Abstract
Anaphylaxis is a rapid, life-threatening hypersensitivity reaction. Until recently, it was mainly attributed to histamine released by mast cells activated by allergen crosslinking (XL) of FcεRI-bound allergen-specific IgE. However, recent reports established that anaphylaxis could also be triggered by basophil, macrophage and neutrophil secretion of platelet activating factor subsequent to FcγR stimulation by IgG/Ag complexes. We have investigated the contribution of Fyn and Lyn tyrosine kinases to FcγRIIb and FcγRIII signaling in the context of IgG-mediated passive systemic anaphylaxis (PSA). We found that mast cell IgG XL induced Fyn, Lyn, Akt, Erk, p38 and JNK phosphorylation. Additionally, IgG XL of mast cells, basophils and macrophages resulted in Fyn- and Lyn-regulated mediator release in vitro. FcγR–mediated activation was enhanced in Lyn-deficient (KO) cells, but decreased in Fyn KO cells, compared to wild type cells. More importantly, Lyn KO mice displayed significantly exacerbated PSA features while no change was observed for Fyn KO mice, compared to wild type littermates. Intriguingly, we establish that mast cells account for the majority of serum histamine in IgG-induced PSA. Taken together, our findings establish pivotal roles for Fyn and Lyn in the regulation of PSA and highlight their unsuspected functions in IgG-mediated pathologies.
INTRODUCTION
The initiation of an allergic response is commonly attributed to mast cell activation via their high affinity IgE receptor (FcεRI) (1, 2). In some cases, the exposure to IgE-bound allergen causes rapid and exacerbated hypersensitivity reactions called anaphylaxis. Consequently, the role of mast cells in allergy, inflammatory diseases and immune homeostasis has been most extensively studied downstream of IgE receptor signaling (3–9), although interesting studies reported that mast cells can also be activated by their IgG receptor (10). Data generated from murine models demonstrate that an anaphylactic reaction can be induced through the IgG-FcγR pathway (11–14). In this “alternative” pathway of anaphylaxis, FcγR activation of basophils, macrophages, and neutrophils elicits anaphylaxis symptoms, including a drop in core body temperature.
FcεRI activation involves a plethora of signaling molecules, including Fyn and Lyn kinases. Lyn kinase, which is expressed as 53 and 56 kDa isoforms due to alternative splicing (15–17), is expressed in nearly all immune cells, except the T cell lineage. For example, the association of Lyn kinase with the B cell antigen receptor (BCR) (18) and the high affinity IgE receptor, FcεRI (19), places Lyn in a critical position to control inflammation. Because of the known stimulatory roles for Src family kinases, it was assumed that Lyn possessed pro-stimulatory roles in signaling. Consistent with this, Lyn knockout (KO) mast cells were found to have poor calcium flux responses after IgE-mediated activation (20). However, further study showed that Lyn deficiency exacerbated IgE-mediated mast cell activation and anaphylaxis (21). These apparently contradictory data were explained by the ability of Lyn to recruit several inhibitory proteins, including SHIP-1, SHP-2, DOK-1, and CBP (22–25). These negative regulators reduce signaling through other Src family members, including PI-3 kinase and the Ras-MAPK cascade. Hence, Lyn appears to be a negative regulator of the mast cell response.
Fyn on the other hand is a 59 kDa Src-family protein tyrosine kinase involved in mast cell degranulation and cytokine release (26, 27). Once phosphorylated, Fyn activates the adaptor protein Gab2, initiating the phosphatidylinositol-3’ kinase (PI3K) pathway. This enhances calcium flux through a TRPC1-mediated process leading to cortical F-actin depolymerization (28). In addition, Fyn enhances transcription factor activity, degranulation and cytokine release (2, 21). Fyn activation is currently viewed as a positive regulator of IgE-mediated inflammatory signals, similar to the well-documented Syk kinase pathway.
IgG receptors (FcgR) share a common gamma subunit with FcεRI, and share similar signaling pathways. Although the importance of Syk kinase in IgG signaling has been demonstrated (29, 30) little is known about Src family kinase functions. In this study we investigated the importance of the Lyn and Fyn kinases in FcγR signaling using mast cells, basophils, and macrophages. Our findings show that Fyn, Lyn, Akt, Erk, p38 and JNK are activated upon FcγR stimulation, and that Fyn and Lyn regulate FcγR-mediated degranulation, cytokine and chemokine release not only in mast cells, but also basophils and macrophages. Furthermore, we demonstrate that mast cells account for the total amount of circulating histamine during FcγR-induced passive systemic anaphylaxis (PSA), which is regulated by Fyn and Lyn. Moreover, we show that Lyn, but not Fyn kinase, is a major regulator of IgG-mediated PSA. These results bring new insights to the function of Fyn and Lyn kinases downstream of FcγR stimulation.
MATERIALS AND METHODS
Animals
C57BL/6x129sv wild type (WT), C57BL/6x129sv Fyn-deficient (KO), 129sv WT and 129sv Lyn KO inbred strains were described previously (21, 26). Mast cell-deficient Wsh−/− and their C57Bl/6 control mice were purchased from The Jackson Laboratory. All mice were used at a minimum of 9 weeks of age, and all experiments received approval from the Virginia Commonwealth University institutional animal care and use committee (IACUC).
Cytokines and reagents
Cytokines and ELISA assay kits were purchased from PeproTech (Rocky Hill, NJ). The Bio-plex Pro cytokine assay kits were purchased from BioRad (Hercules, CA). Histamine and leukotriene C4 (LTC4) ELISA kits were purchased from Cayman Chemicals (Ann Arbor, MI). Cytokine measurements were performed as per the manufacturer’s directions. Antibodies specific for tyrosine-phosphorylated (pY) Lyn and total Lyn were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies specific for the tyrosine-phosphorylated form of the Src kinase activation loop (pY416) and for Fyn were purchased from Cell Signaling Technologies (Danvers, MA). Anti-mouse FcεRIα was purchased from eBioscience (San Diego, CA). Rat anti-mouse FcγRII/RIII (2.4G2), purified mouse IgE, rat anti-mouse IgE, rat IgG isotype control, and rat IgG anti-c-kit (CD117) were purchased from BD Pharmingen (San Diego, CA).
Cells
Mouse bone marrow-derived mast cells (BMMCs) were derived by culture in complete RPMI (cRPMI) 1640 medium (Invitrogen Life Technologies, Carlsbad, CA) containing 10% heat-inactivated FBS, 2 mM L-glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin, 1mM sodium pyruvate, and 1 mM HEPES (all from Biofluids, Rockville, MD), supplemented with IL-3–containing supernatant from WEHI-3 cells and stem cell factor (SCF)-containing supernatant from BHK-MKL cells. The final concentration of IL-3 and SCF was adjusted to 1 ng/ml and 10 ng/ml, respectively, as measured by ELISA. Mouse bone marrow-derived basophils were derived by culturing bone marrow cells for six days in cRPMI 1640 medium supplemented with 5 ng/ml recombinant IL-3 (PeproTech, Rocky Hill, NJ). At day 6, they were analyzed and sorted by flow cytometry on the basis of FcεRI-positive and Kit-negative characteristics. Mouse bone marrow-derived macrophages were derived by culturing bone marrow cells for eight days in cRPMI supplemented with 50 ng/ml MCSF (PeproTech).
Flow cytometry
Mice were bled via tail vein nick. Cells were labeled following RBC lysis and filtration through 40µm cell strainers. Antibodies included unlabeled 2.4G2, FITC-conjugated anti-mouse c-Kit (2B8) and Gr-1 (RB6-6B2); PE-conjugated anti-FcεRIα (MAR-1) and Gr-1 (RB6-8C5); Alexa 647- conjugated anti-mouse CD49b (DX5) and B220 (RA3-6B2); PE-Cy7 conjugated anti-mouse CD3 (145-2C11) and CD11b (M1/70) from BioLegend; and PE-conjugated anti-Siglec-F (E50-2440) from BD Biosciences. Flow cytometric analysis was performed using a Canto (BD Biosciences) and data analysis was conducted with FlowJo software (Tree Star).
Western blot
Cells were dissociated in RIPA (Radio-Immunoprecipitation Assay) buffer and Western blotting was performed using 50µg total cell lysate per sample. Proteins were loaded and separated over an 8–16% or 4–20% gradient SDS-polyacrylamide gel (Bio-Rad, Hercules, CA). Proteins were transferred to nitrocellulose membranes (Pall Corporation, Ann Arbor, MI), and blocked for 60 minutes in Blotto B buffer (Rockland Immunochemicals, Gilbertsville, PA) plus 0.1% Tween-20. Blots were incubated in a solution of TBS supplemented with 0.1% Tween-20 and 5% BSA (TBST), with the indicated antibodies overnight at 4°C with gentle rocking. Blots were washed six times for 10 minutes each in TBST, followed by incubation in Blotto B containing a 1:5,000 dilution of HRP linked anti-IgG matched to the relevant species, from Cell Signaling (Danvers, MA). Size estimates for proteins were obtained using molecular weight standards from Bio-Rad (Hercules, CA).
Passive Systemic Anaphylaxis
Mice were injected intravenously with 200µl of PBS containing 5mg of histamine, 700ng of PAF or 500µg of rat anti-mouse CD16/CD32, clone 2.4G2 (13, 31). The body temperature of each animal was measured using a rectal microprobe (Physitemp Instruments). Mice were then euthanized, and blood was collected by cardiac puncture to prepare serum.
PAF acyl hydrolase (AH) Activity Assays
Substrate (50µM 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine with 0.05 µCi of hexadecyl-2-acetyl-sn-glyceryl-3-phosphocholine, 1-O-[acetyl-(N)-3H] (NEN/PerkinElmer; 13.5 Ci/mmol) added as a tracer), was combined with Serum and incubated for 30 minutes at 37°C to determine PAFAH enzymatic activity. PAFAH activity was expressed as release of [3H] acetate, using a method that we described previously (32). 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine (PAF) was purchased from Avanti Polar Lipids (Alabaster, AL).
Statistical Analysis
Data are presented as the mean plus or minus SEM of at least 3 independent experiments. Comparisons were made by the 2-tailed Student t test for independent samples. P values less than 0.05 were considered statistically significant. Analysis was performed with GraphPad Prism software.
RESULTS
FcγR stimulation induces rapid Fyn, Lyn, Akt, Erk, p38 and JNK activation in bone marrow-derived mast cells
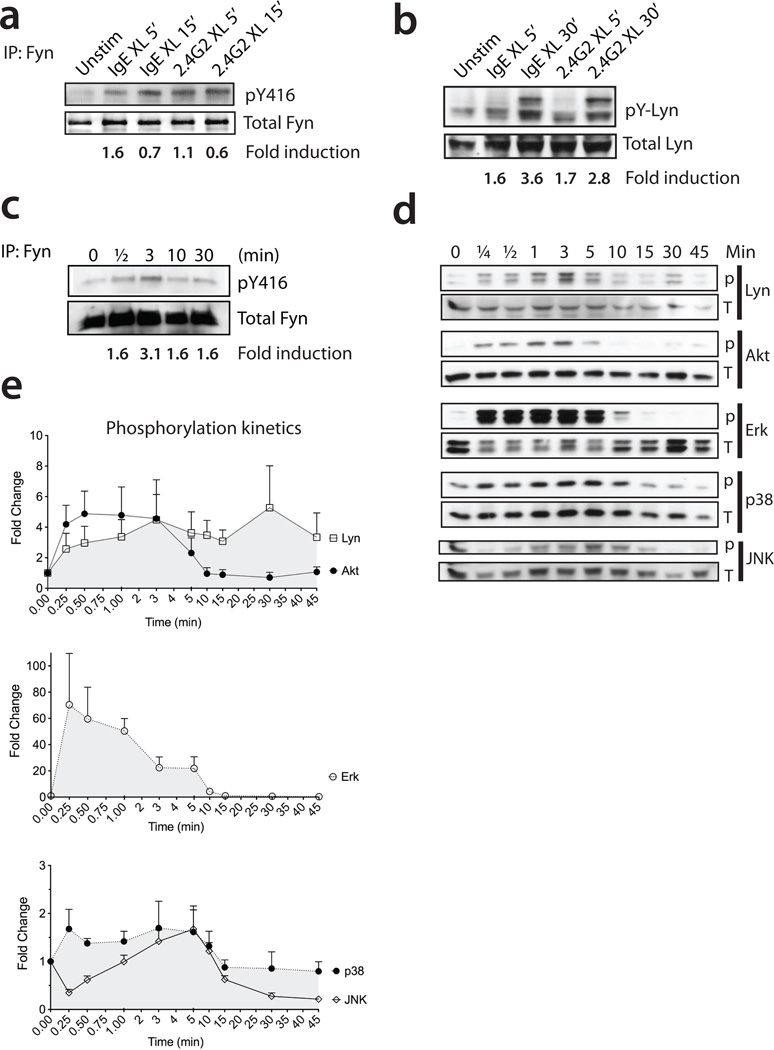
To compare the effect of the IgE versus IgG receptor stimulation on the phosphorylation state of Fyn kinase, bone marrow-derived mast cells were stimulated in vitro with IgE plus antigen or 2.4G2 plus anti-IgG (referred to as IgE or 2.4G2 crosslinking (XL) respectively). Fyn kinase was then immunoprecipitated and proteins separated by SDS-PAGE. The phosphorylated form of Fyn was detected by immunoblotting for the activated loop of the Src kinase family on residue 416 (pY416) (Figure 1a). Our results show that Fyn kinase (59kDa) is rapidly activated downstream of both the IgE and the IgG receptors. Quantification of western blot signal intensity by densitometry after adjusting for loading showed that FcγR and FcεRI activate Fyn to a similar extent. Similar to Fyn, we found that both Lyn kinase isoforms (53 and 56 kD) (17) were also activated following 2.4G2 XL (Figure 1b). FcγR-mediated Lyn activation was nearly as strong as IgE-mediated effects as attested by densitometry analysis of band intensity.
Figure 1. FcγR stimulation activates Fyn, Lyn, Akt, Erk, p38 and JNK in bone marrow-derived mast cells (BMMCs).
BMMCs were sensitized with either anti-DNP IgE (10 µg/ml) or rat anti-mouse FcγRII/III 2.4G2 (10 µg/ml) and stimulated with DNP-HSA (100ng/ml) (IgE XL) or goat anti-rat IgG (10 µg/ml) (2.4G2 XL) respectively for the indicated times. (a) Fyn immunoprecipitation and western blotting with anti-Src kinase activation loop antibody (pY416). Total Fyn expression is shown as a loading control. The image is representative of 3 independent experiments. Data shown are mean ± SEM. The fold induction was quantified by densitometry analysis of band intensity with Image J software. The mean fold change is relative to the control cells receiving media alone as stimulus. (b) Western blot for activated Lyn, detected with anti-phosphotyrosyl Lyn antibody. Total Lyn expression represents the loading control. The image is representative of 3 independent experiments. (c) Fyn was immunoprecipitated and activated Fyn was detected by an anti-Src kinase activated loop antibody (pY416) after 2.4G2 XL. Total Fyn expression is shown as a loading control. (d) Representative immunoblot of the phosphorylation kinetics of Lyn, Akt, Erk, p38 and JNK after 2.4G2 XL. Total protein expression is shown as loading control. (e) Phosphorylation kinetics of activated Lyn, Akt, Erk, p38 and JNK upon 2.4G2 XL. The densitometry analysis of band intensity was calculated using the Image J software. Fold change is relative to the control cells incubated in media alone. The graph summarizes 3 independent experiments. Data shown are mean ± SEM.
Intracellular signaling events occurring in BMMCs after FcεRI activation of mast cells has been intensively studied and is well established. Since our results show that comparable activation levels of Fyn and Lyn can be achieved through FcγR or FcεRI stimulation, we next decided to investigate the kinetics of phosphorylation events subsequent to Fyn and Lyn activation. We found that Fyn activation after 2.4G2 crosslinking occurred rapidly, with maximal phosphorylation observed within 3 minutes (Figure 1c). In addition, FcγR activation of mast cells triggered the activation of Akt, Erk, p38 and JNK (Figure 1d). A representative densitometry analysis of band intensity shows the phosphorylation kinetics of these signaling molecules (Figure 1e), with ERK phosphorylation being the strongest, reaching about 70-fold increase in less than a minute after the initiation of 2.4G2 XL. Our results showed parallel kinetics for Fyn, Akt and Erk during the early time points of FcγR activation of mast cells, with only Lyn displaying sustained phosphorylation over about 30 minutes. Collectively, our results show that comparable Fyn and Lyn activation can be achieved through FcγR or FcεRI stimulation. In addition, FcγR-mediated signals activated similar downstream targets to those previously shown for FcεRI signaling (26, 33–39), especially the robust and early Akt and ERK signaling.
Effects of Fyn or Lyn deficiency downstream of mast cell IgG receptor signaling
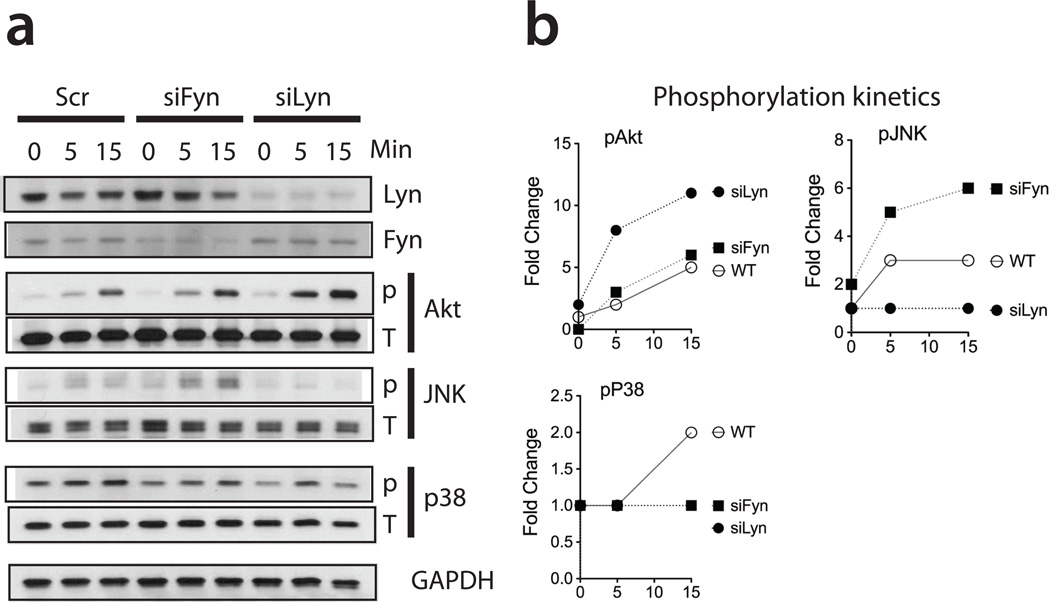
Fyn and Lyn kinases act as opposite regulators of IgE-induced mast cell activation (21), (26, 27). We therefore investigated their roles in IgG-mediated BMMC activation. In order to fairly compare how downstream signaling molecules are affected by Fyn or Lyn deficiency, we depleted these kinases with specific siRNA on C57BL6 mast cells, given that Fyn KO and Lyn KO were generated on different backgrounds (B6.129sv and 129sv, respectively). Fyn KO BMMCs displayed increased phospho-JNK while no change was observed for phospho-Akt and phospho-p38 subsequent to IgG XL (Figure 2a, 2b). On the other hand, Lyn-deficient BMMCs displayed significantly high phospho-Akt while there was no change in JNK or p38 phosphorylation status in comparison to WT BMMCs. Complementing the data in Figure 1, these data demonstrate that Fyn and Lyn regulate particular signaling pathways upon IgG XL in mast cells by targeting different downstream signaling molecules.
Figure 2. Effects of Fyn or Lyn deficiency on mast cell FcγR stimulation.
Fyn or Lyn kinases were knocked down by siRNA in BMMCs, which were then stimulated with 2.4G2 XL for the indicated times. (a) Representative image of Lyn, Fyn, phospho-Akt, phospho-JNK, phospho-p38 immunoblot after SDS-page western blot. Total Fyn, Lyn, Akt, JNK, p38 and GAPDH lanes are shown as loading controls. (b) Phosphorylation kinetics of activated Akt, p38 and JNK upon 2.4G2 XL. The densitometry analysis of band intensity was calculated using the Image J software. Fold change is relative to the control cells incubated in media alone.
Fyn kinase deficiency diminishes FcγR-mediated activation of mast cells, basophils, and macrophages
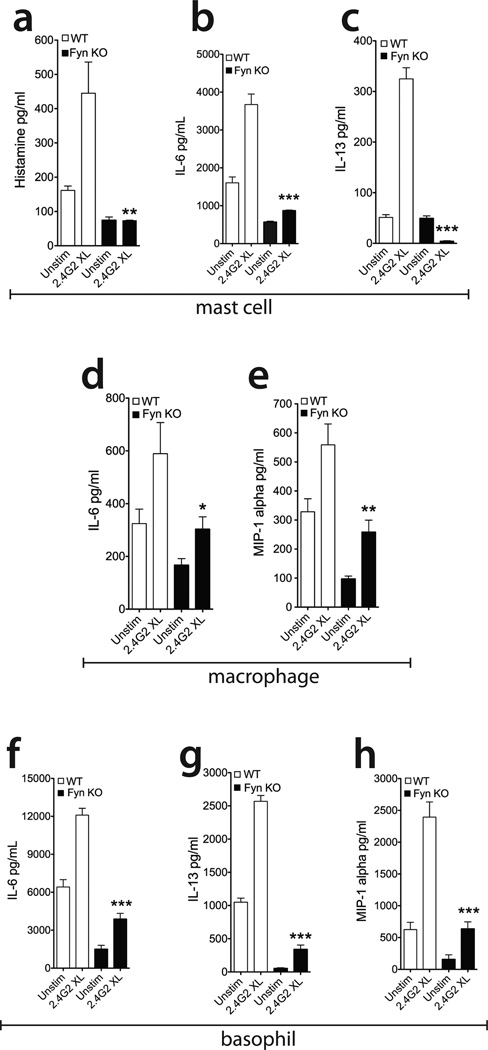
To determine the functional importance of Fyn kinase downstream of FcγR signaling, BMMCs derived from WT or Fyn KO bone marrow progenitors were stimulated by 2.4G2 crosslinking, and cell culture supernatants were collected to measure degranulation and cytokine release. Fyn deficiency significantly decreased histamine release (Figure 3a), and profoundly reduced IL-6 (Figure 3b) and IL-13 (Figure 3c) levels. There were no striking differences in leukotriene C4 (LTC4), MIP-1 alpha or TNF-alpha secretion (data not shown). We used the calcium ionophore ionomycin as a positive control for all of our cytokine and mediator release measurements in this study and observed that the deficiency in either Fyn or Lyn did not significantly impact the amount of mediators secreted (data not shown). These data mirror previous reports of the importance for Fyn kinase in IgE-mediated stimulation (21, 26), expanding its significance to FcγR stimulation.
Figure 3. Fyn kinase deficiency diminishes mast cell, basophil and macrophage FcγR-mediated activation.
(a, b, c) WT and Fyn KO BMMC were activated with 2.4G2 XL as described in Figure 1 for 1 hour to measure early phase mediators (Histamine) or 18 hours for cytokine release, analyzed by ELISA. (d, e) WT and Fyn KO bone marrow-derived macrophages were activated for 18 hours, and cell supernatant was analyzed by ELISA. (f, g, h) WT and Fyn KO bone marrow-derived basophils were activated for 18 hours and cell supernatant analyzed by ELISA. Data shown are mean ± SEM of at least 3 independent experiments done in triplicate. *p<0.05; **p<0.001; ***p<0.0001 based on Student’s t test Fyn WT to Fyn KO cells.
Basophils and macrophages share FcγRIIb/III expression with mast cells, and are also involved in innate immunity. In macrophages, FcγRs receptors regulate a variety of functions, such as macrophage activation or inhibition as well as opsonization of antibody-neutralized pathogens (40–44). Recent studies have identified the pivotal importance of basophils and macrophages in IgG-related allergic and anaphylactic reactions (13, 45–51). To determine the functional relevance of Fyn kinase downstream of FcγR signaling in basophils and macrophages, these cells were derived from WT and Fyn KO bone marrow as described in Materials and Methods. Interestingly, after 2.4G2 crosslinking, we observed that Fyn KO basophils and macrophages displayed a significant decrease in secreted cytokines and chemokines in comparison to the WT cultures (Figures 3d-h). These data demonstrate that Fyn kinase is required for optimal FcγR-mediated activation basophils and macrophages in addition to mast cells.
Lyn kinase deficiency enhances FcγR-mediated activation of mast cells, basophils and macrophages
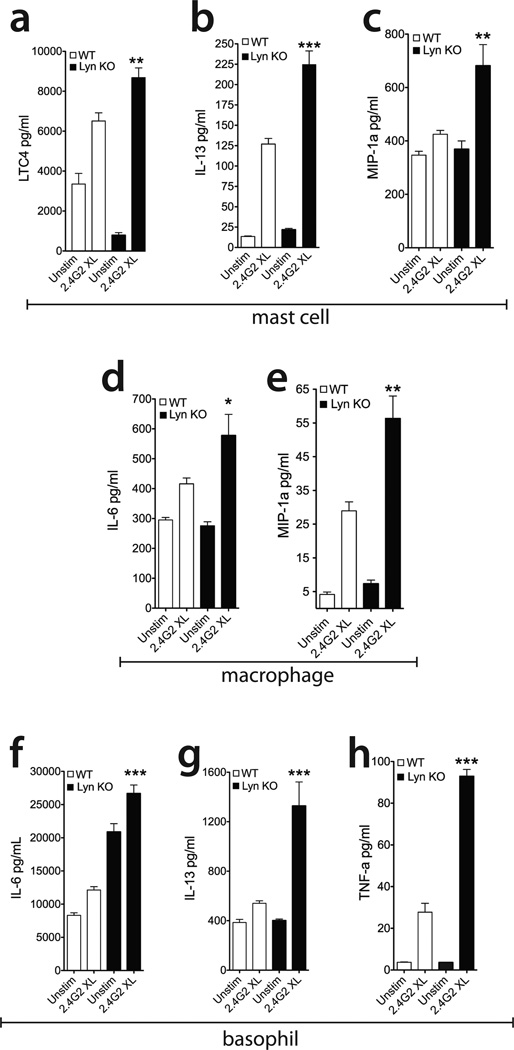
To determine the role of Lyn in FcγR signaling, wild type and Lyn KO mast cells were stimulated by 2.4G2 crosslinking. We found that Lyn KO BMMCs displayed a significant increase in the secretion of early phase mediators such as Leukotriene C4 (LTC4) (Figure 4a). Additionally, the amount of IL-13 and MIP-1α released was significantly increased in Lyn KO BMMCs compared to the control cells (Figure 4b, c). However, we did not observe a significant increase in histamine and other cytokines including TNF-alpha (data not shown). These results support the idea that Lyn kinase selectively regulates the amount of mediators released upon FcγR stimulation in BMMCs. Similar results were observed in macrophages (Figures 4d, e) and basophils (Figures 4f-h), where Lyn deficiency also selectively enhanced FcγR-mediated cytokine/chemokine release. Taken together, our findings support that Fyn and Lyn kinases are key opposing regulators of FcγR-dependent mast cell, basophil and macrophage activation.
Figure 4. Lyn kinase deficiency enhances FcγR-mediated mast cell, basophil, and macrophage activation.
(a, b, c) WT and Lyn KO BMMC were activated with 2.4G2 XL as described in Figure 1 for 1 hour to measure early phase mediators (Leukotriene C4) or 18 hours for cytokine release, and analyzed by ELISA. (d, e) WT and Lyn KO bone marrow-derived macrophages were activated for 18 hours, and cell supernatant was analyzed by ELISA. (f, h) WT and Lyn KO bone marrow-derived basophils were activated for 18 hours as described above, and cell supernatant was analyzed by ELISA. Data shown are mean ± SEM of at least 3 independent experiments done in triplicate. *p<0.05; **p<0.001; ***p<0.0001 based on Student’s t test WT to Lyn KO cells.
Regulatory functions of Fyn and Lyn kinases during FcγR-induced passive systemic anaphylaxis
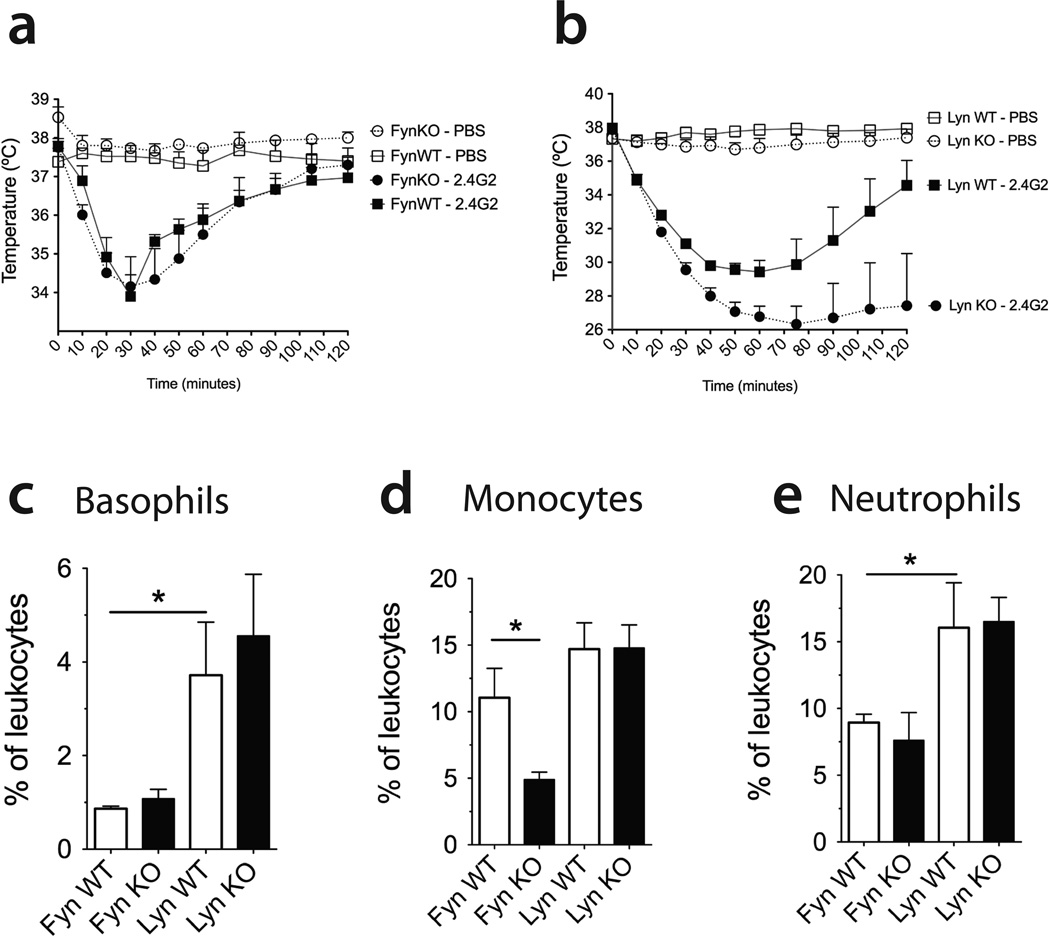
The importance of Fyn and Lyn in FcγR signaling prompted us to investigate their regulatory roles in vivo, in the context of passive systemic anaphylaxis (PSA). We performed an IgG-mediated PSA assay using intravenously injected 2.4G2 (31). Recent studies have reported that mast cells are not required for IgG-mediated PSA, and identified basophils, macrophages and neutrophils as the key players (11, 13, 14). Both Fyn KO age and gender-matched littermate mice developed anaphylaxis as assessed by decreased core body temperature, followed by a recovery period of approximately 2 hours. Unlike in vitro FcγR stimulation (Figure 3), Fyn deficiency did not convey protection from anaphylaxis severity (Figure 5a).
Figure 5. Lyn but not Fyn kinase deficiency is pivotal to IgG-induced passive systemic anaphylaxis.
(a,b) PSA was induced with 500µg of 2.4G2 injected i.v.. Changes in the core body temperature were measured by rectal probe [Fyn WT (PBS, n=4; 2.4G2, n=6), Fyn KO (PBS, n=3; 2.4G2, n=5), Lyn WT (PBS, n=3; 2.4G2, n=3), Lyn KO (PBS, n=3; 2.4G2, n=4)]. Data shown represent the mean ± SE. (c) Blood basophils (IgE+CD49b+), (d) monocytes (CD11b+Gr1−) and (e) neutrophils (CD11b+Gr1+) counts for Fyn (WT, n=8; KO, n=8) and Lyn (WT, n=8; KO, n=8) mice. Data shown represent the mean ± SEM.
In contrast, 2.4G2-induced PSA was exacerbated in Lyn KO mice compared to their wild type littermates (Figure 5b), correlating with our in vitro observations with mast cells, basophils and macrophages (observed in Figure 4). Despite a dramatic (11°C) drop in body temperature, none of the Lyn KO mice died in this experiment. Also, we did not observe any changes in circulating cytokines (Bioplex for GM-CSF, IL-1b, IL-4, IL-5, IL-6, IL-10, IL-13, IL-17a, MCP-1, MIP-1α, MIP-1β and TNF) in the sera of mice 2 hours after 2.4G2-induced PSA (data not shown). In general, 129Sv mice (Lyn WT and KO) displayed a lower nadir than their Fyn WT and KO counterparts. This could be due to increased blood basophil and neutrophils on this genetic background (Figure 5c-e). We found that Fyn or Lyn deficiency did not affect circulating B cell, T cell or eosinophil numbers (data not shown).
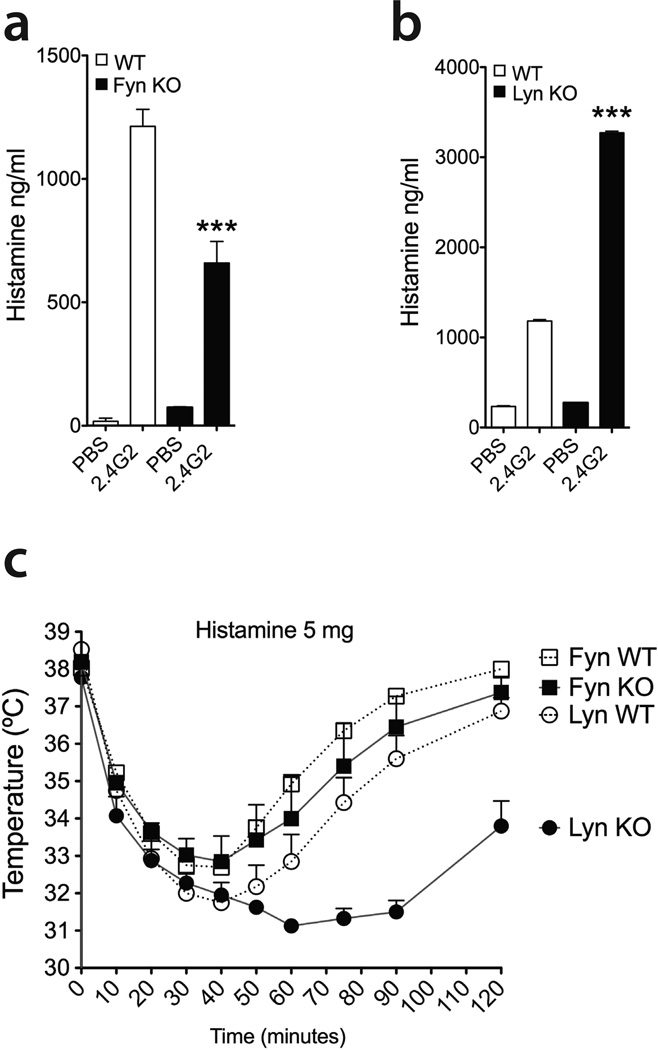
Histamine and platelet-activating factor (PAF) are very potent mediators causing bronchoconstriction, vascular leak and vasodilation, three features observed during human and mouse systemic anaphylaxis (13, 31, 49, 52, 53). Due to a paucity of sensitive assays for measuring PAF, and given that both PAF and histamine cause anaphylaxis, we next investigated the effects of 2.4G2-induced PSA on circulating histamine levels in the context of Fyn or Lyn deficiency. Our results showed that the serum of Fyn KO mice contained significantly lower histamine levels than their wild type counterparts (Figure 6a). In contrast, Lyn KO mice displayed elevated serum histamine compared to their littermates, in agreement with their exacerbated PSA phenotype (Figure 6b).
Figure 6. Fyn and Lyn kinases regulate the amount of serum histamine during IgG-induced PSA.
(a, b) Circulating histamine levels 2 hours after PSA induction [Fyn WT (PBS, n=4; 2.4G2, n=6), Fyn KO (PBS, n=3; 2.4G2, n=5), Lyn WT (PBS, n=3; 2.4G2, n=3), Lyn KO (PBS, n=3; 2.4G2, n=4)]. Data shown represent the mean ± SE. (c) Fyn KO (n=4) and Lyn KO (n=4) mice or control littermates (Fyn, n=4; Lyn, n=4) were injected i.v. with 5 mg of histamine and core body temperature was measured by rectal microprobe. Data shown represent the mean ± SEM.
To assess the impact of Fyn and Lyn kinase deficiency in the vascular sensitivity to histamine, we intravenously injected 5 mg of histamine into Fyn KO, Lyn KO and their wild type counterparts (Figure 6c). While Fyn KO mice exhibited anaphylactic responses mirroring their littermates, Lyn KO mice were more responsive than controls when given the same amount of histamine, suggesting that Lyn deficiency might also affect the vasculature in addition to regulating the amount of released histamine.
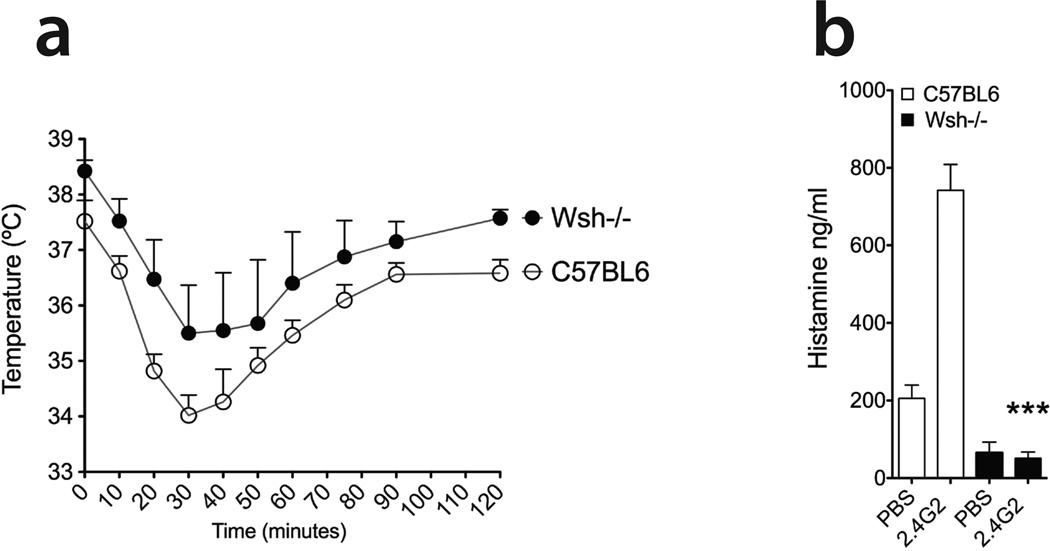
Mast cells account for the amount of serum histamine released in IgG-induced PSA
Mast cells and basophils degranulate and release histamine once activated through their FcγR. Our results show that the amount of histamine released in the serum during IgG-induced PSA is regulated in a Fyn and Lyn dependent-manner (Figures 6a, 6b). However, the cell population accounting for the amount of released histamine remained unclear. To address this question, we induced PSA by 2.4G2 intravenous injection into wild type or mast cell-deficient mice (Wsh−/−) mice, and monitored the drop in core body temperature. As seen in Figure 7a, Wsh−/− mice developed PSA to a similar extent as their WT controls (C57BL6). Not surprisingly, mast cell-deficient mice had little serum histamine two hours after the induction of PSA in comparison to their controls (Figure 7b). Our data demonstrate that mast cells account for the majority of histamine released during IgG-induced PSA.
Figure 7. Mast cells account for the majority of serum histamine during IgG-induced PSA.
(a) PSA was induced in mast cell-deficient mice (Wsh−/−, n=4) as well as their WT controls (C57BL6, n=5) by intravenous injection of 500µg of 2.4G2. The core body temperature was monitored by rectal microprobe. Data shown are the mean ± SEM. Two hours after the induction of PSA in (a), blood was collected from mice, serum prepared and the amount of circulating histamine was assessed by ELISA (b). Data shown are the mean ± SE.
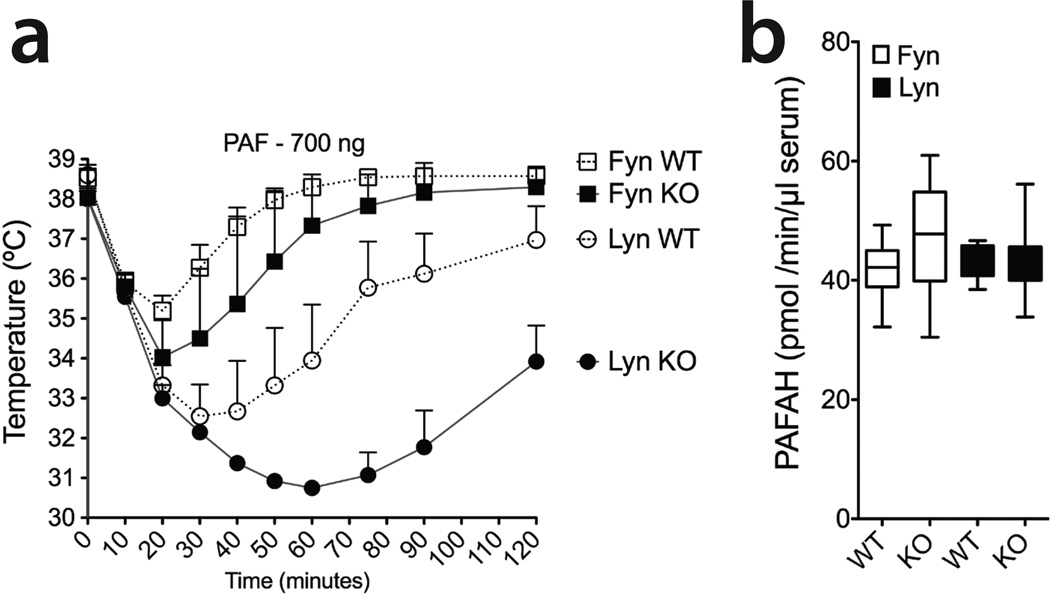
PAF injection recapitulates IgG-mediated PSA in Fyn and Lyn KO mice while the deficiency in neither Fyn nor Lyn affects PAF acytylhydrolase (PAFAH)
PAF is known to be the main mediator regulating IgG-induced PSA (11–14). To assess its importance in our model, we intravenously injected PAF (700ng) into Fyn KO, Lyn KO, and matched wild type mice (Figure 8a). PAF administration recapitulated IgG-mediated PSA kinetics in Fyn and Lyn KO (Figures 4a, 4b). Furthermore, we observed that Lyn deficiency not only worsened anaphylaxis severity (Figures 5b, 6c, 8a), but also lengthened the recovery time to approximately 5 hours, instead of the 2 hours observed with littermate controls (data not shown). These findings could be explained by increased leakage from endothelial cells, or altered decay rates for these anaphylactic mediators. Since PAF is the key factor in IgG-mediated anaphylaxis, we assessed serum activity levels of its inactivating enzyme, PAF acetylhydrolase (PAFAH). As shown in Figure 8b, steady-state PAFAH serum enzymatic activity was unaffected by Lyn or Fyn deficiency. Collectively, these data suggest that Lyn deficiency exacerbates responses to both histamine and PAF. Paired with enhanced macrophage and basophil responsiveness to IgG, the loss absence of Lyn kinase appears to exaggerate the severity and recovery time of anaphylaxis.
Figure 8. Intravenous administration of PAF recapitulates IgG-induced PSA kinetics while the deficiency in neither Fyn nor Lyn affects PAF acetylhydrolase (PAFAH).
(a) PSA was induced by intravenously delivery of 700 ng PAF in Fyn KO (n=3) and Lyn KO (n=4) as well as their littermate controls (Fyn, n=4; Lyn, n=4). Core body temperature was measured by rectal microprobe. Data shown represent the mean ± SE. (b) Enzymatic activity of serum levels of PAF acetylhydrolase (PAFAH) in Fyn and Lyn KO mice and their control littermates (n≥ 9 per group). Data shown represent the mean ± SEM.
DISCUSSION
Recently, the Src family kinases Lyn and Fyn have been found to exert opposing effects on IgE-mediated mast cell activation (21, 26, 54), but little is known about the role of these enzymes in IgG signals. Our findings show that FcγR triggers Fyn and Lyn kinase activation similar to FcεRI crosslinkage in mast cells (Figure 1), which might be explained by the fact that these two receptor families share the common γ chain (55). Furthermore, Fyn and Lyn appear important for the regulation of IgG-induced degranulation and cytokine production, suggesting an important role in mast cell functions. Here we show that Lyn deficiency significantly increases Akt phosphorylation subsequent to mast cell IgG receptor stimulation. These data mirror previous observations of FcεRI-mediated mast cell activation from Kitaura et al. (33). In that report, IgE XL of Lyn KO mast cells induced increased Akt phosphorylation, correlating with enhanced NF-κB, NF-AT and AP-1 transcriptional activation and subsequently elevated IL-2 and TNF production. Taken together, these data demonstrate that Lyn and Fyn kinase play important and similar roles in FcεRI and FcγRIII activation of mast cells. The fact that Fyn and Lyn deficiency similarly altered FcγR signaling in basophils and macrophages (Figures 3, 4) supports the importance of these antagonistically-paired Src-family kinases in IgG-mediated immune responses.
The onset and the regulation of anaphylaxis has been exclusively attributed to mast cells, delineating the generally known classical pathway of anaphylaxis involving IgE-mediated FcεRI mast cell activation and subsequent histamine release (1). In addition to this well-characterized pathway, recent reports in the literature demonstrate that in a murine model, the anaphylactic reaction can be triggered by an IgG-FcγR pathway. Basophils, macrophages and recently neutrophils (14) – but not mast cells – have been identified as crucial players in this “alternative pathway of anaphylaxis”, since mast cell-deficient mice demonstrated IgG-induced anaphylaxis. In contrast, in vivo depletion of basophils with the monoclonal antibody Ba103 (12, 13), or inhibiting macrophage function via gadolinium injection (11) markedly decreased the severity of FcγRIII-mediated anaphylaxis. A recent report further showed that FcγRIV-neutrophil-induced anaphylaxis also occurs, as mice deficient in FcγRI/FcγRIIB/FcγRIIIA/FcεRI/FcεRII−/− (5KO mice) still developed IgG-mediated anaphylaxis (14). In this alternative form of anaphylaxis, platelet-activating factor (PAF), rather than histamine, has been indicated as a pivotal mediator. While PAF serum levels are quite challenging to measure, the in vivo administration of a PAF antagonist protected mice from developing IgG- but not IgE-mediated anaphylaxis (13). Thus it is thought that IgG-induced anaphylaxis operates through FcγR–mediated activation of basophils, neutrophils and macrophages, with subsequent PAF release triggering vasodilation, vascular fluid leak, and loss of core body temperature (11, 13, 14, 48).
Our data support and extend the understanding of IgG-mediated anaphylaxis at the molecular signaling level. First, we found that Fyn or Lyn deletion has opposing effects on histamine release and cytokine secretion during IgG activation of mast cells, basophils, and macrophages in vitro. Interestingly, although the effects on histamine release were consistent in vivo, they did not predict the severity of FcγR-induced PSA. While Lyn KO mice showed increased histamine and worsened hypothermia, Fyn KO had diminished histamine but no change in hypothermia versus respective WT mice. Additionally, although histamine and PAF are responsible for anaphylaxis, we found that mast cells were mainly responsible for the amount of circulating histamine present in the serum during the course of FcγR-induced PSA (Figure 7), extending the involvement of mast cells in IgG-related pathologies. It is interesting to note that a recent study found histamine produced by non-mast cell sources in a model of contact hypersensitivity (56), suggesting that in vivo histamine production varies with the eliciting stimulus. Based on previous reports (11–14), the drop in body temperature observed in mast cell-deficient animals over the course of anaphylaxis should be attributed to PAF released by basophils, macrophages and neutrophils after FcγRIII activation. Furthermore, we did not observe any significant difference in perivascular cell infiltration and pulmonary edema between Fyn KO, Lyn KO and their controls subsequent to 2.4G2-induced PSA (data not shown).
The exacerbated response of Lyn KO mice could be ascribed to many factors: increased PAF secretion, decreased PAF metabolism, or enhanced PAF signaling in vascular endothelium. We found no changes in PAFAH activity, suggesting that reduced PAF catabolism is not an explanation. Furthermore, we found no overt changes in circulating basophils, monocyte, or neutrophils in Lyn KO mice. It is also possible that Lyn KO endothelial cells, just like mast cells, basophils and macrophages, are hyper-responsive to any stimuli involving Lyn kinase. In fact, Kuruvilla et al. have demonstrated that Fyn and Lyn are phosphorylated upon PAF receptor activation, leading to phosphorylation of the p85 regulatory subunit of PI3K (57). In addition, Yu et al. showed that phospho-tyrosine activated Lyn kinase co-localized with PI3K in the lipid body fraction of PMN leukocytes subsequent to PAF receptor stimulation (58). Taken together, and in line with our findings, we therefore speculate that Lyn KO endothelial cells are hyper-responsive to PAF stimulation, triggering a longer and more severe vasodilation episode in comparison to Lyn-sufficient cells.
Based on our data, we can hypothesize that patients with altered Lyn function or expression could be hyperresponsive to IgE- and IgG-mediated pathology. There is precedence for this, as the majority of systemic lupus erythematosus (SLE) patients have been shown to have reduced Lyn expression in two clinical studies (59, 60). While SLE is regarded as an IgG immune complex-mediated disease, Charles et al. recently showed that basophils activated by IgE may participate in the onset or the exacerbation of lupus-like symptoms in a mouse model (61). In the same report, this group demonstrated that aged Lyn KO mice displayed increased basophil numbers in the lymph nodes, blood and spleen. Furthermore, Lyn KO mice display significantly elevated circulating autoantibodies which, in conjunction with many other factors, lead to the development of SLE-like symptoms and contribute to the elevated mortality in this population (62–64). Combined with our data, Lyn deficiency is postulated to worsen either IgG- or IgE-mediated inflammation, which could contribute to the development of SLE in aged mice.
Several studies have reported the presence of allergen-specific IgG in allergic individuals. The involvement of this immunoglobulin isotype in the onset and the development of the allergic reaction remains poorly understood (65, 66). However, Bandukwala et al. used a murine model to demonstrate the importance of FcγRI and FcγRIII in airway inflammation and hyperresponsiveness. This study showed that C57BL/6 mice that were sensitized with noninfectious parasitic Schistosoma mansoni eggs and challenged with soluble egg antigen displayed airway inflammation, including eosinophil infiltration and severe peribronchial and perivascular inflammation. This response was greatly decreased in FcγRI−/− or FcγRIII−/− mice (67). This group also demonstrated that deficiency of FcγRIII, but not FcγRI, reduced lung resistance upon methacholine challenge, compared to littermate controls. Thus, in addition to the well-established role of IgE-FcεRI in the onset and the development of allergy and hypersensitivity, this study provides evidence that FcγRIII activation can participate in airway disease pathogenesis in a murine model. Combined with our data, these findings support the theory that the exacerbated 2.4G2-mediated PSA we noted in Lyn KO mice is elicited by FcγRIII, as previously supported by Ravetch et al. as well as Daeron et al. (68–71).
The high affinity (FcγRI) and low affinity (FcγRIII) IgG receptors are known to be important in the activation of numerous cell types of the immune system and in the phagocytosis of opsonized microbes. In contrast, one of the members of the FcγR family, FcγRIIb, has emerged as an inhibitory receptor (31, 72, 73). Using a mouse model of allergic asthma, Dharajiya et al. demonstrated that in addition to upregulating the expression of FcγRIIb in CD14+MHCII+ mononuclear as well as in CD11b+ cells in the lungs, ragweed extract (RWE) challenge also led to signs of severe allergic asthma-like symptoms in FcγRIIb-deficient mice, delineated by increased airway eosinophilia, mucin production and allergen-specific IgE. Furthermore, a tremendous increase in macrophage, eosinophil and lymphocyte recruitment was observed in the broncho-alveolar lavage fluid of RWE-challenged FcγRIIb KO mice in comparison to wild type controls (74). These data, as well as many others, consolidate the idea that IgG and Fcγ receptors play important roles in the regulation of immune system homeostasis. Our data support the hypothesis that Fyn and Lyn kinases are pivotal antagonistic regulators of this paired IgG receptor system.
The data presented demonstrate that Fyn and Lyn kinases are activated during IgG-mediated signaling and have opposing regulatory functions in mast cells, basophils and macrophages. In addition to Fyn and Lyn activation, we found that FcγR stimulation also led to the phosphorylation of Akt, Erk, p38 and JNK. Further, we also uncover the unsuspected contribution of mast cells as major producers of serum histamine during IgG-induced PSA, regulated in a Fyn- and Lyn-dependent manner. More importantly, we show that overall Lyn but not Fyn kinase regulates the severity of IgG-induced passive systemic anaphylaxis, by enhancing the amount of vasoactive mediators secreted and exacerbating endothelial cell responsiveness to PAF and histamine. In line with previous studies, our findings extend the understanding of IgG-related pathologies and demonstrate the pivotal role of Lyn kinase as the key regulator of IgG-mediated inflammation.
ACKNOWLEDGEMENTS
This work was supported by the intramural research program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (J. Rivera), and NIH grants 1R01AI59638 and U19AI077435 to J. Ryan, KO1AR053186 to C.A. Oskeritzian, and VA Merit Grant to F. Finkelman.
Footnotes
The authors have no conflicting financial interests
REFERENCES
- 1.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 2.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin TR, Galli SJ, Katona IM, Drazen JM. Role of mast cells in anaphylaxis. Evidence for the importance of mast cells in the cardiopulmonary alterations and death induced by anti-IgE in mice. J Clin Invest. 1989;83:1375–1383. doi: 10.1172/JCI114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zabel BA, Nakae S, Zuniga L, Kim JY, Ohyama T, Alt C, Pan J, Suto H, Soler D, Allen SJ, Handel TM, Song CH, Galli SJ, Butcher EC. Mast cell-expressed orphan receptor CCRL2 binds chemerin and is required for optimal induction of IgE-mediated passive cutaneous anaphylaxis. J Exp Med. 2008;205:2207–2220. doi: 10.1084/jem.20080300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan JJ, Fernando JF. Mast cell modulation of the immune response. Curr Allergy Asthma Rep. 2009;9:353–359. doi: 10.1007/s11882-009-0052-z. [DOI] [PubMed] [Google Scholar]
- 6.Gilfillan AM, Rivera J. The tyrosine kinase network regulating mast cell activation. Immunol Rev. 2009;228:149–169. doi: 10.1111/j.1600-065X.2008.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivera J, Olivera A. A current understanding of Fc epsilon RI-dependent mast cell activation. Curr Allergy Asthma Rep. 2008;8:14–20. doi: 10.1007/s11882-008-0004-z. [DOI] [PubMed] [Google Scholar]
- 8.Rivera J, Fierro NA, Olivera A, Suzuki R. New insights on mast cell activation via the high affinity receptor for IgE. Adv Immunol. 2008;98:85–120. doi: 10.1016/S0065-2776(08)00403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daeron M, Bonnerot C, Latour S, Fridman WH. Murine recombinant Fc gamma RIII, but not Fc gamma RII, trigger serotonin release in rat basophilic leukemia cells. J Immunol. 1992;149:1365–1373. [PubMed] [Google Scholar]
- 11.Strait RT, Morris SC, Yang M, Qu XW, Finkelman FD. Pathways of anaphylaxis in the mouse. J Allergy Clin Immunol. 2002;109:658–668. doi: 10.1067/mai.2002.123302. [DOI] [PubMed] [Google Scholar]
- 12.Obata K, Mukai K, Tsujimura Y, Ishiwata K, Kawano Y, Minegishi Y, Watanabe N, Karasuyama H. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood. 2007;110:913–920. doi: 10.1182/blood-2007-01-068718. [DOI] [PubMed] [Google Scholar]
- 13.Tsujimura Y, Obata K, Mukai K, Shindou H, Yoshida M, Nishikado H, Kawano Y, Minegishi Y, Shimizu T, Karasuyama H. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. 2008;28:581–589. doi: 10.1016/j.immuni.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Jonsson F, Mancardi DA, Kita Y, Karasuyama H, Iannascoli B, Van Rooijen N, Shimizu T, Daeron M, Bruhns P. Mouse and human neutrophils induce anaphylaxis. J Clin Invest. 2011;121:1484–1496. doi: 10.1172/JCI45232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanley E, Ralph S, McEwen S, Boulet I, Holtzman DA, Lock P, Dunn AR. Alternatively spliced murine lyn mRNAs encode distinct proteins. Mol Cell Biol. 1991;11:3399–3406. doi: 10.1128/mcb.11.7.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yi TL, Bolen JB, Ihle JN. Hematopoietic cells express two forms of lyn kinase differing by 21 amino acids in the amino terminus. Mol Cell Biol. 1991;11:2391–2398. doi: 10.1128/mcb.11.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alvarez-Errico D, Yamashita Y, Suzuki R, Odom S, Furumoto Y, Yamashita T, Rivera J. Functional analysis of Lyn kinase A and B isoforms reveals redundant and distinct roles in Fc epsilon RI-dependent mast cell activation. J Immunol. 2010;184:5000–5008. doi: 10.4049/jimmunol.0904064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamanashi Y, Kakiuchi T, Mizuguchi J, Yamamoto T, Toyoshima K. Association of B cell antigen receptor with protein tyrosine kinase Lyn. Science. 1991;251:192–194. doi: 10.1126/science.1702903. [DOI] [PubMed] [Google Scholar]
- 19.Eiseman E, Bolen JB. Engagement of the high-affinity IgE receptor activates src protein-related tyrosine kinases. Nature. 1992;355:78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- 20.Kawakami Y, Kitaura J, Satterthwaite AB, Kato RM, Asai K, Hartman SE, Maeda-Yamamoto M, Lowell CA, Rawlings DJ, Witte ON, Kawakami T. Redundant and opposing functions of two tyrosine kinases, Btk and Lyn, in mast cell activation. J Immunol. 2000;165:1210–1219. doi: 10.4049/jimmunol.165.3.1210. [DOI] [PubMed] [Google Scholar]
- 21.Odom S, Gomez G, Kovarova M, Furumoto Y, Ryan JJ, Wright HV, Gonzalez-Espinosa C, Hibbs ML, Harder KW, Rivera J. Negative regulation of immunoglobulin E-dependent allergic responses by Lyn kinase. J Exp Med. 2004;199:1491–1502. doi: 10.1084/jem.20040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satterthwaite AB, Lowell CA, Khan WN, Sideras P, Alt FW, Witte ON. Independent and opposing roles for Btk and lyn in B and myeloid signaling pathways. J Exp Med. 1998;188:833–844. doi: 10.1084/jem.188.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeshita H, Taniuchi I, Kato J, Watanabe T. Abrogation of autoimmune disease in Lyn-deficient mice by the mutation of the Btk gene. Int Immunol. 1998;10:435–444. doi: 10.1093/intimm/10.4.435. [DOI] [PubMed] [Google Scholar]
- 24.Xu Y, Harder KW, Huntington ND, Hibbs ML, Tarlinton DM. Lyn tyrosine kinase: accentuating the positive and the negative. Immunity. 2005;22:9–18. doi: 10.1016/j.immuni.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Hibbs ML, Harder KW, Armes J, Kountouri N, Quilici C, Casagranda F, Dunn AR, Tarlinton DM. Sustained activation of Lyn tyrosine kinase in vivo leads to autoimmunity. J Exp Med. 2002;196:1593–1604. doi: 10.1084/jem.20020515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parravicini V, Gadina M, Kovarova M, Odom S, Gonzalez-Espinosa C, Furumoto Y, Saitoh S, Samelson LE, O'Shea JJ, Rivera J. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat Immunol. 2002;3:741–748. doi: 10.1038/ni817. [DOI] [PubMed] [Google Scholar]
- 27.Gomez G, Gonzalez-Espinosa C, Odom S, Baez G, Cid ME, Ryan JJ, Rivera J. Impaired FcepsilonRI-dependent gene expression and defective eicosanoid and cytokine production as a consequence of Fyn deficiency in mast cells. J Immunol. 2005;175:7602–7610. doi: 10.4049/jimmunol.175.11.7602. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki R, Liu X, Olivera A, Aguiniga L, Yamashita Y, Blank U, Ambudkar I, Rivera J. Loss of TRPC1-mediated Ca2+ influx contributes to impaired degranulation in Fyn-deficient mouse bone marrow-derived mast cells. J Leukoc Biol. 2010 doi: 10.1189/jlb.0510253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crowley MT, Costello PS, Fitzer-Attas CJ, Turner M, Meng F, Lowell C, Tybulewicz VL, DeFranco AL. A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages. J Exp Med. 1997;186:1027–1039. doi: 10.1084/jem.186.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lach-Trifilieff E, Menear K, Schweighoffer E, Tybulewicz VL, Walker C. Syk-deficient eosinophils show normal interleukin-5-mediated differentiation, maturation, and survival but no longer respond to FcgammaR activation. Blood. 2000;96:2506–2510. [PubMed] [Google Scholar]
- 31.Finkelman FD. Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol. 2007;120:506–515. doi: 10.1016/j.jaci.2007.07.033. quiz 516-507. [DOI] [PubMed] [Google Scholar]
- 32.Al-Darmaki S, Schenkein HA, Tew JG, Barbour SE. Differential expression of platelet-activating factor acetylhydrolase in macrophages and monocyte-derived dendritic cells. J Immunol. 2003;170:167–173. doi: 10.4049/jimmunol.170.1.167. [DOI] [PubMed] [Google Scholar]
- 33.Kitaura J, Asai K, Maeda-Yamamoto M, Kawakami Y, Kikkawa U, Kawakami T. Akt-dependent cytokine production in mast cells. J Exp Med. 2000;192:729–740. doi: 10.1084/jem.192.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu M, Liu Y, Koonpaew S, Granillo O, Zhang W. Positive and negative regulation of FcepsilonRI-mediated signaling by the adaptor protein LAB/NTAL. J Exp Med. 2004;200:991–1000. doi: 10.1084/jem.20041223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon M, Vanes L, Geahlen RL, Tybulewicz VL. Distinct roles for the linker region tyrosines of Syk in FcepsilonRI signaling in primary mast cells. J Biol Chem. 2005;280:4510–4517. doi: 10.1074/jbc.M410326200. [DOI] [PubMed] [Google Scholar]
- 36.Xiao W, Nishimoto H, Hong H, Kitaura J, Nunomura S, Maeda-Yamamoto M, Kawakami Y, Lowell CA, Ra C, Kawakami T. Positive and negative regulation of mast cell activation by Lyn via the FcepsilonRI. J Immunol. 2005;175:6885–6892. doi: 10.4049/jimmunol.175.10.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barbu EA, Zhang J, Siraganian RP. The limited contribution of Fyn and Gab2 to the high affinity IgE receptor signaling in mast cells. J Biol Chem. 285:15761–15768. doi: 10.1074/jbc.M110.109413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olenchock BA, Guo R, Silverman MA, Wu JN, Carpenter JH, Koretzky GA, Zhong XP. Impaired degranulation but enhanced cytokine production after Fc epsilonRI stimulation of diacylglycerol kinase zeta-deficient mast cells. J Exp Med. 2006;203:1471–1480. doi: 10.1084/jem.20052424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Zhu M, Nishida K, Hirano T, Zhang W. An essential role for RasGRP1 in mast cell function and IgE-mediated allergic response. J Exp Med. 2007;204:93–103. doi: 10.1084/jem.20061598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bohnsack JF, Kleinman HK, Takahashi T, O'Shea JJ, Brown EJ. Connective tissue proteins and phagocytic cell function. Laminin enhances complement and Fc-mediated phagocytosis by cultured human macrophages. J Exp Med. 1985;161:912–923. doi: 10.1084/jem.161.5.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aderem AA, Wright SD, Silverstein SC, Cohn ZA. Ligated complement receptors do not activate the arachidonic acid cascade in resident peritoneal macrophages. J Exp Med. 1985;161:617–622. doi: 10.1084/jem.161.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 43.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 44.Gao JJ, Diesl V, Wittmann T, Morrison DC, Ryan JL, Vogel SN, Follettie MT. Regulation of gene expression in mouse macrophages stimulated with bacterial CpG-DNA and lipopolysaccharide. J Leukoc Biol. 2002;72:1234–1245. [PubMed] [Google Scholar]
- 45.Kawakami T. Basophils now enhance memory. Nat Immunol. 2008;9:720–721. doi: 10.1038/ni0708-720. [DOI] [PubMed] [Google Scholar]
- 46.Galli SJ, Franco CB. Basophils are back! Immunity. 2008;28:495–497. doi: 10.1016/j.immuni.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 47.Mukai K, Matsuoka K, Taya C, Suzuki H, Yokozeki H, Nishioka K, Hirokawa K, Etori M, Yamashita M, Kubota T, Minegishi Y, Yonekawa H, Karasuyama H. Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity. 2005;23:191–202. doi: 10.1016/j.immuni.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 48.Mukai K, Obata K, Tsujimura Y, Karasuyama H. New insights into the roles for basophils in acute and chronic allergy. Allergol Int. 2009;58:11–19. doi: 10.2332/allergolint.08-RAI-0059. [DOI] [PubMed] [Google Scholar]
- 49.Karasuyama H, Mukai K, Tsujimura Y, Obata K. Newly discovered roles for basophils: a neglected minority gains new respect. Nat Rev Immunol. 2009;9:9–13. doi: 10.1038/nri2458. [DOI] [PubMed] [Google Scholar]
- 50.Hida S, Yamasaki S, Sakamoto Y, Takamoto M, Obata K, Takai T, Karasuyama H, Sugane K, Saito T, Taki S. Fc receptor gamma-chain, a constitutive component of the IL-3 receptor, is required for IL-3-induced IL-4 production in basophils. Nat Immunol. 2009;10:214–222. doi: 10.1038/ni.1686. [DOI] [PubMed] [Google Scholar]
- 51.Charles N, Watford WT, Ramos HL, Hellman L, Oettgen HC, Gomez G, Ryan JJ, O'Shea JJ, Rivera J. Lyn kinase controls basophil GATA-3 transcription factor expression and induction of Th2 cell differentiation. Immunity. 2009;30:533–543. doi: 10.1016/j.immuni.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khodoun M, Strait R, Orekov T, Hogan S, Karasuyama H, Herbert DR, Kohl J, Finkelman FD. Peanuts can contribute to anaphylactic shock by activating complement. J Allergy Clin Immunol. 2009;123:342–351. doi: 10.1016/j.jaci.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hernandez-Hansen V, Smith AJ, Surviladze Z, Chigaev A, Mazel T, Kalesnikoff J, Lowell CA, Krystal G, Sklar LA, Wilson BS, Oliver JM. Dysregulated FcepsilonRI signaling and altered Fyn and SHIP activities in Lyn-deficient mast cells. J Immunol. 2004;173:100–112. doi: 10.4049/jimmunol.173.1.100. [DOI] [PubMed] [Google Scholar]
- 55.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 56.Dudeck A, Dudeck J, Scholten J, Petzold A, Surianarayanan S, Kohler A, Peschke K, Vohringer D, Waskow C, Krieg T, Muller W, Waisman A, Hartmann K, Gunzer M, Roers A. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity. 2011;34:973–984. doi: 10.1016/j.immuni.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 57.Kuruvilla A, Pielop C, Shearer WT. Platelet-activating factor induces the tyrosine phosphorylation and activation of phospholipase C-gamma 1, Fyn and Lyn kinases, and phosphatidylinositol 3-kinase in a human B cell line. J Immunol. 1994;153:5433–5442. [PubMed] [Google Scholar]
- 58.Yu W, Cassara J, Weller PF. Phosphatidylinositide 3-kinase localizes to cytoplasmic lipid bodies in human polymorphonuclear leukocytes and other myeloid-derived cells. Blood. 2000;95:1078–1085. [PubMed] [Google Scholar]
- 59.Liossis SN, Solomou EE, Dimopoulos MA, Panayiotidis P, Mavrikakis MM, Sfikakis PP. B-cell kinase lyn deficiency in patients with systemic lupus erythematosus. J Investig Med. 2001;49:157–165. doi: 10.2310/6650.2001.34042. [DOI] [PubMed] [Google Scholar]
- 60.Flores-Borja F, Kabouridis PS, Jury EC, Isenberg DA, Mageed RA. Decreased Lyn expression and translocation to lipid raft signaling domains in B lymphocytes from patients with systemic lupus erythematosus. Arthritis Rheum. 2005;52:3955–3965. doi: 10.1002/art.21416. [DOI] [PubMed] [Google Scholar]
- 61.Charles N, Hardwick D, Daugas E, Illei GG, Rivera J. Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat Med. 2010;16:701–707. doi: 10.1038/nm.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, Stacker SA, Dunn AR. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 63.Nishizumi H, Horikawa K, Mlinaric-Rascan I, Yamamoto T. A double-edged kinase Lyn: a positive and negative regulator for antigen receptor-mediated signals. J Exp Med. 1998;187:1343–1348. doi: 10.1084/jem.187.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu CC, Yen TS, Lowell CA, DeFranco AL. Lupus-like kidney disease in mice deficient in the Src family tyrosine kinases Lyn and Fyn. Curr Biol. 2001;11:34–38. doi: 10.1016/s0960-9822(00)00024-5. [DOI] [PubMed] [Google Scholar]
- 65.Pepys J, Parish WE, Stenius-Aarniala B, Wide L. Clinical correlations between long-term (IgE) and short-term (IgG S-TS) anaphylactic antibodies in atopic and 'non-atopic' subjects with respiratory allergic disease. Clin Allergy. 1979;9:645–658. doi: 10.1111/j.1365-2222.1979.tb00491.x. [DOI] [PubMed] [Google Scholar]
- 66.Out TA, van de Graaf EA, van den Berg NJ, Jansen HM. IgG subclasses in bronchoalveolar lavage fluid from patients with asthma. Scand J Immunol. 1991;33:719–727. doi: 10.1111/j.1365-3083.1991.tb02546.x. [DOI] [PubMed] [Google Scholar]
- 67.Bandukwala HS, Clay BS, Tong J, Mody PD, Cannon JL, Shilling RA, Verbeek JS, Weinstock JV, Solway J, Sperling AI. Signaling through Fc gamma RIII is required for optimal T helper type (Th)2 responses and Th2-mediated airway inflammation. J Exp Med. 2007;204:1875–1889. doi: 10.1084/jem.20061134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hazenbos WL, Gessner JE, Hofhuis FM, Kuipers H, Meyer D, Heijnen IA, Schmidt RE, Sandor M, Capel PJ, Daeron M, van de Winkel JG, Verbeek JS. Impaired IgG-dependent anaphylaxis and Arthus reaction in Fc gamma RIII (CD16) deficient mice. Immunity. 1996;5:181–188. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- 69.Dombrowicz D, Flamand V, Miyajima I, Ravetch JV, Galli SJ, Kinet JP. Absence of Fc epsilonRI alpha chain results in upregulation of Fc gammaRIII-dependent mast cell degranulation and anaphylaxis. Evidence of competition between Fc epsilonRI and Fc gammaRIII for limiting amounts of FcR beta and gamma chains. J Clin Invest. 1997;99:915–925. doi: 10.1172/JCI119256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miyajima I, Dombrowicz D, Martin TR, Ravetch JV, Kinet JP, Galli SJ. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and Fc gammaRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE- or IgG1-dependent passive anaphylaxis. J Clin Invest. 1997;99:901–914. doi: 10.1172/JCI119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ujike A, Ishikawa Y, Ono M, Yuasa T, Yoshino T, Fukumoto M, Ravetch JV, Takai T. Modulation of immunoglobulin (Ig)E-mediated systemic anaphylaxis by low-affinity Fc receptors for IgG. J Exp Med. 1999;189:1573–1579. doi: 10.1084/jem.189.10.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 10:440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sarmay G, Koncz G, Gergely J. Human type II Fcgamma receptors inhibit B cell activation by interacting with the p21(ras)-dependent pathway. J Biol Chem. 1996;271:30499–30504. doi: 10.1074/jbc.271.48.30499. [DOI] [PubMed] [Google Scholar]
- 74.Dharajiya N, Vaidya SV, Murai H, Cardenas V, Kurosky A, Boldogh I, Sur SA. FcgammaRIIb inhibits allergic lung inflammation in a murine model of allergic asthma. PLoS One. 5:e9337. doi: 10.1371/journal.pone.0009337. [DOI] [PMC free article] [PubMed] [Google Scholar]