Abstract
Antibody-dependent cellular cytotoxicity (ADCC) is potentially an effective adaptive immune response to HIV infection. However, little is understood about the role of ADCC in controlling chronic infection in the small number of long-term slow-progressors (LTSP) who maintain a relatively normal immunological state for prolonged periods of time. We analysed HIV-specific ADCC responses in sera from 139 HIV+ subjects not on antiretroviral therapy. Sixty-five subjects were LTSP, who maintained a CD4 T-cell count > 500/μl for over 8 years after infection without antiretroviral therapy and 74 were non-LTSP individuals. The ADCC responses were measured using an natural killer cell activation assay to overlapping HIV peptides that allowed us to map ADCC epitopes. We found that although the magnitude of ADCC responses in the LTSP cohort were not higher and did not correlate with CD4 T-cell depletion rates, the LTSP cohort had significantly broader ADCC responses compared with the non-LTSP cohort. Specifically, regulatory/accessory HIV-1 proteins were targeted more frequently by LTSP. Indeed, three particular ADCC epitopes within the Vpu protein of HIV were recognized only by LTSP individuals. Our study provides evidence that broader ADCC responses may play a role in long-term control of HIV progression and suggests novel vaccine targets.
Keywords: antibody-dependent cellular cytotoxicity, Env, HIV, slow-progressor, Vpu
Introduction
Partial protection from infection was achieved in the recent RV144 HIV vaccine efficacy trial.1 Despite inducing only narrow neutralizing antibody responses and very modest cytotoxic T-lymphocyte responses, non-neutralizing antibodies were induced by this regimen2 and such antibodies may have played a role in the protective immunity observed.3
Non-neutralizing antibodies could contribute to the control or elimination of a viral infection by multiple mechanisms including antibody-dependent cellular cytotoxicity (ADCC), phagocytosis of infected cells upon opsonization, and activation of the classical pathway of complement. ADCC involves the activation of FcγR-bearing effector cells, such as natural killer (NK cells), with the Fc portion of antibodies specific for antigens expressed on the surface of target cells. Activation of NK cells results in both lysis of the target cell and secretion of effector cytokines. As the ADCC antibody specificity need not be restricted to rarely targeted neutralizing epitopes, ADCC responses may increase the breadth of beneficial antibody responses.
There is a body of evidence demonstrating improved control of HIV in humans and of simian immunodeficiency virus (SIV) in macaques with the development of ADCC antibodies.4–9 Hessell et al.10 showed that an HIV-specific neutralizing antibody mutated in the Fc position was no longer able to elicit Fc-mediated functions, such as ADCC, and that the efficacy in preventing simian/human immunodeficiency virus (SHIV) infection of macaques was significantly decreased, suggesting that the ADCC function is important for the protection afforded by neutralizing antibodies.
There is a more limited understanding of the role of ADCC in the small subset of HIV-infected subjects who naturally control chronic infection, although the role of cytotoxic T lymphocytes and neutralizing antibodies has been extensively studied.11–24 We previously detected ADCC-mediated NK-cell activation in a small cohort of six subjects with slow HIV progression, but found no clear correlation with the magnitude of the ADCC response and control of HIV. A recent study of 22 subjects indicated that elite controllers of HIV infection (subjects with consistent plasma HIV levels of < 50 copies/ml) have higher levels of ADCC antibodies than viraemic subjects, with an absence of correlation between cytotoxic T lymphocytes and neutralizing antibodies.6 Whether these results are generalized across larger numbers of long-term slow-progressors (LTSP) subjects is not clear. In addition, the HIV epitopes targeted by efficient ADCC are unknown but would logically be interesting vaccine targets.
We analysed ADCC responses using an assay studying antibody-mediated interferon- γ (IFN-γ) and CD107a expression of NK cells. We studied serum samples from 139 HIV-infected subjects not on anti-retroviral therapy; 65 subjects were LTSP who maintained a CD4 T-cell count of > 500/μl for at least 8 years after infection and the remaining 74 subjects were non-LTSP. We found that ADCC responses in LTSP subjects were broadly reactive against multiple HIV proteins and that LTSP subjects disproportionally targeted three specific ADCC epitopes within Vpu (viral protein U).
Materials and methods
Study subjects
The characteristics of the 139 subjects are shown in Table 1. All subjects were HIV-infected and not on anti-retroviral therapy at the time of sampling. Subjects enrolled in both cohorts provided written informed consent and the relevant human research ethics committees approved all studies. Subjects were recruited both through the Long-term non-progressor network co-ordinated by the Kirby Institute, Sydney, Australia and through the Melbourne Sexual Health Centre, Australia. Sixty-five of the subjects met the pre-defined criteria as LTSPs, being HIV-positive for more than 8 years without anti-retroviral therapy and maintaining a peripheral CD4+ T-cell count above 500 cells/μl. There were no viral load entry criteria. The remaining 74 subjects did not meet the criteria for LTSP (i.e. had not maintained CD4 T-cell counts > 500 cells/μl for 8 years). For both cohorts, serum for ADCC testing was derived from the earliest time-point available. To assess the impact of ADCC responses on future CD4 T-cell count decline, we obtained all CD4 count data collected in routine care up to either the last day of follow up or the last available CD4 count before starting therapy.
Table 1.
Clinical characteristics of the study cohorts
| LTSP | Non-LTSP | P-value | |
|---|---|---|---|
| Number of subjects | 65 | 74 | |
| Median age (years) | 41 | 37 | NS |
| Male/female | 63/2 | 68/6 | NS |
| CD4 count at entry, cells/μl | 673 (572–889)1 | 494 (370–687) | 0·0001 |
| Viral load at entry, copies/ml | 2529 (457–9000) | 25 100 (5060–68 775) | 0·0004 |
| CCR5Δ32 heterozygotes | 16/452 | ND | NA |
| HLA B27 or B57 | 23/563 | ND | NA |
LTSP, long-term slow-progressors.
Median, interquartile range.
CCR5Δ32 screening was performed on 45 of the LTSP subjects. There were no CCR5Δ32 homozygotes.
HLA-B typing was performed on 56 of the LTSP subjects.
ADCC intracellular cytokine staining assay
The ADCC intracellular cytokine staining-based assay was used to analyse cytokine production and degranulation of ADCC-activated NK cells as described previously.25 Briefly, 150 μl of healthy donor whole blood and 50 μl of patient serum was incubated at 37° with either HIV peptide pools, individual 15 mer peptides, or gp140 Env proteins (1 μg/ml) for 5 hr in the presence of Brefeldin A and monensin (10 μg/ml; Sigma, St. Louis, MO). Following incubation, CD3− CD2+ CD56+ NK lymphocytes were analysed for the expression of intracellular IFN-γ and the degranulation marker CD107a. Fluorescent antibodies used for these experiments were CD3 (catalogue# 347344, fluorescent label: Peridinin chlorophyll protein), CD2 (556611, FITC), CD56 (555516, phycoerythrin), CD107a (624078, allophycocyanin), IFN-γ (557995, Alexa700) all obtained from BD Biosciences (San Jose, CA). We define NK cells in this assay as CD56+ CD2+ CD3− because CD16, although a useful NK-cell marker, is an Fc receptor bound by antibody in the ADCC process. To be classified as being positive for NK-cell-mediated activation, a response had to fulfil two criteria. First, NK cell CD107a and IFN-γ expression was more than three times that of unstimulated NK cells incubated with subject sera but without antigens. Second, a positive response was greater than the mean plus two standard deviations above the response of 10 separate sera samples from HIV-negative donors assayed with each of the peptide pools. The ADCC responses were detected using Consensus subtype B HIV overlapping 15 mer peptides supplied by the National Institutes of Health AIDS reagent repository. The pools were divided into Env, RTV pool (which spans the Rev, Tat and Vpu regulatory proteins), VVN pool (which spans Vpr, Vif and Nef proteins) and two pools spanning Pol proteins – Pol1, Pol2. ADCC responses to pools of 15 mer peptides overlapping by 11 amino acids were further mapped to single 15 mer peptides. We chose not to analyse ADCC responses against Gag peptides because both a pilot study and a previous study26 showed only rare ADCC responses to Gag and the volume of sera available was limited.
Results
Magnitude of ADCC responses does not correlate with CD4 T-cell decline in LTSP
Sixty-five LTSP anti-retroviral therapy-naive HIV-infected subjects were recruited based on the maintenance of CD4 T-cell counts above 500/μl for over 8 years after infection, and 74 non-LTSP subjects who did not meet the LTSP criteria were also recruited (Table 1). As expected, the 65 LTSP subjects had a lower median HIV viral load at study entry and higher CD4 T-cell counts (Table 1).
Most studies have correlated the magnitude of ADCC responses to rates of progression of HIV infection (reviewed in ref. 9); however, there have been limited studies performed on larger numbers of LTSP subjects. The magnitudes of ADCC responses against either gp140 or HIV-1 protein overlapping peptide pools were measured based on the proportion of NK cells activated by sera using the same healthy donor source of NK cells as illustrated in Fig. 1(a). We detected ADCC-mediated NK-cell activation across most (50 of 65) subjects in the LTSP cohort. The ADCC responses were most common against gp140 protein and Env peptides (47 and 40 subjects, respectively), with smaller numbers targeting the RTV, VVN pool or Pol peptide pools (Fig. 1b). The magnitude of the NK-cell activation mediated by ADCC was plotted against the decline in CD4 T cells over time. We found no correlation between the magnitude of the responses against any of the HIV-1 antigens studied and the change in CD4 T-cell percentage over time. Correlations between ADCC responses to gp140 protein or the RTV peptide pool and CD4 T-cell decline are shown in Fig. 1(c). A similar lack of correlation was observed with the magnitude of the ADCC to Env, Pol and RTV peptide pools and CD4 T-cell loss over time (P > 0·3, log-rank test).
Figure 1.
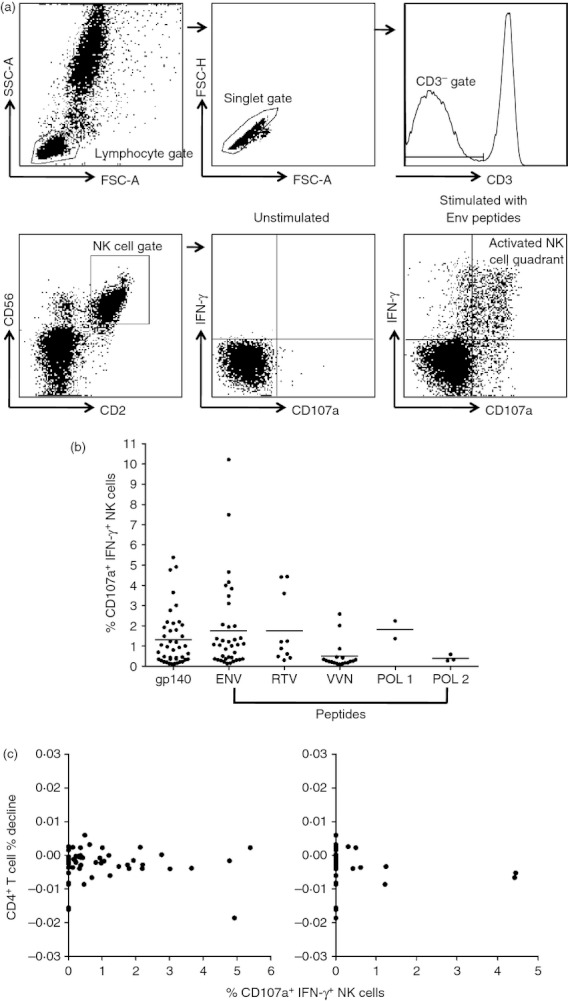
Magnitude of antibody-dependent cellular cytotoxicity (ADCC) responses within the long-term slow-progressors (LTSP) cohort. (a) Gating strategy for the intracellular cytokine staining (ICS) ADCC assays. The gating strategy for the ICS assay first gates on lymphocytes in the forward/side scatter and then CD3− CD2+ CD56+ natural killer (NK) lymphocytes analysed for dual intracellular interferon-γ (IFN-γ) and surface CD107a expression. (b) The magnitude of ADCC-mediated NK cell IFN-γ and CD107a expression across the LTSP cohort were measured for the various peptide pools. Every dot represents a subject and only subjects that induced an ADCC response above background levels (more than three times unstimulated sera and > 2 SD over HIV-negative controls) are depicted. (c) CD4 percentage changes over time in correlation with the magnitude of ADCC response within the LTSP cohort for the gp140 protein (left panel); and the RTV peptide pool (which spans the Rev, Tat and Vpu regulatory proteins; right panel).
Comparison of HIV-specific ADCC responses in the LTSP and non-LTSP cohorts
Antibody-dependent cellular cytotoxicity immunity against HIV is generally assessed against Env proteins; however, we detected a surprising number of ADCC responses targeting non-Env-overlapping HIV peptides. The significance of these ADCC responses is unclear. We compared the presence of HIV-specific ADCC responses against multiple HIV proteins in LTSP sera with that in non-LTSP sera using the intracellular cytokine staining-based ADCC assay described above. The ADCC responses targeting the trimeric gp140 protein and Env peptides were not significantly more common in the LTSP cohort (P > 0·1, analysis of variance Fig. 2a). However, we found that sera from the 65 LTSP subjects more commonly had ADCC-mediated NK-cell activation responses directed to the two pools of regulatory/accessory proteins (RTV peptide pool P = 0·017, VVN pool P = 0·014) compared with sera from the 74 non-LTSP subjects.
Figure 2.
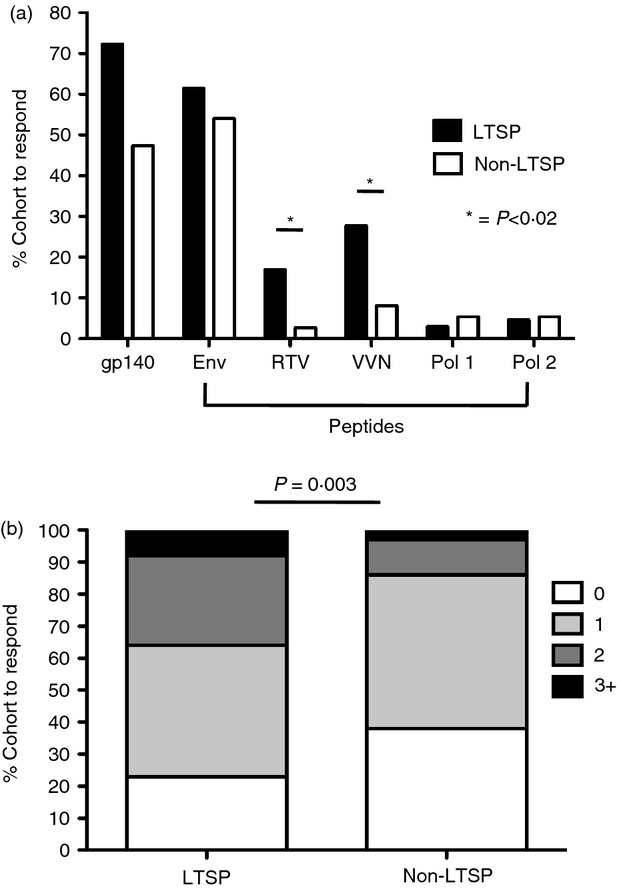
Long-term slow-progressors (LTSP) show more and broader antibody-dependent cellular cytotoxicity (ADCC) responses against HIV peptide pools. (a) Comparison of the percentage of responders that introduce ADCC responses from both cohorts to HIV-1 peptide pools as measured by interferon-γ (IFN-γ) and CD107a expression from gated natural killer (NK) cells. Significant differences are indicated by asterisks (P < 0·02). (b) Comparison of the percentage of responders from both cohorts that produce ADCC responses to zero, one or several peptide pools. Differences between the LTSP cohort and the non-LTSP cohort were significant (P = 0·003) in a 4 × 2 Fischer's exact test.
Breadth of immunity is a key issue for T-cell-mediated control of HIV27,28 and is also important for humoral immunity.29 We therefore studied how many HIV-1 peptide pools were targeted by ADCC responses across both cohorts. The proportion of subjects that responded to multiple peptide pools was significantly higher in the LTSP cohort compared with the non-LTSP cohort (P = 0·003 Fisher's exact test, Fig. 2b). For both cohorts a healthy donor was used as a source for the NK cells, thereby excluding the possibility that the differences were the result of a loss of NK-cell function during the progression of disease.
Mapping of ADCC epitopes in LTSP cohort
The ADCC epitopes more commonly targeted by LTSP subjects could represent interesting vaccine antigens. We therefore undertook to map ADCC epitopes in the LTSP cohort. We focused on identifying epitopes within the RTV pool because we had limited amounts of stored sera and the magnitude of responses against this pool tended to be high (Fig. 1b).
The ADCC responses to the RTV pool were mapped to several specific peptides. First, the RTV pool was broken down into the constituent pools, Rev, Tat and Vpu, and the ADCC responses to these pools were analysed (Fig. 3a). In each case the ADCC responses targeted Vpu. ADCC responses to the 19 overlapping peptides comprising the Vpu peptide pool were measured and then responses were measured to three smaller pools of six or seven Vpu peptides (Fig. 3b). ADCC responses to individual Vpu peptides were then studied to identify the epitope (Figs 3c and 3d shows two separate subjects).
Figure 3.
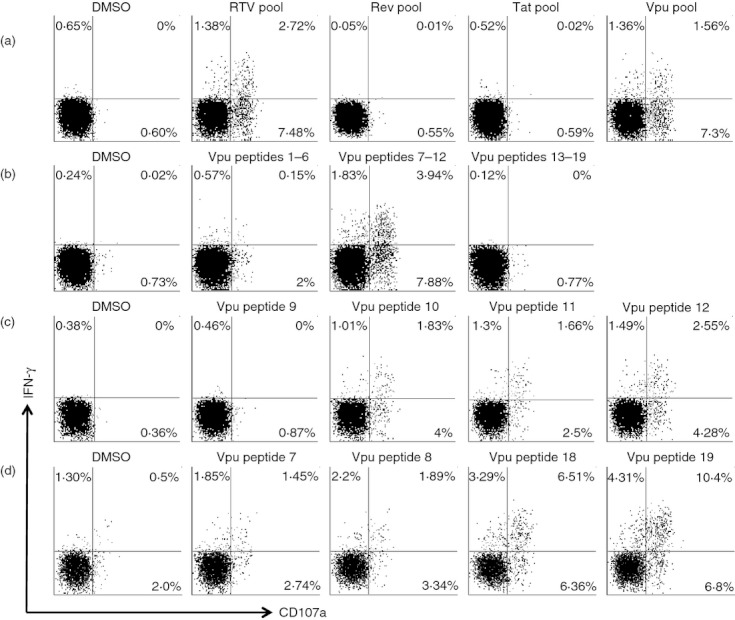
Mapping of antibody-dependent cellular cytotoxicity (ADCC) epitopes in Vpu. Representative flow cytometry plots of ADCC-mediated natural killer (NK) cell activation [interferon-γ (IFN-γ) and CD107a expression] are shown for sera from long-term slow-progressors (LTSP) subjects responding to Vpu peptides. The top panel (a) shows a response to the RTV (Rev, Tat, Vpu) peptide pool which is mapped to the Vpu pool. The second row (b) shows the Vpu peptide pool response from (a) that is mapped to sub-pools of six or seven peptides each. The third row (c) shows the Vpu response from (a) and (b) that is mapped to individual overlapping peptides 10–12. The fourth row (d) shows another serum from another LTSP subject with Vpu epitopes mapped to two other overlapping peptide epitopes.
A total of seven subjects in the LTSP cohort and no subjects in the non-LTSP cohort had ADCC responses mapped within the RTV peptide pool (Table 2). Three epitopes within the Vpu pool were targeted by seven subjects, with six of these seven subjects targeting multiple Vpu peptides (an example of a subject targeting two Vpu epitopes is shown in Fig. 3d). We found that the three overlapping peptide epitopes identified (peptides 7–8: VVWTIVFIEYRKILRQRKI, peptides 10–12: ILRQRKIDRLIDRIRERAEDSGN and peptides 18–19: SALVEMGHHAPWDVDDL) in Vpu were targeted at higher frequencies by LTSP compared with subjects from the non-LTSP cohort. No responses to other peptides within Vpu were identified. The Vpu epitope VVWTIVFIEYRKILRQRKI was targeted by five of the 65 subjects of the LTSP cohort and by no subjects in the non-LTSP cohort (P = 0·02, Table 2). The Vpu epitopes ILRQRKIDRLIDRIRERAEDSGN and SALVEMGHHAPWDVDDL were both targeted by four of the 65 subjects from the LTSP cohort and by no subjects in the non-LTSP cohort (P = 0·045).
Table 2.
Vpu epitopes targeted by long-term slow-progressors (LTSP)
| Overlapping 15 mer peptides | Sequence | LTSP cohort | Non-LTSP cohort | P-value (Fisher's exact test) |
|---|---|---|---|---|
| Vpu 7,8 | VVWTIVFIEYRKILRQRKI | 5/65 (7·7%) | 0/74 (0%) | 0·020 |
| Vpu 10,11,12 | ILRQRKIDRLIDRIRERAEDSGN | 4/65 (6·2%) | 0/74 (0%) | 0·045 |
| Vpu 18,19 | SALVEMGHHAPWDVDDL | 4/65 (6·2%) | 0/74 (0%) | 0·045 |
Discussion
The ADCC responses to HIV are induced early during infection and several studies have shown that ADCC is associated with protection from SIV disease in macaques,4,30 delayed progressive HIV infection in humans,6,8 protection from HIV-1 infection in intravenous drug users,31 and lower genital HIV viral loads.32 The specificities of ADCC responses associated with slower HIV-1 progression are unclear but of direct relevance as vaccine targets.
In this study we investigated ADCC immune responses in HIV-infected subjects with LTSP. ADCC responses to multiple HIV peptide pools were significantly more common in LTSP subjects than in non-LTSP subjects. Specifically, we found that peptides spanning regulatory/accessory proteins of HIV were targeted more frequently by LTSPs. Through a process of mapping ADCC epitopes, we found that three specific ADCC epitopes in Vpu were targeted in seven out of 65 individuals in the LTSP subjects and none of the non-LTSP subjects.
Why would Vpu be targeted by ADCC and would this be relevant in HIV-infected cells? Vpu is a multifunctional protein that is expressed within the cell membrane and at least part of the protein may be accessible to ADCC antibodies.33–37 It will be important in future studies to assess whether purified or monoclonal Vpu epitope-specific ADCC antibodies can recognize virus-infected cells. Vpu is expressed early in the HIV replication cycle and ADCC-mediated NK-cell recognition of Vpu-expressing cells may limit release of new virions. We speculate that if Vpu can be presented in a manner that elicits functional and effective ADCC responses, then the Vpu ADCC epitopes that we describe could be interesting vaccine antigens. Interestingly, a study by Chen et al. in 200338 suggested that Vpu-specific antibody responses detected by Western blot were associated with slower disease progression.
An important caveat of this work is that our mapping of ADCC responses was limited to linear peptide epitopes that could be defined with individual peptides. Conformational ADCC epitopes within Vpu and other HIV proteins recognized by LTSP subjects would also be of considerable interest, but such epitopes are more difficult to map. Further, the number of LTSP subjects that generated Vpu peptide-specific ADCC responses was modest (seven of the 65 subjects, 10·8%). However, this might be expected because multiple other mechanisms, such as HLA class I molecules and CCR5 deletions, have been associated with slow HIV progression.39,40 Indeed, 35% of the LTSP subjects tested were CCR5Δ32 heterozygotes and 41% of the LTSP subjects tested had either HLA B27 or B57 alleles.
It is possible that ADCC responses targeting common epitopes in Env or other HIV-1 proteins are also associated with slowly progressive HIV. The C1 region of Env has recently been shown to be a common target of ADCC antibodies41 and we recently showed that ADCC epitopes within C1 can force immune escape.42 Our ability to fully map Env-specific ADCC in the LTSP cohort was limited by the volumes of sera available from the LTSP cohort and the large number of overlapping peptides spanning Env. This is a subject of ongoing research. The large diversity of infecting Env strains, the ability of Env to readily escape antibody responses, and the limited apparent fitness costs of Env variants potentially makes Env a less attractive target than more conserved HIV proteins.42–45
Although this study identifies an immune response associated with slow HIV progression, this does not prove that this response is causally linked to slow progression. LTSP subjects are by definition infected for long periods of time and the anti-HIV ADCC responses may broaden over time unrelated to the control of viraemia. Previous smaller studies suggest broadening of ADCC responses over time.46,47 However, we are now in a position to definitively test the protective effects of these Vpu ADCC antibodies in passive transfer studies in macaques subsequently challenged with chimeric SHIV expressing HIV-1 Vpu. Previous passive transfer experiments using neutralizing antibodies have suggested an important additive role for ADCC functions,10,48 but the utility of ADCC antibodies without neutralizing activity in protecting macaques from SHIV infection is not known.
In conclusion, we studied HIV-specific ADCC responses in a large cohort of LTSP subjects. We found that ADCC responses to specific Vpu epitopes are over-represented in LTSP subjects and warrant further study as vaccine antigens.
Acknowledgments
The Antibody-Dependent Cellular Cytotoxicity study collaboration group includes physician and nurses who helped to recruit subjects for the study: T. Read, M. Chen, C. Fairley, T. Schmidt, C. Bradshaw, R. Moore, K. Fethers, J. Silvers and H. Kent from the Melbourne Sexual Health Centre; R. McFarlane, D. Baker, M. McMurchie, East Sydney Doctors; S. Pett, A. Carr, St Vincent's Hospital Sydney; R. Finlayson, Taylor Square Clinic; Don Smith, Albion St Centre; T.M. Soo, Interchange General Practice Canberra; M. Kelly, J. Patten, AIDS Medical Centre Brisbane; B. Anderson, St Leonard's Medical Centre; S. Marlton, Port Kembla Sexual Health Clinic; D. Smith, Lismore Sexual Health; M. Bloch, Holdsworth House General Practice; N. Doong, Dr Doong's Surgery; N. Roth, Prahran Market Clinic and A. Shaik for the curation of the database. We are grateful to all the individuals who participated in the study for their assistance. This work was financially supported by NHMRC awards 510448 and 455350, ARC award LP0991498, the Australian Centre for HIV and Hepatitis Virology Research, The Royal Australasian College of Physicians, The Ramaciotti Foundation, and National Institutes of Health award R21AI081541.
Disclosures
The authors declare no competing interests.
Authors contribution
L.W., A.C., G.I., M.P. and M.N. performed ADCC assays; J.A. analysed data, L.W., I.S. and S.K. conceived the study and wrote the manuscript; D.C., A.K., I.S. and ADCC study collaboration recruited subjects and provided samples. All authors read and approved the final manuscript.
References
- 1.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 2.Karnasuta C, Paris RM, Cox JH, et al. Antibody-dependent cell-mediated cytotoxic responses in participants enrolled in a phase I/II ALVAC-HIV/AIDSVAX B/E prime-boost HIV-1 vaccine trial in Thailand. Vaccine. 2005;23:2522–9. doi: 10.1016/j.vaccine.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 3.Haynes BF, Gilbert PB, McElrath MJ, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–86. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomez-Roman VR, Patterson LJ, Venzon D, Liewehr D, Aldrich K, Florese R, Robert-Guroff M. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J Immunol. 2005;174:2185–9. doi: 10.4049/jimmunol.174.4.2185. [DOI] [PubMed] [Google Scholar]
- 5.Xiao P, Zhao J, Patterson LJ, et al. Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J Virol. 2010;84:7161–73. doi: 10.1128/JVI.00410-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambotte O, Ferrari G, Moog C, et al. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS. 2009;23:897–906. doi: 10.1097/QAD.0b013e328329f97d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baum LL, Cassutt KJ, Knigge K, et al. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol. 1996;157:2168–73. [PubMed] [Google Scholar]
- 8.Ahmad R, Sindhu ST, Toma E, Morisset R, Vincelette J, Menezes J, Ahmad A. Evidence for a correlation between antibody-dependent cellular cytotoxicity-mediating anti-HIV-1 antibodies and prognostic predictors of HIV infection. J Clin Immunol. 2001;21:227–33. doi: 10.1023/a:1011087132180. [DOI] [PubMed] [Google Scholar]
- 9.Chung A, Rollman E, Johansson S, Kent SJ, Stratov I. The utility of ADCC responses in HIV infection. Curr HIV Res. 2008;6:515–19. doi: 10.2174/157016208786501472. [DOI] [PubMed] [Google Scholar]
- 10.Hessell AJ, Hangartner L, Hunter M, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–4. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 11.Carrington M, Dean M, Martin MP, O'Brien SJ. Genetics of HIV-1 infection: chemokine receptor CCR5 polymorphism and its consequences. Hum Mol Genet. 1999;8:1939–45. doi: 10.1093/hmg/8.10.1939. [DOI] [PubMed] [Google Scholar]
- 12.Carrington M, O'Brien SJ. The influence of HLA genotype on AIDS. Annu Rev Med. 2003;54:535–51. doi: 10.1146/annurev.med.54.101601.152346. [DOI] [PubMed] [Google Scholar]
- 13.de Roda Husman AM, Koot M, Cornelissen M, et al. Association between CCR5 genotype and the clinical course of HIV-1 infection. Ann Intern Med. 1997;127:882–90. doi: 10.7326/0003-4819-127-10-199711150-00004. [DOI] [PubMed] [Google Scholar]
- 14.Kirchhoff F, Greenough TC, Brettler DB, Sullivan JL, Desrosiers RC. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med. 1995;332:228–32. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 15.Migueles SA, Sabbaghian MS, Shupert WL, et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci USA. 2000;97:2709–14. doi: 10.1073/pnas.050567397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navis M, Schellens I, van Baarle D, Borghans J, van Swieten P, Miedema F, Kootstra N, Schuitemaker H. Viral replication capacity as a correlate of HLA B57/B5801-associated nonprogressive HIV-1 infection. J Immunol. 2007;179:3133–43. doi: 10.4049/jimmunol.179.5.3133. [DOI] [PubMed] [Google Scholar]
- 17.Cao Y, Qin L, Zhang L, Safrit J, Ho DD. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995;332:201–8. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 18.Carotenuto P, Looij D, Keldermans L, de Wolf F, Goudsmit J. Neutralizing antibodies are positively associated with CD4+ T-cell counts and T-cell function in long-term AIDS-free infection. AIDS. 1998;12:1591–600. doi: 10.1097/00002030-199813000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Cecilia D, Kleeberger C, Munoz A, Giorgi JV, Zolla-Pazner S. A longitudinal study of neutralizing antibodies and disease progression in HIV-1-infected subjects. J Infect Dis. 1999;179:1365–74. doi: 10.1086/314773. [DOI] [PubMed] [Google Scholar]
- 20.Pilgrim AK, Pantaleo G, Cohen OJ, et al. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term-nonprogressive infection. J Infect Dis. 1997;176:924–32. doi: 10.1086/516508. [DOI] [PubMed] [Google Scholar]
- 21.Doria-Rose NA, Klein RM, Manion MM, et al. Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. J Virol. 2009;83:188–99. doi: 10.1128/JVI.01583-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Euler Z, van Gils MJ, Bunnik EM, Phung P, Schweighardt B, Wrin T, Schuitemaker H. Cross-reactive neutralizing humoral immunity does not protect from HIV type 1 disease progression. J Infect Dis. 2010;201:1045–53. doi: 10.1086/651144. [DOI] [PubMed] [Google Scholar]
- 23.Simek MD, Rida W, Priddy FH, et al. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol. 2009;83:7337–48. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Gils MJ, Euler Z, Schweighardt B, Wrin T, Schuitemaker H. Prevalence of cross-reactive HIV-1-neutralizing activity in HIV-1-infected patients with rapid or slow disease progression. AIDS. 2009;23:2405–14. doi: 10.1097/QAD.0b013e32833243e7. [DOI] [PubMed] [Google Scholar]
- 25.Stratov I, Chung A, Kent SJ. Robust NK cell-mediated human immunodeficiency virus (HIV)-specific antibody-dependent responses in HIV-infected subjects. J Virol. 2008;82:5450–9. doi: 10.1128/JVI.01952-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isitman G, Chung AW, Navis M, Kent SJ, Stratov I. Pol as a target for antibody dependent cellular cytotoxicity responses in HIV-1 infection. Virology. 2011;412:110–16. doi: 10.1016/j.virol.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goepfert PA, Lumm W, Farmer P, et al. Transmission of HIV-1 Gag immune escape mutations is associated with reduced viral load in linked recipients. J Exp Med. 2008;205:1009–17. doi: 10.1084/jem.20072457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McElrath MJ, De Rosa SC, Moodie Z, et al. HIV-1 vaccine-induced immunity in the test-of-concept step study: a case–cohort analysis. Lancet. 2008;372:1894–905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheid JF, Mouquet H, Feldhahn N, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–40. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 30.Banks ND, Kinsey N, Clements J, Hildreth JE. Sustained antibody-dependent cell-mediated cytotoxicity (ADCC) in SIV-infected macaques correlates with delayed progression to AIDS. AIDS Res Hum Retroviruses. 2002;18:1197–205. doi: 10.1089/08892220260387940. [DOI] [PubMed] [Google Scholar]
- 31.Scott-Algara D, Truong LX, Versmisse P, et al. Cutting edge: increased NK cell activity in HIV-1-exposed but uninfected Vietnamese intravascular drug users. J Immunol. 2003;171:5663–7. doi: 10.4049/jimmunol.171.11.5663. [DOI] [PubMed] [Google Scholar]
- 32.Nag P, Kim J, Sapiega V, et al. Women with cervicovaginal antibody-dependent cell-mediated cytotoxicity have lower genital HIV-1 RNA loads. J Infect Dis. 2004;190:1970–8. doi: 10.1086/425582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subbramanian RA, Cohen EA. Molecular biology of the human immunodeficiency virus accessory proteins. J Virol. 1994;68:6831–5. doi: 10.1128/jvi.68.11.6831-6835.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crise B, Buonocore L, Rose JK. CD4 is retained in the endoplasmic reticulum by the human immunodeficiency virus type 1 glycoprotein precursor. J Virol. 1990;64:5585–93. doi: 10.1128/jvi.64.11.5585-5593.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jabbar MA, Nayak DP. Intracellular interaction of human immunodeficiency virus type 1 (ARV-2) envelope glycoprotein gp160 with CD4 blocks the movement and maturation of CD4 to the plasma membrane. J Virol. 1990;64:6297–304. doi: 10.1128/jvi.64.12.6297-6304.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nomaguchi M, Fujita M, Adachi A. Role of HIV-1 Vpu protein for virus spread and pathogenesis. Microbes Infect. 2008;10:960–7. doi: 10.1016/j.micinf.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Ruiz A, Guatelli JC, Stephens EB. The Vpu protein: new concepts in virus release and CD4 down-modulation. Curr HIV Res. 2010;8:240–52. doi: 10.2174/157016210791111124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen YM, Rey WY, Lan YC, Lai SF, Huang YC, Wu SI, Liu TT, Hsiao KJ. Antibody reactivity to HIV-1 Vpu in HIV-1/AIDS patients on highly active antiretroviral therapy. J Biomed Sci. 2003;10:266–75. doi: 10.1007/BF02256062. [DOI] [PubMed] [Google Scholar]
- 39.Navis M, Miedema F, Schuitemaker H. Cytotoxic T lymphocyte responses in HIV-1-infected long-term nonprogressors: lessons for vaccine design. Future HIV Ther. 2008;2:351–61. [Google Scholar]
- 40.Paxton WA, Kang S. Chemokine receptor allelic polymorphisms: relationships to HIV resistance and disease progression. Semin Immunol. 1998;10:187–94. doi: 10.1006/smim.1998.0132. [DOI] [PubMed] [Google Scholar]
- 41.Ferrari G, Pollara J, Kozink D, et al. A HIV-1 gp120 envelope human monoclonal antibody that recognizes a C1 conformational epitope mediates potent ADCC activity and defines a common ADCC epitope in human HIV-1 serum. J Virol. 2011;85:7029–36. doi: 10.1128/JVI.00171-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung AW, Isitman G, Navis M, Kramski M, Center RJ, Kent SJ, Stratov I. Immune escape from HIV-specific antibody-dependent cellular cytotoxicity (ADCC) pressure. Proc Natl Acad Sci USA. 2011;108:7505–10. doi: 10.1073/pnas.1016048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peut V, Campbell S, Gaeguta A, et al. Balancing reversion of cytotoxic T-lymphocyte and neutralizing antibody escape mutations within human immunodeficiency virus type 1 Env upon transmission. J Virol. 2009;83:8986–92. doi: 10.1128/JVI.00599-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peut V, Kent SJ. Utility of human immunodeficiency virus type 1 envelope as a T-cell immunogen. J Virol. 2007;81:13125–34. doi: 10.1128/JVI.01408-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peut V, Kent SJ. Substantial envelope-specific CD8 T-cell immunity fails to control SIV disease. Virology. 2009;384:21–7. doi: 10.1016/j.virol.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 46.Chung AW, Navis M, Isitman G, et al. Activation of NK cells by ADCC responses during early HIV infection. Viral Immunol. 2011;24:171–5. doi: 10.1089/vim.2010.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johansson SE, Rollman E, Chung AW, et al. NK cell function and antibodies mediating ADCC in HIV-1-infected viremic and controller patients. Viral Immunol. 2011;24:359–68. doi: 10.1089/vim.2011.0025. [DOI] [PubMed] [Google Scholar]
- 48.Hessell AJ, Poignard P, Hunter M, et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009;15:951–4. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


