Abstract
Parkinson’s disease is characterized by abundant α-Synuclein (α-Syn) neuronal inclusions known as Lewy-bodies and Lewy-neurites, and the massive loss of midbrain dopamine neurons. However, a cause-and-effect relationship between Lewy inclusion formation and neurodegeneration remains unclear. Here we found that in wildtype non-transgenic mice a single intrastriatal inoculation of synthetic α-Syn fibrils led to the cell-to-cell transmission of pathologic α-Syn and Parkinson’s-like Lewy pathology in anatomically interconnected regions. Lewy pathology accumulation resulted in progressive loss of dopamine neurons in the substantia nigra pars compacta, but not in the adjacent ventral tegmental area, and was accompanied by reduced dopamine levels culminating in motor deficits. This recapitulation of a neurodegenerative cascade thus establishes a mechanistic link between transmission of pathologic α-Syn and the cardinal features of Parkinson’s disease.
Parkinson’s disease (PD) is a multisystem neurodegenerative disorder characterized by two major disease processes: the accumulation of intraneuronal Lewy-bodies/Lewy-neurites (LBs/LNs) containing misfolded fibrillar α-Syn, and the selective degeneration of midbrain dopamine (DA) neurons in the substantia nigra pars compacta (SNpc) leading to bradykinesia, tremor, and postural instability (1). The etiology of these processes remains unclear, although in familial PD, autosomal dominant α-Syn gene mutations or amplifications directly link α-Syn dysfunction to disease causation (2, 3). Moreover, despite the observation that α-Syn pathology is present in virtually all sporadic and familial PD patients (4) and that its distribution correlates with clinical symptoms (5–7), the precise relationship between α-Syn misfolding and DA neuron loss remains controversial because neither transgenic nor neurotoxin-based animal models of PD fully recapitulate the DA neuron degeneration, motor deficits, and the LB/LN-like α-Syn pathology seen in PD (8, 9).
Converging lines of evidence indicate that misfolded fibrillar forms of neurodegenerative disease-related proteins self-propagate and spread between interconnected central nervous system (CNS) regions, suggesting that cell-to-cell transmission of pathological proteins contributes to disease progression (10–15). Indeed, the pattern of LB/LN accumulation in human PD is highly stereotypical, consistent with propagation of disease over functional networks, suggesting that the transmission of pathologic α-Syn and its corruption of endogenous protein plays a central role in PD pathogenesis and progression (16, 17). Moreover, reports that embryonic mesencephalic neuron grafts in PD patients develop LBs many years after grafting (18, 19) further support the transmissibility of pathologic α-Syn. Consistent with this hypothesis, intracerebral inoculation of α-Syn pre-formed fibrils (PFFs) comprised of recombinant protein accelerates the onset of neurological symptoms and death in transgenic mice overexpressing human A53T mutant α-Syn by enhancing the conversion of endogenous α-Syn into pathological LBs/LNs (20). While these data implicate α-Syn misfolding and fibrillization as triggering events upstream of neuronal dysfunction/death, minimal DA cell loss was observed, possibly reflecting alterations in the pattern of α-Syn transgene expression and the rapid demise of the inoculated mice.
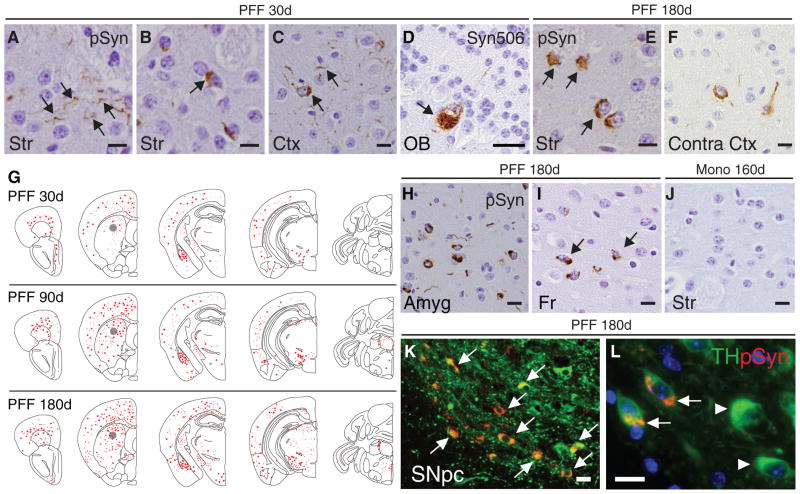
Because synthetic α-Syn PFFs efficiently seed the aggregation and fibrillization of soluble endogenous α-Syn in primary neuronal cultures generated from wildtype (wt) mice (21), we asked if transmission of LB/LN pathology might occur in wt mice in vivo. To answer this, we first determined whether PFFs assembled from recombinant mouse α-Syn initiate pathological α-Syn conversion when introduced into young wt (C57BL6/C3H) mice by stereotaxic injections targeting the dorsal striatum (Fig. S1), a region receptive to PFF uptake (20) and interconnected with multiple CNS nuclei, including midbrain DA neurons. Deposits of hyperphosphorylated α-Syn (pSyn), a marker of human LBs/LNs (22), were visible at the injection site within 30 days of a single unilateral PFF inoculation (Fig. 1A,B,G). Intraneuronal α-Syn accumulations, primarily diffuse LN- and LB-like inclusions, were also present in several areas directly interconnected to the striatum that accumulate LBs in human synucleinopathies, most prominently in cortical layers IV-V, and olfactory bulb (Fig. 1C,D), confirming the pathological conversion of endogenous mouse α-Syn in wt mice. At 30 days post-injection (dpi), pSyn positive LB-like accumulations were exclusively ipsilateral to the injection site with the exception of the amygdala, to which the striatum projects bilaterally (23), suggesting that cell-to-cell transmission followed inter-neuronal connectivity.
Fig. 1. Intrastriatal inoculation of synthetic mouse α-Syn PFFs seed the aggregation of endogenous mouse Syn in wt mice.
Pathology in brains of C57BL6/C3H F1 mice following a single unilateral injection of mouse α-Syn PFFs into the dorsal striatum. (A–D) Accumulation of α-Syn in neuritic processes or as pale cytoplasmic inclusions in striatum (Str) and neocortex (Ctx), and olfactory mitral neurons (OB) ipsilateral to the injection at 30d. Black arrows highlight pathology revealed by immunostaining using anti-pSyn (A–C) or Syn506 (D). (E,F) LB-like inclusions in striatum and contralateral neocortex at 180d post-injection with PFFs. (G) CNS distribution of pSyn accumulations of mice that received a single inoculation of PFFs in the dorsal striatum (indicated by grey circles). Representative maps of LB/LN-like pathology (red dots and stipples, respectively) in the PFF-injected hemisphere are shown for mice sacrificed at 30d, 90d, or 180d post-injection. (H) α-Syn pathology in amygdala (Amyg), and (I) in frontal cortex (Fr). (J) pSyn staining in ipsilateral striatum 160d post-injection with monomeric recombinant α-Syn. (K) Double-immunostaining for pSyn (red) and TH (green) in a PFF-injected animal sacrificed at 180d showing LB-like α-Syn pathology in ipsilateral SNpc. (L) High magnification revealing colocalization of pSyn inclusions to DA neurons (white arrows) and reduced TH immunoreactivity compared to unaffected DA neurons (white arrowheads). Images are representative of 3–7 animals examined per group (see Table S1). Scale bars: 10 μm (A–C, E–F, H–L); 25 μm (D).
LB/LN-pathology within affected regions showed markedly increased pSyn immunoreactivity in mice examined 90 and 180 dpi (Fig. 1E,F,G). Additional populations, such as the contralateral neocortex, also developed LBs/LNs indicative of progressive spread to CNS regions (Fig. 1F–I, Table S1). Mapping of pSyn pathology in mice at 30, 90, and 180 dpi (Fig. 1G) revealed a time-dependent dissemination of LBs/LNs between 30–180 dpi with sequential involvement of populations initially unaffected at 30 dpi including ventral striatum, thalamus, and occipital cortex, along with commissural and brainstem fibers. Although inclusions were absent from multiple regions including hippocampus, septum, and cerebellum (Fig. 1G and Table S1), stereotaxic inoculation of equal quantities of PFFs into the hippocampus resulted in massive accumulation of α-Syn pathology (Fig.S2), indicating that neurons in this region also are susceptible to transmitted pathologic α-Syn. Thus, propagation of LBs/LNs is connectivity-dependent and provides an explanation for the non-uniform distribution of LBs/LNs observed in α-Syn PFF-seeded animals. In contrast, monomeric α-Syn or PBS injections did not result in α-Syn pathology at any time point examined (Fig. 1J and Fig.S2).
Striatal PFF inoculation led to abundant α-Syn pathology in tyrosine hydroxylase (TH) positive SNpc DA neurons (Fig. 1K,L) that project to medium spiny neurons of the dorsal striatum and whose selective degeneration is a major contributor to motor deficits in PD (24). Furthermore, α-Syn pathology in PFF-inoculated mice colocalized with key markers of LBs, including ubiquitin, heat-shock protein 90, and the amyloid-binding dye thioflavin S, indicating that they share common properties with authentic LBs/LNs in human PD (Fig.S3).
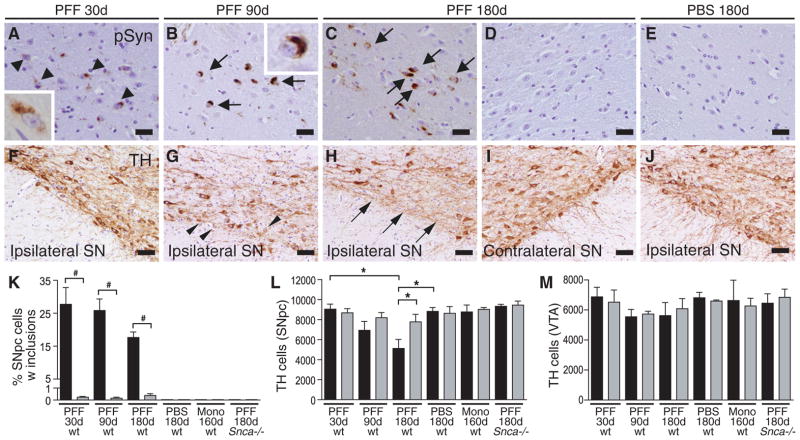
SNpc α-Syn pathology developed progressively following PFF injection, evolving from pale cytoplasmic accumulations at 30 dpi to dense perinuclear LB-like inclusions by 90 and 180 dpi, particularly among ventromedial SNpc populations (Fig. 2A–C). Because the nigrostriatal pathway is exclusively unilateral, LB/LN pathology in the SNpc was confined to the injected hemisphere at all time points examined (Figs.1G and 2A–D). In addition to being absent in ventral tegemental (VTA) neurons, α-Syn pathology was not observed in DA-expressing olfactory glomerular interneurons and arcuate cells of the hypothalamus (Fig.S4). Similarly, norepinephrine (locus coeruleus) and serotonergic (Raphe nucleus) populations also remained unaffected at 180 dpi, suggesting that monoaminergic neurons are not equally susceptible to pathological α-Syn spread.
Fig. 2. Seeded α-Syn pathology leads to progressive DA system degeneration.
(A–D) pSyn immunostaining in SNpc of mice sacrificed at 30d, 90d, or 180d following striatal PFF injection. (A) Diffuse perinuclear pSyn inclusions (black arrowheads) at 30d post-injection. (B,C) Dense LB-like inclusions (black arrows) at 90d and 180d post-injection. Absence of pSyn pathology in SNpc contralateral to the PFF injection site (D) and in ipsilateral SNpc of PBS-injected mouse (E) at 180d. (F–I) TH-immunostaining of SNpc at 30d, 90d and 180d following inoculation with Syn PFFs. Arrowheads in (G) indicate neurons with reduced TH staining in the ipsilateral SNpc at 90d post-injection. (H,I) Ventral SNpc, ipsilateral and contralateral to the site of PFF injection at 180d. Arrows point to DA neuron loss. (J) PBS-injected control at 180d post-injection. (K) Percentage of SNpc TH-neurons containing pSyn-immunoreactive inclusions for each treatment group. Data for treated ipsilateral (black) and contralateral (grey) hemispheres are shown. #p<0.001 paired t-test (N = 3–5 animals per group). (L,M) Quantification of TH-immunoreactive neurons in the SNpc and VTA of mice after intrastriatal PFF-, monomer- or PBS-injection. Data represent mean number of cells per region +/− SEM (N = 3–4 animals per group). *P<0.05 one-way ANOVA. Scale bars: 25 μm (A–D);50 μm (E–J).
α-Synuclein pathology in the SNpc was accompanied by the gradual and unilateral loss of TH-immunoreactivity and neurons (Fig. 2F–I), suggesting that intraneuronal α-Syn inclusions lead to DA neuron loss. 27.7% of SNpc DA neurons colocalized with pSyn pathology at 30 dpi (Fig. 2K), a stage at which SNpc and VTA DA neuron number were similar in both hemispheres (Fig. 2L,M). SNpc but not VTA DA neurons developed LBs/LNs, confirming transmission of misfolded α-Syn via network projections and suggesting that connectivity is a determinant of susceptibility. The proportion of inclusion-bearing SNpc DA neurons did not increase further, but instead declined over time. There was a concomitant decrease of SNpc DA neurons by 15% and 35% at 90 and 180 dpi, respectively, suggesting that LBs/LNs form prior to SNpc DA neuron loss. Moreover, DA neurons in the contralateral SNpc and the VTA of both hemispheres (where inclusions were present in <0.2% of neurons; Fig. 2K) were indistinguishable from those in PBS- or monomer-injected controls, non-injected age-matched mice, and PFF-injected Snca−/− mice, all of which did not develop α-Syn deposits (Fig. 2D,K–M). Thus, accumulation of pathologic α-Syn appears to be upstream of and directly linked to SNpc DA neuron loss.
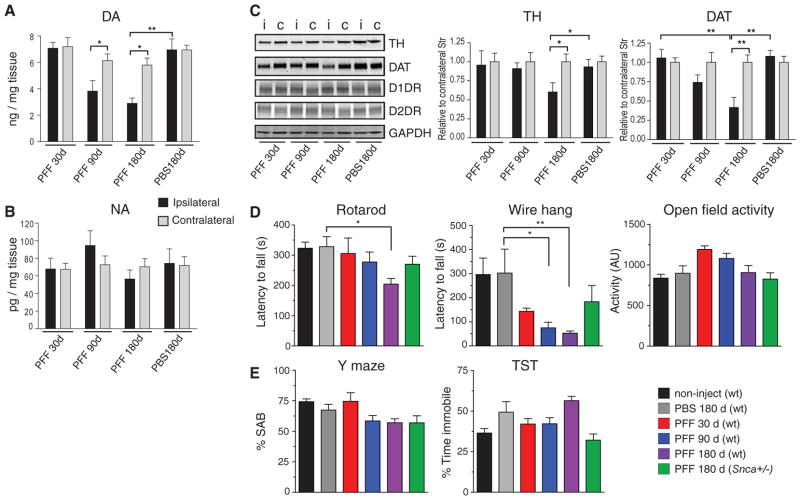
Concomitant with the progressive loss of SNpc neurons, striatal concentrations of DA and its metabolites in the injected hemisphere showed progressive reductions following PFF-inoculations, whereas contralateral striatal DA levels remain unaltered, and noradrenaline (NA) levels were unchanged bilaterally (Figs.3A,B and S5A). Immunoblot analyses confirmed reductions in TH and dopamine transporter (DAT) expression in the ipsilateral striatum (Fig. 3C) consistent with the loss of nigrostriatal DA innervation. Despite the loss of DA terminals, D1 and D2 dopamine receptor levels remained constant (Fig. 3C, and S5C), suggesting the preservation of striatal medium spiny projection neurons.
Fig. 3. Decreased striatal DA and motor deficits in mouse α-Syn PFF-injected wt mice.
DA (A) and NA (B) concentrations in dorsal striata of PFF-injected wt mice and PBS-treated controls measured at the indicated time points. Mean +/− SD are shown (N = 3–6 animals per group. *P<0.01; **P<0.001 one-way ANOVA. (C) Immunoblot analysis of striata from PFF- and PBS-treated animals, using antibodies against TH, DAT, D1 dopamine receptor (D1DR), and D2 dopamine receptor (D2DR). GAPDH is shown as a loading control. Mean intensity values are shown for TH and DAT (N = 3–4 striata per marker). Black and grey bars denote ipsilateral and contralateral regions, respectively. *P<0.05; **P<0.001, one-way ANOVA. (D,E) Behavioral assessment of wt mice 30d, 90d, or 180d (N = 6, 9, 17, and 6, respectively) following a single unilateral inoculation of α-Syn PFFs into the striatum. PFF-injected Snca+/− mice (N = 4), as well as age-matched non-injected (N = 20) and PBS-injected (N = 8) wt animals are also shown. Results of animals on the rotarod test (D, left panel) and wire hang test (D, middle panel) show progressive deficits in PFF-injected but not control mice. (E) Performance on the Y-maze and tail suspension test. Data are mean values +/− SD. Differences were established using one-way ANOVA (P=0.0012) with Tukey post-hoc test (*P<0.05; **P<0.01).
Given the PD-like Lewy pathology, SNpc neuron loss, and reduced striatal DA levels, PFF-inoculated wt mice might be expected to exhibit altered motor function, as in PD. Although no gross motor or behavioral abnormalities were observed up to 180 dpi, rotarod testing revealed significant and progressive performance deterioration in PFF-injected animals, indicative of impaired motor co-ordination and balance (Fig. 3D, left). Furthermore, mice with α-Syn pathology performed poorly on the wire hang test, a complementary measure of motor strength and co-ordination, declining 53% from baseline at 30 dpi and 81% at 180 dpi (Fig. 3D, middle). However, open field activity remained unchanged, indicating overall motor activity was not significantly altered (Fig. 3D, right).
As previously noted, intrastriatal PFF-inoculated mice did not develop hippocampal pathology (Fig. 1G), and no changes in memory function were observed as assessed using the Y-maze (Fig. 3E). This corroborates our interpretation that declining neuronal function is correlated with increasing α-Syn pathology in affected neurons. Moreover, in keeping with the relative sparing of mesolimbic projections, no significant differences were found between PFF-treated and control mice in the tail suspension test (Fig. 3E, right) a measure of depression-like behavior. The correlation between α-Syn pathology and neuronal deficits was also seen in striatal PFF-inoculated Snca+/− mice, where at 180 dpi both SNpc α-Syn pathology and motor impairments were attenuated relative to wt animals (Figs.3D,E and S6A). Finally, striatal inoculation into wt CD1 (Fig.S6B) andC57BL6/SJL mice (Table S1) also resulted in transmission of α-Syn pathology, suggesting that cell-to-cell spread of LBs/LNs is independent of mouse genetic background.
In summary, we demonstrate that a single intrastriatal injection of synthetic misfolded α-Syn seeds into wt mice initiates a neurodegenerative cascade characterized by the accumulation of intracellular LB/LN pathology, selective loss of SNpc DA neurons, and impaired motor coordination. Thus, α-Syn pathology is sufficient to induce the cardinal behavioral and pathological features of sporadic PD.
Supplementary Material
Acknowledgments
We thank M. Byrne, J. Levitan, I. Song, and J. Yeh for technical assistance, and L. Kwong, H. Tran, and K. Brunden for their comments. This work was funded by NIH NS053488, the JPB and Benaroya Foundations, and the Jeff and Anne Keefer Fund. KCL is supported in part by the University of Pennsylvania ITMAT.
Footnotes
The data reported in this paper are presented in the main text and Supporting Online Material. Materials and methods are available as supplementary material on Science Online.
References and Notes
- 1.Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114:2283. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 2.Simon-Sanchez J, et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41:1308. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singleton AB, et al. alpha-synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 4.Poulopoulos M, Levy OA, Alcalay RN. The neuropathology of genetic Parkinson’s disease. Mov Disord. 2012;27:831. doi: 10.1002/mds.24962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spillantini MG, et al. alpha-synuclein in Lewy bodies. Nature. 1997;388:839. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 6.Baba M, et al. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am J Pathol. 1998;152:879. [PMC free article] [PubMed] [Google Scholar]
- 7.Braak H, Braak E. Pathoanatomy of Parkinson’s disease. J Neurol. 2000;247(Suppl 2):II3. doi: 10.1007/PL00007758. [DOI] [PubMed] [Google Scholar]
- 8.Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson’s disease. Neuron. 2010;66:646. doi: 10.1016/j.neuron.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannon JR, Greenamyre JT. Neurotoxic in vivo models of Parkinson’s disease recent advances. Prog Brain Res. 2010;184:17. doi: 10.1016/S0079-6123(10)84002-6. [DOI] [PubMed] [Google Scholar]
- 10.Meyer-Luehmann M, et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313:1781. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- 11.Clavaguera F, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Calignon A, et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron. 2012;73:685. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen C, et al. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121:715. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desplats P, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci US A. 2009;106:13010. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aguzzi A, Rajendran L. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron. 2009;64:783. doi: 10.1016/j.neuron.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Angot E, Steiner JA, Hansen C, Li JY, Brundin P. Are synucleinopathies prion-like disorders? Lancet Neurol. 2010;9:1128. doi: 10.1016/S1474-4422(10)70213-1. [DOI] [PubMed] [Google Scholar]
- 17.Braak H, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 18.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14:504. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 19.Kordower JH, Chu Y, Hauser RA, Olanow CW, Freeman TB. Transplanted dopaminergic neurons develop PD pathologic changes: a second case report. Mov Disord. 2008;23:2303. doi: 10.1002/mds.22369. [DOI] [PubMed] [Google Scholar]
- 20.Luk KC, et al. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med. 2012;209:975. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volpicelli-Daley LA, et al. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujiwara H, et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 23.Pan WX, Mao T, Dudman JT. Inputs to the dorsal striatum of the mouse reflect the parallel circuit architecture of the forebrain. Front Neuroanat. 2012;4:147. doi: 10.3389/fnana.2010.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fahn S. Description of Parkinson’s disease as a clinical syndrome. Ann NY Acad Sci. 2003;991:1–14. 1. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- 25.Abeliovich A, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 26.Luk KC, et al. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci US A. 2009;106:20051. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conway KA, Harper JD, Lansbury PT. Fibrils formed in vitro from alpha-synuclein and two mutant forms linked to Parkinson’s disease are typical amyloid. Biochemistry. 2000;39:2552. doi: 10.1021/bi991447r. [DOI] [PubMed] [Google Scholar]
- 28.Murray J, IV, et al. Role of alpha-synuclein carboxy-terminus on fibril formation in vitro. Biochemistry. 2003;42:8530. doi: 10.1021/bi027363r. [DOI] [PubMed] [Google Scholar]
- 29.Duda JE, et al. Immunohistochemical and biochemical studies demonstrate a distinct profile of alpha-synuclein permutations in multiple system atrophy. J Neuropathol Exp Neurol. 2000;59:830. doi: 10.1093/jnen/59.9.830. [DOI] [PubMed] [Google Scholar]
- 30.Fu Y, et al. A cytoarchitectonic and chemoarchitectonic analysis of the dopamine cell groups in the substantia nigra, ventral tegmental area, and retrorubral field in the mouse. Brain Struct Funct. 2012;217:591. doi: 10.1007/s00429-011-0349-2. [DOI] [PubMed] [Google Scholar]
- 31.Yoshitake T, et al. High-sensitive liquid chromatographic method for determination of neuronal release of serotonin, noradrenaline and dopamine monitored by microdialysis in the rat prefrontal cortex. Journal of neuroscience methods. 2004;140:163. doi: 10.1016/j.jneumeth.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 32.Dai Y, Dudek NL, Li Q, Fowler SC, Muma NA. Striatal expression of a calmodulin fragment improved motor function, weight loss, and neuropathology in the R6/2 mouse model of Huntington’s disease. J Neurosci. 2009;29:11550. doi: 10.1523/JNEUROSCI.3307-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santa-Maria I, et al. GSPE interferes with tau aggregation in vivo: implication for treating tauopathy. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carroll JC, et al. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci. 2007;27:13357. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao S, et al. Insertion mutation at the C-terminus of the serotonin transporter disrupts brain serotonin function and emotion-related behaviors in mice. Neuroscience. 2006;140:321. doi: 10.1016/j.neuroscience.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 36.Carroll JC, Rosario ER, Villamagna A, Pike CJ. Continuous and cyclic progesterone differentially interact with estradiol in the regulation of Alzheimer-like pathology in female 3xTransgenic-Alzheimer’s disease mice. Endocrinology. 2010;151:2713. doi: 10.1210/en.2009-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carroll JC, et al. Sex differences in beta-amyloid accumulation in 3xTg-AD mice: role of neonatal sex steroid hormone exposure. Brain Res. 2010;1366:233. doi: 10.1016/j.brainres.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carroll JC, Pike CJ. Selective estrogen receptor modulators differentially regulate Alzheimer-like changes in female 3xTg-AD mice. Endocrinology. 2008;149:2607. doi: 10.1210/en.2007-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King DL, Arendash GW. Behavioral characterization of the Tg2576 transgenic model of Alzheimer’s disease through 19 months. Physiology & behavior. 2002;75:627. doi: 10.1016/s0031-9384(02)00639-x. [DOI] [PubMed] [Google Scholar]
- 40.Giasson BI, et al. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science. 2003;300:636. doi: 10.1126/science.1082324. [DOI] [PubMed] [Google Scholar]
- 41.Waxman EA, Duda JE, Giasson BI. Characterization of antibodies that selectively detect alpha-synuclein in pathological inclusions. Acta Neuropathologica. 2008;116:37. doi: 10.1007/s00401-008-0375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giasson BI, et al. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 43.Waxman EA, Giasson BI. Specificity and regulation of casein kinase-mediated phosphorylation of alpha-synuclein. J Neuropathol Exp Neurol. 2008;67:402. doi: 10.1097/NEN.0b013e3186fc995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.