Abstract
Alzheimer’s disease (AD) is accompanied by smell dysfunction, as measured by psychophysical tests. Currently it is unknown whether AD-related alterations in central olfactory system neural activity, as measured by functional magnetic resonance imaging (fMRI), are detectable beyond those observed in healthy elderly. Moreover, it is not known whether such changes are correlated with indices of odor perception and dementia. To investigate these issues, twelve early stage AD patients and thirteen non-demented controls underwent fMRI while being exposed to each of three concentrations of lavender oil odorant. All participants were administered the University of Pennsylvania Smell Identification Test (UPSIT), the Mini-Mental State Examination (MMSE), the Mattis Dementia Rating Scale-2 (DRS-2), and the Clinical Dementia Rating Scale (CDR). The Blood oxygen level-dependent (BOLD) signal at primary olfactory cortex (POC) was weaker in AD than in HC subjects. At the lowest odorant concentration, the BOLD signals within POC, hippocampus, and insula were significantly correlated with UPSIT, MMSE, DRS-2, and CDR scores. The BOLD signal intensity and activation volume within the POC increased significantly as a function of odorant concentration in the AD group, but not in the control group. These findings demonstrate that olfactory fMRI is sensitive to the AD-related olfactory and functional cognitive decline.
Keywords: olfaction, Alzheimer’s disease, functional magnetic resonance imaging, microsmia, primary olfactory cortex
1. INTRODUCTION
Significant compromise in the ability to detect, recognize, and remember odorants has been reported in older persons, and most notably in patients with Alzheimer’s disease (AD) (Mesholam et al., 1998; Doty, 2003; Albers et al., 2006; Doty, 2008). In the case of AD, the olfactory deficits are present early in the course of the disease (Doty et al., 1987; Nordin and Murphy, 1996) and precede the onset of classic disease symptoms, likely reflecting an early ‘preclinical’ period of disease development (Doty, 2003). Indeed, olfactory dysfunction is disproportionately found in individuals at risk for AD, including relatives of AD patients and individuals with mild cognitive impairment who eventually develop AD (Doty, 1991, 2003, and 2008).
In AD, these sensory-perceptual changes are complimented by neuropathological changes within olfaction-related brain regions. Neurofibrillary tangles, one of the hallmark pathologic indicators of AD, have been identified within the olfactory bulb, olfactory tract, anterior olfactory nucleus, entorhinal cortex, and amygdala (Ohm and Braak, 1987; Price et al., 1991; Attems and Jellinger, 2006). Severity of dementia has been correlated with the number of neurofibrillary tangles within such regions, including the transentorhinal and entorhinal cortices (Price et al., 1991). Recent neuropathological studies suggest that AD-related pathology may begin within olfaction-related structures, such as the anterior olfactory nucleus and the transentorhinal cortex, subsequently spreading to additional brain regions and ultimately encompassing multiple areas of the brain (Braak and Braak, 1996; Braak and Braak, 1998).
A primary goal of this study was to determine whether AD-related alterations in central olfactory system neural activity, as measured by blood oxygen level-dependent (BOLD) signal changes detected by functional magnetic resonance imaging (fMRI), are detectable beyond those observed in healthy elderly. Although several fMRI studies have reported decreased odorant-induced activation of central olfactory structures in older persons (Suzuki et al., 2001; Yousem et al., 1999; Wang et al., 2005), none has addressed the issue of whether such activation is further reduced or altered otherwise in persons with AD. A second goal of this work was to determine whether the BOLD signal in central olfactory structures is correlated with scores on the University of Pennsylvania Smell Identification Test, a standardized test of odor perception that is sensitive to AD-related changes. While one study using positron emission tomography (PET) reported an association between smell identification scores and odor-induced activity within the right piriform cortex in a combined group of AD and healthy subjects (Kareken et al., 2001), it is not known whether fMRI is similarly sensitive to such relationships. Verification of this association using a different functional imaging modality would strengthen the view that such odor-induced responses truly reflect odor-related perceptual phenomena. Related to this, we also sought to determine if any olfactory fMRI activations are correlated with standard clinical examination scores of dementia. A third goal of our study was to determine the influences of odorant concentration on the fMRI response in AD patients. Since AD patients are rarely anosmic, we hypothesized that greater BOLD signal changes would be induced at higher odorant concentrations.
In this study, a group of AD patients and a group of healthy non-demented senior controls underwent fMRI while being exposed to each of three different concentrations of lavender oil (0.10%, 0.32% & 1.0%). All participants were administered the University of Pennsylvania Smell Identification Test (UPSIT) (Doty et al., 1984) and clinical neurocognitive dementia examinations, which including the Mini-Mental State Examination (MMSE) (Folstein et al., 1975), the Mattis Dementia Rating Scale-2 (DRS-2) (Mattis, 1988), and the Clinical Dementia Rating Scale (CDR) (Morris, 1993), a composite index based on medical history, interview, and cognitive testing results. We compared the fMRI activation to the different odorant concentrations between the AD and healthy controls. In addition, we correlated each subject's activation in major olfactory brain structures with their own UPSIT scores and neurocognitive dementia scores.
2. RESULTS
2.1. Subjects
Twelve 55 to 84 years old patients with early AD and probable AD [74.5 ± 7.5 years (mean ± SD), 5 men and 7 women] and thirteen aged 54 to 82 years healthy controls (HC) (67.8 ± 9.8 years, 8 men and 5 women) participated. A summary of the demographic, cognitive, and olfactory test scores is presented in Table 1. The patient and control groups did not differ with respect to age and gender. However, as expected, the AD sample scored significantly lower on the UPSIT and neurocognitive dementia examinations, i.e., MMSE, DRS-2 and CDR.
Table 1.
Demographic information, UPSIT and neurocognitive test results
| AD (n = 12) Mean ± SD (range) |
HC (n = 13) Mean ± SD (range) |
|
|---|---|---|
| Age | 74.5 ± 7.5 (55–84) | 67.8 ± 9.8 (54–82) |
| Education | 12.8 ± 1.9 (9–16)** | 15.5 ± 1.8 (12–17) |
| Gender | 5 men and 7 women | 8 men and 5 women |
| UPSIT | 20.6 ± 6.8 (10–31)*** (n = 11) |
34.1 ± 3.5 (29–39) |
| MMSE | 20.5 ± 4.5 (13–30)*** | 28.8 ± 1.1 (27–30) |
| CDR | 0.9 ± 0.2 (0.5–1)*** | 0.0 ± 0.0 (0) |
| DRS-2 | 114.8 ± 11.9 (91–132)*** | 140.4 ± 2.0 (136–143) |
| Attention | 34.1 ± 2.8 (27–37)* | 36.6 ± 0.5 (36–37) |
| Initiation/Perseveration | 26.9 ± 4.4 (22–37)*** | 36.5 ± 1.4 (32–37) |
| Construction | 5.5 ± 1.0 (3–6) | 6.0 ± 0.0 (6–6) |
| Conceptualization | 34.5 ± 3.9 (26–39) | 37.0 ± 1.5 (34–39) |
| Memory | 13.8 ± 4.3 (8–23)*** | 24.2 ± 0.8 (23–25) |
AD = Alzheimer’s disease; HC = healthy control; M = mean; SD = standard deviation; UPSIT = University of Pennsylvania Smell Identification Test; MMSE = Mini-Mental State Examination; CDR = Clinical Dementia Rating; DRS-2 = Mattis Dementia Rating Scale-2, including five subcategories: Attention, Initiation/Perseveration, Construction, Conceptualization, and Memory. One AD subject did not finish the whole UPSIT test. One subject in the AD group that had a MMSE score of 30, a CDR score of 0.5 and a DRS score of 132 was clinically diagnosed as a probable AD. Two years later, this subject’s MMSE, CDR, and DRS scores were 27, 0.5 and 130.
p < 0.001;
p < 0.01;
p < 0.05.
2.2. Healthy Control fMRI Activations
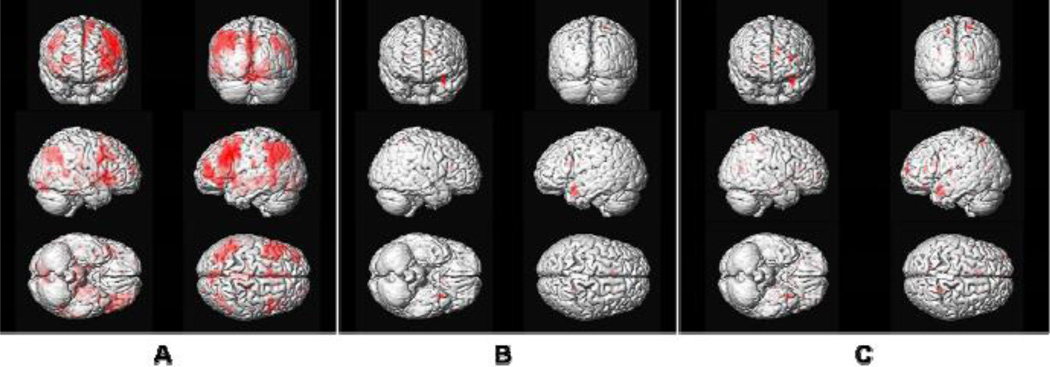
The average olfactory activation map for the lowest concentration of lavender in the HC sample is shown in Figure 1A. Activations were observed in multiple olfactory structures, including the POC, insula, orbitofrontal cortex, and hippocampus. In addition to olfactory structures, there was significant recruitment of the right anterior cingulate and multiple bilateral cortical association areas. The latter recruitments occurred most prominently in prefrontal cortex, lateral temporal cortex, and the supramarginal gyrus of the parietal lobe. Although these are not considered olfactory areas per se, they are interconnected with the activated olfactory regions delineated above, particularly the amygdala, orbitofrontal cortex, and hippocampus. Table 2 provides a summary of these cluster locations and sizes. No significant lateralization effect was observed at p threshold 0.005. However, greater left hemisphere activations were observed with more relaxed statistical threshold.
Figure 1.
3-D representation of average brain activation maps in response to the odor lavender. (A) healthy control group (one-sample t-test, voxel-wise threshold p < 0.005, uncorrected with extent threshold = 6) at lowest and first exposed odorant concentration (0.10%); (B) early AD group at same statistical threshold (one-sample t-test, voxel-wise threshold p < 0.005, uncorrected with extent threshold = 6) and at highest odorant concentration exposure (1.0%); (C) AD group with more relaxed statistical threshold (one-sample t-test, voxel-wise threshold p < 0.01, uncorrected with extent threshold = 0) and at highest odorant concentration exposure (1.0%).
Table 2.
Olfactory activations responding to the 0.10% lavender in healthy controls
| MNI Coordinates | Activation | ||||
|---|---|---|---|---|---|
| Area | x | y | z | size (voxels) | t-value |
| L POC | −22 | −6 | −15 | 172 | 3.94 |
| R POC | 16 | −4 | −10 | 62 | 3.24 |
| L Hippocampus | −24 | −12 | −20 | 121 | 3.69 |
| R Hippocampus | 16 | −8 | −15 | 16 | 3.05 |
| L insula | −42 | 12 | 0 | 72 | 3.74 |
| R insula | 38 | −4 | 5 | 78 | 3.74 |
| L orbitofrontal cortex | −34 | 34 | −15 | 181 | 4.09 |
| R orbitofrontal cortex | 48 | 34 | −10 | 22 | 3.44 |
| L prefrontal cortex | −38 | 18 | 35 | 966 | 5.70 |
| R prefrontal cortex | 54 | 6 | 30 | 296 | 5.09 |
| L Temporal lateral gyrus | −62 | −28 | −10 | 38 | 3.45 |
| R Temporal lateral gyrus | 46 | −26 | −10 | 7 | 3.04 |
| L Supramarginal gyrus | −54 | −22 | 20 | 29 | 3.16 |
| R Supramarginal gyrus | 56 | −22 | 20 | 15 | 3.22 |
| R Anterior Cingulate | 8 | 38 | 10 | 13 | 3.33 |
One-sample t-test, voxel-wise threshold p < 0.005, uncorrected with extent threshold = 6. There was no significant olfactory activation for the AD group at this odorant concentration level. L = left; R = right; POC = primary olfactory cortex. Coordinates are given in the left-posterior-inferior (LPI) system.
Activations to the higher odorant concentrations in the healthy controls, although present, were less robust than those observed for 0.10% concentration (see Table 3 for the respective activation volumes at the POC, hippocampus and insula).
Table 3.
Region-of-interest activations responding to the three odorant concentrations in early Alzheimer’s disease (AD) and healthy control (HC)
| AD (n = 12) |
HC (n = 13) |
||||
|---|---|---|---|---|---|
| Activation size |
p-value SVC |
Activation size |
p-value SVC |
||
| 0.10% Lavender |
POC | 0 | N/A | 234 | 0.002 |
| Hippocampus | 0 | N/A | 137 | 0.004 | |
| Insula | 0 | N/A | 150 | 0.018 | |
| 0.32% Lavender |
POC | 0 | N/A | 32 | 0.13 |
| Hippocampus | 0 | N/A | 0 | N/A | |
| Insula | 21 | 0.12 | 18 | 0.14 | |
| 1.0% Lavender |
POC | 57 | 0.05 | 58 | 0.05 |
| Hippocampus | 0 | N/A | 12 | 0.39 | |
| Insula | 0 | N/A | 0 | N/A | |
Average activation size (voxels) listed are at threshold p < 0.005 (one-sample t-test), small volume corrected (SVC). POC = primary olfactory cortex.
2.3. AD fMRI Activations
Unlike the HC sample, few significant clusters were found in the AD patients at the lowest odorant concentration (0.10% lavender) using the same statistical threshold. Although, as hypothesized, increasing odorant concentration slightly enhanced the BOLD signal, only a small number of additional voxels in these brain structures were activated. Figures 1B and 1C show the 3-D rendered activations of the AD sample at the highest lavender concentration employed (1.0% lavender), with more rigorous (voxel-wise p < 0.005, uncorrected with extent threshold = 6) and more relaxed (voxel-wise p < 0.01, uncorrected with extent threshold = 0) statistical thresholds. Activations minimally involved olfactory structures, e.g., POC, hippocampus, and several scattered cortical regions.
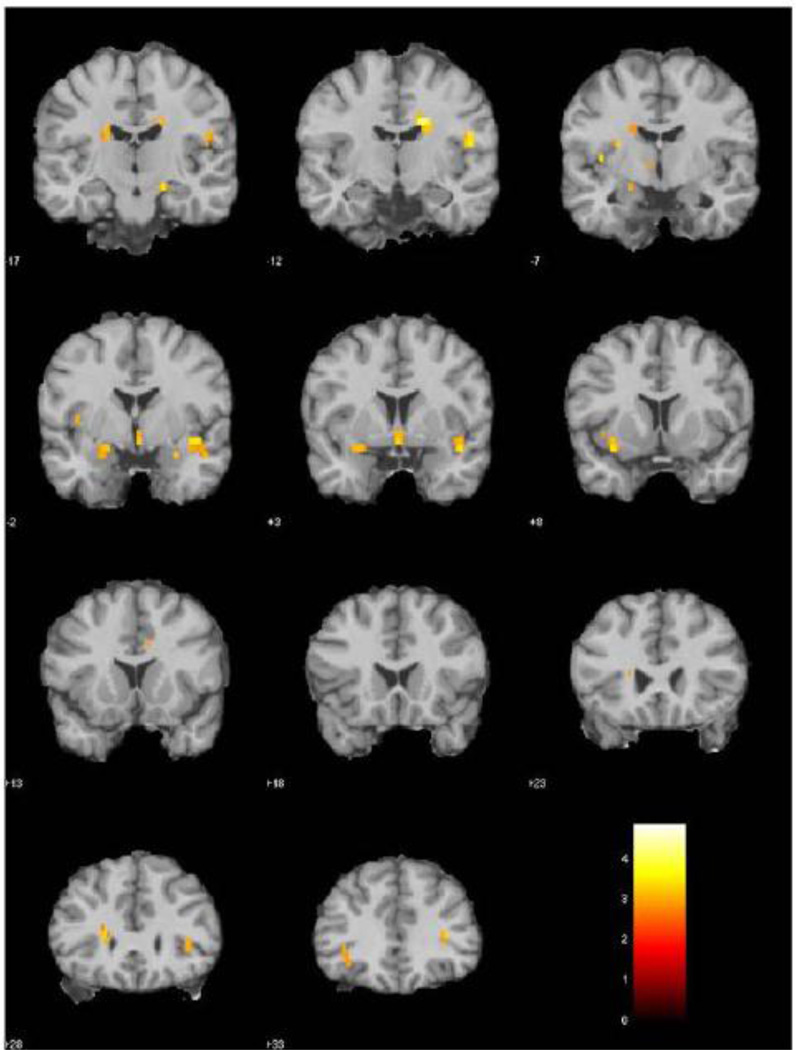
We specifically contrasted the HC and AD fMRI activations for the lowest concentration of lavender (two-sample t-test, voxel-wise threshold p < 0.005, uncorrected with extent threshold = 6). Results of the t-test are shown in coronal sections in Figure 2. Compared to the AD group, the HC sample showed significantly stronger activations within the POC, hippocampus, and insula. These structures receive projections from the olfactory bulb and are known to be involved in olfactory-associated processing. Activation differences were also detected in the hypothalamus and septum, brain regions that are interconnected with the amygdala and hippocampus that mediate important roles in homeostasis, appetite and emotional reactivity.
Figure 2.
fMRI activation differences between healthy control (n = 13) and early AD groups (n = 12) for odorant lavender at 0.10% concentration (two-sample t-test, voxel-wise threshold p < 0.005, uncorrected with extent threshold = 6). The HC group had significantly stronger activation in areas including the POC, hippocampus, insula, thalamus, and hypothalamus.
2.4. Comparison of AD and Healthy Control Activations across Odorant Concentrations
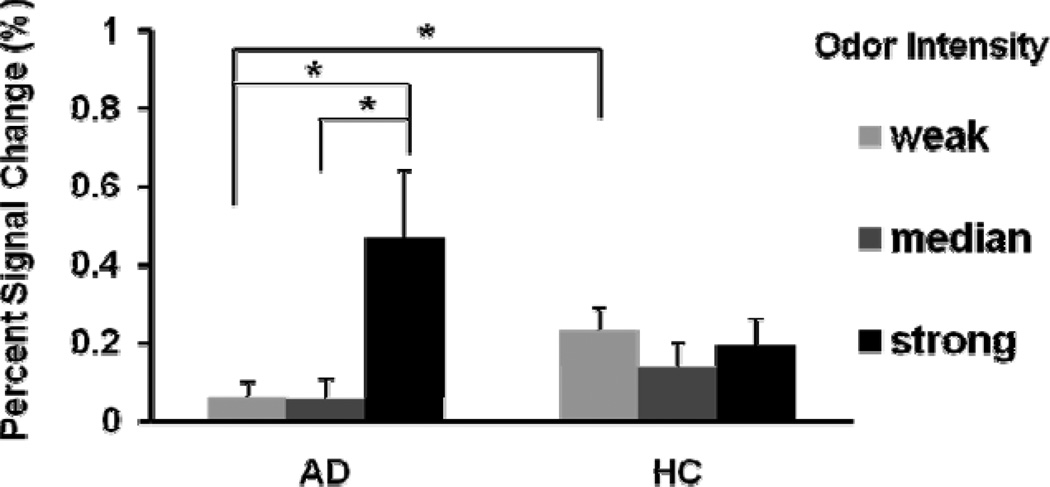
Region of interest (ROI) analysis on POC, hippocampus, and insula was conducted for comparison of activation-inactivation patterns across the three lavender odorant concentrations. Table 3 provides clusters sizes in number of voxels and the corresponding t-values of control and AD activations in the POC, hippocampus and insula for each concentration. The cluster sizes in these olfactory ROIs in the controls showed a general trend of decreased recruitment as the odorant concentration increased from low to median to high (0.10% to 1.0% lavender). POC activation did remain detectable but at comparatively reduced levels. In contrast, the AD group did not show significant activation in the olfactory ROIs responding to the low odorant concentration (0.10% lavender), but showed some modest activation in insula responding to the medium odorant concentration and in POC responding to the high odorant concentration. Correspondingly, as shown in Figure 3 the BOLD signal magnitude at POC responding to the three odorant concentrations exhibited significant differences between AD and HC groups. For HC group, the BOLD signal at POC did not vary significantly across the three odorant concentrations (paired t-test, p’s > 0.10). However, for AD group the average BOLD signal responding to the high odorant concentration was significantly stronger than those to the weak and median odorant concentration (paired t-test, p’s = 0.017 and 0.025 respectively). The BOLD signal responding to the low odorant concentration was significantly higher for HC than that for AD group (two-sample t-test, p = 0.020).
Figure 3.
The BOLD signal intensity at POC responding to different odorant concentrations. There was no significant difference of BOLD signal intensity between the three odorant concentrations. However, the BOLD signal was significant stronger responding to the high odorant concentration than responding to the two lower odorant concentrations (two-sample t-test, p = 0.029 and 0.029). The BOLD signal in the HC group responding to the low odorant concentration is greater than that in the AD group (two-sample t-test, p = 0.020). Vertical bars represent standard error of the mean.
2.5. Correlation between BOLD Activation and UPSIT Scores
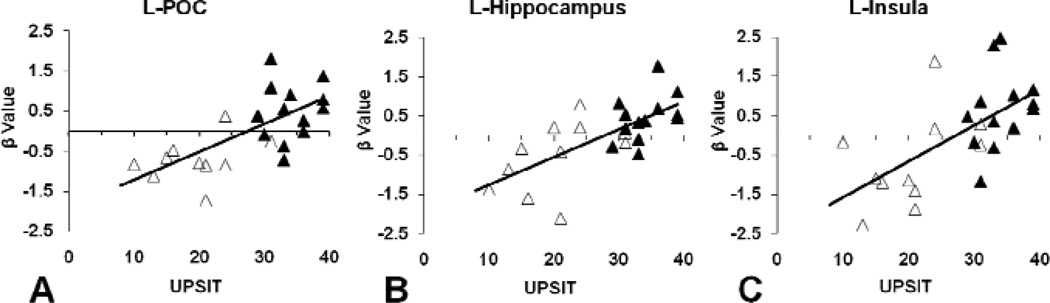
The correlations of UPSIT scores with fMRI brain responses (represented by the β values from SPM estimation) to odor stimulation at the low odorant concentration (0.10% lavender) within the left POC, hippocampus, and insula for the whole study sample are shown in Figure 4. In a similar fashion, within the AD group, the brain response to weak odor stimulation in left body of hippocampus and bilateral insula were significantly correlated with UPSIT scores (respective r’s = 0.726, 0.614, and 0.605, p’s = 0.011, 0.044, and 0.049), and the response in left POC and left head of hippocampus showed a trend toward correlation (respective r’s = 0.573 and 0.589, p’s = 0.065 and 0.056). In the HC group, brain response in left head of hippocampus was significantly correlated with UPSIT scores when aging effect was controlled (r = 0.601, p = 0.039). These results confirm that olfactory BOLD responses in these specific brain structures are highly correlated with olfactory identification abilities. No significant correlations were observed at the subsequent higher odorant concentrations.
Figure 4.
Correlation between fMRI brain response to the low concentration of lavender odorant (0.10%) and UPSIT score. The fMRI brain response was represented by the normalized β values calculated from SPM estimation using: Y = β·X+ε, where Y is the fMRI BOLD signal, X is the fMRI stimulation model, and ε is the estimation error. A: left POC; B: left hippocampus; and C: left insula. Open shapes represent early AD group and closed shapes represent healthy control group; dark line represents linear regression. Significant positive linear correlations between brain response and UPSIT score were observed (A: r = 0.671, p < 0.001; B: r = 0.695, p < 0.001; C: r = 0.628, p = 0.001).
2.6. Correlations between Neurocognitive Dementia Scores, UPSIT, and Olfactory fMRI
Within the whole study cohort, age was significantly correlated with UPSIT score but not with MMSE, CDR and DRS-2 scores, while educational level was significantly correlated with MMSE, CDR and DRS-2 scores, but not with UPSIT score (Table 4). UPSIT score was significantly correlated with MMSE, CDR, and DRS-2 scores (total score and memory sub-score) within the whole study cohort whether the influences from age and educational level were controlled or not. Accordingly, at the lowest odorant concentration level, olfactory brain responses (represented by the β values from SPM estimation) in the POC, hippocampus, and insula were significantly correlated with MMSE, DRS-2 (total score and memory sub-score), and CDR scores (Table 4). However, these activations were not correlated with either age or educational levels.
Table 4.
The correlation between olfactory activation (0.10% lavender) and UPSIT, neurocognitive test (MMSE, CDR and DRS-2) scores
| Age | Education | UPSIT | MMSE | CDR | L-POC | L- Hippo-campus |
L-Insula | |
|---|---|---|---|---|---|---|---|---|
| Age | −.243 | −.078 | −.355 | |||||
| (.252) | (.718) | (.088) | ||||||
| Education | −.007 | .211 | .148 | .381 | ||||
| (.973) | (.323) | (.490) | (.066) | |||||
| UPSIT | .671*** | .695*** | .628*** | |||||
| −.498* | .362 | (<.001) | (<.001) | (.001) | ||||
| (.013) | (.082) | .654^*** | .759^*** | .556^** | ||||
| (.001) | (<.001) | (.006) | ||||||
| MMSE | −.163 | .451* | .574** | .655*** | .511* | .446* | ||
| (.447) | (.027) | (.003) | (.001) | (.011) | (.029) | |||
| CDR | .304 | −.444* | −.829*** | −.864*** | −.689*** | −.625*** | −.531** | |
| (.149) | (.030) | (<.001) | (<.001) | (<.001) | (.001) | (.008) | ||
| DRS-2 [Total Score] |
−.288 | .634*** | .688*** | .843*** | −.804*** | .685*** | .429* | .555** |
| (.172) | (.001) | (<.001) | (<.001) | (<.001) | (<.001) | (.036) | (.005) | |
| Memory Sub-score | −.323 | .558** | .689*** | .897*** | −.870*** | .619*** | .504* | .525** |
| (.123) | (.005) | (<.001) | (<.001) | (<.001) | (.001) | (.012) | (.008) | |
Numbers are correlation coefficient r (p-value). One AD subject that did not finish UPSIT was not included.
p < 0.001;
p < 0.01;
p < 0.05.
partial correlation coefficient (p-value) with the age effect corrected.
3. DISCUSSION
The present study unequivocally demonstrates that fMRI is sensitive to changes in olfactory function due to AD. Relative to healthy control subjects of similar age, the BOLD signals in the POC, hippocampus, and insula regions were markedly reduced in the AD patients. Furthermore, increasing the odorant concentration ten-fold induced only slightly more activation in some brain regions. The fact that any additional recruitment of activity could be induced is in accord with studies indicating that total anosmia in AD is the exception, not the rule, implying at least some residual capacity is available for such recruitment (Doty et al., 1987).
The high correlations between UPSIT scores and odor-induced BOLD signal within POC, hippocampus, and insula suggest that the BOLD signal in these structures can be used as a surrogate for odor identification ability. These effects occurred regardless of age and were most marked when data from AD and healthy control subjects were combined. However, this relationship was still strong when the AD and control groups were evaluated separately. These observations comport well with those from a PET study in which an r = 0.51 correlation was found between the percent change in right piriform cerebral blood flow and UPSIT scores in a group of 7 AD and 8 healthy controls (Kareken et al., 2001). Taken together, these studies indicate that functional measures within the POC and related regions are a reflection of odor perception. Although olfactory deficits in AD were well-established behaviorally, little is known about its underlining functional pathology relating to the disease process inside the brain. In order to achieve this goal, we believe, as the first step, it is necessary to characterize and quantify the functional changes associated with olfactory deficits in the key brain structures that are subject to early attack by the disease.
The experimental data presented in this study allow us to evaluate the feasibility for using olfactory fMRI as a marker for diagnosis and evaluation of AD. Strong correlations among the olfactory and neurocognitive dementia measures (MMSE, DRS-2, and CDR scores) were present in our study cohort. Thus, the UPSIT scores correlated significantly with the neurocognitive and dementia scores (r = 0.57 – 0.83). Odor-induced fMRI activations in the POC, hippocampus, and insula also correlated significantly with such scores (r = 0.43 – 0.69), strongly suggesting that the olfactory system is involved in early AD pathology. These findings are in accord with several previous studies on this topic. For example, Serby et al. reported a significant correlation between UPSIT and MMSE scores in 55 AD patients and 57 healthy controls (Serby et al., 1991). Larsson et al. reported similar observations from a study cohort of 11 AD patient and 11 control subjects using a 20-item odor identification test (Larsson et al., 1999).
The odorant concentration effects identified in this study were likely the result of the increasing odorant concentrations (i.e., low, medium, and high) in the experimental paradigm. The fact that the association between the olfactory fMRI activation and UPSIT scores was only significant at the lowest odorant concentration likely reflected both the initial sensory-perceptual effects of this first odor exposure and habituation of the BOLD signal at the later medium and high odorant concentrations within the healthy controls. Although our study paradigm was designed to control habituation by presenting the lower odorant concentrations first and by employing relatively long interstimulus intervals, habituation appeared to have occurred in the healthy controls. It is well known that the piriform cortex rapidly habituates to odorants, an effect conceivably related to inhibition within the primary olfactory cortex (Sobel et al., 2000). Such a process presumably requires a large complement of active neurons. However, such habituation was not evident in the BOLD data of the AD patients. They failed, in contrast to the controls, to show activations at the lowest odorant concentration and generated only slightly increased BOLD signal at the higher concentrations. Although unlikely, other factors such as non-monotonic relationships that exist between detection performance and odorant concentration for some odorants (Moulton, 1960; Moulton and Marshall, 1976; Doty, 1991) may also have contributed to the concentration-related effect we observed. From a clinical point of view, the different odorant-concentration patterns of BOLD responses observed in the AD and control subjects may prove to be a sensitive index for detecting AD and delineating its severity.
Despite the fact that psychophysical testing is inexpensive and sensitive to the olfactory deficits observed in early AD, functional imaging has the potential for detecting alterations in brain activity patterns that are not easily detectable by such means, including associations among the activity of differing brain regions whose interconnections become compromised (Andrews-Hanna et al., 2007). Psychophysical and neuropsychological measures become compromised or even invalidated as patients become more demented, and fMRI studies employing cognitive stimulation paradigms in even pre-symptomatic AD can be confounded with strong compensatory activation patterns, creating difficulties for data interpretation (Johnson et al., 2000; Small et al., 2000). Thus, olfactory fMRI, as demonstrated in this study, may provide an effective means of assessing the integrity of sectors of the brain that progressively degenerate with minimal requirements placed on the cognitive abilities of the subject. Given that the fMRI results correlate significantly with measures of neurocognition and dementia severity in early AD, fMRI may well provide a viable neurobiological benchmark of overall disease progression. Our results indicate, however, that stimulus concentration and multiple concentration presentations may be key factors in assessing odor-induced BOLD responses to this end.
There are some limitations of the techniques we used in this study. Signal loss and geometric distortion in echo-planar imaging make co-registration between functional images and high resolution anatomical images problematic. We used SPM templates for the normalization instead of using a group-averaged anatomical image. Furthermore, our predefined ROIs were based on a standardized anatomical atlas, not individual anatomies, which may vary significantly across subjects. It should be noted that the anatomical structures listed in Table 2 yielded by SPM provide a general description of olfactory activation clusters in the respective brain region shown in Fig. 1. In this study we focused on primary olfactory cortex, hippocampus and insula cortex, all can be adequately resolved by our methodology. Although our analysis is well within the norms of current fMRI research field, some weight must be given to spatial approximations inherent in standard fMRI analysis tools. Our analyses did not include segmentation or volumetric measurement and comparisons. Significant atrophy of medial temporal lobe structures in AD, e.g., hippocampus, is well known. The BOLD signal can be associated with atrophy (Dickerson et al., 2004; Johnson et al., 2000). Hippocampus volumetric atrophy in AD has been associated with poor olfactory identification ability (Murphy et al., 2003). However, the effect of local volumetric atrophy on the decreased olfactory activation in AD has not been studied. Hippocampal atrophy has been reported to vary among AD patients (Lehtovirta et al., 1996) and normal older people (Soininen et al., 1995). Similar to the wide variation of olfactory activation within the patient or control group, a significant volumetric variation can be expected in the POC and other olfactory brain structures. In addition, given the very small and highly variable groups, the study may have been under-powered to detect group differences at higher lavendar concentrations. One valuable outcome of this research is the acquisition of such a data set that can be used for power calculations for further study design.
In summary, the present study demonstrates that significant AD-related changes in odor-related BOLD signal responding to different odorant concentrations are present beyond those observed in healthy elderly. It also demonstrates that such alterations are significantly correlated with UPSIT, MMSE, DRS-2, and CDR scores, validating the value and sensitivity of olfactory fMRI in testing the olfactory and cognitive decline due to AD pathology.
4. EXPERIMENTAL PROCEDURES
4.1. Subjects
The AD patients were recruited from the local clinics and the healthy controls were recruited from the local community by advertisement. All of the subjects provided written informed consent prior to participation, in accord with the requirements of the Institutional Review Board of the Pennsylvania State University College of Medicine. The study participants underwent screening prior to admission into the study to rule out otorhinolaryngical, neurological or psychiatric conditions other than AD that might adversely influence the study findings. The screening also included a clearance for any potential complications specific for olfactory fMRI studies (e.g., claustrophobia, metal implants).
The AD patients met the NINCDS-ADRDA criteria for probable early AD and were clinically diagnosed by an AD specialist. Most of the AD patients took cholinesterase inhibitors: Aricept (donepezil HCl, 6 patients), Reminyl (galantamine hydrobromider, 3 patients) and Exelon (rivastigmine tartrate, 1 patient). These cholinesterase inhibitors have been reported to slow the progression of Alzheimer's symptoms. There has been no report about their effect on human olfaction function. There was no significant difference of age, gender, educational level, UPSIT score, MMSE, CDR, and DRS-2 scores between cholinesterase inhibitor-taken patients and cholinesterase inhibitor-not-taken patients in this study. Four of the patients took Namenda (memantine HCl, an N-methyl-D-aspartate receptor antagonist). Namenda is indicated for the treatment of moderate to severe dementia of AD. There has been no report about its effect on human olfaction function. There was no significant difference of age, gender, educational level, UPSIT score, MMSE, CDR, and DRS-2 scores between Namenda-taken patients and Namenda-not-taken patients in this study.
4.2. Data acquisition
On the same day of olfactory fMRI study, all the participants received University of Pennsylvania Smell Identification Test and neurocognitive evaluations, which including MMSE, CDR and DRS-2 (except one AD patient did not finish UPSIT).
4.2.1. Olfactometer
Odorants were presented to the subject via a custom-built olfactometer incorporating elements of a design described by Lorig et al. (Lorig et al., 1999). It consisted of a series of solenoids, flow meters, and Teflon tubes that communicated with odorant saturation chambers located next to the MRI scanner. A constant airflow of 8 L/min was maintained in the output line. The solenoid valves were synchronized by transistor-transistor logic signals incorporated within the MRI pulse-timing program. This device, which minimized the dead space between the odorant saturators and the point of bilateral odorant delivery ~ 100 cm from the nostrils, allowed for rapid switching between the odorant concentrations and medical grade air without confounding tactile or thermal cues.
4.2.2. Olfactory Stimuli
Lavender is one of the most effective olfactory stimulant with very minimal or no propensity to stimulate the trigeminal system (Allen, 1936; Patton, 1960). It is generally perceived by North American individuals as being pleasant and familiar. Three concentrations of lavender oil (Quest International, Mount Olive, NJ, USA), 0.10%, 0.32% and 1.0% of lavender in 1,2-propanediol (Sigma, St. Louis, MO, USA), were chosen as stimuli based upon their effectiveness in inducing fMRI activation in young adults (Grunfeld et al., 2005). The odorants were presented birhinally in 6-second blocks separated by 42 second intervals of clean odorless air. Each concentration was presented three times in succession, beginning with the weakest. The respiratory rate of participants was synchronized with image acquisition and odor presentation and closely monitored. Audio breathing instructions were given to participants at a rate of 10 cycles per minute (3 second “Breathe In” and 3 second “Breathe Out”). There was no training for the subjects to get familiar to the stimulation paradigm before the experiment. The subjects were instructed to follow the auditory instructions for breathing and to breathe regularly. During the execution of the fMRI paradigm, before odorant delivery, there were 30 seconds for the subjects to get familiar with the auditory respiration instructions. Respiration and sniffing patterns were monitored via a pneumatic respiration sensor.
4.2.3. fMRI Data Acquisition
Magnetic susceptibility artifacts are notoriously strong in the base of the brain where major olfactory brain structures are present. In this study, several measures have been used to mitigate the artifacts, which included parallel imaging (SENSE), a large acquisition bandwidth, a strong slice selection gradient, standardized head placement and shimming routine. Except orbitofrontal area, the fMRI data from POC and hippocampus are adequate for our analysis. In a separate study (data not provided in this manuscript), we have verified the reproducibility of using this fMRI method to acquire olfactory brain activations.
The fMRI experiment was performed on a 3 T MR system (Intera, Philips Medical Systems, Best, Netherlands). A six-element sensitivity-encoding (SENSE) head coil was used for signal reception. The subjects were positioned in the supine position in a dark environment with their heads elevated 10o–15o from the horizontal level. The heads were fit into a head restrainer to minimize motion and to provide precise positioning and comfort. The standardized head positioning and shimming routine is important for reduction of data variability associated with magnetic susceptibility artifact in the base of the brain.
Functional images were acquired from the entire brain using an echo planar imaging method with a SENSE factor = 2, repetition time / echo time / flip angle (TR / TE / FA) = 3000 ms / 35 ms / 90°, field of view (FOV) = 230 mm × 230 mm, acquisition bandwidth = 204 kHz, acquisition matrix = 80 × 80, 30 axial slices, slice thickness = 4 mm with no distance between slices, reconstructed image resolution = 1.8 mm × 1.8 mm × 4 mm, number of average = 1, repetition = 177, acquisition time = 8 min 51 sec.
4.2.4. Anatomical Data Acquisition
Anatomical images were acquired with a three-dimensional MPRAGE method with TR / TE / FA = 9.798 ms / 4.6 ms / 8°, FOV = 256 mm × 204 mm × 140 mm, matrix = 256 × 205 × 140, reconstructed image resolution = 1 mm × 1 mm × 1 mm, number of average = 2 and 2D fast spin-echo T2-weighted images (TR / TE / FA = 22796 ms / 78 ms / 90°, FOV = 256 mm × 200 mm, acquisition matrix = 256 × 231, 150 axial slices and slice thickness = 1 mm with no distance between slices, reconstructed image resolution = 1 mm × 1 mm × 1 mm, and number of average = 1).
4.3. Data Analysis
4.3.1. fMRI Data Processing
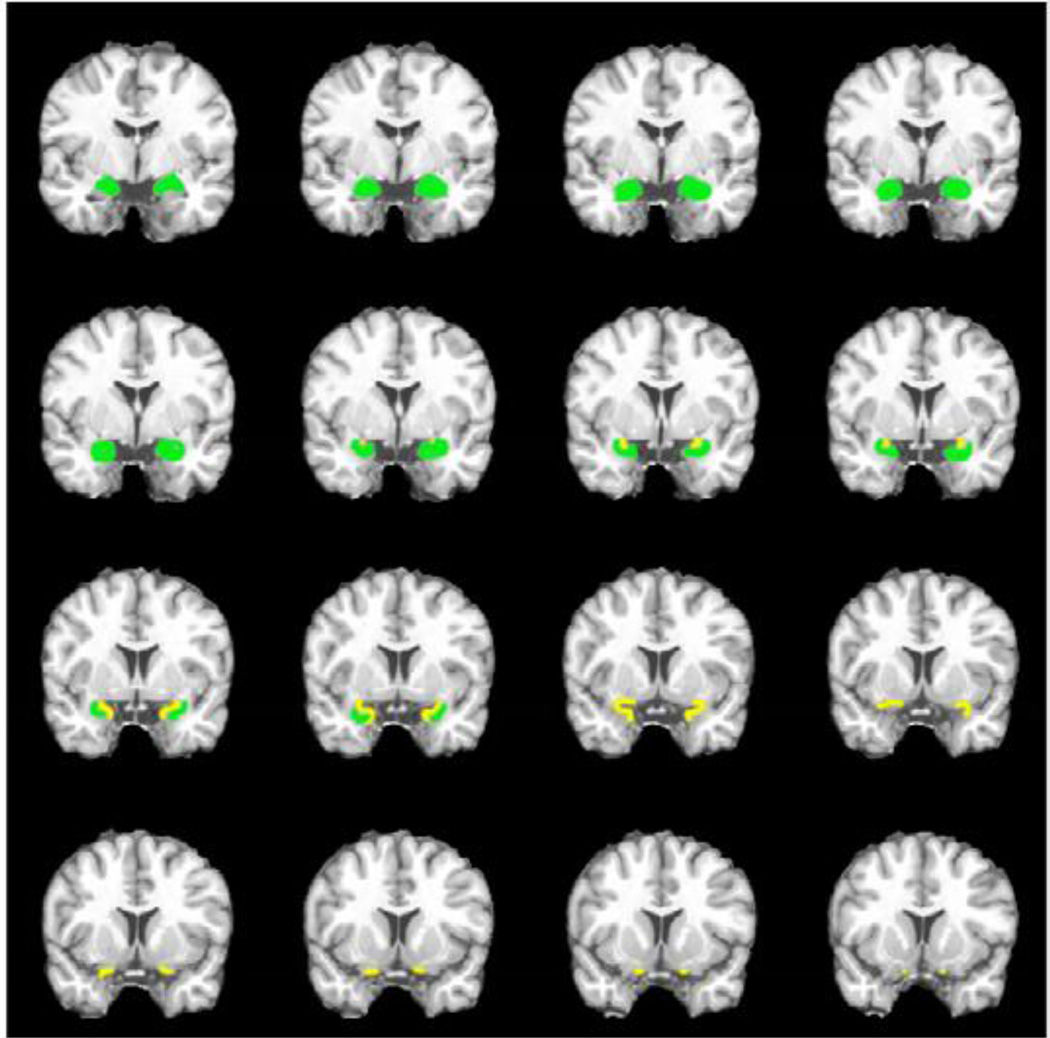
The fMRI data were processed with SPM2 software [Wellcome Department of Imaging Neuroscience, University London College, UK (Friston et al., 1994)]. The first ten images of each fMRI data set were discarded to remove the initial transit signal fluctuations. The subsequent images were re-aligned within the session to remove any minor head movements. The T1-weighted high-resolution anatomical images were co-registered and spatially normalized to the Montreal Neurological Institute (MNI) brain template (Collins et al., 1998) in a spatial resolution of 1 mm × 1 mm × 1 mm. The time-course images were spatially normalized using the same normalization parameters in a spatial resolution of 2 mm × 2 mm × 5 mm, and then smoothed with an 8 × 8 × 12.5 mm3 (full width at half maximum) Gaussian smoothing kernel. A statistical parametric map was generated for each subject under each odorant concentration condition by fitting the stimulation paradigm to the functional data, convolved with hemodynamic response function and time derivative. The voxels representing the active structures were overlaid on the 3-D T1-weighted anatomical image in MNI’s coordinates. Group analysis was undertaken to generate an average activation map (one-sample t-test; statistical thresholds set at p < 0.005) for each study group and each odorant concentration. Region of interest analyses in the major olfactory brain structures such as the primary olfactory cortex (POC), hippocampus, and insula were conducted to compare the AD and control olfactory activations across odorant concentrations. POC structures include the piriform cortex and closely associated areas of the anterior olfactory nucleus, the anterior perforated substance, and the olfactory tubercle, as well as the anterior portion of periamygdaloid cortex and amygdala. In the standard MNI space, POC covers about twelve 1-mm-thick normalized coronal slices. It begins in the posterior orbitofrontal cortex and continues caudally through the most rostral-medial aspects of the temporal lobe to the level posterior to the optic chiasm. Figure 5 shows the illustration of POC and amygdala in the standard MNI space. We defined two 34 mm × 22 mm × 20 mm ROIs for the left and right POC centered at MNI −24 0 −17 and 24 0 −17. The ROIs for left and right hippocampus and insula were copied from Anatomical Automatic Labeling (AAL) template (Tzourio-Mazoyer et al., 2002).
Figure 5.
Illustration of POC (yellow) and amygdala (green) in a standard MNI brain on coronal slices. y coordinate = −5 to 10 mm.
4.3.2. Statistical Analysis
Two-sample t-tests and ANCOVAs with age as a covariate were used to compare the olfactory brain activations between early AD and HC groups (minimum statistical threshold p < 0.005). Regression analysis was used to identify how the BOLD response from the three odorant concentrations related to UPSIT scores, with age as a covariate (minimum threshold p < 0.005), since UPSIT scores are known to be correlated with age (Doty et al., 1984). Analyses were completed for the whole cohort as well as the AD and HC groups separately. Region of interest analysis was conducted on BOLD responses to the three odorant concentrations in relationship to olfaction scores (UPSIT) and neurocognitive scores (MMSE, CDR, and DRS-2).
ACKNOWLEDGMENTS
This investigation was made possible with support from the G.M. Leader Family Foundation and NIH grants R01 EB00454, R01 AG027771 and R01 AG17496. It was also funded, in part, under a grant with the Pennsylvania Department of Health using Tobacco Settlement Funds. The Department specifically disclaims responsibility for any analyses, interpretations or conclusions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Albers MW, Tabert MH, Devanand DP. Olfactory dysfunction as a predictor of neurodegenerative disease. Curr Neurol Neursci Rep. 2006;6:379–386. doi: 10.1007/s11910-996-0018-7. [DOI] [PubMed] [Google Scholar]
- Allen WF. Studies on the level of anesthesia for the olfactory and trigeminal respiratory reflexes in dogs and rabbits. Am J Physiol. 1936;115:579–587. [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attems J, Jellinger KA. Olfactory tau pathology in Alzheimer disease and mild cognitive impairment. Clin Neuropathol. 25:265–271. [PubMed] [Google Scholar]
- Braak H, Braak E. Evolution of the neuropathology of Alzheimer's disease. Acta Neurol Scand. 1996;165:3–12. doi: 10.1111/j.1600-0404.1996.tb05866.x. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Evolution of neuronal changes in the course of Alzheimer's disease. J Neural Trans. 1998;53:127–140. doi: 10.1007/978-3-7091-6467-9_11. [DOI] [PubMed] [Google Scholar]
- Collins DL, Zijdenbos AP, Kollokian V, Sled JG, Kabani NJ, Holmes CJ, Evans AC. Design and construction of a realistic digital brain phantom. IEEE Trans Med Imag. 1998;17:463–468. doi: 10.1109/42.712135. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, Sperling RA. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL. Olfactory system. In: Getchell TV, Doty RL, Bartoshuk LM, Snow JB Jr, editors. Smell and Taste in Health and Disease. New York: Raven Press; 1991. pp. 175–203. [Google Scholar]
- Doty RL. Odor perception in neurodegenerative diseases. In: Doty RL, editor. Handbook of Olfaction and Gustation. 2nd ed. New York: Marcel Dekker; 2003. pp. 479–502. [Google Scholar]
- Doty RL. The olfactory vector hypothesis of neurodegenerative disease: Is it viable? Ann Neurol. 2008;63:7–15. doi: 10.1002/ana.21327. [DOI] [PubMed] [Google Scholar]
- Doty RL, Reyes PF, Gregor T. Presence of both odor identification and detection deficits in Alzheimer's disease. Brain Res Bull. 1987;18:597–600. doi: 10.1016/0361-9230(87)90129-8. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-Mental State": A practical method for grading the cognitive state of patients for the clinician. J Psychiat Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Jezzard P, Turner R. Analysis of functional MRI time-series. Hum Brain Mapp. 1994;1:153–171. [Google Scholar]
- Grunfeld R, Wang J, Meadowcroft M, Ansel L, Sun X, Eslinger PJ, Connor JR, Smith MB, Yang Q. The responsiveness of fMRI signal to odor concentration. Chem. Senses. 2005;30:A237–A238. [Google Scholar]
- Johnson SC, Saykin AJ, Baxter LC, Flashman LA, Santulli RB, McAllister TW, Mamourian AC. The relationship between fMRI activation and cerebral atrophy: Comparison of normal aging and Alzheimer disease. Neuroimage. 2000;11:179–187. doi: 10.1006/nimg.1999.0530. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Doty RL, Moberg PJ, Mosnik D, Chen SH, Farlow MR, Hutchins GD. Olfactory-evoked regional cerebral blood flow in Alzheimer's disease. Neuropsychology. 2001;15:18–29. doi: 10.1037//0894-4105.15.1.18. [DOI] [PubMed] [Google Scholar]
- Larsson M, Semb H, Winblad B, Amberla K, Wahlund LO, Bäckman L. Odor identification in normal aging and early Alzheimer's disease. Neuropsychology. 1999;13:47–53. doi: 10.1037//0894-4105.13.1.47. [DOI] [PubMed] [Google Scholar]
- Lehtovirta M, Soininen H, Laakso MP, Partanen K, Helisalmi S, Mannermaa A, Ryynänen M, Kuikka J, Hartikainen P, Riekkinen PJ. SPECT and MRI analysis in Alzheimer’s disease: relation to apolipoprotein E ε4 allele. J Neurol Neurosurg Psychiatry. 1996;60:644–649. doi: 10.1136/jnnp.60.6.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorig TS, Elmes DG, Zald DH, Pardo JV. A computer-controlled olfactometer for fMRI and electrophysiological studies of olfaction. Behav Res Meth Instr Comp. 1999;31:370–375. doi: 10.3758/bf03207734. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale-2: Professional Manual. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- Mesholam RI, Moberg PJ, Mahr RN, Doty RL. Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer's and Parkinson's diseases. Arch Neurol. 1988;55:84–90. doi: 10.1001/archneur.55.1.84. [DOI] [PubMed] [Google Scholar]
- Moulton DG. Studies in olfactory acuity 3. Relative detectability of n-aliphatic acetates by the rat. Quart J Exp Psychol. 1960;12:203–213. [Google Scholar]
- Moulton DG, Marshall DA. The performance of dogs in detecting a-ionone in the vapor phase. Journal of Comparative Physiology. 1976;110:287–306. [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR) - Current Version and Scoring Rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Murphy C, Jernigan TL, Fennema-Notestine C. Left hippocampal volume loss in Alzheimer's disease is reflected in performance on odor identification: a structural MRI study. J Int Neuropsychol Soc. 2003;9:459–471. doi: 10.1017/S1355617703930116. [DOI] [PubMed] [Google Scholar]
- Nordin S, Murphy C. Impaired sensory and cognitive olfactory function in questionable Alzheimer's disease. Neuropsychology. 1996;10:113–119. [Google Scholar]
- Ohm TG, Braak H. Olfactory bulb changes in Alzheimer's disease. Acta Neuropathol. 1987;73:365–369. doi: 10.1007/BF00688261. [DOI] [PubMed] [Google Scholar]
- Patton HD. Taste, olfaction and visceral sensation. In: Ruth TC, Fulton JF, editors. Medical Physiology and Biophysics. Philadelphia: Saunders; 1960. [Google Scholar]
- Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer's disease. Neurobiol Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- Serby M, Larson P, Kalkstein DS. The nature and course of olfactory deficits in Alzheimer's disease. Am J Psychiatry. 1991;148:357–360. doi: 10.1176/ajp.148.3.357. [DOI] [PubMed] [Google Scholar]
- Serby M, Mohan C, Aryan M, Williams L, Mohs RC, Davis KL. Olfactory identification deficits in relatives of Alzheimer's disease patients. Biological Psychiatry. 1996;39:375–377. doi: 10.1016/0006-3223(95)00472-6. [DOI] [PubMed] [Google Scholar]
- Small GW, Ercoli LM, Silverman DH, Huang SC, Komo S, Bookheimer SY, Lavretsky H, Miller K, Siddarth P, Rasgon NL, Mazziotta JC, Saxena S, Wu HM, Mega MS, Cummings JL, Saunders AM, Pericak-Vance MA, Roses AD, Barrio JR, Phelps ME. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer's disease. Proc Nat Acad Sci USA. 2000;97:6037–6042. doi: 10.1073/pnas.090106797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Zhao Z, Desmond JE, Glover GH, Sullivan EV, Gabrieli JD. Time course of odorant-induced activation in the human primary olfactory cortex. J Neurophysiol. 2000;83:537–551. doi: 10.1152/jn.2000.83.1.537. [DOI] [PubMed] [Google Scholar]
- Soininen H, Partanen K, Pitkanen A, Hallikainen M, Hanninen T, Helisalmi S, Mannermaa A, Ryynänen M, Koivisto K, Riekkinen P. Decreased hippocampal volume asymmetry on MRIs in nondemented elderly subjects carrying the apolipoprotein E ε4 allele. Neurology. 1995;45:391–392. doi: 10.1212/wnl.45.2.391. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Critchley HD, Suckling J, Fukuda R, Williams SC, Andrew C, Howard R, Ouldred E, Bryant C, Swift CG, Jackson SH. Functional magnetic resonance imaging of odor identification: the effect of aging. J Gerontol A Biol Sci Med Sci. 2001;56:M756–M760. doi: 10.1093/gerona/56.12.m756. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labelling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Wang J, Eslinger PJ, Smith MB, Yang QX. Functional Magnetic Resonance Imaging Study of Human Olfaction and Normal Aging. J Gerontol A Biol Sci Med Sci. 2005;60:510–514. doi: 10.1093/gerona/60.4.510. [DOI] [PubMed] [Google Scholar]
- Yousem DM, Maldjian JA, Hummel T, Alsop DC, Geckle RJ, Kraut MA, Doty RL. The effect of age on odor-stimulated functional MR imaging. Amer J Neuroradiol. 1999;20:600–608. [PMC free article] [PubMed] [Google Scholar]