even with 50% of patients adherent to triple therapy, universal triple therapy for those with advanced fibrosis cost $73,200/QALY compared to standard therapy with 70% adherence. For mild fibrosis, universal triple therapy in this scenario cost >$150,000/QALY compared to standard therapy.
Risk of Developing Hepatocellular Carcinoma
There is limited evidence suggesting that there is a risk of developing hepatocellular carcinoma from more advanced fibrosis stages even after achieving SVR post-treatment. To explore this, we conducted a sensitivity analysis in which we assumed that those with F4 fibrosis who were successfully treated had an annual rate of developing hepatocellular carcinoma that was 20% of that for F4 prior to treatment. The results did not alter the main conclusions (Appendix Table 8).
Other Sensitivity Analyses
Additional sensitivity analyses considering non-liver related mortality can be found in Appendix Table 9; retreatment can be found in Appendix Table 10; and subgroup analyses by race, sex, age, IL-28B genotype, and fibrosis can be found in Appendix Table 11.
Probabilistic Sensitivity Analyses
In probabilistic sensitivity analyses, we used the boceprevir cost (Appendix Figure 7). For patients with advanced fibrosis, using either universal triple therapy or IL-28B guided triple therapy was optimal 98% of the time at a willingness-to-pay threshold of $50,000/QALY and 100% at $100,000/QALY. For patients with mild fibrosis, using either universal triple therapy or IL-28B guided triple therapy was optimal 18% of time at $50,000/QALY and 95% at $100,000/QALY. Conversely, at a $50,000/QALY threshold, standard therapy was optimal 82% of the time for mild fibrosis but only 2% of the time for advanced fibrosis.
Discussion
For treatment-naïve patients with chronic genotype 1 HCV mono-infections, universal triple therapy yields greater health benefits than both standard therapy and IL-28B guided triple therapy. Although it also increases total costs, universal triple therapy provides reasonable value for money, costing approximately $50,000/QALY compared to IL-28B guided triple therapy for patients with advanced fibrosis, when it is available for $1,100 per week. Universal triple therapy becomes less cost-effective when the cost of the protease inhibitors is higher, or when adherence rates are substantially lower for triple therapy compared with standard therapy. For patients with mild fibrosis, universal triple therapy at a cost of $1,100 per week is not cost-effective even at $100,000/QALY, but IL-28B guided triple therapy costs $62,900/QALY compared to standard therapy.
Due to the high cost and risk of side-effects of protease inhibitors, some health systems may consider reserving them for second-line rather than a first-line treatment, especially for patients with milder fibrosis. However, for patients with advanced fibrosis, in our main analyses, we find universal, first-line triple therapy reaches conventional levels of cost-effectiveness. We explored the cost-effectiveness of treating all patients with standard therapy and reserving protease inhibitors for second-line treatment and found that this strategy is less effective and more costly than other strategies that employ triple therapy as a first-line for at least some patients (Appendix II).
Despite the highly anticipated benefits of the new protease inhibitors, there is interest within the medical community in tailoring HCV treatment to specific patient characteristics. We find IL-28B genotype may be useful for such stratification, and our findings are consistent with the recommendations on IL-28B genotyping in the new American Association for the Study of Liver Diseases treatment guidelines (17).
Our main analysis did not directly compare boceprevir to telaprevir. We considered a general protease inhibitor in comparison to standard two-drug therapy and IL-28B guided triple therapy. We varied the general protease inhibitor’s costs, treatment algorithms, and effectiveness to be similar to either boceprevir or telaprevir. It is important to note that key differences, especially the difference in cost, are relevant for systems considering whether to include one or both new drugs in their formularies. The per-week cost of telaprevir is four-times greater than that of boceprevir, and depending on treatment duration, the total drug cost for a complete course of telaprevir can be 140% to 190% of boceprevir. The effectiveness of telaprevir appears higher than boceprevir in separate clinical trials (SVR of 75% vs. 68%), although the studies’ populations may not be comparable as there were also differences in the observed effectiveness in the respective placebo arms. The difference between the two drugs has not been evaluated in a head-to-head comparison. The types and rates of side-effects are different between the two drugs, but the overall costs from side-effects appear similar. Modeled as a general protease inhibitor, we found universal triple therapy to be cost-effective compared to standard therapy for those with advanced fibrosis (costing approximately $50,000/QALY or less). However, when compared directly to each other, the increase in telaprevir’s effectiveness relative to boceprevir needed to achieve conventional levels of cost-effectiveness may well be substantially higher than that observed in the ADVANCE trial (6). Clinical trials comparing the two drugs directly are clearly needed to address comparative effectiveness. A model to evaluate the cost-effectiveness of these drugs head-to-head would need to include more detailed modeling of adverse events which would in turn requires substantially more information about treatment patterns in real-world clinical practice.
Since the protease inhibitors we evaluated were only recently approved by the Food and Drug Administration, the feasibility of implementation of response guided therapy recommendations in routine practice, the treatment effectiveness and adherence to treatment are unknown. Adherence is especially important to treatment effectiveness and cost-effectiveness. If treatment adherence to triple therapy is lower than that of standard therapy, IL-28B guided therapy may be the optimal strategy.
While the main analysis focuses on initial treatment of chronic HCV infections, important questions about retreating those who fail initial treatment remain. Our analysis does not primarily address this topic as data and studies of effectiveness in this setting are forthcoming. We explored this issue finding that IL-28B guided triple therapy with retreatment for patients who fail standard therapy is cost-effective in the advanced fibrosis group (Appendix II). However, given the lack of evidence, we did not consider strategies involving retreatment with triple therapy after initial failure from triple therapy. Clinicians will clearly require a strategy for managing patients who fail triple therapy, especially if triple therapy failures are more likely the result of poor adherence or toxicities. Importantly, HCV viral resistance to protease inhibitors can also alter the effectiveness in retreatment, though data on this are still emerging.
Our analysis has several limitations. IL-28B genotyping is a relatively new approach to identifying patients’ likely response to standard HCV therapy. Additional studies will provide further confidence of IL-28B’s predictive value. We did not include reductions in HCV transmission due to successful treatment and thus may underestimate benefits associated with improving SVR rates such as those from triple therapy. A substantial minority of patients infected with chronic HCV is also co-infected with hepatitis B virus and/or HIV. Our results are limited to mono-infected individuals and should only be interpreted in the context of this population because studies evaluating the effectiveness of new protease inhibitors in HIV coinfected persons are on-going. Additionally, challenges with standard HCV/HIV drug interactions are already known, and HCV protease inhibitor interactions are emerging (52, 53). Future analyses should address the complex management issues surrounding treatment decisions for those with co-infections.
New HCV protease inhibitors show great promise in increasing treatment effectiveness for HCV genotype 1 infected patients, even though their additional benefits come with increased side effects and treatment costs. Our study supports the important role of protease inhibitors in treating chronic HCV for patients with advanced fibrosis as part of a first-line regimen. Management of chronic HCV in the U.S. could be improved by a shift towards triple drug strategies that are response guided, provided that the price of protease inhibitors and adherence to taking them are maintained at reasonable levels.
Supplementary Material
Acknowledgments
The authors thank Dr. Paul Barnett and Dr. Steven Asch for extensive and helpful comments on the manuscript.
Financial Support: Ms. Liu wassupported by a St anford Graduate Fellowship. Ms. Cipriano was supported by the Social Science and Humanities Research Council of Canada. Dr. Goldhaber-Fiebertwas supported in part by an NIH NIA Career Development Award (K01 AG037593-01A1: PI: Goldhaber-Fiebert). Drs. Holodniy and Owens are supported by the Department of Veterans Affairs, and supported in part by R01 DA15612-016.
Footnotes
Author Contributions:
Conception and design: S. Liu, L.E. Cipriano, M. Holodniy, D.K. Owens, J.D. Goldhaber-Fiebert.
Analysis and interpretation of the data: S. Liu, L.E. Cipriano, M. Holodniy, D.K. Owens, J.D. Goldhaber-Fiebert.
Drafting of the article: S. Liu, L.E. Cipriano, J.D. Goldhaber-Fiebert.
Critical revision of the article for important intellectual content: S. Liu, L.E. Cipriano, M. Holodniy, D.K. Owens, J.D. Goldhaber-Fiebert.
Final approval of the article: S. Liu, L.E. Cipriano, M. Holodniy, D.K. Owens, J.D. Goldhaber-Fiebert.
Collection and assembly of data: S. Liu, L.E. Cipriano, M. Holodniy
Protocol: Not applicable
Statistical Code: For further information and availability, please contact authors.
Data: For further information and availability, please contact authors.
Potential Financial Conflicts of Interest: Noneto report
Contributor Information
Shan Liu, Email: shanliu@stanford.edu.
Lauren E. Cipriano, Email: cipriano@stanford.edu.
Mark Holodniy, Email: mark.holodniy@va.gov.
Douglas K. Owens, Email: owens@stanford.edu.
Jeremy D. Goldhaber-Fiebert, Email: jeremygf@stanford.edu.
References
- 1.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, Management, and Treatment of Hepatitis C: An Update. Hepatology. 2009;49(4):1335–74. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Annals of Internal Medicine. 2006;144(10):705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 3.Nainan OV, Alter MJ, Kruszon-Moran D, Gao FX, Xia G, McQuillan G, et al. Hepatitis C virus genotypes and viral concentrations in participants of a general population survey in the United States. Gastroenterology. 2006;131(2):478–84. doi: 10.1053/j.gastro.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 4.McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, et al. Peginterferon Alfa-2b or Alfa-2a with Ribavirin for Treatment of Hepatitis C Infection. New England Journal of Medicine. 2009;361(10):580. 1027. doi: 10.1056/NEJMoa0808010. [DOI] [PubMed] [Google Scholar]
- 5.Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for Untreated Chronic HCV Genotype 1 Infection. New England Journal of Medicine. 2011;364(13):1195–206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 364(25):2405–16. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 7.Pollack A. The New York Times. 2011. Second Drug Wins Approval for Treatment of Hepatitis C. [Google Scholar]
- 8.Ge DL, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461(7262):399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nature Genetics. 2009;41(10):1105–U81. doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- 10.Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nature Genetics. 2009;41(10):1100–U74. doi: 10.1038/ng.447. [DOI] [PubMed] [Google Scholar]
- 11.Rauch A, Kutalik Z, Descombes P, Cai T, Di Iulio J, Mueller T, et al. Genetic Variation in IL28B Is Associated With Chronic Hepatitis C and Treatment Failure: A Genome- Wide Association Study. Gastroenterology. 2010;138(4):1338–U173. doi: 10.1053/j.gastro.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 12.Thompson AJ, Muir AJ, Sulkowski MS, Ge D, Fellay J, Shianna KV, et al. Interleukin- 28B Polymorphism Improves Viral Kinetics and Is the Strongest Pretreatment Predictor of Sustained Virologic Response in Genotype 1 Hepatitis C Virus. Gastroenterology. 2010;139(1):120–U78. doi: 10.1053/j.gastro.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Merck & Co Inc. FDA Antiviral Drugs Advisory Committee Meeting Boceprevir Capsules (NDA 202–258, March 29 2011) Briefing Document. Silver Spring: Food and Drug Administration; [3 November 2011]. Accessed at http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AntiviralDrugsAdvisoryCommittee/UCM252343.pdf. [Google Scholar]
- 14.Vertex Pharmaceuticals. Telaprevir NDA 201–917. Silver Spring: Food and Drug Administration; Apr 28, 2011. [3 November 2011]. Telaprevir 375-mg Film-Coated Tablet for the Treatment of Genotype 1 Chronic Hepatitis C, Antiviral Drugs Advisory Committee Briefing Document. Accessed at http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AntiviralDrugsAdvisoryCommittee/UCM252562.pdf. [Google Scholar]
- 15.Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Cost-effectiveness of treatment for chronic hepatitis C infection in an evolving patient population. Jama-Journal of the American Medical Association. 2003;290(2):228–37. doi: 10.1001/jama.290.2.228. [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Schwarzinger M, Carrat F, Goldhaber-Fiebert JD. Cost-Effective Alternatives to Liver Biopsy in the Management of Chronic Hepatitis C, Poster. Society for Medical Decision Making, 2010 Annual Meeting; October 24, 2010; Toronto, Ontario, Canada. SMDM; 2010. [Google Scholar]
- 17.Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54(4):1433–44. doi: 10.1002/hep.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craxi A. EASL Clinical Practice Guidelines: Management of hepatitis C virus infection. J Hepatol. 2011 doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Gold MR. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 20.Siddiqui FA, Ehrinpreis MN, Janisse J, Dhar R, May E, Mutchnick MG. Demographics of a large cohort of urban chronic hepatitis C patients. Hepatology International. 2008;2(3):376–81. doi: 10.1007/s12072-008-9086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Empirically calibrated model of hepatitis C virus infection in the United States. American Journal of Epidemiology. 2002;156(8):761–73. doi: 10.1093/aje/kwf100. [DOI] [PubMed] [Google Scholar]
- 22.Hutton DW, Tan D, So SK, Brandeau ML. Cost-effectiveness of screening and vaccinating Asian and pacific islander adults for hepatitis B. Annals of Internal Medicine. 2007;147(7):460–9. doi: 10.7326/0003-4819-147-7-200710020-00004. [DOI] [PubMed] [Google Scholar]
- 23.Arias E. National Vital Statistics Reports, United States Life Tables. Center for Disease Control, Division of Vital Statistics; 2006. [PubMed] [Google Scholar]
- 24. [1 June 2011];United Network for Organ Sharing. Accessed at http://www.unos.org/ on.
- 25.SEER. [1 June 2011];SEER Cancer Stat Fact Sheets. Accessed at http://seer.cancer.gov/statfacts/ on.
- 26.Fattovich G, Giustina G, Favarato S, Ruol A, Macarri G, Orlandi F, et al. A survey of adverse events in 11241 patients with chronic viral hepatitis treated with alfa interferon. Journal of Hepatology. 1996;24(1):38–47. doi: 10.1016/s0168-8278(96)80184-x. [DOI] [PubMed] [Google Scholar]
- 27.Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, Albrecht J. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology. 2003;38(3):645–52. doi: 10.1053/jhep.2003.50364. [DOI] [PubMed] [Google Scholar]
- 28.Birnkrant D. Advisory Committee Briefing Document for NDA 201-917 Telaprevir 375 mg tablets. Silver Spring: Department of Health & Human Services, Public Health Service, Food and Drug Administration; [1 June 2011]. Apr 1, 2011. Accessed at http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AntiviralDrugsAdvisoryCommittee/UCM252561.pdf. [Google Scholar]
- 29.Levin J. Telaprevir in Combination with Peginterferon alfa-2a and Ribavirin in Genotype 1 HCV Treatment-Naïve patients: Final results of Phase 3 ADVANCE Study. 61th Annual Meeting of the American Association for the Study of Liver Diseases; 2010; Boston, MA. Hynes Convention Center. NATAP; [Google Scholar]
- 30.Background Materials for Boceprevir Advisory Committee Division of Antiviral Products (DAVP) Silver Spring: Food and Drug Administration; [1 June 2011]. Apr 27, 2011. Accessed at http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/drugs/AntiviralDrugsAdvisoryCommittee/ucm252341.pdf. [Google Scholar]
- 31.Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, et al. Telaprevir for Retreatment of HCV Infection. New England Journal of Medicine. 2011;364(25):2417– 28. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 32.Nyman JA, Barleen NA, Dowd BE, Russell DW, Coons SJ, Sullivan PW. Quality-of-life weights for the US population - Self-reported health status and priority health conditions, by demographic characteristics. Medical Care. 2007;45(7):618–28. doi: 10.1097/MLR.0b013e31803dce05. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Medical Decision Making. 2006;26(4):410–20. doi: 10.1177/0272989X06290495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grieve R, Roberts J, Wright M, Sweeting M, DeAngelis D, Rosenberg W, et al. Cost effectiveness of interferon or peginterferon with ribavirin for histologically mild chronic hepatitis C. Gut. 2006;55(9):1332–8. doi: 10.1136/gut.2005.064774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherman KE, Sherman SN, Chenier T, Tsevat J. Health values of patients with chronic hepatitis C infection. Archives of Internal Medicine. 2004;164(21):2377–82. doi: 10.1001/archinte.164.21.2377. [DOI] [PubMed] [Google Scholar]
- 36.Chong CAKY, Gulamhussein A, Heathcote EJ, Lilly L, Sherman M, Naglie G, et al. Health-state utilities and quality of life in hepatitis C patients. American Journal of Gastroenterology. 2003;98(3):630–8. doi: 10.1111/j.1572-0241.2003.07332.x. [DOI] [PubMed] [Google Scholar]
- 37.McLernon DJ, Dillon J, Donnan PT. Health-state utilities in liver disease: A systematic review. Medical Decision Making. 2008;28(4):582–92. doi: 10.1177/0272989X08315240. [DOI] [PubMed] [Google Scholar]
- 38.Meara E, White C, Cutler DM. Trends in medical spending by age, 1963–2000. Health Affairs. 2004;23(4):176–83. doi: 10.1377/hlthaff.23.4.176. [DOI] [PubMed] [Google Scholar]
- 39.Thomson Corporation. Red book : pharmacy's fundamental reference. Montvale, NJ: Thomson PDR; 2009. p. v. [Google Scholar]
- 40.Mitra D, Davis KL, Beam C, Medjedovic J, Rustgi V. Treatment Patterns and Adherence among Patients with Chronic Hepatitis C Virus in a US Managed Care Population. Value in Health. 2010 doi: 10.1111/j.1524-4733.2009.00691.x. [DOI] [PubMed] [Google Scholar]
- 41.Federal Supply Schedule, Drug Pharmaceutical Prices. United States Department of Veterans Affairs; 2011. http://www.pbm.va.gov/DrugPharmaceuticalPrices.aspx. [Google Scholar]
- 42.Stephens CJM, Carter J, Gao X, Haider S, Rustgi VK. Adverse Event-Related Treatment Costs Associated with Protease Inhibitor-Based Combination Therapy for Hepatitis C. The Liver Meeting 2010 (AASLD); September 6, 2010; AASLD ePoster; 2010. http://trs.scivee.tv/node/4033?destination=node%2F4033. [Google Scholar]
- 43.Poret AW, Ozminkowski R, Goetzel R, Pew J, Balent J. Cost Burden of Illness for Hepatitis C Patients with Employer-Sponsored Health Insurance. Disease Management. 2002;5(2):95–107. [Google Scholar]
- 44.Armstrong EP, Charland SL. Burden of illness of hepatitis C from a managed care organization perspective. Current Medical Research and Opinion. 2004;20(5):671–9. doi: 10.1185/030079904125003485. [DOI] [PubMed] [Google Scholar]
- 45.Bennett WG, Inoue Y, Beck JR, Wong JB, Pauker SG, Davis GL. Estimates of the costeffectiveness of a single course of interferon-alpha 2b in patients with histologically mild chronic hepatitis C. Annals of Internal Medicine. 1997;127(10):855. doi: 10.7326/0003-4819-127-10-199711150-00001. [DOI] [PubMed] [Google Scholar]
- 46.Bedossa P, Carrat F. Liver biopsy: The best, not the gold standard. Journal of Hepatology. 2009;50(1):1–3. doi: 10.1016/j.jhep.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 47.Shaheen AAM, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: A systematic review of diagnostic test accuracy. American Journal of Gastroenterology. 2007;102(11):2589–600. doi: 10.1111/j.1572-0241.2007.01466.x. [DOI] [PubMed] [Google Scholar]
- 48.Rockey DC, Bissell DM. Noninvasive measures of liver fibrosis. Hepatology. 2006;43(2):S113–S20. doi: 10.1002/hep.21046. [DOI] [PubMed] [Google Scholar]
- 49.AHFS. Drug Information 2009. 1. Bethesda, MD: American Society of Health-System Pharmacists; 2009. [Google Scholar]
- 50.Holtz-Eakin D. [Accessed on 1 September, 2011];A CBO Paper on Prices for Brand-Name Drugs Under Selected Federal Programs. 2005 at http://www.cbo.gov/ftpdocs/64xx/doc6481/06-16-PrescriptDrug.pdf.
- 51.US Department Of Labor Bureau of Labor Statistics. [3 November 2011];Consumer Price Index. Accessed at ftp://ftp.bls.gov/pub/special.requests/cpi/cpiai.txt on.
- 52.Kasserra C, Hughes E, Treitel M, et al. Clinical Pharmacology of BOC: Metabolism, Excretion, and Drug-Drug Interactions. 18th Conference on Retroviruses and Opportunistic Infections (CROI 2011); Boston. February 27-March 2, 2011; p. Abstract 118. [Google Scholar]
- 53.Heeswijk RV, Vandevoorde A, Boogaerts G, et al. Pharmacokinetic Interactions between ARV Agents and the Investigational HCV Protease Inhibitor TVR in Healthy Volunteers. 18th Conference on Retroviruses and Opportunistic Infections (CROI 2011); Boston. February 27-March 2, 2011; p. Abstract 119. [Google Scholar]
- 54.El-Kamary SS, Jhaveri R, Shardell MD. All-Cause, Liver-Related, and Non-Liver- Related Mortality Among HCV-Infected Individuals in the General US Population. Clin Infect Dis. 2011;53(2):150–7. doi: 10.1093/cid/cir306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9(6):509–16. e1. doi: 10.1016/j.cgh.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 56.Jensen DM, Marcellin P, Freilich B, Andreone P, Di Bisceglie A, Brandao-Mello CE, et al. Re-treatment of patients with chronic hepatitis C who do not respond to peginterferon-alpha2b: a randomized trial. Ann Intern Med. 2009;150(8):528–40. doi: 10.7326/0003-4819-150-8-200904210-00007. [DOI] [PubMed] [Google Scholar]
- 57.Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 364(13):1207–17. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barton GR, Briggs AH, Fenwick EA. Optimal cost-effectiveness decisions: the role of the cost-effectiveness acceptability curve (CEAC), the cost-effectiveness acceptability frontier (CEAF), and the expected value of perfection information (EVPI) Value Health. 2008;11(5):886–97. doi: 10.1111/j.1524-4733.2008.00358.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.