Abstract
Study Objectives:
The magnitude of the post-apnea/hypopnea ventilatory overshoot following arousal may perpetuate subsequent respiratory events in obstructive sleep apnea (OSA) patients, potentially contributing to the disorder's severity. As acetazolamide can reduce apnea severity in some patients, we examined the effect of acetazolamide on the ventilatory response to spontaneous arousals in CPAP-treated OSA patients.
Design:
We assessed the ventilatory response to arousal in OSA patients on therapeutic CPAP before and after administration of acetazolamide for 7 days.
Setting:
Sleep research laboratory.
Participants:
12 (7M/5F) CPAP-treated OSA patients.
Interventions:
Sustained-release acetazolamide 500 mg by mouth twice daily for one week.
Measurements and Results:
A blinded investigator identified spontaneous arousals (3-15 s) during NREM sleep. Breath-by-breath measurements of minute ventilation, end-tidal CO2, tidal volume, expiratory/inspiratory-time, and total breath duration were determined (4-s intervals) 32 s prior and 60 s following each arousal. Acetazolamide significantly increased resting ventilation (7.3 ± 0.2 L/min versus 8.2 ± 0.4 L/min; P < 0.05) and attenuated the percent increase in ventilation following arousal by ~2.5 fold (122.0% ± 4.4% versus 108.7% ± 3.5% pre-arousal level; P < 0.05). There was a positive correlation between the mean increase in ventilatory response to arousal and mean AHI (r2 = 0.44, P = 0.01). However, absolute peak levels of ventilation following arousal remained unchanged between conditions (8.8 ± 0.4 L/min versus 8.9 ± 0.1 L/min).
Conclusions:
Acetazolamide substantially attenuates the increase in ventilation following spontaneous arousal from sleep in OSA patients. This study suggests an additional mechanism by which acetazolamide may contribute to the improvement in ventilatory instability and OSA severity. The data also provide support for reinforcing the importance of ventilatory control in OSA pathogenesis.
Citation:
Edwards BA; Connolly JG; Campana LM; Sands SA; Trinder JA; White DP; Wellman A; Malhotra A. Acetazolamide attenuates the ventilatory response to arousal in patients with obstructive sleep apnea. SLEEP 2013;36(2):281-285.
Keywords: Respiratory control, arousals, acetazolamide, obstructive sleep apnea, lung, ventilation
INTRODUCTION
Obstructive sleep apnea (OSA) is a disorder which affects at least 2% to 4% of middle-aged adults1 and is characterized by repetitive occlusion of the pharyngeal airway during sleep. Airway obstruction leads to increasing respiratory efforts until the airway reopens and breathing is restored, often following arousal from sleep. These transient events expose patients to intermittent hypoxemia, hypercapnia, fluctuations in intrathoracic pressure, and surges in sympathetic activity, all yielding important deleterious consequences.
Although several factors that contribute to the pathogenesis of OSA have been identified, the contribution that ventilatory control instability (often referred to as a high loop gain [LG]) plays in OSA pathogenesis has been debated; with some authors suggesting that high LG may be an important cause of sleep apnea, whereas others have suggested that the presence of recurrent apnea may elevate LG (i.e., a high LG is a consequence rather than cause of disease).2,3 A recent investigation in our laboratory used the ventilatory stimulant acetazolamide (a carbonic anhydrase inhibitor) to lower LG among patients with treated OSA.4 We observed that approximately halving LG pharmacologically was associated with a 50% reduction in OSA severity, suggesting a causal link between LG and OSA. These data raised questions regarding acetazolamide's mechanism(s) of action, specifically how does lowering LG improve OSA?
In our previous publication, we reported that acetazolamide reduced the number of hypopneas during the night. One explanation for this observation is the fact that acetazolamide lowers the efficiency of CO2 excretion (i.e., plant gain), thereby lowering LG without altering upper airway physiology or respiratory arousal threshold. In the presence of a reduced LG, swings in ventilation and blood gases are attenuated, potentially allowing for stable breathing without arousal. Furthermore, our measure of ventilatory instability in the absence of arousal (i.e., dynamic LG) was also reduced. Taken together, these improvements in LG potentially explain the observed reduction in AHI with acetazolamide. However, this explanation neglects the potential importance of sleep state instability in promoting recurrent apnea. Thus, we have acknowledged the need for closer scrutiny of the arousals from sleep in people with OSA. Indeed, the level of hyperventilation and subsequent hypocapnia that occurs following arousal has been suggested as a mechanism that perpetuates subsequent respiratory events, potentially contributing to disease severity. According to modeling studies, a reduction in LG should make it harder for arousal to promote fluctuations in chemical drive,5 possibly by a diminished ventilatory overshoot following arousal. However this concept has not been examined in subjects with OSA to date. On the basis of the above logic, we examined the effect of acetazolamide on the magnitude of the ventilatory response to spontaneous arousal (VRA) from sleep. We tested the hypothesis that lowering LG via acetazolamide would produce a reduction in the VRA. Such a finding would provide a further potential mechanism for how acetazolamide improves ventilatory stability and OSA severity.4
METHODS
Participants
We retrospectively analyzed the VRA of the 12 OSA patients (7M/5F: mean age 49.8 ± 2.1 y) who participated in our previous experiment examining the effect of acetazolamide administration on the physiological traits causing OSA.4 All patients had a history of OSA (AHI > 10 events/h) and were compliant CPAP users (> 5 h/night for ≥ 2 months prior to the study).
Experimental Design and Set-up
Full details of the open label experimental design and set-up have been described previously, although none of the findings from the current analysis have been reported.4 Briefly, patients underwent a clinical polysomnogram (PSG) off CPAP to assess OSA severity followed by a research PSG to measure 4 OSA traits (consecutive nights) under both baseline and acetazolamide conditions. The order of the baseline and acetazolamide studies was randomly assigned with ≥ 1-week washout between conditions. During the treatment arm, subjects took acetazolamide (500 mg by mouth twice daily) for 7 days, and the clinical and research PSGs were performed on days 6-7.
For the clinical PSG a standard clinical montage, including an electroencephalogram, submental and leg electromyogram, electrocardiogram, nasal pressure and thermistor, and arterial oxygen saturation, was used to assess sleep and respiration. Sleep state and respiratory events were scored by a blinded sleep technician according to standard criteria.6 During the research PSG, subjects were asked to sleep in the supine position on a level of CPAP sufficient to eliminate snoring, flow limitation, and hypopneas (therapeutic CPAP). Airflow was measured using a nasal mask (Respironics, Murrysville, PA) attached to a pneumotachometer (model 3700A; Hans Rudolph, Kansas City, MO). The mask was attached to a positive/negative pressure source (Phillips-Respironics, Murrysville, PA) used to regulate the level of CPAP. A port in the mask (Validyne, Northridge, CA) facilitated measurement of mask pressure. CO2 was recorded at the nostril via a capnograph (Vacumed, Ventura, CA). All signals were sampled at 125 Hz and displayed using Nihon Kohden (Tokyo, Japan) and Spike2 software (Cambridge Electronic Design, Cambridge, UK).
Data Analysis
A single investigator blinded to treatment protocol identified spontaneous arousals for analysis from the research PSG. Criteria for spontaneous arousal followed pre-established guidelines7; arousals had to be 3-15 s in duration during stage 2-4 NREM sleep and be preceded and followed by ≥ 1 min of stable NREM sleep. Given that our previous protocol routinely performed 3 min “drops” in CPAP throughout the night to subtherapeutic levels, only the spontaneous arousals that occurred while on therapeutic CPAP were included in the analysis. Arousals were discarded if a mask leak, a change in the level of CPAP, or mouth expiration occurred within 1 min either side of the arousal. Breath-by-breath measurements of inspired minute ventilation (), end-tidal CO2 (PCO2), tidal volume (VT), expiratory time (TE), inspiratory time (TI), and total breath duration were interpolated at 4-s intervals for 32 s prior and 60 s following each arousal (start arousal = time zero). HR was measured on a beat-by-beat basis and was interpolated at 1-s intervals for 30 s prior and 60 s following arousal.
Statistics
Paired t-tests were used to assess the effect of acetazolamide on baseline ventilatory and cardiovascular parameters. Repeated measures ANOVA were used to compare changes in all variables for 60 s following arousal from NREM sleep with a Student-Newman-Keuls post hoc test when significant ANOVA effects were found. Linear regression was used to assess the relationship between the VRA and AHI. All tests were performed using SigmaPlot (Systat Software, CA). P < 0.05 was considered significant. Values are presented as means ± SEM.
RESULTS
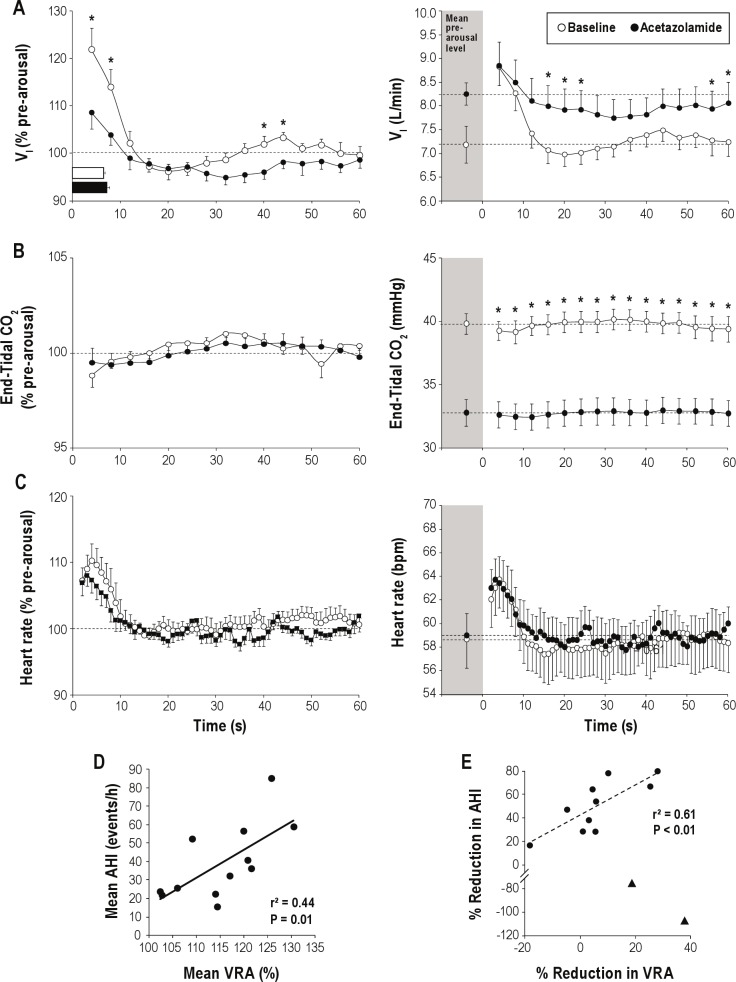
Compared to baseline, administration of acetazolamide did not alter the number (7.3 ± 1.2 versus 7.8 ± 1.5) or duration (7.2 ± 0.5 s versus 8.3 ± 0.6 s) of arousals included in the analysis, and the therapeutic level of CPAP used in both studies did not differ (10.4 ± 0.5 cm H2O versus 10.6 ± 0.6 cm H2O). The effects of acetazolamide on all cardiorespiratory variables are depicted in Figure 1 and are expressed as both a percentage of pre-arousal levels (left column) as well in absolute values (right column). Figure 1A (left column) demonstrates that when compared to the pre-arousal, acetazolamide significantly reduced the magnitude of the first breath following arousal (122.0 ± 4.4 % pre-arousal levels versus 108.7 ± 3.5 % pre-arousal levels; P < 0.001), which was attributed to the reduced VT (data not shown). Furthermore, there was a secondary overshoot during baseline conditions (40-44 sec) that was abolished with acetazolamide. However, when ventilation was examined in absolute terms (not %), the peak following arousal was not different off and on acetazolamide (8.8 ± 0.4 L/min versus 8.9 ± 0.5 L/min; Figure 1A right column). Acetazolamide appeared to attenuate the reduction in PCO2 following arousal (-0.5 ± 0.3 mm Hg versus −0.2 ± 0.2 mm Hg; Figure 1B), but this failed to reach significance (P = 0.29). However, when the CO2 on the first breath following arousal was compared to pre-arousal levels (paired t-test), there was a significant reduction off acetazolamide (39.8 ± 0.8 mm Hg versus 38.9 ± 0.5 mm Hg; P = 0.05) that was not evident on acetazolamide (32.8 ± 1.0 mm Hg versus 32.6 ± 1.0 mm Hg; P = 0.27).
Figure 1.
The effect of acetazolamide on (A), end-tidal PCO2 (B), and heart rate (C) are presented as both percent change from baseline (left column) and absolute values (right column) following spontaneous arousal from NREM sleep on optimal CPAP. Dashed lines represent average pre-arousal levels. Arousal durations are shown by horizontal bars in Panel A. Error bars are presented as SEM. *Indicates P ≤ 0.05 between conditions at equivalent times. Relationship between the magnitude of the ventilatory response to arousal (VRA: expressed as a percentage of resting ventilation) and AHI, averaged from baseline and acetazolamide conditions (D). Notably, the size of the VRA positively correlated with apnea severity (r2 = 0.44; P = 0.01). Interestingly, the reduction in the magnitude of the VRA positively correlated with the improvement (reduction) in AHI in responders (E) (solid circles) to acetazolamide treatment (r2 = 0.61; P < 0.01), a relationship that was not present when non-responders (triangles) were included, potentially highlighting the multi-factorial nature of OSA pathogenesis.
The heart rate response to arousal, when expressed either in terms of percent change from baseline or in absolute values, was unaffected by administration of acetazolamide (Figure 1C). Although there was no relationship between the VRA and AHI under baseline or acetazolamide conditions, when the data from both the baseline and acetazolamide conditions were averaged, there was a positive correlation between the VRA (% pre-arousal levels) and the AHI (Figure 1D; P = 0.01). Interestingly, when we separated the data into those who responded to treatment (defined as those whose AHI was reduced with acetazolamide) compared to non-responders (defined as those whose AHI worsened with acetazolamide), there was a positive correlation in the percent reduction in the VRA and the reduction in AHI in the responders (Figure 1E; P < 0.01).
DISCUSSION
The major novel finding of our study was that the ventilatory response to spontaneous arousal in a group of CPAP-treated OSA patients was attenuated by administration of acetazolamide. Interestingly, we observed that the magnitude of the VRA was positively correlated with AHI. Previous studies have shown that acetazolamide can improve ventilatory instability during sleep, which is considered the mechanism underlying improvements in central and obstructive sleep apnea. This study suggests a novel additional mechanism by which acetazolamide improves ventilatory instability is the substantial (between 2-3 fold) reduction in the VRA. These findings are of particular interest, as our data add strength to the concept that the magnitude of the VRA may contribute to the perpetuation of subsequent respiratory events and OSA pathogenesis.
The mechanisms that determine the magnitude of the VRA have been attributed to a combination of; (1) the sudden removal of the sleep-induced increase in upper airway resistance, (2) a reflex “startle-like” mechanism that is independent of ventilatory sensitivity during wakefulness and, (3) the restoration of the waking chemical drive to the level associated with the increased PCO2 level.8–10 Certainly, any differences in upper airway resistance are unlikely to have played a role in the observed reduction in ventilatory responses, as all spontaneous arousals were measured while the subjects were on therapeutic CPAP. It is difficult to assess definitively whether acetazolamide altered a “startle-like” component of the ventilatory response or the “degree of alertness” achieved during spontaneous arousal. However, we believe important differences are unlikely, as the duration of the spontaneous arousals were similar, as were the heart rate responses to arousal.
Could the attenuation of the VRA with acetazolamide be explained by wake-versus-sleep differences in chemical drive associated with the prevailing PCO2 level during sleep? That is, with acetazolamide, is there a reduced jump in ventilation with the transition from sleep to wake (at constant CO2) due to greater disparities between awake and sleep ventilatory controller curves (ventilation versus PCO2)? On first inspection, this explanation appears unlikely, since there was no effect of acetazolamide on the wake-sleep difference in ventilation (1.6 ± 0.4 L/min versus 2.1 ± 0.6 L/min; baseline vs. acetazolamide; P = 0.33) and CO2 (1.3 ± 0.3 mm Hg versus 0.9 ± 0.4 mm Hg; P = 0.27). This concept is also supported by the findings of Trinder et al.11 who previously demonstrated that the magnitude of the VRA does not seem to differ as a function of the PCO2 at the time of the arousal. However, it is known from animal studies that acetazolamide slows the rate of change in ventilation to both hypoxic and hypercapnic challenges by 50% to 75%. Such a reduction would increase the amount of time required for a new elevated PCO2 (in the case of hypercapnic stimulus) to be matched by a corresponding level of ventilatory output.12,13 Thus, a plausible mechanism by which acetazolamide attenuates the VRA is by decreasing the speed (increasing the time constant) of the neural transduction process that occurs at chemoreceptors or their integration at the brainstem. Upon arousal from sleep, ventilation starts to rise towards to wakefulness levels associated with the prevailing sleep CO2 level. Whether or not this wakefulness level of ventilation is actually achieved may depend on the time constant of the neural response to the existing PCO2 relative to the duration of the arousal. For a time constant of 15 s, switching to a wake controller for 8 s (a typical arousal duration) would raise ventilation by 41% of the maximum wakefulness amount. By contrast, a 2-fold or 4-fold increase in time constant as observed previously12,13 would only allow time for ventilation to rise to 23% or 12% of maximum (2-3 fold reduction). Thus, the reported reduction in the speed of transduction (2- to 4-fold increase in time constant) is consistent with the size of the reduction in VRA observed in the current study. Given the speculative nature of this hypothesis, this concept clearly warrants further investigation.
The role of arousal in OSA has been the subject of intense interest. The immediate impact of arousal is to restore wakefulness pharyngeal tone and patency and thereby prevent asphyxia, which may be necessary to avoid severe hypoxia. More recently, we observed that some patients progressively recruit upper airway muscle activity over frequent recurrent arousals,14 leading to persistent improvement in pharyngeal dilator muscle activity and periods of stable breathing. On the other hand, when arousal induces a considerable ventilatory response, it certainly promotes dynamic ventilatory instability,5 which may contribute to OSA severity in certain individuals. In our study, we provide new evidence that acetazolamide reduces ventilatory instability in the form of a lowered VRA, and the abolition of the secondary overshoot in ventilation following arousal; the latter is consistent with our previous finding that dynamic LG is reduced with acetazolamide (measured in the absence of arousal). Whether or not the acetazolamide-induced improvement in OSA relies on the reduction in the VRA and improved dynamic ventilatory instability remains unclear but is likely to depend on the prevailing ventilatory instability and upper airway mechanics. Nonetheless, based on our observation that the magnitude of the arousal response within an individual correlates with OSA severity, our study suggests that the VRA may influence OSA severity.
CONCLUSIONS
In summary, acetazolamide reduced the ventilatory response to spontaneous arousal, a response that was predictive of OSA severity in our CPAP-treated patients. This study suggests that a novel additional mechanism by which acetazolamide may improve ventilatory instability is by reducing the magnitude of the ventilatory response to arousal. Our data add strength to the concept that the magnitude of the ventilatory overshoot following arousal may contribute to the perpetuation of subsequent respiratory events and OSA pathogenesis; however further investigation is needed.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Wellman is a consultant for Philips Respironics, SOVA Pharmaceuticals and Apnex Medical. Dr. White is the chief medical officer for Philips Respironics. Dr. Malhotra is a consultant for Philips Respironics, SHC, SGS, Apnex Medical, Pfizer and Apnicure. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Miss Lauren Hess and Mrs. Karen Stevenson for their laboratory assistance. Dr. Edwards was supported by the Thoracic Society of Australia and New Zealand/Allen and Hanbury's Respiratory Research Fellowship during data collection and is currently supported by the National Health and Medical Research Council of Australia's CJ Martin Overseas Biomedical Fellowship (1035115). Dr. Sands is supported by an American Heart Association fellowship (11POST7360012). This work was supported by the National Institutes of Health: R01 HL090897-01A2, K24 HL 093218-01A1, P01 HL 095491, R01HL110350, UM1HL108724, R01- AG035117 and R01 HL085188 as well as the American Heart Association: 0840159N, 0575028N. The Harvard Catalyst Clinical Research Center is funded by UL1 RR 025758-01.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CPAP
continuous positive airway pressure
- HR
heart rate
- LG
loop gain
- OSA
obstructive sleep apnea
- PSG
polysomnography
- VRA
ventilatory response to arousal
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Salloum A, Rowley JA, Mateika JH, Chowdhuri S, Omran Q, Badr MS. Increased propensity for central apnea in patients with obstructive sleep apnea: effect of nCPAP. Am J Respir Crit Care Med. 2010;181:189–93. doi: 10.1164/rccm.200810-1658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loewen A, Ostrowski M, Laprairie J, et al. Determinants of ventilatory instability in obstructive sleep apnea: inherent or acquired? Sleep. 2009;32:1355–65. doi: 10.1093/sleep/32.10.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards BA, Sands SA, Eckert DJ, et al. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol. 2012;590:1199–211. doi: 10.1113/jphysiol.2011.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khoo MC, Koh SS, Shin JJ, Westbrook PR, Berry RB. Ventilatory dynamics during transient arousal from NREM sleep: implications for respiratory control stability. J Appl Physiol. 1996;80:1475–84. doi: 10.1152/jappl.1996.80.5.1475. [DOI] [PubMed] [Google Scholar]
- 6.American Academy of Sleep Medicine. International classification of sleep disorders, revised: diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 7.Jordan AS, McEvoy RD, Edwards JK, et al. The influence of gender and upper airway resistance on the ventilatory response to arousal in obstructive sleep apnoea in humans. J Physiol. 2004;558:993–1004. doi: 10.1113/jphysiol.2004.064238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillipson EA. Control of breathing during sleep. Am Rev Respir Dis. 1978;118:909–39. doi: 10.1164/arrd.1978.118.5.909. [DOI] [PubMed] [Google Scholar]
- 9.Khoo MC, Shin JJ, Asyali MH, Kim TS, Berry RB. Ventilatory dynamics of transient arousal in patients with obstructive sleep apnea. Respir Physiol. 1998;112:291–303. doi: 10.1016/s0034-5687(98)00041-3. [DOI] [PubMed] [Google Scholar]
- 10.Horner RL, Rivera MP, Kozar LF, Phillipson EA. The ventilatory response to arousal from sleep is not fully explained by differences in CO(2) levels between sleep and wakefulness. J Physiol. 2001;534:881–90. doi: 10.1111/j.1469-7793.2001.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trinder J, Ivens C, Kleiman J, Kleverlaan D, White DP. The cardiorespiratory activation response at an arousal from sleep is independent of the level of CO2. J Sleep Res. 2006;15:174–82. doi: 10.1111/j.1365-2869.2006.00513.x. [DOI] [PubMed] [Google Scholar]
- 12.Iturriaga R, Mokashi A, Lahiri S. Dynamics of carotid body responses in vitro in the presence of CO2-HCO3-: role of carbonic anhydrase. J Appl Physiol. 1993;75:1587–94. doi: 10.1152/jappl.1993.75.4.1587. [DOI] [PubMed] [Google Scholar]
- 13.Coates EL, Li AH, Nattie EE. Acetazolamide on the ventral medulla of the cat increases phrenic output and delays the ventilatory response to CO2. J Physiol. 1991;441:433–51. doi: 10.1113/jphysiol.1991.sp018760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan AS, Eckert DJ, Wellman A, Trinder JA, Malhotra A, White DP. Termination of respiratory events with and without cortical arousal in obstructive sleep apnea. Am J Respir Crit Care Med. 2011;184:1183–91. doi: 10.1164/rccm.201106-0975OC. [DOI] [PMC free article] [PubMed] [Google Scholar]