Abstract
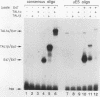
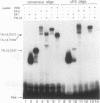
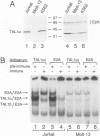
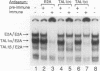
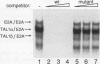
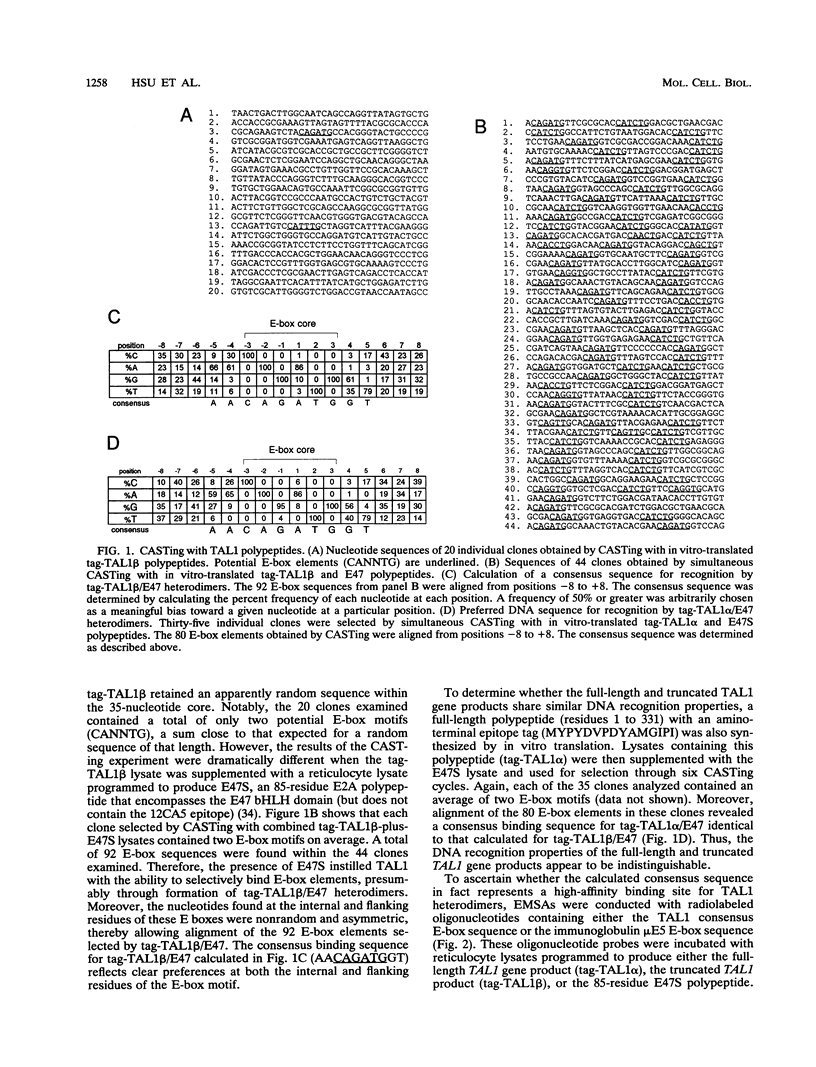
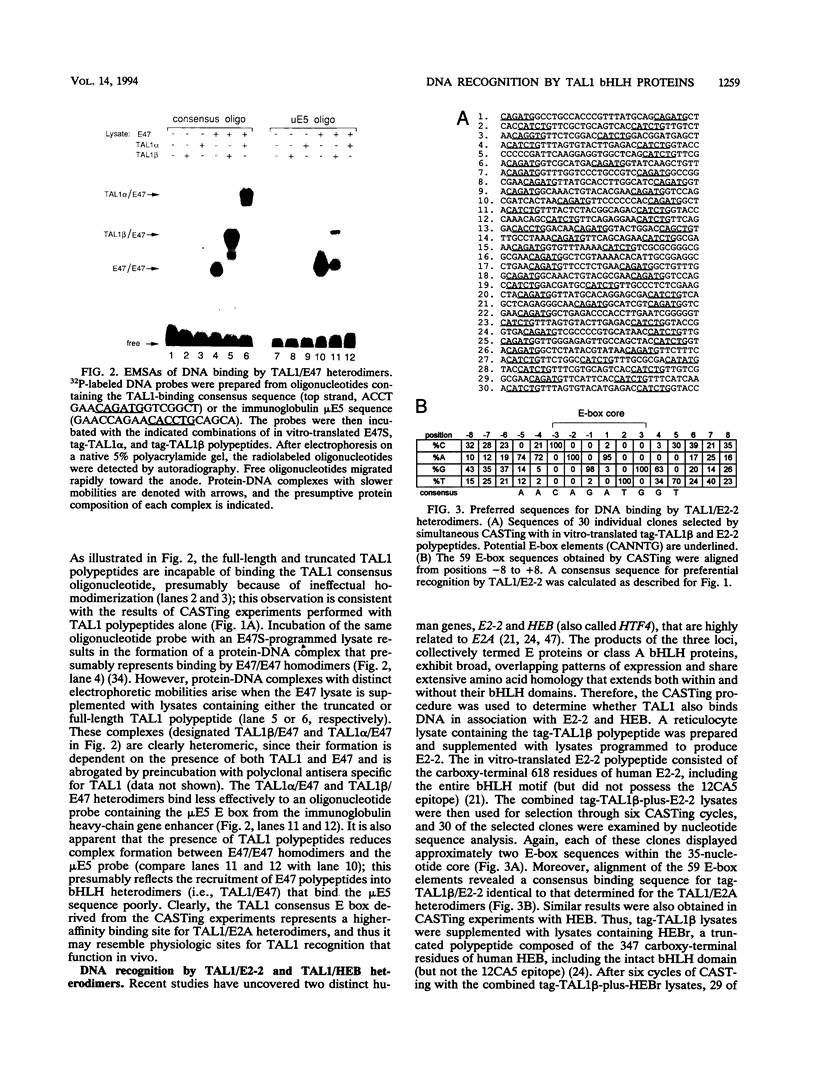
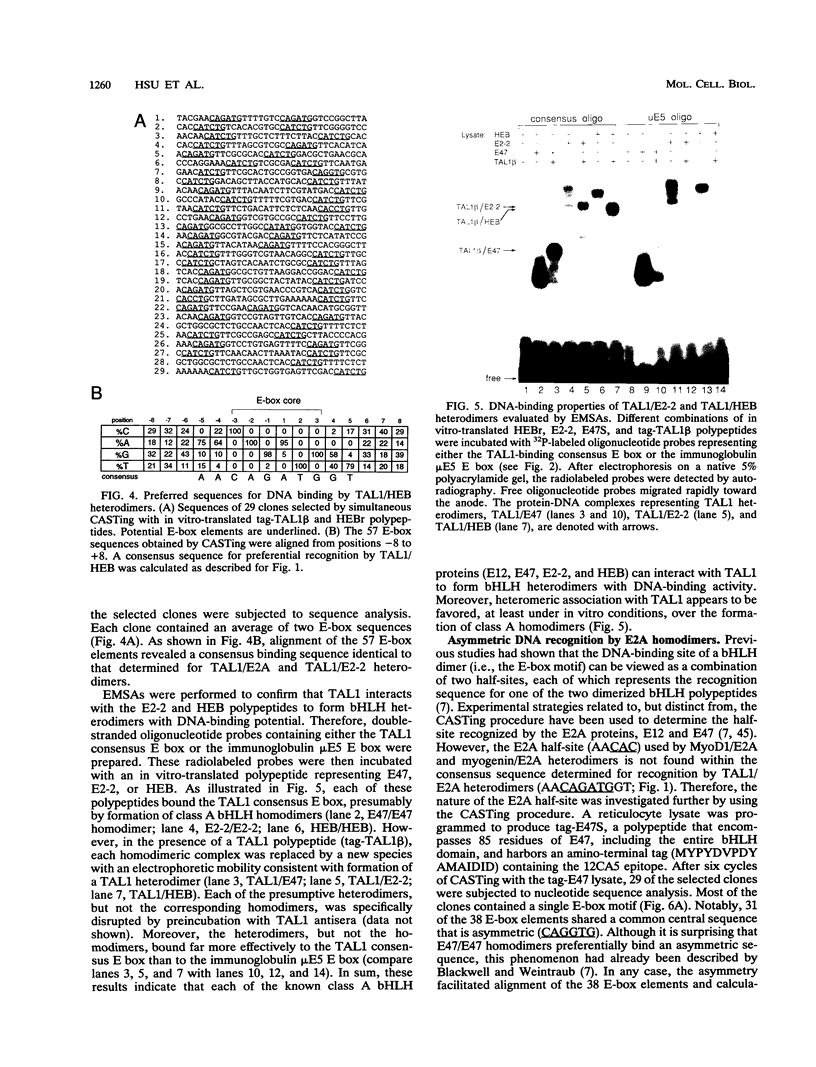
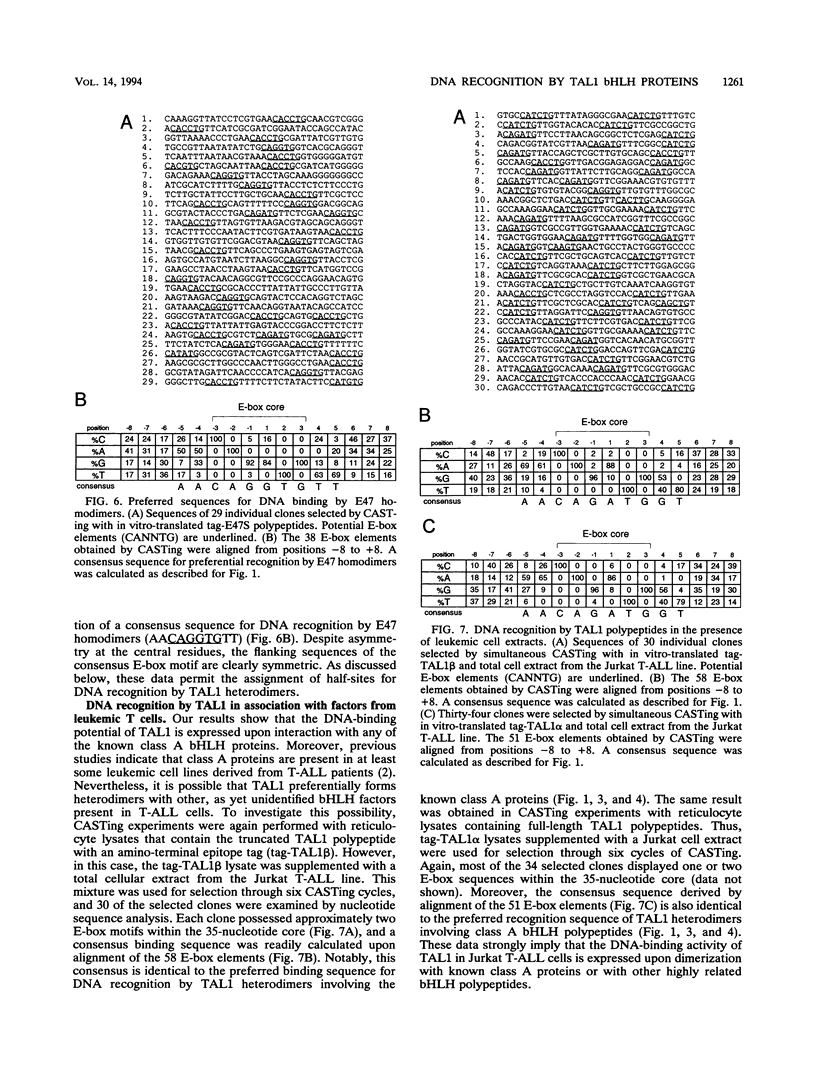
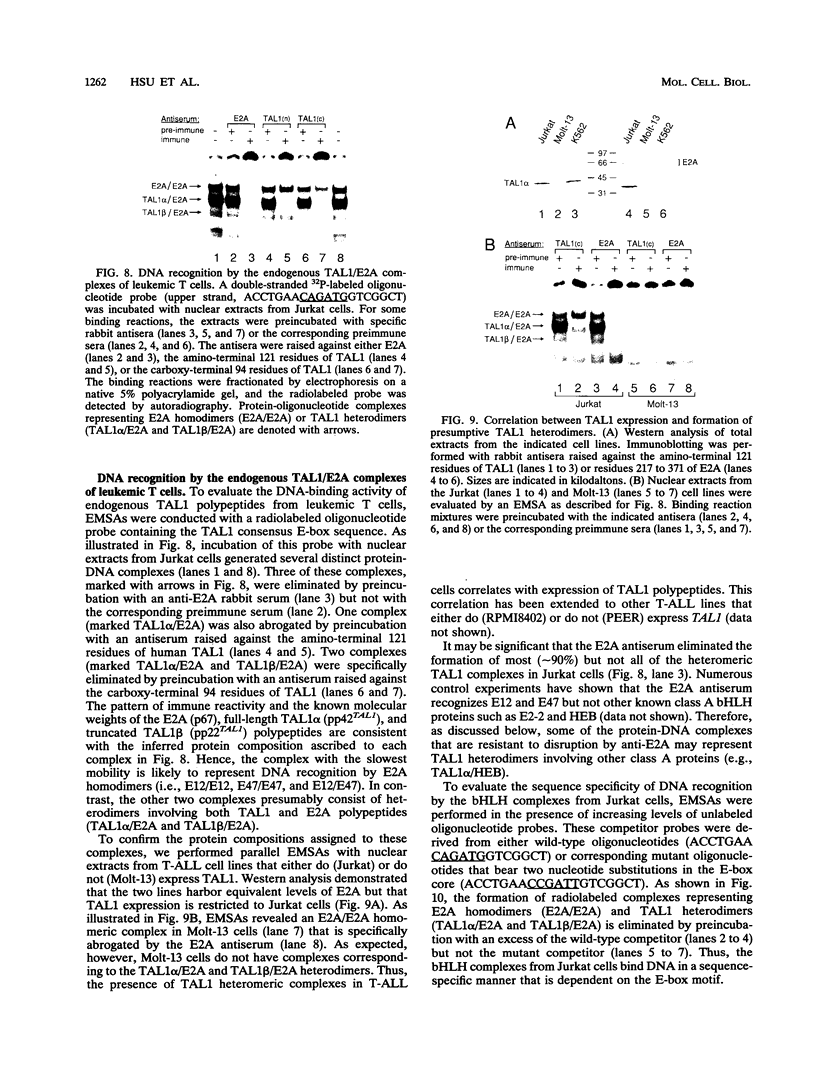
Tumor-specific activation of the TAL1 gene is the most common genetic alteration seen in patients with T-cell acute lymphoblastic leukemia. The TAL1 gene products contain the basic helix-loop-helix (bHLH) domain, a protein dimerization and DNA-binding motif common to several known transcription factors. A binding-site selection procedure has now been used to evaluate the DNA recognition properties of TAL1. These studies demonstrate that TAL1 polypeptides do not have intrinsic DNA-binding activity, presumably because of their inability to form bHLH homodimers. However, TAL1 readily interacts with any of the known class A bHLH proteins (E12, E47, E2-2, and HEB) to form heterodimers that bind DNA in a sequence-specific manner. The TAL1 heterodimers preferentially recognize a subset of E-box elements (CANNTG) that can be represented by the consensus sequence AACAGATGGT. This consensus is composed of half-sites for recognition by the participating class A bHLH polypeptide (AACAG) and the TAL1 polypeptide (ATGGT). TAL1 heterodimers with DNA-binding activity are readily detected in nuclear extracts of Jurkat, a leukemic cell line derived from a patient with T-cell acute lymphoblastic leukemia. Hence, TAL1 is likely to bind and regulate the transcription of a unique subset of subordinate target genes, some of which may mediate the malignant function of TAL1 during T-cell leukemogenesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aplan P. D., Lombardi D. P., Ginsberg A. M., Cossman J., Bertness V. L., Kirsch I. R. Disruption of the human SCL locus by "illegitimate" V-(D)-J recombinase activity. Science. 1990 Dec 7;250(4986):1426–1429. doi: 10.1126/science.2255914. [DOI] [PubMed] [Google Scholar]
- Bain G., Gruenwald S., Murre C. E2A and E2-2 are subunits of B-cell-specific E2-box DNA-binding proteins. Mol Cell Biol. 1993 Jun;13(6):3522–3529. doi: 10.1128/mcb.13.6.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bash R. O., Crist W. M., Shuster J. J., Link M. P., Amylon M., Pullen J., Carroll A. J., Buchanan G. R., Smith R. G., Baer R. Clinical features and outcome of T-cell acute lymphoblastic leukemia in childhood with respect to alterations at the TAL1 locus: a Pediatric Oncology Group study. Blood. 1993 Apr 15;81(8):2110–2117. [PubMed] [Google Scholar]
- Begley C. G., Aplan P. D., Denning S. M., Haynes B. F., Waldmann T. A., Kirsch I. R. The gene SCL is expressed during early hematopoiesis and encodes a differentiation-related DNA-binding motif. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10128–10132. doi: 10.1073/pnas.86.24.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard O., Guglielmi P., Jonveaux P., Cherif D., Gisselbrecht S., Mauchauffe M., Berger R., Larsen C. J., Mathieu-Mahul D. Two distinct mechanisms for the SCL gene activation in the t(1;14) translocation of T-cell leukemias. Genes Chromosomes Cancer. 1990 Jan;1(3):194–208. doi: 10.1002/gcc.2870010303. [DOI] [PubMed] [Google Scholar]
- Bernard O., Lecointe N., Jonveaux P., Souyri M., Mauchauffé M., Berger R., Larsen C. J., Mathieu-Mahul D. Two site-specific deletions and t(1;14) translocation restricted to human T-cell acute leukemias disrupt the 5' part of the tal-1 gene. Oncogene. 1991 Aug;6(8):1477–1488. [PubMed] [Google Scholar]
- Blackwell T. K., Weintraub H. Differences and similarities in DNA-binding preferences of MyoD and E2A protein complexes revealed by binding site selection. Science. 1990 Nov 23;250(4984):1104–1110. doi: 10.1126/science.2174572. [DOI] [PubMed] [Google Scholar]
- Bodine D. M., Ley T. J. An enhancer element lies 3' to the human A gamma globin gene. EMBO J. 1987 Oct;6(10):2997–3004. doi: 10.1002/j.1460-2075.1987.tb02605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L., Cheng J. T., Chen Q., Siciliano M. J., Crist W., Buchanan G., Baer R. Site-specific recombination of the tal-1 gene is a common occurrence in human T cell leukemia. EMBO J. 1990 Oct;9(10):3343–3351. doi: 10.1002/j.1460-2075.1990.tb07535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Cheng J. T., Tasi L. H., Schneider N., Buchanan G., Carroll A., Crist W., Ozanne B., Siciliano M. J., Baer R. The tal gene undergoes chromosome translocation in T cell leukemia and potentially encodes a helix-loop-helix protein. EMBO J. 1990 Feb;9(2):415–424. doi: 10.1002/j.1460-2075.1990.tb08126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J. T., Hsu H. L., Hwang L. Y., Baer R. Products of the TAL1 oncogene: basic helix-loop-helix proteins phosphorylated at serine residues. Oncogene. 1993 Mar;8(3):677–683. [PubMed] [Google Scholar]
- Disela C., Glineur C., Bugge T., Sap J., Stengl G., Dodgson J., Stunnenberg H., Beug H., Zenke M. v-erbA overexpression is required to extinguish c-erbA function in erythroid cell differentiation and regulation of the erbA target gene CAII. Genes Dev. 1991 Nov;5(11):2033–2047. doi: 10.1101/gad.5.11.2033. [DOI] [PubMed] [Google Scholar]
- Ferré-D'Amaré A. R., Prendergast G. C., Ziff E. B., Burley S. K. Recognition by Max of its cognate DNA through a dimeric b/HLH/Z domain. Nature. 1993 May 6;363(6424):38–45. doi: 10.1038/363038a0. [DOI] [PubMed] [Google Scholar]
- Field J., Nikawa J., Broek D., MacDonald B., Rodgers L., Wilson I. A., Lerner R. A., Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988 May;8(5):2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger L. R., Kagan J., Christopher G., Kurtzberg J., Hershfield M. S., Nowell P. C., Croce C. M. Involvement of the TCL5 gene on human chromosome 1 in T-cell leukemia and melanoma. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5039–5043. doi: 10.1073/pnas.86.13.5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk W. D., Ouellette M., Wright W. E. Molecular biology of myogenic regulatory factors. Mol Biol Med. 1991 Apr;8(2):185–195. [PubMed] [Google Scholar]
- Funk W. D., Pak D. T., Karas R. H., Wright W. E., Shay J. W. A transcriptionally active DNA-binding site for human p53 protein complexes. Mol Cell Biol. 1992 Jun;12(6):2866–2871. doi: 10.1128/mcb.12.6.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golemis E. A., Speck N. A., Hopkins N. Alignment of U3 region sequences of mammalian type C viruses: identification of highly conserved motifs and implications for enhancer design. J Virol. 1990 Feb;64(2):534–542. doi: 10.1128/jvi.64.2.534-542.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991 Feb 1;192(2):262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Henthorn P., Kiledjian M., Kadesch T. Two distinct transcription factors that bind the immunoglobulin enhancer microE5/kappa 2 motif. Science. 1990 Jan 26;247(4941):467–470. doi: 10.1126/science.2105528. [DOI] [PubMed] [Google Scholar]
- Hession C., Osborn L., Goff D., Chi-Rosso G., Vassallo C., Pasek M., Pittack C., Tizard R., Goelz S., McCarthy K. Endothelial leukocyte adhesion molecule 1: direct expression cloning and functional interactions. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1673–1677. doi: 10.1073/pnas.87.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H. L., Cheng J. T., Chen Q., Baer R. Enhancer-binding activity of the tal-1 oncoprotein in association with the E47/E12 helix-loop-helix proteins. Mol Cell Biol. 1991 Jun;11(6):3037–3042. doi: 10.1128/mcb.11.6.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J. S., Olson E. N., Kingston R. E. HEB, a helix-loop-helix protein related to E2A and ITF2 that can modulate the DNA-binding ability of myogenic regulatory factors. Mol Cell Biol. 1992 Mar;12(3):1031–1042. doi: 10.1128/mcb.12.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang L. Y., Siegelman M., Davis L., Oppenheimer-Marks N., Baer R. Expression of the TAL1 proto-oncogene in cultured endothelial cells and blood vessels of the spleen. Oncogene. 1993 Nov;8(11):3043–3046. [PubMed] [Google Scholar]
- Kadesch T. Consequences of heteromeric interactions among helix-loop-helix proteins. Cell Growth Differ. 1993 Jan;4(1):49–55. [PubMed] [Google Scholar]
- Kamps M. P., Murre C., Sun X. H., Baltimore D. A new homeobox gene contributes the DNA binding domain of the t(1;19) translocation protein in pre-B ALL. Cell. 1990 Feb 23;60(4):547–555. doi: 10.1016/0092-8674(90)90658-2. [DOI] [PubMed] [Google Scholar]
- Kopito R. R., Andersson M. A., Lodish H. F. Multiple tissue-specific sites of transcriptional initiation of the mouse anion antiport gene in erythroid and renal cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7149–7153. doi: 10.1073/pnas.84.20.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassar A. B., Davis R. L., Wright W. E., Kadesch T., Murre C., Voronova A., Baltimore D., Weintraub H. Functional activity of myogenic HLH proteins requires hetero-oligomerization with E12/E47-like proteins in vivo. Cell. 1991 Jul 26;66(2):305–315. doi: 10.1016/0092-8674(91)90620-e. [DOI] [PubMed] [Google Scholar]
- Lee K. A., Bindereif A., Green M. R. A small-scale procedure for preparation of nuclear extracts that support efficient transcription and pre-mRNA splicing. Gene Anal Tech. 1988 Mar-Apr;5(2):22–31. doi: 10.1016/0735-0651(88)90023-4. [DOI] [PubMed] [Google Scholar]
- Mellentin J. D., Smith S. D., Cleary M. L. lyl-1, a novel gene altered by chromosomal translocation in T cell leukemia, codes for a protein with a helix-loop-helix DNA binding motif. Cell. 1989 Jul 14;58(1):77–83. doi: 10.1016/0092-8674(89)90404-2. [DOI] [PubMed] [Google Scholar]
- Metz R., Ziff E. The helix-loop-helix protein rE12 and the C/EBP-related factor rNFIL-6 bind to neighboring sites within the c-fos serum response element. Oncogene. 1991 Dec;6(12):2165–2178. [PubMed] [Google Scholar]
- Mouthon M. A., Bernard O., Mitjavila M. T., Romeo P. H., Vainchenker W., Mathieu-Mahul D. Expression of tal-1 and GATA-binding proteins during human hematopoiesis. Blood. 1993 Feb 1;81(3):647–655. [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Vaessin H., Caudy M., Jan L. Y., Jan Y. N., Cabrera C. V., Buskin J. N., Hauschka S. D., Lassar A. B. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989 Aug 11;58(3):537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- Nelson C., Shen L. P., Meister A., Fodor E., Rutter W. J. Pan: a transcriptional regulator that binds chymotrypsin, insulin, and AP-4 enhancer motifs. Genes Dev. 1990 Jun;4(6):1035–1043. doi: 10.1101/gad.4.6.1035. [DOI] [PubMed] [Google Scholar]
- Nielsen A. L., Pallisgaard N., Pedersen F. S., Jørgensen P. Murine helix-loop-helix transcriptional activator proteins binding to the E-box motif of the Akv murine leukemia virus enhancer identified by cDNA cloning. Mol Cell Biol. 1992 Aug;12(8):3449–3459. doi: 10.1128/mcb.12.8.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N. MyoD family: a paradigm for development? Genes Dev. 1990 Sep;4(9):1454–1461. doi: 10.1101/gad.4.9.1454. [DOI] [PubMed] [Google Scholar]
- Rabbitts T. H. Translocations, master genes, and differences between the origins of acute and chronic leukemias. Cell. 1991 Nov 15;67(4):641–644. doi: 10.1016/0092-8674(91)90057-6. [DOI] [PubMed] [Google Scholar]
- Reddy P. M., Shen C. K. Protein-DNA interactions in vivo of an erythroid-specific, human beta-globin locus enhancer. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8676–8680. doi: 10.1073/pnas.88.19.8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Sun X. H., Copeland N. G., Jenkins N. A., Baltimore D. Id proteins Id1 and Id2 selectively inhibit DNA binding by one class of helix-loop-helix proteins. Mol Cell Biol. 1991 Nov;11(11):5603–5611. doi: 10.1128/mcb.11.11.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T. K., Turner D., Rupp R., Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991 Feb 15;251(4995):761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- Wright W. E., Binder M., Funk W. Cyclic amplification and selection of targets (CASTing) for the myogenin consensus binding site. Mol Cell Biol. 1991 Aug;11(8):4104–4110. doi: 10.1128/mcb.11.8.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Brown L., Yang C. Y., Tsan J. T., Siciliano M. J., Espinosa R., 3rd, Le Beau M. M., Baer R. J. TAL2, a helix-loop-helix gene activated by the (7;9)(q34;q32) translocation in human T-cell leukemia. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11416–11420. doi: 10.1073/pnas.88.24.11416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Babin J., Feldhaus A. L., Singh H., Sharp P. A., Bina M. HTF4: a new human helix-loop-helix protein. Nucleic Acids Res. 1991 Aug 25;19(16):4555–4555. doi: 10.1093/nar/19.16.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zon L. I., Orkin S. H. Sequence of the human GATA-1 promoter. Nucleic Acids Res. 1992 Apr 11;20(7):1812–1812. doi: 10.1093/nar/20.7.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]