Abstract
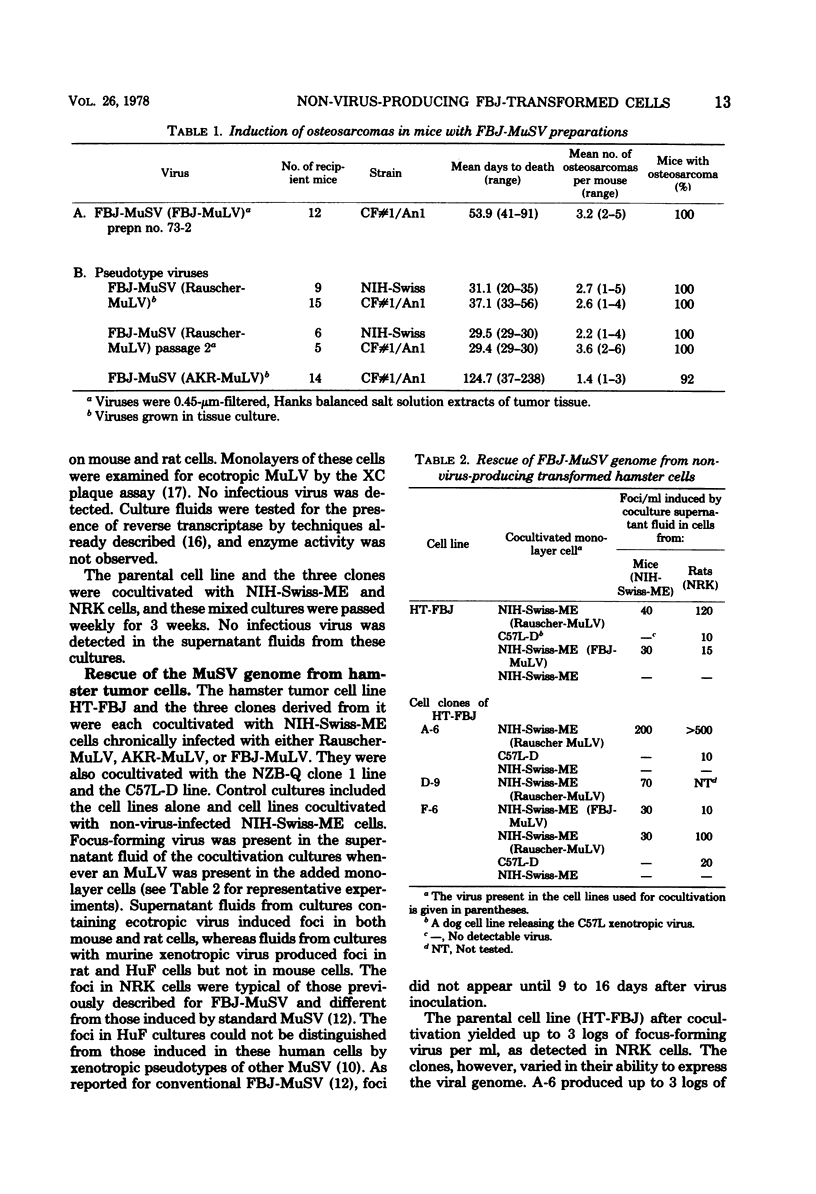
Hamster and rat cell lines have been established that have been transformed by FBJ murine sarcoma virus (FBJ-MuSV) but that do not produce virus. The hamster cell line originated from an osteosarcoma that appeared in a hamster inoculated at birth with an extract of a CFNo1 mouse FBJ-osteosarcoma. The rat cell line was obtained by transferring the FBJ-MuSV genome to normal rat kidney cells in the absence of the FBJ type C virus (FBJ-MuLV), which, usually in high concentration, accompanies the FBJ-MuSV. Both transformed hamster and rat cell lines contain the FBJ-MuSV genome, which can be rescued by ecotropic and xenotropic murine type C viruses. This rescued genome produces characteristic FBJ-MuSV foci in tissue culture and, in appropriate animal hosts, induces osteosarcomas typical of those induced by FBJ-MuSV. FBJ-MuSV was isolated originally from a parosteal osteosarcoma that occurred naturally in a mouse. Since there was no previous history of passage of the agent through any other animal species, these non-virus-producing hamster and rat cells transformed by FBJ-MuSV should be very helpful in molecular studies examining the origin of spontaneous sarcoma genomes in mice.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnstein P., Levy J. A., Oshiro L. S., Price P. J., Suk W., Lennette E. H. Recovery of murine xenotropic type-C virus from C57L mice. J Natl Cancer Inst. 1974 Dec;53(6):1787–1792. [PubMed] [Google Scholar]
- Duc-Nguyen H. Enhancing effect of diethylaminoethyl-dextran on the focus-forming titer of a murine sarcoma virus (Harvey strain). J Virol. 1968 Jun;2(6):643–644. doi: 10.1128/jvi.2.6.643-644.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel M. P., Jinkins P. B., Tolle J., Biskis B. O. Serial radiography of virus-induced osteosarcomas in mice. Radiology. 1966 Aug;87(2):333–339. doi: 10.1148/87.2.333. [DOI] [PubMed] [Google Scholar]
- HUEBNER R. J., ARMSTRONG D., OKUYAN M., SARMA P. S., TURNER H. C. SPECIFIC COMPLEMENT-FIXING VIRAL ANTIGENS IN HAMSTER AND GUINEA PIG TUMORS INDUCED BY THE SCHMIDT-RUPPIN STRAIN OF AVIAN SARCOMA. Proc Natl Acad Sci U S A. 1964 May;51:742–750. doi: 10.1073/pnas.51.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huu Duc-Nguyen, Rosenblum E. N., Zeigel R. F. Persistent infection of a rat kidney cell line with Rauscher murine leukemia virus. J Bacteriol. 1966 Oct;92(4):1133–1140. doi: 10.1128/jb.92.4.1133-1140.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A. Demonstration of differences in murine sarcoma virus foci formed in mouse and rat cells under a soft agar overlay. J Natl Cancer Inst. 1971 May;46(5):1001–1007. [PubMed] [Google Scholar]
- Levy J. A., Hartley J. W., Rowe W. P., Huebner R. J. Studies of FBJ osteosarcoma virus in tissue culture. I. Biologic characteristics of the "C"-type viruses. J Natl Cancer Inst. 1973 Aug;51(2):525–539. [PubMed] [Google Scholar]
- Levy J. A., Hartley J. W., Rowe W. P., Huebner R. J. Studies of FBJ osteosarcoma virus in tissue culture. II. Autoinhibition of focus formation. J Natl Cancer Inst. 1975 Mar;54(3):615–619. [PubMed] [Google Scholar]
- Levy J. A. Host range of murine xenotropic virus: replication in avian cells. Nature. 1975 Jan 10;253(5487):140–142. doi: 10.1038/253140a0. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Kazan P., Varnier O., Kleiman H. Murine xenotropic type C viruses I. Distribution and further characterization of the virus in NZB mice. J Virol. 1975 Oct;16(4):844–853. doi: 10.1128/jvi.16.4.844-853.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rowe W. P., Teich N., Hartley J. W. Murine leukemia virus: high-frequency activation in vitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science. 1971 Oct 8;174(4005):155–156. doi: 10.1126/science.174.4005.155. [DOI] [PubMed] [Google Scholar]
- Ross J., Scolnick E. M., Todaro G. J., Aaronson S. A. Separation of murine cellular and murine leukaemia virus DNA polymerases. Nat New Biol. 1971 Jun 9;231(23):163–167. doi: 10.1038/newbio231163a0. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]


