Abstract
Staphylococcus aureus causes purulent skin and soft tissue infections (SSTIs) that frequently reoccur. Staphylococal SSTIs can lead to invasive disease and sepsis, which are among the most significant causes of infectious disease mortality in both developed and developing countries. Human or animal infections with S. aureus do not elicit protective immunity against staphylococcal diseases. Here we review what is known about the immune evasive strategies of S. aureus that enable the pathogen’s escape from protective immune responses. Three secreted products are discussed in detail, staphylococcal protein A (SpA), staphylococcal binder of immunoglobulin (Sbi) and adenosine synthase A (AdsA). By forming a complex with VH3-type IgM on the surface of B cells, SpA functions as a superantigen to modulate antibody responses to staphylococcal infection. SpA also captures pathogen-specific antibodies by binding their Fcγ portion. The latter activity of SpA is shared by Sbi, which also associates with complement factors 3d and factor H to promote the depletion of complement. AdsA synthesizes the immune signaling molecule adenosine, thereby dampening innate and adaptive immune responses during infection. We discuss strategies how the three secreted products of staphylococci may be exploited for the development of vaccines and therapeutics.
Introduction
The Gram-positive pathogen Staphylococcus aureus causes a wide swath of human diseases including skin and soft tissue infections (SSTI) and invasive diseases that lead to bacteremia, sepsis, endocarditis or pneumonia [1]. S. aureus colonizes the skin and nares of 20-30% of the human population [2]. Due to the frequent use of antibiotics, S. aureus strains have evolved resistance against the most abundantly used therapeutics [3]. These drug-resistant strains are historically referred to as methicillin-resistant S. aureus (MRSA) [4]. Infections with methicillin-sensitive S. aureus (MSSA) or MRSA originate both in the community and in hospitals [5,6]. The therapy of severe MRSA infections is complicated by the fact that these strains are susceptible to only few antimicrobials – vancomycin, daptomycin or linezolid [5]. Because of the severity of invasive disease, MRSA infections are associated with a poor outcome even when appropriate antibiotic therapies have been implemented [7,8]. A key feature of staphylococcal SSTI is its recurrence, which occurs in approximately 30% of all cases. These clinical observations as well as experiments with animals that had been repeatedly challenged with S. aureus suggest that infections with this pathogen do not generate protective immune responses [9,10]. The current epidemic of community- and hospital-acquired MRSA infections in developed and developing countries is testimony for the successful spread and immune evasive attributes of this pathogen [11]. Here we review what is known about the immune evasive strategies of S. aureus.
Staphylococcal immune evasion strategies - an overview
Upon entry into subepidermal tissues or blood, S. aureus encounters the cellular and proteinaceous elements of host innate immune defenses. S. aureus is uniquely programmed to compromise the effectiveness of both components by secreting proteins that inhibit complement deposition or activation as well as the chemotaxis of polymorphonuclear leukocytes (neutrophils) [12-17]. Other secreted polypeptides display lytic activities towards neutrophils, the primary line of defense against S. aureus infections (reviewed in reference [16]). Several of these secreted products are encoded by two gene clusters designated Immune Evasion Cluster 1 and 2 (IEC) that appear to have been acquired through integration of prophages [18]. IEC encoded products include superantigen-like proteins that act on human cells or human complement components, not on those of other mammals, suggesting a pathogenic strategy of host specific adaptation [19,20]. Consequently, the contribution of IEC1 and IEC2 to disease pathogenesis has not yet been explored in animal models for S. aureus infection. IEC also encodes staphylokinase, a bacterial plasminogen activator that mediates cleavage of opsonins by activating plasmin [4], and provides resistance towards antimicrobial peptides such as α-defensins [5]. Details on the physiological and molecular attributes of IEC encoded immune evasion strategies and the variation of these genes between S. aureus strains can be found in several excellent reviews and are not described in detail here [17,20,21]. In addition to host-specific virulence factors, staphylococci also deploy universal modulators of inflammation by increasing the abundance of the host signaling molecule adenosine (Figure 1A). This immune evasion strategy was revealed through the discovery of AdsA, a virulence factor that is encoded in the core genome of the chromosome (see below).
Figure 1.
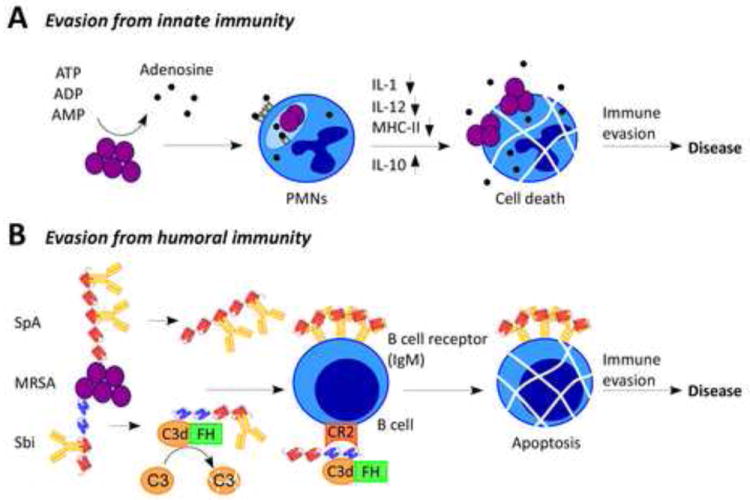
S. aureus escapes innate and adaptive immune responses.
(A) During infection, S. aureus produces adenosine via conversion from AMP, ADP or ATP. Adenosine receptor-mediated signaling on phagocytes results in interference of phagocytic killing of staphylococci by polymorphonuclear leukocytes (PMNs) and may impair adaptive immune responses directed towards staphylococci by professional antigen presenting cells such as macrophages and dendritic cells. Prolonged staphylococcal survival within phagocytic cells may also aid in the pathogen’s escape from innate and adaptive immune responses. (B) Two immunoglobulin binding molecules expressed by S. aureus, staphylococcal protein A (SpA) and S. aureus binder of immunoglobulin (Sbi), inhibit opsonophagocytic clearance of the pathogen. In addition, these proteins have been proposed to block the normal function of B cells, either by inducing B cell apoptosis or by inhibiting receptor interaction of complement factor C3.
Staphylococcal infections in humans result in a transient increase in anti-staphylococcal antibody levels [22-24]. Nevertheless, protective immunity is not observed and recurrent infections occur frequently [1]. Clearly, immunization with defined antigens can elicit protection in animal models of S. aureus disease, suggesting that during infection antibody levels may simply be too low to be protective [25,26]. Two classes of immunomodulatory factors are secreted by S. aureus and compromise the induction of both humoral and cell-mediated immunity: enterotoxins that function as T cell superantigens [27] and the B cell superantigen staphylococcal protein A (SpA) [28]. Injection of either of these factors in purified form into animals is associated with the depletion of T or B cell populations, respectively. The T cell superantigens bind MHC class II determinants on the surface of antigen-presenting cells even in the absence of antigen peptide [29]. This binding triggers functional association with T cell receptors (TCR) on the surface of T helper cells [30,31]. Enterotoxins are commonly expressed by clinical isolates of S. aureus and encoded on mobile genetic elements [32]. Each type of enterotoxin recognizes a specific subset of variable Vβ chains of T-cell receptors via its superantigen domain [33]. While it is clear that enterotoxins such as TSST-1 cause an emetic response when ingested, the contribution of superantigen activity during SSTI or other infections is still poorly understood. In rabbits, where purified T cell superantigens display extraordinary toxicity, at least some of the staphylococcal enterotoxins have been shown to contribute to the pathogenesis of lethal pneumonia and that preexisting immunity can prevent the lethal outcome of these infections [34]. Similarly, humans develop neutralizing antibodies against a T cell superantigen, however cross protection against other staphylococcal T cell superantigens has not been observed [35]. The considerable variability and functional redundancy of staphylococcal enterotoxins and toxic shock syndrome toxins has prevented the development of a superantigen vaccine capable of neutralizing at least several of these T cell superantigens.
Protein A has been shown to bind to the VH3 region of IgM molecules [36] exposed on the surface of B lymphocytes [37] (Figure 1B). VH3 type B-cell receptors (BCR) are present in about 30% of human B cell populations. When injected into mice, purified SpA promotes the proliferation and apoptosis of B cells in both the spleen and the bone marrow [37] (Figure 1B). In mice, B-1 and marginal zone B cells as well as innate B cells, which secrete natural antibodies, are most susceptible to SpA treatment in comparison with B-2 cells or follicular B cells that generate antigen-specific antibodies [28,38]. Once depleted, B cells cannot be replenished readily in SpA-treated animals [37]. SpA interacts with several host proteins and is a key anti-opsonic factor (see below). In agreement with this model, mutants that no longer produce protein A display reduced virulence in multiple animal models for staphylococcal diseases [39-41]. Most relevant to the immune-modulatory attributes of this molecule is the observation that mice infected with spa mutants, but not wild-type S. aureus, elicit protective immunity against secondary infection with wild-type strains in a manner that correlates with the production of anti-staphylococcal antibodies [42]. Like humans, mice with a history of staphylococcal infection have not acquired protective immunity and mount only weak humoral responses to S. aureus infection [42]. These observations support a model whereby S. aureus modulates B cell responses via its SpA surface antigen, a process that may be amenable to therapeutic interference.
Controlling inflammation - adenosine synthase A (AdsA)
Adenosine synthase A (AdsA) is an immune evasion factor that was initially identified in a genetic screen probing for the relative contributions of cell wall anchored surface proteins towards staphylococcal survival in blood [43]. Both wild-type and adsA variants are phagocytosed by neutrophils when inoculated into fresh blood, however wild-type S. aureus survives within neutrophils whereas adsA mutants are killed [43]. Similar to SpA, AdsA is synthesized as a precursor with an N-terminal signal peptide and a C-terminal sorting signal. Following initiation into the secretory pathway, the C-terminal sorting signal is cleaved by sortase and covalently linked to the cell wall envelope of staphylococci [44] (Figure 2). AdsA harbors two 5’-nucleotidase signature sequences and purified recombinant AdsA catalyzes the hydrolysis of phosphate esterified at carbon 5’ of ribose or deoxyribose within purine nucleotides with adenosine monophosphate (AMP) being the preferred substrate [43]. The resulting conversion of adenosine monophosphate (AMP) to adenosine had been observed earlier in 5’-nucleotidases that are present in the cytoplasm of many cells [45]. Following inoculation of staphylococci into blood, AdsA surface protein is responsible for a dramatic increase in the overall abundance of extracellular adenosine, an activity that is absolutely dependent on its 5’-nucleotidase sequences [43].
Figure 2.
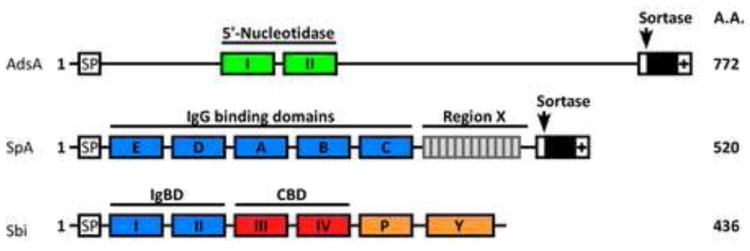
Schematic of staphylococcal immune evasion proteins. AdsA harbors two 5’-nucleotidase motifs and a C-terminal LPXTG motif sorting signal. Protein A (SpA) is comprised of five homologous immunoglobulin binding domains followed by region X, which is the most variable portion of the staphylococcal genome, and by the LPXTG motif sorting signal at the C-terminal end. Sbi is composed of two immunoglobulin binding domains, which are homologous to those of SpA, followed by complement binding domains and uncharacterized Pro / Tyr rich domains.
Adenosine represents the perhaps most potent immunosuppressive signaling molecule. It is formed by cells following hypoxic stress, exposure to reactive oxygen species (ROS) or cell lysis associated with severe cell damage [46,47] (Figure 1A). Extracellular adenosine interacts with one or more of its adenosine receptors - A1, A2A, A2B and A3 - all members of the family of G protein coupled receptors [47,48]. Adenosine receptor interaction triggers anti-inflammatory signaling cascades that cause the inhibition of platelet aggregation [49], of neutrophil superoxide bursts or degranulation [50], of T cell adherence to fibroblasts and interleukin 1α (IL-1) production as well as the increased production of IL-10, an anti-inflammatory cytokine [51]. Thus, adenosine is thought to be the key signal controlling excessive inflammation within mammalian organisms. Staphylococci evolved adsA to provide the adenosine signaling molecule during infection, thereby escaping both innate and adaptive defense mechanisms otherwise essential for the clearance of bacterial pathogens by the host.
The finding that AdsA is necessary for staphylococcal survival within neutrophils provides a rational appreciation for earlier observations, demonstrating that S. aureus escapes the bactericidal attributes of leukocytes [9,52]. Gresham and colleagues demonstrated that the injection of neutrophils harboring S. aureus into the blood stream of naïve mice allowed the pathogen to disseminate within the host and to develop staphylococcal disease [53]. Taken together, these observations suggest a model whereby staphylococci exploit their intracellular location in neutrophils to disseminate from the bloodstream into peripheral tissues. Presumably this journey is completed by triggering neutrophil diapedesis through endothelial barriers [54]. Entry into the neutrophil vehicle comes at a price, as staphylococci have to tame all bactericidal responses of these cells including reactive oxygen species (ROS), hydrolytic enzymes and peptide defensins [54]. There are, however, considerable gains. The intracellular location of staphylococci may provide protection from complement, antibodies and extracellular host defensive peptides as well as the many other cells of the host’s immune system, most notably phagocytes (macrophages and dendritic cells) that can kill bacteria and present antigens to elicit adaptive immune responses [55].
Recent studies proposed that ROS in neutrophils exert not only a canonical bactericidal activity but also activate hydrolytic enzymes such as myeloperoxidase, cathepsin G, elastase, proteinase 3 as well as other hydrolases that degrade bacterial invaders [56]. It is not yet known whether staphylococcal adenosine production in extracellular fluids or within neutrophils can disrupt the formation or maturation of phagolysosomes, interfere with the generation or secretion of bactericidal molecules or induce cellular death mechanisms such as apoptosis or necrosis. Whatever the detailed mechanisms may be, the ability of S. aureus to avoid bactericidal activities of professional phagocytes has profound consequences on the development of humoral immunity. Adenosine decreases the expression of MHC-II on macrophages and dendritic cells and it decreases macrophage production of IL-12, a pivotal stimulus for Th1-type immune responses [48,57,58]. Skewing adaptive immune responses during infection may also precipitate inadequate T cell effector mechanisms, thereby further disabling adaptive immune responses (Figure 1A).
Immunoglobulin binding molecules of S. aureus
Protein A
Staphylococcal protein A (SpA) is an abundant cell wall anchored surface protein, initially discovered as a bacterial trait to precipitate immunoglobulins [59]. Later studies demonstrated that SpA binds tightly to the complement binding (Fcγ) portion of IgG [60] and also stimulates B lymphocyte proliferation [61], provoking their clonal expansion and subsequent cell death [37,62] (Figure 1B). SpA molecules of staphylococcal isolates carry four or five 56-61 residue Ig binding domains (IgBDs) [63]. The IgBDs are followed by the C-terminal region X composed of Xr, a highly repetitive yet variable octapeptide and Xc, a domain of unique sequence that abuts the cell wall anchor structure of SpA [64,65] (Figure 2). The co-crystal structure of one of the IgBD domains of SpA with immunoglobulins revealed that IgBDs may interact simultaneously with two Ig molecules via their Fcγ and F(ab)2 domains [66]. Binding of Fcγ on the cell surface of staphylococci has long been recognized as a strategy to mask underlying surface antigens and inhibit opsonophagocytic killing of staphylococci by PMNs [62]. Mapping of the F(ab)2 domain is in agreement with earlier studies, revealing SpA interaction with VH3 type B cell receptors (BCR) [36]. SpA has also been shown to interact with von Willebrand factor (vWF) [67], gC1qR/p33 [68], and tumor necrosis factor receptor 1 (TNFR1) [69]. The same SpA residues that are involved in Fcγ interaction are also required for association with vWF [66]. vWF is a large multimeric glycoprotein, mediating platelet adhesion following endovascular injury [67]. gC1qR/p33 is a surface protein expressed ubiquitously on B cells and platelets [68]. Platelet activation or interaction is a major determinant of virulence during staphylococcal endocarditis [70]. SpA can also interact with EGFR to activate TACE, a metalloprotease that cleaves TNFR1 from the surface of airway epithelial cells [71]. Released TNFR1 is thought to compete with membrane bound TNFR1 for TNF-α and downregulate the inflammatory response. The intricacies of this network of protein-protein interaction is further complicated by the finding that the Xr module of SpA triggers IFN signaling in lungs during infection of animals [72].
Sbi
Staphylococcal binder of immunoglobulin (Sbi) is a secreted protein that is also associated with the bacterial envelope [73]. Sbi contains two IgBD-like modules that share amino acid sequence homology to the IgBD of SpA followed by two domains responsible for binding of complement factor C3d and factor H (Figure 2). Envelope-associated Sbi interacts mostly with IgG, whereas C3-binding is observed with the secreted forms of the molecule [73]. Sbi was originally identified by panning a phage display library of S. aureus genomic DNA against immobilized human IgG [74]. Like protein A, Sbi carries a variable region at the end of its C-terminus, yet it lacks a C-terminal LPXTG sorting signal [75]. Unlike protein A, the IgBD-like modules of Sbi provide only for Fcγ binding but do not display B cell superantigen activity [75]. Instead, Sbi forms a tripartite complex with complement factor C3d and factor H, a process that leads to the consumption of C3 [76] (Figure 1B). In this manner, Sbi manipulates the molecular link between innate and adaptive immune responses in favor of staphylococcal disease. Complement opsonized antigens or pathogens interact with complement receptors (CR). For example, the deposition of C3d on the surface of a bacterial pathogen promotes interaction of the entire complex with the CR2 receptor, thereby enhancing the specificity of antibody responses by B cells [77]. Biochemical experiments revealed that the Sbi-C3d interaction precludes the association between C3d and CR2 [78]. If so, Sbi likely acts synergistically with protein A by blocking antigen recognition of B cells, whereas SpA triggers expansion and apoptotic collapse of VH3-type B cells that are involved in adaptive immune responses (Figure 1B).
Therapeutic and preventive strategies to tame staphylococcal immune evasion
As is outlined above, staphylococci deploy a wide spectrum of strategies to avoid innate immune attacks such as complement deposition and killing by neutrophils. The complexity and functional redundancy of factors engaged in innate immune evasion renders the development of therapeutic or preventive approaches arduous. Similarly, variability of an immune-modulatory trait among S. aureus strains is an exclusion criterion for vaccine development. In contrast to most genes in the IEC clusters, SpA, Sbi and AdsA are universal traits of all S. aureus strains and may therefore offer opportunities for the development of vaccines or therapeutics [42].
AdsA is a homolog of CD73, the mammalian adenosine synthase (5’nucleotidase) [43]. Although this has not yet been tested, AdsA immunization may elicit antibodies that interfere with CD73 function, thereby promoting inflammatory responses in vaccinees. To establish a rational basis for AdsA vaccines or therapeutics, future work would need to study the specificity of immune responses to this molecule and the ability of specific antibodies to neutralize the bacterial enzyme without affecting the host’s ability to synthesize adenosine.
Wild-type SpA cannot be used as a vaccine antigen because of its B cell superantigen activity [10]. Each SpA IgBD domain adopts a triple α-helical bundle structure [79] and the atomic interactions with Fab VH3 [66] or Fcγ [80] have been elucidated. In a recent study, glutamine 9 and 10 as well as aspartate 36 and 37 were identified as critical for the association of protein A with immunoglobulins [10]. When four substitutions - Q9K, Q10K, D36A, and D37A - were introduced in each of the five IgBDs domains, the new variant, designated SpAKKAA, had lost B cell superantigen and Fcγ binding activities [10]. Immunization of rabbits or mice with SpAKKAA, but not with wild-type SpA, generated high titer antibodies to this antigen. Further, SpAKKAA derived antibodies blocked the association of protein A with immunoglobulin as well as its B cell superantigen activity. Non-toxigenic SpAKKAA elicited protective immunity against S. aureus challenge [10]. The resulting protein A specific antibodies facilitated opsonophagocytic killing of staphylococci in naïve mouse blood [10]. Further, following challenge with S. aureus, animals immunized with SpAKKAA developed humoral immune responses to many different antigens, demonstrating that neutralization of SpA B cell-superantigen activity is paramount to the establishment of immunity [10].
Future prospects
Rapid spread of antibiotic resistance traits among S. aureus isolates as well as the acquisition of enhanced virulence attributes have precipitated a public health crisis that can no longer be addressed with the development of new antibiotics alone [81]. Several recent efforts have been directed towards the development of staphylococcal vaccines [82-86]. Although several staphylococcal antigens have shown promise in preclinical trials, those subjected to protective efficacy evaluation in clinical trials have not yet met the required end points for vaccine licensure [81,87,88]. The S. aureus vaccine field appears to recognize the need for multiple vaccine antigens to neutralize the key virulence strategies of this pathogen [25,26]. Nevertheless, the possibility of boosting the host’s ability to respond to S. aureus infection with protective immune response has not yet been explored. The recent observation that S. aureus spa mutants are unable to prevent animals from mounting a protective immune response correlates well with the know B cell-toxigenic activity of SpA [28,42]. This knowledge may be exploited for the development of new therapies by generating protein A variants that cannot escape host recognition and that elicit both opsonophagocytic and neutralizing antibodies [10].
Highlights.
Staphylococcus aureus causes recurrent infections without eliciting immunity
Escape from protective immunity involves staphylococcal spa, sbi and adsA genes
Interference of B cell development is blocked by antibodies against protein A (SpA)
SpA and Sbi interfere with staphylococcal opsonophagocytic killing
Adenosine synthase (AdsA) dampens immune responses by providing a host signal
Acknowledgments
This work was supported by grants from the National Institute of Allergy and Infectious Diseases (NIAID), Infectious Diseases Branch (AI52474, AI92711 and AI52767). D.M.M. and O.S. acknowledge membership within and support from the Region V “Great Lakes” Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium (NIH Award 1-U54-AI-057153). V.T. acknowledges support from the American Heart Association postdoctoral fellowship 10POST4590023.
Glossary
- AdsA
adenosine synthase A
- 5’-Nucleotidase motif I
ILHTnDiHGrL (a.a. 124-134)
- 5’-Nucleotidase motif II
TdamaVGNHEFD (a.a. 189-201)
- SpA
Staphylococcal protein A
- Sbi
S. aureus binder of IgG
- CBD
complement binding domain
- P
Pro-rich domain
- Y
Tyr-rich domain
Footnotes
Competing interests: The authors declare a conflict of interests as inventors of patent applications that are related to the development of Staphylococcus aureus vaccines and are currently under commercial license.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lowy FD. Staphylococcus aureus infections. New Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Peacock SJ, de Silva I, Lowy FD. What determines nasal carriage of staphylococcus aureus? Trends Microbiol. 2001;9(12):605–610. doi: 10.1016/s0966-842x(01)02254-5. [DOI] [PubMed] [Google Scholar]
- 3.Neu HC. The crisis in antibiotic resistance. Science. 1992;257:1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 4.Rooijakkers SH, van Wamel WJ, Ruyken M, van Kessel KP, van Strijp JA. Anti-opsonic properties of staphylokinase. Microbes Infect. 2005;7(3):476–484. doi: 10.1016/j.micinf.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Jin T, Bokarewa M, Foster T, Mitchell J, Higgins J, Tarkowski A. Staphylococcus aureus resists human defensins by production of staphylokinase, a novel bacterial evasion mechanism. J Immunol. 2004;172(2):1169–1176. doi: 10.4049/jimmunol.172.2.1169. [DOI] [PubMed] [Google Scholar]
- 6.Chambers HF. The changing epidemiology of staphylococcus aureus? Emerg Infect Dis. 2001;7(2):178–182. doi: 10.3201/eid0702.010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, et al. Invasive methicillin-resistant staphylococcus aureus infections in the united states. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 8.Klevens RM, Edwards JR, Gaynes RP, System NNIS. The impact of antimicrobial-resistant, health care-associated infections on mortality in the united states. Clin Infect Dis. 2008;47:927–930. doi: 10.1086/591698. [DOI] [PubMed] [Google Scholar]
- 9.Rogers DE, Tompsett R. The survival of staphylococci within human leukocytes. J Exp Med. 1952;95(2):209–230. doi: 10.1084/jem.95.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10**.Kim HK, Cheng AG, Kim HY, Missiakas DM, Schneewind O. Nontoxigenic protein a vaccine for methicillin-resistant staphylococcus aureus infections in mice. J Exp Med. 2010;207(9):1863–1870. doi: 10.1084/jem.20092514. The authors provide a molecular approach for the development of a protein A antigen that can be used to generate neutralizing antibodies as well as boosts the host immune response toward S. aureus infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy AD, Otto M, Braughton KR, Whitney AR, Chen L, Mathema B, Mediavilla JR, Byrne KA, Parkins LD, Tenover FC, Kreiswirth BN, et al. Epidemic community-associated methicillin-resistant staphylococcus aureus: Recent clonal expansion and diversification. Proc Natl Acad Sci USA. 2008;105:1327–1332. doi: 10.1073/pnas.0710217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hair PS, Ward MD, Semmes OJ, Foster TJ, Cunnion KM. Staphylococcus aureus clumping factor a binds to complement regulator factor i and increases factor i cleavage of c3b. J Infect Dis. 2008;198(1):125–133. doi: 10.1086/588825. [DOI] [PubMed] [Google Scholar]
- 13*.Rooijakkers SH, Wu J, Ruyken M, van Domselaar R, Planken KL, Tzekou A, Ricklin D, Lambris JD, Janssen BJ, van Strijp JA, Gros P. Structural and functional implications of the alternative complement pathway c3 convertase stabilized by a staphylococcal inhibitor. Nat Immunol. 2009;10(7):721–727. doi: 10.1038/ni.1756. Bimolecular C3 convertase (C3bBb) is the central protease complex of the complement system and it is inherently instable This study describes the structure of C3bBb stabilized by SCIN from S aureus and answers some crucial questions related to convertase specificity and function relevant to our understanding of regulation of innate immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Chen H, Ricklin D, Hammel M, Garcia BL, McWhorter WJ, Sfyroera G, Wu YQ, Tzekou A, Li S, Geisbrecht BV, Woods VL, Jr, et al. Allosteric inhibition of complement function by a staphylococcal immune evasion protein. Proc Natl Acad Sci U S A. 2010;107(41):17621–17626. doi: 10.1073/pnas.1003750107. Many staphylococcal virulence factors, including extracellular fibrinogen-binding protein (Efb), target host molecules involved in complement activation or regulation The authors show that Efb interacts with C3b and induces conformational changes through which the active C3 convertase formation is inhibited. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Postma B, Kleibeuker W, Poppelier MJ, Boonstra M, Van Kessel KP, Van Strijp JA, de Haas CJ. Residues 10-18 within the c5a receptor n terminus compose a binding domain for chemotaxis inhibitory protein of staphylococcus aureus. J Biol Chem. 2005;280(3):2020–2027. doi: 10.1074/jbc.M412230200. [DOI] [PubMed] [Google Scholar]
- 16**.Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005;3:948–958. doi: 10.1038/nrmicro1289. An outstanding review that summarizes both pathogenic and immunomodulatory strategies of S. aureus. [DOI] [PubMed] [Google Scholar]
- 17.Lambris JD, Ricklin D, Geisbrecht BV. Complement evasion by human pathogens. Nat Rev Microbiol. 2008;6(2):132–142. doi: 10.1038/nrmicro1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Wamel WJ, Rooijakkers SH, Ruyken M, van Kessel KP, van Strijp JA. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of staphylococcus aureus are located on beta-hemolysin-converting bacteriophages. J Bacteriol. 2006;188(4):1310–1315. doi: 10.1128/JB.188.4.1310-1315.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel D, Wines BD, Langley RJ, Fraser JD. Specificity of staphylococcal superantigen-like protein 10 toward the human igg1 fc domain. J Immunol. 2010;184(11):6283–6292. doi: 10.4049/jimmunol.0903311. [DOI] [PubMed] [Google Scholar]
- 20.Langley R, Patel D, Jackson N, Clow F, Fraser JD. Staphylococcal superantigen super-domains in immune evasion. Crit Rev Immunol. 2010;30(2):149–165. doi: 10.1615/critrevimmunol.v30.i2.40. [DOI] [PubMed] [Google Scholar]
- 21.Laarman A, Milder F, van Strijp J, Rooijakkers S. Complement inhibition by gram-positive pathogens: Molecular mechanisms and therapeutic implications. J Mol Med (Berl) 2010;88(2):115–120. doi: 10.1007/s00109-009-0572-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holtfreter S, Nguyen TT, Wertheim H, Steil L, Kusch H, Truong QP, Engelmann S, Hecker M, Volker U, van Belkum A, Broker BM. Human immune proteome in experimental colonization with staphylococcus aureus. Clin Vaccine Immunol. 2009;16(11):1607–1614. doi: 10.1128/CVI.00263-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dryla A, Prustomersky S, Gelbmann D, Hanner M, Bettinger E, Kocsis B, Kustos T, Henics T, Meinke A, Nagy E. Comparison of antibody repertoires against staphylococcus aureus in healthy individuals and in acutely infected patients. Clin Diagn Lab Immunol. 2005;12:387–398. doi: 10.1128/CDLI.12.3.387-398.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobsson G, Colque-Navarro P, Gustafsson E, Andersson R, Mollby R. Antibody responses in patients with invasive staphylococcus aureus infections. Eur J Clin Microbiol Infect Dis. 2010;29(6):715–725. doi: 10.1007/s10096-010-0919-x. [DOI] [PubMed] [Google Scholar]
- 25.Bubeck Wardenburg J, Missiakas DM, Schneewind O. Vaccines for staphylococcus aureus infections. In: Levine MM, Kaper JB, Rappuoli R, Liu MF, Good AL, editors. New generation vaccines. Informa Health Care; Washington, D.C.: 2008. [Google Scholar]
- 26.Cheng AG, Schneewind O, Missiakas D. Nosocomial infections: Staphylococcus aureus. In: Rappuoli R, Bagnoli F, editors. Vaccine design: Innovative approaches and novel strategies. Caister Academic Press; 2011. [Google Scholar]
- 27.McCormick JK, Yarwood JM, Schlievert PM. Toxic shock syndrome and bacterial superantigens: An update. Annu Rev Microbiol. 2001;55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- 28.Silverman GJ, Goodyear CS. Confounding b-cell defences: Lessons from a staphylococcal superantigen. Nat Rev Immunol. 2006;6(6):465–475. doi: 10.1038/nri1853. [DOI] [PubMed] [Google Scholar]
- 29.Jardetzky TS, Brown JH, Gorga JC, Stern LJ, Urban RG, Chi YI, Stauffacher C, Strominger JL, Wiley DC. Three-dimensional structure of a human class ii histocompatibility molecule complexed with superantigen. Nature. 1994;368:11–718. doi: 10.1038/368711a0. [DOI] [PubMed] [Google Scholar]
- 30.Proft T, Fraser JD. Bacterial superantigens. Clin Exp Immunol. 2003;133(3):299–306. doi: 10.1046/j.1365-2249.2003.02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Llewelyn M, Cohen J. Superantigens: Microbial agents that corrupt immunity. Lancet Infect Dis. 2002;2(3):156–162. doi: 10.1016/s1473-3099(02)00222-0. [DOI] [PubMed] [Google Scholar]
- 32.Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, Heffernan H, Liassine N, Bes M, Greenland T, Reverdy ME, Etienne J. Community-acquired methicillin-resistant staphylococcus aureus carrying panton-valentine leukocidin genes: Worldwide emergence. Emerg Infect Dis. 2003;9(8):978–984. doi: 10.3201/eid0908.030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi Y, Lafferty JA, Clements JR, Todd JK, Gelfand EW, Kappler J, Marrack P, Kotzin BL. Selective expansion of t cells expressing v beta 2 in toxic shock syndrome. J Exp Med. 1990;172(3):981–984. doi: 10.1084/jem.172.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strandberg KL, Rotschafer JH, Vetter SM, Buonpane RA, Kranz DM, Schlievert PM. Staphylococcal superantigens cause lethal pulmonary disease in rabbits. J Infect Dis. 2010;202(11):1690–1697. doi: 10.1086/657156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holtfreter S, Bauer K, Thomas D, Feig C, Lorenz V, Roschack K, Friebe E, Selleng K, Lovenich S, Greve T, Greinacher A, et al. Egc-encoded superantigens from staphylococcus aureus are neutralized by human sera much less efficiently than are classical staphylococcal enterotoxins or toxic shock syndrome toxin. Infect Immun. 2004;72(7):4061–4071. doi: 10.1128/IAI.72.7.4061-4071.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sasso EH, Silverman GJ, Mannik M. Human igm molecules that bind staphylococcal protein a contain vhiii h chains. J Immunol. 1989;142(8):2778–2783. [PubMed] [Google Scholar]
- 37.Goodyear CS, Silverman GJ. Staphylococcal toxin induced preferential and prolonged in vivo deletion of innate-like b lymphocytes. Proc Nat Acad Sci USA. 2004;101:11392–11397. doi: 10.1073/pnas.0404382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viau M, Longo NS, Lipsky PE, Zouali M. Staphylococcal protein a deletes b-1a and marginal zone b lymphocytes expressing human immunoglobulins: An immune evasion mechanism. J Immunol. 2005;175(11):7719–7727. doi: 10.4049/jimmunol.175.11.7719. [DOI] [PubMed] [Google Scholar]
- 39.Jonsson P, Lindberg M, Haraldsson I, Wadstrom T. Virulence of staphylococcus aureus in a mouse mastitis model: Studies of alpha hemolysin, coagulase, and protein a as possible virulence determinants with protoplast fusion and gene cloning. Infect Immun. 1985;49(3):765–769. doi: 10.1128/iai.49.3.765-769.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel AH, Nowlan P, Weavers ED, Foster T. Virulence of protein a-deficient and alpha-toxin-deficient mutants of staphylococcus aureus isolated by allele replacement. Infect Immun. 1987;55(12):3103–3110. doi: 10.1128/iai.55.12.3103-3110.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Cheng AG, Kim HK, Burts ML, Krausz T, Schneewind O, Missiakas DM. Genetic requirements for staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 2009;23(10):3393–3404. doi: 10.1096/fj.09-135467. Using a mouse model of infection, the authors identify staphylococcal components required for abscess formation and persistence in host This is the first study to systematically examine virulence factors critical for staphylococcal abscess formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim HK, Kim HY, Schneewind O, Missiakas D. Identifying protective antigens of staphylococcus aureus, a pathogen that suppresses host immune responses. FASEB J. 2011 doi: 10.1096/fj.11-187963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43**.Thammavongsa V, Kern JW, Missiakas DM, Schneewind O. Staphylococcus aureus synthesizes adenosine to escape host immune responses. J Exp Med. 2009;206(11):2417–2427. doi: 10.1084/jem.20090097. Identification of adenosine synthesis as a novel virulence mechanism used by S. aureus to escape killing by the host immune system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazmanian SK, Liu G, Ton-That H, Schneewind O. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science. 1999;285:760–763. doi: 10.1126/science.285.5428.760. [DOI] [PubMed] [Google Scholar]
- 45.Zimmermann H. 5’-nucleotidase: Molecular structure and functional aspects. Biochem J. 1992;285:345–365. doi: 10.1042/bj2850345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cronstein BN, Levin RI, Belanoff J, Weissmann G, Hirschhorn R. Adenosine: An endogenous inhibitor of neutrophil-mediated injury to endothelial cells. J Clin Invest. 1986;78:760–770. doi: 10.1172/JCI112638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thiel M, Caldwell CC, Sitkovsky MV. The critical role of adenosine a2a receptors in downregulation of inflammation and immunity in the pathogenesis of infectious diseases. Microb Infect. 2003;5:515–526. doi: 10.1016/s1286-4579(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 48.Panther E, Idzko M, Herouy Y, Rheinen H, Gebicke-Haerter PJ, Mrowietz U, Dichmann S, Norgauer J. Expression and function of adenosine receptors in human dendritic cells. FASEB J. 2001;15:1963–1970. doi: 10.1096/fj.01-0169com. [DOI] [PubMed] [Google Scholar]
- 49.Kitakaze M, Hori M, Kamada T. Role of adenosine and its interaction with alpha adrenoceptor activity in ischaemic and reperfusion injury of the myocardium. Cardiovasc Res. 1993;27(1):18–27. doi: 10.1093/cvr/27.1.18. [DOI] [PubMed] [Google Scholar]
- 50.Kaufmann I, Hoelzl A, Schliephake F, Hummel T, Chouker A, Lysenko L, Peter K, Thiel M. Effects of adenosine on functions of polymorphonuclear leukocytes from patients with septic shock. Shock. 2007;27(1):25–31. doi: 10.1097/01.shk.0000238066.00074.90. [DOI] [PubMed] [Google Scholar]
- 51.Csoka B, Nemeth ZH, Virag L, Gergely P, Leibovich SJ, Pacher P, Sun CX, Blackburn MR, Vizi ES, Deitch EA, Hasko G. A2a adenosine receptors and c/ebpbeta are crucially required for il-10 production by macrophages exposed to escherichia coli. Blood. 2007;110(7):2685–2695. doi: 10.1182/blood-2007-01-065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voyich JM, Braughton KR, Sturdevant DE, Whitney AR, Said-Salim B, Porcella SF, Long RD, Dorward DW, Gardner DJ, Kreiswirth BN, Musser JM, et al. Insights into mechanisms used by staphylococcus aureus to avoid destruction by human neutrophils. J Immunol. 2005;175(6):3907–3919. doi: 10.4049/jimmunol.175.6.3907. [DOI] [PubMed] [Google Scholar]
- 53.Gresham HD, Lowrance JH, Caver TE, Wilson BS, Cheung AL, Lindberg FP. Survival of staphylococcus aureus inside neutrophils contributes to infection. J Immunol. 2000;164(7):3713–3722. doi: 10.4049/jimmunol.164.7.3713. [DOI] [PubMed] [Google Scholar]
- 54.Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 55.Garzoni C, Kelley WL. Staphylococcus aureus: New evidence for intracellular persistence. Trends Microbiol. 2009;17(2):59–65. doi: 10.1016/j.tim.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 56.Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Edwards CK, 3rd, Watts LM, Parmely MJ, Linnik MD, Long RE, Borcherding DR. Effect of the carbocyclic nucleoside analogue mdl 201,112 on inhibition of interferon-gamma-induced priming of lewis (lew/n) rat macrophages for enhanced respiratory burst and mhc class ii ia+ antigen expression. J Leukoc Biol. 1994;56(2):133–144. doi: 10.1002/jlb.56.2.133. [DOI] [PubMed] [Google Scholar]
- 58.Hasko G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, Marton A, Szabo C. Adenosine inhibits il-12 and tnf-[alpha] production via adenosine a2a receptor-dependent and independent mechanisms. FASEB J. 2000;14(13):2065–2074. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 59.Jensen K. A normally occuring staphylococcus antibody in human serum. Acta Path Microbiol Scandin. 1958;44:421–428. doi: 10.1111/j.1600-0463.2007.apm_731a.x. [DOI] [PubMed] [Google Scholar]
- 60.Lindmark R, Thoren-Tolling K, Sjoquist J. Binding of immunoglobulins to protein a and immunoglobulin levels in mammalian sera. J Immunol Methods. 1983;62(1):1–13. doi: 10.1016/0022-1759(83)90104-7. [DOI] [PubMed] [Google Scholar]
- 61.Forsgren A, Svedjelund A, Wigzell H. Lymphocyte stimulation by protein a of staphylococcus aureus. Eur J Immunol. 1976;6(3):207–213. doi: 10.1002/eji.1830060312. [DOI] [PubMed] [Google Scholar]
- 62.Peterson PK, Verhoef J, Sabath LD, Quie PG. Effect of protein a on staphylococcal opsonization. Infect Immun. 1977;15(3):760–764. doi: 10.1128/iai.15.3.760-764.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sjödahl J. Repetitive sequences in protein a from staphylococcus aureus. Arrangement of five regions within the protein, four being highly homologous and fc-binding. Eur J Biochem. 1977;73:343–351. doi: 10.1111/j.1432-1033.1977.tb11324.x. [DOI] [PubMed] [Google Scholar]
- 64.Guss B, Uhlén M, Nilsson B, Lindberg M, Sjöquist J, Sjödahl J. Region x, the-cell-wall-attachment part of staphylococcal protein a. Eur J Biochem. 1984;138:413–420. doi: 10.1111/j.1432-1033.1984.tb07931.x. [DOI] [PubMed] [Google Scholar]
- 65.Schneewind O, Fowler A, Faull KF. Structure of the cell wall anchor of surface proteins in staphylococcus aureus. Science. 1995;268:103–106. doi: 10.1126/science.7701329. [DOI] [PubMed] [Google Scholar]
- 66.Graille M, Stura EA, Corper AL, Sutton BJ, Taussig MJ, Charbonnier JB, Silverman GJ. Crystal structure of a staphylococcus aureus protein a domain complexed with the fab fragment of a human igm antibody: Structural basis for recognition of b-cell receptors and superantigen activity. Proc Nat Acad Sci USA. 2000;97:5399–5404. doi: 10.1073/pnas.97.10.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hartleib J, Kohler N, Dickinson R, Chhatwal G, Sixma J, Hartford O, Foster TJ, Peters G, Kehrl B, Herrmann M. Protein a is the von willebrand factor binding protein of staphylococcus aureus. Blood. 2000;96:2149–2156. [PubMed] [Google Scholar]
- 68.Nguyen T, Ghebrehiwet B, Peerschke E. Staphylococcus aureus protein a recognizes platelet gc1qr/p33: A novel mechanism for staphylococcal interactions with platelets. Infect Immun. 2000;68:2061–2068. doi: 10.1128/iai.68.4.2061-2068.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gomez MI, Lee A, Reddy B, Muir A, Soong G, Pitt A, Cheung A, Prince A. Staphylococcus aureus protein a induces airway epithelial inflammatory responses by activating tnfr1. Nature Med. 2004;10(8):842–848. doi: 10.1038/nm1079. [DOI] [PubMed] [Google Scholar]
- 70.Sullam PM, Bayer AS, Foss WM, Cheung AL. Diminished platelet binding in vitro by staphylococcus aureus is associated with reduced virulence in a rabbit model of infective endocarditis. Infect Immun. 1996;64:4915–4921. doi: 10.1128/iai.64.12.4915-4921.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gómez MI, O’Seaghdha M, Prince AS. Staphylococcus aureus protein a activates tace through egfr-dependent signaling. EMBO J. 2007;26:701–709. doi: 10.1038/sj.emboj.7601554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martin FJ, Gomez MI, Wetzel DM, Memmi G, O’Seaghdha M, Soong G, Schindler C, Prince A. Staphylococcus aureus activates type i ifn signaling in mice and humans through the xr repeated sequences of protein a. J Clin Invest. 2009;119(7):1931–1939. doi: 10.1172/JCI35879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith EJ, Visai L, Kerrigan SW, Speziale P, Foster TJ. The sbi protein is a multifunctional immune evasion factor of staphylococcus aureus. Infect Immun. 2011;79(9):3801–3809. doi: 10.1128/IAI.05075-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang L, Jacobsson K, Vasi J, Lindberg M, Frykberg L. A second igg-binding protein in staphylococcus aureus. Microbiology. 1998;144(Pt 4):985–991. doi: 10.1099/00221287-144-4-985. [DOI] [PubMed] [Google Scholar]
- 75.Atkins KL, Burman JD, Chamberlain ES, Cooper JE, Poutrel B, Bagby S, Jenkins AT, Feil EJ, van den Elsen JM. S. Aureus igg-binding proteins spa and sbi: Host specificity and mechanisms of immune complex formation. Mol Immunol. 2008;45(6):1600–1611. doi: 10.1016/j.molimm.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 76.Haupt K, Reuter M, van den Elsen J, Burman J, Halbich S, Richter J, Skerka C, Zipfel PF. The staphylococcus aureus protein sbi acts as a complement inhibitor and forms a tripartite complex with host complement factor h and c3b. PLoS Pathog. 2008;4(12):e1000250. doi: 10.1371/journal.ppat.1000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Toapanta FR, Ross TM. Complement-mediated activation of the adaptive immune responses: Role of c3d in linking the innate and adaptive immunity. Immunol Res. 2006;36(1-3):197–210. doi: 10.1385/IR:36:1:197. [DOI] [PubMed] [Google Scholar]
- 78.Isenman DE, Leung E, Mackay JD, Bagby S, van den Elsen JM. Mutational analyses reveal that the staphylococcal immune evasion molecule sbi and complement receptor 2 (cr2) share overlapping contact residues on c3d: Implications for the controversy regarding the cr2/c3d cocrystal structure. J Immunol. 2010;184(4):1946–1955. doi: 10.4049/jimmunol.0902919. [DOI] [PubMed] [Google Scholar]
- 79.Deisenhofer J. Crystallographic refinement and atomic models of a human fc fragment and its complex with fragment b of protein a from staphylococcus aureus at 2.9- and 2.8-a resolution. Biochemistry. 1981;20:2361–2370. [PubMed] [Google Scholar]
- 80.Gouda H, Shiraishi M, Takahashi H, Kato K, Torigoe H, Arata Y, Shimada I. Nmr study of the interaction between the b domain of staphylococcal protein a and the fc portion of immunoglobulin g. Biochemistry. 1998;37(1):129–136. doi: 10.1021/bi970923f. [DOI] [PubMed] [Google Scholar]
- 81.Projan SJ, Nesin M, Dunman PM. Staphylococcal vaccines and immunotherapy: To dream the impossible dream? Curr Opin Pharmacol. 2006;6:473–479. doi: 10.1016/j.coph.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 82.Kuklin NA, Clark DJ, Secore S, Cook J, Cope LD, McNeely T, Noble L, Brown MJ, Zorman JK, Wang XM, Pancari G, et al. A novel staphylococcus aureus vaccine: Iron surface determinant b induces rapid antibody responses in rhesus macaques and specific increased survival in a murine s. Aureus sepsis model. Infect Immun. 2006;74:2215–2223. doi: 10.1128/IAI.74.4.2215-2223.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fattom AI, Horwith G, Fuller S, Propst M, Naso R. Development of staphvax, a polysaccharide conjugate vaccine against s. Aureus infection: From the lab bench to phase iii clinical trials. Vaccine. 2004;22:880–887. doi: 10.1016/j.vaccine.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 84.Maira-Litran T, Kropec A, Goldmann D, Pier GB. Biologic properties and vaccine potential of the staphylococcal poly-n-acetylglucosamine surface polysaccharide. Vaccine. 2004;22:872–879. doi: 10.1016/j.vaccine.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 85.Stranger-Jones YK, Bae T, Schneewind O. Vaccine assembly from surface proteins of staphylococcus aureus. Proc Natl Acad Sci U S A. 2006;103(45):16942–16947. doi: 10.1073/pnas.0606863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bubeck Wardenburg J, Schneewind O. Vaccine protection against staphylococcus aureus pneumonia. J Exp Med. 2008;205:287–294. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, Yeoh H, Law D, Robbins JB, Schneerson R, Muenz L, et al. Use of a staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med. 2002;346:491–496. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 88.DeJonge M, Burchfield D, Bloom B, Duenas M, Walker W, Polak M, Jung E, Millard D, Schelonka R, Eyal F, Morris A, et al. Clinical trial of safety and efficacy of inh-a21 for the prevention of nosocomial staphylococcal bloodstream infection in premature infants. J Pediatr. 2007;151(3):260–265. 265 e261. doi: 10.1016/j.jpeds.2007.04.060. [DOI] [PubMed] [Google Scholar]


