Abstract
Graft-versus-host disease (GVHD) is a frequent and severe complication following hematopoietic cell transplantation. Natural CD4+25+ regulatory T cells (nTregs) have proven highly effective in preventing GVHD and autoimmunity in murine models. Yet, clinical application of nTregs has been severely hampered by their low frequency and unfavorable ex vivo expansion properties. Previously, we demonstrated that umbilical cord blood (UCB) nTregs could be purified and expanded in vitro using GMP reagents; however, the initial number of nTregs in UCB units is limited, and average yield after expansion was only 1×109 nTregs. Therefore, we asked whether yield could be increased by using peripheral blood (PB), which contains far larger quantities of nTregs. PB nTregs were purified under GMP conditions and expanded 80-fold to yield 19×109 cells using anti-CD3 antibody loaded, cell-based artificial antigen presenting cells (aAPCs) that expressed the high affinity Fc receptor and CD86. A single re-stimulation increased expansion to ~3,000-fold and yield to >600×109 cells, while maintaining FoxP3 expression and suppressor function. nTreg expansion was ~50 million-fold when flow-sort purified nTregs were re-stimulated four times with aAPCs. Indeed, cryopreserved donor nTregs re-stimulated four times significantly reduced GVHD lethality induced by the infusion of human T cells into immune deficient mice. The capability to efficiently produce donor cell banks of functional nTregs could transform the treatment of GVHD and autoimmunity by providing an off-the-shelf, cost-effective, and proven cellular therapy.
Introduction
Acute graft-versus-host disease (GVHD) is a major cause of morbidity and mortality after hematopoietic cell transplantation(1). Natural regulatory T-cells (nTregs) express the transcription factor FoxP3 and are required for immune self-tolerance(2). In murine models, adoptive transfer of nTregs prevents GVHD and donor bone marrow graft rejection, as well as speeds immune recovery in GVHD-prone animals(3-5), making Tregs an attractive therapeutic tool for preventing and/or treating disease in humans(6-9). However, clinical testing has been hampered by low nTreg frequency (1-2%) in peripheral blood (PB)(10), contamination with non-Tregs, CD25+ T-effector or -memory cells(7, 11), and the lack of availability of good manufacturing practice (GMP)-compatible procedures for nTreg purification. Maximizing yield is also critical, as murine studies find high Treg doses (~1:1 with donor T-cells) are required to efficiently and reproducibly suppress GVHD(5).
Previously, we found that nTregs were more readily purified from umbilical cord blood (UCB) than PB due to the relative paucity of CD25+ non-Tregs in UCB; these cells could be expanded several hundred-fold ex vivo using anti-CD3/CD28 monoclonal antibody (mAb)-coated microbeads and IL-2(11, 12). These studies allowed us to initiate the world’s first clinical trial to study the safety of ex-vivo expanded nTregs. Transferred nTregs remained Foxp3+ and could be tracked in blood for up to 14 days. No adverse effects were observed and a trend towards a lower incidence of acute grade II-IV GVHD was observed, but the maximum cell dose was limited by insufficient and variable nTreg expansion rates for some UCB units(13). In other prior studies, we have shown that stimulation of UCB nTregs with cell-based aAPCs increases expansion (~4-fold) over bead-based aAPCs, but this increase alone would not have much effect on clinical nTreg dose. although this degree of expansion was less than desired. Because the nTreg number in UCB are limited and the dose-limiting toxicity was not reached, other nTreg sources need to be explored to determine the maximal efficacy of single or multiple dose nTreg therapy.
Despite non-Treg contaminants, isolation of PB nTregs offers several advantages over UCB nTregs, including increased nTreg number, continued donor availability for additional isolations, and use of autologous cells. PB nTregs can be successfully purified using cell sorting (14, 15), and expanded ~80-fold in vitro. However, cell sorting is a challenging GMP procedure, and overall nTreg yield from PB obtained with this isolation and expansion approach is not greatly increased over that from UCB. Re-stimulation increased total expansion to ~1,000-fold but cultures frequently lost Foxp3 expression and suppressive function concomitant with the appearance of effector T cells secreting IL-2 and IFNγ (16, 17). Although nTregs can also be purified using mAb-coated magnetic beads and ~30-fold more CD25high cells can be isolated from PB than UCB (~150×106 as compared with ~5×106, respectively), bead-purified nTregs contain higher numbers of CD25lo cells are less pure than those obtained by flow cytometry sorting (18, 19). Thus, the mTOR inhibitor, rapamycin, which preferentially inhibits cytokine responses in and survival of T-effector/memory cells as compared with nTregs is often added to bead-purified expansion cultures, albeit at the expense of a 5-10 fold reduction in nTreg expansion (20-23).
Here we show, using GMP-grade reagents, that repetitive nTreg stimulation with cell-based aAPCs massively increases nTreg yield while maintaining Foxp3 and suppressive function. Expanded cells expressed nTreg specific markers (Foxp3 and LAP), displayed Treg-specific demethylation in the Foxp3 gene, and contained very few IL-2, IFNγ, or IL-17 secreting cells. Despite four re-stimulations and expansion of >50-million fold, fresh and cryopreserved nTregs each were capable of suppressing lethality in a xenogeneic model of GVHD. These findings advance the clinical utility of expanded nTregs for the prevention and/or treatment of GVHD following blood and marrow transplantation, solid organ rejection, and autoimmune disease.
Results
PB nTregs can be purified and expanded using GMP reagents and protocols
Although our phase I studies showed UCB nTreg cellular therapy to be well-tolerated, a dose-limiting toxicity of nTregs was not reached, possibly due to limitations in nTreg expansion rates, and GVHD was significantly reduced but not eliminated as compared to historical controls(13). To determine whether nTreg yield could be increased if the source was changed from UCB to PB, we purified cells from leukapheresis products using a two-step protocol using GMP antibody-coated magnetic beads, whereby CD4+ cells were enriched by depleting cells expressing CD8, CD14, and CD19, followed by selection of CD25high cells (Fig. 1A). The starting purity of PB nTregs was assessed by flow cytometry using a phenotype that displays potent suppressive capacity (CD4+127−Foxp3+, Fig. S1A)(24), and was comparable to previous observations for UCB nTregs (95±1% CD4+, of which 66±2% were CD127−Foxp3+). Of the non-CD4+ cells in either cellular preparation, <1% were positive for CD8, −14, −19 (Fig. S1A). The average yield of PB nTregs after expansion (233±31×106 cells) was ~40-fold higher than with UCB nTregs(13).
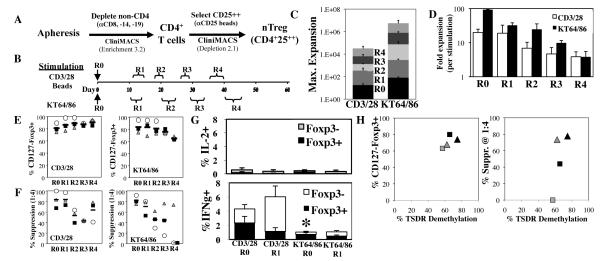
Fig. 1. Re-stimulation greatly increases PB nTreg expansion, and cell-based aAPCs are more effective than bead-based aAPCs.
nTregs were purified from peripheral blood leukapheresis products and expanded using GMP anti-CD3/CD28 mAb-coated beads or an anti-CD3 mAb-loaded cell line (KT64/86). (A) GMP purification schema. (B) Schema showing time course of experiment and ranges for size-based re-stimulation (R0=no re-stimulation, R1= one re-stimulation, etc.). Fold nTreg expansion (average ± SEM); Total (C) or following each stimulation (D). (E) Percentage of cultured cells (CD4-gated) that are CD127−Foxp3+ after each stimulation. (F) Percent suppression of in vitro, anti-CD3-mediated CD8 T cell proliferation at 1:4 (nTreg:PBMNC) as determined by CFSE dye dilution. (G) nTregs from each stimulation were re-stimulated with PMA and ionomycin for 4 hours in the presence of brefeldin A, and the percentage of cells secreting IL-2 or IFNγ was determined by flow cytometry. (H) Bead-purified PB nTregs re-stimulated 3 or 4 times (black and gray symbols, respectively) with anti-CD3 mAb-loaded KT 64/86 cells were harvested and genomic DNA was purified. Foxp3 TSDR demethylation status was assessed using bisulfite sequencing and is compared to nTreg purity (percentage of CD4+ cells that are CD127-Foxp3+) or percent suppression at a 1:4 ratio of nTregs:PBMNCs. Averages are for three independent experiments, individual symbols in (E) and (F) represent independent experiments. Individual brackets indicate the range of days for each stimulus. * represents p<0.05.
Purified cells were stimulated with clinical-grade anti-CD3/28 mAb-coated beads or KT64/86 cells, a recently GMP licensed cell-based aAPC expressing CD86 (a CD28 ligand) and CD64 (the high affinity Fc receptor) due to lentiviral gene transfer. KT64/86 cells were loaded with anti-CD3 mAb. IL-2 (300U/ml) and rapamycin (109 nM) were added to all cultures (Fig. 1B). As reported (25), stimulation with KT64/86 cells versus anti-CD3/28 mAb-coated beads increased PB nTreg expansion by ~5-fold (82±11 vs. 18±5-fold, respectively) (Fig. 1C and D). More robust expansion observed with KT64/86 cells versus anti-CD3/28 mAb-coated beads was associated with increased overall viability (94.3±0.5% vs. 90.0±0.3%, p≤0.001) and decreased Granzyme B production (p<0.02) (Fig. S1B-D). nTreg cultures stimulated once with anti-CD3/28 mAb-coated beads or KT64/86 maintained a nTreg phenotype (97±2 or 99.5±0.3% CD4+ of which 81±5% or 84±7% were CD127−Foxp3+, respectively) and in vitro function (84±12% or 83±6% inhibition of CFSE-labeled CD8 T-cell proliferation after anti-CD3 stimulation at a 1:4 ratio of nTreg:PB mononuclear cells, PBMNCs) (Fig. 1E and F). These data demonstrate that suppressive, Foxp3+ nTregs can be expanded from PB using GMP procedures, and shows stimulation with cell-based aAPC is superior to anti-CD3/28 mAb-coated beads. However, because PB nTreg expanded 5-10 fold less than UCB nTregs expanded without rapamycin, overall nTreg yield was not substantially increased.
Re-stimulation greatly increases nTreg expansion
To maximize yield, we re-stimulated GMP bead-purified nTregs grown in rapamycin after they had returned to resting size (≤8.5μm Fig. S1E), which we have shown maximizes CD4+ T cell expansion (26). nTregs stimulated with KT64/86 cells were found to have a higher peak cell size as compared to anti-CD3/28 mAb-coated beads (Fig. S1F). Re-stimulation with anti-CD3/28 mAb-coated beads or KT64/86 cells increased expansion 18- and 36-fold, respectively, to a total of 330- or 3,000-fold over input cell number (Fig. 1C and D). Cultures remained >65% FoxP3+ and suppressed in vitro T-cell proliferation >50% @ ratios of 1:4 (nTregs:PBMNCs) (Fig. 1E and F). Of the non-CD4+ cells expanded with either stimulus, <1% were positive for CD8, −14, −19, or −56 (Fig. S1G).
Others have shown that nTreg re-stimulation in the absence of rapamycin results in up to 30% cells that secrete either IL-2 and/or IFNγ, two cytokines that could potentially exacerbate GVHD(16, 17). Therefore, we quantified the number of IL-2- and IFNγ-secreting cells by intracellular cytokine staining after PMA/Ionomycin stimulation of bead-purified nTreg cultured with anti-CD3/28 mAb-coated beads or KT64/86 cells and rapamycin. As shown in Figure 1G, <1% of cells expanded with either anti-CD3/28 mAb-coated beads or KT64/86 cells secreted IL-2. Although less than 6% of cells in any culture expanded with either aAPCs or rapamycin were IFNγ+, significantly fewer IFNγ+ cells were found in nTreg cultures expanded with KT64/86 than CD3/28 beads (1% vs. 4% and 1% vs. 6% ± re-stimulation, respectively).
Multiple re-stimulations lead to reduced suppressive function despite the presence of Foxp3
To determine whether bead-purified nTregs could be expanded even further, we stimulated the above cultures another three times (4 re-stimulations total) (Fig. 1C). In contrast to anti-CD3/28 mAb-coated bead expanded cultures, whose peak size declined after each stimulation and was <9.0μm after the fourth re-stimulation, peak size after KT64/86 cell stimulation remained high at ~9.5μm (Fig. S1E and F). In addition, the fold expansion induced by successive stimulations with anti-CD3/28 mAb coated beads decreased more rapidly than with KT64/86 cells, ultimately resulting in 200-fold lower total expansion than with KT64/86 cells (25,000-fold vs. ~5 million-fold, respectively) (Fig. 1C and D). nTregs re-stimulated with anti-CD3/28 mAb-coated beads remained >80% Foxp3+, and although expression gradually decreased in KT64/86 cell expanded nTregs, FoxP3 remained >60% after the fourth re-stimulation (Fig. 1E). nTregs expanded with anti-CD3/28 mAb-coated beads also had higher Foxp3 on a per cell basis than those expanded with KT64/86 cells (Fig. S1H and I). Despite achieving Foxp3 levels previously associated with significant suppressive function by expanded UCB nTregs(13), <50% suppression of anti-CD3 mAb-driven CD8 T-cell proliferation at a 1:4 nTreg:PBMNC ratio was observed in 2/2 and 1/3 cultures re-stimulated 3 or 4 times with anti-CD3/28 mab-coated beads, respectively; and 2/3 and 2/3 with KT64/86 cells (Fig. 1F).
Stable expression of Foxp3 is a trait of natural, but not induced, Tregs and is conferred through epigenetic modification of the Foxp3 gene at the Treg-specific demethylated region (TSDR) (27). To assess the methylation status of the Foxp3 gene in re-stimulated nTreg, DNA from cultures receiving 3 or 4 re-stimulations was purified, bisulfite modified, sequenced, and the average % methylation of 11 CpG sites contained in the TSDR determined. Because Foxp3 is on the X chromosome and becomes hyper-methylated during X-inactivation, the data shown are restricted to male samples. An evaluation of two informative samples, Fig. 1H suggests that TSDR demethylation status is proportional to Foxp3 and slightly decreases between the third and fourth re-stimulation, although the effects were not significant (r = 0.65, P = 0.35. As observed for Foxp3, TSDR demethylation is not directly proportional to suppressive function (r = 0.75; P = 0.25).
Sort-purified nTregs maintain Foxp3 and suppressive function after multiple stimulations
Decreased suppressive function could be caused by contaminating cells that become amplified after re-stimulation and acquire FoxP3 during the process of massive cell expansion. Therefore, PB nTregs were purified by flow cytometry sorting and re-stimulated with KT64/86 cells in the presence or absence of rapamycin (Fig. 2A). To enable more meaningful comparisons of the various re-stimulations, we also developed freeze/thaw conditions that allow expanded nTregs to maintain phenotype and suppressive function so that all samples are assayed simultaneously (Fig. S2). The most common strategy for sorting nTreg is to first purify CD4+ cells, and then gate on the 2% of cells with the highest expression of CD25. Although cells purified in this manner are regularly >90% Foxp3+, it is relatively inefficient and only captures ~20% of the total Foxp3+ cells. To maximize yield, we performed an initial purification with magnetic anti-CD25 mAb-coated beads and then sorted for CD4+25hi127− cells, which allowed >25% of sorted cells to be positively selected. This method increased initial nTreg purity from 66±2% CD127−Foxp3+ for bead-based purification to 84±3 (p≤0.003), and resulted in a routine yield of 15-30×106 nTregs from 2×109 PBMNCs (Fig. S3A and B).
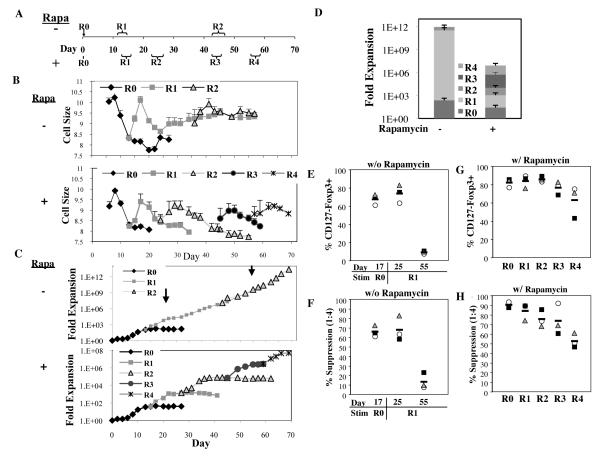
Fig. 2. Sort-purified nTregs maintain Foxp3 and suppressive function after multiple stimulations.
PB nTreg were sort-purified (CD4+25hi127−) and expanded with anti-CD3 mab-loaded KT64/86 in the presence or absence of rapamycin using 4 or 2 re-stimulations, respectively. (A) Schema showing time course of experiment and time ranges for size-based re-stimulation (R0=no re-stimulation, R1= one re-stimulation, etc.), individual brackets indicate the range of days for each stimulus. (B) Average cell size (± SEM) over time for PB nTreg cultures re-stimulated ± rapamycin. Representative examples (C) and average (D) expansion of nTregs ± rapamycin, respectively. Arrows in (C) on days 25 and 55 mark two distinct phases (plateau and growth phase) seen after first re-stimulation of nTreg cultures grown without rapamycin. Average percent CD127-Foxp3+(CD4-gated) or percent suppression of in vitro T cell proliferation at a 1:4 ratio of nTregs:PBMNCs for cultures expanded in the absence (E and F) or presence (G and H) of rapamycin, respectively. Bars represent average; other symbols represent individual experiments.
nTregs stimulated with KT64/86 cells in the absence of rapamycin, known to affect size and proliferation (28), had both a larger peak size (Fig. 2B; 10.4 vs. 9.9μM for without and with rapamycin, respectively; p<0.01), and increased expansion (Figs. 2C and D; 290- vs. 55-fold, respectively). nTregs cultured in the presence or absence of rapamycin were re-stimulated at 8.5μm (day 13±1) and, after 4 days of expansion and size increase, cultures started to decrease in size and stop expanding. However, after day 25, without additional stimulation, cultures grown without rapamycin increased in size to ~9.3μM and started proliferating, impressively expanding over 5×1011-fold throughout the 55 day observation period. Additional re-stimulation did not increase either cell size or maximal expansion. Day 55 cultures contained few CD127-Foxp3+ nTregs and were not suppressive, whereas those harvested on day 25 had still expanded 11,000±2,000-fold, were ≥60% CD127-Foxp3+ and conferred ≥60% suppression of CD8 T-cell proliferation (Figs. 2E and F).
In contrast to cultures established in the absence of rapamycin, sort-purified nTregs expanded with KT64/86 cells in the presence of rapamycin returned to resting size and ceased proliferating after each re-stimulation (Figs. 2B and C). Cumulative expansion after re-stimulation of sort-purified nTregs + rapamycin was >6-fold higher than mAb-coated-bead purified nTregs (31±14×106 vs. 4.7±0.7×106 fold expansion, respectively, p<0.05), due mainly to the fact that the fold-expansion did not decline after each re-stimulation (Fig. S3C). Repeated stimulation caused a gradual decrease in Foxp3 such that after the fourth re-stimulation, 63±12% of cells were CD127−Foxp3+ (Fig. 2G). However, unlike bead-purified, sort-purified nTregs expanded after four repetitive stimulations maintained >50% suppression of T-cell responses for all re-stimulations (Fig. 2H). Table S1 summarizes the in vitro expansion characteristics (yield, Foxp3 expression, Teff contamination and suppressive function) for nTreg derived from UCB and PB with varying culture and re-stimulation conditions.
To determine whether re-stimulation affects the Treg phenotype, the level of several Treg associated markers (including LAP, CD62L, CD27, CCR7 and CD45RA) was assessed on cells receiving either single or multiple stimulations. Although Latency Associated Peptide (LAP), derived from the N-terminal region of TGFß, was expressed on Foxp3+ cells after all 4 re-stimulations (Fig. S4A), CD62L and CD27 staining was lost after 2 and 4 re-stimulations, respectively (Fig. S4B). CCR7 behaved like an activation marker, being more highly expressed at d7 than at resting size (Fig. S4C). However, if nTregs were maintained in culture after returning to basal size, a subpopulation of nTregs spontaneously regained CCR7 staining (Fig. S4D). Although it is not surprising that re-stimulation decreased CD45RA expressed on naïve, resting T cells and Tregs, the finding that cells regained staining after returning to resting size was unanticipated (Fig. S4E, especially re-stimulations 2 and 3).
We next examined changes in surface phenotype and T-cell receptor (TCR) repertoire of the Tregs expanded with one (R0) or a total of 5 stimulations (R4). After multiple rounds of stimulation, the nTreg phenotype changed from CD27+CD45RA−CD57−to CD27−CD45RA−CD57−, suggesting that these cells were undergoing differentiation to a more mature state (Fig. S5). However, an increase in CD57 expression was not noted after expansion, suggesting that the cells did not become terminally differentiated or senescent (29). Finally, TCR Vß usage was essentially unchanged between R0 and R4, suggesting that particular TCR Vß families were not preferentially expanded despite a massive increase in nTreg number during the course of cell culture (Fig. S6).
nTreg cultures re-stimulated with KT64/86 cells in the presence of rapamycin do not secrete IL-2 or effector cytokines
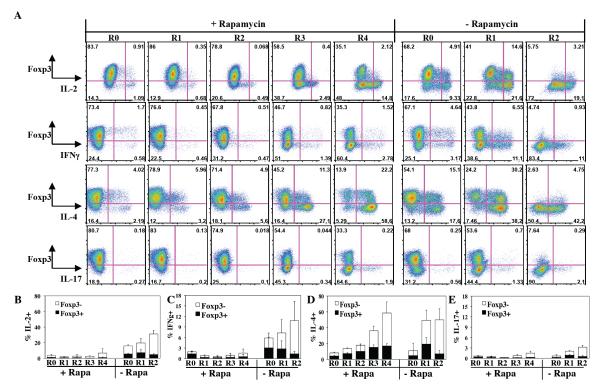
Repetitive stimulation of TH cells in the absence of rapamycin generates effector cells, which secrete cytokines that could exacerbate GVHD. To determine the extent of effector T-cell contamination in our cultures, samples of each re-stimulation from KT64/86 expanded cultures grown with or without rapamycin were stimulated with PMA/Ionomycin and assayed for IL-2, IL-4, IL-17, and IFNγ (Fig. 3A) using intracellular cytokine staining. To make comparisons between various re-stimulations more valid, frozen nTregs representing all conditions were assayed simultaneously, and were co-stained for Foxp3 to differentiate secretion by nTregs vs. non-Treg cells. Adding rapamycin suppressed effector cell generation such that ≤3% of PMA/ionomycin stimulated cells secreted IL-2 and ≤2% secreted IFNγ, as compared to ≥17% and ≥6% for cultures without rapamycin (Figs. 3B and C). In contrast, rapamycin was less effective at inhibiting IL-4 production in Foxp3+ or Foxp3− cells, and the percentage of IL-4+ cells increased with each successive re-stimulation from 8±2% to 58±17% (Fig. 3D). Finally, the total number of IL-17 secreting cells present in cultures of sorted nTregs was consistently low (<3.1%) for all stimulations with or without rapamycin (Fig. 3E).
Fig. 3. nTreg cultures re-stimulated with KT64/86 cells in the presence of rapamycin do not secrete IL-2 or effector cytokines.
PB nTregs were sort-purified and expanded with multiple rounds of stimulation with anti-CD3 mAb-loaded KT 64/86 cells in the presence or absence of rapamycin. The R1 without rapamycin sample corresponds to the d25 time point with high Foxp3 staining. (A) Representative example of cytokine production by Foxp3+ and - cells (CD4−gated). Average (± SEM) % of cells secreting IL-2 (B), IFNγ (C), IL-4 (D), or IL-17 (E). Averages are for three independent experiments.
nTregs expanded with multiple rounds of stimulation ameliorate disease in a xenogeneic model of GVHD
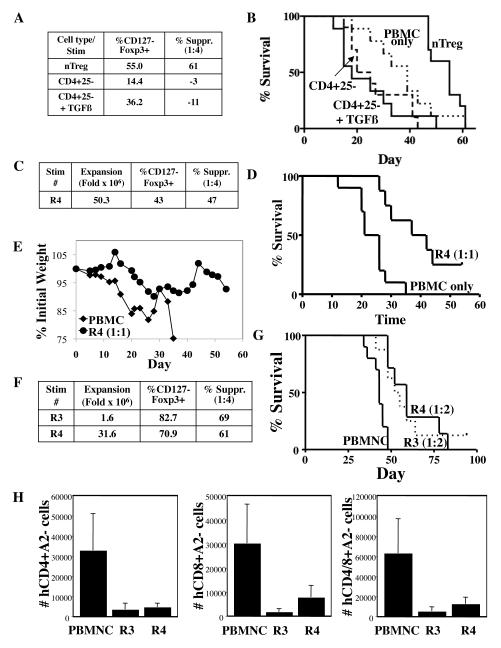
Several groups have reported that nTregs are not terminally differentiated and can be reprogrammed into helper T-cells(30) and Teffs(31,32), which are capable of inducing proinflammatory responses and disease(32). Therefore, we used a xenogeneic model of GVHD, in which nTregs are co-transferred at a 1:1 ratio with allogeneic PBMNCs (30×106 each) into NOD/Scid/γc−/− recipients, to compare the stability and safety of in vitro expanded nTregs versus CD4+25− cells that were re-stimulated 4 times cultured in the absence or presence of TGFß, which was used to induce FoxP3 (Figure 4A). Adoptive transfer of nTregs increased median survival from 39 to 55 days (p<0.01). Transfer of non-Tregs appeared to exacerbate GVHD, even if Foxp3 was induced with TGFß (Figure 4B), whereas Foxp3− cells present in nTreg cultures did not expand or persist long-term and, in contrast to cultures expanded from CD4+25− cells, did not exacerbate GVHD. We also tested the in vivo potency, stability and safety of nTregs expanded 50-million fold with four re-stimulations using KT64/86 cells. Although recipients of PBMNCs rapidly and uniformly succumbed to GVHD, mice given nTregs had a significantly prolonged survival, with 25% of mice surviving to day 55 (Fig. 4D; n = 8-10/group; p<0.05). GVHD amelioration was also indicated by a significant decrease in weight loss between days 14 and 21 (Fig. 4E). The partial protection seen using nTregs in this xenogeneic GVHD model has also been observed using UCB nTregs obtained after a single stimulation with anti-CD3/28 mAb-coated beads, which we have shown to rescue 50% of macrophage-depleted, sublethally irradiated Rag2, γc−/− recipients when infused at a 1:1 ratio with PBMNCs (12).
Fig. 4. PB nTregs expanded over 50 million fold can still ameliorate disease in a xenogeneic model of GVHD, even after freezing and thawing.
(A) Summary of purity (percentage of CD4+ cells that are CD127-Foxp3+) and in vitro suppressive function for in vitro expanded nTregs or CD4+25− cells (grown ± TGFß) after a single stimulation with KT64/86 cells. (B) Kaplan-Meier survival curve comparing NOD/Scid/γc−/− mice receiving human PBMNCs only or co-transferred with nTregs, CD4+25− cells, or CD4+25− cells expanded in TGFß co-transferred at 1:1 (e.g. 30×106 PBMNCs and 30×106 nTregs) (C) Summary of fold expansion, purity (percentage of CD4+ cells that are CD127-Foxp3+), and in vitro suppressive function for nTreg expanded with 4 re-stimulations (R4). (D) Kaplan-Meyer survival curve comparing NOD/Scid/γ−/− mice receiving human PBMNCs ± fresh nTreg re-stimulated 4 times (R4) co-transferred at 1:1. (E) Average weight (percentage of initial) for mice surviving on a given day for different groups of mice. (*) p ≤ 0.05 for fresh nTregs from days 14-21. (F) Summary of fold expansion, purity (percentage of CD4+ cells that are CD127-Foxp3+), and in vitro suppressive function for expanded nTregs re-stimulated 3 or 4 times (R3 and R4, respectively). (G) Kaplan-Meyer survival curve showing survival of mice receiving human PBMNC ± cryopreserved and thawed R3 or R4 nTregs (HLA-A2+) co-transferred at 1:2 (i.e. 15×106 nTregs and 30×106 PBMNCs). n= 10, 8, and 7 for groups PBMNC, R3 nTregs, and R4 nTregs, respectively. (H) Average number (±SEM) of human CD4+HLA-A2−, CD8+HLA-A2− or Total CD4/8A2− cells per μl blood on day 30 for animals in (G).
Since there was a modest decrement in %CD127−FoxP3+ and in vitro suppression of nTregs in the GVHD model, we used a suboptimal ratio of nTregs:PBMNCs (1:2) to help uncover potential differences between nTreg re-stimulated three or four times. Fig. 4F shows that nTregs re-stimulated three or four times maintained their phenotype and in vitro suppressive function after cryopreservation and thawing. Both expanded nTreg preparations significantly reduced GVHD-induced lethality when compared with PBMNC controls, and there was no difference in their relative potency (Fig. 4G; P<0.003 and 0.001 vs. PBMNC controls for three or four re-stimulations, respectively). Expansion of PB-derived CD4+ and CD8+ T-cells is predictive of GVHD severity and Fig. 4H shows that, like UCB nTregs, co-transfer of re-stimulated PB nTregs significantly reduced the number of GVHD-causing T-cells on day 30 post-transfer.
Discussion
The therapeutic potential of nTregs to prevent or cure multiple autoimmune diseases or GVHD in murine or xenogeneic models has been well documented (3-5). Two critical obstacles to overcome before implementing this therapy in humans are generating sufficient cell numbers and demonstrating their in vivo safety and stability. Here we show that sort-purified nTregs could be expanded at least 50 million fold by repetitive stimulation with cell-based aAPCs while maintaining suppressive function in vitro and in vivo. Addition of rapamycin minimized contamination with Th1 inflammatory cytokine secreting cells, but not Th2 cells, which skew immunity away from inflammatory responses. Re-stimulated nTregs differentiated from a CD27+ memory phenotype to CD27− memory phenotype, but, importantly, did not adopt a senescent (CD57+) phenotype (33). The lack of Vß skewing in the T cell receptor repertoire indicates that massively expanded nTregs retain a broad spectrum of reactivities and are not transformed.
Maximizing nTreg expansion, while minimizing loss of suppressive function and contamination with non-Tregs, is critical for establishing an nTreg cellular therapy. Three studies have shown that nTregs can be expanded >1000-fold if re-stimulated in the absence of rapamycin, but in each case cultures contained high numbers of IL-2- and IFNγ-secreting cells that were both Foxp3− and + (16, 17, 34). We confirmed these data and found that nTreg cultures eventually lost Foxp3 and suppressive function in the absence of rapamycin. Loss of Foxp3 correlated with an increased ratio of cycling (i.e. Ki-67+) Foxp3− cells (Fig. S7), suggesting loss of purity is due to the outgrowth of Foxp3− cells as opposed to conversion of Foxp3+ cells as suggested by one report (16).
We previously demonstrated the increased stimulatory capacity of cell-based aAPCs allowed PB nTregs to be expanded 1000-fold with a single re-stimulation, even in the presence of rapamycin, and nTreg expanded with aAPCs were equal to anti-CD3/28 mAb-coated beads expanded cells at suppressing xenogeneic GVHD (Fig. S1K and (25)). For these initial studies, re-stimulation was performed at the growth plateau phase, but the high variability (day 8 to 12) and difficulty of determining this timepoint are not conducive to clinical production. Re-stimulation on a specific day is optimal for clinical trials. However, although studies without rapamycin showed re-stimulation on day 7 increased expansion, we observed no increase in expansion with day 7 re-stimulation (n=3) with bead-purified nTreg stimulated with anti-CD3/28 mAb-coated beads (25- vs. 18-fold for with or without day 7 re-stimulation, respectively). Although re-stimulation based upon cell size resulted in more variability in the day of optimal re-stimulation than would be the case at a single time point, such an approach identified a time range (day 13±1) more suitable for clinical re-stimulation.
All nTreg cultures contain some level of Foxp3− cells, which have the potential, especially after re-stimulation, to become effector T-cells and exacerbate disease. Although nTreg cultures re-stimulated in the absence of rapamycin contained high numbers of IL-2- and IFNγ-secreting cells, the number of these cells did not increase with re-stimulation in the presence of rapamycin. Furthermore, when transferred in vivo, Foxp3− cells present in nTreg cultures did not expand or persist long-term and, in contrast to cultures expanded from CD4+25− cells, did not exacerbate GVHD. In addition, studies show rapamycin temporally imparts Foxp3 expression and Treg-like activity to effector T-cells, which can re-acquire T-effector cell function if rapamycin is removed (35). LAP expression differentiates activated nTregs from stimulated CD4+25− T-cells expressing Foxp3 spontaneously or after exposure to TGFß or rapamycin (17). Even after four re-stimulations, most Foxp3+ cells expressed LAP even 7 days after re-stimulation, showing that the cultures remain primarily nTregs. Furthermore, cultures expanded over 1 million-fold maintained nTreg-specific demethylation in the Foxp3 gene. Murine T-cells expanded in rapamycin are Th2 skewed, secrete IL-4 and IL-10 and, after adoptive transfer, decrease allospecific IFNγ secretion and ameliorate disease in a murine model of GVHD (36). Interestingly although rapamycin almost completely inhibited the differentiation of IL-2- and IFNγ-secreting cells in our cultures of human cells, the effect on IL-4 was not complete, and >50% of cells secreted IL-4 (both Foxp3+ and FoxP3– cells) after the fourth re-stimulation.
Murine and human nTregs are not terminally differentiated, and can be reprogrammed to secrete IL-17 in vitro or in vivo when activated in the presence of IL-6 (31, 32, 37). Adoptive transfer of reprogrammed murine nTregs induced autoimmune diabetes but, unlike their human counterparts, these cells also produced IFNγ and TNFα. It is not known whether reprogrammed human nTregs will cause disease, since only ~5% of nTregs become IL-17+ in vitro (37) and these retain suppressive function (31). Several findings from this study suggest nTreg reprogramming may not be a grave issue in developing a cellular therapy for in vitro expanded nTregs. First, IL-17 was undetectable in the supernatants of all re-stimulation samples cultured with rapamycin (limit of detection 0.3pg/ml). Second, the number of expanded cells that were IL-17+ cells was very low (<2% total and ≤0.5% Foxp3+IL-17+) and, even more important, did not increase significantly over the 4 re-stimulation cycles (Fig. S1J). Although the likelihood for in vivo reprogramming of nTregs and especially expanded nTregs may be context dependent, the high degree of TSDR demethylation of these cells may provide some degree of resistance to the reprogramming process.
In summary, the degree of nTreg expansion reported here could lead to the widespread application of nTreg cellular therapy for GVHD and graft rejection through the creation of an off-the-shelf therapy using nTreg banks generated from HLA-typed donors with known safety and potency records. The massive expansion observed with repetitive polyclonal stimulation should also allow relatively rare, auto-antigen-specific nTreg clones to be expanded to treat autoimmune diseases. Ultimately, this strategy could be applied to expansion of antigen-specific nTregs, which are more effective than polyclonal Tregs at suppressing disease. This strategy is potentially preferable to using Tregs induced in vitro by FoxP3 gene transfer or other conditions that favor FoxP3 expression. Furthermore, if increased purity and/or suppressive function is required, nTregs could be re-isolated after expansion using a protocol described recently by Shevach’s group based upon LAP expression (17). Although GMP sorting can be challenging for many institutions, re-stimulation-driven expansion could produce sufficient numbers of cells in a small number of sorts to support the creation of a master cell bank that would contain matches for multiple patients. Finally, an nTreg master cell bank would be an effective treatment for multiple diseases because, as shown here, nTregs suppress third-party responses and ameliorate disease without long-term persistence and are also able to maintain suppressive function after freeze/thaw.
Materials and Methods
Treg Isolation and Culture
For all experiments, non-mobilized peripheral blood leukapheresis products were collected from normal adult volunteers using FDA approved/cleared apheresis instruments. Written informed consent was obtained from all subjects with approval from the University of Minnesota Institutional Review Board. nTregs were purified using GMP magnetic beads or by sorting and cultured as in the Supplemental Methods. Where indicated, rapamycin (Rapammune, Wyeth-Ayerst) at 109 nM was added on day 0 and with subsequent media supplementation. Cell size and viability were determined by ViCell (Beckman Coulter).
For mAb-bead-based nTreg purification, CD4+ T-cells were enriched by MACS (all beads from Miltenyi Biotec) by depleting non-CD4s with GMP-grade mAb-coated microbeads (cocktail of CD8, CD14, CD19±CD56, 7.5ml each/Apheresis product) in combination with a CliniMACS (Depletion 2.1 – Max TNC = 2.0×1010). Unbound cells were washed and CD25high Tregs were subsequently purified by positive selection using GMP-grade anti-CD25 mAb-coated microbeads (7.5ml/Apheresis product) and CliniMACS (Enrichment 3.2). CD8−/CD14−/CD19−/CD25− cells were subsequently enriched for CD3+ feeder cells using GMP-grade anti-CD3 microbeads. All bead incubations were performed as specified by the manufacturer (i.e. 30 minutes at room temperature for GMP-grade beads). All washes were performed at 300 × for 10 minutes at room temperature.
nTregs were sort purified from peripheral blood mononuclear cells (PBMNCs; Ficoll–Hypaque, Amersham Biosciences, Uppsala, Sweden) in a two step procedure in which CD25+ cells were initially enriched from PBMNCs by AutoMACS (PosselD2) with GMP-grade anti-CD25 microbeads (75μl/2×108 cells). CD25high cells were stained with CD4, −8, −25 and −127 and sorted via FACSAria as CD4+, 8−, 25++, 127−. Note that the bead bound and fluorochome conjugated anti-CD25 antibodies recognize different epitopes.
Purified CD4+CD25+ cells were cultured with either GMP anti-CD3/CD28 mAb-coated Dynabeads (26) (3:1 bead:cell) or with K562 cell lines engineered to express CD86 and the high affinity Fc Receptor (CD64) (36) (2:1 nTreg:KT), which had been irradiated with 10,000 cGray and incubated with anti-CD3 (Orthoclone OKT3, Janssen-Cilag). In some experiments, nTreg were stimulated with KT64/86 cells that were pre-loaded, irradiated, and frozen (1:1 nTreg:KT). Irradiated feeder cells (2600 rads, CD8−/CD14−/CD19−/CD25−/CD3+) were added to CD3/28 bead cultures at 1:1 feeder:Treg. nTregs were cultured in X-Vivo-15 media (BioWhittaker) supplemented with 10% human AB serum (Valley Biomedical), GlutaMAX (Gibco) and N-acetylcysteine (USP). Recombinant IL-2 (300 IU/ml, Chiron) was added on day 2 and maintained for culture duration. Cultures were maintained at 0.3 – 0.5×106 viable NC/ml every 2-3 days.
Intracellular cytokine staining
Fresh or frozen nTregs were cultured in supplemented X-Vivo 15 for 4 hours±PMA (2 pg/ml) and Ionomycin (1 μg/ml) in the presence of Brefeldin A (100 ng/ml) (all Sigma). Frozen/thawed samples were cultured for 1 hour at 37° prior to re-stimulation. Cells were then harvested and stained for CD4, 25, Foxp3 and cytokine (IL-2, -4, -17, and IFNγ) or Granzyme B using the standard Foxp3 intracellular staining kit.
Suppression Assays
The in vitro suppressive capacity of expanded nTregs was assessed with a 5-carboxyfluorescein diacetate succinimide ester (CFSE) inhibition assay as previously published. Briefly, PBMNC were purified, labeled with CFSE (InVitrogen), and stimulated with anti-CD3 mAb-coated beads (Dynal) ± cultured nTreg (1:2-1:32 nTregs:PBMNCs). On day 4, cells were stained with antibodies to CD4 and 8 and proliferation, data analyzed using FlowJo (8.8.7), and suppression was determined from the Division Index (Treestar). nTregs suppressed CD4 and 8 responses equivalently (Fig. S8), and only CD8 data is presented. Xenogeneic GVHD experiments were performed as in 25, and are described in Supplemental Methods.
Statistical Analysis
Data were analyzed by ANOVA or student’s t-test. Probability (P) values ≤0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We would like to thank Christine Nelson for excellent assistance with animal husbandry.
Funding: This work was supported in part by research grants from the Children’s Cancer Research Fund and Blood and Marrow Transplant Research Fund to KLH, Leukemia and Lymphoma Translational Research (grant R6029-07), R37 HL56067, and P01 AI056299 to BRB, P01 CA067493 and N01HB037164 to BRB, JEW, and JSM; support from Miltenyi Biotec to BRB and JEW, a grant from Becton-Dickinson to JEW; and support from the JDRF Collaborative Centers for Cell Therapy and the JDRF Center on Cord Blood Therapies for Type 1 Diabetes to JLR and CHJ.
Footnotes
Author contributions: K.L.H. designed the research, performed the experiments, interpreted the data, and wrote the paper. S.C.M., D.K.S., C.M.S. performed the experiments, interpreted the data, and assisted with the paper. D.S., D.M.K. performed the research. J. S. M., J. E. W., D.H.M., J.S.B., B.L.L., C.H.J. and J.L.R. designed the research, and wrote the paper. B.R.B. designed the research, interpreted the data, and wrote the paper.
Competing interests: J.L.R., C.H.J, and J.E.W. have research funding from Becton Dickinson and C.H.J. and B.R.B. were previously scientific consultants for Becton Dickinson, although this funding did not conflict with this manuscript. K.L.H., J.L.R., C.H.J. and B.R.B. are authors on U.S. provisional patent application number 61/322, 186, “Methods to expand a T regulatory cell master cell bank.” The other authors declare that they have no competing interests.
References and Notes
- 1.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 2.Wildin RS, Freitas A. IPEX and FOXP3: clinical and research perspectives. Journal of Autoimmunity. 2005;25(Suppl):56. doi: 10.1016/j.jaut.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389. doi: 10.1084/jem.20020399. published online EpubAug 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shevach EM, DiPaolo RA, Andersson J, Zhao DM, Stephens GL, Thornton AM. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol Rev. 2006;212:60. doi: 10.1111/j.0105-2896.2006.00415.x. published online EpubAug. [DOI] [PubMed] [Google Scholar]
- 5.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493. doi: 10.1182/blood.v99.10.3493. published online EpubMay 15. [DOI] [PubMed] [Google Scholar]
- 6.Gavin M, Rudensky A. Control of immune homeostasis by naturally arising regulatory CD4+ T cells. Current Opinion in Immunology. 2003;15:690. doi: 10.1016/j.coi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 7.June CH, Blazar BR. Clinical application of expanded CD4+25+ cells. Semin Immunol. 2006;18:78. doi: 10.1016/j.smim.2006.01.006. published online EpubApr. [DOI] [PubMed] [Google Scholar]
- 8.Piccirillo CA, Shevach EM. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Seminars in Immunology. 2004;16:81. doi: 10.1016/j.smim.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585. doi: 10.1038/nri2138. published online EpubAug. [DOI] [PubMed] [Google Scholar]
- 10.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245. doi: 10.4049/jimmunol.167.3.1245. published online EpubAug 1. [DOI] [PubMed] [Google Scholar]
- 11.Godfrey WR, Ge YG, Spoden DJ, Levine BL, June CH, Blazar BR, Porter SB. In vitro-expanded human CD4(+)CD25(+) T-regulatory cells can markedly inhibit allogeneic dendritic cell-stimulated MLR cultures. Blood. 2004;104:453. doi: 10.1182/blood-2004-01-0151. published online EpubJul 15. [DOI] [PubMed] [Google Scholar]
- 12.Hippen KL, Harker-Murray P, Porter SB, Merkel SC, Londer A, Taylor DK, Bina M, Panoskaltsis-Mortari A, Rubinstein P, Van Rooijen N, Golovina TN, Suhoski MM, Miller JS, Wagner JE, June CH, Riley JL, Blazar BR. Umbilical cord blood regulatory T-cell expansion and functional effects of tumor necrosis factor receptor family members OX40 and 4-1BB expressed on artificial antigen-presenting cells. Blood. 2008;112:2847. doi: 10.1182/blood-2008-01-132951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, Defor T, Levine BL, June CH, Rubinstein P, McGlave PB, Blazar BR, Wagner JE. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117:1061. doi: 10.1182/blood-2010-07-293795. published online EpubJan 20 (blood-2010-07-293795 [pii]10.1182/blood-2010-07-293795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann P, Eder R, Boeld TJ, Doser K, Piseshka B, Andreesen R, Edinger M. Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood. 2006;108:4260. doi: 10.1182/blood-2006-06-027409. published online EpubDec 15. [DOI] [PubMed] [Google Scholar]
- 15.Karakhanova S, Munder M, Schneider M, Bonyhadi M, Ho AD, Goerner M. Highly efficient expansion of human CD4+CD25+ regulatory T cells for cellular immunotherapy in patients with graft-versus-host disease. Journal of Immunotherapy. 2006;29:336. doi: 10.1097/01.cji.0000203080.43235.9e. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann P, Boeld TJ, Eder R, Huehn J, Floess S, Wieczorek G, Olek S, Dietmaier W, Andreesen R, Edinger M. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur J Immunol. 2009;39:1088. doi: 10.1002/eji.200838904. published online EpubApr. [DOI] [PubMed] [Google Scholar]
- 17.Tran DQ, Andersson J, Hardwick D, Bebris L, Illei GG, Shevach EM. Selective expression of latency-associated peptide (LAP) and IL-1 receptor type I/II (CD121a/CD121b) on activated human FOXP3+ regulatory T cells allows for their purification from expansion cultures. Blood. 2009;113:5125. doi: 10.1182/blood-2009-01-199950. published online EpubMay 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann P, Boeld TJ, Eder R, Albrecht J, Doser K, Piseshka B, Dada A, Niemand C, Assenmacher M, Orso E, Andreesen R, Holler E, Edinger M. Isolation of CD4+CD25+ regulatory T cells for clinical trials. Biology of Blood & Marrow Transplantation. 2006;12:267. doi: 10.1016/j.bbmt.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Peters JH, Hilbrands LB, Koenen HJ, Joosten I. Ex vivo generation of human alloantigen-specific regulatory T cells from CD4(pos)CD25(high) T cells for immunotherapy. PLoS ONE [Electronic Resource] 2008;3:e2233. doi: 10.1371/journal.pone.0002233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338. doi: 10.4049/jimmunol.177.12.8338. published online EpubDec 15. [DOI] [PubMed] [Google Scholar]
- 21.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743. doi: 10.1182/blood-2004-10-3932. published online EpubJun 15. [DOI] [PubMed] [Google Scholar]
- 22.Coenen JJ, Koenen HJ, van Rijssen E, Hilbrands LB, Joosten I. Rapamycin, and not cyclosporin A, preserves the highly suppressive CD27+ subset of human CD4+CD25+ regulatory T cells. Blood. 2006;107:1018. doi: 10.1182/blood-2005-07-3032. published online EpubFeb 1. [DOI] [PubMed] [Google Scholar]
- 23.Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J Immunol. 2007;178:320. doi: 10.4049/jimmunol.178.1.320. published online EpubJan 1. [DOI] [PubMed] [Google Scholar]
- 24.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701. doi: 10.1084/jem.20060772. published online EpubJul 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golovina TN, Mikheeva T, Suhoski MM, Aqui NA, Tai VC, Shan X, Liu R, Balcarcel RR, Fisher N, Levine BL, Carroll RG, Warner N, Blazar BR, June CH, Riley JL. CD28 costimulation is essential for human T regulatory expansion and function. Journal of Immunology. 2008;181:2855. doi: 10.4049/jimmunol.181.4.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine BL, Bernstein WB, Connors M, Craighead N, Lindsten T, Thompson CB, June CH. Effects of CD28 costimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J Immunol. 1997;159:5921. published online EpubDec 15. [PubMed] [Google Scholar]
- 27.Huehn J, Polansky JK, Hamann A. Epigenetic control of FOXP3 expression: the key to a stable regulatory T-cell lineage? Nat Rev Immunol. 2009;9:83. doi: 10.1038/nri2474. published online EpubFeb (nri2474 [pii] 10.1038/nri2474) [DOI] [PubMed] [Google Scholar]
- 28.Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167:6869. doi: 10.4049/jimmunol.167.12.6869. published online EpubDec 15. [DOI] [PubMed] [Google Scholar]
- 29.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711. doi: 10.1182/blood-2002-07-2103. published online EpubApr 1 (10.1182/blood-2002-07-2103 2002-07-2103 [pii]) [DOI] [PubMed] [Google Scholar]
- 30.Sharma MD, Hou DY, Baban B, Koni PA, He Y, Chandler PR, Blazar BR, Mellor AL, Munn DH. Reprogrammed foxp3(+) regulatory T cells provide essential help to support cross-presentation and CD8(+) T cell priming in naive mice. Immunity. 2010;33:942. doi: 10.1016/j.immuni.2010.11.022. published online EpubDec 14 (S1074-7613(10)00452-8 [pii] 10.1016/j.immuni.2010.11.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beriou G, Costantino CM, Ashley CW, Yang L, Kuchroo VK, Baecher-Allan C, Hafler DA. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113:4240. doi: 10.1182/blood-2008-10-183251. published online EpubApr 30 (blood-2008-10-183251 [pii] 10.1182/blood-2008-10-183251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radhakrishnan S, Cabrera R, Schenk EL, Nava-Parada P, Bell MP, Van Keulen VP, Marler RJ, Felts SJ, Pease LR. Reprogrammed FoxP3+ T regulatory cells become IL-17+ antigen-specific autoimmune effectors in vitro and in vivo. J Immunol. 2008;181:3137. doi: 10.4049/jimmunol.181.5.3137. published online EpubSep 1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211:214. doi: 10.1111/j.0105-2896.2006.00391.x. published online EpubJun (IMR391 [pii] 10.1111/j.0105-2896.2006.00391.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Putnam AL, Brusko TM, Lee MR, Liu W, Szot GL, Ghosh T, Atkinson MA, Bluestone JA. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes. 2009;58:652. doi: 10.2337/db08-1168. published online EpubMar (db08-1168 [pii] 10.2337/db08-1168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valmori D, Tosello V, Souleimanian NE, Godefroy E, Scotto L, Wang Y, Ayyoub M. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J Immunol. 2006;177:944. doi: 10.4049/jimmunol.177.2.944. published online EpubJul 15. [DOI] [PubMed] [Google Scholar]
- 36.Foley JE, Mariotti J, Ryan K, Eckhaus M, Fowler DH. Th2 cell therapy of established acute graft-versus-host disease requires IL-4 and IL-10 and is abrogated by IL-2 or host-type antigen-presenting cells. Biol Blood Marrow Transplant. 2008;14:959. doi: 10.1016/j.bbmt.2008.06.007. published online EpubSep (S1083-8791(08)00250-4 [pii] 10.1016/j.bbmt.2008.06.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koenen HJPM, Smeets RL, Vink PM, van Rijssen E, Boots AMH, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood %R 10.1182/blood-2008-01-133967. 2008;112:2340. doi: 10.1182/blood-2008-01-133967. published online EpubSeptember 15, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Scheinberg P, Melenhorst JJ, Brenchley MM, Hill BJ, Hensel NF, Chattopadhyay PK, Roederer M, Picker LJ, Price DA, Barrett J, Douek DC. The transfer of adaptive immunity to CMV during hematopoietic stem cell transplantation is dependent on the specificity and phenotype ofCMV-specific T cells in the donor. Blood 10.1182/blood-2009-04-214684. 2009;114:5071. doi: 10.1182/blood-2009-04-214684. published online Epub September 25,2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.