Abstract
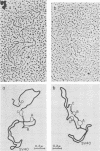
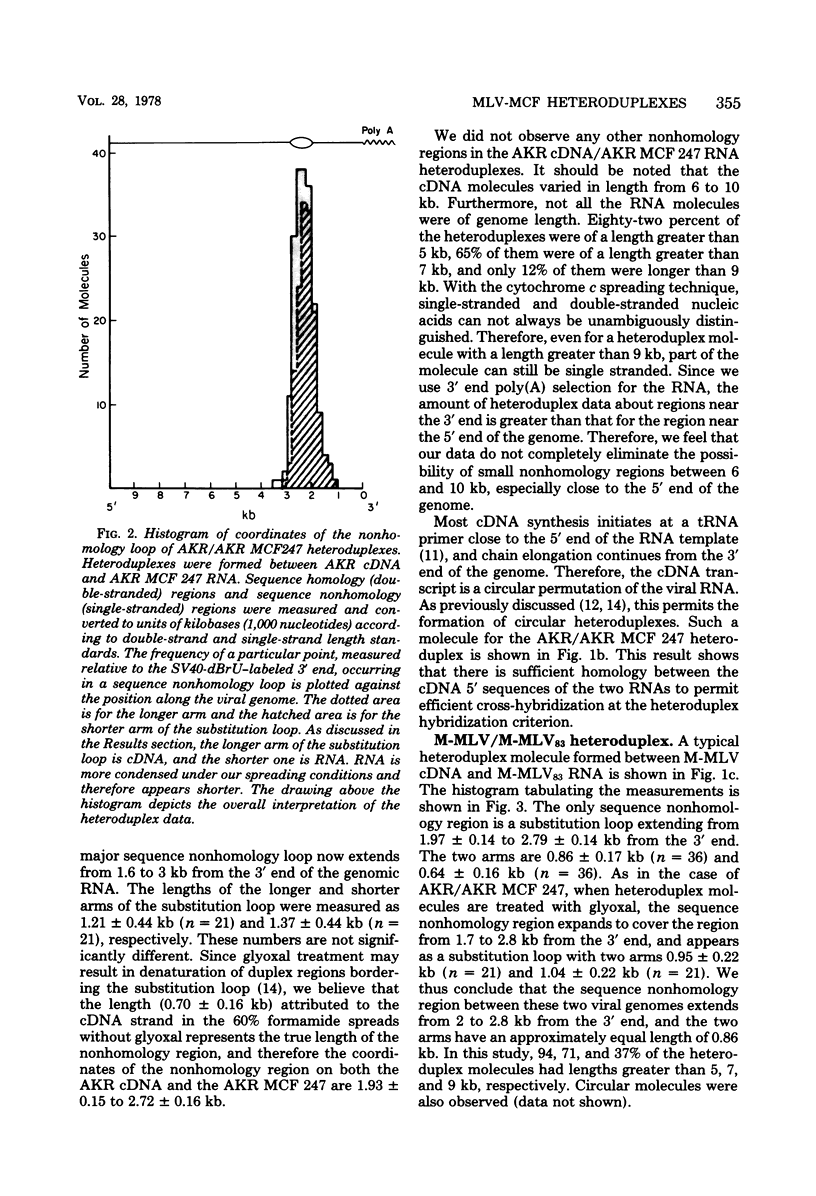
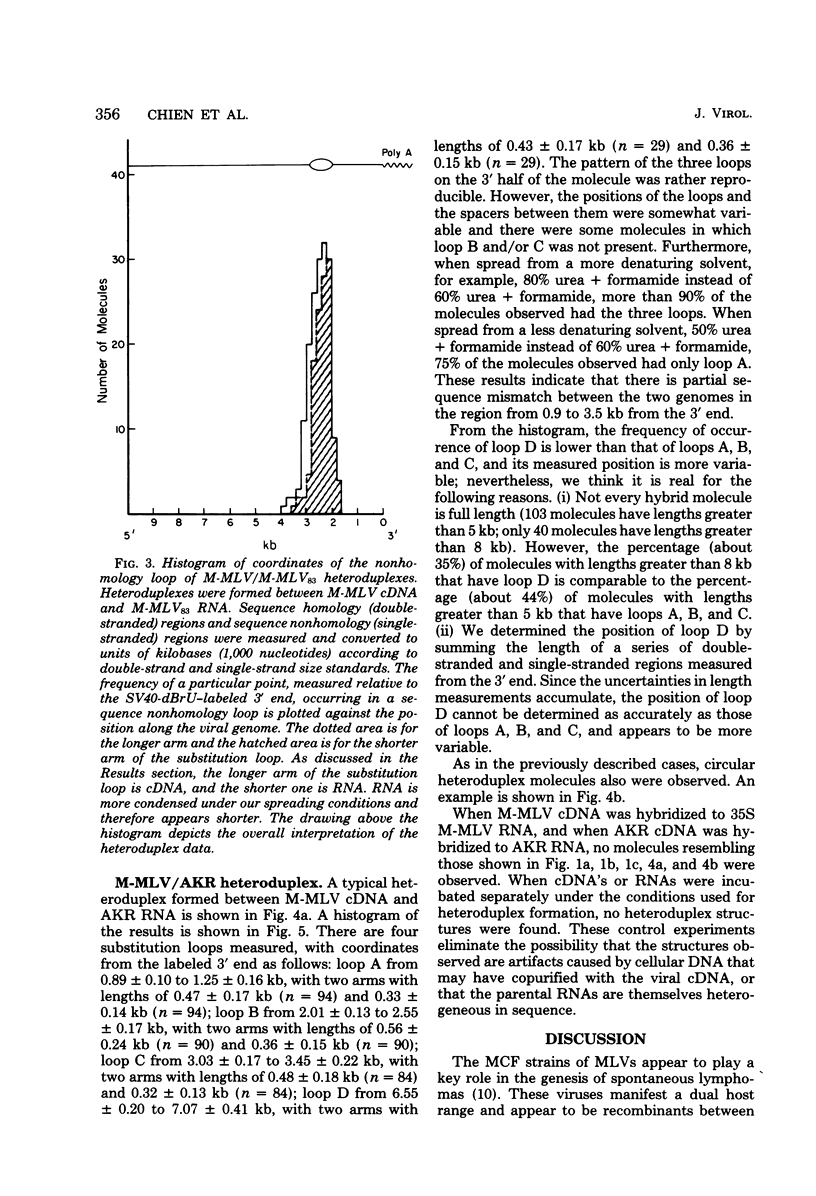
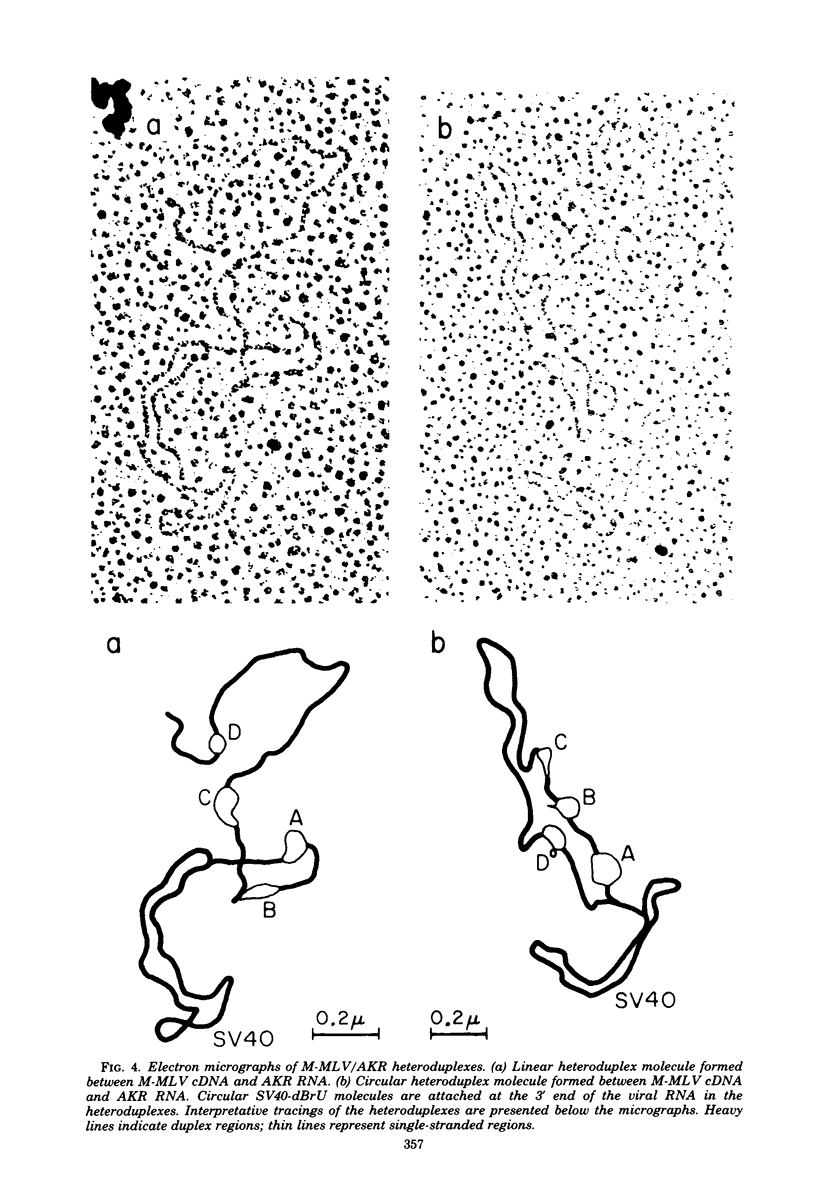
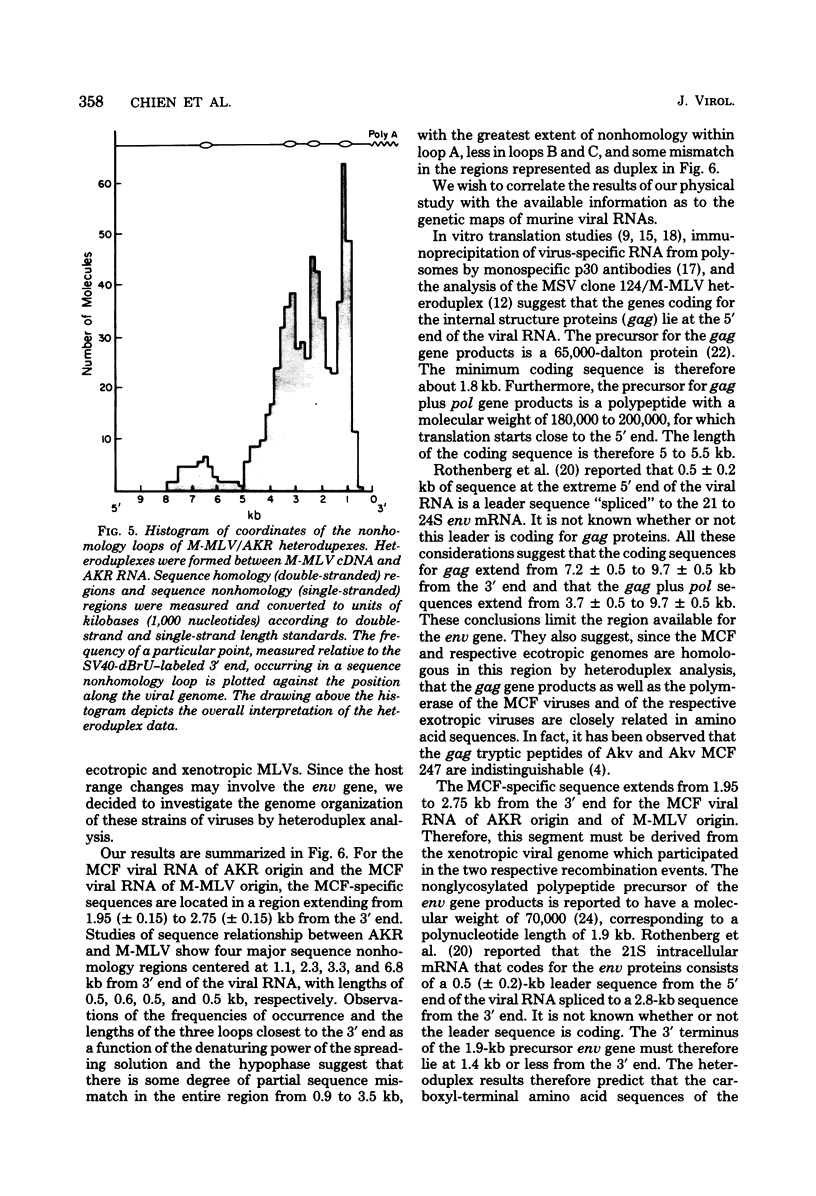
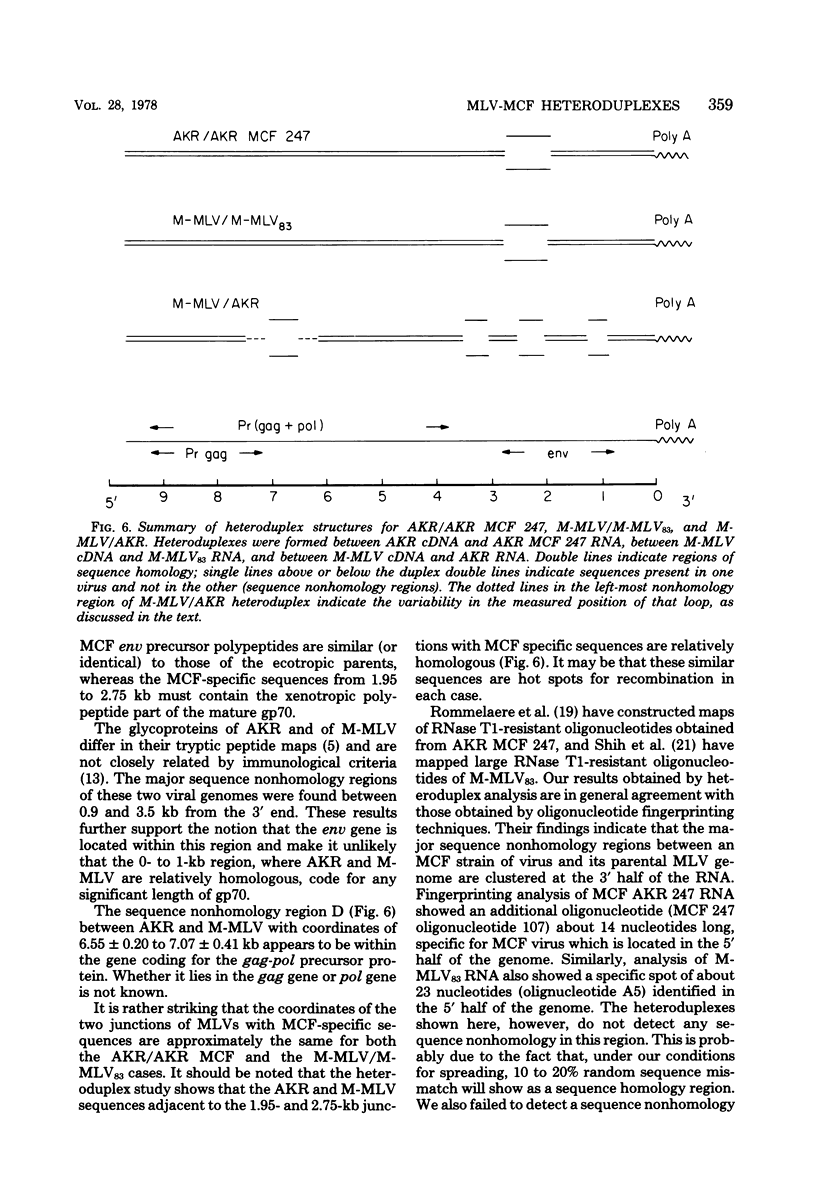
The sequence relationships betwen AKR ecotropic virus and an AKR-derived "mink cell focus-inducing" (MCF) isolate (AKR MCF 247), between Moloney murine leukemia virus (M-MLV) and an M-MLV MCF isolate (M-MLV83), and between AKR and M-MLV were studied by electron microscopic heteroduplex analysis. The MCF-specific sequences were found to map from 1.95 kilobases (kb) to 2.75 kb (+/- 0.15 kb) from the 3' end of the RNAs for both MCF isolates. The major sequence nonhomology regions between AKR and M-MLV lie between 0.9 and 3.5 kb from the 3' end. However, the AKR and M-MLV sequences immediately adjacent to the 1.95- and 2.75-kb junctions with MCF-specific sequences are relatively similar in AKR and M-MLV. Our results suggest that the env gene of MLVs maps from 1 kb to 3 kb from the 3' end of the genomic RNA and that the carboxyl end of the glycoprotein of each MCF strain is similar (or identical) to that of its ecotropic parent.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bender W., Davidson N. Mapping of poly(A) sequences in the electron microscope reveals unusual structure of type C oncornavirus RNA molecules. Cell. 1976 Apr;7(4):595–607. doi: 10.1016/0092-8674(76)90210-5. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Gautsch J. W., Jensen F. C., Lerner R. A., Hartley J. W., Rowe W. P. Biochemical evidence that MCF murine leukemia viruses are envelope (env) gene recombinants. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4676–4680. doi: 10.1073/pnas.74.10.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Jensen F. C., Bryant M. L., Lerner R. A. Polymorphism of the major envelope glycoprotein (gp70) of murine C-type viruses: virion associated and differentiation antigens encoded by a multi-gene family. Nature. 1977 May 5;267(5606):23–28. doi: 10.1038/267023a0. [DOI] [PubMed] [Google Scholar]
- Fan H., Baltimore D. RNA metabolism of murine leukemia virus: detection of virus-specific RNA sequences in infected and uninfected cells and identification of virus-specific messenger RNA. J Mol Biol. 1973 Oct 15;80(1):93–117. doi: 10.1016/0022-2836(73)90235-0. [DOI] [PubMed] [Google Scholar]
- Fan H., Paskind M. Measurement of the sequence complexity of cloned Moloney murine leukemia virus 60 to 70S RNA: evidence for a haploid genome. J Virol. 1974 Sep;14(3):421–429. doi: 10.1128/jvi.14.3.421-429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger P. J., Nomura S., Bolognesi D. P. A novel murine oncornavirus with dual eco- and xenotropic properties. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5150–5155. doi: 10.1073/pnas.72.12.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielkens A. L., Van Zaane D., Bloemers H. P., Bloemendal H. Synthesis of Rauscher murine leukemia virus-specific polypeptides in vitro. Proc Natl Acad Sci U S A. 1976 Feb;73(2):356–360. doi: 10.1073/pnas.73.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine W. A., Kleid D. G., Panet A., Rothenberg E., Baltimore D. Ordered transcription of RNA tumor virus genomes. J Mol Biol. 1976 Sep 5;106(1):109–131. doi: 10.1016/0022-2836(76)90303-x. [DOI] [PubMed] [Google Scholar]
- Hu S., Davidson N. A heteroduplex study of the sequence relationships between the RNAs of M-MSV and M-MLV. Cell. 1977 Mar;10(3):469–477. doi: 10.1016/0092-8674(77)90034-4. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Denny T. P., Bolognesi D. P. Purification and serological characterization of the major envelope glycoprotein from AKR murine leukemia virus and its reactivity with autogenous immune sera from mice. J Virol. 1976 Mar;17(3):727–736. doi: 10.1128/jvi.17.3.727-736.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghans R. P., Hu S., Knight C. A., Davidson N. Heteroduplex analysis of avian RNA tumor viruses. Proc Natl Acad Sci U S A. 1977 Feb;74(2):477–481. doi: 10.1073/pnas.74.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Olshevsky U., Lodish H. F., Baltimore D. Translation of murine leukemia virus RNA in cell-free systems from animal cells. J Virol. 1976 May;18(2):627–635. doi: 10.1128/jvi.18.2.627-635.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung H. J., Hu S., Bender W., Bailey J. M., Davidson N., Nicolson M. O., McAllister R. M. RD-114, baboon, and woolly monkey viral RNA's compared in size and structure. Cell. 1976 Apr;7(4):609–620. doi: 10.1016/0092-8674(76)90211-7. [DOI] [PubMed] [Google Scholar]
- Mueller-Lantzsch N., Fan H. Monospecific immunoprecipitation of murine leukemia virus polyribosomes: identification of p30 protein-specific messenger RNA. Cell. 1976 Dec;9(4 Pt 1):579–588. doi: 10.1016/0092-8674(76)90040-4. [DOI] [PubMed] [Google Scholar]
- Philipson L., Andersson P., Olshevsky U., Weinberg R., Baltimore D., Gesteland R. Translation of MuLV and MSV RNAs in nuclease-treated reticulocyte extracts: enhancement of the gag-pol polypeptide with yeast suppressor tRNA. Cell. 1978 Jan;13(1):189–199. doi: 10.1016/0092-8674(78)90149-6. [DOI] [PubMed] [Google Scholar]
- Rommelaere J., Faller D. V., Hopkins N. Characterization and mapping of RNase T1-resistant oligonucleotides derived from the genomes of Akv and MCF murine leukemia viruses. Proc Natl Acad Sci U S A. 1978 Jan;75(1):495–499. doi: 10.1073/pnas.75.1.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Donoghue D. J., Baltimore D. Analysis of a 5' leader sequence on murine leukemia virus 21S RNA: heteroduplex mapping with long reverse transcriptase products. Cell. 1978 Mar;13(3):435–451. doi: 10.1016/0092-8674(78)90318-5. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O., Troxler D. H., Coffin J. M., Scolnick E. M. Mapping host range-specific oligonucleotides within genomes of the ecotropic and mink cell focus-inducing strains of Moloney murine leukemia virus. J Virol. 1978 Apr;26(1):71–83. doi: 10.1128/jvi.26.1.71-83.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler D. H., Lowy D., Howk R., Young H., Scolnick E. M. Friend strain of spleen focus-forming virus is a recombinant between ecotropic murine type C virus and the env gene region of xenotropic type C virus. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4671–4675. doi: 10.1073/pnas.74.10.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M. Genome organization of RNA tumor viruses. I. In vitro synthesis of full-genome-length single-stranded and double-stranded viral DNA transcripts. J Virol. 1978 Jun;26(3):615–629. doi: 10.1128/jvi.26.3.615-629.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Ven W. J., Onnekink C., Vermorken A. J., Bloemers H. P. Effect of impaired glycosylation on the synthesis of envelope proteins of Rauscher murine leukemia virus. Virology. 1977 Oct 15;82(2):334–344. doi: 10.1016/0042-6822(77)90008-3. [DOI] [PubMed] [Google Scholar]