The introduction of HIV-1 protease inhibitors (PIs) into highly active antiretroviral therapy (HAART) in late 1996 had a critical impact in reducing HIV-related morbidity and mortality.[1] Indeed, HAART treatment regimens with PIs initially suppressed HIV-1 replication in patients to undetectable HIV-1 RNA levels in plasma.[2] However, one of the serious shortcomings of current treatment is the rapid emergence of drug-resistant strains of HIV.[3] There is an urgent need of improved PIs for the treatment of the increasing number of treatment-experienced patients harboring multidrug-resistant HIV-1 strains.[4,5]
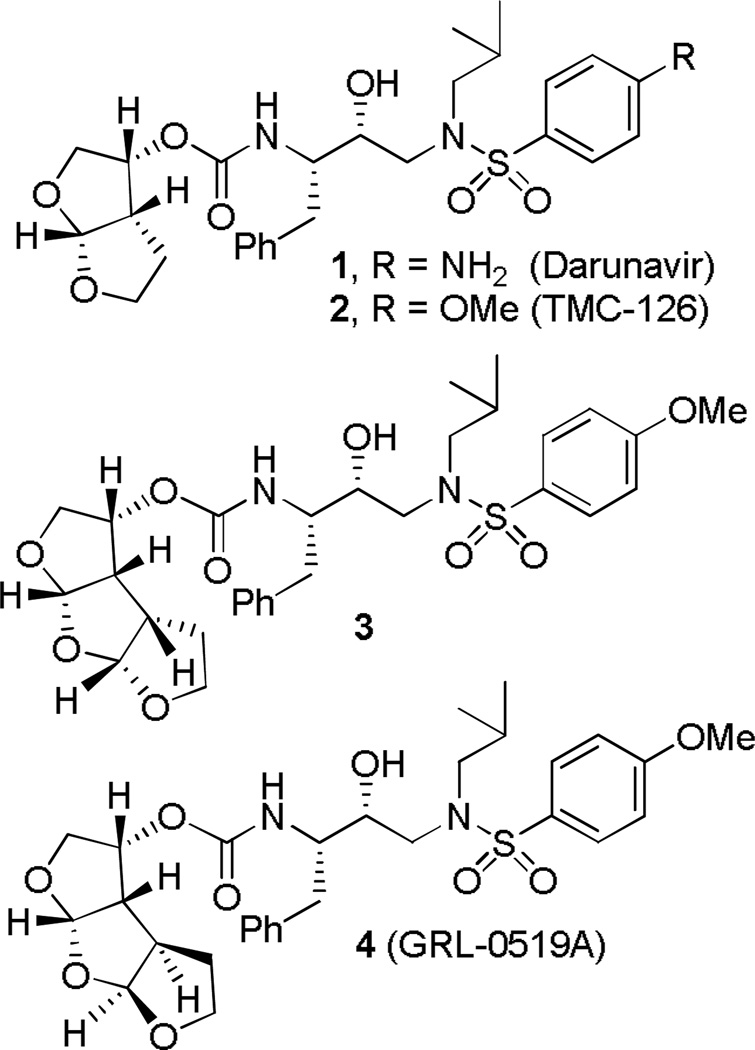
We recently reported a number of PIs incorporating structure-based designs of novel nonpeptide ligands/scaffolds targeting the HIV-1 protease substrate binding site.[6–17] One of the PIs, darunavir (1, DRV, Figure 1), was first approved for HIV/AIDS patients harboring drug-resistant HIV that do not respond to other antiretroviral drugs.[11] Recently, DRV has received full approval for all HIV/AIDS patients including children infected with HIV-1.[12] DRV incorporated a stereochemically defined fused bis-tetrahydrofuran (bis-THF) as the P2-ligand.[7,8] The X-ray structures of both 1-bound and 2-bound HIV-1 protease complexes revealed extensive protein-ligand hydrogen bonding interactions involving the backbone of HIV-1 protease throughout the active site.[13,14] In particular, both oxygens of the P2-bis-THF ligand are involved in hydrogen bonding with Asp-29 and Asp-30 backbone NH’s.[15] In addition, the bicyclic ligand appears to fill in the hydrophobic pocket at the S2-subsite. The P2-bis-THF is responsible for both DRV’s (1) and TMC-126’s (2) superior drug-resistance properties. For our preliminary investigation, we choose to incorporate the p-methoxysulfonamide as the P2’-ligand due to its marked hydrogen bonding interaction in the S2’-site. To combat drug resistance, our inhibitor design strategies focused on maximizing inhibitor interactions with the HIV-1 protease active-site, particularly to promote extensive hydrogen bond interactions with the protein backbone atoms.[15] We have recently shown that enhancing ‘backbone binding’ led to the design of PIs that maintain full potency against a panel of multidrug-resistant HIV-1 variants.[17] Based upon examination of the protein-ligand X-ray structure of DRV-bound HIV-1 protease, we have further speculated that the incorporation of another tetrahydrofuran ring on the bis-THF ligand would provide additional ligand-binding site interactions. Particularly, it appears that ligand oxygens can effectively maintain backbone hydrogen bonding with Asp29 and Asp30 as well as fill in the hydrophobic pocket effectively. This in turn could further improve drug-resistance properties of the PIs. Such oxatricyclic ligand could have a number of possible stereochemical motifs, including a syn-syn-syn (SSS-type) and a syn-anti-syn (SAS-type) isomers. Our X-ray structure-based preliminary modeling suggested that the SAS-type ligand-based inhibitor 4 would make enhanced interactions in the S2-subsite when compared to SSS-derived inhibitor 3 (Figure 1).
Figure 1.
Structure of darunavir (1) and PIs 2–4.
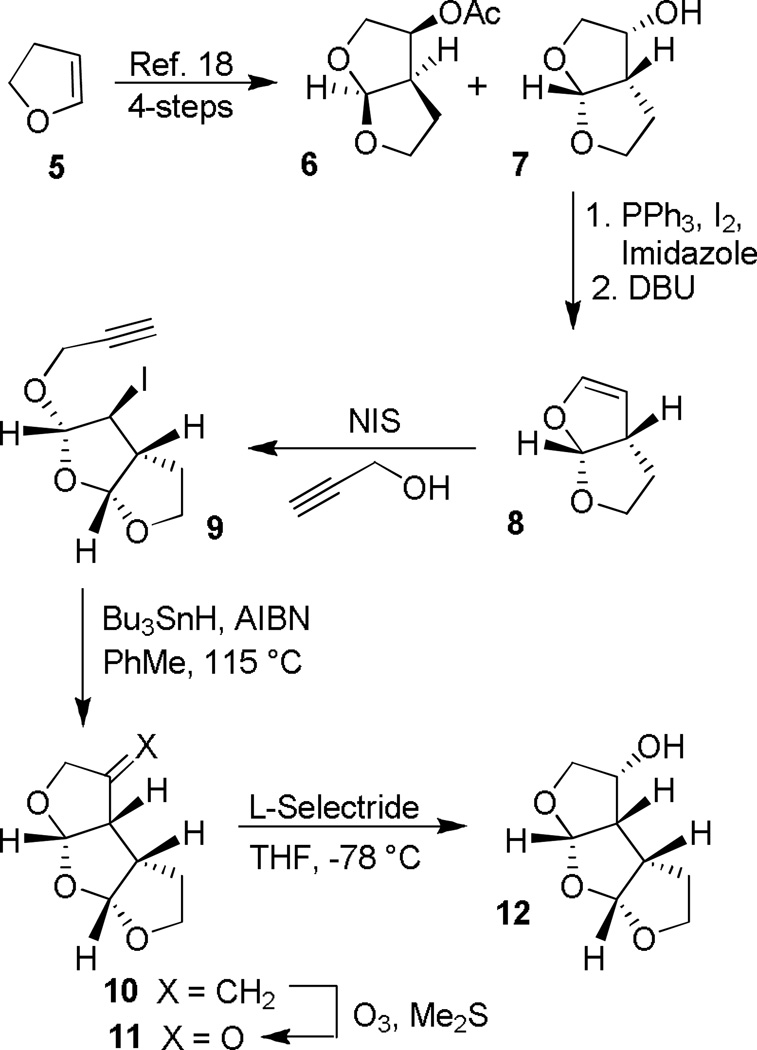
The synthesis of the oxatricyclic tris-THF ligand with a syn-syn-syn (SSS)-configuration was carried out as shown in Scheme 1. Optically active bis-THF derivatives 6 and 7 were conveniently prepared in multi gram quantities from dihydrofuran 5 in a three-step sequence followed by lipase-catalyzed optical resolution as described previously.[18,19] Optically active alcohol 7 was treated with triphenylphosphine and iodine in the presence of imidazole to provide the corresponding iodide. Treatment of the resulting iodide with DBU furnished volatile bicyclic enol-ether 8. Reaction of 8 with N-iodosuccinimide (NIS) and propargyl alcohol in CH2Cl2 at 0 °C to 23 °C afforded iodoether 9 in 58% yield, over three steps.[18] Radical cyclization of 9 with nBu3SnH in the presence of AlBN in toluene at reflux provided tricyclic olefin 10. The relative stereochemistry of the tricyclic core was established by extensive NMR experiments (COSY, NOESY). Ozonlytic cleavage of the olefin, followed by L-selectride reduction of the resulting ketone furnished endo-alcohol 12 as a single isomer (by 1H-NMR analysis).
Scheme 1.
Synthesis of the Syn-Syn-Syn-tris-THF Ligand 12.
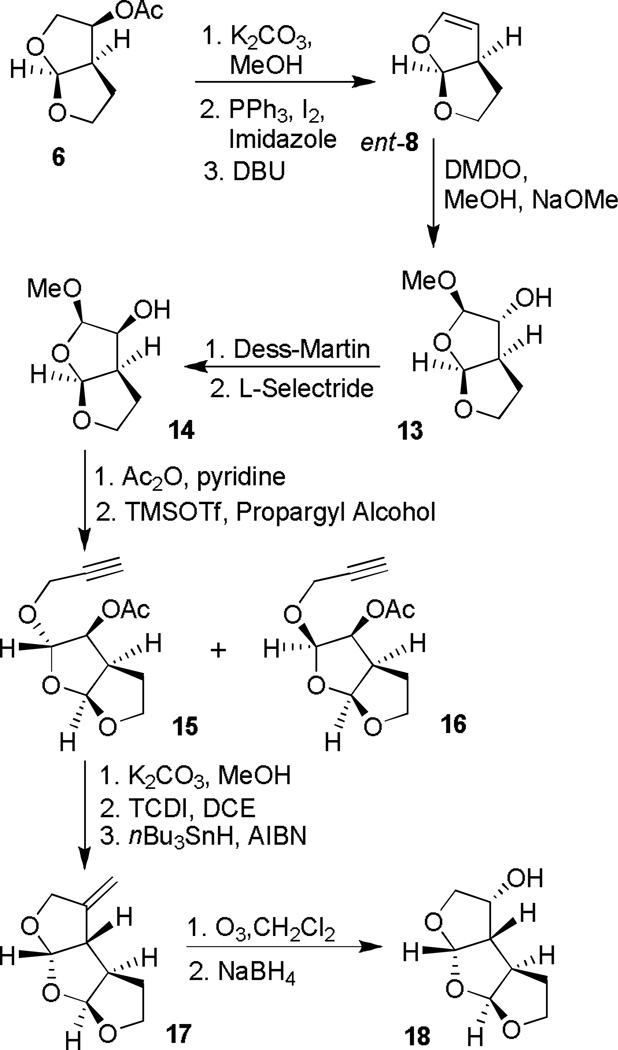
The synthesis of tris-THF ligand with a syn-anti-syn ring fusion is shown in Scheme 2. Optically active (3S, 3aR, 6aS)-bis-THF alcohol ent-7 was readily obtained by saponification of acetate derivative 6. It was converted to bicyclic enol ether ent-8 as described above. This enol ether was exposed to acetone-free dimethyl dioxirane (DMDO)[20] in CH2Cl2 at −78°C to provide the corresponding epoxide. Treatment of the resulting epoxide with a catalytic sodium methoxide (10%) in methanol provided epoxide opened product 13 as a single diastereomer in 96% yield.[21] The depicted stereochemistry of 13 was fully supported by extensive NMR studies. Dess-Martin oxidation[22] of 13 followed by L-selectride reduction of the resulting ketone provided endo-alcohol 14 as a single isomer in 68% yield over two steps. To append the third oxacyclic ring, alcohol 14 was first acylated and the resulting acetate was treated with TMSOTf and propargyl alcohol which afforded acetals 15 and 16 as a 4:1 mixture of diastereomers. The isomers could not be separated at this stage, however, removal of acetate provided the corresponding alcohols which were readily separated by column chromatography. The major diastereomeric alcohol was converted to tricyclic olefin 17 in a two step sequence involving: (1) reaction of alcohol with thiocarbonyldiimidazole (TCDl) in dichloroethane (DCE) to provide the corresponding thiocarbamate and (2) treatment of the resulting thiocarbamate with nBu3SnH in the presence of AlBN in toluene at reflux to provide olefin 17 in 75% yield over two-steps.[23] The stereochemistry of olefin 17 was established by 1H-NMR studies. Olefin 17 was converted to endo-alcohol 18 by ozonolytic cleavage of the olefin followed by NaBH4 reduction of the resulting ketone to furnish 18 as a single isomer. Mosher ester analysis[24] revealed high optical purity (99% ee).
Scheme 2.
Synthesis of Syn-Anti-Syn-tris-THF alcohol 18
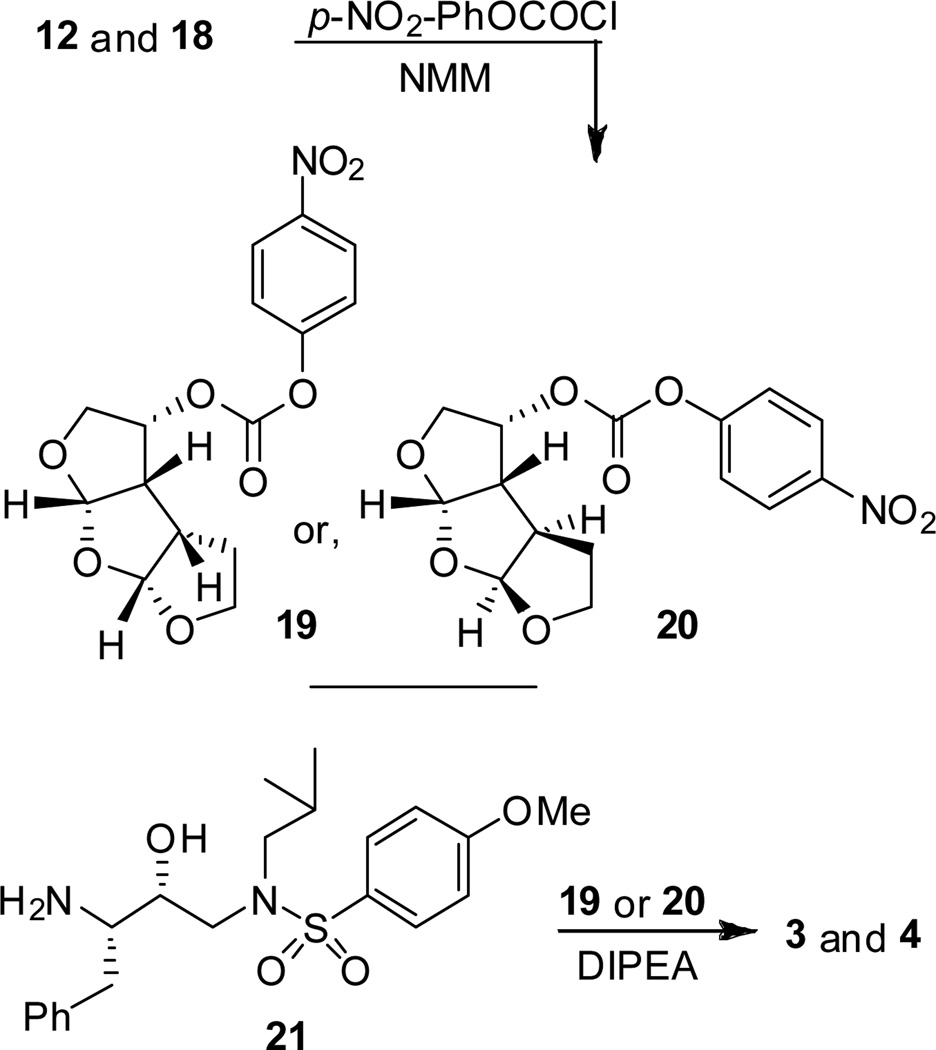
The synthesis of tris-THF containing PIs is shown in Scheme 3. Optically active tris-THF ligand alcohols 12 and 18 were converted to the corresponding p-nitrophenyl carbonates 19 and 20 by treatment with p-nitrophenyl chloroformate and N-methylmorpholine (NMM). Treatment of amine 21,[25] synthesized previously, with activated carbonate 19 or 20 in the presence of diisopropylethylamine (DIPEA) provided PIs 3 and 4 in 65% yield.
Scheme 3.
Synthesis of inhibitors 3 and 4.
Inhibitor 3 has shown a potent inhibitory activity (Ki = 60 pM). In comparison, inhibitor 4 (GRL-0519A, Figure 1) has displayed a Ki of 5.9 pM, a 10-fold improvement over 3. Both PIs 3 (IC50 = 3.5 nM) and 4 (IC50 = 1.8 nM) have also shown excellent antiviral activity against the wild-type HIV-1LAV. PI 4 (GRL-0519A) was most potent against a laboratory HIV-1 strain, HIV-1LAI, with an EC50 value of 1.8 nM and a favorable cytotoxicity profile (CC50: 44.6 µM) as examined using MT-2 cells as target cells, producing a selectivity index (CC50/EC50) of 24,778.
We next examined GRL-0519A against a variety of primary HIV-1 strains which were isolated from those with AIDS who had failed a number of anti-HIV therapeutic regimens after receiving 9 to 11 anti-HIV-1 drugs over the past 32 to 83 months and proved to be highly resistant to multiple PIs.[9, 26] These primary strains contained 9 to 14 amino acid substitutions in the protease-encoding region of the HIV-1 genome which have been reported to be associated with HIV-1 resistance against various PIs.[27] The substitutions identified included Leu-10→Ile (L10I; 6 of 6 isolates), M46I/L (6 of 6), I54V (4 of 6), L63P (6 of 6), A71V/T (5 of 6), V82A (6 of 6), and L90M (4 of 6) (see the footnote to Table 1).
Table 1.
Antiviral activity of GRL-0519A (4), amprenavir, and darunavir against multi-drug resistant clinical isolates in PHA-PBMs (EC50, µM)
| Virus | GRL-0519A (4) | Amprenavir | Darunavir |
|---|---|---|---|
| HIV-1ERS104pre (wild-type: X4) | 0.0006 ± 0.0002 | 0.032 ± 0.006 | 0.005 ± 0.002 |
| HIV-1MDR/B (X4) | 0.0043 ± 0.0012 (7) | 0.521 ± 0.203 (16) | 0.028 ± 0.008 (6) |
| HIV-1MDR/C (X4) | 0.0009 ± 0.0002 (2) | 0.357 ± 0.040 (11) | 0.011 ± 0.004 (2) |
| HIV-1MDR/G (X4) | 0.0027 ± 0.0012 (5) | 0.485 ± 0.073 (15) | 0.031 ± 0.002 (6) |
| HIV-1MDR/TM (X4) | 0.0022 ± 0.0001 (4) | 0.488 ± 0.009 (15) | 0.031 ± 0.002 (6) |
| HIV-1MDR/MM (R5) | 0.0027 ± 0.0006 (5) | 0.291 ± 0.128 (9) | 0.016 ± 0.006 (3) |
| HIV-1MDR/JSL (R5) | 0.0028 ± 0.0001 (5) | 0.419 ± 0.122 (13) | 0.024 ± 0.007 (5) |
The amino acid substitutions identified in the protease-encoding region of HIV-1ERS104pre, HIV-1B, HIV-1C, HIV-1G, HIV-1TM, HIV-1MM, HIV-1JSL compared to the consensus type B sequence cited from the Los Alamos database include L63P; L10I, K14R, L33I, M36I, M46I, F53I, K55R, I62V, L63P, A71V, G73S, V82A, L90M, I93L; L10I, I15V, K20R, L24I, M36I, M46L, I54V, I62V, L63P, K70Q,V82A, L89M; L10I, V11I, T12E, I15V, L19I, R41K, M46L, L63P, A71T, V82A, L90M; L10I, K14R, R41K, M46L, I54V, L63P, A71V, V82A, L90M, I93L; L10I, K43T, M46L, I54V, L63P, A71V, V82A, L90M, Q92K; and L10I, L24I, I33F, E35D, M36I, N37S, M46L, I54V, R57K, I62V, L63P, A71V, G73S, V82A, respectively. HIV-1ERS104pre served as a source of wild-type HIV-1. The EC50 values were determined by using PHA-PBMs as target cells and the inhibition of p24 Gag protein production by each drug was used as an endpoint. The numbers in parentheses represent the fold changes of EC50 values for each isolate compared to the IC50 values for wild-type HIV-1ERS104pre. All assays were conducted in duplicate, and the data shown represent mean values (± 1 standard deviations) derived from the results of two or three independent experiments.
Darunavir (DRV) was more potent against wild-type HIV-1ERS104pre with an EC50 value of 0.005 µM compared to amprenavir (APV),[28] whose EC50 value was 0.032 µM. DRV was moderately less active against a panel of multi-drug resistant HIV-1 variants with EC50 values ranging from 0.011 to 0.031 µM. The fold-difference of EC50 values was 2 to 6 relative to its EC50 value against HIV-1ERS104pre. APV was less active against the variants with EC50 values and fold-differences ranging between 0.291 to 0.521 µM and 9 to 16, respectively. GRL-0519A exerted the greatest potency among the three agents against wild-type HIV-1ERS104pre with an EC50 value of 0.6 nM. GRL-0519A was less potent against the variants with EC50 values ranging from 0.9 to 4.3 nM and the fold-difference was 2 to 7 compared to wild-type HIV-1 (Table 1). It should be noted, however, that GRL-0519A was more potent than DRV by a factor of 5.9 to 14 when absolute concentrations of EC50 values were compared.
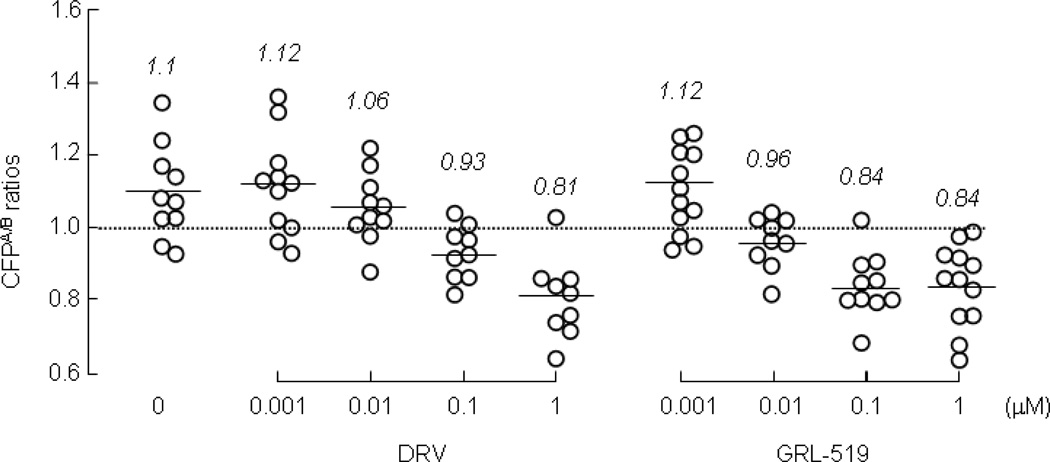
Dimerization of HIV-1 protease subunits is an essential process for the acquisition of proteolytic activity of HIV-1 protease, which plays a critical role in the maturation and replication of the virus.[29] We previously demonstrated that a group of compounds including DRV and TPV inhibit the dimerization of HIV-1 protease monomers subunits as examined using the FRET-based HIV-1 expression assay employing cyan and yellow fluorescent protein-tagged protease monomers, while other conventional FDA-approved protease inhibitors (PIs), such as amprenavir (APV), failed to block the dimerization [30]. As shown in Figure 2, the average CFPA/B ratio determined in the cells transfected and cultured in the absence of drug was 1.1 ± 0.13, indicating that protease dimerization efficiently occurred. In the same assay, in the presence of 0.1 and 1 µM DRV, the average CFPA/B ratios determined were 0.93 ± 0.07 and 0.81 ± 0.11, respectively, indicating that DRV effectively inhibited protease dimerization at those two concentrations. However, the average ratios were greater than 1.0 at 0.001 and 0.01 µM, indicating that no dimerization inhibition occurred at those low DRV concentrations, in line with the data we previously reported [30]. We next examined whether GRL-0519A blocked protease dimerization under exactly the same conditions in the FRET-based HIV-1 expression assay. When the ratios were determined in the cells transfected and cultured in the presence of 0.01, 0.1, and 1 µM GRL-0519A, the average values were 0.96 ± 0.07, 0.84 ± 0.09, and 0.84 ± 0.11, respectively, indicating that GRL-0519A blocked protease dimerization more potently, by at least a factor of 10-fold, as compared to DRV.
Figure 2.
Inhibition of HIV-1 protease dimerization by GRL-0519A. Changes in emission intensity ratios in the presence of DRV or GRL-0519A are shown. COS7 cells were co-transfected with a pair of HIV-PRCFP and HIV-PRYFP, and CFPA/B ratios were determined. The mean values of the ratios are shown as bars. The results of statistical evaluation of the changes in the CFPA/B ratios determined in the presence or absence of DRV or GRL-0519A are as follows: the CFPA/B ratios in the absence of drug (CFPA/BNo Drug) vs the CFPA/B ratios in the presence of 0.001 µM DRV (CFPA/B0.001 DRV), p=0.94; CFPA/BNo Drug vs CFPA/B0.01 DRV, p=0.15; CFPA/BNo Drug vs CFPA/B0.1 DRV, p=0.0042; CFPA/BNo Drug vs CFPA/B1.0 DRV, p=0.0004; CFPA/BNo Drug vs CFPA/B0.001 GRL-519, p=0.947; CFPA/BNo Drug vs CFPA/B0.01 GRL-519, p=0.0077; CFPA/BNo Drug vs CFPA/B0.1 GRL-519, p=0.0003; CFPA/BNo Drug vs CFPA/B1.0 GRL-519, p=0.0002; CFPA/B0.01 DRV vs CFPA/B0.01 GRL-519, p=0.013. Panel A: HIVWT vs rHIVWTpro75/219gag, p=0.53; HIVWT vs rHIVWTpro12/75/219/309/409gag, p=0.0080; HIVWT vs rHIVWTpro219/409gag, p=0.22; rHIVWTpro75/219gag vs rHIVWTpro12/75/219/309/409gag, p=0.0065; rHIVWTpro75/219gag vs rHIVWTpro219/409gag, p=0.15; rHIVWT pro12/75/219/309/409gag vs rHIVWTpro219/409gag, p=0.0018. Panel B: HIVWT vs rHIVWTpro75/219gag, p=0.65; HIVWT vs rHIVWTpro12/75/219/309/409gag, p<0.0001; HIVWT vs rHIVWTpro219/409gag, p<0.0001;
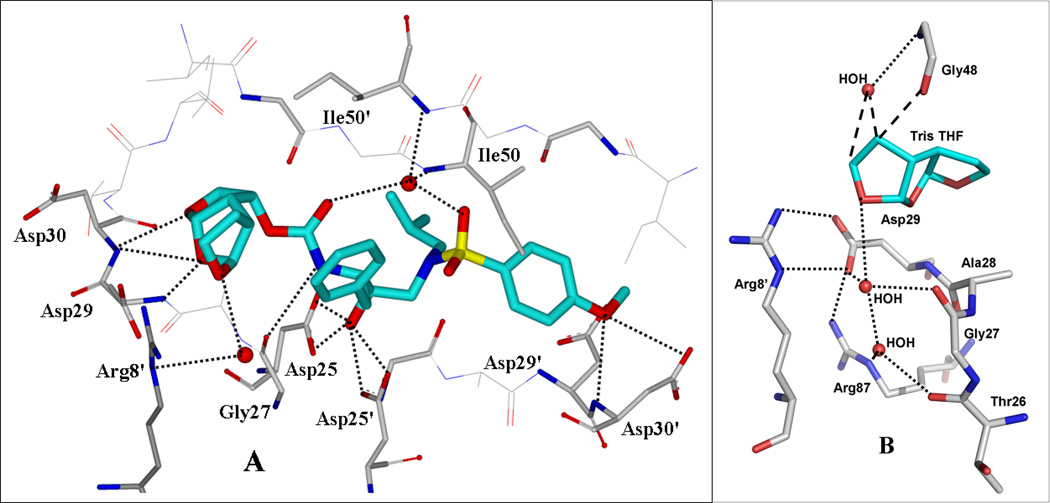
The X-ray crystal structure of the wild type HIV-1 protease co-crystallized with inhibitor GRL-0519A was refined at the near-atomic resolution of 1.27 Ǻ (PDB ID 3OK9). The structure comprises the protease dimer and the inhibitor in two orientations related by a 180° rotation with 55/45% occupancies. The protease dimer structure is essentially identical to that in the protease-darunavir complex[31] with an RMSD of 0.16 Ǻ on Cα atoms. The inhibitor is bound in the active site cavity via a series of hydrogen bond interactions and weaker CH…O interactions with the main chain atoms of HIV-1 protease (Figure 3A). The major conformation of the inhibitor forms hydrogen bonding interactions of its urethane NH with the carbonyl oxygen of Gly27. The oxymethyl oxygen of the tris-THF interacts with the amide of Asp30, and the second THF oxygen forms hydrogen bonds with the amides of Asp29, Asp30 and the carboxylate side chain of Asp30. In addition, water-mediated interactions connect the inhibitor carbonyl oxygen and sulfonamide oxygen with the amides of Ile50 and 50’ in the flaps, and link the inhibitor P2’ aromatic ring with the amide of Asp30’. Similar interactions are shared by the protease-darunavir complex and are considered responsible for the high affinity of darunavir for HIV protease and its potency against drug resistant HIV-1 variants.[31]
Figure 3.
A. GRL-0519A-bound X-ray structure of HIV-1 protease. The major orientation of the inhibitor is shown. The inhibitor carbon atoms are shown in cyan, water molecules are red spheres, and the hydrogen bonds are indicated by dotted lines. B. Polar interactions of third THF with the protease. The hydrogen bonds are indicated by dotted lines and CH…O interactions by dashed lines.
The majority of differences are confined to the interactions of the third THF moiety in GRL-0519A. The third THF ring forms CH…O interactions with Gly48 in the protease flap and water-mediated hydrogen bonds with conserved residues at the dimer interface as shown in Figure 3B. The third THF ring forms CH…O and water-mediated interactions with the carbonyl and amide of Gly48 that may stabilize the flexible flap conformation. The oxygen at the opposite side of the THF ring forms a hydrogen bond with a conserved water molecule and stabilizes a network of hydrogen bonds and ionic interactions connecting Arg8’ from the other subunit with the side chains of Asp29 and Arg87, as well as with the main chain carbonyl oxygens of Thr26 and Gly27 in the characteristic catalytic triplet (Asp25-Thr26-Gly27) of aspartic proteases. The ionic and hydrogen bonding interactions of Arg8’ with Asp29 and Arg87 are conserved among HIV protease structures and form important components of the dimer interface.[32, 33] Also, the oxygen of the third THF ring interacts with a semicircular network of three conserved water molecules that surround the guanidine side chain of Arg8’. The hydrogen bond and van der Waals interactions of the third THF restrain the Arg8’ side chain in a single conformation, and thus preventing the formation of an alternate conformation observed in the darunavir complex. Furthermore, the third THF ring packs neatly against the P1-phenyl group of the inhibitor with better internal hydrophobic contacts than are possible for bis-THF in darunavir. The ring stereochemistry (SSS-type) in the tris-THF ligand of 3 would not be able to make similar hydrophobic contacts. Thus, the tris-THF ring of GRL-0519A (4) fills the substrate binding cavity better than the bis-THF ligand of darunavir, anchors Arg87 at the dimer interface, and forms new CH…O and conserved water mediated interactions with the flaps and the base of the substrate binding cavity.
In conclusion, we designed and synthesized novel oxatricyclic [3(R), 3aS, 4aS, 6aR, 7aS] and [3(R), 3aS, 4aR, 6aS, 7aS]-ligands and incorporated these ligands in the (R)-hydroxyethyl sulfonamide isostere.[16] The orientation, the ring size, and stereochemistry, all are critical to potency of the oxatricyclic ligand-derived inhibitors. The ligands were synthesized with defined stereochemistry in optically active forms. Incorporation of a syn-syn-syn-fused tris-THF led to inhibitor 3 with a significant reduction in enzyme inhibitory and antiviral potency compared to DRV. However, the tris-THF ligand with a syn-anti-syn fusion, provided inhibitor GRL-0519A (4) with remarkable enzyme inhibitory and antiviral activity. Of particular interest, GRL-0519A displayed potent activity against a variety of multidrug-resistant clinical HIV-1 strains with EC50 values ranging from 0.6–4.3 nM, nearly a 10-fold improvement over darunavir. Also, GRL-0519A more potently blocked protease dimerization by at least a factor of 10-fold compared to DRV. An X-ray structure of GRL-0519A-bound HIV-1 protease revealed molecular insight into the ligand-binding site interactions responsible for its potency against wild-type and mutant viruses. It appears that the first and second THF rings of the tris-THF ligand with a syn-anti-syn configuration maintain key backbone hydrogen bonding interactions similar to the bis-THF ligand of darunavir. The third THF ring oxygen makes water-mediated hydrogen bonds with Asp29, Arg8’, and Gly27. In addition, the ring fills in the hydrophobic pocket in the S2-subsite and also stacks nicely behind the P1-phenylmethyl substituent at the S1-subsite. Therefore, this line of basic design strategy has been shown to be extremely powerful and is well worth of further experimentation.
Supplementary Material
Acknowledgements
The research was supported by grants from the National Institutes of Health (GM53386, AKG and GM62920, IW). This work was also supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health and in part by a Grant-in-aid for Scientific Research (Priority Areas) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Monbu Kagakusho), a Grant for Promotion of AIDS Research from the Ministry of Health, Welfare, and Labor of Japan (Kosei Rohdosho: H15-AIDS-001), and the Grant to the Cooperative Research Project on Clinical and Epidemiological Studies of Emerging and Reemerging Infectious Diseases (Kumamoto University) of Monbu-Kagakusho.
Footnotes
Supporting information for this article is available on the WWW under http://www.chemmedchem.org or from the author.
References
- 1.Flexner C. N. Engl. J. Med. 1998;338:1281–1292. doi: 10.1056/NEJM199804303381808. [DOI] [PubMed] [Google Scholar]
- 2.Sepkowitz KA. N. Engl. J. Med. 2001;344:1764–1772. doi: 10.1056/NEJM200106073442306. [DOI] [PubMed] [Google Scholar]
- 3.Waters L, Nelson M. Int. J. Clin. Pract. 2007;61:983–990. doi: 10.1111/j.1742-1241.2007.01383.x. [DOI] [PubMed] [Google Scholar]
- 4.Pillay D, et al. AIDS. 2006;20:21–28. [Google Scholar]
- 5.Yerly S, Kaiser L, Race E, Bru JP, Clavel F, Perrin L. Lancet. 1999;354:729–733. doi: 10.1016/S0140-6736(98)12262-6. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh AK, et al. Bioorg. Med. Chem. Lett. 1998;8:687–690. doi: 10.1016/s0960-894x(98)00098-5. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh AK, et al. Farmaco. 2001;56:29–32. doi: 10.1016/s0014-827x(01)01025-4. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh AK, et al. ChemMedChem. 2006;1:939–950. doi: 10.1002/cmdc.200600103. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimura K, et al. J. Virol. 2002;76:1349–1358. doi: 10.1128/JVI.76.3.1349-1358.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koh Y, Nakata H, Maeda K. Antimicrob. Agents. Chemother. 2003;47:3123–3129. doi: 10.1128/AAC.47.10.3123-3129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FDA approved Darunavir on June 23, 2006: FDA approved new HIV treatment for patients who do not respond to existing drugs. Please see: http://www.fda.gov/bbrs/topics/NEWS/2006/NEW01395.htmL
- 12.On October 21, 2008, FDA granted traditional approval to Prezista (darunavir), co-administered with ritonavir and with other antiretroviral agents, for the treatment of HIV-1 infection in treatment-experienced adult patients. In addition to the traditional approval, a new dosing regimen for treatment-naïve patients was approved.
- 13.Tie Y, et al. Proteins. 2007;67:232–242. doi: 10.1002/prot.21304. [DOI] [PubMed] [Google Scholar]
- 14.Kovalevsky AY, et al. J. Med. Chem. 2006;49:1379–1387. doi: 10.1021/jm050943c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh AK, Chapsal BD, Weber IT, Mitsuya H. Acc. Chem. Res. 2008;41:78–86. doi: 10.1021/ar7001232. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh AK, et al. J. Med. Chem. 2006;49:5252–5261. doi: 10.1021/jm060561m. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh AK, et al. J. Med. Chem. 2009;52:3902–3914. doi: 10.1021/jm900303m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh AK, Chen Y. Tetrahedron Lett. 1995;36:505–508. doi: 10.1016/0040-4039(94)02296-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh AK, Kincaid JF, Walters DE. J. Med. Chem. 1996;39:3278–3290. doi: 10.1021/jm960128k. [DOI] [PubMed] [Google Scholar]
- 20.Gibert M, et al. Tetrahedron. 1997;53:8643–8650. [Google Scholar]
- 21.Boulineau FP, Wei A. Org. Lett. 2004;6:119–121. doi: 10.1021/ol036183m. [DOI] [PubMed] [Google Scholar]
- 22.Dess DB, Martin JC. J. Am. Chem. Soc. 1991;113:7277–7287. [Google Scholar]
- 23.Corey EJ, Ghosh AK. Tetrahedron Lett. 1988;29:3205–3208. doi: 10.1016/0040-4039(88)85122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dale JA, et al. J. Org. Chem. 1969;34:2543–2549. [Google Scholar]
- 25.Ghosh AK, et al. J. Med. Chem. 2008;51:6021–6033. doi: 10.1021/jm8004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshimura K, et al. Proc. Natl. Acad. Sci. USA. 1999;96:8675–8680. doi: 10.1073/pnas.96.15.8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Mendoza C, Soriano V. Curr. Drug. Metab. 2004;5:321–328. doi: 10.2174/1389200043335522. [DOI] [PubMed] [Google Scholar]
- 28.Kim EE, et al. J. Am. Chem. Soc. 1995;117:1181–1182. [Google Scholar]
- 29.Wlodawer A, et al. Science. 1989;245:616–621. doi: 10.1126/science.2548279. [DOI] [PubMed] [Google Scholar]
- 30.Koh Y, et al. J. Mol. Biol. 2007;282:28709–28720. doi: 10.1074/jbc.M703938200. [DOI] [PubMed] [Google Scholar]
- 31.Tie Y, et al. J. Mol. Biol. 2004;338:341–352. doi: 10.1016/j.jmb.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 32.Ishima R, et al. Proteins. 2010;78:1015–1025. doi: 10.1002/prot.22625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber IT, et al. J. Biol. Chem. 1990;265:10492–10496. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.