Abstract
Background
Health behaviors are known risk factors for colorectal cancer and are more common in low socioeconomic status (SES) populations. We evaluated the extent to which behavioral risk factors and body mass index (BMI) explain SES disparities in colorectal cancer incidence, overall and by tumor location.
Methods
We analyzed prospective National Institutes of Health-AARP Diet and Health Study data on 506 488 participants who were recruited in 1995–1996 from six US states and two metropolitan areas and followed through 2006. Detailed baseline data on risk factors for colorectal cancer, including health behaviors, were obtained using questionnaires. SES was measured by self-reported education and census-tract data. The outcome was primary incident invasive colorectal adenocarcinoma. Poisson regression was used to estimate the association between SES and risk of incident colorectal cancer, with adjustment for age, sex, race and ethnicity, family history, and state of residence. The model estimates were used to derive percentage mediation by behavioral risk factors; bias-corrected 95% confidence intervals were obtained through bootstrap techniques.
Results
Seven-thousand six-hundred seventy-six participants developed colorectal cancer during follow-up. SES differences in prevalence of physical inactivity, unhealthy diet, smoking, and unhealthy weight each explained between 11.3% (BMI) and 21.6% (diet) of the association between education and risk of colorectal cancer and between 8.6% (smoking) and 15.3% (diet) of the association between neighborhood SES and risk of colorectal cancer. Health behaviors and BMI combined explained approximately 43.9% (95% CI = 35.1% to 57.9%) of the association of education and 36.2% (95% CI = 28.0% to 51.2%) of the association of neighborhood SES with risk of colorectal cancer. The percentage explained by all factors and BMI combined was largest for right colon cancers and smallest for rectal cancers.
Conclusion
A substantial proportion of the socioeconomic disparity in risk of new-onset colorectal cancer, and particularly of right colon cancers, may be attributable to the higher prevalence of adverse health behaviors in low-SES populations.
Low socioeconomic status (SES) is a risk factor for the development of colorectal cancer (1,2), and unhealthy but modifiable behaviors such as physical inactivity and unhealthful dietary habits are believed to contribute to this disparity (1,3). In the United States, behavioral risk factors and obesity are more common in low-SES groups than in affluent populations (1,4–11), and unhealthy lifestyles may account for up to 70% of colorectal cancers (12). Epidemiological and biological investigations have shown that unhealthy lifestyles and their ensuing health consequences, such as obesity, affect molecular pathways involved in colorectal carcinogenesis (13–15). However, to date, no large prospective study has quantified the extent to which behavioral risk factors and obesity explain the disproportionately high risk of colorectal cancer in low-SES populations.
In a study that analyzed data from 46 128 women in the Nurses’ Health Study (1986–2006), Kim et al. evaluated the relationship between behavioral risk factors, a census block-group SES score, and risk of colorectal cancer (2). They observed that the greater prevalence of some risk-promoting health behaviors in low-SES neighborhoods partly explained the association between neighborhood poverty and increased risk of rectal cancers; however, disparities in health behaviors were associated with SES-related disparities in colon cancer only among nurses with a college degree. They did not find statistically significant mediation by levels of physical activity, which was surprising because of consistent reports that physical inactivity increases risk of colorectal cancer (14–16). There were some limitations of that report that warrant further study: it was restricted to female nurses (thus a narrow SES band), it could not examine individual-level SES, and it did not quantify the proportion of the risk of colorectal cancer incidence attributable to health behaviors. Thus, the extent to which differences in behavioral risk factors contribute to socioeconomic disparities in incident colorectal cancer, characterized at both the individual and neighborhood levels, remains unclear. Providing such evidence is important because healthy behaviors have many health benefits that include cancer prevention and are promising targets for reducing disparities in multiple chronic conditions (17,18).
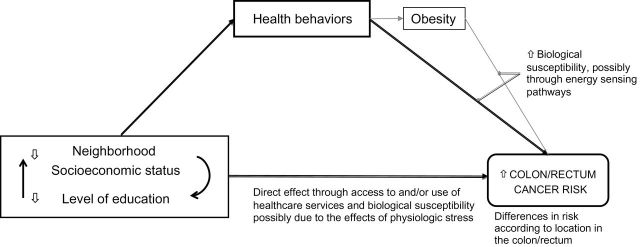
We previously analyzed data from 506 488 men and women in the prospective National Institutes of Health-AARP Diet and Health (NIH-AARP) Study and found that SES disparities in risk of colorectal cancer were attenuated by adjustment for behavioral factors (1). In this study, we extended that analysis by quantifying the contribution of behavioral risk factors and obesity, separately and together, to SES disparities in risk of colorectal cancer. Individual-level and neighborhood SES factors are intertwined but are each independently associated with risk of colorectal cancer (1). Thus, conceptually, we based this analysis on the interrelationships among neighborhood SES, individual-level SES, health behaviors, obesity, and risk of colorectal cancer (Figure 1). Obesity may be on the causal pathway between health behaviors and risk of colorectal cancer. We therefore evaluated its incremental effect on behavioral risk factors (19). Because of potential biological differences between right and left colon cancers (20,21), we also performed the analysis according to tumor location.
Figure 1.
Conceptual model of the relationships among socioeconomic factors, health behaviors, and colorectal cancer risk. Neighborhood socioeconomic status refers to the racial composition and socioeconomic context within a given unit of aggregation for area-level socioeconomic measures. The health behaviors examined are physical activity, dietary pattern, and smoking. This grouping does not imply a causal relationship between smoking and obesity.
Methods
Study Design and Population
Data for this study were obtained from the ongoing prospective NIH-AARP Study, which has previously been described in detail (22). The cohort comprised 566 401 men and women who were ages 50–71 years at the time of recruitment into the study between October 1995 and May 1996. Study participants were drawn from six US states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) and two metropolitan areas (Atlanta, GA, and Detroit, MI). For this analysis, we excluded a total of 59 913 participants who had invalid census data (n = 705), did not accrue follow-up time or withdrew from the study (n = 947), had a history of colorectal cancer at baseline (n = 4 497), or had missing data on education, race, smoking, physical activity, or body mass index (BMI; n = 53 764). The study was approved by the National Cancer Institute Special Studies Institutional Review Board.
Data Collection
Participants completed a mailed questionnaire at baseline (1995–1996) about several variables that are related to risk of colorectal cancer, including age, sex, race and ethnicity, educational level, health behaviors, and history of colorectal cancer in a first-degree relative. Incident cancers were identified by linking participants to states’ tumor registry data. The NIH-AARP study tracked participants’ place of residence and found that about 95% of the subjects in this cohort continued to reside in their original tumor registry area through December 2000 (22).
Ascertainment of Colorectal Cancer Incidence
The primary outcome for this study was primary incident invasive adenocarcinoma of the colon or rectum during the follow-up period. The time to colorectal cancer diagnosis for each participant was calculated from the date of receipt of the baseline questionnaire at the study center. Each participant was followed until he or she moved out of a registry area or December 31, 2006, whichever occurred first. Participants who were diagnosed with new-onset colorectal cancer during the follow-up period were identified from tumor registry data using International Classification of Diseases for Oncology (ICDO) codes. We restricted our outcome definition to adenocarcinomas because they are believed to develop through the adenoma–carcinoma pathway and are the targets of prevention through healthy behavior. We used the ICDO codes to categorize the segment of the colon/rectum where a tumor was found (location) as: 1) “right” colon if proximal to the splenic flexure, 2) “left” colon if in or distal to the splenic flexure including the sigmoid colon, 3) rectal, or 4) unspecified.
Socioeconomic Indicators
The predictor of interest in our analysis was SES, which was measured at both the individual and neighborhood levels. We collected information about “the highest grade or level of schooling completed.” Possible responses were less than 8 years, 8–11 years, 12 years or completed high school, post–high school training other than college (eg, vocational or technical training), some college, college graduate, or postgraduate. The first two categories were combined as “less than 12 years of schooling” in our analyses.
The residential address of each study participant at baseline was linked to socioeconomic variables from the 2000 decennial census at the census-tract level. Principal component analysis was then used to compute a census-tract socioeconomic deprivation index (neighborhood SES), which has previously been described in detail (9). Briefly, the index was derived from the percentage in the census-tract of persons who 1) had less than high school education, 2) were unemployed, 3) were non-Hispanic blacks, 4–5) were in managerial jobs, separately for men and women, and of households, 6) below 1999 federal poverty levels, 7) on public assistance, or 8) with annual income less than $30 000, 9) with no car, or 10) headed by a woman with dependent children. The index was standardized by dividing it by the square root of the factor’s variance (or Eigenvalue) (23). Higher deprivation scores corresponded to higher levels of poverty or unemployment in the particular census tract.
Behavioral Risk Factors and BMI
The candidate mediators in our analyses were dietary pattern, level of physical activity, smoking, and BMI. A validated 124-item food frequency questionnaire assessed components of participants’ usual diet during a previous 12-month period (24). A Mediterranean diet score (range 0–9) was used as our measure of dietary patterns. Conformity to the Mediterranean dietary pattern is based on the reported intakes of vegetables, legumes, fruit and nuts, fish and seafood, cereals, meat and meat products, dairy products, ratio of monounsaturated to saturated fat, and alcoholic beverages (25). Thus, this measure incorporates several of the dietary components known to be associated with risk of colorectal cancer, accounts for the complex interrelationships of nutrients in individual dietary components, and is strongly predictive of risk of colorectal cancer (26). The results of the analyses using either the Mediterranean diet score or individual dietary components of the index were similar. This finding supported our use of the dietary pattern analysis approach, which is also easier to interpret and communicate to the public. Physical activity was measured by the frequency of vigorous physical activity that lasted at least 20 minutes and was categorized as never, rarely, 1–3 times per month, 1–2 times per week, 3–4 times per week, or 5 or more times per week (ie, active daily). Data on smoking included whether a participant had ever smoked more than 100 cigarettes during his or her lifetime, and if so, the typical number of cigarettes smoked per day and current smoking status (22).
BMI was calculated from self-reported body weight (lbs) and height (ft-inches) at baseline and was categorized as less than 20.0, 20.0–22.4, 22.5–24.9, 25.0–27.4, 27.5–29.9, 30.0–31.9, 32.0–33.9, or 34kg/m2 or more. We combined the first two categories as less than 22.5 in some analyses because their associations with risk of colorectal cancer were of similar magnitude, and the sample sizes were relatively small. In 1996–1997, the NIH-AARP Study collected data on participants’ weight at ages 18, 35, and 50 years. However, these additional measurements yielded results similar to the baseline BMI.
Statistical Analysis
The χ2 test was used to compare categorical variables according to levels of educational achievement or quintiles of neighborhood SES deprivation. Analysis of variance was used for continuous variables. Poisson regressions (with robust variance estimation) were used to obtain colorectal cancer incidence rate ratios (IRRs) and 95% confidence intervals (CIs) (27). Poisson models were a good fit for the data. All P values were two-sided.
We performed analyses for all colorectal cancers and then separately according to cancer location. We examined for two-way interactions among the SES factors, health behaviors, and BMI using the Wald χ2 with a Bonferroni correction. We further evaluated statistically significant interactions in terms of the effect on the predicted number of incident cancers from the regression models. Although we found a statistically significant interaction between physical activity and diet, inclusion of the interaction terms in the models did not substantively change our results and was therefore not included in the estimations.
In our analyses, we first examined the association between each measure of SES and risk of colorectal cancer, adjusted for age, sex, race and ethnicity, family history of colorectal cancer, and state of residence; the covariates were entered in the models as categorical variables except age, a continuous variable (Model 1). We further evaluated the risk of colorectal cancer associated with each behavioral factor (physical activity, diet, and smoking) or BMI adjusted for the covariates in Model 1. We then constructed two additional models to assess the effect of health behaviors on the association between SES and colorectal cancer risk (Figure 1): Model 2 added all the behavioral risk factors to Model 1, and Model 3 further adjusted for BMI. Education and neighborhood SES were analyzed in separate models, each as a categorical variable.
The above modeling scheme was used to compute the magnitude by which the behavioral risk factors and BMI explained SES-related differences in the risk of incident colorectal cancer. For the mediation analyses, both education and neighborhood SES (standardized census-tract socioeconomic deprivation score) were analyzed as continuous variables. Linearity assumptions were reasonable as evaluated with graphical plots and the Wald χ2 test for trend: for neighborhood SES, we used deciles of the standardized score. We also examined linearity assumptions for the deprivation score by evaluating a quadratic term of the score, which was not statistically significant.
The percentage mediation was calculated with the formula, (β[SES]−β[SES](M)])/β[SES] * 100, where β is the regression coefficient for education or neighborhood SES without (β (SES)) and with (β [SES(M)]) the health behaviors and/or BMI as shown in the conceptual model in Figure 1 (28). Bias-corrected CIs for the percentage mediation were obtained through bootstrap techniques with 1000 replications. According to Shrout and Bolger, we set the minimum possible lower CI to 0% and the maximum to 100% (28). All analyses were performed using STATA Release 12.
Results
First, we listed the distribution of the cohort according to self-reported education and neighborhood SES (Table 1). High-risk health behaviors were more common in low-SES groups. Compared with participants in high-SES groups (education and neighborhood), a higher proportion of participants in low-SES groups had low Mediterranean diet scores, unhealthy weight, or were physically inactive. Of the 7676 participants who were diagnosed with invasive primary colorectal adenocarcinoma during the follow-up period, 3420 had cancers that were diagnosed in the right colon, 2050 in the left colon, 1991 in the rectum, and 215 in unspecified locations.
Table 1.
Characteristics of the cohort according to levels of neighborhood socioeconomic deprivation: NIH-AARP Study, 1995–2006
| Characteristic, % | Neighborhood socioeconomic deprivation index* | Highest level of educational achievement* | ||||
| First quintile, n=150 389 | Third Quintile, n=111 485 | Fifth quintile, n=34 653 | <12 years, n=31 333 | Post–high school, n=51 612 | Postgraduate, n=103 164 | |
| Age, y | ||||||
| <55 | 14.6 | 12.3 | 13.0 | 6.6 | 13.2 | 15.8 |
| 55–59 | 23.7 | 21.5 | 22.6 | 17.0 | 21.8 | 23.9 |
| 60–64 | 27.7 | 28.0 | 29.1 | 28.0 | 27.8 | 27.5 |
| 65–69 | 30.6 | 34.2 | 31.8 | 42.8 | 33.4 | 29.5 |
| ≥70 | 3.4 | 3.9 | 3.5 | 5.5 | 3.8 | 3.3 |
| Women, % | 35.5 | 40.8 | 49.4 | 39.0 | 43.0 | 30.8 |
| Race or ethnicity | ||||||
| Non-Hispanic white | 95.6 | 94.6 | 71.9 | 89.0 | 93.6 | 93.5 |
| Non-Hispanic black | 1.2 | 2.5 | 22.0 | 6.2 | 3.4 | 3.1 |
| Hispanic | 1.3 | 1.7 | 4.0 | 3.5 | 1.8 | 1.3 |
| Others | 2.0 | 1.2 | 2.1 | 1.3 | 1.3 | 2.1 |
| Family history of colorectal cancer | 6.3 | 6.2 | 5.3 | 4.8 | 6.1 | 6.8 |
| Smoking | ||||||
| Never smoked | 38.4 | 34.5 | 36.4 | 26.4 | 32.3 | 45.3 |
| Former ≤ 20 cigs/ day | 29.9 | 28.6 | 27.6 | 29.8 | 29.6 | 28.5 |
| Former > 20 cigs/ day | 22.6 | 23.2 | 18.9 | 25.4 | 23.3 | 19.2 |
| Current ≤ 20 cigs/ day | 6.1 | 8.6 | 11.6 | 11.9 | 9.2 | 4.6 |
| Current > 20 cigs/ day | 3.1 | 5.1 | 5.5 | 6.5 | 5.6 | 2.4 |
| Mediterranean diet score | ||||||
| 0–1 (lowest) | 4.5 | 6.3 | 6.7 | 8.8 | 6.7 | 4.6 |
| 2 | 9.3 | 12.1 | 12.8 | 15.6 | 12.4 | 9.5 |
| 3 | 15.7 | 18.4 | 19.5 | 21.7 | 18.9 | 16.3 |
| 4 | 20.4 | 21.5 | 21.9 | 21.7 | 21.6 | 20.7 |
| 5 | 20.6 | 19.1 | 18.5 | 16.9 | 19.0 | 20.6 |
| 6 | 16.1 | 13.2 | 12.5 | 9.7 | 12.9 | 15.7 |
| 7 | 9.4 | 6.8 | 6.0 | 4.3 | 6.3 | 8.9 |
| 8–9 (highest) | 4.1 | 2.5 | 2.0 | 1.2 | 2.2 | 3.7 |
| Physical activity | ||||||
| Never | 3.0 | 5.2 | 7.9 | 11.7 | 4.6 | 2.1 |
| Rarely | 11.4 | 14.6 | 18.3 | 19.4 | 15.1 | 9.6 |
| 1–3 times per month | 13.3 | 13.8 | 14.6 | 13.1 | 14.6 | 12.7 |
| 1–2 times per week | 22.5 | 21.5 | 19.9 | 17.9 | 22.4 | 22.2 |
| 3–4 times per week | 29.1 | 26.1 | 22.8 | 20.8 | 25.2 | 30.5 |
| 5 or more times per week | 20.7 | 18.7 | 16.5 | 17.1 | 18.1 | 22.9 |
| Body mass index, kg/m2 | ||||||
| <20.0 | 3.4 | 3.0 | 3.0 | 10.6 | 13.1 | 15.5 |
| 20.0–22.4 | 12.5 | 10.0 | 8.9 | 17.6 | 20.6 | 24.5 |
| 22.5–24.9 | 24.4 | 20.5 | 17.9 | 25.0 | 25.7 | 26.7 |
| 25.0–27.4 | 26.9 | 25.9 | 23.3 | 17.9 | 16.9 | 15.6 |
| 27.5–29.9 | 15.6 | 16.9 | 16.8 | 10.2 | 9.2 | 7.5 |
| 30.0–31.9 | 7.5 | 9.3 | 10.1 | 6.9 | 5.4 | 4.3 |
| 32.0–33.9 | 4.3 | 5.7 | 6.9 | 11.7 | 8.9 | 5.9 |
| ≥34.0 | 5.4 | 8.8 | 13.1 | 10.6 | 13.1 | 15.5 |
* All two-sided Pearson χ2 P values from χ2 tests for trend were less than .001. Only the lowest, middle, and highest categories of education and neighborhood socioeconomic status (SES) are shown. The distribution of characteristics among participants in the categories omitted for education or neighborhood SES followed the pattern of the categories shown. For example, the percentage most physically active was as follows: 16.9% in participants with a high school education (n = 101 580), 18.2% in people with some college (n = 120 507), and 20.1% in college graduates (n = 98 292), and 19.2% in the second neighbor SES quartile (n = 132 342) and 18.2% in the third quartile (n = 77 619).
Associations With Risk of Incident Colorectal Cancer
Next, we analyzed the colorectal cancer IRRs for behavioral risk factors after adjustment for age, sex, race and ethnicity, family history of colorectal cancer, and state of residence (Table 2). The behavioral risk factors were each strongly predictive of risk of colorectal cancer in a dose–response manner. For example, people in very low conformity with a Mediterranean dietary pattern (score of <2) had a 91% greater risk of colorectal cancer incidence (IRR = 1.91, 95% CI = 1.61 to 2.26) compared with those with the highest scores. Physical inactivity, compared with being active daily, was associated with a 44% greater risk of colorectal cancer (IRR = 1.44, 95% CI = 1.29 to 1.61), and a BMI of 34kg/m2 or more, compared with a BMI of less than 20kg/m2, was associated with a 55% greater risk of colorectal cancer (IRR = 1.55, 95% CI = 1.41 to 1.71).
Table 2.
Incidence of invasive adenocarcinoma of the colon or rectum according to behavioral risk factors and body mass index in the NIH-AARP study, 1995–2006
| Participants | ||||
| Colorectal cancer risk (CRC) factors | No. | No. with CRC | Incidence (per 10 000 person-years)* | Adjusted incidence rate ratio (95% CI)† |
| Smoking | ||||
| Never smoked | 182 924 | 2342 | 13.1 | 1.00 (referent) |
| Former smoker (≤20 cigs/day) | 146 121 | 2185 | 15.6 | 1.11 (1.05 to 1.18) |
| Former smoker (>20 cigs/day) | 114 853 | 2192 | 20.7 | 1.41 (1.33 to 1.50) |
| Current smoker (≤20 cigs/day) | 40 103 | 628 | 17.3 | 1.40 (1.28 to 1.52) |
| Current smoker (>20 cigs/day) | 22 487 | 329 | 16.7 | 1.37 (1.22 to 1.53) |
| Mediterranean diet score | ||||
| 0–1 (lowest adherence) | 28 956 | 542 | 20.3 | 1.91 (1.61 to 2.26) |
| 2 | 56 447 | 964 | 18.3 | 1.71 (1.46 to 2.01) |
| 3 | 89 735 | 1488 | 17.6 | 1.64 (1.40 to 1.92) |
| 4 | 106 591 | 1656 | 16.4 | 1.51 (1.29 to 1.77) |
| 5 | 99 541 | 1432 | 15.1 | 1.37 (1.17 to 1.61) |
| 6 | 71 460 | 946 | 13.8 | 1.23 (1.05 to1.45) |
| 7 | 38 603 | 476 | 12.8 | 1.12 (0.94 to 1.33) |
| 8–9 (highest adherence) | 15 155 | 172 | 11.7 | 1.00 (referent) |
| Physical activity | ||||
| Never | 23 242 | 410 | 20.6 | 1.44 (1.29 to 1.61) |
| Rarely | 70 530 | 1176 | 18.3 | 1.34 (1.24 to 1.45) |
| 1–3 times per month | 70 032 | 1076 | 16.3 | 1.22 (1.13 to 1.32) |
| 1–2 times per week | 109 898 | 1666 | 15.9 | 1.15 (1.07 to 1.24) |
| 3–4 times per week | 135 540 | 1963 | 15.0 | 1.04 (0.97 to 1.12) |
| ≥5 times per week | 97 246 | 1385 | 14.7 | 1.00 (referent) |
| Body mass index, kg/m2 | ||||
| <20.0 | 16 022 | 189 | 12.9 | 1.00 (referent) |
| 20.0–22.4 | 54 712 | 654 | 12.6 | 0.95 (0.81 to 1.12) |
| 22.5–24.9 | 109 740 | 1502 | 14.4 | 0.99 (0.85 to 1.15) |
| 25.0–27.4 | 131 721 | 1974 | 15.7 | 1.05 (0.91 to 1.22) |
| 27.5–29.9 | 83 251 | 1427 | 18.1 | 1.22 (1.05 to 1.42) |
| 30.0–31.9 | 44 209 | 742 | 17.7 | 1.24 (1.06 to 1.46) |
| 32.0–33.9 | 26 560 | 481 | 19.3 | 1.38 (1.17 to 1.64) |
| ≥34.0 | 40 273 | 707 | 18.9 | 1.49 (1.27 to 1.75) |
* The incidence rates were estimated from Poisson regression models that were adjusted for age and sex.
† Models for the incidence rate ratios were adjusted for age, sex, race and ethnicity, family history of CRC in a first-degree relative, and state of residence, which were entered as categorical variables in the models except age (continuous, measured from the baseline date).
We also performed ad hoc analyses to assess the combined effects of health behaviors and SES (separately for educational level and neighborhood SES) on overall colorectal cancer risk controlling for confounders in Model 1 (data not shown). Compared with the group with the highest SES and the healthiest behavior, as SES and healthy behaviors decreased, except for smoking exposure, the risk of colorectal cancer increased. For instance, compared with participants who had a postgraduate education plus a diet score of 8–9, those who were least educated and had a diet score of less than 2 were at substantially higher risk of colorectal cancer (IRR = 2.56, 95% CI = 1.75 to 3.75). Also, the least-educated, most physically inactive participants were at much higher risk of colorectal cancer (IRR = 1.90, 95% CI = 1.48 to 2.45) compared with the most-educated persons who were active daily. Similarly, the least-educated smokers were at more risk than the most-educated nonsmokers (IRR = 1.67, 95% CI = 1.17 to 2.36), and less-educated obese participants (BMI 34kg/m2 or more) were at more risk than well-educated participants with BMI of 20kg/m2 or less (IRR = 2.24, 95% CI = 1.72 to 2.92).
There were similar patterns with respect to neighborhood SES and behavioral factors. Compared with participants from highest-SES neighborhoods who had diet score of 8 or 9, or were active daily, healthy-weight, or nonsmokers, colorectal cancer risk was much higher for persons in the fifth quintile of neighborhood SES with a diet score of less than 2 (IRR = 1.99, 95% CI = 1.36 to 2.92), were physically inactive (IRR = 1.78, 95% CI = 1.32 to 2.39), obese (IRR = 2.20, 95% CI = 1.74 to 2.78), or current smokers (IRR = 1.12, 95% CI = 0.73 to 1.72), respectively.
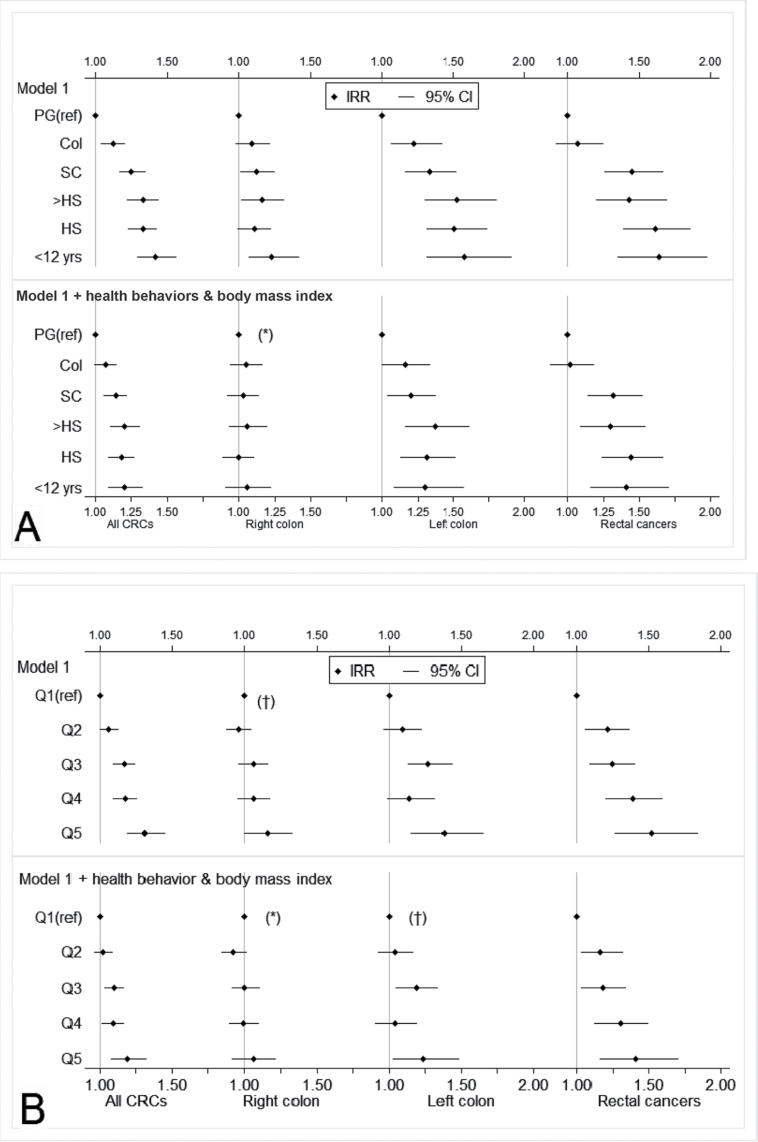
Next, we computed the colorectal cancer IRRs according to levels of each SES measure and by tumor location, with and without adjustment for behavioral risk factors and BMI (Figure 2 and Supplementary Table 1, available online). Lower educational level or neighborhood SES was associated with a higher cancer risk irrespective of tumor location. Adjustment for behavioral factors attenuated the magnitude of the associations. Associations for all colorectal cancers considered together remained statistically significant principally because of the robust associations with left colon and rectal cancers.
Figure 2.
Associations between socioeconomic measures and colorectal cancer incidence according to tumor location before and after adjustment for behavioral factors and BMI: NIH-AARP Study, 1995–2006. A) Association of educational level with colorectal cancer incidence. The top panel shows incidence rate ratios (IRR) and 95% confidence intervals (CIs) for colorectal cancer risk by tumor location for persons with varying levels of education (PG = postgraduate, Col = college degree, SC = some college, >HS = more than high school, HS = high school diploma) before adjustment, and the bottom panel shows the same associations after adjustment for health behaviors (Mediterranean diet score, physical activity, and smoking) and body mass index. B) Association of neighborhood SES with colorectal cancer incidence. The top panel shows incidence rate ratios (IRR) and 95% confidence intervals (CIs) for colorectal cancer risk by tumor location for persons in varying levels of neighborhood deprivation (by quintile: Q1, least deprived to Q5, most deprived). The vertical lines represent an IRR of 1.0 (referent). All Wald χ2 two-sided P values for trend are less than .01 except those marked by an asterisk (* means ≤.05) or a dagger († means >.05). Model 1 was adjusted for age, sex, race and ethnicity, family history of colorectal cancer in a first-degree relative, and state of residence, which were entered as categorical variables in the models except age (continuous, measured from the baseline date).
Mediation of SES Disparities by Behavioral Factors
Next, we analyzed the contribution of health behaviors to differences in the risk of colorectal cancer by SES, separately for individual level education and neighborhood SES (Table 3). For the analyses on all colorectal cancers, differences in the prevalence of individual health behaviors by SES explained between 11.3% (BMI) and 21.6% (diet) of the association between education and colorectal cancer risk. Diet, physical activity, and smoking together explained about 37.3% (95% CI = 29.9% to 49.8%) of the higher risk of colorectal cancer associated with lower educational achievement. These behavioral factors and BMI together explained 43.9% (95% CI = 35.1% to 57.9%) of the colorectal cancer risk disparity by education. Individually, the behavioral factors explained between 8.6% (smoking) and 15.3% (diet) of the association between neighborhood SES and colorectal cancer risk: combined, they explained about 26.4% (95% CI = 20.2% to 37.5%), and together with BMI, they explained 36.2% of the risk of colorectal cancer by neighborhood SES (95% CI = 28.0% to 51.2%).
Table 3.
Association between neighborhood deprivation, education, and incidence of colorectal cancer explained by behavioral factors and body mass index, NIH-AARP Study, 1995–2006
| Percentage of the association between SES and colorectal cancer risk explained (95% CI) | ||||
| Mediators according to SES measure | Any colorectal cancer | Rectal cancers | Left colon cancers | Right colon cancers |
| Educational level | ||||
| Referent Model* | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Separate effects of each factor† | ||||
| Effect of smoking history | 12.9 (9.6 to 41.2) | 8.8 (5.3 to 13.2) | 7.7 (3.9 to 13.2) | 28.0 (14.0 to 100) |
| Effect of Mediterranean diet score | 21.6 (16.4 to 28.9) | 13.8 (9.1 to 20.6) | 17.9 (11.3 to 27.3) | 45.6 (23.4 to 100) |
| Effect of physical activity | 11.9 (8.3 to 17.2) | 4.9 (1.0 to 9.2) | 11.2 (6.1 to 19.2) | 26.7 (11.6 to 95.0) |
| Effect of body mass index (BMI) | 11.3 (8.2 to 14.9) | 3.2 (0.6 to 6.3) | 12.0 (8.0 to 18.7) | 28.0 (13.2 to 95.5) |
| Combined effect of all health behaviors | 37.3 (29.9 to 49.8) | 22.7 (13.9 to 31.4) | 30.3 (21.1 to 48.9) | 78.9 (40.1 to 100) |
| Combined effect of all health behaviors and BMI | 43.9 (35.1 to 57.9) | 24.2 (16.4 to 34.1) | 37.8 (26.3 to 54.6) | 95.0 (50.1 to 100) |
| Neighborhood SES | ||||
| Referent Model* | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| Separate effects of each factor† | ||||
| Effect of smoking history | 8.6 (6.0 to 12.7) | 5.3 (2.6 to 8.8) | 5.3 (1.6 to 11.7) | 18.3 (8.3 to 100) |
| Effect of Mediterranean diet score | 15.3 (11.1 to 21.9) | 9.9 (6.7 to 16.3) | 14.9 (8.9 to 27.5) | 28.6 (13.2 to 100) |
| Effect of physical activity | 10.7 (7.8 to 15.4) | 4.8 (1.7 to 8.7) | 11.7 (6.3 to 22.7) | 21.2 (8.1 to 96.8) |
| Effect of BMI | 14.7 (10.7 to 22.1) | 4.4 (1.4 to 8.6) | 16.9 (10.4 to 30.4) | 34.6 (16.7 to 100) |
| Combined effect of all health behaviors | 26.4 (20.2 to 37.5) | 15.3 (10.0 to 23.7) | 24.6 (16.1 to 50.3) | 51.3 (23.8 to 100) |
| Combined effect of all health behaviors and BMI | 36.2 (28.0 to 51.2) | 17.6 (11.5 to 27.5) | 36.2 (23.3 to 67.6) | 75.4 (36.5 to 100) |
* Referent models (separately for self-reported education and neighborhood SES) were adjusted for age, sex, race and ethnicity, family history of colorectal cancer, and state of residence, which were entered as categorical variables in the models except age (continuous, measured from the baseline date). Models on the effects of each behavioral factor or BMI (kg/m2) added the respective variable to the referent model.
† Health behaviors refer to Mediterranean diet score, physical activity, and smoking.
The extent to which each of the behavioral risk factors and BMI explained the association of education with incidence of colorectal cancer was substantially larger for right colon cancers (26.7% for physical activity to 45.6% for diet) than for left colon cancers (7.7% for smoking to 17.9% for diet) and rectal cancers (3.2% for BMI to 13.8% for diet) (see Supplementary Figure 1, available online). When considered together, the behavioral risk factors explained about 78.9% of the association between education and colorectal cancer incidence for right-sided colon cancers, 30.3% for left colon cancers, and 22.7% for rectal cancers (Table 3 and Supplementary Table 2, available online). The behavioral factors and BMI combined explained about 95.0% of the association between education and right colon cancer risk, 37.8% for left colon cancers, and 24.2% for rectal cancers. Similarly, the extent to which diet, physical activity, and smoking explain the association of neighborhood SES with cancer risk was larger for right colon cancers (from 18.3% for smoking to 34.6% for BMI) than for left colon cancers (from 5.3% for smoking to 16.9% for BMI) and rectal cancers (from 4.4% for BMI to 9.9% for diet): the percentage mediated by the health behaviors combined was 51.3% for right colon cancers, 24.6% for left colon cancers, and 15.3% for rectal cancers. These health behaviors and BMI combined explained about 75.4% of the association between neighborhood SES and risk of right colon cancer, 36.2% for left colon cancers, and 17.6% for rectal cancers. However, the confidence intervals for right colon cancers were wide because of the relatively weak association with SES (see Supplementary Table 1, available online).
Discussion
This study analyzed a large prospective US cohort with more than 10 years of follow-up and found that the disproportionately high risk of potentially preventable colorectal cancers among people with low SES was explained, in part, by a higher prevalence of obesity, unhealthy dietary patterns, physical inactivity, and smoking in low-SES populations. We found that each of the individual adverse health behaviors explained between 9% and 22% of SES disparities in overall colorectal cancer incidence, with different patterns observed for individual-level and neighborhood measures. Together, higher prevalence of behavioral risk factors in the low-SES groups explained about 37.3% (95% CI = 29.9% to 49.8%) of the excess incidence of colorectal cancer attributable to lower educational achievement and 26.4% (95% CI = 20.2% to 37.5%) of the excess incidence of colorectal cancer among people living in poorer areas. Further, differences in BMI by SES contributed modestly to the disparity in colorectal cancer risk. Thus, behavioral risk factors and BMI combined explained about 43.9% (95% CI = 35.1% to 57.9%) of the excess risk of colorectal cancer associated with lower educational achievement and 36.2% (95% CI = 28.0% to 51.2%) of the colorectal cancer risk associated with living in poorer areas. The percentage of SES disparity explained by behavioral risk factors was larger for colon cancers, particularly those of the right colon (although the confidence intervals were wide), than it was for rectal cancers. The most consistent factor contributing to the observed SES disparity in risk of colorectal cancer was diet. Nonconformity with the Mediterranean dietary patterns alone explained about one-fifth of SES-related differences in incidence for all colorectal cancers, one-third for right colon cancers, nearly one-fifth for left colon cancers, and about one-tenth for rectal cancers.
This study’s findings add to the many confirmed benefits of healthy behavior for disease prevention and longevity (17) and support the use of strategies to promote healthy behavior to reduce SES-related disparities in colorectal cancer incidence. Our findings also support the recommendations of groups that advocate a healthy lifestyle to reduce the risk of colorectal cancer (29,30). However, as an observational study, our findings suggest but do not definitively prove causal relationships between SES, behavioral factors, obesity, and the risk of colorectal cancer.
This is the largest cohort study to date (n = 506 488) and the first study of both sexes to examine the association of behavioral factors with both self-reported educational achievement and neighborhood SES. Thus, there are no similar studies for direct comparison. Some previous studies did show that adjustment for behavioral risk factors attenuates the association between SES and risk of colorectal cancer (1,2,31). Kim et al. (2), using data from the Nurses’ Health Study, found statistically significant indirect pathways through red meat intake, alcohol intake, and BMI for the relationship between neighborhood SES and rectal cancer, but indirect pathways for colon cancers were only statistically significant among female nurses with a college degree. Our study applied a different analytic approach and found statistically significant mediation of self-reported education- or neighborhood SES-related colorectal cancer risk by all of the behavioral factors examined, although the proportion attributable to behavioral factors, particularly physical activity and BMI, was relatively small for rectal cancer risk. We found that behavioral factors were stronger mediators of self-reported education- or neighborhood SES-related colorectal cancer risk for right colon cancers than for left colon cancers, and we did not find a statistically significant interaction between self-reported education and neighborhood SES. Our study also was performed on a larger cohort with a broader range of SES and employed a dietary pattern analysis approach to incorporate the complexity of dietary components.
There is consistent evidence from a variety of investigations that support our observation of the substantial role that behavioral factors and BMI play in SES-related disparities in the incidence of colorectal cancer. Studies have shown that healthful dietary patterns such as the Mediterranean diet have anticarcinogenic properties (13,32), and physical activity has been found to reduce the risk of colorectal cancer (14,15). Obesity is postulated to promote carcinogenesis (15,33), and smoking is believed to increase risk of colorectal cancer by promoting the formation and growth of adenomas (34). These factors, especially obesity, diet, and physical activity, may affect colorectal pathogenesis through 5′-adenosine monophosphate-activated protein kinase (AMPK) signaling pathways (35,36). AMPK is a cellular energy and nutrient sensor that suppresses cell proliferation including activation of p53, a tumor suppressor (35,36). AMPK is suppressed by obesity, physical inactivity, and poor diet.
Behavioral factors are likely to act together in complex, synergistic, and mutually reinforcing ways to account for at least some of the SES variations in incidence of colorectal cancer seen in our study. In this analysis, as has been reported in some previous studies, there was a strong relationship between these health behavioral factors and risk of colorectal cancer, and these behavioral risk factors and obesity are more common in low-SES populations. The risk of colorectal cancer associated with both SES and health behaviors combined was larger than either factor alone. The complex relationship between SES, behavioral risk factors, BMI, and colorectal cancer risk is also suggested by the modest incremental effect of BMI and the interactions observed between physical activity and diet.
The finding that the proportion of SES-related risk of right-sided colon cancers mediated by health behaviors was about four times that for rectal cancers dovetails into evidence of biological or etiological differences between right and left colon cancers (20,21,37). Our results also show that differences in behavioral risk factors and obesity do not account for all of the excess risk of rectal and left colon cancers associated with low SES. It follows that other factors, such as access to and use of health care services, as noted in our conceptual model (Figure 1), may contribute to the SES effect. People from low-SES populations have lower rates of colorectal cancer screening (38,39). Screening has been shown to reduce the risk of incidence of colorectal cancer (40,41), and there is evidence that screening may be more effective for left colon and rectal cancers than for the right colon cancers (42). Thus, the differences in the magnitude of the relationships based on the location of the tumors suggest that the unexplained proportions may be because of differences in utilization of colorectal cancer screening. However, the extent to which differential use of screening contributes to SES disparities according to tumor location, independent of behavioral factors, needs further study with prospectively collected data on screening use. Because our analyses estimated percentage changes in regression coefficients due to the mediators, our inability to account for use of colorectal cancer screening, although a limitation, is unlikely to have affected the findings.
There are other limitations of this study. The neighborhood socioeconomic measures were collected from census data in 2000, which were linked to addresses in 1995 or 1996. Thus, our analyses did not take into account changes from baseline in place of residence or health behaviors. This limitation may have affected our results, but the effect is likely to be nondifferential.
In conclusion, adverse health behaviors contribute to the risk of colorectal cancer (18). This study showed that over one-third of the excess risk of invasive adenocarcinoma of the colon and rectum resulting from low SES could be explained by differences in exposure to behavioral risk factors, particularly an unhealthy diet. A healthful lifestyle has numerous health benefits (17) and may avert up to 70% of new-onset colorectal cancers in the United States (12). Our findings suggest that interventions that achieve long-term sustained healthy diets, regular physical activity, nonsmoking status, and a healthy weight in low-SES individuals and communities could reduce SES disparities in colorectal cancer risk. However, future prospective studies are needed to confirm if such long-term interventions reduce the risk of colorectal cancer in low-SES populations.
Funding
This report was supported in part by awards from the National Cancer Institute at the National Institutes of Health (K01CA127118 and U01 CA151736 to CAD, U54CA091431-09S1 to AOL, and R01 CA137750 to MS). This analysis was conducted in collaboration with intramural scientists at the National Cancer Institute, who participated in the analysis and interpretation of the data, the writing of the manuscript, and the decision to submit it for publication. However, the contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the National Cancer Institute. The authors have no conflicts of interest.
Notes
Cancer incidence data from the Atlanta metropolitan area were collected by the Georgia Center for Cancer Statistics, Department of Epidemiology, Rollins School of Public Health, Emory University. Cancer incidence data from California were collected by the California Department of Health Services, Cancer Surveillance Section. Cancer incidence data from the Detroit metropolitan area were collected by the Michigan Cancer Surveillance Program, Community Health Administration, State of Michigan. The Florida cancer incidence data used in this report were collected by the Florida Cancer Data System (FCDC) under contract with the Florida Department of Health (FDOH). The views expressed herein are solely those of the authors and do not necessarily reflect those of the FCDC or FDOH. Cancer incidence data from Louisiana were collected by the Louisiana Tumor Registry, Louisiana State University Medical Center in New Orleans. Cancer incidence data from New Jersey were collected by the New Jersey State Cancer Registry, Cancer Epidemiology Services, New Jersey State Department of Health and Senior Services. Cancer incidence data from North Carolina were collected by the North Carolina Central Cancer Registry. Cancer incidence data from Pennsylvania were supplied by the Division of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, Pennsylvania. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions. Cancer incidence data from Arizona were collected by the Arizona Cancer Registry, Division of Public Health Services, Arizona Department of Health Services. Cancer incidence data from Texas were collected by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services.
We are indebted to the participants in the NIH-AARP Diet and Health Study for their outstanding cooperation. We also thank Sigurd Hermansen and Kerry Grace Morrissey from Westat for ascertainment and management of study outcomes and Michael Spriggs and Leslie Carroll at Information Management Services for data support.
References
- 1. Doubeni CA, Laiyemo AO, Major JM, Schootman M, Lian M, Park Y, et al. Socioeconomic status and the risk of colorectal cancer: an analysis of over one-half million adults in the National Institutes of Health-AARP Diet and Health Study Cancer. 2012. 118(14):3636–3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim D, Masyn KE, Kawachi I, Laden F, Colditz GA. Neighborhood socioeconomic status and behavioral pathways to risks of colon and rectal cancer in women. Cancer 2010. 116(17):4187––4196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer 2007. 110(10):2119––2152 [DOI] [PubMed] [Google Scholar]
- 4. Lynch JW, Kaplan GA, Salonen JT. Why do poor people behave poorly? Variation in adult health behaviours and psychosocial characteristics by stages of the socioeconomic lifecourse. Soc Sci Med 1997. 44(6): 809––819 [DOI] [PubMed] [Google Scholar]
- 5. Nicklett EJ, Szanton S, Sun K, et al. Neighborhood socioeconomic status is associated with serum carotenoid concentrations in older, community-dwelling women. J Nutr 2011. 141(2):284––289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diez-Roux AV, Nieto FJ, Caulfield L, Tyroler HA, Watson RL, Szklo M. Neighbourhood differences in diet: the Atherosclerosis Risk in Communities (ARIC) Study. J Epidemiol Community Health 1999. 53(1):55––63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stringhini S, Sabia S, Shipley M, et al. Association of socioeconomic position with health behaviors and mortality. JAMA 2010. 303(12):1159––1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lantz PM, House JS, Lepkowski JM, Williams DR, Mero RP, Chen J. Socioeconomic factors, health behaviors, and mortality: results from a nationally representative prospective study of US adults. JAMA 1998. 279(21):1703––1708 [DOI] [PubMed] [Google Scholar]
- 9. Doubeni CA, Schootman M, Major JM, et al. Health status, neighborhood socioeconomic context, and premature mortality in the United States: The National Institutes of Health-AARP Diet and Health Study. Am J Public Health 2012. 102(4):680––688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang VW, Lauderdale DS. Income disparities in body mass index and obesity in the United States, 1971–2002. Arch Intern Med 2005. 165(18):2122––2128 [DOI] [PubMed] [Google Scholar]
- 11. Drewnowski A, Specter SE. Poverty and obesity: the role of energy density and energy costs. Am J Clin Nutr 2004. 79(1):6––16 [DOI] [PubMed] [Google Scholar]
- 12. Willett WC. Diet and cancer: an evolving picture. JAMA 2005. 293(2):233––234 [DOI] [PubMed] [Google Scholar]
- 13. Ferguson LR. Recent advances in understanding of interactions between genes and diet in the etiology of colorectal cancer. World J Gastrointest Oncol 2010. 2(3):125––129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Halle M, Schoenberg MH. Physical activity in the prevention and treatment of colorectal carcinoma. Dtsch Arztebl Int 2009. 106(44):722––727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qasim A, O’Morain C. Primary prevention of colorectal cancer: are we closer to reality? Eur J Gastroenterol Hepatol 2010. 22(1):9––17 [DOI] [PubMed] [Google Scholar]
- 16. Winzer BM, Whiteman DC, Reeves MM, Paratz JD. Physical activity and cancer prevention: a systematic review of clinical trials. Cancer Causes Control 2011. 22(6):811––826 [DOI] [PubMed] [Google Scholar]
- 17. Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA 2004. 291(10):1238––1245 [DOI] [PubMed] [Google Scholar]
- 18. Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010. 116(3):544––573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med 1995. 122(5):327––334 [DOI] [PubMed] [Google Scholar]
- 20. Deng G, Kakar S, Tanaka H, et al. Proximal and distal colorectal cancers show distinct gene-specific methylation profiles and clinical and molecular characteristics. Eur J Cancer 2008. 44(9):1290––1301 [DOI] [PubMed] [Google Scholar]
- 21. Freeman HJ. Heterogeneity of colorectal adenomas, the serrated adenoma, and implications for screening and surveillance. World J Gastroenterol 2008. 14(22):3461––3463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol 2001. 154(12):1119––1125 [DOI] [PubMed] [Google Scholar]
- 23. Messer LC, Laraia BA, Kaufman JS, et al. The development of a standardized neighborhood deprivation index. J Urban Health 2006. 83(6):1041––1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thompson FE, Kipnis V, Midthune D, et al. Performance of a food-frequency questionnaire in the US NIH-AARP (National Institutes of Health-American Association of Retired Persons) Diet and Health Study. Public Health Nutr 2008. 11(2):183––195 [DOI] [PubMed] [Google Scholar]
- 25. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 2003. 348(26):2599––2608 [DOI] [PubMed] [Google Scholar]
- 26. Reedy J, Mitrou PN, Krebs-Smith SM, et al. Index-based dietary patterns and risk of colorectal cancer: the NIH-AARP Diet and Health Study. Am J Epidemiol 2008. 168(1):38––48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frome EL. The analysis of rates using Poisson regression models. Biometrics 1983. 39(3):665––674 [PubMed] [Google Scholar]
- 28. Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods 2002. 7(4):422––445 [PubMed] [Google Scholar]
- 29. Kushi LH, Byers T, Doyle C, et al. ; American Cancer Society 2006 Nutrition and Physical Activity Guidelines Advisory Committee American Cancer Society Guidelines on Nutrition and Physical Activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin 2006. 56(5):254–2–81; quiz 313 [DOI] [PubMed] [Google Scholar]
- 30. World Cancer Research Fund and American Institute for Cancer Research Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective World Cancer Research Fund and American Institute for Cancer Research: Washington: DC; 2007. [Google Scholar]
- 31. Simon MS, Thomson CA, Pettijohn E, et al. Racial differences in colorectal cancer incidence and mortality in the Women’s Health Initiative. Cancer Epidemiol Biomarkers Prev 2011. 20(7):1368––1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim YS, Milner JA. Dietary modulation of colon cancer risk. J Nutr 2007. 137(11 suppl):2576S––2579S [DOI] [PubMed] [Google Scholar]
- 33. Sung MK, Bae YJ. Linking obesity to colorectal cancer: application of nutrigenomics. Biotechnol J 2010. 5(9):930––941 [DOI] [PubMed] [Google Scholar]
- 34. Zisman AL, Nickolov A, Brand RE, Gorchow A, Roy HK. Associations between the age at diagnosis and location of colorectal cancer and the use of alcohol and tobacco: implications for screening. Arch Intern Med 2006. 166(6):629––634 [DOI] [PubMed] [Google Scholar]
- 35. Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation—AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol (Lond) 2006. 574(pt 1):63––71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ashrafian H. Cancer’s sweet tooth: the Janus effect of glucose metabolism in tumorigenesis. Lancet 2006. 367(9510):618––621 [DOI] [PubMed] [Google Scholar]
- 37. Rex DK, Eid E. Considerations regarding the present and future roles of colonoscopy in colorectal cancer prevention. Clin Gastroenterol Hepatol 2008. 6(5):506––514 [DOI] [PubMed] [Google Scholar]
- 38. Doubeni CA, Laiyemo AO, Klabunde CN, Young AC, Field TS, Fletcher RH. Racial and ethnic trends of colorectal cancer screening among Medicare enrollees. Am J Prev Med 2010. 38(2):184––191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Doubeni CA, Laiyemo AO, Reed G, Field TS, Fletcher RH. Socioeconomic and racial patterns of colorectal cancer screening among Medicare enrollees in 2000 to 2005. Cancer Epidemiol Biomarkers Prev 2009. 18(8):2170––2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Atkin WS, Edwards R, Kralj-Hans I, et al. ; UK Flexible Sigmoidoscopy Trial Investigators Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 2010. 375(9726):1624––1633 [DOI] [PubMed] [Google Scholar]
- 41. Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med 2000. 343(22):1603––1607 [DOI] [PubMed] [Google Scholar]
- 42. Brenner H, Hoffmeister M, Arndt V, Stegmaier C, Altenhofen L, Haug U. Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study. J Natl Cancer Inst 2010. 102(2):89––95 [DOI] [PubMed] [Google Scholar]