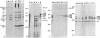Abstract
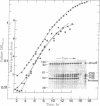
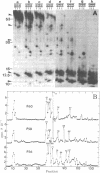
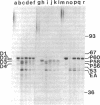
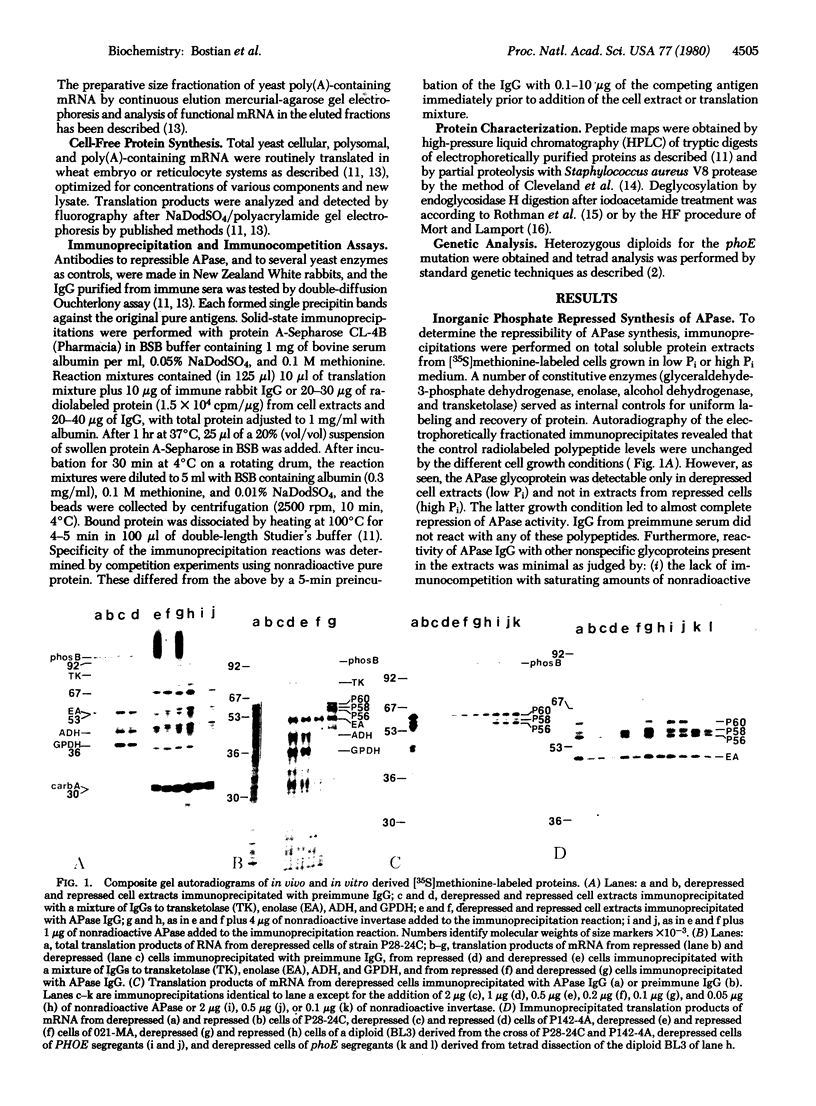
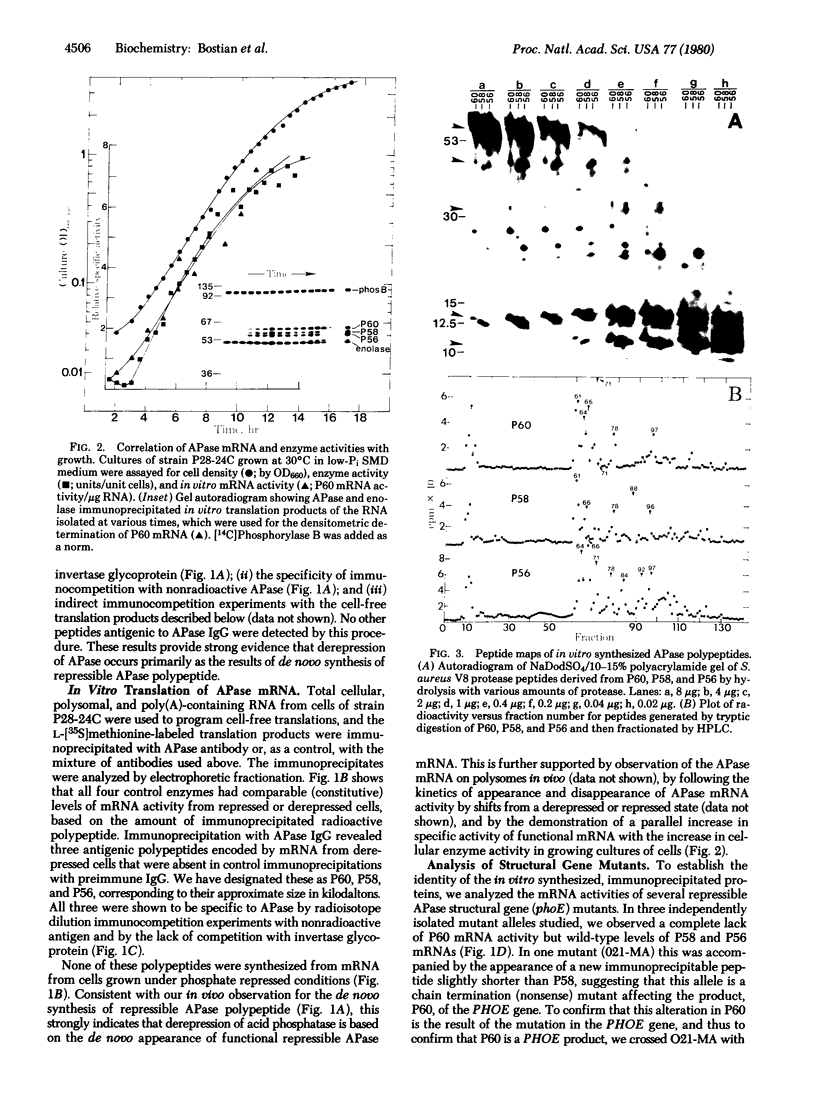
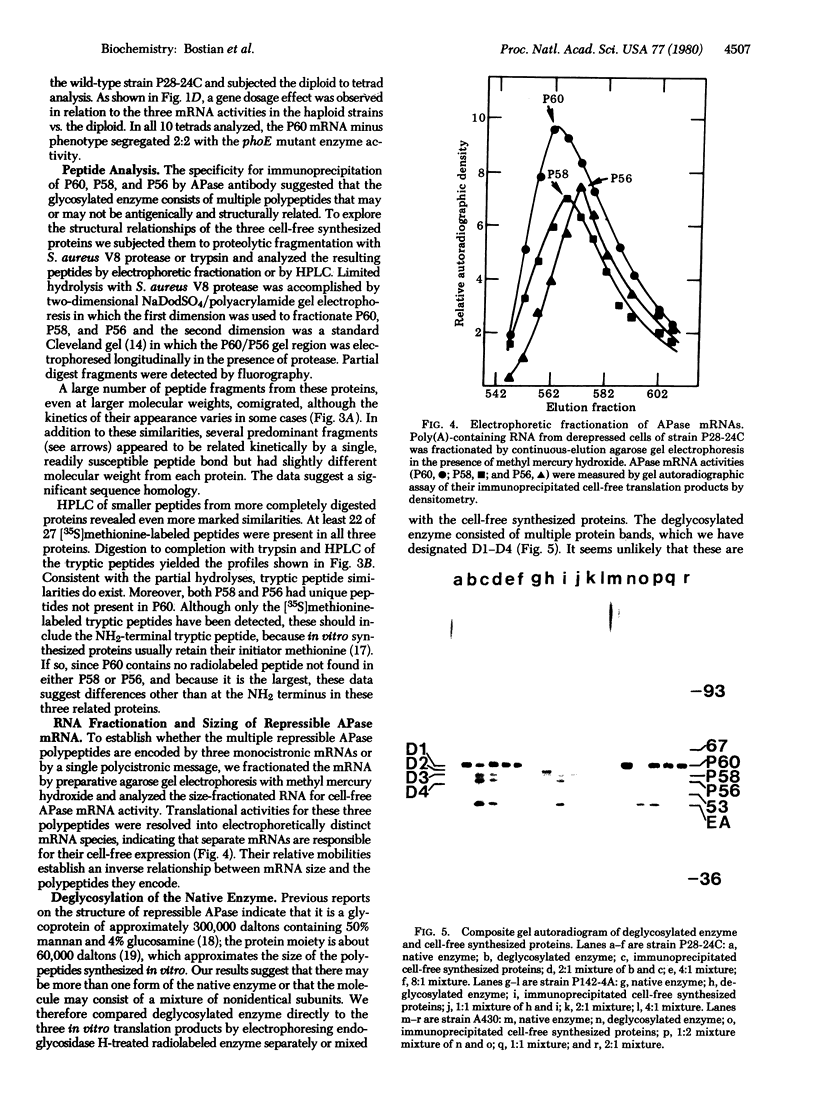
Antibodies to repressible nonspecific acid phosphatase [APase; orthophosphoric-monoester phosphohydrolase (acid optimum), EC 3.1.3.2] purified from Saccharomyces cerevisiae were used to detect the in vitro products of APase mRNA. Immunoprecipitation of cell-free synthesized protein and of in vivo enzyme from cell extracts has shown that derepression of enzyme synthesis in situ is the result of de novo appearance of functional mRNA followed by de novo protein synthesis. At least three unique APase polypeptides are synthesized in vitro from separate mRNAs and appear to be glycosylated in vivo to form secreted enzyme.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boer P., Steyn-Parvé E. P. Isolation and purification of an acid phosphatase from baker's yeast (Saccharomyces cerevisiae). Biochim Biophys Acta. 1966 Nov 15;128(2):400–402. doi: 10.1016/0926-6593(66)90189-5. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Hopper J. E., Rogers D. T., Tipper D. J. Translational analysis of the killer-associated virus-like particle dsRNA genome of S. cerevisiae: M dsRNA encodes toxin. Cell. 1980 Feb;19(2):403–414. doi: 10.1016/0092-8674(80)90514-0. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Lee R. C., Halvorson H. O. Preparative fractionation of nucleic acids by agarose gel electrophoresis. Anal Biochem. 1979 May;95(1):174–182. doi: 10.1016/0003-2697(79)90201-x. [DOI] [PubMed] [Google Scholar]
- Carey N. Unsuspected relatives of the ovalbumin gene. Nature. 1979 May 10;279(5709):101–102. doi: 10.1038/279101a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- GAREN A., ECHOLS H. Genetic control of induction of alkaline phosphatase synthesis in E. coli. Proc Natl Acad Sci U S A. 1962 Aug;48:1398–1402. doi: 10.1073/pnas.48.8.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansche P. E., Beres V., Lange P. Gene duplication in Saccharomyces cerevisiae. Genetics. 1978 Apr;88(4 Pt 1):673–687. [PMC free article] [PubMed] [Google Scholar]
- Lingappa V. R., Devillers-Thiery A., Blobel G. Nascent prehormones are intermediates in the biosynthesis of authentic bovine pituitary growth hormone and prolactin. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2432–2436. doi: 10.1073/pnas.74.6.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnemans W. A., Boer P., Elbers P. F. Localization of acid phosphatase in Saccharomyces cerevisiae: a clue to cell wall formation. J Bacteriol. 1977 Aug;131(2):638–644. doi: 10.1128/jb.131.2.638-644.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlewood B. S., Chia W., Metzenberg R. L. Genetic control of phosphate-metabolizing enzymes in Neurospora crassa: relationships among regulatory mutations. Genetics. 1975 Mar;79(3):419–434. doi: 10.1093/genetics/79.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loppes R. A mutation altering some properties of the neutral phosphatase in Chlamydomonas reinhardi: possible post-translational modification of phosphatase structure. J Bacteriol. 1978 Aug;135(2):551–558. doi: 10.1128/jb.135.2.551-558.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matur A., Berry D. R. The use of step enzymes as markers during meiosis and ascospore formation in Saccharomyces cerevisiae. J Gen Microbiol. 1978 Dec;109(2):205–213. doi: 10.1099/00221287-109-2-205. [DOI] [PubMed] [Google Scholar]
- Mort A. J., Lamport D. T. Anhydrous hydrogen fluoride deglycosylates glycoproteins. Anal Biochem. 1977 Oct;82(2):289–309. doi: 10.1016/0003-2697(77)90165-8. [DOI] [PubMed] [Google Scholar]
- Nakata A., Yamaguchi M., Izutani K., Amemura M. Escherichia coli mutants deficient in the production of alkaline phosphatase isozymes. J Bacteriol. 1978 Apr;134(1):287–294. doi: 10.1128/jb.134.1.287-294.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1858–1862. doi: 10.1073/pnas.76.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Katz F. N., Lodish H. F. Glycosylation of a membrane protein is restricted to the growing polypeptide chain but is not necessary for insertion as a transmembrane protein. Cell. 1978 Dec;15(4):1447–1454. doi: 10.1016/0092-8674(78)90068-5. [DOI] [PubMed] [Google Scholar]
- Schurr A., Yagil E. Regulation and characterization of acid and alkaline phosphatase in yeast. J Gen Microbiol. 1971 Mar;65(3):291–303. doi: 10.1099/00221287-65-3-291. [DOI] [PubMed] [Google Scholar]
- Schweingruber M. E., Schweingruber A. M. Posttranslational regulation of repressible acid phosphatase in yeast. Mol Gen Genet. 1979 Jun 20;173(3):349–351. doi: 10.1007/BF00268647. [DOI] [PubMed] [Google Scholar]
- Seargeant L. E., Stinson R. A. Evidence that three structural genes code for human alkaline phosphatases. Nature. 1979 Sep 13;281(5727):152–154. doi: 10.1038/281152a0. [DOI] [PubMed] [Google Scholar]
- Shimada Y., Shinmyo A., Enatsu T. Purification and properties of one component of acid phosphatase produced by Aspergillus niger. Biochim Biophys Acta. 1977 Feb 9;480(2):417–427. doi: 10.1016/0005-2744(77)90034-1. [DOI] [PubMed] [Google Scholar]
- TONINO G. J., STEYN-PARVE E. P. Localization of some phosphatases in yeast. Biochim Biophys Acta. 1963 Mar 12;67:453–469. doi: 10.1016/0006-3002(63)91851-1. [DOI] [PubMed] [Google Scholar]
- To-E A., Ueda Y., Kakimoto S. I., Oshima Y. Isolation and characterization of acid phosphatase mutants in Saccharomyces cerevisiae. J Bacteriol. 1973 Feb;113(2):727–738. doi: 10.1128/jb.113.2.727-738.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh-E A., Nakamura H., Oshima Y. A gene controlling the synthesis of non specific alkaline phosphatase in Saccharomyces cerevisiae. Biochim Biophys Acta. 1976 Mar 25;428(1):182–192. doi: 10.1016/0304-4165(76)90119-7. [DOI] [PubMed] [Google Scholar]
- Toh-e A., Kakimoto S. Genes coding for the structure of the acid phosphatases in Saccharomyces cerevisiae. Mol Gen Genet. 1975 Dec 30;143(1):65–70. doi: 10.1007/BF00269421. [DOI] [PubMed] [Google Scholar]
- Toh-e A., Kobayashi S., Oshima Y. Disturbance of the machinery for the gene expression by acidic pH in the repressible acid phosphatase system of Saccharomyces cerevisiae. Mol Gen Genet. 1978 Jun 14;162(2):139–149. doi: 10.1007/BF00267870. [DOI] [PubMed] [Google Scholar]
- Van Rijn H. J., Boer P., Steyn-Parvé E. P. Biosynthesis of acid phosphatase of baker's yeast. Factors influencing its production by protoplasts and characterization of the secreted enzyme. Biochim Biophys Acta. 1972 May 12;268(2):431–441. doi: 10.1016/0005-2744(72)90339-7. [DOI] [PubMed] [Google Scholar]