Abstract
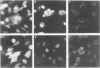
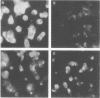
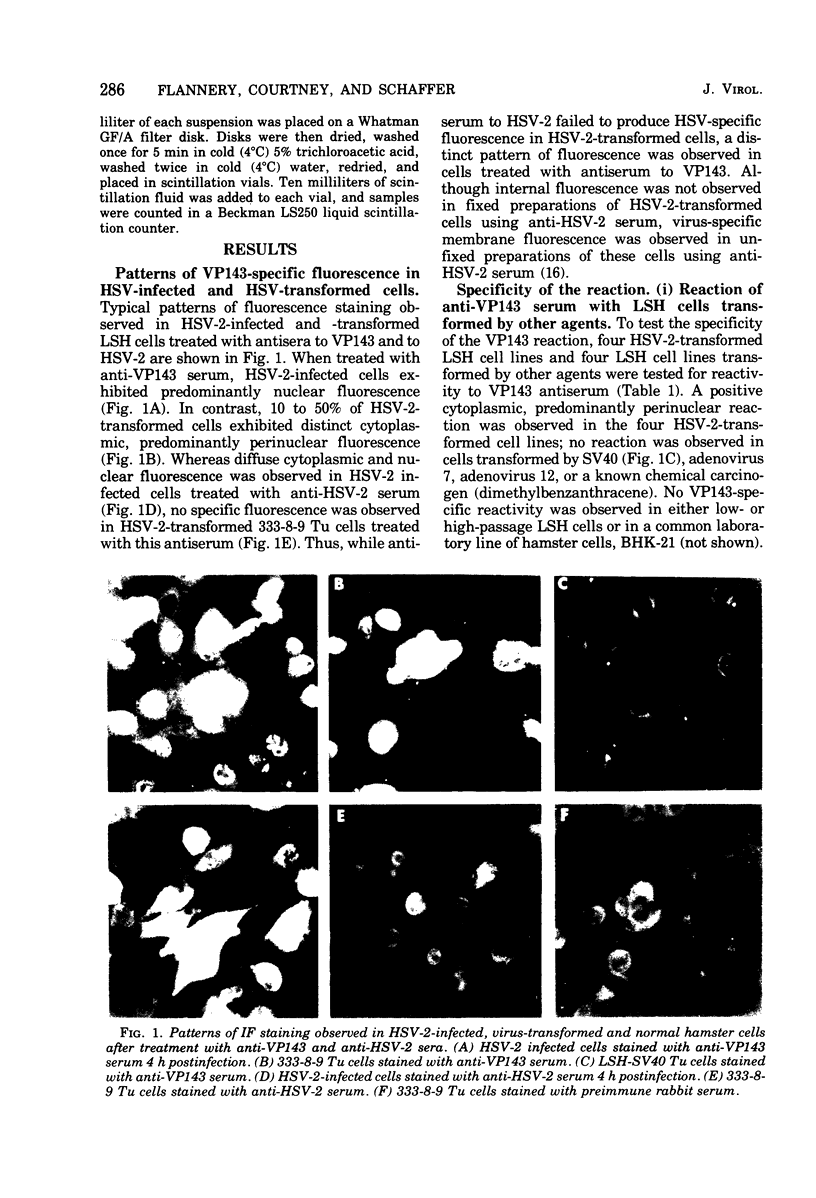
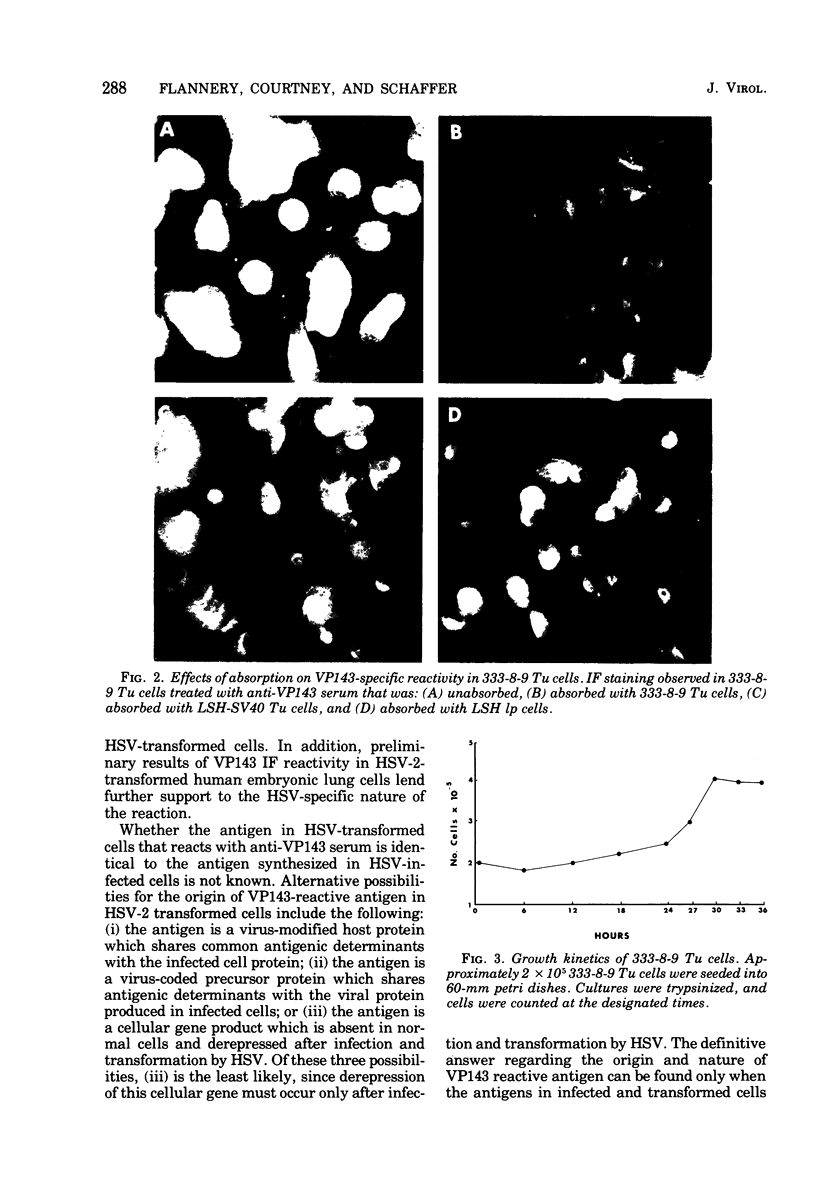
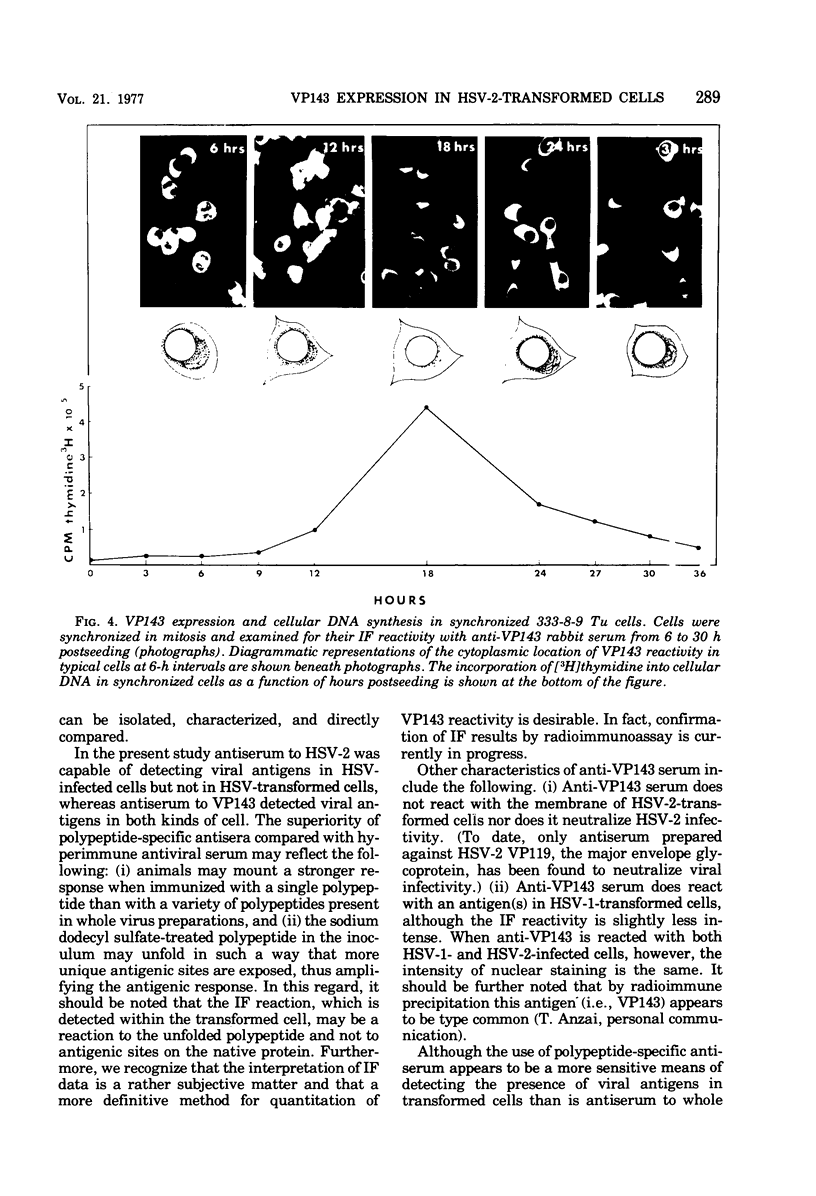
Hyperimmune rabbit antiserum to an early, nonstructural herpes simplex virus type 2 (HSV-2)-induced polypeptide (VP143) reacted in immunofluorescence tests with a variety of cell lines transformed by HSV-2. Cytoplasmic fluorescence was observed in 10 to 50% of HSV-2-transformed cells, whereas no fluorescence was observed in cells transformed by other oncogenic DNA viruses or by a chemical carcinogen. VP143-specific reactivity could be absorbed from anti-VP143 serum with HSV-2-transformed cells but not with cells transformed by other agents. When HSV-2-transformed cells were synchronized in mitosis and examined at various times postmitosis for VP143-specific fluorescence, the expression of VP143 was shown to be cell cycle dependent.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd A. L., Orme T. W. Transformation of mouse cells after infection with ultraviolet irradiation-inactivated herpes simplex virus type 2. Int J Cancer. 1975 Oct 15;16(4):526–538. doi: 10.1002/ijc.2910160403. [DOI] [PubMed] [Google Scholar]
- Collard W., Thornton H., Green M. Cells transformed by human Herpesvirus type 2 transcribe virus-specific RNA sequences shared by Herpesvirus types 1 and 2. Nat New Biol. 1973 Jun 27;243(130):264–266. doi: 10.1038/newbio243264a0. [DOI] [PubMed] [Google Scholar]
- Courtney R. J., Benyesh-Melnick M. Isolation and characterization of a large molecular-weight polypeptide of herpes simplex virus type 1. Virology. 1974 Dec;62(2):539–551. doi: 10.1016/0042-6822(74)90414-0. [DOI] [PubMed] [Google Scholar]
- Davis D. B., Kingsbury D. T. Quantitation of the viral DNA present in cells transformed by UV-irradiated herpes simplex virus. J Virol. 1976 Mar;17(3):788–793. doi: 10.1128/jvi.17.3.788-793.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derge J. G., Martos L. M., Tagamets M. A., Chang S. Y., Chakrabarty M. Identification of a critical period during the S phase for activation of the Epstein-Barr virus by 5-iododeoxyuridine. Nat New Biol. 1973 Aug 15;244(137):214–217. doi: 10.1038/newbio244214a0. [DOI] [PubMed] [Google Scholar]
- Duff R., Rapp F. Oncogenic transformation of hamster embryo cells after exposure to inactivated herpes simplex virus type 1. J Virol. 1973 Aug;12(2):209–217. doi: 10.1128/jvi.12.2.209-217.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff R., Rapp F. Properties of hamster embryo fibroblasts transformed in vitro after exposure to ultraviolet-irradiated herpes simplex virus type 2. J Virol. 1971 Oct;8(4):469–477. doi: 10.1128/jvi.8.4.469-477.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esparza J., Purifoy D. J., Schaffer P. A., Benyesh-Melnick M. Isolation, complementation and preliminary phenotypic characterization of temperature-sensitive mutants of herpes simplex virus type 2. Virology. 1974 Feb;57(2):554–565. doi: 10.1016/0042-6822(74)90194-9. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Locker H., Cox B., Roizman B., Rapp F. Herpes simplex virus DNA in transformed cells: sequence complexity in five hamster cell lines and one derived hamster tumor. J Virol. 1976 Jun;18(3):885–893. doi: 10.1128/jvi.18.3.885-893.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberger J. S., Aaronson S. A. Cycloheximide induction of xenotropic type C virus from synchronized mouse cells: metabolic requirements for virus activation. J Virol. 1975 Jan;15(1):64–70. doi: 10.1128/jvi.15.1.64-70.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampar B., Derge J. G., Showalter S. D. Enhanced activation of the repressed Epstein-Barr viral genome by inhibitors of DNA synthesis. Virology. 1974 Mar;58(1):298–301. doi: 10.1016/0042-6822(74)90164-0. [DOI] [PubMed] [Google Scholar]
- Hampar B., Lenoir G., Nonoyama M., Derger J. G., Chang S. Cell cycle dependence for activation of Epstein-Barr virus by inhibitors of protein synthesis or medium deficient in arginine. Virology. 1976 Feb;69(2):660–668. doi: 10.1016/0042-6822(76)90494-3. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. C., Kleinman L. F., Black P. H. Cell cycle dependence of simian virus 40 induction from transformed hamster cells by ultraviolet irradiation. Virology. 1975 Nov;68(1):215–220. doi: 10.1016/0042-6822(75)90162-2. [DOI] [PubMed] [Google Scholar]
- Kimura S., Esparza J., Benyesh-Melnick M., Schaffer P. A. Enhanced replication of temperature-sensitive mutants of herpes simplex virus type 2 (HSV-2) at the nonpermissive temperature in cells transformed by HSV-2. Intervirology. 1974;3(3):162–169. doi: 10.1159/000149752. [DOI] [PubMed] [Google Scholar]
- Kimura S., Flannery V. L., Levy B., Schaffer P. A. Oncogenic transformation of primary hamster cells by herpes simplex virus type 2 (hsv-2) and an hsv-2 temperature-sensitive mutant. Int J Cancer. 1975 May 15;15(5):786–798. doi: 10.1002/ijc.2910150510. [DOI] [PubMed] [Google Scholar]
- Kraiselburd E., Gage L. P., Weissbach A. Presence of a herpes simplex virus DNA fragment in an L cell clone obtained after infection with irradiated herpes simplex virus I. J Mol Biol. 1975 Oct 5;97(4):533–542. doi: 10.1016/s0022-2836(75)80057-x. [DOI] [PubMed] [Google Scholar]
- Kucera L. S., Gusdon J. P. Transformation of human embryonic fibroblasts by photodynamically inactivated herpes simplex virus, type 2 at supra-optimal temperature. J Gen Virol. 1976 Feb;30(2):257–261. doi: 10.1099/0022-1317-30-2-257. [DOI] [PubMed] [Google Scholar]
- Kutinová L., Vonka V., Broucek J. Increased oncogenicity and synthesis of herpesvirus antigens in hamster cells exposed to herpes simplex type-2 virus. J Natl Cancer Inst. 1973 Mar;50(3):759–766. doi: 10.1093/jnci/50.3.759. [DOI] [PubMed] [Google Scholar]
- Macnab J. C., Timbury M. C. Complementation of ts mutants by a herpes simplex virus ts-transformed cell line. Nature. 1976 May 20;261(5557):233–235. doi: 10.1038/261233a0. [DOI] [PubMed] [Google Scholar]
- Macnab J. C. Transformation of rat embryo cells by temperature-sensitive mutants of herpes simplex virus. J Gen Virol. 1974 Jul;24(1):143–153. doi: 10.1099/0022-1317-24-1-143. [DOI] [PubMed] [Google Scholar]
- Minson A. C., Thouless M. E., Eglin R. P., Darby G. The detection of virus DNA sequences in a herpes type 2 transformed hamster cell line (333-8-9). Int J Cancer. 1976 Apr 15;17(4):493–500. doi: 10.1002/ijc.2910170412. [DOI] [PubMed] [Google Scholar]
- Munyon W., Kraiselburd E., Davis D., Mann J. Transfer of thymidine kinase to thymidine kinaseless L cells by infection with ultraviolet-irradiated herpes simplex virus. J Virol. 1971 Jun;7(6):813–820. doi: 10.1128/jvi.7.6.813-820.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahmias A. J., Josey W. E., Naib Z. M., Luce C. F., Guest B. A. Antibodies to Herpesvirus hominis types 1 and 2 in humans. II. Women with cervical cancer. Am J Epidemiol. 1970 Jun;91(6):547–552. doi: 10.1093/oxfordjournals.aje.a121166. [DOI] [PubMed] [Google Scholar]
- Porter D. D., Wimberly I., Benyesh-Melnick M. Prevalence of antibodies to EB virus and other herpesviruses. JAMA. 1969 Jun 2;208(9):1675–1679. [PubMed] [Google Scholar]
- Rawls W. E., Tompkins W. A., Melnick J. L. The association of herpesvirus type 2 and carcinoma of the uterine cervix. Am J Epidemiol. 1969 May;89(5):547–554. doi: 10.1093/oxfordjournals.aje.a120967. [DOI] [PubMed] [Google Scholar]
- Reed C. L., Cohen G. H., Rapp F. Detection of a virus-specific antigen on the surface of herpes simplex virus-transformed cells. J Virol. 1975 Mar;15(3):668–670. doi: 10.1128/jvi.15.3.668-670.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. A., Panem S., Kirsten W. H. Distribution and virogenic effects of 5-bromodeoxyuridine in synchronized rat embryo cells. Proc Natl Acad Sci U S A. 1975 May;72(5):1829–1833. doi: 10.1073/pnas.72.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman S., Zeuthen J., Ringertz N. R. Expression of SV40 T antigen during the cell cycle of sv40-transformed cells. Int J Cancer. 1975 Apr 15;15(4):547–554. doi: 10.1002/ijc.2910150403. [DOI] [PubMed] [Google Scholar]
- TERASIMA T., TOLMACH L. J. Growth and nucleic acid synthesis in synchronously dividing populations of HeLa cells. Exp Cell Res. 1963 Apr;30:344–362. doi: 10.1016/0014-4827(63)90306-9. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Yamanishi K. Transformation of hamster embryo and human embryo cells by temperature sensitive mutants of herpes simplex virus type 2. Virology. 1974 Sep;61(1):306–311. doi: 10.1016/0042-6822(74)90267-0. [DOI] [PubMed] [Google Scholar]