Abstract
Dengue represents a substantial burden in many tropical and sub-tropical regions of the world. We estimated the economic burden of dengue illness in Malaysia. Information about economic burden is needed for setting health policy priorities, but accurate estimation is difficult because of incomplete data. We overcame this limitation by merging multiple data sources to refine our estimates, including an extensive literature review, discussion with experts, review of data from health and surveillance systems, and implementation of a Delphi process. Because Malaysia has a passive surveillance system, the number of dengue cases is under-reported. Using an adjusted estimate of total dengue cases, we estimated an economic burden of dengue illness of US$56 million (Malaysian Ringgit MYR196 million) per year, which is approximately US$2.03 (Malaysian Ringgit 7.14) per capita. The overall economic burden of dengue would be even higher if we included costs associated with dengue prevention and control, dengue surveillance, and long-term sequelae of dengue.
Introduction
Dengue virus infection is the most common arthropod-borne disease of humans and, unlike most infectious diseases, its infection rates are expanding. There are 50–100 million dengue infections each year and approximately 24,000 result in death, mostly in children.1–3 Dengue represents a substantial economic burden to communities and health services in many tropical and sub-tropical regions, and has shown a four-fold increase in the number of cases in the past 30 years.1–7 Southeast Asia has the highest dengue incidence of all regions of the world; cycles of epidemics have affected the region since the 1950s, and their magnitude is increasing.8
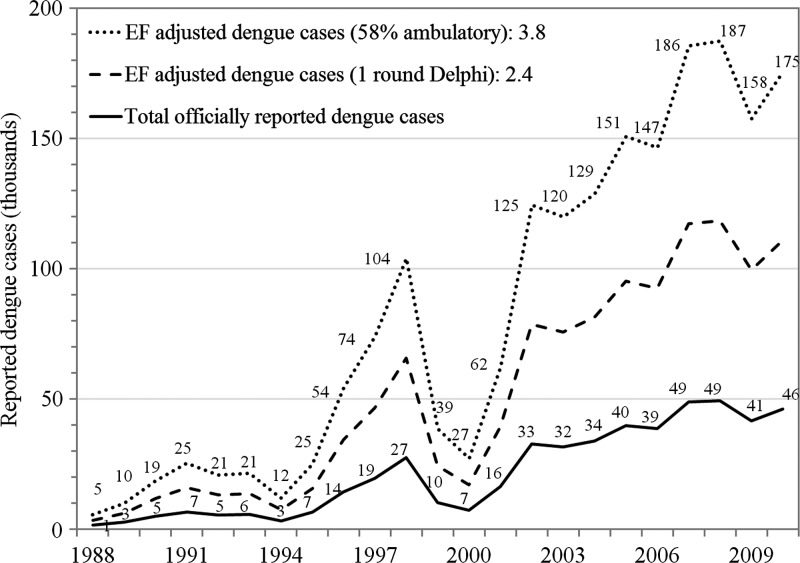
Dengue infection is among the most pervasive public health problems in Malaysia.9,10 The first reported dengue episode in Malaysia was in 1901, and it has spread throughout the whole country.10 Dengue episodes have been tabulated in Malaysia since 1974, and reported dengue cases have increased over past decade (Figure 1). Since the 1980s, the peak age incidence of dengue has shifted from children to young adults. A prospective study in Malaysia showed that dengue has a considerable adverse impact on social function, vitality, and wellbeing, and represents a 60% lost in quality of life (QoL) at its worst point.11
Figure 1.
Reported and projected cases of dengue in Malaysia, 1988–2010. Reported cases were obtained from the Ministry of Health Malaysia18 and the World Health Organization Regional Office for the Western Pacific.19 EF = adjusted dengue cases based on workshop estimates.
To set health policy priorities and to inform decision-makers about the implementation of existing and new technologies to control dengue illness, policy makers and international donors require information about the economic burden of dengue.12,13 We adjusted previous estimates of economic burden of dengue to 2009 U.S. dollars by using the U.S. gross domestic product (GDP) deflator.14 Using the average of reported dengue episodes during 2001–2005, Suaya and others7 estimated an annual economic burden of dengue (±SE) of US$3.1 (±0.2), US$41.9 (±4.28), and US$52.5 (±11.4) million in Cambodia, Malaysia, and Thailand, respectively. Results from a comprehensive study of dengue costs in Malaysia (2000–2005) and Thailand (2002–2007), including costs of dengue illness, vector control, and government research and development related to dengue, gave annual costs of dengue illness of US$134 million in Malaysia and US$136 million in Thailand.15 Results from a study based on Khon Kaen Hospital, Thailand, suggested that the national economic burden of dengue in Thailand was US$173 million.6
However, accuracy of past estimates is limited because of incomplete data. The total incidence of symptomatic dengue illness is not fully captured by passive surveillance systems, which usually under-report the total number of cases.16–21 Malaysia, like most countries, uses a passive surveillance system to capture the incidence of dengue illness.22 Although this approach is helpful to identify outbreaks and to examine time trends, passive surveillance has several limitations. Diagnoses are difficult because of the variety of manifestations of dengue infections. Disease notification relies on health care professionals, and there are differences in reporting from public and private healthcare facilities and between epidemic and non-epidemic periods. Also, the definition of a reported dengue case varies.22–24 We overcame these limitations by merging multiple data sources to refine our estimates of the total number and the unit costs of dengue cases in Malaysia. We used World Health Organization (WHO) case definitions of dengue fever and dengue hemorrhagic fever.25
Materials And Methods
Overview.
We calculated the economic burden of dengue from a societal perspective as the number of cases per year multiplied by the direct and indirect costs per case. Because dengue is a reportable illness, the number of cases officially reported to Malaysia Ministry of Health (MoH) gives a rough approximation of the true number. In 2009, that number was 41,454 cases (Ministry of Health Malaysia, unpublished data). We used 2009 as the reference year because it was the year for which we obtained the most comprehensive surveillance and cost data, and it is a good representation of recent reported dengue cases. Although using the average of a series of years could have been useful if detailed data were available, the number of cases reported in 2009 was close to the averages for 2006–2010 (44,879 cases) and 2001–2010 (37,866 cases) (Figure 1).
To address possible under-reporting of cases, we first obtained estimates of expansion factors from previous studies and the Malaysia Foreign Worker Medical Examination Monitoring Agency (FOMEMA). An expansion factor (EF) is the number by which reported cases need to be multiplied to obtain the true number of cases. Using this information, we then implemented a two-stage Delphi process to estimate specific EFs for Malaysia. The first stage was part of a one-day workshop on the burden of dengue in Malaysia, facilitated by the MoH, and the second was a follow-up by phone and e-mail a few weeks later.26 To estimate the unit costs of dengue cases, we combined a publication from the University of Malaya, data from special studies, national health accounts, and inflation adjustments. Because we knew that each of the datasets would have some limitations, we tried to design methods at the outset that would compensate for them. We used Crystal Ball software for a probabilistic analysis of the total costs of dengue illness in the sensitivity analysis.27 We report our results in U.S. dollars by using the exchange rate of July 1, 2009 (1 Malaysian Ringgit = US$0.284).28
Estimating the number of and treatment sites of dengue cases.
We classified cases of dengue officially reported by the MoH in 2009 into ambulatory and hospitalized cases, and then subdivided them between public and the private sectors according to the location from which the case was reported. Although dengue is a reportable disease in Malaysia, under-reporting inevitably leads to a lower estimation of the real number of cases. To estimate the total number of cases, we had to first estimate the corresponding EFs.
Estimates of EF in Southeast Asia.
An EF can be derived by dividing the best estimate of the total number of dengue illness cases in a specified population in one year by the number of reported cases considered dengue illness (whether they actually were laboratory confirmed or real dengue illness) in that population in that year. Some authors have used EFs obtained from studies in the Western Hemisphere to estimate the burden of disease in countries in Asia,29,30 Given the differences in epidemiology and surveillance systems, we believe that it is preferable to rely on studies from the same region. We have previously conducted a systematic literature review (1995–2011) and identified nine published papers reporting original, empirically derived EFs or the necessary data to estimate the total incidence of dengue illness in four countries in Southeast Asia.21 We reviewed some of these papers during the workshop. We used and commended the cohort study from Kamphaeng Phet, Thailand, to illustrate the use of EFs to estimate the total cases of dengue.31,32
FOMEMA system.
Another source of evidence about under-reporting of dengue in Malaysia came from an examination of the FOMEMA system. FOMEMA aims to reduce the spread of communicable diseases by routinely testing foreign workers in Malaysia for six communicable diseases upon arrival and annually thereafter.33,34
The numbers of cases of communicable diseases identified that were notified in 2009 to the MoH from this testing are considerably fewer than the actual positive cases screened for all of these conditions.26 To illustrate this finding, we can consider the case of malaria. Like dengue, malaria is a common arthropod-borne disease of humans. Symptomatic malaria produces high fever and body pain, considerably reducing the ability of the patient to work. On the basis of analysis of FOMEMA data, the estimated EF for malaria is approximately 8. If malaria is assumed to be the most relevant disease to compare with the dengue situation, we would expect to see only approximately 10–12% of dengue cases being reported to the MoH. The EF for all six communicable diseases in FOMEMA is 52 cases of infection for each reported case, indicating substantial under-reporting by private physicians. These numbers were not used directly to estimate the EF for dengue. Rather, they were presented as evidence for under-reporting of communicable diseases in Malaysia, and considered by the experts attending the workshop as they estimated EFs for dengue.
Laboratory tests in the private sector.
Another way of estimating the total dengue cases is by analyzing the number of laboratory tests for dengue requested from the private sector. Following economic growth and expansion in Malaysia in recent decades35 the country's private health care sector has grown substantially since the early 1980s.36
Pantai Holdings, which owns seven large hospitals, is a major stakeholder in the private health sector in Malaysia. Laboratories in Pantai hospitals implemented and analyzed approximately 20% of the total private sector tests of suspected dengue patients according to information that Pantai laboratory staff received from the country's main supplier of dengue test kits. We projected the total numbers of tests in the private sector by multiplying the number from Pantai hospitals by 5 to account for their 20% market share.
The total number of true dengue cases receiving a laboratory test is probably somewhere between the total number of positive laboratory test results and the number of tests performed on patients who showed probable symptoms of dengue because a negative test result does not necessarily rule out dengue infection.37,38 An illness can be reported as dengue based on a clinical diagnosis. Laboratory tests are not required and are not always performed because of cost or time required. Public hospitals are more likely than private hospitals to report a case as probable dengue, rather than obtaining a laboratory confirmation.
Using the proportion of persons with dengue seen in the private sector relative to the public sector, we could make an estimate from the Pantai Holdings data of the total number of suspected and actual dengue cases in the country. Most (60%) hospitals in Malaysia are private, but only 24% of beds, 26% of admissions, and 13% of hospital ambulatory visits are in the private sector.26,39,40 Based on the shares of acute care hospital beds in 2005 that were private (30% of urban beds and 10% of rural beds) and that dengue is over-represented in urban areas, we estimated that 26% of hospitalized dengue patients are probably in private hospitals.26
Delphi process.
A Delphi process uses expert knowledge systematically to solve complex issues when there are insufficient data. It aims to achieve consensus on a specific matter through several rounds of consultation.41 Our Delphi process had two rounds followed by an adjustment process for internal consistency. The first round was carried out during a one-day workshop facilitated by the Malaysian MoH on December 6, 2010, in Putrajaya, Malaysia. Experts from the academic, public, and private sectors were asked to fill in a survey giving their best estimates for the EFs for dengue cases in Malaysia. We requested EFs for hospitalized and ambulatory cases in the public sector, and hospitalized and ambulatory cases in the private sector. Participants were asked to reach this estimate based on the information discussed above, which was presented and discussed at the workshop, bearing in mind which evidence, data, or specific values they believed were more reliable indicators. The second round of the Delphi process was carried out by e-mail and telephone a few weeks after the workshop, and enabled us to further refine our estimates of total dengue cases. We sent participants a report and a narrated presentation with improved results from the workshop, together with a request to re-estimate under-reporting for public and private health sectors, and also the share of dengue cases that are ambulatory. Participants are listed in the acknowledgments.
Understanding that private ambulatory facilities generally refer patients to private hospitals, and public facilities refer patients to public hospitals, we assumed that the ratio of public to private hospitalized cases is the same as the ratio of public to private ambulatory cases. We calculated multiplicative adjustment factors for mean Delphi EFs for ambulatory cases in the public and private sectors. These factors made the distribution of dengue illness between hospitalized and ambulatory modalities consistent with the estimated share of ambulatory cases from the second round of the Delphi process.
Estimating the unit cost of dengue cases.
Complete and reliable information on the unit costs of providing inpatient and outpatient medical care in the public and private sectors was not readily available. We combined a variety of sources of cost information in our estimates of dengue burden in Malaysia, mostly focusing on costs of service provision in public healthcare facilities.
Costs of service provision in public hospitals.
We used three sources of data for estimating costs in the public sector. First, we estimated the unit costs of inpatient and outpatient hospital services in the University of Malaya Medical Center (UMMC), a national referral hospital that was used in an eight-country comparative study of dengue costs in 2005.7 We used these data on the operating expenditures and workload of UMMC in 2005 and updated them using 2009 data. The 2009 unit cost estimates included salaries for academic clinicians not captured in the 2005 study. These clinicians were paid directly by the university and information concerning their salaries was not kept by the hospital. Information on academic staffing and their salaries were obtained directly from the university. We then assumed that 60% of the time of UMMC clinicians was spent on patient care, and the rest was spent on research and teaching duties (Table 1).7,42
Table 1.
Estimation of unit costs at the University of Malaya Medical Center, 2005 and 2009*
| Row | Item | Source | UMMC, 2005 | UMMC, 2009 |
|---|---|---|---|---|
| 1 | Admissions | Hospital report† | 41,000 | 46,977 |
| 2 | No. registered beds (official) | Hospital report | 875 | 983 |
| 3 | Occupancy rate | Hospital report | 92% | 69% |
| 4 | Occupied beds | 2 × 3 | 805 | 681 |
| 5 | Annual bed days | 4 × 365 | 293,825 | 248,645 |
| 6 | Ambulatory clinic visits | Hospital report | 491,000 | 776,420 |
| 7 | Emergency visits | Hospital report | 68,000 | 103,442 |
| 8 | Total ambulatory visits | 6 + 7 | 559,000 | 879,862 |
| 9 | Relative cost: visit/inpatient day | Shepard et al. | 0.20 | 0.20 |
| 10 | Ambulatory bed-day equivalents | 8 × 9 | 111,800 | 175,972 |
| 11 | Total bed day equivalents | 5 + 10 | 405,625 | 424,617 |
| 12 | Operating expenditure, US$ million | Hospital report | 73 | 112‡ |
| 13 | Cost per bed day equivalent, US$ | 12/11 | 181 | 263 |
| 14 | Cost per ambulatory visit, US$ | 13 × 9 | 36 | 53 |
Second, we used a study that examined the financial performance of six public district hospitals, including estimates of inpatient and outpatient unit costs (year 2001).43 We used data from the World Health Organization CHOosing Interventions Which Are Cost-Effective (WHO-CHOICE) project.44 Presumably because of the lack of suitable data, Malaysia was not included among the 49 countries in the WHO-Choice database of unit hospital costs. Using econometric modeling, the project predicted the unit hospital costs for countries in which data were not available. The WHO-CHOICE project has made available online predicted estimates for costs per bed day and costs per outpatient visit in public hospitals in Malaysia for 2005.44 These estimates included capital costs but excluded costs of provision of drugs, diagnostic tests, and procedures in the hospitals.
We combined data from the 2009 UMMC estimates (Table 1) and the WHO-CHOICE to derive unit cost estimates associated to dengue illness.7,44 We chose these studies because both were available from international peer-reviewed publications that used more recent data than the six district hospitals study. We used the latter study only in the sensitivity analysis.43 The estimation process of the average unit cost per bed day and average cost per outpatient visit is shown in Table 2. As part of the expert workshop in Malaysia, we first agreed on the distribution of facility types by their total hospital beds in the country (Table 2). We assumed that the share of hospitalized and ambulatory dengue patients who seek care in each hospital type is proportional to the total number of beds the facility has. The tertiary-level beds made up 50% of all hospital beds in the country. Secondary and primary level hospital beds made up 30% and 20% of the total, respectively. We used the WHO-CHOICE estimates to derive the ratio of WHO-CHOICE unit cost for secondary-level and primary-level hospitals to the cost for tertiary-level hospitals (Table 2). The unit cost per bed day in the primary-level hospital was 56% of the costs in the tertiary-level hospital. We then applied these cost-ratios to the 2009 UMMC unit cost estimates and combined these figures with the distribution of hospital beds by facility type (Table 2) to derive the weighted average of the unit cost per bed day and per outpatient visit in a public hospital in Malaysia in 2009 of US$219.16 and US$41.96, respectively.7,44
Table 2.
Estimation of average unit costs per bed-day and per outpatient visit in a public hospital in Malaysia, 2009
| Item and type of hospital | Estimated % of beds by hospital type* | Unit cost (2005 US$) | Ratio: cost of hospital type:tertiary hospital | Unit cost per bed-day (2009 US$) |
|---|---|---|---|---|
| Per bed-day | ||||
| Primary-level hospital | 20 | 32.74 | 0.56 | 147.84 |
| Secondary-level hospital | 30 | 42.72 | 0.73 | 192.89 |
| Tertiary-level hospital | 50 | 58.35 | 1.00 | 263.45 |
| Average cost per bed-day | 219.16 | |||
| Per outpatient visit | ||||
| Primary-level hospital | 20 | 11.46 | 0.48 | 25.23 |
| Secondary-level hospital | 30 | 16.26 | 0.68 | 35.54 |
| Tertiary-level hospital | 50 | 24.05 | 1.00 | 52.58 |
| Average cost per outpatient visit | 41.96 | |||
As a sensitivity analysis, we combined two new sets of estimates with our best estimate by using a triangular distribution, the approach in previously published estimates.7,16 One set of estimates used the WHO-CHOICE costs with the six district hospital costs.43,44 This set generated unit cost estimates for hospital services of US$209.19 per bed day and US$55.59 per outpatient visit (2009 US$), which are approximately 5% lower for inpatient services and 32% higher per outpatient visit than the unit costs obtained from UMMC estimates. The other set of estimates used a weighted average between the UMMC based estimates and the six district hospital based estimates. Because we considered the UMMC estimate more accurate than that from the six public hospitals, we gave them relative weights of 0.67 and 0.33, respectively, to generate our last estimate (US$212.51 per bed day and US$51.05 per outpatient visit).
Costs of service provision in public clinics.
We derived the unit costs of visits to public clinics from an unpublished cost study on the provision of outpatient services in 11 public clinics in Kedah by Lim.45 The unit cost per visit (US$2.94 when inflated to 2009 values)14 was derived mainly from the operating budget of the clinics involved. This figure was confirmed by participants in the workshop who had prior experience managing district health care. Although WHO-CHOICE also provided estimates for unit costs of public clinic visits, workshop participants did not believe that they were applicable to dengue treatment in the public sector. The WHO-CHOICE estimates applied to 20-minute visits, which are considerably longer than typical public sector visits in Malaysia, and estimates were mostly extrapolated from private sector static health facilities data.
Costs of service provision in private hospitals.
We used data from the Malaysia National Health and Morbidity Survey II to estimate private hospital costs.46 This survey included more than 13,600 households and approximately 60,000 persons. The survey contained data on reported inpatient and outpatient out-of-pocket expenditures (OOP) for public and private facilities. We examined a subset of persons who reported OOP only for private care, with no recourse for reimbursement or payment from any third party payer (e.g., employer, health insurance). Assuming that the OOP payment approximates the costs (and profit) at these private hospitals, we estimated the costs per bed-day in a private hospital in Malaysia and the unit cost of an outpatient visit.
Using the mean values to estimate the costs, we found that the data suggested that the estimate of OOP per bed-day in a private hospital (US$249.24) was approximately 14% higher than the weighted estimate we obtained for a bed-day in a public hospital (US$219.16) (Table 2). However, it was within the range of bed-day costs for different public hospital types (US$147.84 in a primary level hospital and US$263.45 in a tertiary level hospital) (Table 2). One possible interpretation of this difference in costs is that 90% of the capacity of private hospitals in Malaysia is in urban rather than rural areas.
To obtain an estimate of the cost per outpatient visit, we took a weighted average between OOP per hospital outpatient visit and OOP per general practitioner outpatient visit. The cost for OOP per outpatient hospital visit is US$23.76, which is approximately 43% lower than our estimate for outpatient visits to a public hospital (US$41.96) (Table 2).
Indirect costs.
The main source of indirect cost is work-time lost (i.e., productivity loss) caused by illness. The indirect costs include those incurred by the patient and also include relatives' time spent at home caring for the patient and in trips to the hospital.47 By dividing total indirect cost by the number of days affected in the data for Malaysia of Suaya and others,7 we found that the average indirect cost per day was approximately 1.5 times the minimum wage. This estimate includes the productivity loss of both the patient and relatives, and the corresponding days of school and work days based on the age distribution of cases. In 2011, Malaysia required a minimum wage of US$199 per month for private security guards.48 However, policy debates examining wages in all sectors and have mentioned a benchmark wage of US$199 per month.49 We used this benchmark as a reasonable economic value each day lost to dengue and based our calculations of indirect costs on it. We also used the study by Suaya and others to estimate the total number of days lost to dengue fever in Malaysia (averaging 11.2 days lost per ambulatory episode and 16.2 days lost per hospitalized episode, including patient and household impact).7
To include economic costs associated with deaths, we used the human capital approach based on productivity lost,50 and estimated the years of premature life lost based on life expectancy using WHO life tables for Malaysia.51 We used the age distribution of the 92 reported deaths related to dengue in the year 2009 (Ministry of Health Malaysia, unpublished data, 2010). Although not discussed in the workshop, a paper from Indonesia showed under-reporting in the region for dengue-related deaths, even from major hospitals.18 Recent studies have also shown associations between dengue illness and other severe health complications,52–58 and we would expect some of the resulting deaths not to be reported as dengue. Because of the paucity of data, we assumed that the overall under-reporting of dengue-related deaths is equal to the under-reporting of hospitalized cases from the public and private sectors, and that the deaths in the public and private sectors are proportional to their shares of dengue cases. To be conservative, we assumed that all workers earn minimum wage and that students stay in school until they are 17 years of age. We calculated the costs of school days lost using the estimates of Suaya and others (2009 US$5.05 per day),7 and adjusted for time preferences using an annual discount rate of 3%.
Overall cost per case.
We made several assumptions to estimate the total costs per dengue case. First, because regional breakdowns were not available, and dengue is primarily urban, we assumed that the duration of dengue cases from the UMMC data is on average representative of the national data.7 Second, we assumed that the national impact on patients and households per dengue case is the same as that estimated from UMMC data. This seems plausible because the ratio of dengue cases in adults to those in children was estimated at 2.3 in UMMC is similar to the ratio of 1.93 we derived from using the incidence data of dengue fever and dengue hemorrhagic fever data during 2003–2005.12 Third, we assumed that all dengue cases affect productive persons and valued their time lost at the minimum wage if they are adults or the cost per day of school if they are children. Fourth, to update the indirect daily costs of dengue for persons with hospitalized and ambulatory cases from the study by Suaya and others,7 we assumed that the relationship between the minimum wage and the indirect costs in both settings has not changed since 2005. Fifth, we assumed that the indirect daily costs of dengue cases are the same for the private and public sectors. This is a conservative assumption because we would expect wealthier persons to receive treatment predominantly at private facilities, which is likely to raise the average indirect costs in this setting. We do not have the required data to test this hypothesis and make a more accurate estimate. Finally, we assume that the impact of dengue on total days lost is caused by the average duration of an acute dengue case. However, we did not include any costs associated with long-term consequences of dengue, a possible chronic sequela of illness referred to as post-infectious fatigue syndrome.59–63
Sensitivity analysis.
The probabilistic sensitivity analysis of total costs includes the simultaneous variation of five categories of parameters summarized in Table 3. We computed 20,000 Monte Carlo simulations for each parameter. Iterations drew random values from the distribution of each input.7,27,43,44
Table 3.
Summary of parameters varied simultaneously in sensitivity analysis, Malaysia*
| Parameter | Best estimate | Sensitivity analysis on parameter | Source or reference | |||
|---|---|---|---|---|---|---|
| Distribution | Minimum | Central | Maximum | |||
| EF of hospitalized cases (public sector) | 1.30 | Triangular | 1.00 | 1.30 | 2.00 | Delphi, round 2 |
| EF of hospitalized cases (private sector) | 2.45 | Triangular | 1.30 | 2.45 | 5.00 | Delphi, round 2 |
| Proportion of ambulatory treatment of dengue | 0.58 | Triangular | 0.30 | 0.58 | 0.95 | Delphi, round 2 |
| Bed-day cost hospitalized dengue (public sector) | 219.16 | Triangular | 209.19 | 215.84 | 219.16 | 7, 43, 44 |
| Visit cost ambulatory dengue (public sector) | 41.96 | Triangular | 41.96 | 46.50 | 55.59 | 7, 43, 44 |
| Days lost per episode (hospitalized) | 16.2 | Normal | NA | 16.2 (mean) | 0.59 (SE) | 7 |
| Days lost per episode (ambulatory) | 11.2 | Normal | NA | 11.2 (mean) | 0.41 (SE) | 7 |
| No. of deaths from dengue | 92 | Poisson | NA | 92 | NA | Ministry of Health† |
EF = expansion factor; NA = not applicable; SE = standard error. Our cost estimates were derived using only the best estimate for these parameters.
No. deaths correspond to reported deaths in 2009 (Ministry of Health Malaysia, unpublished data).
Results
Total number of cases.
In the second round of the Delphi process, 10 of 14 participants gave estimates for EFs and the percentage of ambulatory cases. The respondents came from varied settings (four from academia, two from the Ministry of Health, and four from the private sector). Their EFs averaged 1.30 (range = 1.0–2.0) for public sector hospitalized cases and 2.45 (range = 1.3–5.0) for private hospitalized cases.
In the second round of the Delphi process, we addressed ambulatory EFs indirectly through the share of cases treated in the ambulatory sector. In that round, respondents' estimated percentage of dengue cases treated entirely in the ambulatory sector averaged 58% (range = 30–95%). Thus, the triangular distribution in the sensitivity analysis on the proportion of dengue cases that are ambulatory used these results as most likely, minimal, and maximal values, respectively. Corresponding adjusted EFs for each of the four categories of dengue patients using the mean values of the Delphi process and the resulting dengue cases adjusted using EF is shown in Table 4. The EF-adjusted dengue cases satisfy our consistency checks in that the ambulatory shares for public patients (50,186 of 86,527) and private patients (40,955 of 70,613) both equal 58%. The upper line in Figure 1 shows the EF-adjusted dengue cases by year.
Table 4.
Derivation of dengue cases in Malaysia using expansion factors (2009), by sector and setting*
| Sector | Hospitalized cases | Ambulatory cases | Overall |
|---|---|---|---|
| Adjusted EFs (combining mean factors, 58% share ambulatory) | |||
| Public | 1.30 | 43.08† | 2.97 |
| Private | 2.45 | 178.84† | 5.73 |
| Both‡ | 1.65 | 65.38† | 3.79 |
| No. reported dengue cases | |||
| Public | 27,955 | 1,165 | 29,120 |
| Private | 12,105 | 229 | 12,334 |
| Total | 40,060 | 1,394 | 41,454 |
| Adjusted dengue cases using EFs (58% share ambulatory) | |||
| Public | 36,341 | 50,186 | 86,527 |
| Private | 29,658 | 40,955 | 70,613 |
| Total | 65,999 | 91,141 | 157,140 |
| Row % | 42.00 | 58.00 | 100.00 |
EF = expansion factor.
EFs were estimated by comparing the EF adjusted cases and reported cases, assuming that the total ratio of public/private ambulatory cases is the same as this ratio for hospitalized cases. The EFs for ambulatory cases were indirectly derived in two steps. First, we obtained EFs for hospitalized cases in the private and public sectors (second column) and the share of the total cases that were ambulatory (58%) from the Delphi process. Second, we derived EFs for ambulatory cases by dividing the estimate of total ambulatory cases for each sector by the officially reported cases.
The both row and overall column are derived by comparing the projected and reported numbers.
Total costs of ambulatory and hospitalized dengue cases.
Estimated total costs of ambulatory and hospitalized dengue cases in the public and private sectors using EF adjusted dengue cases (considering a 58% share of ambulatory cases; Table 4) is shown in Table 5. The annual burden of dengue in Malaysia was US$55.83 million (Malaysian Ringgit, MYR196.44). Of this burden, 45.1% was from the private sector and 54.9% was from the public sector; 51.2% corresponded to non-fatal hospitalized cases of dengue, 34.3% to ambulatory cases, and 14.5% corresponded to indirect costs associated to deaths; 33.0% of the total costs were direct costs (e.g., hospital services) and 67.0% were indirect costs (e.g., productivity loss).
Table 5.
Cost of dengue illness by sector, setting, and component, Malaysia*
| Sector | Ambulatory | Hospitalized | Indirect deaths | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Indirect | Direct | Total | Indirect | Direct | Total | |||
| Estimated costs per case (2009 US$) | ||||||||
| Public | 176.36 | 41.96 | 218.32 | 200.55 | 219.16 | 419.71 | 53,336.51 | 343.50 |
| Private | 176.36 | 23.76 | 200.11 | 200.55 | 249.24 | 449.79 | 53,336.51 | 345.57 |
| Total | 176.36 | 33.78 | 210.14 | 200.55 | 232.68 | 433.22 | 53,336.51 | 344.43 |
| Estimated aggregate costs from EF-adjusted dengue cases (58% ambulatory; 2009 US$1,000s) | ||||||||
| Public | 8,850 | 2,106 | 10,956 | 7,288 | 7,965 | 15,253 | 4,451 | 30,660 |
| Private | 7,223 | 973 | 8,196 | 5,947 | 7,392 | 13,339 | 3,633 | 25,168 |
| Total | 16,073 | 3,079 | 19,152 | 13,235 | 15,357 | 28,592 | 8,084 | 55,828 |
| Range† | (8,940–117,670) | (23,900–42,600) | (6,470– 9,790) | (44,300–153,200) | ||||
EF = expansion factor. The unit cost of death reported is the average cost; the actual values were estimated on the basis of the age distribution of reported deaths caused by dengue in 2009 (Ministry of Health Malaysia, unpublished data).
Malaysia has a population of approximately 27.5 million persons. Thus, the per capita cost of dengue illness was approximately US$2.03 (MYR7.14). Because the GDP per capita of Malaysia was approximately US$6,830 in 2009 US dollars (MYR24,032), the cost of dengue illness represented 0.03% of the per capita GDP.64 Based on trends in the most recent published National Health Accounts for Malaysia, the country spent approximately US$363.80 (MYR1,280.09) per person per year on health care.65 Thus, the direct cost of dengue represented 0.18% of the country's total health expenditures.
The 13 states and 3 federal territories in Malaysia vary considerably in size and population, ranging from the federal territory of Putrajaya with approximately 68,000 persons to the federal state of Selangor with approximately almost 5.5 million persons.66 The biggest urban area is the metropolitan area of Kuala Lumpur. Using data of the reported cases of dengue from the Department of Statistics, Malaysia,66 and assuming that the costs are proportional to the number of dengue cases, we found that Kuala Lumpur represented approximately 9% of the total costs of dengue fever in Malaysia.
Sensitivity analysis.
In our sensitivity analysis, the factor with the largest impact on the variation of total cost of dengue in Malaysia was the share of ambulatory cases (75.0% of the total variance), followed by the EFs for dengue reported in private hospitals (17.8% of total variance), the EFs for dengue reported in public hospitals (6.6%), the total number of deaths (0.2%), the total days lost because of ambulatory treatment of dengue infection (0.2%), and the total days lost because of hospitalization (0.1%). Because the estimates of direct costs derived from UMMC and the six district hospital study were similar, their contribution to the total variation of costs was negligible. To illustrate this point, when we estimated public sector unit costs data from the six district hospitals instead of UMMC 2009 data, we found that the total annual economic burden of dengue illness in Malaysia is US$54.45 million, which is only approximately a 2% change from our original value. If we varied all these parameters simultaneously, the 95% certainty level (centered on the median) for the total economic burden of dengue was US$44.30–$153.20 million, and the interquartile range was US$56.05–$80.60 million. Although the mean values of costs we report are based on our best assumptions and data, the width of the certainty levels and interquartile range illustrates the uncertainty of estimates of economic burden when using incomplete data.
Discussion
Our findings suggest that there is substantial under-reporting of symptomatic dengue fever in Malaysia, and that the economic burden of dengue fever in Malaysia is considerable. Combining multiple sources of data is critical to achieve reliable estimates of the total cases and economic burden of dengue fever. The EFs we used to adjust for under-reporting were estimated on the basis of several data sources, including existing literature, expert knowledge, and laboratory evidence. The Delphi process led to an overall EF of 3.79, adjusted using the mean value of 58% share of ambulatory cases. This EF is well in the range of those of published studies from Southeast Asia (EF = 3.1–9.1), and lower than the average from these studies (7.8 times as many cases of dengue as those officially reported).21 However, our estimates are probably conservative, understating the overall burden of dengue in Malaysia, for several reasons.
First, a thorough study of EFs in Thailand and Cambodia with EFs of 8.7 and 9.1, respectively,19 was not available at the time of the workshop. If that study had been available, the EF estimates from the Delphi process might have been somewhat higher.
Second, our estimates include only the acute phase of dengue illness. Recent evidence indicates that dengue causes a substantial reduction in QoL of patients during the course of their infection. A previous study in Malaysia found that the reduction in QoL for dengue patients lasted longer than the duration of the fever.11 For some patients, symptoms are more persistent but we do not know for how long these symptoms might last, and how they affect the QoL of a patient. Dengue chronic fatigue refers to the long-term consequences of dengue fever,62 but only a few published studies have examined this phenomenon. A study of adults in Cuba suggests that 57% of the symptomatic dengue patients reported having persistent symptoms for two years after their infection.59 Other studies suggest that 25% of discharged patients had symptoms after two months (Singapore),63 47% of patients had symptoms after 6 months (Cuba),60 and 8.5% of patients reported having difficulty in their daily activities after 2 months and 5.1% after 6 months (Brazil).63 If dengue chronic fatigue is as common as these studies suggest, then our calculations of the total economic burden of dengue in Malaysia would be considerably underestimated.
Third, our indirect cost estimates related to productivity loss, resulting from illness and premature death, were based on minimum wage instead of the more commonly used GDP or gross national income per capita (approximately US$580 monthly output per capita in 2009).14 We chose to base our cost estimates on the minimum wage to best fit the types of time lost. Among patients, some of the time loss from dengue is from leisure and from children who are not in the labor market. Among family care givers, families can generally choose which member takes care of a sick household member, or does not enter the labor market so as to be available when needed. An economically rational family would ask the lowest paid household member to take that task. We thought minimum wage might better reflect the productivity of homemakers who choose not to enter the labor market, or who are caregivers of sick children. Using minimum wage also enabled us to make our cost estimates comparable to previous studies in Malaysia.7
Fourth, our cost estimates do not include the costs of prevention, surveillance, and dengue vector control activities. These activities measurably increase the total economic burden of dengue, as suggested by other studies. For example they added 39% to the estimated economic burden of dengue in Thailand,6 43% in Panama,67 and 20% in Puerto Rico.68 There are vector control units administered by the Malaysian MoH and by local councils (city hall, city councils, and municipal councils). There are yet other costs not considered, such as the impact of dengue illness on tourism or the effects of the seasonal clustering of dengue on health systems.69 Despite this limitation, our cost estimates improve previous estimates.
Our best estimate of the annual costs of dengue illness per year (US$55.83 million) is between previous estimates by Suaya and others7 (US$41.92 million) and Lim and others15 (US$134.40 million). The estimate of Suaya and others is based on the average of yearly reported dengue cases in 2001–2005 without adjustment for under-reporting. Because our conservative estimate (the lower bound of our certainty range was US$44.3 million) is based on lower ranges of expansion factors and other parameters, it is similar to that of Suaya and others.7 Also, the unit costs in the study by Suaya and others are based only on data from a national referral hospital (UMMC). We overcame the limitations in their study by using EFs and adjusting UMMC cost data by facility type using WHO-CHOICE estimates.44 These adjustments decreased the cost per bed-day by 17% and per outpatient visit by 20% compared with using only UMMC data.
The estimate of Lim and others15 (US$134.40 million) is considerably higher than our best estimate because, in addition to the cost of illness, those authors include costs of vector control activities and research and development. Lim and others15 estimated an overall EF of 3.23, which was similar to our estimated EF of 3.79, but their estimated overall unit cost was higher (US$449.95 versus US$344.43 in our study). Lim and others15 used the average of reported dengue for 2002–2007 (37,793 cases versus 41,454 in our study). From the values reported by Lim and others,15 we derived the component of dengue costs in their study caused by illness. This result (US$54.93 million) is virtually identical to our estimate (US$55.83 million). When we adjusted for GDP deflators in Malaysia and each year's exchange rate,26,66 we found that our total cost estimate was equivalent to US$71.05 million in 2012 US dollars (2012 MYR218.27 million).
More generally, limited data required to extrapolate EFs and costs across calendar years, age groups, locations, and countries create substantial uncertainty in our estimates. This variation is reflected in our wide sensitivity analysis. Some limitations and areas of uncertainty deserve special attention.
First, the accuracy of the Delphi estimates depends on the quality of the evidence examined and the combination of knowledge from the group of experts. We examined evidence of under-reporting from a wide variety of sources, including published literature reporting serologic and capture–recapture original studies in the region, and unpublished data from the public and the private sectors. We included several experts from academic, public, and private sectors, and achieved consensus through two rounds of consultation and adjustments for internal consistency. We believe that our estimates for EF and the share of ambulatory cases are as accurate as available evidence allowed at the time the workshop took place. We do not recommend a Delphi panel as a substitute for gathering more original data through well-designed capture–recapture or cohort studies. However, such studies have time and resource demands that are at least an order of magnitude greater than organizing a Delphi panel and, to our knowledge, none existed in Malaysia. A rigorous Delphi process is a good alternative when facing complex health issues with budget and time constraints.
The EF for dengue episodes in the private sector is probably most critical because there is a paucity of data. To check our estimates, we examined data from private laboratories. A total of 22,725 patients were tested for dengue infection in Pantai hospitals in 2009, and 4,531 were positive for dengue. Projecting to the overall private sector overall of Malaysia, we estimated that 113,625 suspected dengue cases occurred in 2009, of which 22,655 were laboratory confirmed. The reported number of dengue cases in the private sector was approximately 12,000 (Table 4), which was half the figure we projected from the data of Pantai Holdings. Even the value of approximately 25,000 might hide a level of under-representation because single-sample serologic tests might show false-negative results (e.g., the test was conducted early in the illness, the sample was not kept sufficiently cold or processed promptly, or the infection was a secondary one).62 Workshop participants believed that the rate of false-negative results might be approximately 50%. We expect that some cases are not tested, particularly those who are adults; thus, our estimates are consistent. Correcting for this rate of false-negative results would increase the projected number of dengue cases in the private sector to approximately 50,000 (approximately 25,000 × 2). Assuming that reporting of hospitalized cases is accurate (EF = 1), then the remaining cases of dengue in the private sector, approximately 38,000 (approximately 50,000–12,000) cases should be ambulatory, resulting in a high EF for hospitalized cases of approximately 166 (EF ambulatory private sector is 38,000 of 229 or 166). Interestingly, this number is close to the estimate of an EF of approximately 179 for ambulatory cases in the private sector we calculated using the estimates from the Delphi process (Table 4).
There is another confounding factor in estimating the number of cases: in the private sector a number of laboratory-confirmed dengue patients are referred to the public sector after a positive result. This factor might lead to the above estimation of EF being inflated relative to the actual situation. Although such cases will be reported twice, this approximately 25,000 might still be an over-expansion. The EFs for the private sector, and particularly for ambulatory cases, remain an area of considerable uncertainty. A comprehensive literature review of EFs in Southeast Asia showed no results for private sector or ambulatory EFs for dengue.21
Second, our estimates of unit costs of inpatient and outpatient could be further refined by distinguishing not only by facility type (based on the number of beds), but also by region, GDP, population, number of specialist physicians, and healthcare. Unfortunately, these data are not readily available.
In conclusion, information about economic burden is needed for setting health policy priorities and deciding about the implementation of existing and new technologies, but accurate estimation is difficult because of incomplete data. We overcame this limitation by drawing on multiple data sources. Although hospital-based reporting appeared relatively complete, the data indicate that in the ambulatory and private sectors there is considerable under-reporting of dengue cases. After adjusting for under-reporting we found that dengue imposes a considerable economic burden in Malaysia. Our results and previous studies suggest that health policies aimed at controlling dengue efficiently would most likely be economically valuable.
ACKNOWLEDGMENTS
We thank the Ministry of Health, Malaysia, for sponsoring the Burden of Dengue Workshop on December 6, 2010, in Putrajaya, and also those who participated: Dr. Satwant al Singh, Dr. Jeremy Brett, Ms. Sharon Chiang, Dr. Chee Kheong Chong, Dr. Laurent Coudeville, Dr. Ahmad Faudzi bin Yusoff, Dr. B. K. Ho, Dr. Shree Jacob, Dr. Ahamad Jusoh, Dr. P. Ravi Raviwharmman, Ms. Puan Shanaliza Sulaiman, Dr. Jameela Zainuddin, Dr. Rosemary Susan Lees, Dr. Lucy Lum, Dr. Chiu Wan Ng, and Dr. Donald Shepard; Dr. Abdul Hasan, Dr. Lokman Hakim B. Sulaiman, Dr. Noor Azimah bt. Hassan, and Stanley Ho for advice; Clare L. Hurley for editorial assistance; and three anonymous reviewers for constructive suggestions.
Footnotes
Financial support: This study was supported by an agreement from Sanofi Pasteur to Brandeis University.
Disclosure: Three participants in the workshop and Delphi process (Jeremy Brett, Laurent Coudeville, and Shree Jacob) were employees of Sanofi-Pasteur, a company currently developing a dengue vaccine.
Authors' addresses: Donald S. Shepard, Eduardo A. Undurraga, and Yara Halasa, Schneider Institutes for Health Policy, Heller School, Brandeis University, Waltham, MA, E-mails: shepard@brandeis.edu, eundurra@brandeis.edu, and yara@brandeis.edu. Rosemary Susan Lees, Centre for Research in Biotechnology for Agriculture, University of Malaya, Kuala Lumpur, Malaysia, E-mail: rosemarylees@um.edu.my. Lucy Chai See Lum and Chiu Wan Ng, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia, E-mails: lumcs@ummc.edu.my and chiuwan.ng@ummc.edu.my.
References
- 1.Guzman A, Isturiz RE. Update on the global spread of dengue. Int J Antimicrob Agents. 2010;36:S40–S42. doi: 10.1016/j.ijantimicag.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 2.Ooi EE, Goh KT, Gubler DJ. Denque prevention and 35 years of vector control in Singapore. Emerg Infect Dis. 2006;12:887–893. doi: 10.3201/10.3201/eid1206.051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halstead SB. Dengue. Lancet. 2007;370:1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 4.Gubler DJ, Meltzer M. Advances in Virus Research. Volume 53. San Diego, CA: Academic Press Inc; 1999. Impact of dengue/dengue hemorrhagic fever on the developing world; pp. 35–70. [DOI] [PubMed] [Google Scholar]
- 5.Shepard DS, Suaya JA. Cost-Effectiveness of a dengue vaccine in Southeast Asia and Panama: preliminary estimates. In: Preedy VR, Watson RR, editors. Handbook of Disease Burdens and Quality of Life Measures. New York: Springer; 2009. pp. 1281–1296. [Google Scholar]
- 6.Kongsin S, Jiamton S, Suaya J, Vasanawathana S, Sirisuvan P, Shepard D. Cost of dengue in Thailand. Dengue Bull. 2010;34:77–88. [Google Scholar]
- 7.Suaya JA, Shepard DS, Siqueira JB, Martelli CT, Lum LCS, Tan LH, Kongsin S, Jiamton S, Garrido F, Montoya R, Armien B, Huy R, Castillo L, Caram M, Sah BK, Sughayyar R, Tyo KR, Halstead SB. Cost of dengue dases in eight countries in the Americas and Asia: a prospective study. Am J Trop Med Hyg. 2009;80:846–855. [PubMed] [Google Scholar]
- 8.Ooi EE, Gubler DJ. Dengue in Southeast Asia: epidemiological characteristics and strategic challenges in disease prevention. Cad Saude Publica. 2009;25:S115–S124. doi: 10.1590/s0102-311x2009001300011. [DOI] [PubMed] [Google Scholar]
- 9.Holmes EC, Tio PH, Perera D, Muhi J, Cardosa J. Importation and co-circulation of multiple serotypes of dengue virus in Sarawak, Malaysia. Virus Res. 2009;143:1–5. doi: 10.1016/j.virusres.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Poovaneswari S. Dengue situation in Malaysia. Malays J Pathol. 1993;15:3–7. [PubMed] [Google Scholar]
- 11.Lum LCS, Suaya JA, Tan LH, Sah BK, Shepard DS. Quality of life of dengue patients. Am J Trop Med Hyg. 2008;78:862–867. [PubMed] [Google Scholar]
- 12.Ministry of Health Malaysia Vector Borne Disease Control Section. 2010. http://www.dph.gov.my/vektor/eng/kes_dd_tahunan.htm Available at. Accessed May 1, 2010.
- 13.World Health Organization . Data Query by Country—Global Health Atlas: Denguenet. Geneva: World Health Organization; 2010. [Google Scholar]
- 14.International Monetary Fund . World Economic Outlook Database. World Economic and Financial Surveys. Washington, DC: International Money Fund; 2011. [Google Scholar]
- 15.Lim LH, Vasan SS, Birgelen L, Murtola TM, Gong H-F, Field RW, Mavalankar DV, Ahmad NW, Hakim LS, Murad S, Wan NC, Lum LC, Suaya JA, Shepard DS. Immediate cost of dengue to Malaysia and Thailand: an estimate. Dengue Bull. 2010;34:65–76. [Google Scholar]
- 16.Shepard DS, Coudeville L, Halasa YA, Zambrano B, Dayan GH. Economic impact of dengue illness in the Americas. Am J Trop Med Hyg. 2011;84:200–207. doi: 10.4269/ajtmh.2011.10-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vong S, Ngan C, Buchy P, Khieu V, Huy R, Duong V, Ong S, Duong S, Chang MS, Xu ZY, Margolis HS. Estimating the incidence of dengue fever in Cambodia: results of a capture recapture analysis. Am J Trop Med Hyg. 2007;77:26. [Google Scholar]
- 18.Chairulfatah A, Setiabudi D, Agoes R, van Sprundel M, Colebunders R. Hospital based clinical surveillance for dengue haemorrhagic fever in Bandung, Indonesia 1994–1995. Acta Trop. 2001;80:111–115. doi: 10.1016/s0001-706x(01)00180-2. [DOI] [PubMed] [Google Scholar]
- 19.Wichmann O, Yoon IK, Vong S, Limkittikul K, Gibbons RV, Mammen MP, Ly S, Buchy P, Sirivichayakul C, Buathong R, Huy R, Letson GW, Sabchareon A. Dengue in Thailand and Cambodia: an assessment of the degree of underrecognized disease burden based on reported cases. PLoS Negl Trop Dis. 2011;5:e996. doi: 10.1371/journal.pntd.0000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vong S, Khieu V, Glass O, Ly S, Duong V, Huy R, Ngan C, Wichmann O, Letson GW, Margolis HS, Buchy P. Dengue incidence in urban and rural Cambodia: results from population-based active fever surveillance, 2006–2008. PLoS Negl Trop Dis. 2010;4:e903. doi: 10.1371/journal.pntd.0000903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Undurraga EA, Halasa YA, Shepard DS. Expansion factors: a key step in estimating dengue burden and costs in Southeast Asia. Am J Trop Med Hyg. 2011;85:318. [Google Scholar]
- 22.Gubler DJ. How effectively is epidemiological surveillance used for dengue programme planning and epidemic response? Dengue Bull. 2002;26:96–106. [Google Scholar]
- 23.Ooi EE, Gubler DJ, Nam VS. Report of the Scientific Working Group Meeting on Dengue. Geneva: 2007. Dengue research needs related to surveillance and emergency response; pp. 124–133. October 1–5, 2006. [Google Scholar]
- 24.Deen JL, Harris E, Wills B, Balmaseda A, Hammond SN, Rocha C, Dung NM, Hung NT, Hien TT, Farrar JJ. The WHO dengue classification and case definitions: time for a reassessment. Lancet. 2006;368:170–173. doi: 10.1016/S0140-6736(06)69006-5. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization—Regional Office for South-East Asia . Comprehensive Guidelines for Prevention and Control of Dengue and Dengue Haemorrhagic Fever. Revised and Expanded. Geneva: World Health Organization; 2011. p. 67. [Google Scholar]
- 26.Shepard D, Lees R, Ng CW, Undurraga EA, Halasa YA, Lum L. Burden of dengue in Malaysia: Report from Ministry of Health Workshop. Putrajaya, Malaysia: Schneider Institutes for Health Policy, Brandeis University, & University of Malaya; 2011. http://people.brandeis.edu/∼shepard/dengue_in_Malaysia_V42.pdf December 6, 2010. Available at. Accessed August 1, 2012. [Google Scholar]
- 27.Decisioneering Inc . Crystal Ball 2000.2. User Manual. Denver, CO: Decisioneering Inc; 2001. [Google Scholar]
- 28.X-Rates Currency Calculator. 2011. http://www.x-rates.com/ Available at. Accessed March 18, 2011.
- 29.Clark DV, Mammen MP, Nisalak A, Puthimethee V, Endy TP. Economic impact of dengue fever/dengue hemorrhagic fever in Thailand at the family and population levels. Am J Trop Med Hyg. 2005;72:786–791. [PubMed] [Google Scholar]
- 30.Garg P, Nagpal J, Khairnar P, Seneviratne SL. Economic burden of dengue infections in India. Trans R Soc Trop Med Hyg. 2008;102:570–577. doi: 10.1016/j.trstmh.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Anderson KB, Chunsuttiwat S, Nisalak A, Mammen MP, Libraty DH, Rothman AL, Green S, Vaughn DW, Ennis FA, Endy TP. Burden of symptomatic dengue infection in children at primary school in Thailand: a prospective study. Lancet. 2007;369:1452–1459. doi: 10.1016/S0140-6736(07)60671-0. [DOI] [PubMed] [Google Scholar]
- 32.Endy TP, Chunsuttiwat S, Nisalak A, Libraty DH, Green S, Rothman AL, Vaughn DW, Ennis FA. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol. 2002;156:40–51. doi: 10.1093/aje/kwf005. [DOI] [PubMed] [Google Scholar]
- 33.FOMEMA FOMEMA: Caring for Malaysia. 2010. http://hugelane.com/Fomema%20Corporate%20Website.htm Available at. Accessed August 1, 2012.
- 34.Leong CC. Pre-employment medical examination of Indonesian domestic helpers in a private clinic in Johor Bahru. An eight year review. Med J Malaysia. 2006;5:592–598. [PubMed] [Google Scholar]
- 35.United States Department of State . Background Note: Malaysia. 2010. http://www.state.gov/r/pa/ei/bgn/2777.htm Available at. Accessed December 24, 2010. [Google Scholar]
- 36.Leng CH. The Emergence of a Transnational Healthcare Service Industry in Malaysia. Singapore: Asia Research Institute, National University of Singapore; 2006. Working Paper Series. [Google Scholar]
- 37.Guzman MG, Jaenisch T, Gaczkowski R, Vo TT, Sekaran SD, Kroeger A, Vazquez S, Ruiz D, Martinez E, Mercado JC, Balmaseda A, Harris E, Dimano E, Leano PS, Yoksan S, Villegas E, Benduzu H, Villalobos I, Farrar J, Simmons CP. Multi-country evaluation of the sensitivity and specificity of two commercially-available NS1 ELISA assays for dengue diagnosis. PLoS Negl Trop Dis. 2010;4(pii):e811. doi: 10.1371/journal.pntd.0000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dussart P, Petit L, Labeau B, Bremand L, Leduc A, Moua D, Matheus S, Baril L. Evaluation of two new commercial tests for the diagnosis of acute dengue virus infection using NS1 antigen detection in human serum. PLoS Negl Trop Dis. 2008;2(pii):e280. doi: 10.1371/journal.pntd.0000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ministry of Health Malaysia . Health Facts 2008. Kuala Lumpur, Malaysia: Health Informatics Center, Planning and Development Division; 2009. p. 12. [Google Scholar]
- 40.Ministry of Health Malaysia . In: Health Facts 2009. Center HI, editor. Kuala Lumpur, Malaysia: Health Informatics Center, Planning and Development Division; 2010. p. 14. [Google Scholar]
- 41.Delbecq AL, Van de Ven AH, Gustafson DH. Group Techniques for Program Planning: A Guide to Nominal Group and Delphi Processes. Glenview, IL: Scott, Foresman and Company; 1975. [Google Scholar]
- 42.Shepard DS, Hodgkin D, Anthony YE. Analysis of Hospital Costs: A Manual for Managers. Geneva: World Health Organization; 2000. [Google Scholar]
- 43.Sabrina AR. Kuala Lumpur, Malaysia: Department of Social and Preventive Medicine, Faculty of Medicine. University of Malaya; 2006. (Unit cost of patient care in district hospitals in the Ministry of Health, Malaysia). [Google Scholar]
- 44.World Health Organization Choosing Interventions that are Cost Effective (WHO-CHOICE) 2011. http://www.who.int/choice/en/ Available at. Accessed January 2011. [DOI] [PMC free article] [PubMed]
- 45.Lim KJ. Costing of OPD Services at Main Health Clinics. Malaysia: Ministry of Health; 1995. Kedah Darul Aman. [Google Scholar]
- 46.Institute of Public Health . The National Health and Morbidity Survey II (NHMS II) Kuala Lumpur, Malaysia: Ministry of Health Malaysia; 1996. [Google Scholar]
- 47.Drummond MF, Sculpher MJ, Torrance G, O'Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. New York: Oxford University Press; 2005. [Google Scholar]
- 48.Berita National Malaysia (Bernama) Minimum Wage Ruling for Security Guards Takes Effect. Kuala Lumpur, Malaysia: January 12, 2011. http://thestar.com.my/news/story.asp?file=/2011/1/12/nation/20110112151455&sec=nation The Star Online. Available at. Accessed August 1, 2012. [Google Scholar]
- 49.Anis MN. Single Minimum Wage Proposal for All Sectors, Locals and Foreign Workers. The Star Online. Kuala Lumpur; Malaysia: February 14, 2011. http://thestar.com.my/news/story.asp?sec=nation&file=/2011/2/14/nation/20110214163954 Available at. Accessed August 1, 2012. [Google Scholar]
- 50.World Health Organization WHO . Guide for Standardization of Economic Evaluations of Immunization Programmes. Initiative for Vaccine Research, Immunizations, Vaccines and Biologicals. Geneva: World Health Organization; 2008. p. 116. [Google Scholar]
- 51.World Health Organization Life Tables for WHO Member States for Year 2009. 2009. http://www.who.int/healthinfo/statistics/mortality_life_tables/en/ Available at. Accessed September 22, 2011.
- 52.Dinh TT, Le TT, Tran TH, Nguyen TH, Nguyen NV, Pham TD, Nguyen TC, Simmons C, Wills B. Liver involvement associated with dengue infection in adults in Vietnam. Am J Trop Med Hyg. 2010;83:774–780. doi: 10.4269/ajtmh.2010.10-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laoprasopwattana K, Pruekprasert P, Dissaneewate P, Geater A, Vachvanichsanong P. Outcome of dengue hemorrhagic fever-caused acute kidney injury in Thai children. J Pediatr. 2010;157:303–309. doi: 10.1016/j.jpeds.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 54.Seet RC, Lim EC, Wilder-Smith EP. Acute transverse myelitis following dengue virus infection. J Clin Virol. 2006;35:310–312. doi: 10.1016/j.jcv.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 55.Seet RC, Quek AM, Lim EC. Symptoms and risk factors of ocular complications following dengue infection. J Clin Virol. 2007;38:101–105. doi: 10.1016/j.jcv.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 56.Davis JS, Bourke P. Rhabdomyolysis associated with dengue virus infection. Clin Infect Dis. 2004;38:E109–E111. doi: 10.1086/392510. [DOI] [PubMed] [Google Scholar]
- 57.Lim M, Goh H. Rhabfomyolysis following dengue virus infection. Singapore Med J. 2005;46:645–646. [PubMed] [Google Scholar]
- 58.Hutspardol S, Prommalikit O, Upiya N, Chataroopwijit J, Khemakanok K, Assadamongkol K. Heavy proteinuria following dengue hemorrhagic fever. Southeast Asian J Trop Med Public Health. 2011;42:579–582. [PubMed] [Google Scholar]
- 59.Garcia G, Gonzalez N, Perez AB, Sierra B, Aguirre E, Rizo D, Izquierdo A, Sanchez L, Diaz D, Lezcay M, Pacheco B, Hirayama K, Guzman MG. Long-term persistence of clinical symptoms in dengue-infected persons and its association with immunological disorders. Int J Infect Dis. 2011;15:E38–E43. doi: 10.1016/j.ijid.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 60.Gonzalez D, Martinez R, Castro O, Serrano T, Portela D, Vazquez S, Kouri G, Guzman MG. Evaluation of some clinical, humoral, and immunological parameters in patients of dengue haemorrhagic fever six months after acute illness. Dengue Bull. 2005;29:79–84. [Google Scholar]
- 61.Kularatne S. Survey on the management of dengue infection in Sri Lanka: opinions of physicians and pediatricians. Southeast Asian J Trop Med Public Health. 2005;36:1198–1200. [PubMed] [Google Scholar]
- 62.Seet RC, Quek AM, Lim EC. Post-infectious fatigue syndrome in dengue infection. J Clin Virol. 2007;38:1–6. doi: 10.1016/j.jcv.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 63.Teixeira LD, Lopes JS, Martins AG, Campos FA, Miranzi SD, Nascentes GA. Persistence of dengue symptoms in patients in Uberaba, Minas Gerais State, Brazil. Cad Saude Publica. 2010;26:625–630. doi: 10.1590/s0102-311x2010000300019. [DOI] [PubMed] [Google Scholar]
- 64.World Bank GDP Per Capita (Current US$) 2011. http://data.worldbank.org/indicator/NY.GDP.PCAP.CD Available at. Accessed October 17, 2011.
- 65.Ministry of Health Malaysia . Health Expenditures Report (2007–2008). Malaysia National Health Accounts Project. Putrajaya, Malaysia: Malaysia National Health Accounts Unit, Planning and Development Division, Ministry of Health Malaysia; 2009. [Google Scholar]
- 66.Department of Statistics Malaysia The Source of Malaysia's Official Statistics. http://www.statistics.gov.my/portal/ Available at. Accessed January 2, 2011.
- 67.Armien B, Suaya JA, Quiroz E, Sah BK, Bayard V, Marchena L, Campos C, Shepard DS. Clinical characteristics and national economic cost of the 2005 dengue epidemic in Panama. Am J Trop Med Hyg. 2008;79:364–371. [PubMed] [Google Scholar]
- 68.Halasa YA, Shepard DS, Zeng W. Aggregate economic cost of dengue in Puerto Rico. Am J Trop Med Hyg. 2012;86:745–752. doi: 10.4269/ajtmh.2012.11-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuo HI, Chang CL, Huang BW, Chen CC, McAleer M. Estimating the impact of avian flu on international tourism demand using panel data. Tour Econ. 2009;15:501–511. [Google Scholar]