Abstract
Since the 19th century, many studies have enlightened the role of inflammation in atherosclerosis, changing our perception of “vessel plaque due to oxidized lipoproteins”, similar to a “rusted pipe”, towards a disease with involvement of many cell types and cytokines with more complex mechanisms. Although “physical activity” and “physical exercise” are two terms with some differences in meaning, compared to sedentary lifestyle, active people have lower cardiovascular risk and lower inflammatory markers. Activities of skeletal muscle reveal “myokines” which have roles in both the immune system and adipose tissue metabolism. In vitro and ex-vivo studies have shown beneficial effects of exercise on inflammation markers. Meanwhile in clinical studies, some conflicting results suggested that type of activity, exercise duration, body composition, gender, race and age may modulate anti-inflammatory effects of physical exercise. Medical data on patients with inflammatory diseases have shown beneficial effects of exercise on disease activity scores, patient well-being and inflammatory markers. Although the most beneficial type of activity and the most relevant patient group for anti-inflammatory benefits are still not clear, studies in elderly and adult people generally support anti-inflammatory effects of physical activity and moderate exercise could be advised to patients with cardiovascular risk such as patients with metabolic syndrome.
Keywords: inflammation, physical activity, atherosclerosis, obesity, myokines, adipokines, insulin resistance, metabolic syndrome
Introduction
The inflammatory hypothesis of atherosclerosis emerged in the 19th century as inflammation-based arterial changes for atherogenesis [1]. As it became clear within the century, the process starts with injury to the endothelium, orchestrated by endothelial cells, smooth muscle cells, platelets, lymphocytes, monocytes and macrophages, and all of these cells generate many molecules such as cytokines, growth factors, eicosanoids, proteases and reactive oxygen species, and provoke acute and eventually chronic inflammation within the vessel wall, not only as just “plaque within the lumen”, within a complex process involving many humoral and hormonal factors [2–5]. The role of chronic inflammation in propagation from atherogenesis to thrombotic events described in the medical literature led clinicians to use inflammatory markers to evaluate disease activity, in particular leukocyte count, high sensitivity C-reactive protein (hsCRP), interleukins (IL-6, IL-18), and soluble CD40 ligand [6].
Inflammation is also revealed in conditions increasing cardiovascular disease risk such as insulin resistance, visceral obesity, metabolic syndrome and type 2 diabetes, with higher proinflammatory cytokines secreted by macrophages infiltrating visceral fat [7–12]. A similar increase in cardiovascular risk has been associated with physical inactivity [13–15], even independently from body mass index [16]. In this era of increasing inactivity with age even among children [17], medical data show that besides the potential to raise mood and the effect on endorphin and coupled nitric oxide pathways [18], physical activity causes more complex interactions between organ systems, including anti-inflammatory pathways. Both “physical exercise” and “physical activity” (PA) refer to voluntary movements expending more calories than a resting position, but physical exercise is a form of PA that is specifically planned, structured, repetitive and regular, to improve cardiovascular-respiratory fitness, muscle power and endurance, flexibility, agility, balance and/or body composition [19]. Compared with a sedentary lifestyle, insufficiently active lifestyle and “weekend warrior”-style high activity pursuits, regular physical exercise (spending ≥ 1000 kcal/week) provides the best decreases in mortality risk [20]. Meanwhile, meta-analytic results revealed that PA formulated on the basis of fitness activities provides significant cardiovascular benefit [21]. Since in the medical literature these two terms are used interchangeably, our review involves effects of both PA and exercise.
Hence the aim of our review is to evaluate the relationship between PA and inflammation, in relationship to cardiovascular disease risk and other inflammatory diseases. Therefore a PubMed/ Embase search was performed up to June 2012 using combinations of “Physical activity, exercise, physical exercise” and “inflammation, inflammatory disease, cytokines, CRP” with each of the following key words: cardiovascular disease, coronary disease, atherosclerosis, obesity, metabolic syndrome, diabetes, prediabetes, impaired glucose tolerance (IGT), hypertension, cancer, asthma, chronic obstructive pulmonary disease (COPD), renal disease, renal failure, and rheumatologic diseases. Preclinical studies, randomised controlled trials, original papers, review articles and case reports are included in the present review. References of these articles were scrutinised for relevant articles.
Physical activity, myokines and inflammation
As adipose tissue, muscle tissue was also suggested to be an “endocrine organ” with myokines providing cross-talk between adipose tissue, the immune system, hypothalamus and muscle cells [16]. Fischer et al. reported a 100-fold increase in IL-6 after acute exercise [22] which was not preceded by an increase in tumour necrosis factor α (TNF-α) as in sepsis [23]. Meanwhile the exercise duration and involved muscle mass was directly related to the degree of this post-exercise IL-6 amplitude [22–24].
Interleukin-6 increases hepatic glucose production during exercise and lipolysis in adipose tissue [23]. Increase in IL-6 also enhances insulin action and sensitivity [25] unlike TNF-α-induced insulin resistance [26]. Absence of classical proinflammatory cytokines (TNF-α and IL-1β) in the exercise-induced cytokine cascade causes an increase of IL-6, IL-1ra, IL-19 and sTNF-R [27], creating an anti-inflammatory environment. It appears that exercise inhibits TNF-α directly by IL-6 [28] and indirectly via epinephrine [29]. The down-regulation of TNF-α induced by skeletal-muscle-derived IL-6 may also participate in mediating the atheroprotective effect of PA [30].
Other myokines that increase after exercise are IL-8 and IL-15 [31]. IL-15 was suggested to have an anabolic role and decrease adipose tissue mass [32], and IL-8 may play a role in angiogenesis [33].
Besides actions of myokines, physical exercise causes laminar shear stress activation and down-regulates endothelial angiotensin II type 1 receptor (AT1R) expression, causing decreased reactive oxygen species (ROS) generation, preserving NO availability and, consequently, having anti-atherogenic effects [30].
Habitual PA and exercise training decreases TNF-α and resistin levels and increase adiponectin levels [34]. In fact, high adiponectin and low hsCRP levels may have a relationship with resolution of metabolic syndrome [35].
On the other hand, impaired PA causes insulin resistance via genes involved in inflammation and endoplasmic reticulum stress, and impaired expression of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) [36]. PPAR-β/δ activation blocks inflammation in myocytes [37]. As one of the key regulatory factors in active skeletal muscle, PGC-1α may also be a link between metabolism, inflammation and skeletal muscle activity [38].
Another link between PA and insulin resistance may be its association with satiety hormones. In a small study carried out on children, increased PA caused increased obestatin, a decreased ghrelin to obestatin ratio, and increased leptin and soluble leptin receptor [39]. Meanwhile, in premenopausal women, exercise with a diet programme decreased ghrelin and ICAM-1 levels, and increased plasma adiponectin [40].
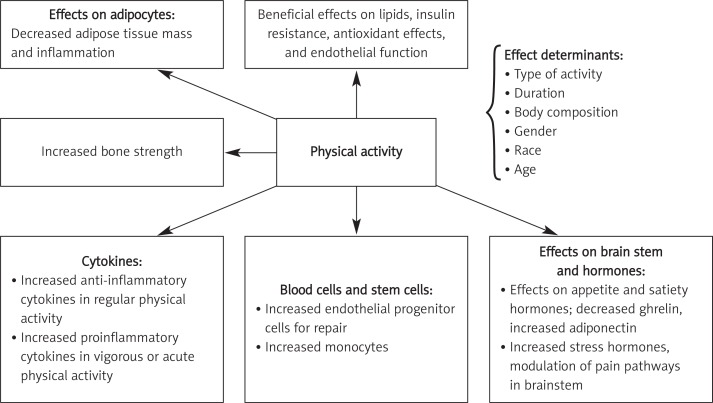
Thus, there are many different pathways in the mechanism of anti-inflammatory action of PA, and although some systemic effects are known, mediators of these effects are not clearly demonstrated yet (Figure 1).
Figure 1.
Systemic effects of physical activity and main determinants
Effects of physical activity on inflammation in cardiovascular and non-cardiovascular diseases
In vitro and ex-vivo studies
In diet-induced obese rats, both acute and chronic exercise blunt Toll-like receptor-4 (TLR-4) signalling and cause improved post-receptor insulin action [41]. Exercise reduces protein tyrosine kinase phosphatase 1B activity and insulin receptor substrate 1 serine phosphorylation, with concomitant reduction in c-jun N-terminal kinase activities in the muscle of diet-induced obese rats [42].
Studies in Zucker diabetic fatty rats, a rodent model of type 2 diabetes, show that 10 weeks of exercise as 5 km/day running significantly decreased IL-6, haptoglobin, malondialdehyde levels and JNK phosphorylation, and also decreased hepatic phosphoenolpyruvate carboxykinase levels and Ser(307)-phosphorylated insulin receptor substrate-1. All these changes indicate decreased JNK activity and decreased hyperglycemia [43]. This model of rats also showed increased adiponectin and decreased CRP levels after regular exercise [44]. Treadmill exercise may also decrease CRP in renal proximal tubules and increase IL-10. It also restores renal dopamine D1 receptor functions in rats [45], suggesting interaction of exercise with inflammatory cytokines and kidneys.
Exhaustive exercise and endurance exercise training differently modify the physiological status of the body, and therefore may have different anti-inflammatory results. Studies on rats revealed that endurance training increased the rate of tricarboxylic acid cycle and antioxidant activity whereas exhaustive exercise increased urea markers and inflammation in rat liver tissue [46].
In diet-induced obese mice, physical exercise decreases expression of TNF-α, MCP-1, PAI-1 and IKK-β in adipose tissue but not in liver [47]; therefore there may be cross-talk between muscle and adipose tissue just after muscle activity, causing changes directly in adipose tissue.
In contrast, overtraining may activate pro-inflammatory cytokines. In fact, overtrained groups of rats showed elevated levels of IL-10 and IL-6 in adipose tissue, accompanied by increased TLR-4 and NFkBp65 compared to control and trained groups [48]. Training may provide necessary changes to adapt to exercise and to trigger mechanisms against inflammation that will occur after muscle activity. Acute exercise causes endoplasmic reticulum stress (detected as increased mRNA levels and x-box binding proteins), and increases inflammatory markers (e.g. IL-6, TNF-α) and oxidative stress (detected as increased metallothionein 1F, metallothionein 1H, and NADPH oxidase) [49]. That means the effects of acute exercise are extremely different from those of chronic training [50].
In mice with a high fat diet exercise ameliorates the progression of endothelial dysfunction and decreases atherosclerotic areas. Meanwhile it has anti-inflammatory effects such as decreased IL-6 and macrophage chemoattractant protein-1 and higher adiponectin levels [51]. Thus, exercise may be beneficial by mechanisms other than anti-inflammatory effects against atherosclerosis and obesity.
Besides adipocytes, satiety hormones, markers of atherosclerosis, endothelial cells, bone tissue and kidneys, PA even may affect blood cells. Endurance exercise may also affect behaviour of blood cells, increasing tissue factor activity of lipopolysaccharide-stimulated monocytes, IL-8 increase and increased lipopolysaccharide-induced thromboxane B2, these increases being more prominent after a second bout of exercise [52]. Vigorous exercises such as marathon running increase neutrophilia, and also anti-inflammatory and antioxidant defences were activated to prevent exercise-induced oxidative stress [53], together with hormonal responses, e.g. acute growth hormone release accompanying IL-6 increase and later catecholamines possibly suppressing neutrophil responses [54, 55].
Clinical evidence
Studies on general population
Physical activity and cardiorespiratory fitness are consistently associated with 6-35% lower CRP levels, and longitudinal training studies have demonstrated reductions in CRP concentrations of 16% to 41%, an effect that may be independent of baseline levels of CRP, body composition or weight loss, indicating that the PA and CRP relationship is dose-dependent [56]. In a review evaluating 19 articles on the acute inflammatory response to exercise, 18 on cross-sectional comparisons of subjects by PA levels, and 5 examining prospectively the effects of exercise training on the inflammatory process, it was concluded that short-term exercise produces an inflammatory response but long-term training has anti-inflammatory effects [57].
In a study involving 13,748 participants, leisure time PA was associated with lower fibrinogen and white blood cell counts and higher albumin concentrations [58]. Besides beneficial effects on inflammatory markers, PA causes better haemostasis [59].
In a Finnish study, 3803 adults were evaluated for effects of physical exercise on CRP levels [60]. After adjustment for age, PA and CRP levels were inversely associated for both men and women, and after adjustment for all other factors, this relationship was present only for women. In the EPIC-Norfolk Prospective study, people with an active lifestyle had significantly lower CRP levels than inactive ones [61]. In a further study carried out on 796 healthy subjects, fibrinogen and IL-6 were related to PA, and CRP levels were inversely related to activity after adjustments for body mass index, waist-to-hip ratio, smoking, hypertension, diabetes and lipids [62].
In elderly subjects, high-volume regular PA was found to be associated with lower levels of IL-6 and higher levels of IL-10 [63]. Similar results were also seen in the study of Reuben et al., with 870 elderly people [64]. In a further cohort of 5888 elderly people lower concentrations of CRP, white blood cells, fibrinogen, and factor VIII activity were associated with higher PA quartiles [65]. Therefore probably elderly people may particularly benefit from PA.
Race and gender may also be important in anti-inflammatory effects of PA; in the study of Majka et al., there was a tendency to have lower hsCRP levels by PA tertiles only in black and white men, but not in any female groups [66]. In the PLAY study conducted in South Africa, 193 black children were evaluated for their CRP levels in different activity categories. “Fit” children had lower CRP, and especially in girls higher PA groups showed lower CRP levels [67]. In a cross-sectional study of more than 3000 Chinese urban men, CRP was tested and obesity, smoking and alcohol intake were associated with high CRP levels whereas high PA was inversely associated [68].
Studies on populations with high cardiovascular risk
In the ATTICA study conducted in the Attica region of Greece, 1514 men and 1528 women with metabolic syndrome were evaluated for their self-reported PA status and inflammatory and coagulation markers [69]. Serum CRP, white blood cells, serum amyloid A protein, and fibrinogen were significantly lower in physically active patients than in inactive ones.
Four weeks of physical exercise training in impaired glucose tolerance and type 2 diabetic patients improved plasma adipokine concentrations and CRP concentrations but the effect on IL-6 was not significant [70].
Moreover, 152 sedentary, obese or overweight postmenopausal women who were free of chronic inflammatory diseases were tested for PA energy expenditure (PAEE), total energy expenditure (TEE) and resting energy expenditure (REE), and the relationships between these PA markers and serum hsCRP, haptoglobin, soluble tumor necrosis factor-α receptor 1 (sTNFR1), interleukin-6, orosomucoid and white blood cells were evaluated [71]. While TNF-1 was positively correlated with TEE and REE, PAEE was found to be an independent predictor of hsCRP and haptoglobin.
In a study carried out on hypertensive patients, sequential physical training decreased body mass index, waist circumference and blood pressure, and improved glycemia and lipemia, but a significant reduction in hsCRP levels was only significant in metabolic syndrome patients [72], suggesting a link between hsCRP and metabolic parameters, rather than PA itself. But probably the type of exercise and patient group may cause differences in anti-inflammatory results of physical exercise; in a small group of type 2 diabetics, leptin, resistin and IL-6 decreased after 12 months of aerobic and aerobic + resistance exercise, and IL-1β, TNF-α and IFN-γ decreased whereas IL-4 and IL-10 increased in aerobic and resistance exercise groups, independent of weight loss [73]. Also angiographically documented coronary disease patients benefit from leisure time PA regarding its beneficial effects on inflammatory markers [74].
Similar to patients with coronary artery disease, patients with peripheral arterial disease also benefit from anti-inflammatory effects of PA. A higher level of activity was found to be associated with lower sVCAM, D-dimer, homocysteine, CRP and sICAM levels in these patients [75].
In a further small sample size study in obese people practising exercise together with diet, anti-inflammatory effects were only observed in adipose tissue, and not in skeletal muscle [76]. Therefore body fat distribution may also affect anti-inflammatory effects of exercise.
In most clinical studies PA was evaluated by self-reported questionnaire or pedometers. There are few interventional studies performed in healthy people or different groups of patients with selected types of regular exercise showing beneficial effects on inflammatory markers (Table I).
Table I.
Types of physical activity in prospective clinical studies on inflammation-related parameters
| Activity | Patients | Reference | Result |
|---|---|---|---|
| Treadmill exercise for 3 months | Patients with intermittent claudication (n = 82). Sixty-seven claudicants and 15 controls | Tisi, et al. 1997 [122], | Beneficial |
| Individually trained treadmill exercise programmes, mean 2.5 h/week | High cardiovascular risk people (n = 43) | Smith, et al. 1999 [123] | Beneficial |
| Long distance running – 9 months of training, mean distance increased from 31 ±9 km to 53 ±15 km | Healthy subjects (n = 14) | Mattusch, et al. 2000 [124] | Beneficial |
| Jogging and aerobic dancing | Adults over 17 years (n = 4072) | King, et al. 2003 (NHANES III study, after adjustments) [97] | Beneficial |
| Individually tailored moderate intensity resistance training for upper and lower extremity large muscles, group walking and hiking | Middle-aged overweight subjects (n = 522) | Lindström, et al. 2003 (Finnish Diabetes PreventionStudy) [125] | Beneficial |
| Low- to moderate-intensity aerobic exercise | Healthy men (n = 140) | Rauramaa, et al. 2004 (DNASCO study) [94] | Beneficial |
| Four weeks of aerobic exercise training | Normal, impaired glucose tolerance and type 2 diabetic patients (n = 60) | Oberbach, et al. 2006 [70] | Beneficial |
| Individually tailored aerobic exercise | Post-acute myocardial infarction patients (n = 60) | Balen, et al. 2008 [126] | Beneficial |
| Sequential training from 3 METs/week to 6 METs/week | Overweight patients (n = 80) | Cicero, et al. 2009 [72] | Beneficial |
| Submaximal single-leg ergometer test for 20 min/day | Chronic obstructive lung disease patients (n = 25). Fifteen lung disease patients and 10 controls | Mercken, et al. 2009 [104] | Beneficial |
| Gradually increasing walking by 3000 steps/day on 5 days of the week, for 12 weeks | Healthy males (n = 48), 24 exercising subjects and 24 controls | Gray, et al. 2009 [127] | Not beneficial |
| 10-30 min stationary cycling at an intensity of 12-16 out of 20 at the rate of perceived exertion(RPE) on Borg scale in aerobic group and using ankle weights for knee extension, hip abduction and flexions at an intensity of 15-17 out of 20 at the RPE on Borg scale in resistance group | Haemodialysis patients (n = 21). Seven patients in aerobic resistance, 7 in interdialytic exercise and 7 in control groups | Afshar, et al. 2010 [118] | Beneficial |
| Twice-a-week supervised aerobic and resistance training plus structured exercise counselling | Sedentary type 2 diabetics and metabolic syndrome patients (n = 606) | Balducci, et al. 2010(IDES study) [128] | Beneficial |
| Minimum 30 min of aerobic exercise, 5-6 times/month | Hypercholesterolaemic men (n = 157) | Sjögren, et al. 2010 [85] | Beneficial |
| Bicycle home ergometer, home-based exercise plan | Patients who underwent percutaneous coronary intervention (n = 62), 33 patients taking exercise and 29 sedentary | Astengo, et al. 2010 [129] | Not beneficial |
| Moderate intensity aerobic activity for 12 months | Elderly nondisabled men and women (n = 368) | Beavers, et al. 2010 [130] | Not beneficial |
| 40-minute walking for 5 days per week | Coronary heart failure patients (n = 28). Eighteen patients in exercise and 10 patients in non-exercise group | Tsarouhas, et al. 2011 [121] | Beneficial |
Effects of excessive physical activity
Although it was known that vigorous PA may trigger an inflammatory response in muscle tissue, in a study carried out on 520 adolescents, vigorous PA was associated with decreased CRP levels in boys [77]. High endurance physical exercise in non-athlete adults increases CRP and TNF-α and IL-6 changes may not be significant [78]. Different levels of bench press exercise intensity were not associated with changes in IL-6, IL-1β and TNF-α levels, although maximal creatinine kinase levels were reached [79].
Prolonged vigorous exercise such as the spartathlon (246 km continuous running) causes an acute inflammatory response in muscle tissue, marked increases in plasma levels of CRP, IL-6, SAA, MCP-1, IL-8, sVCAM-1, sICAM-1, thrombomodulin (sTM) and NT-pro-BNP, and increased endothelial progenitor cells as a repair mechanism [80]. Similar results of increased inflammatory markers were also observed in marathon and ultra-marathon (200 km) runners and triathlon racers [81–83].
Conflicting results of clinical studies
Apart from this large body of evidence supporting the positive effect of PA on inflammation, other studies have reported neutral, partial or opposite effects. For instance, the PREPARE (Pre-diabetes Risk Education and Physical Activity Recommendation and Encouragement) programme randomized trial did not reveal anti-inflammatory effects of ambulation [84]. In another recent study, physical exercise per se did not have any benefit regarding sVCAM, sICAM or IL-6, but when combined with diet, exercise caused significant reductions in sICAM and sE-selectin [85]. In elderly patients, 12-month moderate intensity PA significantly lowered IF-8 levels, but no effect was observed on other inflammatory markers such as TNF-α or soluble receptors of IL-1 or 6 [86].
The “Physical Activity as a preventive agent of the Development of Overweight, Obesity, Allergies, Infections, and Cardiovascular Risk Factors in adolescents” study (La Actividad Fisica como Agente Preventivo del Desarrollo de Sobrepeso, Obesidad, Alergias, Infecciones y Factores de Riesgo Cardiovasular en Adolescentes, AFINOS) evaluated 192 adolescents [87] and controlled effects of 7 days’ PA measured by accelerometer on CRP, IL-6 and complement factors C3 and C4. Independent from HOMA-IR, only body fat was related to CRP, and PA measures were not independently associated with inflammatory markers. Similarly, in the European Young Heart Study, CRP and C3 were negatively correlated with cardiovascular fitness but inversely related to body fat mass [88].
The Inflammation and Exercise (INFLAME) study was conducted to test whether diet or PA can reduce CRP in individuals with increased CRP levels. A 4-month period of physical training was started for sedentary people and there was no significant difference between exercising and sedentary groups regarding CRP when adjusted for gender and body weight [89]. Also data from 950 people evaluated in the National Health and Nutrition Survey (1999-2002) showed no beneficial association between reported PA rates and CRP [90].
In another study carried out on 892 male subjects, a questionnaire was used to detect levels of PA and after adjustments for personal characteristics there was no relationship between PA status and CRP or serum amyloid A or fibrinogen levels [91]. In a study with elderly subjects, interestingly, PA was correlated with lower levels of inflammatory markers (e.g. CRP, IL-6 and TNF-α) but this association was also observed in people who do not exercise but take antioxidants [92].
In a study involving 109 healthy men and women, body mass index was related to hsCRP levels, but PA was not [93].
Meanwhile, in the “DNA Polymorphism and Carotid Atherosclerosis” (DNASCO) study investigating whether PA slows progression of atherosclerosis and effects of genetic factors, hsCRP levels were not significantly lower in the exercising group [94]. In another study carried out on obese people, PA was only inversely related to C-peptide and insulin levels, whereas other inflammatory markers such as CRP, IL-6, and soluble TNF receptors 1 and 2 were not affected [95].
Therefore although large, population-based cohort studies support anti-inflammatory effects of PA, data from large, randomized, controlled trials have conflicting results [96]. Besides age, gender and duration, type of PA (for example aerobic dancing vs weight lifting or gardening) is probably important [97].
Studies on non-cardiovascular inflammatory diseases
In a systematic review, 19 studies in children and adults with chronic inflammatory diseases were collected and acute and chronic effects of physical exercise on inflammatory markers were tested [98]. Acute exercise increases inflammatory markers but training decreases them, but the results depend on the nature of the PA.
Rheumatological diseases – Anti-inflammatory effects of physical exercise were first noticed in chronic inflammatory diseases such as autoimmune arthritis or chronic obstructive lung disease [99]. It was found that physical exercise decreases IL-6 and CRP levels in these patients [99].
In studies carried out on rheumatoid arthritis patients, the number of CD4(+) cells in synovial fluid decreases after moderate physical exercise [100]. The role of exercise in synovial inflammation was also similar in vascular inflammation in these patients [101].
Respiratory diseases – together with pharmacological treatment, exercise reduces oxidative stress and airway inflammation and increases antioxidant enzyme activities in asthmatic children [102, 103], whereas tests in COPD patients showed increased oxidative stress without an increase in inflammatory markers after acute exercise (e.g. continuous single leg exercise) [104]. The degree of PA is also associated with lower hs-CRP and IL-6 levels in COPD patients [105]. Similarly, physical training in asthmatic patients leads to decreased serum hs-CRP levels and better pulmonary function [106]. In the recent prospective cohort study of Waschki et al., PA was shown to be the strongest predictor of all-cause mortality in COPD patients [107].
Exercise training may be protective in lung ischaemia, protecting against ischaemia-reperfusion injury by improving pulmonary vascular permeability, as shown in rats [108].
Cancers – Metabolic syndrome and related parameters are clearly associated with colon cancer [109] and breast cancer incidence [110].
Since PA decreases insulin resistance, obesity, CRP and estrone levels, its effects in breast cancer were hypothesized and studied at the beginning of this century [111]. Physical exercise was found to have slight to moderate effects on improving some biomarkers in breast and colon cancer patients including insulin, leptin, estrogens, inflammation, immune function and apoptosis regulation [112]. In fact, both acute and chronic exercise alter the number of circulating cells of the innate immune system; for example, it is agreed that lymphocytosis occurs during and after acute exercise, and mobilization of T and B cells is largely influenced by catecholamines [113]. Among nearly 73 studies examining the effects of exercise on breast cancer risk, there is a 25% average risk reduction among physically active women compared to the least active ones [114]. Besides breast cancer, lung cancer also has a strong correlation with decreased PA; PA reduces lung cancer by 20-30% in women and 20-50% in men, possibly via improved pulmonary function, reduced concentrations of carcinogenic agents in the lungs, enhanced immune function, reduced inflammation, enhanced DNA repair capacity, changes in growth factor levels and possible gene-PA interactions [115]. Similar mechanisms may also be involved in the preventive effect of physical exercise against colon cancer [116].
Chronic renal failure – In chronic renal failure patients, especially those under haemodialysis treatment, who usually have a low PA, the available studies are low in number, and the effects of malnutrition generally prevent evaluation of the effects of PA [117, 118]. However, in a recent study, self-reported PA levels were inversely related to the hsCRP serum level [119].
Chronic heart failure – In New York Heart Association grade II-II heart failure patients evaluated by peak before and after a 12-week physical training programme in a cross-over design, a significant correlation between training-induced oxygen consumption and reduction in soluble intercellular adhesion molecule-1 (sICAM) and soluble vascular cell adhesion molecule-1 (VCAM-1) levels was found, reflecting beneficial monocyte-endothelial cell-macrophage interaction in these patients [120]. Better asymmetric dimethyl arginine (ADMA) and homocysteine levels were also observed in the same group of patients after a standardized exercise programme [121].
Conclusions
Despite increasing evidence of an inflammation modulatory effect of PA, much research is needed to better understand which kind of activity or exercise is associated with the largest anti-inflammatory effect, which kind of patients could benefit most from this approach, and whether the PA-related decrease of systemic inflammation is associated with an improvement in cardiovascular prognosis. The current data suggest that moderate PA could have some anti-inflammatory effects in both adult and elderly healthy subjects as well as in patients with cardiovascular risk factors such as metabolic syndrome. The mechanisms involved in its anti-inflammatory action and knowledge about determinants of physical exercise are not clearly elucidated yet. Meanwhile, prospective clinical studies with PA interventions to study anti-inflammatory effects are still too low in number to decide about the type of activity for specific groups of patients.
References
- 1.Mayerl C, Lukasser M, Sedivy R, et al. Atherosclerosis research from past to present on the tract of two pathologists with opposing views, Carl von Rokitanski and Rudolf Wirchow. Virch Arch. 2006;449:96–103. doi: 10.1007/s00428-006-0176-7. [DOI] [PubMed] [Google Scholar]
- 2.Lamon BD, Hajjar DP. Inflammation at the molecular interface of atherogenesis. An anthropological journey. Am J Pathology. 2008;173:1253–64. doi: 10.2353/ajpath.2008.080442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libby P. Inflammation and the cardiovascular disease mechanisms. Am J Nutr. 2006;83(Suppl):456S–60S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 4.Ertek S, Akgül E, Cicero AF, et al. 25-Hydroxy vitamin D levels and endothelial vasodilator function in normotensive women. Arch Med Sci. 2012;8:47–52. doi: 10.5114/aoms.2012.27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ertek S, Francesco Cicero A, Erdoğan G. The relationship between calcium metabolism, insulin-like growth factor-1 and pulse pressure in normotensive, normolipidaemic and non-diabetic patients. Arch Med Sci. 2011;7:776–80. doi: 10.5114/aoms.2011.25551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pickard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008;54:2438. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- 7.Schuster DP. Obesity and the development of type 2 diabetes: the effects of fatty tissue inflammation. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2010;3:253–62. doi: 10.2147/dmsott.s7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paoletti R, Bolego C, Poli A, Cignarella A. Metabolic syndrome, inflammation and atherosclerosis. Vasc Health Risk Manag. 2006;2:145–52. doi: 10.2147/vhrm.2006.2.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derosa G, Cicero AF, Scalise F, et al. Metalloproteinase-2 and -9 in diabetic and nondiabetic subjects during acute coronary syndromes. Endothelium. 2007;14:45–51. doi: 10.1080/10623320601177064. [DOI] [PubMed] [Google Scholar]
- 10.Derosa G, D'Angelo A, Scalise F, et al. Comparison between metalloproteinases-2 and -9 in healthy subjects, diabetics, and subjects with acute coronary syndrome. Heart Vessels. 2007;22:361–70. doi: 10.1007/s00380-007-0989-6. [DOI] [PubMed] [Google Scholar]
- 11.Haffner SM. Abdominal adiposity and cardiometabolic risk: do we have all the answers? Am J Med. 2007;120:S10–6. doi: 10.1016/j.amjmed.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Bays HE. Sick fat”, metabolic disease and atherosclerosis. Am J Med. 2009;122:S26–37. doi: 10.1016/j.amjmed.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Nocon N, Hiermann T, Muller-Riemenschineider F, Thalau F, Roll S, Willich SN. Association of physical activity with all-cause and ardiovascular mortality: a systemic review and meta-analysis. Eur J Cardiovasc Prev Rehab. 2008;15:239–46. doi: 10.1097/HJR.0b013e3282f55e09. [DOI] [PubMed] [Google Scholar]
- 14.Tuomiletho J, Lindstrom J, Eriksson JL, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with mpaired glucose tolerance. N Eng J Med. 2001;344:1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 15.Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer. 2009;100:611–6. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedersen BK. The diseasome of physical inactivity and the role of myokines in muscle-fat cross-talk. J Physiol. 2009;587:5559–68. doi: 10.1113/jphysiol.2009.179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simeunovic S, Milincic Z, Nikolic D, et al. Physical activity evaluation in Yugoslav Study of the Precursors of Atherosclerosis in School Children – YUSAD study. Arch Med Sci. 2010;6:874–8. doi: 10.5114/aoms.2010.19294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esch T, Stefano GB. Endogenous reward mechanisms and their importance in stress reduction, exercise and the brain. Arch Med Sci. 2010;6:447–55. doi: 10.5114/aoms.2010.14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fletcher GF, Balady G, Blair SN, et al. Statement on exercise: benefits and recommendations for physical activity programs for all Americans. Circulation. 1996;94:857–62. doi: 10.1161/01.cir.94.4.857. [DOI] [PubMed] [Google Scholar]
- 20.Lee IM, Sesso HD, Oquma Y, Paffenbarger RS., Jr The “weekend-warrior” and risk of mortality. Am J Epidemiol. 2004;160:636–41. doi: 10.1093/aje/kwh274. [DOI] [PubMed] [Google Scholar]
- 21.Williams PT. Physical fitness and activity as separate heart disease risk factors. Med Sci Sports Exerc. 2001;33:754–61. doi: 10.1097/00005768-200105000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer CP. Interleukin-6 in acute exercise and training: what is the biological relevance? Exercise Immunol Rev. 2006;12:6–33. [PubMed] [Google Scholar]
- 23.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle derived interleukin-6. Physiol Rev. 2008;88:1379–406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 24.Kamiłski KA, Jasiewicz M, Knapp M, et al. Short period of exercise causes rapid increase of serum interleukin 6 with no effect on its soluble receptor. Arch Med Sci. 2009;5:364–70. [Google Scholar]
- 25.Wojtaszewski JF, Hansen BF, Gade Kiens B, et al. Insulin signalling and insulin sensitivity after exercise in human skeletal muscle. Diabetes. 2000;49:325–31. doi: 10.2337/diabetes.49.3.325. [DOI] [PubMed] [Google Scholar]
- 26.Hotlamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signalling from the insulin receptor. Proc Natl Acad Sci USA. 1994;91:4854–8. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ostrowski K, Schjerling P, Pedersen BK. Physical activity and plasma IL-6 levels in humans: effect of intensity of exercise. Eur J Appl Physiol. 2000;83:512–5. doi: 10.1007/s004210000312. [DOI] [PubMed] [Google Scholar]
- 28.Mizuhara H, O'Neill E, Seki N, et al. T-cell activation associated hepatic injury, mediation tumor necrosis factors and protection by interleukin-6. J Exp Med. 1994;179:1529–37. doi: 10.1084/jem.179.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Poll T, Coyle SM, Barbosa K, Braxton CC, Lowry SF. Epinephrine inhibits tumor necrosis factor-alpha and potentiates interleukin-10 production during human endotoxemia. J Clin Invest. 1996;97:713–9. doi: 10.1172/JCI118469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szostak J, Laurant P. The forgotton face of regular physical exercise: a natural antiatherogenic activity. Clin Sci (Lond) 2011;121:91–106. doi: 10.1042/CS20100520. [DOI] [PubMed] [Google Scholar]
- 31.Pinto A, Di Raimondo D, Tuttolomondo A, Buttà C, Milio G, Licata G. Effects of physical exercise on inflammatory markers of atherosclerosis. Curr Pharm Des. 2012 Feb 29; doi: 10.2174/138161212802481192. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Quinn LS, Strait-Bodey L, Anderson BG, Argiles JM, Havel PJ. Intereukin-15 stimulates adiponectin secretion by 3T3-L1 adipoctyes: evidence for a skeletal muscle to fat signalling pathway. Cell Biol Int. 2005;29:449–57. doi: 10.1016/j.cellbi.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Frydelund-Larsen , Penkowa M, Akerstrom T, Zankari A, Nielsen S, Pedersen BK. Exercise induces interleukin-8 receptor (CXCR2) expression in human skeletal muscle. Exp Physiol. 2007;92:233–40. doi: 10.1113/expphysiol.2006.034769. [DOI] [PubMed] [Google Scholar]
- 34.Rubin DA, Hackney AC. Inflammatory cytokines and metabolic risk factors during growth and maturation: influence of physical activity. Med Sport Sci. 2010;55:43–55. doi: 10.1159/000321971. [DOI] [PubMed] [Google Scholar]
- 35.Ahonen TM, Saltevo JT, Kautiainen HJ, Kumpusalo EA, Vanhala MJ. The association of adiponectin and low-grade inflammation with the course of metabolic syndrome. Nutr Metab Cardiovasc Dis. 2012;22:285–91. doi: 10.1016/j.numecd.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Alibegovic AC, Sonne MP, Højbjerre L, et al. Insulin resistance induced by physical inactivity is associated with multiple transcriptional changes in skeletal muscle in young men. Am J Physiol Endocrinol Metab. 2010;299:E752–63. doi: 10.1152/ajpendo.00590.2009. [DOI] [PubMed] [Google Scholar]
- 37.Alvarez-Guardia D, Palomer X, Coll T, et al. PPARbeta/delta activation blocks lipid-induced inflammatory pathways in mouse heart and human cardiac cells. Biochim Biophys Acta. 2011;1811:59–67. doi: 10.1016/j.bbalip.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Handschin C. PGC-1alpha in muscle links metabolism to inflammation. Clin Exp Pharmacol Physiol. 2009;36:1139–43. doi: 10.1111/j.1440-1681.2009.05275.x. [DOI] [PubMed] [Google Scholar]
- 39.Balagopal PB, Gidding SS, Buckloh LM, et al. Changes in circulating satiety hormones in obese children: randomized controlled physical activity – based intervention study. Obesity. 2010;18:1747–53. doi: 10.1038/oby.2009.498. [DOI] [PubMed] [Google Scholar]
- 40.Ata SM, Vaishnav U, Puglisi M, et al. Macronutrient composition and increased physical activity modulate plasma adipokines and appetite hormones during a weight loss intervention. J Womens Health. 2010;19:139–45. doi: 10.1089/jwh.2009.1472. [DOI] [PubMed] [Google Scholar]
- 41.Oliviera AG, Carvalho BM, Tobar N, et al. Physical exercise reduces circulating lipopolysaccharide and TLR4 activation and improves insulin signalling in tissues of DIO rats. Diabetes. 2011;60:784–96. doi: 10.2337/db09-1907. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Da Silva AS, Pauli JR, Ropelle ER, et al. Exercise intensity, inflammatory signalling, and insulin resistance in obese rats. Med Sci Sports Exerc. 2010;42:2180–8. doi: 10.1249/MSS.0b013e3181e45d08. [DOI] [PubMed] [Google Scholar]
- 43.Király MA, Campbell J, Park E, et al. Exercise maintains euglycemia in association with decreased activation of c-Jun NH2-terminal kinase and serine phosphorylation of IRS-1 in the liver of ZDF rats. Am J Physiol Endocrinol Metab. 2010;298:E671–81. doi: 10.1152/ajpendo.90575.2008. [DOI] [PubMed] [Google Scholar]
- 44.de Lemos ET, Reis F, Baptista S, et al. Exercise training is associated with improved levels of C-reactive protein and adiponectin in ZDF (type 2) diabetic rats. Med Sci Monit. 2007;13:BR 168–74. [PubMed] [Google Scholar]
- 45.Asghar M, George L, Lokhandwala MF. Exercise decreases oxidative stress and inflammation and restores renal dopamine D1 receptor function in old rats. Am J Physiol Renal Physiol. 2007;293:F914–9. doi: 10.1152/ajprenal.00272.2007. [DOI] [PubMed] [Google Scholar]
- 46.Huang CC, Lin WT, Hsu FL, Tsai PW, Hou CC. Metabolomics investigation of exercise modulated changes in metabolism in rat liver after exhaustive and endurance exercises. Eur J Applied Phys. 2010;108:557–66. doi: 10.1007/s00421-009-1247-7. [DOI] [PubMed] [Google Scholar]
- 47.Bradley RL, Jeon JY, Liu FF, Maratos-Flier E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am J Physiol Endocrinol Metab. 2008;295:E586–94. doi: 10.1152/ajpendo.00309.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lira FS, Rosa JC, Pimenel GD, et al. Inflammation and adipose tissue:effects of progressive load training in rats. Lipids Health Dis. 2010;9:109. doi: 10.1186/1476-511X-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim HJ, Jamart C, Deldicque L, et al. Endoplasmic reticulum stress markers and ubiquitin-proteasome pathway activity in response to a 200 km run. Med Sci Sport Exerc. 2011;43:18–25. doi: 10.1249/MSS.0b013e3181e4c5d1. [DOI] [PubMed] [Google Scholar]
- 50.Wilund KR. Is the anti-inflammatory effect of regular exercise responsible for reduced cardiovascular disease? Clin Sci (Lond) 2007;112:543–55. doi: 10.1042/CS20060368. [DOI] [PubMed] [Google Scholar]
- 51.Fukao K, Shimada K, Naito H, et al. Voluntary exercise ameliorates the progression of atherosclerotic lesion formation via anti-inflammatory effects in apoprotein E-deficient mice. J Atheroscler Thromb. 2010;17:1226–36. doi: 10.5551/jat.4788. [DOI] [PubMed] [Google Scholar]
- 52.Degerstrøm J, Østerud B. Increased inflammatory response of blood cells to repeated bout of exercise. Med Sci Sports Exerc. 2006;38:1297–303. doi: 10.1249/01.mss.0000227315.93351.8d. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki K, Nakaji S, Yamada M, et al. Impact of competitive marathon race on systemic cytokine and neutrophil responses. Med Sci Sports Exerc. 2003;35:348–55. doi: 10.1249/01.MSS.0000048861.57899.04. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki K, Totsuka M, Nakaji S, et al. Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics and muscle damage. J Appl Physiol. 1999;87:1360–7. doi: 10.1152/jappl.1999.87.4.1360. [DOI] [PubMed] [Google Scholar]
- 55.Pizza FX, Davis BH, Henrickson SD, et al. Adaptation to eccentric exercise: effect on CD64 and CD11b/CD18 expression. J Appl Physiol. 1996;80:47–55. doi: 10.1152/jappl.1996.80.1.47. [DOI] [PubMed] [Google Scholar]
- 56.Plaisance EP, Grandjean PW. Physical activity and high sensitive C-reactive protein. Sports Med. 2006;36:443–58. doi: 10.2165/00007256-200636050-00006. [DOI] [PubMed] [Google Scholar]
- 57.Kasapis C, Thompson PD. The effects of physical activity on serum CRP and inflammatory markers: a systematic review. J Am Coll Cardiol. 2005;45:1563–9. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 58.Ford ES. Does exercise reduce inflammation? Physical activity and C-reactive protein among US adults. Epidemiology. 2002;13:561–8. doi: 10.1097/00001648-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 59.Wannamethee SG, Lowe GD, Whincup PH, Rumley A, Walker M, Lennon L. Physical activity and hemostatic and inflammatory variables in elderly men. Circulation. 2002;105:1785–90. doi: 10.1161/hc1502.107117. [DOI] [PubMed] [Google Scholar]
- 60.Borodulin K, Laatikainen T, Salomaa V, Jousilahti P. Associations of leisure time physical activity, self-rated physical fitness and estimated aerobic fitness with serum C-reactive protein among 3,803 adults. Atherosclerosis. 2006;185:381–7. doi: 10.1016/j.atherosclerosis.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 61.Boekholdt SM, Sandhu MS, Day NE, et al. Physical activity, C-reactive protein levels and the risk of future coronary artery disease in apparently healthy men and women: the EPIC-Norfolk prospective population study. Eur J Cardiovasc Prev Rehab. 2006;13:970–6. doi: 10.1097/01.hjr.0000209811.97948.07. [DOI] [PubMed] [Google Scholar]
- 62.Autenrieth C, Schneider A, Döring A, et al. Association between different domains of physical activity and markers of inflammation. Med Sci Sports Exerc. 2009;41:1706–13. doi: 10.1249/MSS.0b013e3181a15512. [DOI] [PubMed] [Google Scholar]
- 63.Jankord R, Jemiolo B. Influence of physical activity on serum IL-10 and IL-6 levels in healthy older men. Med Sci Sports Exerc. 2004;36:960–4. doi: 10.1249/01.mss.0000128186.09416.18. [DOI] [PubMed] [Google Scholar]
- 64.Reuben DB, Judd-Hamilton L, Harris TB, Seeman TE. MacArthur Studies of Successful Aging. The associations between physical activity and inflammatory markers in high-functioning older persons: MacArthur Studies of Successful Aging. J Am Geriatr Soc. 2003;51:1125–30. doi: 10.1046/j.1532-5415.2003.51380.x. [DOI] [PubMed] [Google Scholar]
- 65.Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA, Tracy RP. Association between physical activity and markers of inflammation in healthy elderly population. Am J Epidemiol. 2001;153:242–50. doi: 10.1093/aje/153.3.242. [DOI] [PubMed] [Google Scholar]
- 66.Majka DS, Chang RW, Vu TH, et al. Physical activity and high sensitivity C-reactive protein: the multi-ethnic study of atherosclerosis. Am J Prev Med. 2009;36:56–62. doi: 10.1016/j.amepre.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harmse B, Kruger HS. Significant differences between serum CRP levels in children in different categories of physical activity. Cardiovasc J Afr. 2010;21:316–22. doi: 10.5830/CVJA-2010-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Villegas R, Xiang YB, Cai H, et al. Lifestyle determinants of C-reactive protein in middle aged urban Chinese men. Nurt Metab Cardiovasc Dis. 2012;22:223–30. doi: 10.1016/j.numecd.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pitsavos C, Panagiotakos DB, Chrysohoou C, Kavouras S, Stefanadis C. The associations between physical activity, inflammation and coagulation markers, in people with metabolic syndrome. Eur J Cardiovasc Prev Rehab. 2005;12:151–8. doi: 10.1097/01.hjr.0000164690.50200.43. [DOI] [PubMed] [Google Scholar]
- 70.Oberbach A, Tönjes A, Klöting N, et al. Effect of a 4-week physical training program on plasma concentrations of inflammatory markers in patients with abnormal glucose tolerance. Eur J Endocrinol. 2006;154:577–85. doi: 10.1530/eje.1.02127. [DOI] [PubMed] [Google Scholar]
- 71.Lavoie ME, Rabasa-Lhoret R, Doucet E, et al. Association between physical activity, energy expenditure and inflammatory markers in sedentary overweight and obese women. Int J Obes. 2010;34:1387–95. doi: 10.1038/ijo.2010.55. [DOI] [PubMed] [Google Scholar]
- 72.Cicero AF, Derosa G, Bove M, Di Gregori V, Gaddi AV, Borghi C. Effect of sequential training programme on inflammatory, prothrombic and vascular remodelling biomarkers in hypertensive overweight patients with or without metabolic syndrome. Eur J Cardiovasc Prev Rehab. 2009;16:698–704. doi: 10.1097/HJR.0b013e32833158e4. [DOI] [PubMed] [Google Scholar]
- 73.Balducci S, Zanuso S, Nicolucci A, et al. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nurt Metab Cardiovasc Dis. 2010;20:608–17. doi: 10.1016/j.numecd.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 74.Rothenbacher D, Hoffmeister A, Brenner H, Koenig W. Physical activity, coronary heart disease, and inflammatory response. Arch Intern Med. 2003;163:1200–5. doi: 10.1001/archinte.163.10.1200. [DOI] [PubMed] [Google Scholar]
- 75.Craft LL, Guralnik JM, Ferrucci L, et al. Physical activity during daily life and circulating biomarker levels in patients with peripheral arterial disease. Am J Cardiol. 2008;102:1263–8. doi: 10.1016/j.amjcard.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab. 2006;290:E961–7. doi: 10.1152/ajpendo.00506.2005. [DOI] [PubMed] [Google Scholar]
- 77.Sabiston CM, Castonguay A, Low NC, et al. Vigorous physical activity and low grade systemic inflammation in adolescent boys and girls. Int J Pediatr Obes. 2010;5:509–15. doi: 10.3109/17477160903572019. [DOI] [PubMed] [Google Scholar]
- 78.Andersson J, Jansson JH, Hellsten G, Nilsson TK, Hallmans G, Boman K. Effects of heavy endurance physical exercise on inflammatory markers in non-athletes. Atherosclerosis. 2010;209:601–5. doi: 10.1016/j.atherosclerosis.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 79.Uchida MC, Nosaka K, Ugrinowitsch C, et al. Effect of bench press exercise intensity on muscle soreness and inflammatory mediators. J Sports Sci. 2009;27:499–507. doi: 10.1080/02640410802632144. [DOI] [PubMed] [Google Scholar]
- 80.Goussetis E, Spiropoulos A, Tsironi M, et al. Spartathlon, a 246 km foot race: effects of acute inflammation induced by prolonged exercise on circulating progenitor reparative cells. Blood Cells Mol Dis. 2009;42:294–9. doi: 10.1016/j.bcmd.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 81.Kim HJ, Lee YH, Kim CK. Changes in serum cartilage oligomeric matrix protein (COMP), plasma CPK and plasma hs-CRP in relation to running distance in a marathon (42.195 km) and an ultra-marathon race. Eur J Applied Physiol. 2009;105:765–70. doi: 10.1007/s00421-008-0961-x. [DOI] [PubMed] [Google Scholar]
- 82.Suzuki K, Peake J, Nosaka K, et al. Changes in markers of muscle damage, nflammation and HSP 70 after an Ironman Triathlon race. Eur J Applied Physiol. 2006;98:525–34. doi: 10.1007/s00421-006-0296-4. [DOI] [PubMed] [Google Scholar]
- 83.Gomez-Merino D, Drogou C, Guezennec CY, et al. Comparison of systemic cytokine responses after a long distance triathlon and a 100 km-run: relationship to metabolic and inflammatory processes. Eur Cytokine Netw. 2006;17:117–24. [PubMed] [Google Scholar]
- 84.Yates T, Davies MJ, Gorely T, et al. The effect of increased ambulatory activity on markers of chronic low-grade inflammation, evidence from PREPARE programme randomized controlled trial. Diabet Med. 2010;27:1256–63. doi: 10.1111/j.1464-5491.2010.03091.x. [DOI] [PubMed] [Google Scholar]
- 85.Sjögren P, Cederholm T, Heimburger M, et al. Simple advice on lifestyle habits and longterm changes in biomarkers of inflammation and vascular adhesion in healthy middle-aged men. Eur J Clin Nutr. 2010;64:1450–6. doi: 10.1038/ejcn.2010.182. [DOI] [PubMed] [Google Scholar]
- 86.Baevers KM, Hsu FC, Isom S, et al. Long term physical activity and inflammatory biomarkers in odler adults. Med Sci Sports Exerc. 2010;42:2189–96. doi: 10.1249/MSS.0b013e3181e3ac80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martinez-Gomez D, Eisenmann JC, Wärnberg J, et al. Associations of physical activity, cardiorespiratory fitness, and fatness with low-grade inflammation in adolescents: the AFINOS study. Int J Obes (Lond) 2010;34:1501–7. doi: 10.1038/ijo.2010.114. [DOI] [PubMed] [Google Scholar]
- 88.Ruiz JR, Ortega FB, Warnberg J, Sjöström M. Associations of low grade inflammation with physical activity, fitness, and fatness in prepubertal children, European Young Heart Study. Int J Obes. 2007;31:1545–51. doi: 10.1038/sj.ijo.0803693. [DOI] [PubMed] [Google Scholar]
- 89.Church TS, Earnest CP, Thompson AM, et al. Exercise without weight loss does not reduce C-reactive protein: the INFLAME study. Med Sci Sports. 2010;42:708–16. doi: 10.1249/MSS.0b013e3181c03a43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin CY, Chen PC, Kuo HK, Lin LY, Lin JW, Hwang JJ. Effects of obesity, physical activity, and cardiorespiratory fitness on blood pressure, inflammation, and insulin resistance in National Health and Nutrition Survey 1999-2002. Nurt Metab Cardiovasc Dis. 2010;20:713–9. doi: 10.1016/j.numecd.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 91.Verdaet D, Dendale P, De Bacquer D, Delanghe J, Block P, De Backer G. Association between leisure time physical activity and markers of chronic inflammation related to coronary heart disease. Atherosclerosis. 2004;176:303–10. doi: 10.1016/j.atherosclerosis.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 92.Colbert LH, Visser M, Simonsick EM, et al. Physical activity, exercise and inflammatory markers in older adults, findings from the Health, Ageing and Body Composition Study. J Am Geriatr Soc. 2004;52:1098–104. doi: 10.1111/j.1532-5415.2004.52307.x. [DOI] [PubMed] [Google Scholar]
- 93.Rawson ES, Freedson PS, Osganian SK, Matthews CE, Reed G, Ockene IS. Body mass index, but not physical activity, is associated with C-reactive protein. Med Sci Sports Exerc. 2003;35:1160–6. doi: 10.1249/01.MSS.0000074565.79230.AB. [DOI] [PubMed] [Google Scholar]
- 94.Rauramaa R, Halonen P, Väisänen SB, et al. Effects of aerobic physical exercise on inflammationand atherosclerosis in men, the DNASCO study: a six year randomized, controlled trial. Ann Int Med. 2004;140:1007–14. doi: 10.7326/0003-4819-140-12-200406150-00010. [DOI] [PubMed] [Google Scholar]
- 95.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Rimm EB. Leisure time physical activity and reduced plasma levels of obesity-related inflammatory markers. Obes Res. 2003;11:1055–64. doi: 10.1038/oby.2003.145. [DOI] [PubMed] [Google Scholar]
- 96.Beavers KM, Brinkley TE, Nicklas BJ. Effect of exercise training on chronic inflammation. Clin Chim Acta. 2010;411:785–93. doi: 10.1016/j.cca.2010.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.King DE, Carek P, Mainous AG, 3rd, Pearson WS. Inflammatory markers and exercise: differences related to exercise type. Med Sci Sports Exerc. 2003;35:575–81. doi: 10.1249/01.MSS.0000058440.28108.CC. [DOI] [PubMed] [Google Scholar]
- 98.Ploeger HE, Takken T, de Greef MH, Timmons BW. The effects of acute and chronic exercise on inflammatory markers in children and adults with a chronic systemic diseases: a systematic review. Exerc Immunol Rev. 2009;15:6–41. [PubMed] [Google Scholar]
- 99.Nader GA, Lundberg E. Exercise as an anti-inflammatory intervention to combat inflammatory diseases of muscle. Curr Opin Rheumatol. 2009;21:599–603. doi: 10.1097/BOR.0b013e3283319d53. [DOI] [PubMed] [Google Scholar]
- 100.Shephard RJ, Shek PN. Autoimmune disorders, physical activity, and training with particular reference to rheumatoid arthritis. Exerc Immunol Rev. 1997;3:53–67. [PubMed] [Google Scholar]
- 101.Metsios GS, Stavropoulos-Kalinoglou A, Sandoo A, et al. Vascular function and inflammation in rheumatoid arthritis: the role of physical activity. Open Cardiovasc Med J. 2010;4:89–96. doi: 10.2174/1874192401004020089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Onur E, Kabaroglu C, Gunay O, et al. The beneficial effect of physical exercise on anti-oxidant status in asthmatic children. Allergol Immunopathol (Madr) 2011;39:90–5. doi: 10.1016/j.aller.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 103.Mendes FA, Almeida FM, Cukier A, et al. Effects of aerobic training on airway inflammation in asthmatic patients. Med Sci Sports. 2011;43:197–203. doi: 10.1249/MSS.0b013e3181ed0ea3. [DOI] [PubMed] [Google Scholar]
- 104.Mercken EM, Gosker HR, Rutten EP, et al. Systemic and pulmonary oxidative stress after single leg exercise in COPD. Chest. 2009;136:1291–300. doi: 10.1378/chest.08-2767. [DOI] [PubMed] [Google Scholar]
- 105.Watz H, Waschki B, Kirsten A, et al. The metabolic syndrome in patients with chronic bronchitis and COPD: frequency and associated consequences for systemic inflammation and physical inactivity. Chest. 2009;136:1039–46. doi: 10.1378/chest.09-0393. [DOI] [PubMed] [Google Scholar]
- 106.Juvonen R, Bloigu A, Peitso A, et al. Training improves physical fitness and decreases CRP also in asthmatic conscripts. J Asthma. 2008;45:237–42. doi: 10.1080/02770900701883790. [DOI] [PubMed] [Google Scholar]
- 107.Waschki B, Kirsten A, Holz O, et al. hysical activity ist he strongest predictor of all cause mortality in patients with COPD; a prospective cohort study. Chest. 2011;140:331–42. doi: 10.1378/chest.10-2521. [DOI] [PubMed] [Google Scholar]
- 108.Mussi RK, Camargo EA, Ferreira T, et al. Exercise training reduces pulmonary ischemia – reperfusion induced inflammatory responses. Eur Resp J. 2008;31:645–9. doi: 10.1183/09031936.00015607. [DOI] [PubMed] [Google Scholar]
- 109.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86:S836–42. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- 110.Xue F, Michels KB. Diabetes, metabolic syndrome, and breast cancer: a review of the current evidence. Am J Clin Nutr. 2007:S823–35. doi: 10.1093/ajcn/86.3.823S. [DOI] [PubMed] [Google Scholar]
- 111.Neilson HK, Friedenreich CM, Brockton NT, Millikan RC. Physical activity and postmenopausal breast cancer: proposed biologic mechanisms and areas for future research. Cancer Epidemiol Biomarkers Prev. 2009;18:11–27. doi: 10.1158/1055-9965.EPI-08-0756. [DOI] [PubMed] [Google Scholar]
- 112.Winzer BM, Whiteman DC, Reeves MM, Paratz JD. Physical activity and cancer prevention: a systematic review of the clinical trials. Cancer Causes Control. 2011;22:811–26. doi: 10.1007/s10552-011-9761-4. [DOI] [PubMed] [Google Scholar]
- 113.Walsh NP, Gleeson M, Shephard RJ, et al. Position statement. Part 1: Immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- 114.Friedenreich CM. Physical activity and breast cancer: review of the epidemiologic evidence and biologic mechanisms. Recent Results Cancer Res. 2011;188:125–39. doi: 10.1007/978-3-642-10858-7_11. [DOI] [PubMed] [Google Scholar]
- 115.Emaus A, Thune I. Physical acxtivity and lung cancer prevention. Recent Results Cancer Res. 2011;186:101–33. doi: 10.1007/978-3-642-04231-7_5. [DOI] [PubMed] [Google Scholar]
- 116.Hauret KG, Bostick RM, Matthews CE, et al. Physical activity and reduced risk of incident sporadic colorectal adenomas: observational support for mechanisms involving energy balance and inflammation modulation. Am J Epidemiol. 2004;159:983–92. doi: 10.1093/aje/kwh130. [DOI] [PubMed] [Google Scholar]
- 117.Hung AM, Chertow GM, Young BS, Carey S, Johansen KL. Inflammatory markers are unrelated to physical activity, performance and functioning in hemodialysis. Adv Ren Replace. 2003;10:232–40. doi: 10.1053/j.arrt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 118.Afshar R, Shegarfy L, Shavandi N, Sanavi S. Effects of aerobic exercise and resistance training on lipid profiles and inflammation status in patients on maintainance hemodialysis. Indian J Nephrol. 2010;20:185–9. doi: 10.4103/0971-4065.73442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Anand S, Chertow GM, Johansen KL, et al. Association of self-reported physical activity with laboratory markers of nutrition and inflammation: the comprehensive dialysis study. J Ren Nutr. 2011 doi: 10.1053/j.jrn.2010.09.007. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Adamopoulos S, Parissis J, Kroupis C, et al. Physical training reduces peripheral markers of inflammation in patients with chronic heart failure. Eur Heart J. 2001;22:791–7. doi: 10.1053/euhj.2000.2285. [DOI] [PubMed] [Google Scholar]
- 121.Tsarouhas K, Karatzaferi C, Tsitsimpikou C, et al. Effects of walking in heart rate recovery, endothelium modulators and quality of life in patients with heart failure. Eur J Cardiovasc Prev Rehab. 2011 doi: 10.1177/1741826710397099. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 122.Tisi PV, Hulse M, Chulakadabba A, Gosling P, Shearman CP. Exercise training for intermittent claudication: does it adversely affect biochemical markers of the exercise-induced inflammatory response? Eur J Vasc Endovasc Surg. 1997;14:344–50. doi: 10.1016/s1078-5884(97)80283-3. [DOI] [PubMed] [Google Scholar]
- 123.Smith JK, Dykes R, Douglas JE, Krishnaswamy G, Berk S. Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischemic heart disease. JAMA. 1999;281:1722–7. doi: 10.1001/jama.281.18.1722. [DOI] [PubMed] [Google Scholar]
- 124.Mattusch F, Dufaux B, Heine O, Mertens I, Rost R. Reduction of the plasma concentration of C-reactive protein following nine months of endurance training. Int J Sports Med. 2000;21:21–4. doi: 10.1055/s-2000-8852. [DOI] [PubMed] [Google Scholar]
- 125.Lindström J, Louheranta A, Mannelin M, et al. The Finnish Diabetes Prevention Study (DPS). Lifestyle intervention and 3-year results on diet and physical activity. Diab Care. 2003;26:3230–6. doi: 10.2337/diacare.26.12.3230. [DOI] [PubMed] [Google Scholar]
- 126.Balen S, Vukelić-Damijani N, Persić V, et al. Anti-inflammatory effects of exercise training in the early period after myocardial infarction. Coll Antropol. 2008;32:285–91. [PubMed] [Google Scholar]
- 127.Gray SR, Baker G, Wright A, et al. The effect of a 12 week walking intervention on markers of insulin resistance and systemic inflammation. Prev Med. 2009;48:39–44. doi: 10.1016/j.ypmed.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 128.Balducci S, Zanuso S, Nicolucci A, et al. Effect of an intensive exercise intervention strategy on modifiable cardiovascular risk factors in subjects with type 2 diabetes mellitus: a randomized, controlled trial: the Italian Diabetes and Exercise Study (IDES) Arch Intern Med. 2010;170:1794–803. doi: 10.1001/archinternmed.2010.380. [DOI] [PubMed] [Google Scholar]
- 129.Astengo M, Dahl A, Karlsson T, Mattsson- Hulten L, Wiklund O, Wennerblom B. Physical training after percutaneous coronary intervention in patients with stable angina: effects on working capacity, metabolism and markers of inflammation. Eur J Cardiovasc Prev Rehabil. 2010;17:349–54. doi: 10.1097/HJR.0b013e3283336c8d. [DOI] [PubMed] [Google Scholar]
- 130.Beavers KM, Hsu FC, Isom S, et al. Longterm physical activity and inflammatory biomarkers in older adults. Med Sci Sports Exerc. 2010;42:2189–96. doi: 10.1249/MSS.0b013e3181e3ac80. [DOI] [PMC free article] [PubMed] [Google Scholar]