Abstract
The accumulation of aggregated amyloid-β (Aβ) in amyloid plaques is a neuropathological hallmark of Alzheimer's disease (AD). Reactive astrocytes are intimately associated with amyloid plaques; however, their role in AD pathogenesis is unclear. We deleted the genes encoding two intermediate filament proteins required for astrocyte activation—glial fibrillary acid protein (Gfap) and vimentin (Vim)—in transgenic mice expressing mutant human amyloid precursor protein and presenilin-1 (APP/PS1). The gene deletions increased amyloid plaque load: APP/PS1 Gfap−/−Vim−/− mice had twice the plaque load of APP/PS1 Gfap+/+Vim+/+ mice at 8 and 12 mo of age. APP expression and soluble and interstitial fluid Aβ levels were unchanged, suggesting that the deletions had no effect on APP processing or Aβ generation. Astrocyte morphology was markedly altered by the deletions: wild-type astrocytes had hypertrophied processes that surrounded and infiltrated plaques, whereas Gfap−/−Vim−/− astrocytes had little process hypertrophy and lacked contact with adjacent plaques. Moreover, Gfap and Vim gene deletion resulted in a marked increase in dystrophic neurites (2- to 3-fold higher than APP/PS1 Gfap+/+Vim+/+ mice), even after normalization for amyloid load. These results suggest that astrocyte activation limits plaque growth and attenuates plaque-related dystrophic neurites. These activities may require intimate contact between astrocyte and plaque.—Kraft, A. W., Hu, X., Yoon, H., Yan, P., Xiao, Q., Wang, Y., Gil, S. C., Brown, J., Wilhelmsson, U., Restivo, J. L., Cirrito, J. R., Holtzman, D. M., Kim, J., Pekny, M., Lee, J.-M. Attenuating astrocyte activation accelerates plaque pathogenesis in APP/PS1 mice.
Keywords: Alzheimer's disease, GFAP, astrogliosis, intermediate filaments, vimentin
Amyloid plaques are a neuropathological hallmark of Alzheimer's disease (AD). Equally prominent are reactive astrocytes and microglia that surround and intimately associate with amyloid plaques. Traditionally, reactive gliosis in AD and other neurodegenerative disorders was viewed as a component of an inflammatory cascade with neurotoxic consequences (1–3). However, reactive glial cells may play a protective role by suppressing plaque progression. For example, increased gliosis induced by brain infusion of lipopolysaccharide (4) or overexpression of the inflammatory cytokine interleukin-6 (5) or interferon-γ (6) markedly reduces amyloid plaque burden. However, direct evidence that reactive gliosis suppresses plaque accumulation is lacking.
Much attention has focused on microglia in AD pathogenesis. Early ultrastructural studies in human brain specimens suggested that microglia can engulf amyloid-β (Aβ) and sequester it in endosome-like compartments (7). In vitro studies subsequently demonstrated that microglia can internalize and clear Aβ (8). More recent studies in transgenic mouse models of AD have shown that microglia migrate to amyloid plaques and may alter plaque growth dynamics (9–12). However, near-complete ablation of microglia in transgenic mice expressing mutant human amyloid precursor protein (APP) and presenilin 1 (PS1) had no effect on plaque deposition or neuritic dystrophy (13), raising questions about the role of endogenous microglia in plaque clearance.
In comparison to microglia, the role of astrocytes in plaque pathogenesis has been largely understudied. Astrocytes, which constitute 50% of the cells in the cortex (far outnumbering microglia), are critical in brain development, provide metabolic support for neurons, regulate synaptic function, and are important modulators of nervous system injury and repair (14).
Astrocytes are activated in response to acute or chronic central nervous system injury. The activated state is characterized by hypertrophy of proximal processes and up-regulation of the intermediate filament (nanofilament) proteins glial fibrillary acid protein (GFAP) and vimentin (Vim) (15, 16). Absence of GFAP and/or Vim in mice has no major effect under physiological conditions (17–20), reflecting the role of intermediate filament (nanofilament) system as a signaling platform in situations connected with cellular stress (21). However, after brain or spinal cord injury, reactive gliosis and the formation of glial scar are attenuated and the healing process is prolonged (22). On a cellular level, astrocytes of Gfap−/−Vim−/− mice in the injured area do not develop the characteristic hypertrophy of proximal processes (23). They are completely devoid of intermediate filaments since neither of the other two intermediate filament proteins expressed in reactive astrocytes, nestin and synemin, are able to form intermediate filaments in the absence of both GFAP and Vim (20, 22, 24). The astrocyte intermediate filament system has been linked to astrocyte motility (25), viscoelastic properties of astrocytes (26), intracellular vesicle trafficking (27–29), activation of c-fos and Erk (30), resistance to hypoosmotic stress (31), and the efficiency of glutamate transport and gap junctional communication within the astrocyte syncytium (32). Attenuated reactive gliosis in Gfap−/− Vim−/− mice was connected with larger infarctions in a mouse model of focal brain ischemia (32) and facilitated disease progression in a mouse model of Batten's disease (33). Interestingly, Gfap−/−Vim−/− mice exhibit better posttraumatic regeneration of neuronal synapses (23) and axons (34), increased baseline and posttraumatic neurogenesis (35), and improved functional recovery after spinal cord trauma (36). They also support better integration of neural grafts in the retina (37) and neuronal and astrocyte differentiation of neural stem cells transplanted in the hippocampus (38). Thus, as supported also by results from other transgenic models (39, 40), the benefits of reactive gliosis at acute stages of injuries might be balanced against restricted regenerative potential later on.
With the exception of Batten's disease (33), the role of astrocyte intermediate filament proteins in chronic neurodegenerative diseases has not been investigated. In 2003, Wyss-Coray et al. (41) demonstrated that cultured adult astrocytes can migrate to and degrade amyloid plaques in brain slices from aged APP transgenic mice. Moreover, this plaque-degrading activity in astrocytes was subsequently found to be dependent on apolipoprotein E (apoE) expression (42). In the current study, we examined the in vivo role of reactive astrocytes in plaque pathogenesis in APP transgenic mice by deleting Gfap and Vim.
MATERIALS AND METHODS
Transgenic mice
APPswe/PS1ΔE9 transgenic mice (APP/PS1; ref. 43; Jackson Laboratories, Bar Harbor, ME, USA) were crossed with Gfap−/−Vim−/− mice (22). Because the two knockout genes and transgene segregated independently, this cross required 3 generations of crosses to produce APP/PS1 Gfap+/+Vim+/+ and APP/PS1 Gfap−/−Vim−/− mice: 1) APP/PS1+/− Gfap+/+ Vim+/+ × APP/PS1−/− Gfap−/− Vim−/−; 2) APP/PS1+/− Gfap+/− Vim+/− × APP/PS1−/− Gfap+/− Vim+/−; and 3) APP/PS1+/− Gfap−/− Vim−/− × APP/PS1−/− Gfap−/− Vim−/− or APP/PS1+/− Gfap+/+ Vim+/+ × APP/PS1−/− Gfap+/+ Vim+/+. Mice of both genotypes were maintained on the same mixed backgrounds (B6C3 × C57BL 129SV 129Ola).
Tissue preparation
Mice were deeply anesthetized with isoflurane and transcardially perfused with 0.01 M PBS. The brains were removed, and the hemispheres were separated. Left hemispheres were immediately dissected, snap-frozen on dry ice, and stored at −80°C for biochemical analysis. Right hemispheres were fixed in 4% paraformaldehyde for 24 h and transferred to 30% sucrose in 0.2 M PBS. After brains were saturated with fixative, they were snap-frozen on dry ice and stored at −80°C. Coronal sections, 50 μm thick, were made with a sliding microtome and stored in 0.2 M PBS, 30% sucrose, and 30% ethylene glycol at −20°C.
Immunohistochemistry
Sections were permeabilized with 0.3% Tween-20 in Tris-buffered saline (TBS-T20) for 10 min, and endogenous peroxidase activity was quenched by a 10-min treatment of 0.3% H2O2 solution in TBS. Tissue was washed with TBS, blocked with 3% dry milk in TBS-T20 for 1 h, and incubated with primary antibody overnight. A fresh solution of streptavidin and horseradish peroxidase-conjugated biotin (1:400, Vector Laboratories, Burlingame, CA, USA) was applied to tissue for 90 min, followed by 0.025% 3-3′-diaminobenzadine tetrachloride in 0.25% NiCl and 0.05% H2O2 for 15 min. The slices were placed on glass slides, dried overnight, dehydrated, and mounted. Primary antibodies were mouse anti-Aβ antibody (HJ3.4, 1:1000; ref. 44), rabbit anti-reticulon-3 (RTN-3) antibody (1:4000; a gift from Dr. Riqiang Yan, Lerner Research Institute, Cleveland Clinic, Cleveland, OH, USA; ref. 45), and rabbit anti-synaptophysin antibody (1:200; Cell Signaling, Danvers, MA, USA).
Immunofluorescence
After perfusion fixation (as above), brains were postfixed for 4 h in the same fixation solution. Free-floating sections (50 μm) were cut and collected. Rabbit anti-Iba-1 antibody (1:1000; Wako Pure Chemicals Industries, Tokyo, Japan), rabbit anti-glutamine synthetase (GS; 1:4000; Abcam, Cambridge, MA, USA), or mouse anti-neuronal nuclei (NeuN, 1:400; Millipore, Billerica, MA, USA) was applied to the sections overnight at 4°C. Sections were incubated with Alexa Fluor 488-conjugated donkey anti-mouse IgG antibody (Alexa Fluor 488; 1:400; Molecular Probes, Grand Island, NY, USA). Sections were washed, mounted, and examined by confocal microscopy (Carl Zeis, Thornwood, NY, USA).
Plaque staining
Brain slices were mounted on glass slides. Tissue was permeablized with 0.25% Triton for 30 min and stained with X-34 (gift from Robert Mach, Mallinckrodt Institute of Radiology, Washington University School of Medicine, St. Louis, MO, USA) dissolved in a solution of 40% ethanol and 60% water, pH 10, for 20 min. Tissue was then rinsed in distilled water and mounted. For FD Neurosilver staining (MTR Scientific, Ijamsville, MD, USA), fixed sections were stained according to the manufacturer's protocol.
Aβ ELISA
Dissected cortices were homogenized in PBS and then in 5 mM guanidine HCL in TBS (pH 8). After each homogenization step, samples were centrifuged at 12,000 rpm for 20 min, and supernantants were collected for ELISA. Pellets were used for subsequent extraction steps. All ELISA samples were diluted in a final buffer consisting of 0.25% BSA, 500 mM guanidine, and 200 mM Tris-PBS (pH 7.4), with protease inhibitors. Antibodies HJ2 (anti-Aβ35–40) and HJ7.4 (anti-Aβ37–42) were used to capture Aβx-40 and Aβx-42, respectively; biotinylated HJ5.1 (anti-Aβ13–28) was used to detect Aβ (44). Aβ1-x was captured with m266 antibody (anti-Aβ13–28) and detected with biotinylated 3D6 antibody (anti-Aβ1–5) (44). Streptavidin-poly-horseradish peroxidase-40 (Research Diagnostics, Flanders, NJ, USA) was added, and slow ELISA TMB (Sigma, St. Louis, MO, USA) was used to develop the peroxidase. A BioTek 600FL microtiter plate reader (BioTek, Winooski, VT, USA) 600FL was used for colorimetric detection and analysis. Standard curves were generated from synthetic Aβ peptide (American Peptide, Sunnyvale, CA, USA).
Real-time quantitative PCR (qPCR)
Messenger RNA was extracted from frozen cortical tissue using TRIzol, and reverse transcribed with the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Carlsbad, CA, USA). qPCR was performed using the ABI 7500 in the default thermal cycling mode with Power SYBR (Applied Biosystems, Foster City, CA, USA) using the primers shown in Supplemental Table S1. Mouse β-actin was used as a normalization reference. To confirm the specificity of qPCR reactions, dissociation curves were analyzed at the end of qPCR assay. Relative mRNA levels were calculated using the comparative Ct method, and expressed as a percentage of control (APP/PS1 Gfap+/+ Vim+/+).
Western blotting
Dissected cortices were homogenized in 1% Triton X-100 in PBS and centrifuged at 12,000 rpm for 20 min, and supernantants were collected. Protein concentration was determined with the BCA kit (Pierce, Rockford, IL, USA). Equal amounts of protein from each sample were run on 3–8% Tris acetate or 4–12% Bis-Tris XT gels (Bio-Rad, Hercules, CA, USA) and transferred to PVDF membranes with the iBlot dry blotting system (Invitrogen, Grand Island, NY, USA). Membranes were blocked with 5% dry milk in 1% Triton X-100 in TBS for 1 h. Membranes were probed with the following antibodies diluted in 2.5% dry milk in 1% Triton X-100 in TBS overnight: APP and its C-terminal fragment (1:1000; Invitrogen, Grand Island, NY, USA), matrix metalloproteinase 2 (MMP-2; 1:200; Abcam, Cambridge, MA, USA), matrix metalloproteinase 9 (MMP-9; 1:200; Abcam), apoE (1:30000; Calbiochem, Darmstadt, Germany), apolipoprotein J (apoJ; 1:4000; Covance, Princeton, NJ, USA), and S100B (1:200; Dako, Houston, TX, USA). Membranes were washed and probed with biotinylated secondary antibodies (1:5000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and visualized with Pico West (Pierce) or Amersham ECL Advance (GE Healthcare, Piscataway, NJ, USA) kits. Normalized band intensity was quantified with ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA; ref. 44).
In vivo microdialysis
In vivo microdialysis to assess brain interstitial fluid (ISF) Aβ in the hippocampus of awake, freely moving mice was performed as described previously (46), to sample soluble molecules (<30 kDa) in the ISF. Aβ capable of entering the probe has been dubbed “exchangeable Aβ” (eAβ). Under isoflurane anesthesia, guide cannulas (BR-style; Bioanalytical Systems, West Lafayette, IN, USA) were cemented into the left hippocampus (3.1 mm behind the bregma, 2.5 mm lateral to the midline, and 1.2 mm below the dura at a 12° angle). Microdialysis probes (2 mm) were inserted through the guides so the membrane was contained entirely within the hippocampus (BR-2, 38-kDa molecular mass cutoff membrane; Bioanalytical Systems). Microdialysis perfusion buffer was artificial cerebrospinal fluid containing 4% BSA (Sigma) that was filtered through a 0.1-μm membrane. Flow rate was a constant 1.0 μl/min. Samples were collected every 60 min with a refrigerated fraction collector into polypropylene tubes and assessed for Aβ1-x by ELISA (as described above) after each experiment. ISF Aβ half-life was determined as described previously (46). After steady-state ISF Aβ levels were established, mice received an intraperitoneal injection of compound E (20 mg/kg), a potent, blood-brain permeable γ-secretase inhibitor (ref. 47; synthesized by AsisChem, Waltham, MA, USA) to rapidly block Aβ production. Microdialysis samples were collected every 60 min for 6 h and assayed for Aβx-40 by ELISA. The half-life of ISF Aβ was calculated from the slope of the semilog plot of percentage change in Aβ vs. time (46). Only Aβ values that were continually decreasing were included in half-life analysis (the first 3 h after compound E administration).
Viral vectors
An adeno-associated virus 2 (AAV2) construct (Stratagene, Santa Clara, CA, USA) was modified as follows. The 1.2-kb cytomegalovirus promoter and β-globin intron were removed from the plasmid using MluI and SalI restriction sites and replaced with the GFAP promoter and green fluorescent protein (GFP). The construct was transformed into DH5α bacterial cells, which were grown on ampicillin-containing medium overnight. The Hope Center Viral Core (Washington University, St. Louis, MO, USA) generated AAV2 virions containing our construct at 6 × 1011 viral genomes/ml.
Stereotactic injection
AAV-GFAP-GFP (2 μl) was injected into the cortex of 12-mo-old APP/PS1 Gfap+/+Vim+/+ and APP/PS1 Gfap−/−Vim−/− mice (n=3/group) at the following stereotactic coordinates: 2.0, ±1.7, −0.5. The virus was slowly infused through a 30-gauge needle (0.2 μl/min) over 10 min; the syringe was left in place for an additional 10 min before it was slowly withdrawn. In each mouse, the AAV was allowed to incubate for 4 wk.
Plaque quantification
Brain sections (50 μm) were collected every 600 μm from the rostral anterior commissure to the caudal hippocampus. The sections (n=9–10/group) were stained with X-34 or immunostained with HJ3.4 antibodies and imaged with a ×20 0.75 N.A. objective on a NanoZoomer digital scanner (Hamamatsu, Bridgewater, NJ, USA) to create high-resolution digital images (TIFF). Individual images were exported to ImageJ. Cortex and hippocampus were manually delineated, and brightness thresholding was used to delineate plaques. For each slice, the total area of plaque coverage was measured in the cortex and hippocampus, expressed as percentage of total area in each mouse and averaged for each group as described previously (44). For individual plaque analysis of RTN-3 burden and corresponding plaque size, images of randomly selected plaques from stained brain sections were captured using a ×60 1.4 N.A. oil immersion lens on a Nikon Eclipse E800 (Nikon, Tokyo, Japan) with attached Optronics camera (Optronics, Goleta, CA, USA), exported to ImageJ, and quantified by using brightness-thresholding to delineate dystrophic neurites and manual tracing to delineate plaque profiles. Cross-sectional areas of both dystrophic neurites and plaque size were measured and graphed on a scatterplot by genotype. All imaging in this study was performed at room temperature.
Stereology
RTN-3-stained slices were collected every 300 μm (n=5/group). Dystrophic neurites in the cortex were quantified using unbiased stereology (Cavalieri-point counting method) with a ×60 1.4 N.A. oil immersion lens on a Nikon Eclipse E800 with attached Optronics camera as described previously (48).
Astrocyte, microglia, and neuron quantification
Brain sections labeled with GS, Iba-1, or NeuN for immunofluorescence were systematically collected every 300 μm, while AAV-GFAP-GFP-transfected tissue was collected 100 and 200 μm on either side of the injection needle track (4 slices/mouse, n=3/group). Labeled sections were imaged by confocal microscopy (Zeiss Axiovert 200 M with LSM 5 Pa) with a ×40 1.2 N.A water immersion or a ×67 1.4 N.A. oil immersion objective (Zeiss, Thornwood, NY, USA) to image several XY planes along a Z-axis. Images were exported to ImageJ for quantification of cell count and plaque invasion (overlap between cell and plaque staining). Some confocal Z stacks were used to reconstruct 3-dimensional images for rotation and captured on video.
Statistical analysis
Various measures in APP/PS1 Gfap+/+Vim+/+ and APP/PS1 Gfap−/−Vim−/− mice were compared using two-tailed Student's t test when data were normally distributed. Normality was tested with the Kolmogorov-Smirnov test (SigmaStat 2.0 statistical software; Systat Software, Chicago, IL, USA). For data with non-normal distributions or insufficient sample sizes to test normality, the Mann-Whitney U test was performed. The level of significance was set at P ≤ 0.05.
RESULTS
Gfap/Vim deletion exacerbates amyloid plaque pathogenesis
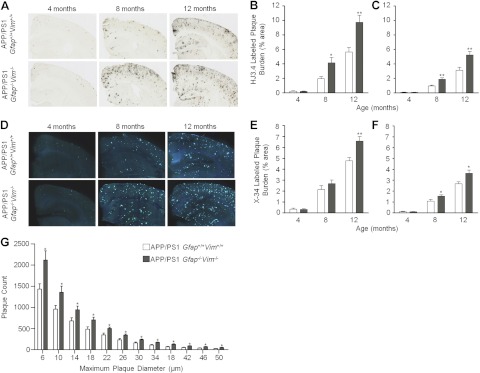
APP/PS1 mice (APPswe PS1ΔE9; ref. 43) were crossed with Gfap−/−Vim−/− mice (20, 22) to produce APP/PS1 Gfap+/+Vim+/+ and APP/PS1 Gfap−/−Vim−/− mice. Mice of both genotypes were grossly normal and fertile, without obvious behavioral or anatomical differences. Histological examination of neurons using cresyl violet staining revealed no qualitative differences in neuronal layers of the cortex or hippocampus (Supplemental Fig. S1). Quantitative analysis using NeuN immunofluorescence in regions of the cortex did not reveal differences in neuronal density (Supplemental Fig. S1I–K). Brain sections from these mice were immunostained with anti-Aβ antibody (HJ3.4) to quantify Aβ plaque load or stained with X-34 to quantify amyloid load. Aβ load and amyloid load were significantly increased in the cortex and hippocampus of APP/PS1 Gfap−/−Vim−/− mice at 8 and 12 but not 4 mo of age (Fig. 1A–F). In APP/PS1 mice, plaque accumulation begins at ∼4 mo of age and increases thereafter. These data suggest that Gfap and Vim deletions do not influence the initial deposition of plaques but exacerbate subsequent plaque accumulation, consistent with the contention that Gfap/Vim deletion selectively affects astrocyte activation—an event that occurs after initial plaque formation. By examining the frequency distribution of plaque sizes in 12-mo-old mice, it is apparent that the increase in plaque load is a result of increased plaque numbers of all sizes (Fig. 1G).
Figure 1.
Deletion of Gfap and Vim accelerates amyloid plaque pathogenesis. A–F) Brain sections from APP/PS1 Gfap+/+Vim+/+ and APP/PS1 Gfap−/−Vim−/− mice were immunostained with anti-Aβ antibodies (A) or stained with X-34 to label plaques (D). Gfap and Vim deletion increased Aβ-immunostained plaque load in the cerebral cortex (B) and hippocampus (C) at 8 and 12 mo of age, but there was no difference at 4 mo in either region. X-34-stained “compact” plaque load was also increased in cerebral cortex (E) and hippocampus (F) in APP/PS1 Gfap−/−Vim−/− mice. G) Size-frequency histogram of X-34-labeled amyloid plaques in 12-mo-old APP/PS1 Gfap+/+Vim+/+ (open bars) and APP/PS1 Gfap−/−Vim−/− (shaded bars) mice. APP/PS1 Gfap−/−Vim−/− mice had more plaques in every size category. Values are expressed as means ± se; n = 8–11 mice/group. *P < 0.05, **P < 0.01.
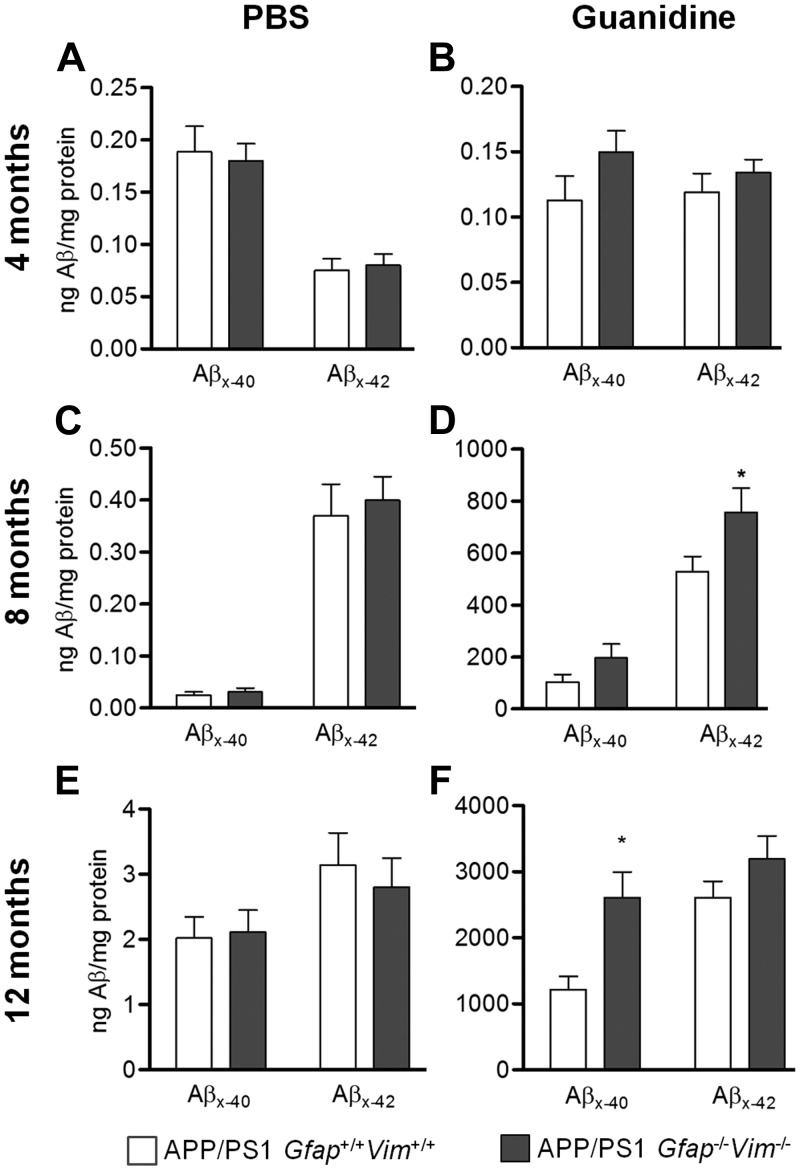
Plaque load was corroborated by Aβ quantification with ELISA after serial extractions of brain tissue with PBS and guanidine. Consistent with the plaque load data, no difference in Aβ levels was detected between genotypes at 4 mo of age. At 8 and 12 mo, APP/PS1 Gfap−/− Vim−/− mice had significantly higher Aβ levels in the guanidine extract but not in the PBS fraction (Fig. 2). These data suggest that Gfap and Vim deletion selectively alters insoluble (plaque-associated) Aβ but not the soluble pools of Aβ in APP/PS1 mice.
Figure 2.
Insoluble Aβ is increased in aged APP/PS1 Gfap−/−Vim−/− mice. Cortices dissected from APP/PS1 Gfap+/+Vim+/+ and APP/PS1 Gfap−/−Vim−/− mice were extracted in PBS (A, C, E) and then in guanidine (B, D, F) at 4 (A, B), 8 (C, D), and 12 (E, F) mo of age. Consistent with plaque load data, Aβx-42 in guanidine extracts was increased in 8-mo-old APP/PS1 Gfap−/−Vim−/− mice, while Aβx-40 demonstrated a trend for increase (P=0.14; D). Similarly, in 12-mo-old mice, Aβx-40 was significantly higher in APP/PS1 Gfap−/−Vim−/− mice, while Aβx-42 was not (F). PBS-soluble fractions showed no difference between genotypes at all ages. n = 7–12 mice/group. *P < 0.05.
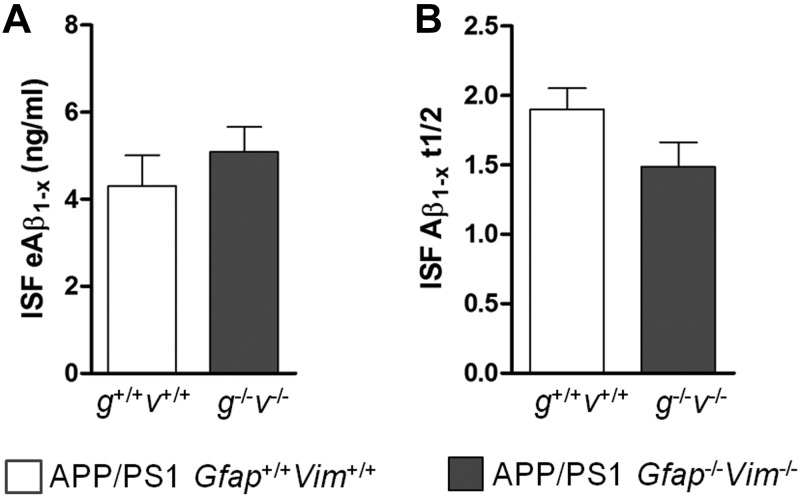
To further examine the effects of Gfap and Vim deletion on Aβ levels in ISF, in vivo microdialysis was performed in 3-mo-old mice. Microdialysis probes were implanted unilaterally in the hippocampus of APP/PS1 Gfap+/+Vim+/+ and APP/PS1 Gfap−/−Vim−/− mice under isoflurane anesthesia. After probe implantation, mice remained awake with freedom of movement throughout the experiment. Steady-state measurements of ISF Aβ levels revealed no difference in mean exchangeable Aβ1-x levels between the APP/PS1 Gfap−/− Vim−/− mice and APP/PS1 Gfap+/+Vim+/+ mice (Fig. 3A). To determine whether the gene deletions affect Aβ turnover, mice were treated with the γ-secretase inhibitor compound E, and Aβ levels in ISF were measured to create a decay curve from which the ISF Aβ half-life was calculated. The ISF Aβ half-life was unchanged by deletion of Gfap and Vim (Fig. 3B). Thus, Gfap and Vim deletion does not alter ISF Aβ levels or metabolism but selectively alters amyloid plaque load.
Figure 3.
ISF Aβ is unchanged by Gfap and Vim deletion. In vivo microdialysis was performed in the hippocampi of APP/PS1 Gfap+/+Vim+/+ and APP/PS1 Gfap−/−Vim−/− mice. A) Steady-state levels of Aβ1-x were similar in mice of both genotypes. B) The half-life of Aβ was also similar, suggesting that the deletions did not alter the Aβ metabolism in these mice.
Gfap/Vim deletion exacerbates neuritic dystrophy
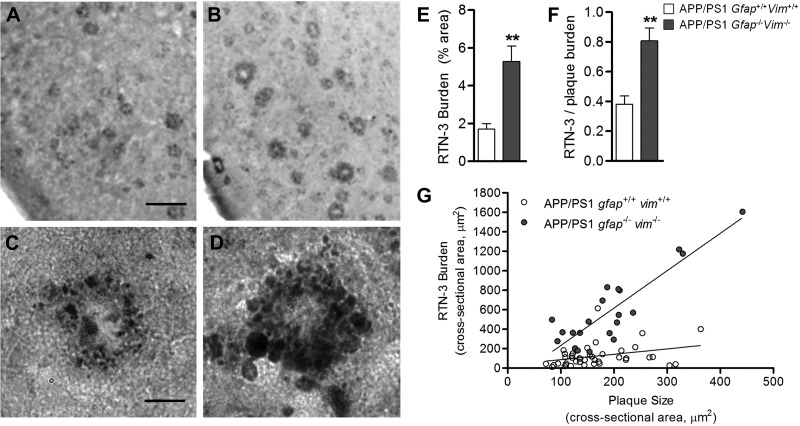
In general, APP transgenic mice do not show significant neuronal cell death or brain atrophy, in contrast to brains of patients with AD (49). However, APP mice develop dystrophic neurites immediately around plaques (50), suggesting that this neuronal pathology is independent of cell death. We examined the impact of Gfap and Vim deletion on neuritic dystrophy using unbiased stereological counts of brains immunostained with RTN-3, a protein that selectively accumulates in dystrophic neurites (45). At 12 mo of age, dystrophic neurites were significantly more abundant in APP/PS1 Gfap−/−Vim−/− mice than in APP/PS1 Gfap+/+Vim+/+ mice (Fig. 4A–E). Because APP/PS1 Gfap−/−Vim−/− mice have greater plaque load than APP/PS1 Gfap+/+Vim+/+ mice, we normalized dystrophic neurite counts to amyloid load and still found a significant increase (Fig. 4F). Furthermore, using a random sampling of plaques from mice of both genotypes, we plotted plaque size vs. dystrophic neurite burden (cross-sectional area). Across all sizes of plaques, dystrophic neurites were greater in APP/PS1 Gfap−/−Vim−/− (Fig. 4G). To verify labeling of dystrophic neurites with RTN-3, we performed colabeling studies using RTN-3 immunostaining and silver staining in adjacent 15-μm brain sections. RTN-3 immunostaining consistently delineated dystrophic neurites demonstrated by silver staining in APP/PS1 mice (Supplemental Fig. S2). These findings suggest that activated astrocytes either protect neurons from developing dystrophic neurites or are involved in the clearance of dystrophyic neurites.
Figure 4.
Gfap and Vim deletion exacerbates neuritic dystrophy in APP/PS1 mice. A–D) RTN-3-labeled dystrophic neurites were compared in the entorhinal cortex of 12-mo-old APP/PS1 Gfap+/+Vim+/+ mice (A, B) and APP/PS1 Gfap−/−Vim−/− mice (C, D). E, F) Deletion of Gfap and Vim increased the burden of RTN-3-labeled dystrophic neurites (E), even when normalized to plaque load (F). G) Individual plaques from mice of both genotypes were randomly imaged; plaque size (cross-sectional area) and RTN-3 burden (cross-sectional area) were measured for each plaque and plotted in a scatterplot, which demonstrates that the dystrophic neurite burden was greater in Gfap−/−Vim−/− mice across all plaque sizes. Linear regression for Gfap+/+Vim+/+ mice: y = 0.55x + 32.51, R2 = 0.77; and for Gfap−/−Vim−/− mice: y = 3.84x − 150.61, R2 = 0.10 (P<0.0001 for difference in slopes). RTN-3 immunostaining colabels silver-stained dystrophic neurites (see Supplemental Fig. S1). Scale bars = 150 μm (A, B); 20 μm (C, D). Values are expressed as means ± se; n = 5 mice/group. **P < 0.01.
Although Gfap/Vim gene deletion exacerbates neuritic dystrophy, we observed no difference in the pattern of synaptophysin immunoreactivity surrounding plaques and no difference in the amount of synaptophysin or PSD95 mRNA between the cortices of each genotype (Supplemental Fig. S3).
Gfap/Vim deletion does not alter expression or processing of APP or Aβ-degrading proteases
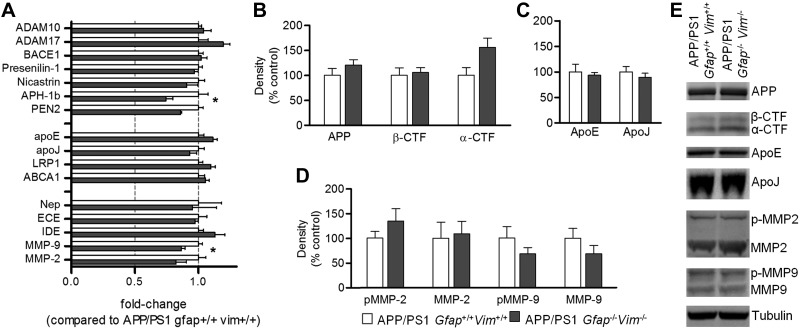
To further understand the molecular mechanisms of the effects of Gfap and Vim deletion on plaque pathogenesis, we examined the expression of genes and proteins involved in Aβ synthesis and degradation. RNA extracted from cortex of 12-mo-old mice was subjected to real-time PCR to compare transcript levels for APP processing secretases [ADAM10, ADAM17, PS1, memapsin-2, nicastrin, γ-secretase subunit anterior pharynx defective 1 homolog B (APH-1b), PEN2], lipoprotein mediators (apoE, apoJ, LRP1, ABCA1), and Aβ-degrading enzymes (neprilysin, endothelin-converting enzyme, insulin-degrading enzyme, MMP-9, MMP-2). Only APH-1b and MMP-9 showed significant, but modest, decreases (20-30% decrease) in mRNA levels in the APP/PS1 Gfap−/−Vim−/− mice (Fig. 5A); transcript levels for all other genes were unchanged. Western blot analysis showed no differences between genotypes in APP expression and C-terminal fragments (Fig. 5B, E), suggesting that Gfap and Vim gene deletion did not alter APP expression or processing. ApoE and apoJ (clusterin) showed no significant differences (Fig. 5C, E). Similarly, MMP-2 and MMP-9 showed no significant changes in APP/PS1 Gfap−/−Vim−/− mice (Fig. 5D, E), although pro-MMP9 and MMP9 showed a decreasing trend.
Figure 5.
Gfap/Vim deletion does not alter expression or processing of APP- or Aβ-degrading proteases. A) RNA extracted from cortex of 12-mo-old mice was subjected to real-time PCR to compare transcript levels for APP processing secretases, lipoprotein mediators, and Aβ-degrading enzymes (as indicated), and expressed as fold-change compared to APP/PS1 Gfap+/+ Vim+/+ mice. Of all transcripts examined, only APH-1a and MMP9 showed differences between genotypes. *P < 0.05. B–D) Protein extracted from cortex of mice was analyzed by Western blot with anti-APP (B); anti-apoE and anti-apoJ (C); and anti-MMP-2 and anti-MMP-9 (D; pMMP-2, pro-MMP-2; pMMP-9, pro-MMP-9) antibodies as indicated. There was no significant difference in expression of any protein examined. Values are means ± se; n = 4–7 mice/group. E) Representative blots comparing APP/PS1 Gfap+/+ Vim+/+ (left lane) to APP/PS1 Gfap−/− Vim−/− (right lane) mice for each of the proteins above.
Astrocytic invasion of plaques is attenuated in APP/PS1 Gfap−/−Vim−/− mice
To examine astrocyte morphology in vivo, mice were injected with AAV2 constructs carrying GFP driven by a Gfap promoter (AAV-GFAP-GFP). Because immunostaining with individual antibodies cannot reveal the full extent of astrocyte morphology (16), we expressed GFP in astrocytes in vivo to observe the complete cellular profile of the cells. Mice received a cortical injection of viral particles at 12 mo of age. After 1 mo, the mice were euthanized and processed for histology.
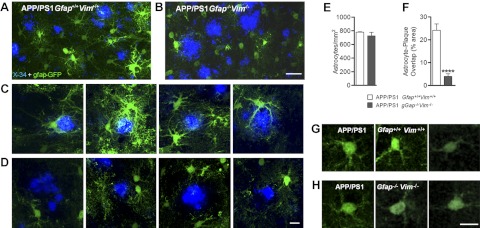
In regions surrounding plaques, astrocytes in APP/PS1 Gfap+/+Vim+/+ mice were hypertrophic with thick proximal processes, characteristic of reactive astrocytes (Fig. 6A, C). Processes were also evident deep within X-34-stained plaques, suggesting process invasion within plaques (Fig. 6C). In contrast, astrocytes from APP/PS1 Gfap−/−Vim−/− mice did not exhibit process hypertrophy, and distal processes appeared as fine strands. Moreover, little overlap between astrocyte and plaque was evident (Fig. 6B, D). In fact, regions immediately adjacent to the plaques were devoid of astrocyte processes, forming a “halo” around plaques (Fig. 6D). This difference in astrocyte interaction with plaques was even more striking in 3-dimensional reconstructions of the confocal images in Fig. 6C, D (Supplemental Videos S1 and S2).
Figure 6.
Gfap−/−Vim−/− astrocytes surrounding plaques lack the reactive phenotype. A–D) Twelve-month-old APP Gfap+/+Vim+/+ mice (A, C) and APP/PS1 Gfap−/−Vim−/− mice (B, D) were injected with AAV-GFAP-GFP to label astrocytes (green) and euthanized 1 mo later. Plaques were labeled with X-34 (blue). Low-power images from APP Gfap+/+Vim+/+ (A) and APP/PS1 Gfap−/− Vim−/− mice (B) reveal a striking difference in cell morphology, which is better appreciated at high power (C, D). While wild-type astrocytes had hypertrophic proximal processes and intimate contact with plaques in APP mice (C), the Gfap−/−Vim−/− astrocytes lack both (D), and see Supplemental Videos S1 and S2. E-F) Astrocyte cell counts revealed no difference between genotypes (E); however, quantification of regions of overlap between astrocyte processes and X-34 staining shows a marked decrease in APP/PS1 Gfap−/−Vim−/− mice (F). Values are expressed as means ± se; n = 58–61 plaques in 3 mice/group. ****P < 0.0001. G, H) Astrocytes distant from plaques of both genotypes were indistinguishable. Scale bars = 50 μm (A, B); 20 μm (C, D, G, H).
Quantitative assessment of astrocyte numbers (GFP-expressing astrocytes) did not reveal a significant difference in the density of astrocytes (n/mm2) between genotypes (Fig. 6E). This finding was confirmed by quantification of glutamine synthetase-immunostained astrocytes (Supplemental Fig. S4), suggesting that deletion of Gfap and Vim alters the activation and morphology of astrocytes without altering their viability. However, in APP/PS1 Gfap−/−Vim−/− mice, there was far less overlap between astrocyte processes and plaque staining than in APP/PS1 Gfap+/+Vim+/+ mice (Fig. 6F). Astrocytes distant from amyloid plaques were indistinguishable between the two genotypes (Fig. 6G, H). These findings suggest that Gfap/Vim deletion results in astrocytes less capable of process hypertrophy and invasion into amyloid plaques. Previous work has shown BDNF expression is increased in amyloid-associated astrocytes (51) and that this may play a role in amyloid associated pathology (52). Quantitative PCR analysis revealed no difference in BDNF message levels (Supplemental Fig. S3G).
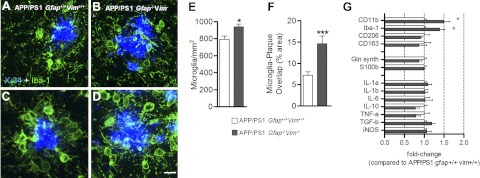
Microglial morphology was also examined by Iba-1 immunostaining. Gross morphological differences between microglia of the two genotypes were not found (Fig. 7A–D). The density of Iba-1-positive microglia (number of cells/area) was slightly higher in APP/PS1 Gfap−/−Vim−/− than in APP/PS1 Gfap+/+Vim+/+ mice (Fig. 7E). In addition, the overlap between microglia and plaques was two-fold greater in APP/PS1 Gfap−/−Vim−/− mice than in APP/PS1 Gfap+/+Vim+/+ mice (Fig. 7F). Thus, microglia seem to be more abundant around plaques in the absence of activated astrocytes. Real-time PCR from the cortex of 12-mo-old mice confirmed an increased gene expression of CD11b and Iba-1 in APP/PS1 Gfap−/−Vim−/− mice; however, expression of astrocyte-specific genes (glutamine synthase and S100b), as well as cytokines and chemokines (IL-1b, IL-6, IL-10, TNF-α, TGF-β, iNOS), was unchanged (Fig. 7G). Despite this increase in microglial density in APP/PS1 Gfap−/−Vim−/− mice, amyloid plaque load was increased.
Figure 7.
Gfap and Vim deletion increase microglial abundance around plaques. A–D) Brain sections from 8-mo-old APP/PS1 Gfap+/+Vim+/+ (A, C) and APP/PS1 Gfap−/−Vim−/− (B, D) mice were immunostained with anti-Iba-1 antibodies (green) and X-34 (blue). E, F) Deletion of Gfap and Vim modestly increased the density of Iba-1-immunostained cells (E) but doubled the overlap between plaque and microglia, suggesting increased interaction (F); n = 3 mice/group. G) RT-PCR quantification of transcripts demonstrates increased expression of CD11b and Iba-1, consistent with the increased immunostaining. Astroglial transcripts (glutamine synthase, S100b) and inflammatory mediators (IL-1b, IL-6, IL-10, TNF-α, TGF-β, and iNOS) were unchanged; n = 4–7 mice/group. Values are expressed as means ± se. *P < 0.05. ***P < 0.001.
DISCUSSION
This study shows that deletion of Gfap and Vim in APP/PS1 mice increases amyloid plaque load. APP expression and processing were unaltered by the gene deletions, and therefore were not the cause of the differences in plaque deposition. Moreover, PBS-soluble and ISF Aβ levels were similar in APP/PS1 mice with and without Gfap and Vim, and the half-life of ISF Aβ was unchanged. These findings suggest that astrocytes influence plaque pathogenesis through direct interaction with plaques rather than through Aβ synthesis or metabolism. In comparing the morphology of astrocytes between APP/PS1 Gfap+/+Vim+/+ and APP/PS1 Gfap−/−Vim−/− mice, a striking difference was noted in astrocytes immediately adjacent to plaques. Gfap−/−Vim−/− astrocytes lacked the hypertrophic processes observed in wild-type astrocytes. In addition, the astrocytes of Gfap−/−Vim−/− mice demonstrated very little interaction with plaques; the intimate interaction observed with APP/PS1 Gfap+/+Vim+/+ astrocytes suggests that processes surround and penetrate plaques. In contrast, astrocytes distant from plaques (nonactivated astrocytes) were indistinguishable between the two genotypes. Thus, activated astrocytes appear to inhibit the appearance and growth of plaques and require intimate interaction with plaques to exert this activity.
The intermediate filament proteins GFAP and Vim are expressed at high levels in activated astrocytes and are critical for the activated phenotype. In several models of acute central nervous system injury, deletion of the genes encoding these proteins suppresses morphological changes associated with astrocyte activation (22, 23, 26, 30, 32, 36). Under baseline conditions, however, Gfap−/−Vim−/− mice have astrocytes of normal appearance and grossly normal brain anatomy (22, 23, 32). In this report, we find that Gfap and Vim gene deletion in APP/PS1 mice does not alter neuronal or astrocytic density, suggesting that the gene deletions do not affect cell viability. Moreover, the expression of synaptic markers synaptophysin and PSD95 (Supplemental Fig. S3G) does not appear to be affected. Thus, Gfap/Vim gene deletion in APP/PS1 mice did not alter synaptic protein expression in the cortex, but led to local exacerbation of neuritic dystrophy.
GFAP is almost exclusively expressed in astrocytes; however, Vim is found in a variety of central nervous system cell types. The deletion of both genes appears to primarily affect astrocytes (for review, see refs. 15, 53). GFAP can form intermediate filament networks by itself, but Vim requires a second intermediate filament protein (e.g., nestin or synemin in astrocytes) to form a network (20, 24). Thus, individual deletion of Gfap or Vim allows formation of intermediate filament networks and astrocyte activation under pathological conditions. Further, the effects of acute central nervous system injury are comparable in Gfap−/−, Vim−/−, and wild-type mice (22, 32). But when Gfap and Vim are both deleted, astrocytes cannot form intermediate filament networks or display reactive morphology (22, 23, 32). Our focused survey of gene expression, examining genes related to Aβ synthesis and degradation, CNS cell-type markers, cytokines, and chemokines, showed little change between APP/PS1 Gfap+/+Vim+/+ and APP/PS1 Gfap−/−Vim−/− mice. Only APH-1, MMP-9, and microglial markers (Iba-1 and CD11b) were altered. Expression of inflammatory mediators was unchanged. Thus, the major change induced by Gfap and Vim gene deletion appeared to be morphology of activated astrocytes without any major transcriptional change identified. We have previously shown that following entorhinal cortex lesion, in the dentate gyrus of the hippocampus, the cellular processes of Gfap−/−Vim−/− astrocytes were shorter and less straight than those of wild-type astrocytes (23).
The intimate contact between astrocyte processes and plaques in wild-type astrocytes was highly diminished in Gfap−/−Vim−/− astrocytes, suggesting that this contact is important for the plaque-suppressing activity. In brain specimens from patients with AD, activated astrocytes are intimately associated with amyloid plaques (54–56), and Aβ has been observed in vesicles within astrocytes (57, 58). Furthermore, ultrastructural studies have shown that astrocytic invasion of plaques is associated with fragmentation of amyloid (56, 59). Transcriptome profiling of different cell types in mouse brain revealed that astrocytes express high levels of phagocytic genes, including Draper/Megf10 and Mertk/integrin αvβ5, which are involved in engulfing debris coated by specific opsins (60).
The phagocytosis of amyloid plaque material by astrocytes is an intriguing possibility, but direct evidence is lacking. Intimate contact between astrocytes and plaques may also be necessary for the delivery of proteins directly into or around nascent plaques. For example, direct delivery of Aβ-degrading proteases or Aβ-binding proteins may be required to limit or restrict plaque appearance and growth effectively. Although we did not find significant differences in the expression of Aβ-degrading proteases or Aβ-binding chaperones (apoE and apoJ) expressed by astrocytes, it is plausible that their direct delivery into or around nascent plaques was impaired in the Gfap−/−Vim−/− astrocytes, resulting in a compromised ability to restrict plaque appearance and growth. We have previously found that the expression of MMP-9 is up-regulated in reactive astrocytes surrounding plaques (61, 62) and that MMP-9 is capable of degrading amyloid fibrils in vitro and amyloid plaques in situ (61). It is likely that Gfap−/−Vim−/− astrocytes may have impaired ability to deliver such proteases directly into the plaque.
Another potential mechanism for the observed effect of Gfap/Vim gene deletion on plaque pathogenesis may be via the interface between astrocytes and the vasculature. Astrocyte endfeet line the abluminal aspect of endothelial cells and are important for the formation and maintenance of the blood-brain-barrier (29). Moreover, astrocytes may play an important role in the transport of Aβ into or out of the CNS through this important interface (63). While Gfap/Vim gene deletion did not alter soluble brain Aβ levels, it is still possible that regulation of influx/efflux of Abeta may be altered by the gene deletions (64).
Dystrophic neurites were more numerous in APP/PS1 Gfap−/−Vim−/− mice than in APP/PS1 Gfap+/+Vim+/+ mice, even after normalization for amyloid load. Moreover, a scatterplot of plaque size vs. dystrophic neurite load showed a greater burden of dystrophic neurites in Gfap−/−Vim−/− mice across all plaque sizes. It is unclear how activated astrocytes modulate neuritic dystrophy. Astrocytes support neuronal function by regulating extracellular concentrations of ions and neurotransmitters (65), especially in response to cellular stress (21). In Gfap−/−Vim−/− astrocytes surrounding plaques, this regulatory function may be impaired by the paucity of their processes, less efficient communication across gap junctions, or reduced glutamate transport (32). Another possibility is that wild-type astrocytes protect neurons by forming better physical barriers around plaques (56) than Gfap−/−Vim−/− astrocytes. However, we cannot discount the possibility that activated astrocytes remove dystrophic neurites, and the observed changes are due to impaired clearance instead of exacerbated neurotoxicity.
Astrocyte density in the brain was the same in APP/PS1 Gfap−/−Vim−/− and APP/PS1 Gfap+/+Vim+/+ mice, suggesting that deletion of Gfap and Vim does not affect astrocyte viability. Interestingly, the gene deletions increased microglial density, perhaps reflecting increased recruitment to the brain from the periphery, increased proliferation of endogenous microglia, or both. The infiltration of plaques by microglia was also increased in these mice. Despite the increases in the number of microglia and in plaque overlap, APP/PS1 Gfap−/−Vim−/− mice still had an increased plaque burden.
The efficacy of microglial clearance of amyloid plaques is unclear. Although microglia can internalize fibrillar Aβ in vitro and in vivo, amyloid fibrils may not be effectively degraded (8, 12). Furthermore, near-complete ablation of microglia in APP mice does not alter plaque load (13). Our data provide additional evidence that microglia may not mediate plaque clearance effectively, at least in the absence of activated astrocytes. The increased microglial response in APP/PS1 Gfap−/−Vim−/− mice may be a compensatory response to impaired astrocytic activation. Alternatively, activated astrocytes may suppress microglial recruitment or activation around amyloid plaques—a function that is impaired in the Gfap−/−Vim−/− astrocytes.
In summary, our studies demonstrate that reactive gliosis around amyloid plaques is important for restricting the appearance and growth of amyloid plaques in APP mice. This activity appears to require intimate contact between activated astrocytes and plaques, although the precise mechanism for this contact-dependent, plaque-suppressing effect is unclear. Activated astrocytes may also influence axonal pathology by inhibiting formation of, or actively clearing, dystrophic neurites. Further investigation is required to elucidate the molecular mechanisms of these effects of reactive astrocytes.
Supplementary Material
Acknowledgments
The authors thank Alec Zhu for help with PCR, histology, and writing of the manuscript.
This work was supported by U.S. National Institutes of Health grants R01 NS48283, R01 NS67905, and P01 NS32636 (to J.-M.L.); R37 AG13956 (to D.M.H.); and P30NS69329 (to J.K.); and grants from the Swedish Medical Research Council (11548), ALF Göteborg, Sten A. Olsson Foundation for Research and Culture, NanoNet COST Action (BM1002), the EU FP 7 Programs EduGlia (237956) and TargetBraIn (279017), the Frimurare Foundation, Wilhelm and Martina Lundgren's Research Foundation, Hjärnfonden, and AFA Insurance (to M.P.).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- AAV
- adeno-associated virus
- Aβ
- amyloid-β
- AD
- Alzheimer's disease
- APH-1b
- γ-secretase subunit anterior pharynx defective 1 homolog B
- apoE
- apolipoprotein E
- apoJ
- apolipoprotein J
- APP
- amyloid precursor protein
- ISF
- interstitial fluid
- GFAP
- glial fibrillary acid protein
- GFP
- green fluorescent protein
- GS
- glutamine synthetase
- MMP-2
- matrix metalloproteinase 2
- MMP-9
- matrix metalloproteinase-9
- NeuN
- neuronal nuclei
- PS1
- presenilin 1
- RTN-3
- reticulon-3
- Vim
- vimentin
REFERENCES
- 1. Mrak R. E., Sheng J. G., Griffin W. S. (1995) Glial cytokines in Alzheimer's disease: review and pathogenic implications. Hum. Pathol. 26, 816–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weisman D., Hakimian E., Ho G. J. (2006) Interleukins, inflammation, and mechanisms of Alzheimer's disease. Vitam. Horm. 74, 505–530 [DOI] [PubMed] [Google Scholar]
- 3. Wyss-Coray T. (2006) Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat. Med. 12, 1005–1015 [DOI] [PubMed] [Google Scholar]
- 4. DiCarlo G., Wilcock D., Henderson D., Gordon M., Morgan D. (2001) Intrahippocampal LPS injections reduce Abeta load in APP+PS1 transgenic mice. Neurobiol. Aging 22, 1007–1012 [DOI] [PubMed] [Google Scholar]
- 5. Chakrabarty P., Jansen-West K., Beccard A., Ceballos-Diaz C., Levites Y., Verbeeck C., Zubair A. C., Dickson D., Golde T. E., Das P. (2009) Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. FASEB J. 24, 548–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chakrabarty P., Ceballos-Diaz C., Beccard A., Janus C., Dickson D., Golde T. E., Das P. (2010) IFN-γ promotes complement expression and attenuates amyloid plaque deposition in amyloid beta precursor protein transgenic mice. J. Immunol. 184, 5333–5343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frackowiak J., Wisniewski H. M., Wegiel J., Merz G. S., Iqbal K., Wang K. C. (1992) Ultrastructure of the microglia that phagocytose amyloid and the microglia that produce β-amyloid fibrils. Acta Neuropathol. 84, 225–233 [DOI] [PubMed] [Google Scholar]
- 8. Paresce D. M., Ghosh R. N., Maxfield F. R. (1996) Microglial cells internalize aggregates of the Alzheimer's disease amyloid β-protein via a scavenger receptor. Neuron 17, 553–565 [DOI] [PubMed] [Google Scholar]
- 9. Mandrekar S., Jiang Q., Lee C. Y., Koenigsknecht-Talboo J., Holtzman D. M., Landreth G. E. (2009) Microglia mediate the clearance of soluble Abeta through fluid phase macropinocytosis. J. Neurosci. 29, 4252–4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jiang Q., Lee C. Y., Mandrekar S., Wilkinson B., Cramer P., Zelcer N., Mann K., Lamb B., Willson T. M., Collins J. L., Richardson J. C., Smith J. D., Comery T. A., Riddell D., Holtzman D. M., Tontonoz P., Landreth G. E. (2008) ApoE promotes the proteolytic degradation of Abeta. Neuron 58, 681–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meyer-Luehmann M., Spires-Jones T. L., Prada C., Garcia-Alloza M., de Calignon A., Rozkalne A., Koenigsknecht-Talboo J., Holtzman D. M., Bacskai B. J., Hyman B. T. (2008) Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer's disease. Nature 451, 720–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bolmont T., Haiss F., Eicke D., Radde R., Mathis C. A., Klunk W. E., Kohsaka S., Jucker M., Calhoun M. E. (2008) Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J. Neurosci. 28, 4283–4292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grathwohl S. A., Kalin R. E., Bolmont T., Prokop S., Winkelmann G., Kaeser S. A., Odenthal J., Radde R., Eldh T., Gandy S., Aguzzi A., Staufenbiel M., Mathews P. M., Wolburg H., Heppner F. L., Jucker M. (2009) Formation and maintenance of Alzheimer's disease beta-amyloid plaques in the absence of microglia. Nat. Neurosci. 12, 1361–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barres B. A. (2008) The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60, 430–440 [DOI] [PubMed] [Google Scholar]
- 15. Pekny M., Nilsson M. (2005) Astrocyte activation and reactive gliosis. Glia 50, 427–434 [DOI] [PubMed] [Google Scholar]
- 16. Wilhelmsson U., Bushong E. A., Price D. L., Smarr B. L., Phung V., Terada M., Ellisman M. H., Pekny M. (2006) Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc. Natl. Acad. Sci. U. S. A. 103, 17513–17518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pekny M., Leveen P., Pekna M., Eliasson C., Berthold C. H., Westermark B., Betsholtz C. (1995) Mice lacking glial fibrillary acidic protein display astrocytes devoid of intermediate filaments but develop and reproduce normally. EMBO J. 14, 1590–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gomi H., Yokoyama T., Fujimoto K., Ikeda T., Katoh A., Itoh T., Itohara S. (1995) Mice devoid of the glial fibrillary acidic protein develop normally and are susceptible to scrapie prions. Neuron 14, 29–41 [DOI] [PubMed] [Google Scholar]
- 19. Colucci-Guyon E., Portier M. M., Dunia I., Paulin D., Pournin S., Babinet C. (1994) Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell 79, 679–694 [DOI] [PubMed] [Google Scholar]
- 20. Eliasson C., Sahlgren C., Berthold C. H., Stakeberg J., Celis J. E., Betsholtz C., Eriksson J. E., Pekny M. (1999) Intermediate filament protein partnership in astrocytes. J. Biol. Chem. 274, 23996–24006 [DOI] [PubMed] [Google Scholar]
- 21. Pekny M., Lane E. B. (2007) Intermediate filaments and stress. Exp. Cell Res. 313, 2244–2254 [DOI] [PubMed] [Google Scholar]
- 22. Pekny M., Johansson C. B., Eliasson C., Stakeberg J., Wallen A., Perlmann T., Lendahl U., Betsholtz C., Berthold C. H., Frisen J. (1999) Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J. Cell Biol. 145, 503–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilhelmsson U., Li L., Pekna M., Berthold C. H., Blom S., Eliasson C., Renner O., Bushong E., Ellisman M., Morgan T. E., Pekny M. (2004) Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J. Neurosci. 24, 5016–5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jing R., Wilhelmsson U., Goodwill W., Li L., Pan Y., Pekny M., Skalli O. (2007) Synemin is expressed in reactive astrocytes in neurotrauma and interacts differentially with vimentin and GFAP intermediate filament networks. J. Cell Sci. 120, 1267–1277 [DOI] [PubMed] [Google Scholar]
- 25. Lepekhin E. A., Eliasson C., Berthold C. H., Berezin V., Bock E., Pekny M. (2001) Intermediate filaments regulate astrocyte motility. J. Neurochem. 79, 617–625 [DOI] [PubMed] [Google Scholar]
- 26. Lu Y. B., Iandiev I., Hollborn M., Korber N., Ulbricht E., Hirrlinger P. G., Pannicke T., Wei E. Q., Bringmann A., Wolburg H., Wilhelmsson U., Pekny M., Wiedemann P., Reichenbach A., Kas J. A. (2011) Reactive glial cells: increased stiffness correlates with increased intermediate filament expression. FASEB J. 25, 624–631 [DOI] [PubMed] [Google Scholar]
- 27. Potokar M., Kreft M., Li L., Daniel Andersson J., Pangrsic T., Chowdhury H. H., Pekny M., Zorec R. (2007) Cytoskeleton and vesicle mobility in astrocytes. Traffic 8, 12–20 [DOI] [PubMed] [Google Scholar]
- 28. Potokar M., Stenovec M., Gabrijel M., Li L., Kreft M., Grilc S., Pekny M., Zorec R. (2010) Intermediate filaments attenuate stimulation-dependent mobility of endosomes/lysosomes in astrocytes. Glia 58, 1208–1219 [DOI] [PubMed] [Google Scholar]
- 29. Vardjan N., Gabrijel M., Potokar M., Svajger U., Kreft M., Jeras M., de Pablo Y., Faiz M., Pekny M., Zorec R. (2012) IFN-gamma-induced increase in the mobility of MHC class II compartments in astrocytes depends on intermediate filaments. J. Neuroinflammation 9, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nakazawa T., Takeda M., Lewis G. P., Cho K. S., Jiao J., Wilhelmsson U., Fisher S. K., Pekny M., Chen D. F., Miller J. W. (2007) Attenuated glial reactions and photoreceptor degeneration after retinal detachment in mice deficient in glial fibrillary acidic protein and vimentin. Invest. Ophthalmol. Vis. Sci. 48, 2760–2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ding M., Eliasson C., Betsholtz C., Hamberger A., Pekny M. (1998) Altered taurine release following hypotonic stress in astrocytes from mice deficient for GFAP and vimentin. Brain Res. Mol. Brain Res. 62, 77–81 [DOI] [PubMed] [Google Scholar]
- 32. Li L., Lundkvist A., Andersson D., Wilhelmsson U., Nagai N., Pardo A. C., Nodin C., Stahlberg A., Aprico K., Larsson K., Yabe T., Moons L., Fotheringham A., Davies I., Carmeliet P., Schwartz J. P., Pekna M., Kubista M., Blomstrand F., Maragakis N., Nilsson M., Pekny M. (2008) Protective role of reactive astrocytes in brain ischemia. J. Cereb. Blood Flow Metab. 28, 468–481 [DOI] [PubMed] [Google Scholar]
- 33. Macauley S. L., Pekny M., Sands M. S. (2011) The role of attenuated astrocyte activation in infantile neuronal ceroid lipofuscinosis. J. Neurosci. 31, 15575–15585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cho K. S., Yang L., Lu B., Feng Ma H., Huang X., Pekny M., Chen D. F. (2005) Re-establishing the regenerative potential of central nervous system axons in postnatal mice. J. Cell Sci. 118, 863–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wilhelmsson U., Faiz M., de Pablo Y., Sjoqvist M., Andersson D., Widestrand A., Potokar M., Stenovec M., Smith P. L., Shinjyo N., Pekny T., Zorec R., Stahlberg A., Pekna M., Sahlgren C., Pekny M. (2012) Astrocytes negatively regulate neurogenesis through the Jagged1-mediated Notch pathway. Stem Cells 30, 2320–2329 [DOI] [PubMed] [Google Scholar]
- 36. Menet V., Prieto M., Privat A., Gimenez y Ribotta M. (2003) Axonal plasticity and functional recovery after spinal cord injury in mice deficient in both glial fibrillary acidic protein and vimentin genes. Proc. Natl. Acad. Sci. U. S. A. 100, 8999–9004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kinouchi R., Takeda M., Yang L., Wilhelmsson U., Lundkvist A., Pekny M., Chen D. F. (2003) Robust neural integration from retinal transplants in mice deficient in GFAP and vimentin. Nat. Neurosci. 6, 863–868 [DOI] [PubMed] [Google Scholar]
- 38. Widestrand A., Faijerson J., Wilhelmsson U., Smith P. L., Li L., Sihlbom C., Eriksson P. S., Pekny M. (2007) Increased neurogenesis and astrogenesis from neural progenitor cells grafted in the hippocampus of GFAP−/− Vim−/− mice. Stem Cells 25, 2619–2627 [DOI] [PubMed] [Google Scholar]
- 39. Okada A., Charron F., Morin S., Shin D. S., Wong K., Fabre P. J., Tessier-Lavigne M., McConnell S. K. (2006) Boc is a receptor for sonic hedgehog in the guidance of commissural axons. Nature 444, 369–373 [DOI] [PubMed] [Google Scholar]
- 40. Herrmann J. E., Imura T., Song B., Qi J., Ao Y., Nguyen T. K., Korsak R. A., Takeda K., Akira S., Sofroniew M. V. (2008) STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J. Neurosci. 28, 7231–7243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wyss-Coray T., Loike J. D., Brionne T. C., Lu E., Anankov R., Yan F., Silverstein S. C., Husemann J. (2003) Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat. Med. 9, 453–457 [DOI] [PubMed] [Google Scholar]
- 42. Koistinaho M., Lin S., Wu X., Esterman M., Koger D., Hanson J., Higgs R., Liu F., Malkani S., Bales K. R., Paul S. M. (2004) Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat. Med. 10, 719–726 [DOI] [PubMed] [Google Scholar]
- 43. Jankowsky J. L., Fadale D. J., Anderson J., Xu G. M., Gonzales V., Jenkins N. A., Copeland N. G., Lee M. K., Younkin L. H., Wagner S. L., Younkin S. G., Borchelt D. R. (2004) Mutant presenilins specifically elevate the levels of the 42 residue β-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum. Mol. Genet. 13, 159–170 [DOI] [PubMed] [Google Scholar]
- 44. Kim J., Castellano J. M., Jiang H., Basak J. M., Parsadanian M., Pham V., Mason S. M., Paul S. M., Holtzman D. M. (2009) Overexpression of low-density lipoprotein receptor in the brain markedly inhibits amyloid deposition and increases extracellular A beta clearance. Neuron 64, 632–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hu X., Shi Q., Zhou X., He W., Yi H., Yin X., Gearing M., Levey A., Yan R. (2007) Transgenic mice overexpressing reticulon 3 develop neuritic abnormalities. EMBO J. 26, 2755–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cirrito J. R., May P. C., O'Dell M. A., Taylor J. W., Parsadanian M., Cramer J. W., Audia J. E., Nissen J. S., Bales K. R., Paul S. M., DeMattos R. B., Holtzman D. M. (2003) In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J. Neurosci. 23, 8844–8853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Grimwood S., Hogg J., Jay M. T., Lad A. M., Lee V., Murray F., Peachey J., Townend T., Vithlani M., Beher D., Shearman M. S., Hutson P. H. (2005) Determination of guinea-pig cortical gamma-secretase activity ex vivo following the systemic administration of a gamma-secretase inhibitor. Neuropharmacology 48, 1002–1011 [DOI] [PubMed] [Google Scholar]
- 48. Holtzman D. M., Bales K. R., Tenkova T., Fagan A. M., Parsadanian M., Sartorius L. J., Mackey B., Olney J., McKeel D., Wozniak D., Paul S. M. (2000) Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 97, 2892–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Irizarry M. C., McNamara M., Fedorchak K., Hsiao K., Hyman B. T. (1997) APPSw transgenic mice develop age-related A beta deposits and neuropil abnormalities, but no neuronal loss in CA1. J. Neuropathol. Exp. Neurol. 56, 965–973 [DOI] [PubMed] [Google Scholar]
- 50. Borchelt D. R., Ratovitski T., van Lare J., Lee M. K., Gonzales V., Jenkins N. A., Copeland N. G., Price D. L., Sisodia S. S. (1997) Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron 19, 939–945 [DOI] [PubMed] [Google Scholar]
- 51. Burbach G. J., Hellweg R., Haas C. A., Del Turco D., Deicke U., Abramowski D., Jucker M., Staufenbiel M., Deller T. (2004) Induction of brain-derived neurotrophic factor in plaque-associated glial cells of aged APP23 transgenic mice. J. Neurosci. 24, 2421–2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Szapacs M. E., Numis A. L., Andrews A. M. (2004) Late onset loss of hippocampal 5-HT and NE is accompanied by increases in BDNF protein expression in mice co-expressing mutant APP and PS1. Neurobiol. Dis. 16, 572–580 [DOI] [PubMed] [Google Scholar]
- 53. Pekny M., Pekna M. (2004) Astrocyte intermediate filaments in CNS pathologies and regeneration. J. Pathol. 204, 428–437 [DOI] [PubMed] [Google Scholar]
- 54. Kato S., Gondo T., Hoshii Y., Takahashi M., Yamada M., Ishihara T. (1998) Confocal observation of senile plaques in Alzheimer's disease: senile plaque morphology and relationship between senile plaques and astrocytes. Pathol. Int. 48, 332–340 [DOI] [PubMed] [Google Scholar]
- 55. Wegiel J., Wang K. C., Tarnawski M., Lach B. (2000) Microglia cells are the driving force in fibrillar plaque formation, whereas astrocytes are a leading factor in plague degradation. Acta Neuropathol. 100, 356–364 [DOI] [PubMed] [Google Scholar]
- 56. Wegiel J., Wang K. C., Imaki H., Rubenstein R., Wronska A., Osuchowski M., Lipinski W. J., Walker L. C., LeVine H. (2001) The role of microglial cells and astrocytes in fibrillar plaque evolution in transgenic APP(SW) mice. Neurobiol. Aging 22, 49–61 [DOI] [PubMed] [Google Scholar]
- 57. Yamaguchi H., Sugihara S., Ogawa A., Saido T. C., Ihara Y. (1998) Diffuse plaques associated with astroglial amyloid β-protein, possibly showing a disappearing stage of senile plaques. Acta Neuropathol. 95, 217–222 [DOI] [PubMed] [Google Scholar]
- 58. Thal D. R., Schultz C., Dehghani F., Yamaguchi H., Braak H., Braak E. (2000) Amyloid β-protein (Abeta)-containing astrocytes are located preferentially near N-terminal-truncated Abeta deposits in the human entorhinal cortex. Acta Neuropathol. 100, 608–617 [DOI] [PubMed] [Google Scholar]
- 59. Wisniewski H. M., Wegiel J. (1991) Spatial relationships between astrocytes and classical plaque components. Neurobiol. Aging 12, 593–600 [DOI] [PubMed] [Google Scholar]
- 60. Cahoy J. D., Emery B., Kaushal A., Foo L. C., Zamanian J. L., Christopherson K. S., Xing Y., Lubischer J. L., Krieg P. A., Krupenko S. A., Thompson W. J., Barres B. A. (2008) A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 28, 264–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yan P., Hu X., Song H., Yin K., Bateman R. J., Cirrito J. R., Xiao Q., Hsu F. F., Turk J. W., Xu J., Hsu C. Y., Holtzman D. M., Lee J. M. (2006) Matrix metalloproteinase-9 degrades amyloid-beta fibrils in vitro and compact plaques in situ. J. Biol. Chem. 281, 24566–24574 [DOI] [PubMed] [Google Scholar]
- 62. Yin K. J., Cirrito J. R., Yan P., Hu X., Xiao Q., Pan X., Bateman R., Song H., Hsu F. F., Turk J., Xu J., Hsu C. Y., Mills J. C., Holtzman D. M., Lee J. M. (2006) Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-beta peptide catabolism. J. Neurosci. 26, 10939–10948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Askarova S., Yang X., Sheng W., Sun G. Y., Lee J. C. (2011) Role of Abeta-receptor for advanced glycation endproducts interaction in oxidative stress and cytosolic phospholipase A(2) activation in astrocytes and cerebral endothelial cells. Neuroscience 199, 375–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zlokovic B. V. (2011) Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat. Rev. Neurosci. 12, 723–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Allen N. J., Barres B. A. (2009) Neuroscience: Glia - more than just brain glue. Nature 457, 675–677 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.