Abstract
Photosynthesis in marine diatoms is a vital fraction of global primary production empowered by CO2-concentrating mechanisms. Acquisition of HCO3− from seawater is a critical primary step of the CO2-concentrating mechanism, allowing marine photoautotrophic eukaryotes to overcome CO2 limitation in alkaline high-salinity water. However, little is known about molecular mechanisms governing this process. Here, we show the importance of a plasma membrane-type HCO3− transporter for CO2 acquisition in a marine diatom. Ten putative solute carrier (SLC) family HCO3− transporter genes were found in the genome of the marine pennate diatom Phaeodactylum tricornutum. Homologs also exist in marine centric species, Thalassiosira pseudonana, suggesting a general occurrence of SLC transporters in marine diatoms. Seven genes were found to encode putative mammalian-type SLC4 family transporters in P. tricornutum, and three of seven genes were specifically transcribed under low CO2 conditions. One of these gene products, PtSLC4-2, was localized at the plasmalemma and significantly stimulated both dissolved inorganic carbon (DIC) uptake and photosynthesis in P. tricornutum. DIC uptake by PtSLC4-2 was efficiently inhibited by an anion-exchanger inhibitor, 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid, in a concentration-dependent manner and highly dependent on Na+ ions at concentrations over 100 mM. These results show that DIC influx into marine diatoms is directly driven at the plasmalemma by a specific HCO3− transporter with a significant halophilic nature.
Keywords: bicarbonate transporter, chromista, marine environment, sodium-dependent
Inorganic carbon entry into algal cells is the primary limiting factor for photosynthesis and requires specific transporters (1). The problem is exacerbated especially in marine environment. Specifically, dissolved CO2 concentrations are low, and the rate of spontaneous CO2 formation from HCO3− is much slower in the ocean relative to freshwater because of the high alkalinity and salinity of seawater (2). Marine diatoms are responsible for one-fifth of global primary productivity and play a key role in global cycles of carbon and other elements (3, 4). The concentration of dissolved CO2 in seawater under the present atmospheric pCO2 (below 15 µM at 20 °C) is much lower than the Km[CO2] of ribulose-1,5-bisphosphate carboxylase/oxygenase in diatom species (5). Marine diatoms are, thus, believed to rely directly or indirectly on the use of abundant levels of seawater HCO3− to support their primary production.
The CO2-concentrating mechanism (CCM) has been studied extensively in cyanobacteria, and molecular characterizations have revealed a set of CCM components that completely account for the strategy of cyanobacterial tolerance of CO2 limitation. Freshwater β-cyanobacteria possess three plasma membrane HCO3− transporters denoted bicarbonate transporter 1, sodium bicarbonate transporter A (SbtA), and bicarbonate transporter A (BicA), all displaying different affinities for HCO3− (6–8). NADPH dehydrogenase CO2 hydration protein complexes at the thylakoid membrane convert diffusing CO2 into HCO3− (9). The accumulated HCO3− is dehydrated into CO2 when HCO3− passes across the shell of the carboxysome, an icosahedrons crystal body in the cell (10), by a function of carbonic anhydrase (CA) (11). The CO2 product is fixed by ribulose-1,5-bisphosphate carboxylase/oxygenase within the carboxysome. It is strongly suggested that the establishment of the cyanobacterial CCM is a newer event relative to the primary endosymbiosis (12), and algal plastids have developed a different type CCM based on the pyrenoid, a protein body found in the most algal chloroplasts (5). The presence of a pyrenoid does not necessarily imply the operation of a CCM (13), but the possibility remains that a pyrenoid is a central component of an active CCM in eukaryotic algae. In the freshwater chlorophyte, Chlamydomonas reinhardtii, chloroplastic CAs, a pyrenoidal component including low-CO2–inducible protein (LCI) B/C, and the membrane transporter, LCI1, are known to facilitate the CCM (14–17). Additionally, the role of extracellular CAs (CAext) in CCM has been controversial in the study of green algae (18).
Physiological evidence supports the occurrence of direct uptakes of CO2 and HCO3− in marine diatom species (19–22). Like other eukaryotic algae, diatoms possess CCM (2, 19, 20, 23), and furthermore, a C4-type biochemical-concentrating system of CO2 was reported also to be a part of the diatom CCM (24). The active HCO3− transport in the CCM also plays an important role in dissipating excess light energy by consuming ATP for a carbon cycling, which is cooperated with a fast efflux of CO2 across the plasmalemma (25). The molecular mechanism of HCO3− acquisition in diatoms is critical information that defines the initial step of the vast marine primary production and the adaptation strategy to environmental stresses, allowing a precise understanding of the diatom carbon acquisition and its response under changing ocean environments. However, there is little molecular evidence on this subject. Especially, systems that work for an efficient mobilization of dissolved inorganic carbon (DIC) at the plasmalemma are perhaps unique in chromista because of their distinct evolutionally history (26–28).
In the present study, we have shown the occurrence of a solute carrier 4 (SLC4) family HCO3− transporter in the marine diatom Phaeodactylum tricornutum and characterized its function.
Results
Candidate HCO3− Transporters in Diatoms.
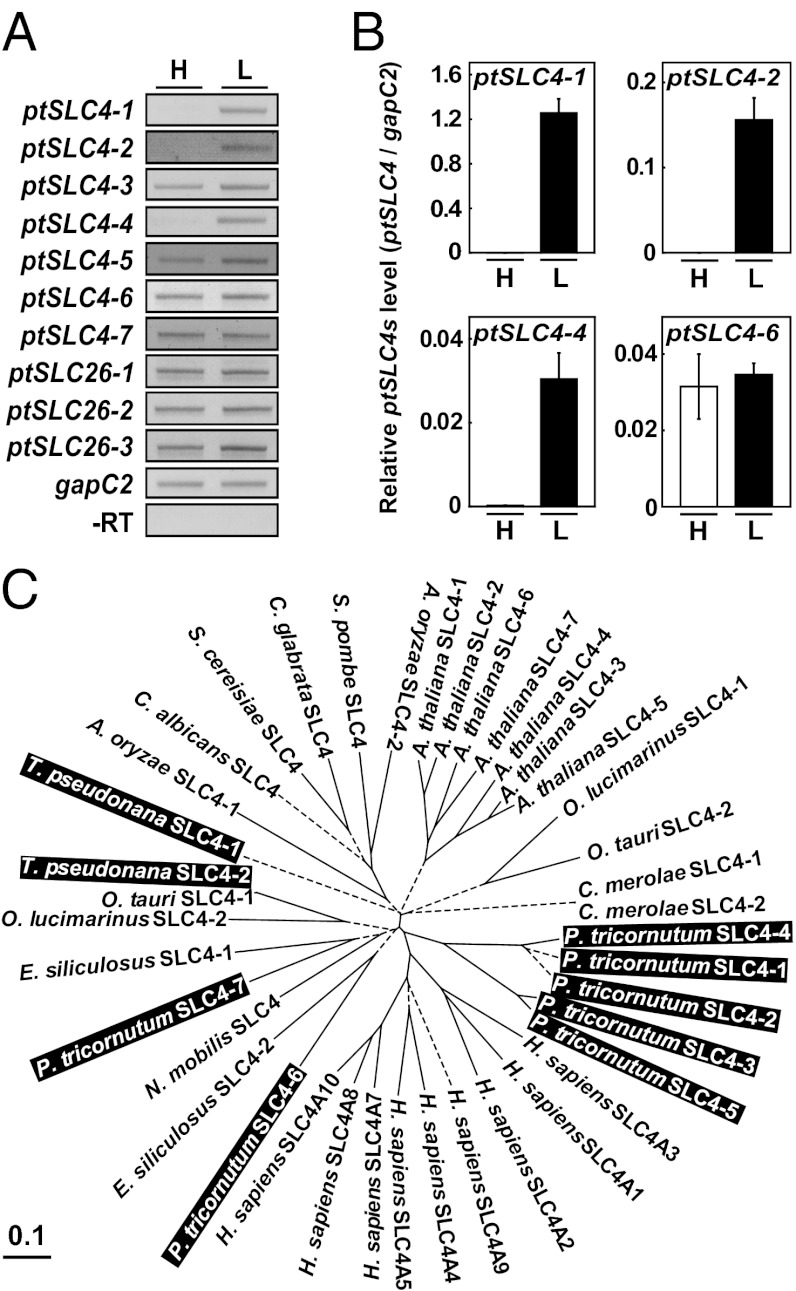
Diatom genomes contain numerous genes that apparently share origins with the mammalian genome, and these genes are believed to be derived from the ancestral eukaryotic host cell (probably a biciliate) of secondary endosymbiosis (26–28). In the genome of P. tricornutum, there are at least 10 candidate HCO3− transporter genes. Of these genes, seven genes were found to be similar to members of the mammalian SLC4 family, and the remainder were similar to the SLC26 family (Fig. 1A). In mammals, SLC4 and SLC26 are unrelated multigene families of HCO3− transporters, which have evolved independently (29, 30). These proteins transport HCO3− across the mammalian plasma membrane and play a critical role in maintaining intracellular and extracellular pH within a narrow range (31), and mutations in these proteins are known to cause various genetic diseases in humans (30).
Fig. 1.
Candidate DIC transporters, putative SLC family genes, and their transcript levels. (A) Apparent expression levels of 10 putative DIC transporter genes in high- (H) and low-CO2–grown cells (L) of P. tricornutum revealed by a semiquantitative RT-PCR. The gapC2 transcript was used as a constitutive internal control. (B) Quantification of transcript levels of three CO2-responsive ptSLC4-1, -2, and -4 in H and L P. tricornutum cells by qRT-PCR. Transcript of one apparently constitutive gene, ptSLC4-6, was also quantified. (C) Phylogenetic trees of SLC4 family genes from mammal, plant, fungi, and eukaryotic algae (Dataset S1). The phylogenetic tree was constructed using the neighbor-joining method. Bootstrap values are above 776 over 1,000 trials (solid lines), with a few exceptions below 775 (dashed lines).
All candidate SLC4 and SLC26 in the P. tricornutum genome were transcriptionally active (Fig. 1A). Of these genes, ptSLC4-5, -6, and -7 were previously predicted to encode HCO3− transporters by Kroth et al. (32). Three SLC4-type genes (ptSLC4-1, ptSLC4-2, and ptSLC4-4) displayed transcriptional activation specifically under atmospheric CO2 condition (low CO2), whereas they were repressed in high CO2 (5% CO2) (Fig. 1 A and B), suggesting that these ptSLC4 products are functional under CO2-limiting conditions. A phylogenetic relationship of SLC4 family genes was analyzed (Fig. 1C, Dataset S1). Together with ptSLC4-3 and ptSLC4-5, aforementioned CO2-responsive genes formed a diatom-specific cluster that likely shares a common origin with Homo sapiens SLC4 genes (supported by a bootstrap value of 776) (Fig. 1C). Interestingly, three CO2-responsive PtSLC4 genes (ptSLC4-1, -2, and -4) were most closely related (Fig. 1C). Three other SLC4 candidates in P. tricornutum (ptSLC4-6 and -7) and Thalassiosira pseudonana (tpSLC4-2) were clustered with heterokont genes, which were also related to the aforementioned human/diatom cluster (supported by a bootstrap value of 983) (Fig. 1C). Conversely, T. pseudonana tpSLC4-1 was not related to any clade with a reliable bootstrap value; hence, its phylogenetic position was not clear (Fig. 1C).
In silico translation of full-length cDNA sequences of the CO2-responsive ptSLC4-1, -2, and -4 revealed them to be 76–82% similar (Fig. S1) and have significant similarity to human SLC4A1 (29%, 29%, and 27% sequence homology, respectively). Moreover, 12 membrane-spanning helices of human SLC4A1 were highly conserved (Fig. 2A and Fig. S1, TM2–TM13). With the exception of the first transmembrane domain, which was unique to PtSLC4-2, a comparison of membrane topology deduced from the translated amino acid sequences revealed a striking similarity between PtSLC4-2 and human SLC4A1 (Fig. 2A). The aforementioned PtSLC4 candidates possessed one or two of the N-glycosylation site N-X-S (Fig. S1, underline), a characteristic of human SLC4A1 and SLC4A4 with a proposed role in promoting protein folding (33, 34). PtSLC4-1 and PtSLC4-2 also possessed amino acid sequences homologous to human SLC4s, Asp-Gly-Asp-Asp (DGDD) and/or Ser-Asp-Asp-Val (SDDV), at their C termini (Fig. 2A and Fig. S1, bold). The C terminus of human SLC4A4 possesses two acidic motifs, Asp-Asn-Asp-Asp (DNDD) and Leu-Asp-Asp-Val (LDDV), which are speculated to form a transport metabolon with CAII (35). The structural properties imply that the transport mechanism of these CO2-responsive PtSLC4s is similar to the mechanism of human SLC4s.
Fig. 2.
Localization of the exogenously introduced PtSLC4-2:GFP fusion. (A) Predicted membrane topology of PtSLC4-2 based on the alignment with human SLC4A1. The numbered cylinders are putative membrane-spanning helices, and the one depicted by the dashed line is the helix unique to PtSLC4-2. Lysine (530) is a PtSLC4-2 counterpart for a putative DIDS-interacting residue, which was predicted in human SLC4A1. DGDD and SDDV are PtSLC4-2 counterparts for CA binding site predicted in human SLC4A1. (B–E) Localization of PtSLC4-2:GFP fusion in P. tricornutum. (B) Light image. (C) Hoechst-stained nucleus (blue) and autofluorescence of the chloroplast (red). (D) GFP signal. (E) Merged image of C and D. (F–H) WT cells. (F) Light image. (G) Nucleus (blue) and chloroplast (red). (H) GFP signal. (I) Z-stacked image with GFP signals (green) and chlorophyll autofluorescence (red). (J) The cross-section at the orange line in I. (Scale bar: 10 µm.)
Localization of Exogenously Introduced PtSLC4-2:GFP Fusion.
The enhanced GFP gene (egfp) was fused immediately downstream of the full-length ptSLC4-2 cDNA and transformed into P. tricornutum cells under the control of the fucoxanthin chlorophyll (Chl) a/c binding protein gene promoter, which functions regardless of [CO2] in the medium (36). Quantitative RT-PCR (qRT-PCR) and Western blotting showed the constitutive expression of the GFP fusion product in the transformants carrying the ptSLC4-2::egfp fusion (SLC4G cells) (Fig. S2 A and B). The expressed GFP fusion was equally localized in the peripheral area of SLC4G cells (Fig. 2 B–E), whereas no GFP signal was observed in WT cells (Fig. 2 F–H). Cross-sections at the distal tip area of fusiform cells showed a distinct ring-shaped pattern of the GFP signal (Fig. 2 I and J), indicating that membrane-spanning helices of PtSLC4-2 target the plasmalemma. We had confirmed the occurrence of GFP signal before all of the following experiments (Fig. S2C). As shown in Fig. 2 D and E, large dots of GFP signal, which were not overlapped with either Hoechst or Mitotacker signals (Fig. S2D), were occasionally observed in SLC4G cells. We assume that this body is a part of the Golgi apparatus with exocytotic vesicles carrying PtSLC4-2:GFP fusion proteins, although details are, so far, not clear.
Influence of Exogenously Introduced PtSLC4-2:GFP on Photosynthetic Parameters.
Because of a down-regulation of the CCM (2), photosynthetic affinity for HCO3− was decreased significantly for WT cells grown in high CO2 with a K0.5[HCO3−] of 515 µM (Table 1, Fig. S3A). Conversely, high-CO2–grown SLC4G cells revealed 2.8-fold lower K0.5[HCO3−] value relative to high-CO2–grown WT cells (Table 1). The [DIC] at the CO2 compensation point was consistent with these changes in affinity, whereas Pmax values were stable in WT and SLC4G cells (Table 1), indicating a significant improvement in photosynthetic affinity caused by constitutive expression of PtSLC4-2 under high-CO2 conditions. Little difference in the photosynthetic parameters was observed between WT and SCL4G cells grown in low CO2 (Table 1), probably because the effect of the boosted PtSLC4-2 expression was veiled by the fully operational CCM components in the low-CO2–acclimated cells.
Table 1.
Photosynthetic parameters in WT and SLC4G cells
| Cells | K0.5 [HCO3−] (µM) | Pmax (µmol mg−1 Chl h−1) | [DIC] at CO2 CP* (µM) |
| WT (H)† | 515 ± 28 (14)‡ | 179 ± 27 (14)‡ | 87 ± 18 (14)‡ |
| SLC4G (H) † | 181 ± 32 (11)‡ | 157 ± 35 (11)‡ | 51 ± 16 (11)‡ |
| WT (L)§ | 15 ± 9 (10)‡ | 180 ± 15 (10)‡ | 4.2 ± 2.6 (10)‡ |
| SLC4G (L)§ | 17 ± 8 (9)‡ | 182 ± 11 (9)‡ | 3.7 ± 1.2 (9)‡ |
*Compensation point.
†High-CO2–grown cells.
‡Number of replicates.
§Low-CO2–grown cells.
DIC Fluxes Across the Plasma Membrane and Gross HCO3− Uptake by PtSLC4-2.
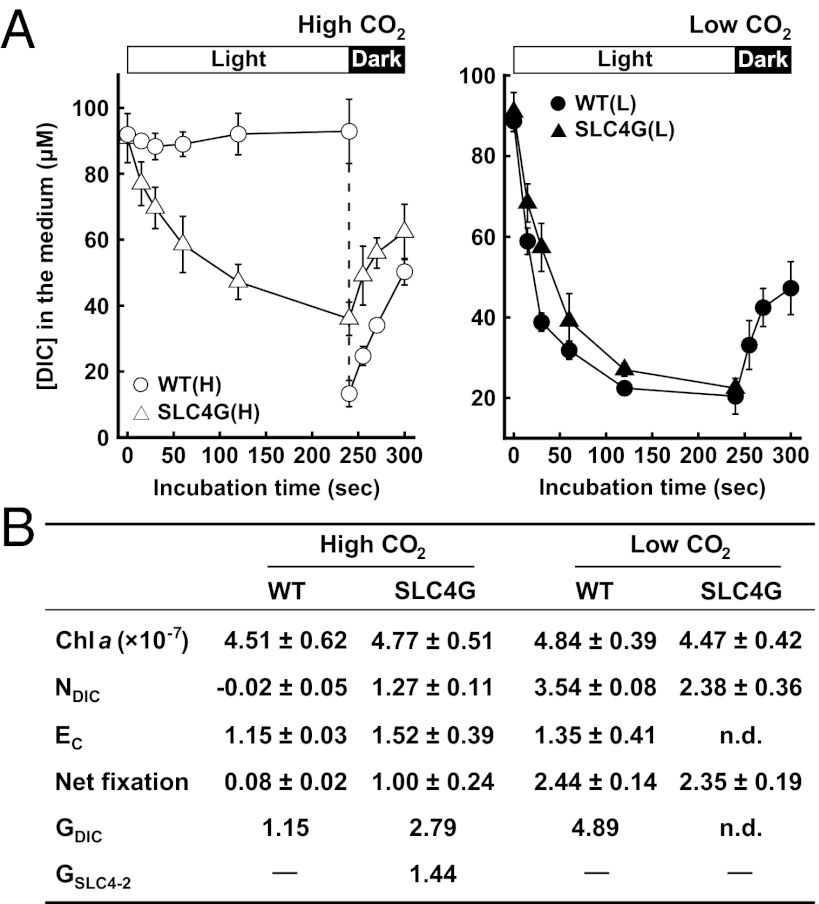
A DIC depletion and evolution time course by P. tricornutum cells was determined by a filtration–centrifugation technique combined with a gas chromatographic (GC) detection of [DIC] (37) (methodological validation is in Fig. S3 B–D). These parameters were measured at an initial external DIC of 100 µM, a concentration well below what is required to saturate photosynthesis at the seawater level pH (thus, it is suitable to compare photosynthetic affinity). WT cells grown in high CO2 showed little net DIC uptake rate (NDIC), which was calculated from the initial rate of DIC depletion from the bulk medium (Fig. 3A, open circles). In contrast, NDIC by low-CO2–grown WT and SLC4G cells was fairly quick (3.54 and 2.38 µmol mg−1 Chl min−1, respectively), with ∼60% DIC taken up from the surrounding seawater in the first 30 s (Fig. 3A, closed symbols). Similarly, high-CO2–grown SLC4G cells revealed fast NDIC (1.27 µmol mg−1 Chl min−1) (Fig. 3A, open triangles). The CO2 efflux rate (Ec) was determined from DIC evolution time course immediately after turning off the light in an assumption that the efflux of HCO3− is negligible relative to the efflux of CO2 (20). High-CO2–grown WT cells showed Ec of 1.15 µmol mg−1 Chl min−1 after turning off the light in a condition where external DIC was quickly replaced by DIC-free medium (Fig. 3 A, open circles, and B). Similar Ec values were obtained with high-CO2–grown SLC4G cells and low-CO2–grown WT cells (Fig. 3 A, open triangles and closed circles, and B) when light was turned off at the stationary phase of each DIC uptake. The Chl a content of SLC4G cells was marginally different from WT cells, irrespective of growth conditions (Fig. 3B). The net rates of CO2 fixation in each cell were determined by the O2 evolution rate at 100 µM DIC in the light (Fig. 3B). Together with the Chl a content and the net fixation rate, aforementioned parameters Ec and NDIC are summarized in Fig. 3B.
Fig. 3.
Determination of DIC flux parameters and the contribution of PtSLC4-2 to gross total DIC uptake. (A) Time course of DIC depletion and evolution by WT (circles) and SLC4G cells (triangles) grown in high (open symbols) and low CO2 (closed symbols). Data are mean ± SD (n = 3–4). (B) Chl a content and DIC flux parameters at 100 μM initial DIC in WT and SLC4G cells grown in high and low CO2. Chl a is in micrograms cell−1. Ec, CO2 efflux rate; GDIC, gross DIC uptake rate; GSLC4-2, gross HCO3− uptake rate by PtSLC4-2; NDIC, net DIC uptake rate; net fixation, net CO2 fixation rate determined by the O2 evolution assay; n.d., not determined. Unit of all flux parameters is micromoles milligrams−1 Chl minute−1.
A DIC flux model across the plasma membrane was constructed according to Badger et al. (38) (Fig. S4), and the model was solved theoretically with the parameters described above (Fig. 3B and SI Results). As a result, a gross DIC uptake (GDIC) in high-CO2–grown SLC4G cells was about 2.4 times and 57% of GDIC of high- and low-CO2–grown WT cells, respectively (Fig. 3B). The contribution of PtSLC4-2 to the gross HCO3− uptake (GSLC4-2) in high-CO2–grown SLC4G cells was, thus, calculated to be 1.44 µmol mg−1 Chl min−1 (Fig. 3B). The DIC accumulation in high-CO2–grown SLC4G cells was about 2.2 times higher than the DIC accumulation of high-CO2–grown WT cells (Fig. S5A), reflecting the difference in GDIC between these cells. The difference in Ec values in Fig. 3 was small, and thus, we used the NDIC value for evaluations of the PtSLC4-2 activity in assays hereafter.
Inhibition of PtSLC4s.
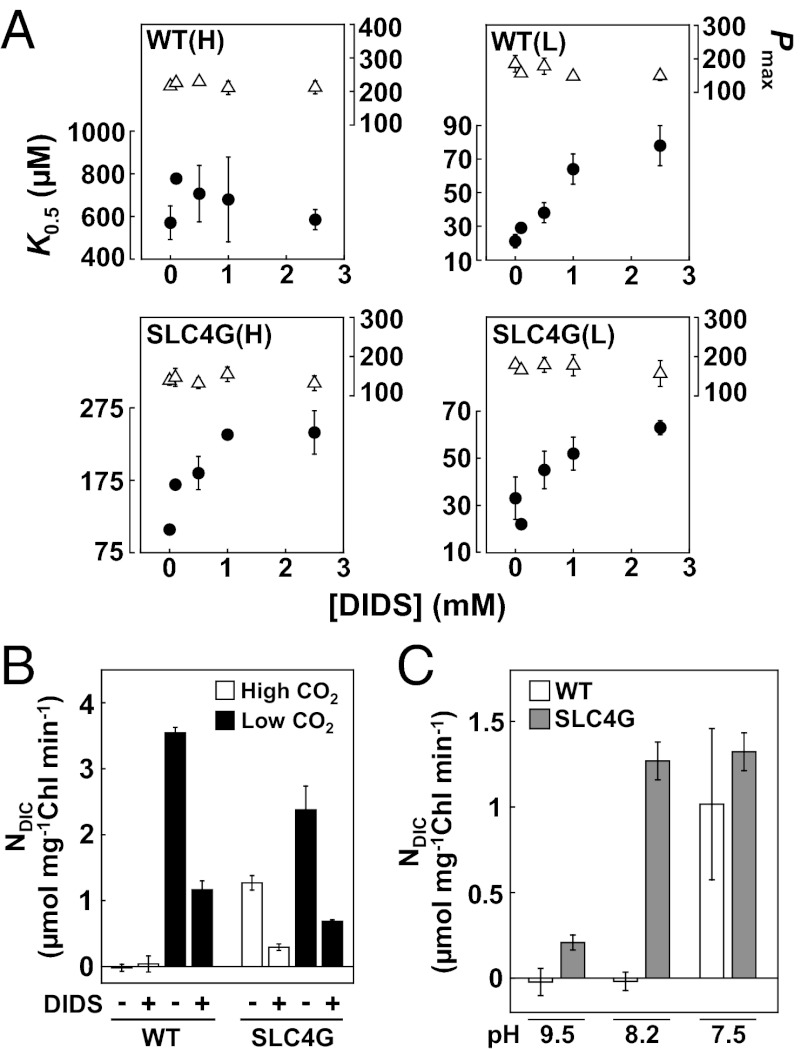
4,4′-Diisothiocyanostilbene-2,2′-disulfonic acid (DIDS), an inhibitor commonly used for mammalian SLC-type HCO3− transporters (39), did not significantly inhibit photosynthetic efficiency in high-CO2–grown WT cells (Fig. 4A). In sharp contrast, DIDS treatment of low-CO2–grown WT cells and SLC4G cells (grown under both high- and low-CO2 conditions) significantly increased the K0.5[HCO3−] values in a dose-dependent manner, whereas Pmax values were unaffected by DIDS (Fig. 4A). The effect of DIDS on K0.5[HCO3−] of low-CO2–grown cells was significant (Fig. 4A), indicating a major role for the DIDS-sensitive SLC-type transporters supporting high-affinity photosynthesis in low-CO2–grown P. tricornutum. The same tendency was apparent in the effect of saturating (3 mM) DIDS on NDIC. There was very little NDIC in high-CO2–grown WT cells, and DIDS exerted little inhibition; however, in low-CO2–grown WT cells, 3 mM DIDS reduced NDIC by 65% (Fig. 4B). By clear contrast, high-CO2–grown SLC4G cells showed the fast NDIC comparable with low-CO2–grown cells, and 67% of this uptake was inhibited by DIDS (Fig. 4B). These results account for the function of the plasma membrane transporter, PtSLC4-2, and perhaps, PtSLC4-1 and -4, which support the majority of the DIC influx into diatoms. Our previous study of the physiological estimate and the molecular localization negated the occurrence of CAext in the strain used in this study (40, 41). Furthermore, treatment by a CAext inhibitor, N-(5-sulfamoyl-1,3,4-thiadiazol-2-yl) acetamide, showed little effect on NDIC (Fig. S5B). The CAext was, thus, excluded from the CCM model in this study.
Fig. 4.
Effects of an inhibitor and pH on the function of PtSLC4-2. (A) Effects of various concentrations of SLC4 inhibitor, DIDS, on photosynthetic parameters of WT and SLC4G cells grown in H and L. K0.5 [HCO3−] (closed circles); Pmax (open triangles) in micromoles O2 milligrams−1 Chl hour−1. Values are mean ± SD of at least three replicates. (B) Effects of DIDS on NDIC of WT and SLC4G cells grown in high and low CO2. (C) Effects of pH on NDIC of WT (white bars) and SLC4G (gray bars) cells grown in high CO2. Initial [DIC] was set at 100 μM for NDIC measurement in B and C. Values are mean ± SD (n = 3).
pH and Substrate Dependency.
There were clear differences in pH dependency of NDIC between high-CO2–grown WT and SLC4G cells. High-CO2–grown WT cells displayed little NDIC in the presence of initial HCO3− of 100 μM in the media with pH 8.2 and 9.5, where there was virtually no CO2 available, whereas NDIC was evident (∼1.0 μmol DIC mg−1 Chl min−1) at pH 7.5 (Fig. 4C, white bars), which is presumably supported by a rapid production of CO2 at this pH. In sharp contrast, high-CO2–grown SLC4G cells showed significant NDIC at pH 9.5 (0.21 µmol DIC mg−1 Chl min−1), which reached a maximum level at pH below 8.2 (1.27–1.32 µmol DIC mg−1 Chl min−1), where HCO3− represented 94% of DIC (Fig. 4C, gray bars), strongly suggesting that HCO3− is preferentially taken up by PtSLC4-2 and functions optimally at the pHs typical of seawater. Together, from this evidence, it is clear that DIC influxes into low-CO2–grown WT cells are primarily supported by direct DIDS-sensitive HCO3− uptake, for which PtSLC4-2 is one of critical components.
Na+ Dependency of PtSLC4-2.
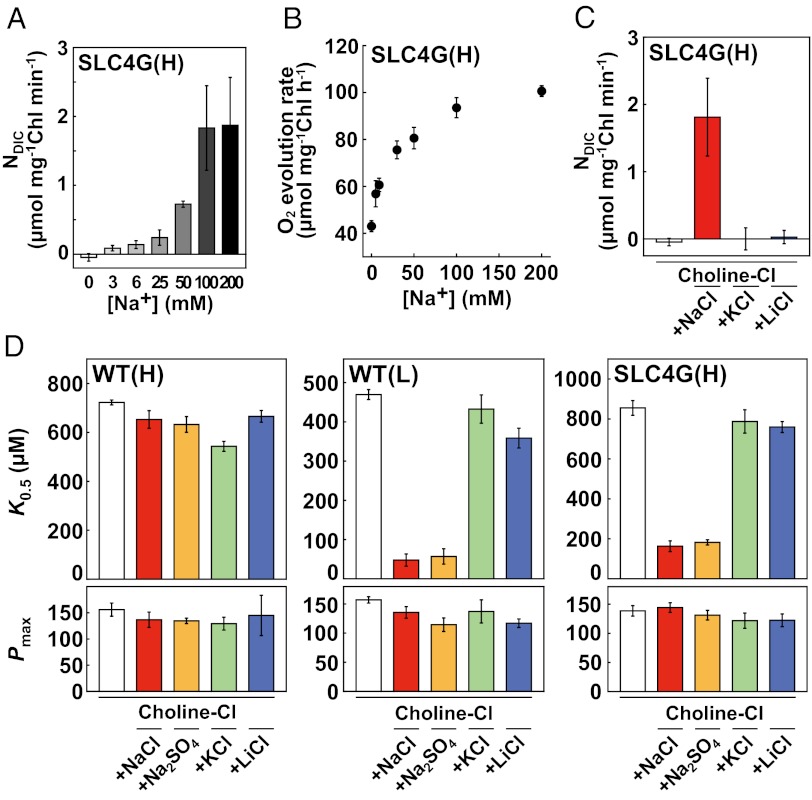
Many mammalian SLC4 transporters are Na+-dependent (39, 42, 43). DIC uptake in high-CO2–grown SLC4G cells was severely inhibited after complete depletion of Na+ ions from F/2ASW in experiments where osmotic pressure was maintained constant with choline-chloride (Fig. 5A). A linear increase in the NDIC of SLC4G cells was associated with increasing [Na+] and became saturated at levels above 100 mM Na+ (Fig. 5A). Oxygen evolution in high-CO2–grown SLC4G cells was also Na+-dependent, with saturation over 100 mM (Fig. 5B). NDIC by PtSLC4-2 was stimulated by Na+, and neither K+ nor Li+ provided functional substitution (Fig. 5C). The same ion selectivity was displayed for photosynthetic parameters. Photosynthetic affinity, K0.5[HCO3−], of high-CO2–grown WT cells was unaffected by [Na+], whereas a marked decrease in K0.5[HCO3−] was evident in both low-CO2–grown WT and high-CO2–grown SLC4G cells in the presence of 100 mM NaCl or 50 mM Na2SO4 (Fig. 5D). This Na+-dependent stimulation of affinity did not occur in the presence of an equivalent concentration of K+ or Li+, and the maximum photosynthetic activity, Pmax, was fairly stable at around 140 μmol mg−1 Chl h−1 in all experiments (Fig. 5D). These data indicate that Na+ is required for the DIC acquisition system and PtSLC4-2 activity.
Fig. 5.
Na+-dependent HCO3− uptake of PtSLC4-2. (A) Na+ dependency of NDIC in high-CO2–grown SLC4G cells. (B) Na+ dependency of the rate of O2 evolution in high-CO2–grown SLC4G cells on the addition of 160 µM HCO3−. (C) NDIC by high-CO2–grown SLC4G cells in the absence and presence of 100 mM NaCl, KCl, or LiCl. (D) Na+ dependency of photosynthetic parameters in WT and SLC4G cells grown in high or low CO2. (Upper) K0.5[HCO3−]. (Lower) Pmax values in micromoles O2 milligrams−1 Chl hour−1. Open bars, no salt control with 365 mM chorine chloride; red, 100 mM NaCl; yellow, 50 mM Na2SO4; green, 100 mM KCl; blue, 100 mM LiCl. Values are mean ± SD (n = 3–5).
Discussion
In this study, we have clearly shown the occurrence of SLC4-type protein and their significant contribution to the CCM in a marine diatom, a secondary endosymbiotic chromalveolata. The mammalian SLC4 family has been subcategorized into several functional groups with potential as transporters for HCO3− and/or CO32−, which are Na+-independent Cl−/HCO3− exchangers, Na+–HCO3− cotransporters, and Na+-driven Cl−/HCO3− exchangers (39, 42–44). Theoretical considerations on DIC fluxes in this study quantified the contribution of PtSLC4-2 (DICSLC4-2) to total uptake, which reached to above 50% of GDIC in high-CO2–grown SLC4G cells (Fig. 3B and SI Results). These theoretical arrangements were based on an assumption that this transporter is specific for HCO3−, and evidence in the present study is likely in favor of this assumption. It is highly probable that the PtSLC4-2 specifically pumps HCO3− from seawater in an Na+-dependent manner and is capable of contributing to total DIC uptake under the CO2-limiting environment. The mode of HCO3− transport by PtSLC4-2 most likely involves Na+–HCO3− cotransport or Na+-driven Cl−/HCO3− exchange, both which would require Na+ ions above 100 mM and thus, is fully active in normal seawater, although the mechanism for transport so far remains to be elucidated.
PtSLC4-2 is unlikely a transporter of high affinity for HCO3−, because the overexpression in high-CO2–grown cells resulted in a moderate improvement of photosynthetic affinity for HCO3− but did not sufficiently mimic the high-affinity state in low-CO2–grown cells (Table 1 and Fig. S3A). A significant difference in the K0.5[HCO3−] values between low-CO2–grown cells (both WT and SLC4G) and high-CO2–grown SLC4G cells under the saturating DIDS concentrations is also indicative of the operation of the additional transport system (Fig. 4A). These results strongly suggest participation of other high-affinity transporters under low-CO2 environment, for which two low-CO2–inducible transporters, SLC4-1 and -4, are so far the likeliest candidates. Relating to this consideration, it is important to note that numerous constitutive-type SLC family proteins are likely to occur in diatoms (Fig. 1). In fact, the DIC flux model and a DIC accumulation assay performed in the present study displayed a considerably high GDIC and an internal DIC accumulation even in high-CO2–grown WT cells (Fig. 3, Fig. S5A, and SI Results), suggesting the operation of both constitutive- and inducible-type DIC transport systems in marine diatoms.
Cyanobacterial BicA (SLC26-type protein) and SbtA occur in freshwater and marine species. BicA and SbtA are found to require about 1.7 and 1.0 mM Na+, respectively, for half-maximal stimulation of their HCO3− transport activity (7, 8). However, a mammalian SLC4-type HCO3− transporter, a muscle-specific Na+–HCO3− cotransporter, expressed in human skeletal muscle requires 24 mM Na+ for half-maximal stimulation (42). This Na+ dependency is quite similar to PtSLC4-2 (28 mM Na+) (Fig. 5B), indicating that they possess considerable halophilic dependency compared with their cyanobacterial counterparts. This halophilic dependency is probably a unique phenomenon to the SLC4 family. Such differences in the Na+ dependency of HCO3− uptake might significantly impinge on the survivability of certain strains and species of algae under environmental fluctuations in oceans.
It is noteworthy that PtSLC4-2 is a plasma membrane-type transporter that enables direct uptake of HCO3− from the bulk seawater, providing a solid molecular example that a direct HCO3− transport at the plasmalemma seems to be the major route for CO2 acquisition in marine diatoms and supporting the past physiological evidences (2, 19–22, 41). A significantly fast DIC efflux immediately after turning off the light (Fig. 3A) strongly suggests an occurrence of the carbon cycling system across the plasma membrane, which would potentially work as an efficient system for the light energy dissipation (25). It is also interesting that diatoms do not contain genes homologous to the other algal transporters, cyanobacterial bicarbonate transporter 1, SbtA, and Chlamydomonas LCI1, presumably because of the evolutionary history of the CCM, which might have independently evolved after the primary endosymbiosis (12). The possibility, thus, remains that aquatic photoautotrophs may have recruited a wide variety of HCO3− transporters from different ancestral symbiotic hosts, generating a diverse system for HCO3− acquisition. Diatoms may use transporters derived from the common ancestor with mammals, which is further supported by a fact that a DIDS is commonly effective on these transporters. These proteins function diversely as key players for the pH homeostasis in our body and the carbon balance in the ocean.
The molecular mechanisms of DIC acquisition in chromalveolates have been almost totally unknown, and the impact of CCMs on global primary productivity is not a part of the current prediction model for the ocean ecosystem (45). Molecular details of DIC uptake, subsequent flux controls of substrate carbon for photosynthesis, and their genetic regulation in marine diatoms in response to environmental factors are potentially vital to the model.
Materials and Methods
Cells and Culture Conditions.
The marine diatom P. tricornutum Bolin (UTEX 642) was obtained from the University of Texas Culture Collection and cultured in artificial seawater, which was supplemented with half-strength Guillard’s f solution (F/2ASW) under continuous illumination (50–75 µmol m−2 s−1) at 20 °C under constant aeration with 5% CO2 or ambient air (0.039% CO2).
Semiquantitative RT-PCR and qRT-PCR.
Total RNA was isolated from high- and low-CO2–grown WT cells using the RNeasy Plant Mini Kit (Qiagen). After the single-strand cDNAs were synthesized, semiquantitative RT-PCR and qRT-PCR were carried out by a set of primers shown in Table S1.
Construct of a Transformation Vector Containing the ptSLC4-2::egfp Fusion.
Inverse PCR was carried out on pFcpApGFP vector (46) as a template with high-fidelity PrimeSTAR HS DNA polymerase (TaKaRa) and a primer pair: egfp Fw: 5′-ATGGTGAGCAAGGGCGAGGAGCTGTTC-3′; fcpAp Rv: 5′-TCGAAACGGCAGACAAATTTGTG-3′. A ptSLC4-2 fragment was amplified from single-strand cDNA synthesized by the SMART RACE cDNA Amplification Kit, phosphorylated, and ligated at the upstream region of egfp in the inverse PCR product described above.
Transformation of WT Cells and Screening of Transformants.
Transformation was carried out as previously described (47), and transformants were screened on agar plates containing 100 µg mL−1 Zeocin. GFP positive clones were further screened from the Zeocin-resistant clones.
Confocal Laser Microscopy.
Cells at midlogarithmic growth phase were harvested and resuspended in a small volume of F/2ASW. The nucleus in the SLC4G cells was stained by 5 μM Hoechst 33342 at room temperature for 30 min. The stained cells were washed three times with F/2ASW. Specimens were observed using a Nikon A-1 confocal microscope.
Determination of Photosynthetic Parameters.
After high- and low-CO2–grown cells at the midlogarithmic growth phase were harvested by centrifugation, cells were washed with DIC-free F/2ASW. Cells were then suspended in the same buffer at a Chl a concentration of 10 µg mL−1 (determined as described in SI Materials and Methods). The rate of photosynthetic O2 evolution at various concentrations of DIC was measured with a Clark-type oxygen electrode (Hanzatech). [DIC] at CO2 compensation point was measured by GC. K0.5 and Pmax values were determined by the least-squares method.
Determination of the DIC Flux Parameters.
Cells were washed and resuspended as described above, except that final Chl a concentration was set at 40 µg mL−1. NDIC and Ec values were determined by a filtration–centrifugation technique using an Eppendorf tube equipped with a filter cartridge. Cell culture maintained at the CO2 compensation point was mixed in the filtrater cartridge with NaHCO3 at a final concentration of 100 µM. The bulk medium was filtered by centrifugation after various reaction times in the light, and [DIC] was determined by GC. DIC depletion rate was calculated as NDIC. Ec was determined as a CO2 evolution rate in the dark. Ec of high-CO2–grown WT cells was determined in the dark immediately after the removal of the external DIC. GDIC and GSLC4-2 values were calculated as described in SI Materials and Methods.
Additional details of materials and methods are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Gale G. Bozzo for critical reading of this manuscript, Ms. Sae Kikutani and Ms. Nobuko Higashiuchi for their technical assistance, and Ms. Miyabi Inoue for her skillful secretarial aid. This work was supported by Grant-in-Aid for Scientific Research B 24310015 (to Y.M.) and Grant-in-Aid for Challenging Exploratory Research 24651119 (to Y.M.) from the Japan Society for the Promotion of Science (JSPS) and the Ministry of Education, Culture, Sports, Science, and Technology (MEXT)-Supported Program for the Strategic Research Foundation (Y.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. AB733620 (ptSLC4-1), AB733621 (ptSLC4-2), AB733622 (ptSLC4-4), AB733623 (ptSLC4-6), and AB733624 (ptSLC4-7)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1216234110/-/DCSupplemental.
References
- 1.Badger MR, Price GD. CO2 concentrating mechanisms in cyanobacteria: Molecular components, their diversity and evolution. J Exp Bot. 2003;54(383):609–622. doi: 10.1093/jxb/erg076. [DOI] [PubMed] [Google Scholar]
- 2.Matsuda Y, Hara T, Colman B. Regulation of the induction of bicarbonate uptake by dissolved CO2 in the marine diatom, Phaeodactylum tricornutum. Plant Cell Environ. 2001;24(6):611–620. [Google Scholar]
- 3.Tréguer P, et al. The silica balance in the world ocean: A reestimate. Science. 1995;268(5209):375–379. doi: 10.1126/science.268.5209.375. [DOI] [PubMed] [Google Scholar]
- 4.Falkowski P, et al. The global carbon cycle: A test of our knowledge of earth as a system. Science. 2000;290(5490):291–296. doi: 10.1126/science.290.5490.291. [DOI] [PubMed] [Google Scholar]
- 5.Badger MR, et al. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Can J Bot. 1998;76(6):1052–1071. [Google Scholar]
- 6.Omata T, et al. Identification of an ATP-binding cassette transporter involved in bicarbonate uptake in the cyanobacterium Synechococcus sp. strain PCC 7942. Proc Natl Acad Sci USA. 1999;96(23):13571–13576. doi: 10.1073/pnas.96.23.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shibata M, et al. Genes essential to sodium-dependent bicarbonate transport in cyanobacteria: Function and phylogenetic analysis. J Biol Chem. 2002;277(21):18658–18664. doi: 10.1074/jbc.M112468200. [DOI] [PubMed] [Google Scholar]
- 8.Price GD, Woodger FJ, Badger MR, Howitt SM, Tucker L. Identification of a SulP-type bicarbonate transporter in marine cyanobacteria. Proc Natl Acad Sci USA. 2004;101(52):18228–18233. doi: 10.1073/pnas.0405211101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maeda S, Badger MR, Price GD. Novel gene products associated with NdhD3/D4-containing NDH-1 complexes are involved in photosynthetic CO2 hydration in the cyanobacterium, Synechococcus sp. PCC7942. Mol Microbiol. 2002;43(2):425–435. doi: 10.1046/j.1365-2958.2002.02753.x. [DOI] [PubMed] [Google Scholar]
- 10.Kerfeld CA, et al. Protein structures forming the shell of primitive bacterial organelles. Science. 2005;309(5736):936–938. doi: 10.1126/science.1113397. [DOI] [PubMed] [Google Scholar]
- 11.So AKC, Cot SSW, Espie GS. Characterization of the C-terminal extension of carboxysomal carbonic anhydrase from Synechocystis sp PCC6803. Funct Plant Biol. 2002;29(3):183–194. doi: 10.1071/PP01179. [DOI] [PubMed] [Google Scholar]
- 12.Badger MR, Hanson D, Price GD. Evolution and diversity of CO2 concentrating mechanisms in cyanobacteria. Funct Plant Biol. 2002;29(3):161–173. doi: 10.1071/PP01213. [DOI] [PubMed] [Google Scholar]
- 13.Genkov T, Meyer M, Griffiths H, Spreitzer RJ. Functional hybrid rubisco enzymes with plant small subunits and algal large subunits: Engineered rbcS cDNA for expression in chlamydomonas. J Biol Chem. 2010;285(26):19833–19841. doi: 10.1074/jbc.M110.124230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitra M, Lato SM, Ynalvez RA, Xiao Y, Moroney JV. Identification of a new chloroplast carbonic anhydrase in Chlamydomonas reinhardtii. Plant Physiol. 2004;135(1):173–182. doi: 10.1104/pp.103.037283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raven JA. CO2-concentrating mechanism: A direct role for thylakoid lumen acidification? Plant Cell Environ. 1997;20(2):147–154. [Google Scholar]
- 16.Yamano T, et al. Light and low-CO2-dependent LCIB-LCIC complex localization in the chloroplast supports the carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Cell Physiol. 2010;51(9):1453–1468. doi: 10.1093/pcp/pcq105. [DOI] [PubMed] [Google Scholar]
- 17.Ohnishi N, et al. Expression of a low CO2-inducible protein, LCI1, increases inorganic carbon uptake in the green alga Chlamydomonas reinhardtii. Plant Cell. 2010;22(9):3105–3117. doi: 10.1105/tpc.109.071811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ynalvez RA, Xiao Y, Ward AS, Cunnusamy K, Moroney JV. Identification and characterization of two closely related β-carbonic anhydrases from Chlamydomonas reinhardtii. Physiol Plant. 2008;133(1):15–26. doi: 10.1111/j.1399-3054.2007.01043.x. [DOI] [PubMed] [Google Scholar]
- 19.Trimborn S, et al. Inorganic carbon acquisition in potentially toxic and non-toxic diatoms: The effect of pH-induced changes in seawater carbonate chemistry. Physiol Plant. 2008;133(1):92–105. doi: 10.1111/j.1399-3054.2007.01038.x. [DOI] [PubMed] [Google Scholar]
- 20.Burkhardt S, Amoroso G, Riebesell U, Sültemeyer D. CO2 and HCO3- uptake in marine diatoms acclimated to different CO2 concentrations. Limnol Oceanogr. 2001;46(6):1378–1391. [Google Scholar]
- 21.Korb RE, Saville PJ, Johnston AM, Raven JA. Sources of inorganic carbon for photosynthesis by three species of marine diatom. J Phycol. 1997;33(3):433–440. [Google Scholar]
- 22.Rost B, et al. Isotope disequilibrium and mass spectrometric studies of inorganic carbon acquisition by phytoplankton. Limnol Oceanogr Methods. 2007;5:328–337. [Google Scholar]
- 23.Johnston AM, Raven JA. Inoragnic carbon accumulation by the marine diatom Phaeodactylum tricornutum. Eur J Phycol. 1996;31(3):285–290. [Google Scholar]
- 24.Reinfelder JR, Kraepiel AML, Morel FMM. Unicellular C4 photosynthesis in a marine diatom. Nature. 2000;407(6807):996–999. doi: 10.1038/35039612. [DOI] [PubMed] [Google Scholar]
- 25.Tchernov D, Silverman J, Luz B, Reinhold L, Kaplan A. Massive light-dependent cycling of inorganic carbon between oxygenic photosynthetic microorganisms and their surroundings. Photosynth Res. 2003;77(2–3):95–103. doi: 10.1023/A:1025869600935. [DOI] [PubMed] [Google Scholar]
- 26.Armbrust EV, et al. The genome of the diatom Thalassiosira pseudonana: Ecology, evolution, and metabolism. Science. 2004;306(5693):79–86. doi: 10.1126/science.1101156. [DOI] [PubMed] [Google Scholar]
- 27.Bowler C, et al. The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature. 2008;456(7219):239–244. doi: 10.1038/nature07410. [DOI] [PubMed] [Google Scholar]
- 28.Cavalier-Smith T. Membrane heredity and early chloroplast evolution. Trends Plant Sci. 2000;5(4):174–182. doi: 10.1016/s1360-1385(00)01598-3. [DOI] [PubMed] [Google Scholar]
- 29.Bonar PT, Casey JR. Plasma membrane Cl−/HCO3− exchangers: Structure, mechanism and physiology. Channels (Austin) 2008;2(5):337–345. doi: 10.4161/chan.2.5.6899. [DOI] [PubMed] [Google Scholar]
- 30.Cordat E, Casey JR. Bicarbonate transport in cell physiology and disease. Biochem J. 2009;417(2):423–439. doi: 10.1042/BJ20081634. [DOI] [PubMed] [Google Scholar]
- 31.Bevensee MO, Schmitt BM, Choi I, Romero MF, Boron WF. An electrogenic Na+-HCO3- cotransporter (NBC) with a novel COOH-terminus, cloned from rat brain. Am J Physiol Cell Physiol. 2000;278(6):C1200–C1211. doi: 10.1152/ajpcell.2000.278.6.C1200. [DOI] [PubMed] [Google Scholar]
- 32.Kroth PG, et al. A model for carbohydrate metabolism in the diatom Phaeodactylum tricornutum deduced from comparative whole genome analysis. PLoS One. 2008;3(1):e1426. doi: 10.1371/journal.pone.0001426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Groves JD, Tanner MJ. Role of N-glycosylation in the expression of human band 3-mediated anion transport. Mol Membr Biol. 1994;11(1):31–38. doi: 10.3109/09687689409161027. [DOI] [PubMed] [Google Scholar]
- 34.Choi I, Hu L, Rojas JD, Schmitt BM, Boron WF. Role of glycosylation in the renal electrogenic Na+-HCO3- cotransporter (NBCe1) Am J Physiol Renal Physiol. 2003;284(6):F1199–F1206. doi: 10.1152/ajprenal.00131.2002. [DOI] [PubMed] [Google Scholar]
- 35.Pushkin A, et al. Molecular mechanism of kNBC1-carbonic anhydrase II interaction in proximal tubule cells. J Physiol. 2004;559(Pt 1):55–65. doi: 10.1113/jphysiol.2004.065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harada H, Nakatsuma D, Ishida M, Matsuda Y. Regulation of the expression of intracellular β-carbonic anhydrase in response to CO2 and light in the marine diatom Phaeodactylum tricornutum. Plant Physiol. 2005;139(2):1041–1050. doi: 10.1104/pp.105.065185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brimingham BC, Colman B. Measurement of carbon dioxide compensation points of freshwater algae. Plant Physiol. 1979;64(5):104–130. doi: 10.1104/pp.64.5.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badger MR, Palmqvist K, Yu J. Measurement of CO2 and HCO3- fluxes in cyanobacteria and microalgae during steady-state photosynthesis. Physiol Plant. 1994;90(3):529–536. [Google Scholar]
- 39.Romero MF, Fulton CM, Boron WF. The SLC4 family of HCO 3 - transporters. Pflugers Arch. 2004;447(5):495–509. doi: 10.1007/s00424-003-1180-2. [DOI] [PubMed] [Google Scholar]
- 40.Tachibana M, et al. Localization of putative carbonic anhydrases in two marine diatoms, Phaeodactylum tricornutum and Thalassiosira pseudonana. Photosynth Res. 2011;109(1–3):205–221. doi: 10.1007/s11120-011-9634-4. [DOI] [PubMed] [Google Scholar]
- 41.Satoh D, Hiraoka Y, Colman B, Matsuda Y. Physiological and molecular biological characterization of intracellular carbonic anhydrase from the marine diatom Phaeodactylum tricornutum. Plant Physiol. 2001;126(4):1459–1470. doi: 10.1104/pp.126.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pushkin A, et al. Cloning, tissue distribution, genomic organization, and functional characterization of NBC3, a new member of the sodium bicarbonate cotransporter family. J Biol Chem. 1999;274(23):16569–16575. doi: 10.1074/jbc.274.23.16569. [DOI] [PubMed] [Google Scholar]
- 43.Wang CZ, Yano H, Nagashima K, Seino S. The Na+-driven Cl-/HCO3- exchanger. Cloning, tissue distribution, and functional characterization. J Biol Chem. 2000;275(45):35486–35490. doi: 10.1074/jbc.C000456200. [DOI] [PubMed] [Google Scholar]
- 44.Sterling D, Casey JR. Transport activity of AE3 chloride/bicarbonate anion-exchange proteins and their regulation by intracellular pH. Biochem J. 1999;344(Pt 1):221–229. [PMC free article] [PubMed] [Google Scholar]
- 45.Raven JA, Giordano M, Beardall J, Maberly SC. Algal and aquatic plant carbon concentrating mechanisms in relation to environmental change. Photosynth Res. 2011;109(1–3):281–296. doi: 10.1007/s11120-011-9632-6. [DOI] [PubMed] [Google Scholar]
- 46.Kitao Y, Harada H, Matsuda Y. Localization and targeting mechanisms of two chloroplastic β-carbonic anhydrases in the marine diatom Phaeodactylum tricornutum. Physiol Plant. 2008;133(1):68–77. doi: 10.1111/j.1399-3054.2008.01053.x. [DOI] [PubMed] [Google Scholar]
- 47.Zaslavskaia LA, et al. Transformation of the diatom Phaeodactylum tricornutum (Bacillariophyceae) with a variety of selectable marker and reporter genes. J Phycol. 2000;36(2):379–386. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.