Abstract
A member of the p53 family, p73, has several isoforms and differentially regulates transcription of genes involved in the control of the cell cycle and apoptosis. We have previously shown efficient and p53-independent, tumor-specific cell death induced by the viral proteins E1A and Apoptin. Here, we demonstrate that the induction of apoptosis by these viral proteins involves activation of TAp73. Both E1A and Apoptin induced expression of endogenous TAp73 and the p53/p73 BH3-only pro-apoptotic target, PUMA, independently of the p53 function. Furthermore, exogenous expression of TAp73 isoforms, particularly TAp73β, sensitized cells to killing by both E1A and Apoptin, while expression of ΔNp73α blocked this activity. Besides, knockout of the p73 regulator, c-Abl, attenuated E1A-induced apoptosis. In accordance with the role of p73 in apoptosis induced by these viral proteins, overexpression of TAp73β strongly induced apoptosis in p53-deficient cancer cells in vitro and in HNSCC xenografts. Using a doxycycline-inducible system, we provide evidence for target selectivity and significant differences in protein stability for specific p73 isoforms, suggesting a diverse and pivotal role for p73 in response to various genotoxic agents. Collectively, our data show that in the absence of the p53 function, viral proteins E1A and Apoptin utilize the p73 pathway to induce efficient tumor cell death.
Keywords: p73, E1A, Apoptin, apoptosis, PUMA
Introduction
The p73 proteins show considerable amino acid identity with p53, particularly in the DNA-binding domain1 and exist as at least seven C-terminal splice variants: p73α-η.2-4 The gene has two distinct promoters resulting in the generation of two types of protein: the transactivation competent (TA) forms and NH2-terminally truncated (ΔN) forms.5 Like p53, TAp73 induces the transcription of genes involved in cell cycle arrest and apoptosis, in response to cellular stress signals.6-9 In contrast, ΔNp73 isoforms have anti-apoptotic potential4,10 and function as dominant negative inhibitors of TAp73 and p53, either by competing with p53 and the TAp73 variants for DNA binding sites, or by forming hetero-complexes with p53 and TAp73.11-13 It has been suggested that the balance between these two proteins plays an important role in tumorigenesis, because increased ΔNp73 expression has been associated with tumor progression and poor prognosis in several human cancers including neuroblastoma, ovarian cancer, lung cancer and hepatocellular carcinoma.5,14-18
Although TAp73 isoforms are able to transactivate many p53 responsive promoters, the efficiencies on different promoters vary among the different isoforms.8 Some studies have demonstrated that p73β is more efficient than p73α in transactivating a number of p53/p73 target genes,19,20 while another study has shown TAp73γ to be the most potent isoform in transactivating Bax and inducing apoptosis.21 Thus, specific p73 isoforms may determine both the level of transactivation of p53/p73 targets, as well as their functional consequences. It has been suggested that the transactivation ability of TAp73 is associated with its protein turnover; p73 transactivation activity was shown to be necessary for rapid p73 turnover and a naturally occurring ΔNp73 was substantially more stable than TAp73.22
We and others have shown the p73 pathway to be important in response to DNA damage induced by chemotherapeutic drugs and by oncogenes such as the early region 1A (E1A) of human adenovirus type 2 and 5.23,24 E1A gene is essential for adenovirus replication and E1A encoded proteins, particularly the two major ones E1A13S and E1A12S, have been shown to regulate the transcription of many cellular target genes to provide a suitable environment for viral replication (reviewed in ref. 25). In cooperation with specific onco-genes, E1A immortalizes primary rodent fibroblasts.26,27 However, no evidence of transformation of human cells by E1A has so far been reported. Indeed, many recent studies have shown E1A to be a powerful pro-apoptotic factor in several types of human malignancies (reviewed in ref. 25).
It has been suggested that growth suppression induced by E1A is mediated via p53 and the p53 regulator p19ARF.28 However, we and others have shown that E1A induces efficient apoptosis in a number of cancer cell lines with mutated or deleted p53.29-31 Furthermore, we have recently shown that the expression of E1A13S efficiently transactivates p73 and induces expression of p53 apoptotic targets, in particular Noxa, in several cancer cell lines that are deficient in p53.24 However, the direct involvement of p73 in cell killing induced by E1A has so far not been demonstrated.
Another viral protein, the chicken anemia viral protein VP3/Apoptin, causes tumor-selective apoptosis in human malignant and transformed cell lines independently of the p53 status.32,33 Apoptin induces apoptosis via the intrinsic/mitochondria apoptotic pathway, requiring the activation of downstream caspases (reviewed in ref. 34). Apoptin has been shown to be phosphorylated by an as yet unknown kinase, on threonine 108, in human cancer cells but negligibly in normal cells.35 This modification is important for function of Apoptin as a tumor-specific apoptosis inducing protein. However, the upstream signaling pathways leading to phosphorylation and cell death induced by Apoptin are currently unknown (reviewed in ref. 34).
In this study, we sought to determine the role of specific p73 isoforms in the p53-independent induction of cell death by the viral proteins, E1A and Apoptin. We observed that both viral proteins induced cellular levels of TAp73 and the p53/p73 target PUMA. TAp73 was crucial for E1A-induced cell death and the induction of its target genes PUMA, Bax and Noxa because all these effects were attenuated by overexpressing the dominant negative form ΔNp73α and/or in c-Abl knockout mouse embryo fibroblasts (MEFs) which are incapable of activating the p73 pathway after DNA damage.36 Remarkably, in the p53-mutated human head and neck squamous cell carcinoma (HNSCC) cell line, H357, activation of TAp73 by Apoptin was associated with the phosphorylation of Apoptin known to be crucial for its tumor killing function. We also show that doxycycline-induced ectopic expression of TAp73 isoforms, but not ΔNp73α, increased the apoptosis sensitivity to both E1A and Apoptin in the p53-deficient Saos-2 osteosarcoma cell line. Consistent with this finding, the strongly pro-apoptotic TAp73β isoform effectively killed p53-deficient head and neck cancer xenografts when injected into the tumor. Investigation of the biochemical function of the specific p73 isoforms demonstrated significant differences in the ability to induce apoptosis, transactivation of downstream targets and protein stability between different p73 isoforms. In summary, our data indicate that p73 isoforms have important and differential roles in apoptosis induced by a variety of genotoxic stimuli including the viral proteins E1A and Apoptin and suggest that TAp73, in particular the β isoform, may have significant therapeutic potential in cancers with p53 aberrations.
Results
p73 isoforms have differential effects on the induction of cell death by E1A or Apoptin
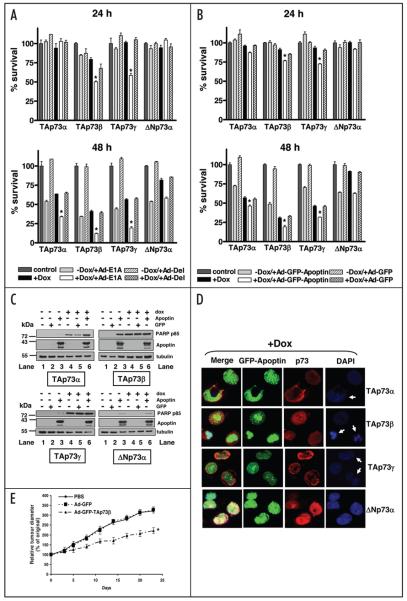
To determine whether p73 isoforms differ in their effects on the induction of cell death by E1A or Apoptin, we used the doxycycline-inducible Saos-2 cells expressing either the dominant negative ΔNp73α or the TAp73 isoforms. The inducible Saos-2 cells expressing TAp73α, β, γ or ΔNp73α were infected with either a recombinant adenovirus vector expressing wild-type E1A (Ad-E1A) or the control virus Ad-Del37 in the presence or absence of p73 inducer, doxycycline. Cell survival was measured by the MTT assay at 24 and 48 h post-infection. 24 h infection with Ad-E1A resulted in a significant reduction of cell survival in Saos-2 cells overexpressing TAp73β (50.4% cell survival) and TAp73γ (59% cell survival), but not in those overexpressing TAp73α. ΔNp73α-expressing cells infected with Ad-E1A had a slightly increased growth rate compared to the control (Fig. 1A, top). 48 h after expression of E1A in Saos-2 cells overexpressing TAp73α, TAp73β and TAp73γ resulted in dramatic cell death, particularly in Saos-2 cells expressing TAp73β and TAp73γ (Fig. 1A, bottom). Survival rates of Saos-2 cells overexpressing TAp73α, TAp73β and TAp73γ infected with Ad-E1A were reduced to 34.1%, 12.2% and 19.3%, respectively. In contrast, almost 60% of Saos-2 cells overexpressing ΔNp73α and infected with Ad-E1A remained viable, indicating that the cells overexpressing TAp73 isoforms, but not ΔNp73α, significantly enhanced E1A-induced cell death (p < 0.001). The control virus Ad-Del had no significant effect on the survival of cells with induced expression of various p73 isoforms.
Figure 1.
p73 isoforms have differential effects on the induction of cell death by E1A or Apoptin. (A) Saos-2 cells inducible for TAp73α, β, γ or ΔNp73α were infected with Ad-E1A or Ad-Del at a MOI of 10 in the presence or absence of doxycycline. Cell survival was measured by the MTT assay 24 (top) and 48 h post-infection (bottom). Results are shown as percentage of viable cells with respect to non-treated cells. All experiments were performed in triplicate, error bars indicate S.E. Asterisk represents significant difference of p less than 0.001 as compared with Ad-E1A-infected cells expressing ΔNp73α. (B) Saos-2 cells inducible for TAp73α, β, γ or ΔNp73α were infected with Ad-GFP-Apoptin or Ad-GFP at a MOI of 50 and 10, respectively, in the presence or absence of doxycycline. Cell survival was measured by the MTT assay 24 (top) and 48 h post-infection (bottom). Results are shown as percentage of viable cells with respect to non-treated cells. All experiments were performed in triplicate, error bars indicate S.E. Asterisk represents significant difference of p less than 0.001 as compared with Ad-GFP-Apoptin-infected cells expressing ΔNp73α. (C) Western blot analysis of Saos-2 cells inducible for TAp73α, β, γ or ΔNp73α infected with indicated recombinant adenoviruses and lysed 24 h post-infection, equal amounts of total protein from each sample was separated by SDS-PAGE, transferred to nitrocellulose membrane and hybridized to different antibodies as described in Materials and Methods. (D) Saos-2 cells inducible for TAp73α, β, γ or ΔNp73α were infected with Ad-GFP-Apoptin at a MOI of 50 in the presence of doxycycline. Cells were fixed 24 h post-infection and stained for p73 isoforms by immunofluorescent staining using mouse anti-HA primary antibody and Texas-Red-labelled secondary anti-mouse antibody. Cells were mounted in DAPI-containing mounting medium. p73 stained red and GFP-Apoptin stained green. Apoptin-p73 co-expressing cells showing apoptotic nuclei are indicated with white arrows. (E) Inhibitory effect of Ad-TAp73β on tumor growth. Five million HSC-3 cells were inoculated subcutaneously into female, nude mice. When the tumor reached 5–6 mm in diameter, mice were injected orthotopically with PBS, 2 × 109 pfu of either Ad-GFP-TAp73β or Ad-GFP according to the schedule described in Materials and Methods. Tumor size was measured twice weekly and expressed as mentioned in Materials and Methods. Experiments were performed twice and data points represent the mean change in tumor size relative to day 0 for each group of animals (n = 7). Error bars indicate S.D. Asterisk represents significant difference of p less than 0.001 as compared with PBS or Ad-GFP group.
We then investigated the effect of different p73 isoforms on the sensitivity of inducible Saos-2 cells to Apoptin. Cells were infected with either adenovirus expressing Apoptin fused to GFP (Ad-GFP-Apoptin) or the control adenovirus expressing GFP (Ad-GFP) (Fig. 1B). Similar to the effect observed with E1A, after 24 h, expression of Apoptin in cells induced for TAp73β and TAp73γ showed a significant reduction of cell survival as compared with Ad-GFP-Apoptin-infected cells induced for ΔNp73α (Fig. 1B, top; p < 0.001). At 48 h post-infection, a significant increase in cell sensitivity to Apoptin-induced cell death was observed in cells expressing TAp73 isoforms, in particular TAp73β (46.3%, 19% and 31.9% survival with the expression of TAp73α, TAp73β and TAp73γ, respectively). Furthermore, cell survival in those cells was significantly less than Ad-GFP-Apoptin-infected cells expressing ΔNp73α (62.8% cell survival, p < 0.001) (Fig. 1B, bottom). The control virus Ad-GFP had no significant effect on the survival of cells expressing various p73 isoforms. Western blot analysis at 24 h post-infection with Ad-GFP-Apoptin in the doxycycline-induced Saos-2 cells expressing TAp73α, TAp73β and TAp73γ, but not ΔNp73α, showed increased levels of the apoptotic marker PARP p85, as compared with non-induced, Ad-GFP-Apoptin-infected cells (Fig. 1C, Lanes 3 and 6). The level of cleaved PARP fragment increased with combined expression of Apoptin with TAp73α (Fig. 1C, upper left, Lanes 4 and 6) but not with TAp73β or TAp73γ (Fig. 1C, upper right and lower left, Lanes 4 and 6). This may be due to the TAp73β or TAp73γ isoforms being more potent inducers of apoptosis than TAp73α, therefore, the fragmentation of PARP reaching the maximum level at this time point. Indirect immunofluorescent study also showed increased nuclear fragmentation by DAPI staining in Saos-2 cells expressing the TAp73 isoforms, but not ΔNp73α (Fig. 1D).
Anti-tumor effects of Ad-TAp73β in head and neck cancer xenografts
The above experiments suggested the TAp73β to be the most potent TAp73 isoform in enhancing sensitivity to E1A or Apoptin-induced cell death; we therefore evaluated the potential therapeutic benefit of TAp73β in p53-mutated HSC-3 head and neck cancer xenografts. We injected HSC-3 cells (5 × 106) into the right flank of nude mice as described in Materials and Methods. Two weeks later, the mice were grouped (n = 7/group) and injected into tumors of 5–6 mm in diameter with either control (PBS), Ad-GFP or adenovirus carrying GFP tagged TAp73β (Ad-GFP-TAp73β) as described in Materials and Methods. As depicted in Figure 1E, tumor growth was significantly reduced in mice treated with Ad-GFP-TAp73β compared to PBS or the control Ad-GFP (p < 0.001).
E1A transactivates p73 and PUMA independently of the p53-binding sites in p53-deficient cancer cells
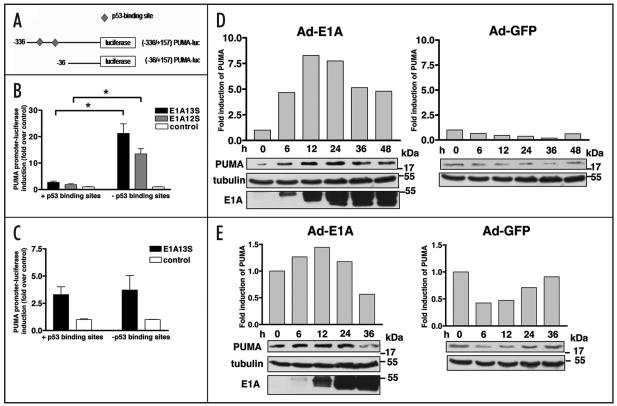
We have previously shown that E1A highly transactivates TAp73 and pro-apoptotic targets such as Noxa, a ‘BH3-only’ member of the Bcl-2 family.24 Here, we have investigated the effect of E1A on the transactivation of another ‘BH3-only’ family member, PUMA, an important p53 target activated by DNA damage.38 Two PUMA luciferase reporter constructs were used; one in which both putative p53-binding sites are intact, and one in which the p53-binding sites were deleted (Fig. 2A). SH-SY5Y neuroblastoma cells with aberrant p53 were co-transfected with PUMA reporters together with E1A13S, E1A12S or empty vector DNA and the luciferase activity was measured 24 h post-transfection. Both E1A13S and E1A12S expression were shown to activate luciferase expression from the PUMA promoter with deleted p53-binding sites by 21 and 13.5 folds, respectively. Unexpectedly, there was only a small increase in the luciferase activity from the PUMA promoter with intact p53-binding sites (2.7 and 1.8 folds by E1A13S and E1A12S, respectively) (Fig. 2B). To verify that the p53-binding independent induction of the PUMA promoter by E1A was not cell-type specific, the two PUMA reporters were expressed in the p53-null lung carcinoma H1299 cells together with E1A13S or empty vector DNA. At 24 h post-transfection, luciferase reporter assay showed a 3.3- to 3.7-fold activation of the PUMA promoter by E1A13S, irrespectively of the presence of the p53-binding sites (Fig. 2C). Taken together, these data indicate that the transactivation of the PUMA promoter by E1A is independent of the p53-binding sites.
Figure 2.
PUMA is induced by E1A. (A) Schematic representation of the human PUMA promoter. (B and C) Major isoforms of E1A activated the promoter of PUMA independently of the p53-binding sites in p53-deficient cancer cells. SH-SY5Y (B) and H1299 cells (C) were co-transfected with the PUMA luciferase reporters, either containing or lacking p53-binding sites, together with empty vector DNA, E1A13S or E1A12S plasmids. The luciferase activity was measured 24 h after transfection. Experiments were performed twice in triplicate, error bars indicate S.E. Asterisk represents significant difference of p less than 0.001. (D and E) E1A upregulated endogenous PUMA. Western blot showed the expression of PUMA, E1A and tubulin in SH-SY5Y (D) and H1299 cells (E) from cell lysates collected at indicated time points after infection with Ad-E1A or Ad-GFP at a MOI of 10. The quantification of the expression of PUMA in SH-SY5Y cells and H1299 cells was determined by using Version 2.0 of Aida 2D Densitometry software and normalized with tubulin and the average values were shown in the histogram.
E1A induces the endogenous expression of PUMA
The transcriptional effect of E1A on the endogenous PUMA gene was further investigated in SH-SY5Y and H1299 cells by Western blotting. Cells were infected with Ad-E1A or control Ad-GFP at a MOI of 10 and were lysed at 0, 6, 12, 24, 36 and 48 h post-infection. PUMA protein expression was examined using a rabbit anti-PUMA antibody. In Ad-E1A-infected SH-SY5Y cells, a 4.7-fold increase in levels of PUMA protein was observed as early as 6 h post-infection compared with the baseline level at 0 h. The highest level of PUMA expression was observed at 12 h post-infection (8.3-fold), after which PUMA protein levels were reduced to 7.7, 5.1 and 4.8 folds at 24, 36 and 48 h, respectively (Fig. 2D, left). In agreement with the reporter gene assay, which showed an approximately three-fold transactivation of the PUMA promoter in H1299 cells (Fig. 2C), Western blot analysis showed a small increase in endogenous PUMA protein levels with the highest level of 1.4 fold at 12 h post-infection. At 36 h post-infection, when extensive apoptosis was clearly detectable, a downregulation of endogenous PUMA protein was detected (Fig. 2E, left). Co-transfection with Ad-GFP control did not result in increased levels of PUMA protein (Fig. 2D and E, right).
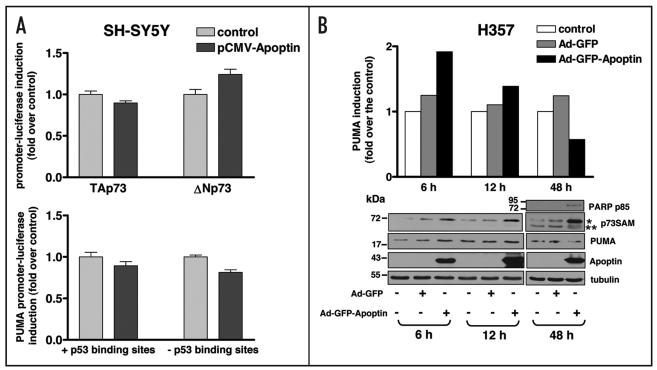
Apoptin induces expression of p73 and its downstream target PUMA
We next investigated whether, similar to E1A, Apoptin was able to induce p73 and its downstream pro-apoptotic targets including PUMA at the transcriptional and protein levels. SH-SY5Y cells were transfected with the luciferase reporter plasmids under the control of the TAp73, ΔNp73 or PUMA promoters in combination with pCMV-Apoptin or the control empty vector plasmid. Apoptin was found to have no effect on the transactivation of all the promoters tested (Fig. 3A). We then examined the effect of Apoptin on the induction of protein levels of p73 and PUMA in p53-mutated HNSCC cells, H357. This cell line was chosen because of its high sensitivity to Apoptin cytotoxicity. H357 cells were left either untreated or infected with Ad-GFP-Apoptin or a control Ad-GFP and total cell lysates were collected at 6, 12 and 48 h post-infection. Western blot analysis using anti-p73α antibody (p73SAM), which has been previously shown to react with only TAp73α and ΔNp73α but not with other p73 isoforms or with TAp63α,39 demonstrated increased levels of endogenous TAp73α as early as 6 h post-infection. The increase in the level of TAp73α was particularly high at 48 h post-infection. Interestingly, the level of anti-apoptotic protein ΔNp73α isoform was significantly reduced at 48 h post-infection (Fig. 3B). The sizes of TAp73α isoform and ΔNp73α isoform detected in H357 cells by anti-p73α antibody were comparable to those observed in Saos-2 cells inducible for TAp73α and ΔNp73α (Fig. 5C).
Figure 3.
p73 and PUMA are not transcriptionally activated but are induced at the protein level by Apoptin. (A) SHSY5Y cells were transfected with the luciferase reporter plasmids for TAp73, ΔNp73 or PUMA, together with empty vector DNA or pCMV-Apoptin plasmid. The luciferase activity was measured 24 h after transfection. Experiments were performed in triplicate, error bars indicate S.E. (B) H357 cells were non-infected or infected with either Ad-GFP-Apoptin or Ad-GFP. Cells were lysed at 6, 12 and 48 h after infection, equal amounts of total protein from each sample was separated by SDS-PAGE, transferred to nitrocellulose membrane and hybridized to different antibodies as described in Materials and Methods. The intensity of tubulin and PUMA bands from the Western blot was measured using Version 2.0 of Aida 2D Densitometry software. The amount of endogenous PUMA protein was normalized with tubulin expression level to correct for loading differences. The fold induction of PUMA expression of H357 cells infected with Ad-GFP-Apoptin or Ad-GFP was calculated with respect to the PUMA expression of control non-infected H357 cells which was set at 1 and the values were shown in the histogram. Asterisks (* and **) next to the p73 blots indicate the positions of TAp73α and ΔNp73a recognized by anti-p73α antibody, respectively.
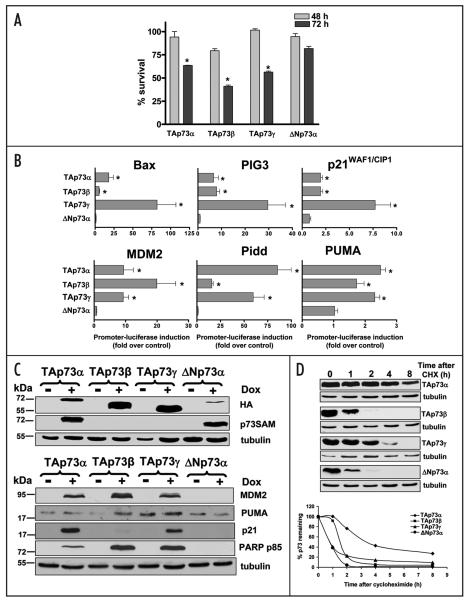
Figure 5.
Specific p73 isoforms have differences in the induction of apoptosis, transactivation ability and protein stability. (A) Saos-2 cells inducible for TAp73α, β, γ and ΔNp73α were treated with 2.5 μg/ml doxycycline. Cell survival was measured by the MTT assay at 48 and 72 h. Results are shown as percentage of viable cells with respect to non-treated cells. Experiments were performed in triplicate, and error bars indicate S.E. Asterisk represents significant difference of p less than 0.001 as compared with cells expressing ΔNp73α. (B) Inducible Saos-2 cell lines containing HA-tagged TAp73α, β, γ or ΔNp73α were induced with doxycycline following transfection with the luciferase reporter plasmids including Bax, PIG3, p21WAF1/CIP1, MDM2, Pidd or PUMA. The luciferase activity was measured 24 h post-transfection. The result is shown as fold induction compared to basal lucifer-ase level in inducible Saos-2 cells without doxycycline treatment after normalized by Renilla value. Experiments were performed in triplicate and were repeated twice. Error bars indicate S.E. Asterisk represents significant difference of p less than 0.05 as compared with cells expressing ΔNp73α. (C) The protein expression of each p73 isoform in inducible Saos-2 cells. Top: TAp73α, β, γ or ΔNp73α-inducible Saos-2 cells were induced with 2.5 μg/ml doxycycline for 24 h. The protein of each p73 isoform obtained was subjected to Western blotting using anti-HA and anti-p73α antibodies. The same blot was stripped and reprobed with anti-tubulin for confirmation of equal protein loading. Bottom: TAp73α, β, γ or ΔNp73α-inducible Saos-2 cells were induced with 2.5 μg/ml doxycycline for 48 h and equal amounts of total protein from each sample was separated by SDS-PAGE, transferred to nitrocellulose membrane and hybridized using antibodies for MDM2, p21WAF1/CIP1, PUMA, PARP p85 and tubulin as described in Materials and Methods. (D) p73 isoforms show different protein stability. Saos-2 cells inducible for TAp73α, TAp73β, TAp73γ or ΔNp73α were induced with 2.5 μg/ml doxycycline for 24 h, followed by treating with 20 μg/ml cycloheximide (CHX). Cells were collected at the indicated time points (0, 1, 2, 4, 8 h) and cell lysates were subjected to Western blotting. The relative amount of p73 protein was evaluated by using Version 2.0 of Aida 2D Densitometry software and normalized to tubulin for correction of the protein loading.
The effect of Apoptin on p73 pro-apoptotic target, PUMA, was also examined. An almost 2-fold induction of endogenous PUMA was observed at 6 h after the expression of Apoptin and the level of PUMA was then reduced; however, remained higher than those in the control non-infected cells or Ad-GFP-infected cells, to 1.4 fold at 12 h post-infection. At 48 h after infection, the level of PUMA was reduced approximately 40% as compared with the control non-infected cells, this coincided with the clear presence of apoptosis at 48 h, detected by cleaved PARP p85 antibody, in cells expressing Ad-GFP-Apoptin (Fig. 3B). Expression of GFP in these cells did not have any significant effect on the protein level of both p73 and PUMA (Fig. 3B).
TAp73-compromised c-Abl knockout cells are resistant to E1A-induced transactivation of TAp73 and p53/p73 apoptotic targets
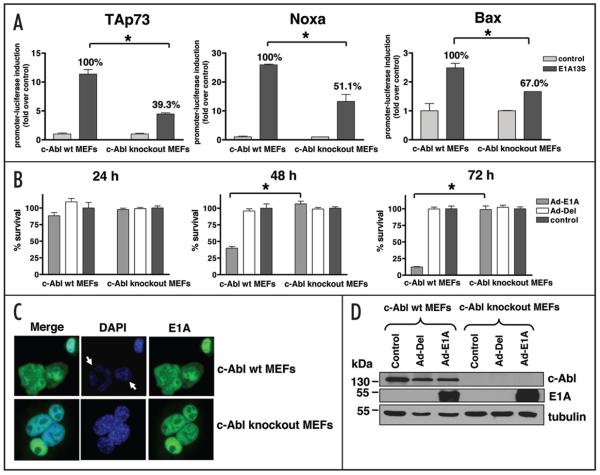
The direct involvement of p73 in E1A-induced apoptosis was investigated using c-Abl knockout MEFs. p73 protein has been shown to be stabilized and activated after DNA damage through a pathway involving the c-Abl tyrosine kinase.36,40,41 c-Abl knockout MEFs are therefore p73 function deficient and do not respond to treatment by chemotherapeutic drugs such as cisplatin.36 c-Abl wild-type and c-Abl knockout MEFs were transfected with the luciferase reporter plasmids for TAp73, Bax or Noxa in combination with a plasmid expressing E1A13S or empty vector DNA. In c-Abl wild-type MEFs, expression of E1A13S resulted in an 11-fold induction of the TAp73 promoter, while in c-Abl knockout cells, expression of E1A13S resulted in only a four-fold induction (a ~60% reduction compared to wild-type cells). Similar results were obtained with the Bax and Noxa reporters, with the ability of E1A to transactivate Bax and Noxa in c-Abl knockout MEFs being reduced by 33% and 49.9%, respectively, as compared with those in c-Abl wild-type cells (Fig. 4A).
Figure 4.
c-Abl knockout cells are resistant to E1A-induced transactivation of TAp73 and p53/p73 pro-apoptotic targets, and to the induction of cell death by E1A. (A) c-Abl wt and c-Abl knockout MEFs were transfected with the luciferase reporter plasmids for TAp73, Bax or Noxa in combination with E1A13S or empty vector DNA. The luciferase activity was measured at 24 h post-transfection and the result is shown as fold induction compared with basal luciferase level after transfection with empty vector DNA. Error bars indicate S.E. Asterisk represents significant difference of p less than 0.05. The percentages indicated in this figure are deduced from the level of promoter activation by E1A in c-Abl wt MEFs, which was arbitrarily set at 100%. (B) c-Abl wt MEFs and c-Abl knockout MEFs were infected with Ad-E1A or Ad-Del at a MOI of 10. Cell survival was measured by the MTT assay at 24, 48 and 72 h post-infection. Results are shown as percentage of viable cells with respect to non-infected cells. Infection of Ad-E1A, not of Ad-Del, resulted in substantial cell death in c-Abl wt MEFs particularly at 48 and 72 h. In contrast, Ad-E1A was not able to kill c-Abl knockout MEFs. Experiments were performed twice in triplicate, error bars indicate S.E. Asterisk represents significant difference of p less than 0.0001. (C) c-Abl wt MEFs and c-Abl knockout MEFs were infected with Ad-E1A at a MOI of 10. Cells were fixed 48 h post-infection and stained for E1A by immunofluorescent staining using mouse anti-E1A clone M58 primary antibody and FITC-labelled secondary anti-mouse antibody. Cells were mounted in DAPI-containing mounting medium. E1A stained green and white arrows indicate E1A-expressing c-Abl wt MEFs showing apoptotic nuclei. (D) c-Abl wt MEFs and c-Abl knockout MEFs were non-infected or infected with either Ad-Del or Ad-E1A. Cells were lysed at 48 h after infection, equal amounts of total protein from each sample was separated by SDS-PAGE, transferred to nitrocellulose membrane and hybridized to different antibodies as described in Materials and Methods.
TAp73 function is required for E1A-induced cell death
We further examined the induction of cell death by E1A in c-Abl wild-type and c-Abl knockout MEFs. Cells were infected with either Ad-E1A or control Ad-Del and survival was measured by the MTT assay at 24, 48 and 72 h post-infection. Substantial reduction in cell survival was observed in c-Abl wild-type MEFs infected with Ad-E1A, with 60% and 90% reduction observed at 48 and 72 h post-infection, respectively. In contrast, c-Abl knockout MEFs appeared to be resistant to E1A-induced apoptosis as almost 100% of cells infected with Ad-E1A survived suggesting that the c-Abl/p73 pathway is crucial for E1A-induced apoptosis (Fig. 4B and Suppl. Fig. 1). To confirm that this was not due to cell-type differences in the efficiency of transfection and expression of the transgene, the expression of E1A in c-Abl wild-type and c-Abl knockout MEFs were detected by immunofluorescent staining and Western blotting (Fig. 4C and D). Both cell lines appeared to have similar levels of E1A expression (detected in green in Fig. 4C) and E1A was expressed mainly in the nucleus in both cell lines. Figure 4C demonstrated apoptotic nuclei detected by DAPI staining in E1A-expressing c-Abl wild-type MEFs whereas normal nuclear morphology was observed in E1A-expressing c-Abl knockout MEFs.
Specific p73 isoforms show important differences in efficiency to induce apoptosis, transactivation of target genes and protein stability
The above results demonstrated that p73 isoforms were different in response to cell death induced by both E1A and Apoptin. We therefore examined the different characteristics of specific p73 isoforms in relation to the ability to induce apoptosis, transactivation of target genes and protein stability among p73 isoforms.
The ability of TAp73 expression in inducing efficient apoptosis in p53-deficient cells was investigated using doxycycline-inducible Saos-2 cells for specific p73 isoforms. The MTT assay (Fig. 5A) showed that 72 h after doxycycline treatment cell viability in the TAp73-positive cells was significantly reduced compared with non-induced or cells expressing ΔNp73α (p < 0.001). TAp73β showed the highest induction of apoptosis (41% cell survival) followed by TAp73γ (56.4% cell survival) and TAp73α (63.5% cell survival). In contrast, ΔNp73α had an insignificant effect on the induction of cell death. Furthermore, we performed the transient transfection study to further confirm the efficient ability of TAp73 in inducing apoptosis in a p53-independent manner. Saos-2 cells were transiently transfected with plasmids encoding TAp73α, β, γ, δ or ΔNp73α and cells were analyzed at 24 and 48 h post-transfection. Indirect immunofluorescence studies showed that each p73 isoform was expressed mainly in the nucleus although cells expressing TAp73α showed cytoplasmic expression at 24 h (Suppl. Fig. 2A and B). All TAp73 isoforms, but not the dominant negative ΔNp73α, significantly induced cell death in Saos-2 cells (p < 0.05). The percentages of apoptotic cells induced by TAp73 isoforms varied from 10.9 to 26.6 (Suppl. Fig. 2A–C).
Specific p53/p73 targets have been shown to be differentially activated by various p73 isoforms.8 In this study we used the inducible Saos-2 cells to investigate the effect of each p73 isoform on the activation of several p53/p73 targets. Saos-2 cells expressing the doxycycline-inducible p73 isoforms were transfected with the luciferase reporter plasmids for Bax, PIG3, Pidd, MDM2, p21WAF1/CIP1 and PUMA and the luciferase activity was measured after 24 h. Expression of each p73 isoform was confirmed by Western blot analysis after 24 h-doxycycline treatment (Fig. 5C).
As shown in Figure 5B, induction of TAp73γ isoform substantially increased Bax and PIG3 promoter activity by 82 and 30 folds, respectively, and resulted in an almost eight-fold increase in the activity of the p21WAF1/CIP1 promoter. TAp73β expression resulted in about 20-fold increase in the MDM2 promoter activity, while the Pidd promoter was highly induced by both TAp73α and TAp73γ by 85 and 60 folds, respectively. Expression of either TAp73α or TAp73γ resulted in a 2.5- and a 2.3-fold induction of the PUMA promoter. In contrast, overexpression of ΔNp73α had no effect on transcription from any of the promoters tested. In agreement with the luciferase reporter assays, Western blot analysis demonstrated the induction of p53 downstream targets including p21WAF1/CIP1, MDM2 and PUMA by all three TAp73 isoforms tested, whereas ΔNp73α expression failed to induce those targets. However, there were some differences in the levels of induction of various targets between the luciferase and Western blotting methods. This could be due to the effect of post-translational modification and/or protein stability of tested targets. All TAp73 isoforms-expressing Saos-2 cells underwent apoptosis but not in cells expressing ΔNp73α (Fig. 5C). In agreement with the MTT assay, the TAp73β and TAp73γ expression were more apoptotic than TAp73α as evident from PARP p85 levels.
Several studies have investigated the mechanisms involved in the regulation of p73 activities and protein stabilities. The stability of p73 proteins, which in most cases have been studied under conditions of transient overexpression, varies among different studies.22,42 We chose to investigate the half-life of TAp73α, TAp73β, TAp73γ and ΔNp73α when expressed from stably transfected inducible constructs in Saos-2 cells.
As shown in Figure 5D, TAp73α had the longest half-life, followed by TAp73β, TAp73γ and ΔNp73α. Over 50% of TAp73α protein remained undegraded at approximately 3.5 h after cycloheximide treatment, while TAp73β had a half-life of almost 1.5 h and TAp73γ and ΔNp73α had turnovers of less than 50 min. At 4 h after cycloheximide treatment, 40% of TAp73α protein remained intact, whereas the other three isoforms were barely detectable.
Discussion
Many viral proteins including E1A and Apoptin have been shown to be effective inducers of death in cancer cells (reviewed in refs. 25 and 34). This is partly due to the fact that these proteins preferentially kill tumor over normal cells. Apoptin is known to induce apoptosis in a variety of tumor cell lines, leaving normal cells mostly unaffected.32,35,43 E1A induces cytotoxicity in tumors which overexpress certain oncogenes such as EGFR and c-erbB2.31,44 The tumor selective toxicity of these viral proteins makes them important candidates for anti-cancer therapy. We and others have shown that the induction of apoptosis by both E1A and Apoptin is independent of the p53 function.30-33 This is an important feature for an efficient tumor killing as many tumors have either lost or mutated p53 expression. However, how exactly E1A and Apoptin induce tumor apoptosis and why these proteins show tumor selectivity remains unknown.
We previously reported that TAp73 function is required for the sensitivity of head and neck cancers to chemotherapy.23 In addition, we found that E1A expression transactivates TAp73 and a series of p53 target genes including p21WAF1/CIP1, Bax and Noxa, independently of the p53 status.24 However, the importance of p73 function in E1A-induced cell death has not yet been elucidated. In this study, we have investigated the role of p73 function in cell death induced by both E1A and Apoptin.
We observed diminished cell death induction by E1A, when p73 was inhibited by the expression of the dominant negative isoform, ΔNp73, or by using c-Abl knockout MEFs in which the function of p73 is impaired. Furthermore, E1A-induced transcriptional activation of TAp73 and p53 pro-apoptotic targets, including Bax and Noxa was significantly reduced in c-Abl knockout cells, suggesting that phosphorylation and hence activation of p73 by c-Abl is important for the transactivation function of E1A. To prove that p73 was necessary for E1A-induced cell death, we started to use p73 knockout MEFs (obtained from Gerry Melino). However, these cells exhibited early senescence resulting in inconclusive data.
We found that in addition to Bax and Noxa, E1A also activated the promoter of the pro-apoptotic BH3-only protein and p53/p73 target PUMA, in p53-deficient cells. To our knowledge, this is the first evidence of a direct transactivation of PUMA by E1A. Interestingly, E1A expression activated the PUMA promoter more efficiently when p53-binding sites were deleted. This was shown in the neuroblastoma SH-SY5Y cell line which expressed wild-type p53 but harbouring a PUMA promoter construct lacking p53-binding sites. By contrast, PUMA promoter activation was similar in p53-deficient H1299 cells irrespective of the presence or absence of p53-binding sites in the PUMA promoter. These data clearly show a p53-independent transactivation of PUMA by E1A. A direct, p53-independent, activation of PUMA by E2F1 has been previously demonstrated.45 In addition, a recent study has shown that the promoter of PUMA contains a set of 10 potential binding sites for Sp family proteins in the proximal region between nucleotides −120 and −8.46 This area partially overlaps with the PUMA promoter construct lacking the p53-binding sites used in our study. Additionally, E1A13S was reported to activate the p21WAF1/CIP1 promoter through the Sp1-binding sites.47 Therefore, the observed p53-independent transactivation of PUMA by E1A may be partially due to the direct interaction of E1A with the Sp1-binding sites in the PUMA promoter. Whether this is the case or E1A indirectly affects the PUMA promoter via other transcriptional regulators remains to be elucidated. In accordance with our promoter transactivation assay, endogenous PUMA protein levels were increased in E1A-infected cells, with the highest level detected in SH-SY5Y cells. In H1299 cells, PUMA induction was lower, which might have been due to our recent finding that E1A was incapable of transactivating p73 in these cells.24 In both cell lines, maximum expression levels of PUMA in response to Ad-E1A infection were observed at 12 h. This time was before the execution of cell death indicating that PUMA induction occurred at an early stage of apoptosis and might therefore be crucial for the E1A-induced apoptotic response. Conversely, when apoptosis was prominent, particularly in H1299 cells, the level of PUMA started to decline. This is in agreement with Rossi et al.48 who suggested that PUMA is induced by p73 before apoptosis, and that it mediates the conformational changes of Bax, resulting in its activation and subsequent mitochondrial translocation. Indeed, both E1A and Apoptin have been shown to induce cell death via the intrinsic mitochondrial pathway.49,50 As other BH3-only proteins PUMA is known to function upstream of Bax and Bak to cause the permeabilization of the mitochondrial outer membrane, leading to cytochrome c release, caspase-3 activation and apoptosis. Thus, PUMA is very likely to be a crucial mediator of the apoptotic response to E1A and Apoptin. Final proof has to be obtained from cell death analysis in PUMA knockout or knockdown (si/shRNA) cells.
Another important finding in our study is that p73 also plays a major role in the p53-independent cell death in response to Apoptin. We and others have shown that Apoptin specifically induces apoptosis in tumor cells,32,35 but the exact death signaling pathway implicated has not yet been uncovered. Apoptosis induction by Apoptin is independent of the functions of p53 and pRB,34 and the mitochondrial pathway requiring caspase-3 rather than caspase-8 seems to be involved.34 Here, we observed that the expression of Apoptin in HNSCC cells resulted in a significant increase in the levels of endogenous TAp73 and a reduction in the expression of ΔNp73. Luciferase reporter assays indicated that, unlike E1A, which transactivated TAp73 and p53 apoptotic targets, Apoptin expression had no significant effect on the p73 promoter. Strikingly, however, Apoptin increased the protein levels of TAp73 and the p53 pro-apoptotic target, PUMA. These data suggested a higher stabilization of TAp73 due to a possible Apoptin-induced post-translational modification rather than a transcriptional control. Furthermore, exogenous expression of TAp73, particularly TAp73β, significantly enhanced Apoptin-induced apoptosis in Saos-2 cells. By contrast, ΔNp73 failed to sensitize cells to cell death induced by Apoptin. Interestingly, we noted an association between TAp73 induction and an increased phosphorylation of Apoptin detected by the phospho-specific anti-Apoptin antibody (Fig. 3B). It will therefore be interesting to find out whether TAp73 is involved in the phosphorylation and activation of Apoptin, thus explaining how p73 sensitizes cells to killing by Apoptin.
As p73 is expressed as different isoforms in both normal and tumor cells, it was important to study how each isoform contributes to cell death induced by E1A and Apoptin. By using a regulated doxycycline-inducible expression system of p73 isoforms in Saos-2 cells, we found that although all three isoforms (α, β, γ) induced apoptosis, the β isoform of TAp73 was the most efficient, as has previously been suggested.51 Western blot analysis revealed that TAp73β was the most potent inducer of PARP cleavage (a read-out for caspase-3/7 activation). Moreover, among the isoforms, TAp73β was the strongest in activating PUMA and decreasing survival based on the MTT assay. Finally, Ad-TAp73β also markedly inhibited the growth of p53-mutated head and neck cancer xenografts, a finding that may be exploited for the development of p73-based drugs for anti-cancer therapy. TAp73 isoforms can transactivate a number of p53 target genes including p21WAF1/CIP1, RGC, MDM2, Bax, cyclin G, GADD45, IGF-BP3 and 14-3-3.6-9 Lee and La Thangue19 demonstrated differences in the transcriptional activity between p73α and β isoforms for cellular targets, including GADD45 and MDM2. In our study, we used transient transactivation assays and doxycycline-inducible expression of specific p73 isoforms to show significant differences in target gene activation between the various p73 isoforms. The TAp73β isoform efficiently activated the MDM2 promoter, while the TAp73γ isoform strongly transactivated p21WAF1/CIP1. The g isoform strongly transactivated Bax and PIG3, while both the TAp73α and the TAp73γ isoforms strongly activated the Pidd promoter, but only weakly activated the PUMA promoter. These data suggest that the promoter selectivity of specific p73 isoforms may be an important determinant for the cellular response to specific genotoxic stresses. Western blot analysis confirmed the induction of p53 target protein levels could be due to the effect of post-translational modifications including protein stability, cell cycle time, etc.
Inconsistent results have been obtained with respect to the stability of different p73 protein isoforms.19,22,42,52 Here, we found by using the doxycycline-inducible expression system that the TAp73α isoform was the most stable protein followed by TAp73β, TAp73γ and ΔNp73α. This is in contrast to Lee and La Thangue19 who reported that p73β is considerably more stable than the α isoform, and that the difference may reside in the COOH-terminal region which appears to be crucial for regulating its protein stability. However, in support of our finding, a recent study has reported that RanBPM binds to the COOH-terminal region of p73α and inhibits its ubiquitination, resulting in its increased stability.53 It was also shown that the ΔN isoforms of p73 are more stable than the TAp73 isoforms.22 However, we found that the ΔNp73 isoform had the shortest protein half-life. In addition, Apoptin-induced cell death correlated with a reduction in the level of ΔNp73α. This is in agreement with the reported observation that, unlike p53 and TAp73, ΔNp73 is selectively and rapidly degraded after DNA damage.54 We think that the differences seen in the stability of p73 isoforms might be due to different cell types and variable experimental conditions. Indeed, the post-translational modifications of p73 are multifactorial, including ubiquitinations, phosphorylations, prolyl-isomerizations, acetylations, neddylations and the recruitment into PML nuclear bodies.17,55 It is so far unclear how all these modifications affect p73 function. Irrespective of this knowledge, there seems to be no clear correlation between the stability of different p73 isoforms and their cooperation with viral proteins in inducing cell death. Further studies on the mechanisms and pathways involved in the control of p73 protein stability are needed in order to understand the importance of p73 isoform-specific stability in response to genotoxic stresses including viral proteins.
In summary, our data show that for their apoptotic response, the viral proteins E1A and Apoptin affect p73 isoforms by different mechanisms. While E1A activates specific p73 isoforms and their transcriptional downstream targets, at both the transcriptional and protein level, Apoptin only affects the protein levels of p73 isoforms, possibly due to protein stabilization. Nevertheless, both viral proteins significantly increase expression of the pro-apoptotic BH3-only protein PUMA, possibly through the activation of TAp73 and independently of the p53 status of the cell. Future studies will be designed to understand the role of PUMA in apoptosis induced by these viral proteins. Interestingly, PUMA has been shown to be a crucial target in the apoptosis induced by HIV,56 which further emphasizes its importance in the response to different viral proteins, with diverse functions. It will be interesting to find out whether p73 and PUMA are involved in the sensitivity of cancer cells to a variety of pro-apoptotic viruses, including oncolytic adenovirus. In conclusion, this study identifies TAp73 and its target PUMA as important, p53-independent, mediators of E1A and Apoptin-induced apoptosis. Moreover, we have established that cellular responses to these viral proteins very much depend on the p73 isoform at play.
Materials and Methods
Cell lines and plasmids
Osteosarcoma cell line Saos-2, wild-type or knockout c-Abl MEFs,57 neuroblastoma SH-SY5Y, non-small cell lung cancer cell line H1299, head and neck cancer cell line HSC-3 and human primary embryonal kidney 293A adenoviral packaging cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FCS, 50 μg/ml streptomycin, 100 μg/ml penicillin and 1 mM sodium pyruvate, all purchased from Sigma (Gillingham, UK). HNSCC cell line H357 was cultured in Nut Mix (Invitrogen, Paisley, UK) supplemented with 5% FCS, 4 mM L-glutamine, 25 μg/l hydrocortisone, 50 μg/ml streptomycin, 100 μg/ml penicillin and 1 mM sodium pyruvate. TAp73α, β, γ and ΔNp73α-inducible Saos-2 cells58 were grown in RPMI medium with 10% tetracycline free FCS, G418 0.5 mg/ml, hygromycin 0.25 mg/ ml and induced with 2.5 μg/ml doxycycline for 24 h. All of the p73 isoforms were haemagglutinin (HA) tagged. Cultures were incubated at 37°C and 5% CO2.
The pcDNA3-TAp73α, β, γ, δ and ΔNp73α, all are HA-tagged, were obtained from Prof. Gerry Melino. pcDNA4-HA-E1A13S and pcDNA4-HA-E1A12S have been described elsewhere.24 The TAp73 and ΔNp73 luciferase reporter plasmids and luciferase reporter constructs of p53/p73 target genes were provided by Prof. Gerry Melino and Prof. Thierry Soussi (Institut Curie and Université P. and M. Curie, Laboratoire de Génotoxicologie des Tumeurs, Paris, France) and have been described elsewhere.24 The PUMA-luciferase constructs were from Prof. Bert Vogelstein (The Howard Hughes Medical Institute and Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore, MD).59 pCMV-Apoptin expression plasmid has been described elsewhere.32 The following replication-incompetent adenoviruses were used: AdE1A (dl324) containing functional E1A (both E1A12S and E1A13S) but with complete deletion of E1B and E3, and Ad-Del (dl312) as a control containing a complete deletion of E1A and E3.37 Ad-GFP-TAp73β expressing TAp73β fused to GFP was obtained from Prof. Karen Vousden (Beatson Institute for Cancer Research, Glasgow, UK). Ad-GFP was obtained from Prof. Bert Vogelstein. Ad-GFP-Apoptin has been described previously.60
Adenovirus amplification and purification
Approximately 1 × 109 293A cells were infected with different adenovirus constructs. Cells were harvested when a complete cytopathic effect was observed, i.e., 95–100% of the cells were rounded and 5–10% were floating. Cells were lifted from the plate by pipetting gently and collected by centrifuging 10 min at 200×g. Purification and titration of the adenovirus was essentially done as described previously.61
MTT cell proliferation assay
Cell survival was measured by the MTT assay as described previously.31
Transient transfection and fluorescence microscopy
Cells were seeded in Falcon 8-well culture slides (Becton Dickinson, Oxford, UK) and transfected at 50–80% confluency with 400 ng plasmid DNA preincubated with 1.4 μl LipofectAMINE 2000 (Invitrogen). Cells were washed in PBS and directly fixed in 2% paraformaldehyde for 30 min and permeabilized in 0.2% Triton X-100 for 15 min at room temperature. p73 expression was detected by anti-HA monoclonal antibody (Sigma) followed by goat anti-mouse IgG (whole antibody) fluorescein isothiocyanate conjugate (FITC) (Sigma). For Saos-2 inducible cells, p73 expression was detected by anti-HA monoclonal antibody (Sigma) followed by horse anti-mouse Texas Red (Vector Laboratories, Peterborough, UK). Subsequently, cells were mounted in Vectashield mounting medium (Vector Laboratories) containing 4′,6-diamidino-2-phenylindole (DAPI), for nuclei staining. Cells were visualized using an Olympus AX70 fluorescence microscope at high power (x400).
Luciferase reporter gene assays
Cells were seeded at 4 × 104 cells per well in 96-well plates. Cells were transfected in the next day with 40 ng/well of the various luciferase reporter plasmid and 200 ng of inducer expression plasmid preincubated with 0.6 μl LipofectAMINE 2000 in 80 μl DMEM without FCS and antibiotics. In doxycycline-inducible Saos-2 cells harbouring the various p73 isoforms, cells were transfected with 40 ng/well of reporter construct followed by treatment with 2.5 μg/ml doxycycline. For normalizing transfection efficiency, 13.2 ng of the pRL-null Renilla luciferase reporter (Promega, Southampton, UK) was transfected into each well. At 24 h post-transfection, the medium was removed and the firefly and Renilla luciferase activities were sequentially assayed using Dual-Glo reagents (Promega) in a Wallac Trilux 1450 luminometer (PerkinElmer Life Sciences, Massachusetts, USA).
Western blot analysis
Cells were washed in cold PBS and lysed in Laemmli Sample buffer (62.5 mM Tris-Cl pH 6.7, 100 mM β-mercaptoethanol, 2% SDS, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 100 μg/ml phenylmethylsulfonylfluoride). The lysate was passed through a 25G needle and boiled for 5 min. Lysates were resolved by gel electrophoresis for 1.5–2 h at 80 volts on 10–12% SDS-polyacrylamide gels. Western blotting was performed as previously described.62 The antibodies used for Western blot analysis were: mouse anti-E1A clone M58 (Pharmingen, BD Biosciences, Oxford, UK), mouse anti-HA (Sigma), rabbit anti-p73α (p73SAM)39 (obtained from Prof. Gerry Melino), mouse anti-Abl (Pharmingen, BD Biosciences), rabbit anti-PUMA (abcam®, Cambridge, UK), mouse anti-tubulin (Sigma), mouse anti-MDM2 (Oncogene Research Products, Calbiochem, Nottingham, UK), mouse anti-p21WAF1/CIP1 clone EA10 (Oncogene Research Products, Calbiochem), rabbit anti-phospho-Apoptin (T108) (anti-108-P) (Eurogentec, Seraing, Belgium), rabbit anti-PARP p85 fragment clone G734A (Promega), secondary anti-mouse (Sigma) and anti-rabbit antibodies linked to horseradish peroxidase (Amersham, UK).
Determination of p73 half-life
p73 protein turnover was determined by adding 20 μg/ml cycloheximide to TAp73α, β, γ or ΔNp73α-inducible Saos-2 cell lines 24 h after induction with 2.5 μg/ml doxycycline. Protein levels were determined by collecting cells at the different time points and performing Western blotting as described above. The relative amount of p73 protein was evaluated by using Version 2.0 of Aida 2D Densitometry software and normalized to tubulin.
Animal study
Six-week-old female BALB/c nude mice were injected with 5 million HSC-3 head and neck cancer cells in 0.1 ml PBS. Cells were injected subcutaneously into the right flank of each mouse. Animals were monitored twice weekly for tumor growth. Two weeks later when tumor size reached 5–6 mm in diameter, mice were placed into groups of seven and were injected orthotopically with either PBS, 2 × 109 pfu of Ad-GFP or Ad-GFP-TAp73β into several areas within the tumor mass. Tumor growth was monitored twice weekly. The tumors were measured with calipers for two perpendicular diameters and tumor size was calculated based on average dimensions.
Statistical analysis
For statistical analyses, the Student's t-test or one-way analysis of variance was carried out. Statistically significant difference was defined as p < 0.05.
Supplementary Material
Acknowledgements
We are grateful to Dr. Ygal Haupt for providing c-Abl knockout MEFs and their wild-type counterparts, Prof. Joe Mymryk for providing E1A13S and E1A12S plasmids, Prof. Thierry Soussi for providing luciferase reporter constructs, Prof. Bert Vogelstein for providing PUMA-luciferase reporter constructs and Ad-GFP, Prof. Karen Vousden for providing Ad-GFP-TAp73β, Profs. Farzin Farzaneh, Christoph Borner and Sydney Shall for the critical and helpful reading of this manuscript. This work was supported by grants from Cancer Research UK and the Rosetrees Trust. Marcella Flinterman is supported by a grant from Cancer Research UK (C1116). Poramaporn Klanrit is supported by a Royal Thai Government Scholarship.
Abbreviations
- Ad
adenovirus
- CHX
cycloheximide
- DAPI
4′,6-diamidino-2-phenylindole
- DMEM
Dulbecco's modified Eagle's medium
- Dox
doxycycline
- E1A
early region 1A
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- GFP
green fluorescent protein
- HA
influenza A virus haemagglutinin
- HNSCC
human head and neck squamous cell carcinoma
- MEFs
mouse embryo fibroblasts
- MOI
multiplicity of infection
- MTT
3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide
- PBS
phosphate-buffered saline
- PUMA
p53 upregulated modulator of apoptosis
- SDS-PAGE
lauryl sulphate-polyacrylamide gel electrophoresis
- wt
wild-type
Footnotes
Note
Supplementary materials can be found at: www.landesbioscience.com/supplement/KlanritCC7-2-Sup.pdf
References
- 1.Kaghad M, Bonnet H, Yang A, Creancier L, Biscan JC, Valent A, et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809–19. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- 2.De Laurenzi V, Costanzo A, Barcaroli D, Terrinoni A, Falco M, Annicchiarico-Petruzzelli M, et al. Two new p73 splice variants, gamma and delta, with different transcriptional activity. J Exp Med. 1998;188:1763–8. doi: 10.1084/jem.188.9.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melino G, De Laurenzi V, Vousden KH. p73: Friend or foe in tumorigenesis. Nat Rev Cancer. 2002;2:605–15. doi: 10.1038/nrc861. [DOI] [PubMed] [Google Scholar]
- 4.Ishimoto O, Kawahara C, Enjo K, Obinata M, Nukiwa T, Ikawa S. Possible oncogenic potential of DeltaNp73: a newly identified isoform of human p73. Cancer Res. 2002;62:636–41. [PubMed] [Google Scholar]
- 5.Grob TJ, Novak U, Maisse C, Barcaroli D, Luthi AU, Pirnia F, et al. Human delta Np73 regulates a dominant negative feedback loop for TAp73 and p53. Cell Death Differ. 2001;8:1213–23. doi: 10.1038/sj.cdd.4400962. [DOI] [PubMed] [Google Scholar]
- 6.Zeng X, Chen L, Jost CA, Maya R, Keller D, Wang X, et al. MDM2 suppresses p73 function without promoting p73 degradation. Mol Cell Biol. 1999;19:3257–66. doi: 10.1128/mcb.19.5.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jost CA, Marin MC, Kaelin WG., Jr p73 is a simian [correction of human] p53-related protein that can induce apoptosis. Nature. 1997;389:191–4. doi: 10.1038/38298. [DOI] [PubMed] [Google Scholar]
- 8.Zhu J, Jiang J, Zhou W, Chen X. The potential tumor suppressor p73 differentially regulates cellular p53 target genes. Cancer Res. 1998;58:5061–5. [PubMed] [Google Scholar]
- 9.Di Como CJ, Gaiddon C, Prives C. p73 function is inhibited by tumor-derived p53 mutants in mammalian cells. Mol Cell Biol. 1999;19:1438–49. doi: 10.1128/mcb.19.2.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pozniak CD, Radinovic S, Yang A, McKeon F, Kaplan DR, Miller FD. An anti-apoptotic role for the p53 family member, p73, during developmental neuron death. Science. 2000;289:304–6. doi: 10.1126/science.289.5477.304. [DOI] [PubMed] [Google Scholar]
- 11.Stiewe T, Theseling CC, Putzer BM. Transactivation-deficient Delta TA-p73 inhibits p53 by direct competition for DNA binding: implications for tumorigenesis. J Biol Chem. 2002;277:14177–85. doi: 10.1074/jbc.M200480200. [DOI] [PubMed] [Google Scholar]
- 12.Fillippovich I, Sorokina N, Gatei M, Haupt Y, Hobson K, Moallem E, et al. Transactivation-deficient p73alpha (p73Deltaexon2) inhibits apoptosis and competes with p53. Oncogene. 2001;20:514–22. doi: 10.1038/sj.onc.1204118. [DOI] [PubMed] [Google Scholar]
- 13.Zaika AI, Slade N, Erster SH, Sansome C, Joseph TW, Pearl M, et al. DeltaNp73, a dominant-negative inhibitor of wild-type p53 and TAp73, is upregulated in human tumors. J Exp Med. 2002;196:765–80. doi: 10.1084/jem.20020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uramoto H, Sugio K, Oyama T, Nakata S, Ono K, Morita M, et al. Expression of deltaNp73 predicts poor prognosis in lung cancer. Clin Cancer Res. 2004;10:6905–11. doi: 10.1158/1078-0432.CCR-04-0290. [DOI] [PubMed] [Google Scholar]
- 15.Casciano I, Mazzocco K, Boni L, Pagnan G, Banelli B, Allemanni G, et al. Expression of DeltaNp73 is a molecular marker for adverse outcome in neuroblastoma patients. Cell Death Differ. 2002;9:246–51. doi: 10.1038/sj.cdd.4400993. [DOI] [PubMed] [Google Scholar]
- 16.Concin N, Becker K, Slade N, Erster S, Mueller-Holzner E, Ulmer H, et al. Transdominant DeltaTAp73 isoforms are frequently upregulated in ovarian cancer. Evidence for their role as epigenetic p53 inhibitors in vivo. Cancer Res. 2004;64:2449–60. doi: 10.1158/0008-5472.can-03-1060. [DOI] [PubMed] [Google Scholar]
- 17.Oberst A, Rossi M, Salomoni P, Pandolfi PP, Oren M, Melino G, et al. Regulation of the p73 protein stability and degradation. Biochem Biophys Res Commun. 2005;331:707–12. doi: 10.1016/j.bbrc.2005.03.158. [DOI] [PubMed] [Google Scholar]
- 18.Muller M, Schilling T, Sayan AE, Kairat A, Lorenz K, Schulze-Bergkamen H, et al. TAp73/DeltaNp73 influences apoptotic response, chemosensitivity and prognosis in hepatocellular carcinoma. Cell Death Differ. 2005 doi: 10.1038/sj.cdd.4401774. [DOI] [PubMed] [Google Scholar]
- 19.Lee CW, La Thangue NB. Promoter specificity and stability control of the p53-related protein p73. Oncogene. 1999;18:4171–81. doi: 10.1038/sj.onc.1202793. [DOI] [PubMed] [Google Scholar]
- 20.Ueda Y, Hijikata M, Takagi S, Chiba T, Shimotohno K. New p73 variants with altered C-terminal structures have varied transcriptional activities. Oncogene. 1999;18:4993–8. doi: 10.1038/sj.onc.1202817. [DOI] [PubMed] [Google Scholar]
- 21.Melino G, Bernassola F, Ranalli M, Yee K, Zong WX, Corazzari M, et al. p73 Induces apoptosis via PUMA transactivation and Bax mitochondrial translocation. J Biol Chem. 2004;279:8076–83. doi: 10.1074/jbc.M307469200. [DOI] [PubMed] [Google Scholar]
- 22.Wu L, Zhu H, Nie L, Maki CG. A link between p73 transcriptional activity and p73 degradation. Oncogene. 2004;23:4032–6. doi: 10.1038/sj.onc.1207538. [DOI] [PubMed] [Google Scholar]
- 23.Bergamaschi D, Gasco M, Hiller L, Sullivan A, Syed N, Trigiante G, et al. p53 polymorphism influences response in cancer chemotherapy via modulation of p73-dependent apoptosis. Cancer Cell. 2003;3:387–402. doi: 10.1016/s1535-6108(03)00079-5. [DOI] [PubMed] [Google Scholar]
- 24.Flinterman M, Guelen L, Ezzati-Nik S, Killick R, Melino G, Tominaga K, et al. E1A activates transcription of p73 and Noxa to induce apoptosis. J Biol Chem. 2005;280:5945–59. doi: 10.1074/jbc.M406661200. [DOI] [PubMed] [Google Scholar]
- 25.Frisch SM, Mymryk JS. Adenovirus-5 E1A: paradox and paradigm. Nat Rev Mol Cell Biol. 2002;3:441–52. doi: 10.1038/nrm827. [DOI] [PubMed] [Google Scholar]
- 26.Houweling A, van den Elsen PJ, van der Eb AJ. Partial transformation of primary rat cells by the leftmost 4.5% fragment of adenovirus 5 DNA. Virology. 1980;105:537–50. doi: 10.1016/0042-6822(80)90054-9. [DOI] [PubMed] [Google Scholar]
- 27.Mymryk JS. Tumor suppressive properties of the adenovirus 5 E1A oncogene. Oncogene. 1996;13:1581–9. [PubMed] [Google Scholar]
- 28.de Stanchina E, McCurrach ME, Zindy F, Shieh SY, Ferbeyre G, Samuelson AV, et al. E1A signaling to p53 involves the p19(ARF) tumor suppressor. Genes Dev. 1998;12:2434–42. doi: 10.1101/gad.12.15.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcellus RC, Teodoro JG, Wu T, Brough DE, Ketner G, Shore GC, et al. Adenovirus type 5 early region 4 is responsible for E1A-induced p53-independent apoptosis. J Virol. 1996;70:6207–15. doi: 10.1128/jvi.70.9.6207-6215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teodoro JG, Shore GC, Branton PE. Adenovirus E1A proteins induce apoptosis by both p53-dependent and p53-independent mechanisms. Oncogene. 1995;11:467–74. [PubMed] [Google Scholar]
- 31.Flinterman M, Gaken J, Farzaneh F, Tavassoli M. E1A-mediated suppression of EGFR expression and induction of apoptosis in head and neck squamous carcinoma cell lines. Oncogene. 2003;22:1965–77. doi: 10.1038/sj.onc.1206190. [DOI] [PubMed] [Google Scholar]
- 32.Guelen L, Paterson H, Gaken J, Meyers M, Farzaneh F, Tavassoli M. TAT-apoptin is efficiently delivered and induces apoptosis in cancer cells. Oncogene. 2004;23:1153–65. doi: 10.1038/sj.onc.1207224. [DOI] [PubMed] [Google Scholar]
- 33.Zhuang SM, Shvarts A, van Ormondt H, Jochemsen AG, van der Eb AJ, Noteborn MH. Apoptin, a protein derived from chicken anemia virus, induces p53-independent apoptosis in human osteosarcoma cells. Cancer Res. 1995;55:486–9. [PubMed] [Google Scholar]
- 34.Tavassoli M, Guelen L, Luxon BA, Gaken J. Apoptin: Specific killer of tumor cells? Apoptosis. 2005;10:717–24. doi: 10.1007/s10495-005-0930-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rohn JL, Zhang YH, Aalbers RI, Otto N, Den Hertog J, Henriquez NV, et al. A tumor-specific kinase activity regulates the viral death protein Apoptin. J Biol Chem. 2002;277:50820–7. doi: 10.1074/jbc.M208557200. [DOI] [PubMed] [Google Scholar]
- 36.Gong JG, Costanzo A, Yang HQ, Melino G, Kaelin WG, Jr., Levrero M, et al. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 1999;399:806–9. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- 37.Yan DH, Chang LS, Hung MC. Repressed expression of the HER-2/c-erbB-2 proto-oncogene by the adenovirus E1a gene products. Oncogene. 1991;6:343–5. [PubMed] [Google Scholar]
- 38.Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–8. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 39.Sayan AE, Paradisi A, Vojtesek B, Knight RA, Melino G, Candi E. New antibodies recognizing p73: comparison with commercial antibodies. Biochem Biophys Res Commun. 2005;330:186–93. doi: 10.1016/j.bbrc.2005.02.145. [DOI] [PubMed] [Google Scholar]
- 40.Agami R, Blandino G, Oren M, Shaul Y. Interaction of c-Abl and p73alpha and their collaboration to induce apoptosis. Nature. 1999;399:809–13. doi: 10.1038/21697. [DOI] [PubMed] [Google Scholar]
- 41.Yuan ZM, Shioya H, Ishiko T, Sun X, Gu J, Huang YY, et al. p73 is regulated by tyrosine kinase c-Abl in the apoptotic response to DNA damage. Nature. 1999;399:814–7. doi: 10.1038/21704. [DOI] [PubMed] [Google Scholar]
- 42.Maisse C, Guerrieri P, Melino G. p73 and p63 protein stability: the way to regulate function? Biochem Pharmacol. 2003;66:1555–61. doi: 10.1016/s0006-2952(03)00511-2. [DOI] [PubMed] [Google Scholar]
- 43.Danen-Van Oorschot AA, Fischer DF, Grimbergen JM, Klein B, Zhuang S, Falkenburg JH, et al. Apoptin induces apoptosis in human transformed and malignant cells but not in normal cells. Proc Natl Acad Sci USA. 1997;94:5843–7. doi: 10.1073/pnas.94.11.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu D, Wolf JK, Scanlon M, Price JE, Hung MC. Enhanced c-erbB-2/neu expression in human ovarian cancer cells correlates with more severe malignancy that can be suppressed by E1A. Cancer Res. 1993;53:891–8. [PubMed] [Google Scholar]
- 45.Hershko T, Ginsberg D. Upregulation of Bcl-2 homology 3 (BH3)-only proteins by E2F1 mediates apoptosis. J Biol Chem. 2004;279:8627–34. doi: 10.1074/jbc.M312866200. [DOI] [PubMed] [Google Scholar]
- 46.Koutsodontis G, Vasilaki E, Chou WC, Papakosta P, Kardassis D. Physical and functional interactions between members of the tumor suppressor p53 and the Sp families of transcription factors: importance for the regulation of genes involved in cell cycle arrest and apoptosis. Biochem J. 2005;389:443–55. doi: 10.1042/BJ20041980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Najafi SM, Li Z, Makino K, Shao R, Hung MC. The adenoviral E1A induces p21WAF1/CIP1 expression in cancer cells. Biochem Biophys Res Commun. 2003;305:1099–104. doi: 10.1016/s0006-291x(03)00905-7. [DOI] [PubMed] [Google Scholar]
- 48.Rossi M, Sayan AE, Terrinoni A, Melino G, Knight RA. Mechanism of induction of apoptosis by p73 and its relevance to neuroblastoma biology. Ann N Y Acad Sci. 2004;1028:143–9. doi: 10.1196/annals.1322.015. [DOI] [PubMed] [Google Scholar]
- 49.Danen-van Oorschot AA, van Der Eb AJ, Noteborn MH. The chicken anemia virus-derived protein apoptin requires activation of caspases for induction of apoptosis in human tumor cells. J Virol. 2000;74:7072–8. doi: 10.1128/jvi.74.15.7072-7078.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fearnhead HO, Rodriguez J, Govek EE, Guo W, Kobayashi R, Hannon G, et al. Oncogene-dependent apoptosis is mediated by caspase-9. Proc Natl Acad Sci USA. 1998;95:13664–9. doi: 10.1073/pnas.95.23.13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deyoung MP, Ellisen LW. p63 and p73 in human cancer: defining the network. Oncogene. 2007;26:5169–83. doi: 10.1038/sj.onc.1210337. [DOI] [PubMed] [Google Scholar]
- 52.Slade N, Zaika AI, Erster S, Moll UM. DeltaNp73 stabilises TAp73 proteins but compromises their function due to inhibitory hetero-oligomer formation. Cell Death Differ. 2004;11:357–60. doi: 10.1038/sj.cdd.4401335. [DOI] [PubMed] [Google Scholar]
- 53.Kramer S, Ozaki T, Miyazaki K, Kato C, Hanamoto T, Nakagawara A. Protein stability and function of p73 are modulated by a physical interaction with RanBPM in mammalian cultured cells. Oncogene. 2005;24:938–44. doi: 10.1038/sj.onc.1208257. [DOI] [PubMed] [Google Scholar]
- 54.Maisse C, Munarriz E, Barcaroli D, Melino G, De Laurenzi V. DNA damage induces the rapid and selective degradation of the DeltaNp73 isoform, allowing apoptosis to occur. Cell Death Differ. 2004;11:685–7. doi: 10.1038/sj.cdd.4401376. [DOI] [PubMed] [Google Scholar]
- 55.Watson IR, Irwin MS. Ubiquitin and ubiquitin-like modifications of the p53 family. Neoplasia. 2006;8:655–66. doi: 10.1593/neo.06439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salomoni P, Cossarizza A. HIV: no PUMA no death? Cell Death Differ. 2004;11:691–2. doi: 10.1038/sj.cdd.4401446. [DOI] [PubMed] [Google Scholar]
- 57.Sionov RV, Coen S, Goldberg Z, Berger M, Bercovich B, Ben-Neriah Y, et al. c-Abl regulates p53 levels under normal and stress conditions by preventing its nuclear export and ubiquitination. Mol Cell Biol. 2001;21:5869–78. doi: 10.1128/MCB.21.17.5869-5878.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakano K, Balint E, Ashcroft M, Vousden KH. A ribonucleotide reductase gene is a transcriptional target of p53 and p73. Oncogene. 2000;19:4283–9. doi: 10.1038/sj.onc.1203774. [DOI] [PubMed] [Google Scholar]
- 59.Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7:673–82. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- 60.Liu X, Elojeimy S, El-Zawahry AM, Holman DH, Bielawska A, Bielawski J, et al. Modulation of ceramide metabolism enhances viral protein apoptin's cytotoxicity in prostate cancer. Mol Ther. 2006;14:637–46. doi: 10.1016/j.ymthe.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 61.Graham FL, Prevec L. Manipulation of Adenovirus vectors. In: Murray EJ, editor. Methods in Molecular Biology. Humana Press; Clifton, NJ: 1991. pp. 109–28. [DOI] [PubMed] [Google Scholar]
- 62.Sartor M, Steingrimsdottir H, Elamin F, Gaken J, Warnakulasuriya S, Partridge M, et al. Role of p16/MTS1, cyclin D1 and RB in primary oral cancer and oral cancer cell lines. Br J Cancer. 1999;80:79–86. doi: 10.1038/sj.bjc.6690505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.