Abstract
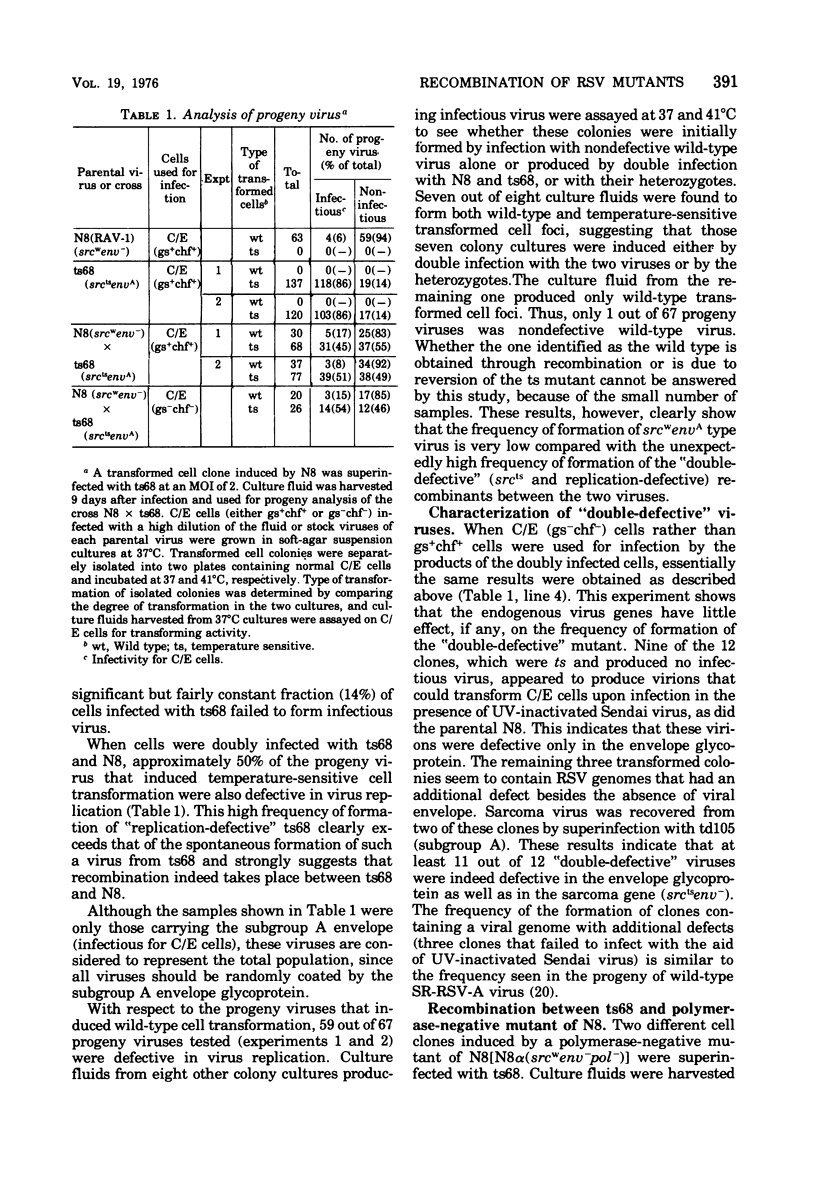
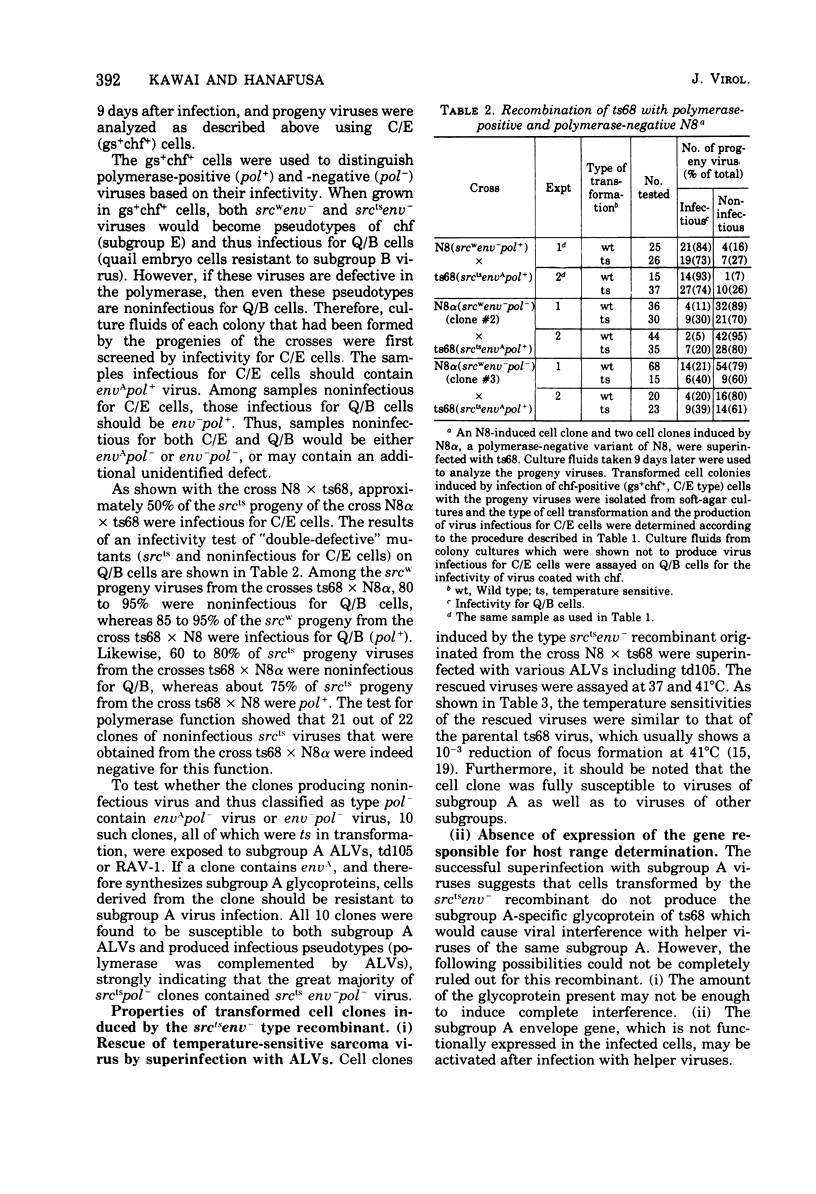
Cells doubly infected with two mutants of the Schmidt-Ruppin strain of Rous sarcoma virus (RSV), ts68, which is temperature sensitive for cell transformation (srcts), and a deletion mutant, N8, which is deficient in the envelope glycoprotein (env-), produced a recombinant which carried the defects of both parents. The frequency of formation of such a recombinant was exceptionally high and made up 45 to 55% of the progeny carrying the srcts marker. By contrast, the reciprocal recombinant, which is wild type in transformation (srcts) and contains the subgroup A envelope glycoprotein (envA), was almost undetectable. This remarkable difference in the frequency of the formation of the two possible recombinants suggests that a unique mechanism may be involved in the genetic interaction of the two virus genomes, one of which has a large deletion. When an RNA-dependent DNA polymerase-negative variant of the N8 (N8alpha) was crinants also became deficient in the polymerase. Cells infected by the srctsenv- recombinant were morphologically normal at the nonpermissive temperature (41 degrees C) and susceptible to all subgroups of RSV. The rate by which the wild-type RSV transformed the recombinant-preinfected cells was indistinguishable from that of transformation of uninfected chicken cells by the same wild-type virus. This indicates that no detectable interference exists at postpenetration stages between the preinfected and superinfecting virus genomes and confirms that the expression of the transformed state is dominant over the suppressed state.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. Tumor viruses: 1974. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1187–1200. doi: 10.1101/sqb.1974.039.01.137. [DOI] [PubMed] [Google Scholar]
- Baluda M. A., Shoyab M., Markham P. D., Evans R. M., Droham W. N. Base sequence complexity of 35S avian myeloblastosis virus RNA determined by molecular hybridization kinetics. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):869–874. doi: 10.1101/sqb.1974.039.01.101. [DOI] [PubMed] [Google Scholar]
- Beemon K., Duesberg P., Vogt P. Evidence for crossing-over between avian tumor viruses based on analysis of viral RNAs. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4254–4258. doi: 10.1073/pnas.71.10.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter M. A., Parsons J. T., Coffin J. M. The nucleotide sequence complexity of avian tumor virus RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3560–3564. doi: 10.1073/pnas.71.9.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biquard J. M., Vigier P. Isolement et étude d'un mutant conditionnel du virus de Rous à capacité transformante thermosensible. C R Acad Sci Hebd Seances Acad Sci D. 1970 Dec 21;271(25):2430–2433. [PubMed] [Google Scholar]
- Cooper P. D., Wyke J. A. The genome of RNA tumor viruses: a functional requirement for a polyploid structure? Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):997–1004. doi: 10.1101/sqb.1974.039.01.114. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Kawai S., Wang L. H., Vogt P. K., Murphy H. M., Hanafusa H. RNA of replication-defective strains of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1569–1573. doi: 10.1073/pnas.72.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. The RNA of influenza virus. Proc Natl Acad Sci U S A. 1968 Mar;59(3):930–937. doi: 10.1073/pnas.59.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields B. N., Joklik W. K. Isolation and preliminary genetic and biochemical characterization of temperature-sensitive mutants of reovirus. Virology. 1969 Mar;37(3):335–342. doi: 10.1016/0042-6822(69)90217-7. [DOI] [PubMed] [Google Scholar]
- HIRST G. K. Genetic recombination with Newcastle disease virus, polioviruses, and influenza. Cold Spring Harb Symp Quant Biol. 1962;27:303–309. doi: 10.1101/sqb.1962.027.001.028. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Hanafusa T. Further studies on RSV production form transformed cells. Virology. 1968 Apr;34(4):630–636. doi: 10.1016/0042-6822(68)90084-6. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Hanafusa H., Miyamoto T., Fleissner E. Existence and expression of tumor virus genes in chick embryo cells. Virology. 1972 Feb;47(2):475–482. doi: 10.1016/0042-6822(72)90283-8. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Miyamoto T., Hanafusa H. A type of chick embryo cell that fails to support formation of infectious RSV. Virology. 1970 Jan;40(1):55–64. doi: 10.1016/0042-6822(70)90378-8. [DOI] [PubMed] [Google Scholar]
- Joho R. H., Billeter M. A., Weissmann C. Mapping of biological functions on RNA of avian tumor viruses: location of regions required for transformation and determination of host range. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4772–4776. doi: 10.1073/pnas.72.12.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Genetic recombination with avian tumor virus. Virology. 1972 Jul;49(1):37–44. doi: 10.1016/s0042-6822(72)80005-9. [DOI] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Isolation of defective mutant of avian sarcoma virus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3493–3497. doi: 10.1073/pnas.70.12.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Plaque assay for some strains of avian leukosis virus. Virology. 1972 Apr;48(1):126–135. doi: 10.1016/0042-6822(72)90120-1. [DOI] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Kawai S., Metroka C. E., Hanafusa H. Complementation of functions required for cell transformation by double infection with RSV mutants. Virology. 1972 Jul;49(1):302–304. doi: 10.1016/s0042-6822(72)80032-1. [DOI] [PubMed] [Google Scholar]
- Kawai S., Yamamoto T. Isolation of different kinds of non-virus producing chick cells transformed by Schmidt-Ruppin strain (subgroup A) of Rous sarcoma virus. Jpn J Exp Med. 1970 Aug;40(4):243–256. [PubMed] [Google Scholar]
- Khoury A. T., Hanafusa H. Synethesis and integration of viral DNA in chicken cells at different time after infection with various multiplicities of avian oncornavirus. J Virol. 1976 May;18(2):383–400. doi: 10.1128/jvi.18.2.383-400.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. S. Rous sarcoma virus: a function required for the maintenance of the transformed state. Nature. 1970 Sep 5;227(5262):1021–1023. doi: 10.1038/2271021a0. [DOI] [PubMed] [Google Scholar]
- Neiman P. E., Purchase H. G., Okazaki W. Chicken leukosis virus genome sequences in DNA from normal chick cells and virus-induced bursal lymphomas. Cell. 1975 Apr;4(4):311–319. doi: 10.1016/0092-8674(75)90151-8. [DOI] [PubMed] [Google Scholar]
- Pons M. W., Hirst G. K. Polyacrylamide gel electrophoresis of influenza virus RNA. Virology. 1968 Feb;34(2):385–388. doi: 10.1016/0042-6822(68)90257-2. [DOI] [PubMed] [Google Scholar]
- Quade K., Smith R. E., Nichols J. L. Evidence for common nucleotide sequences in the RNA subunits comprising Rous sarcoma virus 70 S RNA. Virology. 1974 Sep;61(1):287–291. doi: 10.1016/0042-6822(74)90263-3. [DOI] [PubMed] [Google Scholar]
- Schincariol A. L., Joklik W. K. Early synthesis of virus-specific RNA and DNA in cells rapidly transformed with Rous sarcoma virus. Virology. 1973 Dec;56(2):532–548. doi: 10.1016/0042-6822(73)90056-1. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D., Loh P. Separation of ten reovirus genome segments by polyacrylamide gel electrophoresis. J Virol. 1968 Oct;2(10):986–991. doi: 10.1128/jvi.2.10.986-991.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., Baluda M. A., Evans R. Acquisition of new DNA sequences after infection of chicken cells with avian myeloblastosis virus. J Virol. 1974 Feb;13(2):331–339. doi: 10.1128/jvi.13.2.331-339.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima K., Vogt P. K. Temperature sensitive mutants of an avian sarcoma virus. Virology. 1969 Dec;39(4):930–931. doi: 10.1016/0042-6822(69)90030-0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Vogt P. K., Bishop J. M. Integration of deoxyribonucleic acid specific for Rous sarcoma virus after infection of permissive and nonpermissive hosts. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3067–3071. doi: 10.1073/pnas.70.11.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K. Genetically stable reassortment of markers during mixed infection with avian tumor viruses. Virology. 1971 Dec;46(3):947–952. doi: 10.1016/0042-6822(71)90093-6. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. H., Kawai S., Hanafusa H. Location of envelope-specific and sarcoma-specific oligonucleotides on RNA of Schmidt-Ruppin Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):447–451. doi: 10.1073/pnas.73.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. A., Mason W. S., Vogt P. K. Genetic recombinants and heterozygotes derived from endogenous and exogenous avian RNA tumor viruses. Virology. 1973 Apr;52(2):535–552. doi: 10.1016/0042-6822(73)90349-8. [DOI] [PubMed] [Google Scholar]
- Wyke J. A., Bell J. G., Beamand J. A. Genetic recombination among temperature-sensitive mutnats of Rous sarcoma virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):897–905. doi: 10.1101/sqb.1974.039.01.104. [DOI] [PubMed] [Google Scholar]
- Wyke J. A. Complementation of transforming functions by temperature-sensitive mutants of avian sarcoma virus. Virology. 1973 Jul;54(1):28–36. doi: 10.1016/0042-6822(73)90111-6. [DOI] [PubMed] [Google Scholar]


