Abstract
A unique sensitivity to specific biochemical processes is responsible for selective vulnerability of midbrain dopamine neurons in several diseases. Prior studies have shown these neurons are susceptible to energy failure and mitochondrial dysfunction, oxidative stress, and impaired disposal of misfolded proteins. These neurons also are especially vulnerable to the loss of purine recycling. In the brains of humans or mice with inherited defects of the purine recycling enzyme hypoxanthine-guanine phosphoribosyltransferase (HPRT), the most prominent defect is loss of basal ganglia dopamine. To investigate the nature of the relationship between HPRT deficiency and dopamine, the mouse MN9D dopaminergic neuronal cell line was used to prepare 10 sublines lacking HPRT. The mutant sublines grew more slowly than the parent line, but without morphological signs of impaired viability. As a group, the mutant sublines had significantly lower dopamine than the parent line. The loss of dopamine in the mutants did not reflect impaired energy status, as judged by ATP levels or vulnerability to inhibitors of energy production. Indeed, the mutant lines as a group appeared energetically more robust than the parent line. The loss of dopamine also was not accompanied by enhanced susceptibility to oxidative stress or proteasome inhibitors. Instead, the loss of dopamine reflected only one aspect of a broad change in the molecular phenotype of the cells affecting mRNAs encoding tyrosine hydroxylase, the dopamine transporter, the vesicular monoamine transporter, monoamine oxidase B, catechol-O-methyltransferase, and GTP-cyclohydrolase. These changes were selective for the dopamine phenotype, since multiple control mRNAs were normal. These studies suggest purine recycling is an intrinsic metabolic process of particular importance to the molecular phenotype of dopaminergic neurons independent of previously established mechanisms involving energy failure, oxidative stress, or proteasome dysfunction.
Dopaminergic pathways projecting from the midbrain to the forebrain play a key role in regulating multiple functions including motor activity, motivation, mood, and cognition. Dysfunction of these pathways is linked with several neurological and psychiatric conditions, including Parkinson disease and schizophrenia. Because of the importance of these dopaminergic pathways in several diseases, a great deal of effort has been devoted to characterizing unique properties that affect their function and survival.
Dopaminergic neurons have an unusual vulnerability to mitochondrial dysfunction (Cookson, 2005; Moore et al., 2005; About-Sleiman et al., 2006), and they show particularly robust expression of genes encoding proteins involved in energy metabolism (Greene et al., 2004). Their high energy requirements may reflect the ability of dopamine to inhibit mitochondrial ATP production (Ben-Shachar et al., 1995; Gluck and Zeevalk, 2004). Dopaminergic neurons also are unusually susceptible to oxidative stress, as a result of generation of reactive oxidant molecules during the metabolism of dopamine together with high endogenous iron stores (Fahn and Cohen, 1992; Jenner, 2003). Finally, dopaminergic neurons are especially sensitive to the toxic effects of misfolded proteins or impaired proteasome function (Rideout et al., 2005; McNaught et al., 2006). These processes are not independent, as abnormalities of one affect the others.
Another important influence affecting dopamine neurons involves purine recycling. This influence was first revealed in studies of Lesch-Nyhan disease, a rare genetic disorder due to deficiency of the purine recycling enzyme, hypoxanthine-guanine phosphoribosyltransferase (HPRT). This enzyme recycles hypoxanthine and guanine into nucleotides (Jinnah and Friedmann, 2000). The most severe and consistent abnormality in the hypoxanthine-guanine phosphoribosyltransferase–deficient (HPRT−) human brain is loss of basal ganglia dopamine (Lloyd et al., 1981; Ernst et al., 1996; Wong et al., 1996; Saito et al., 1999; Visser et al., 2000; Jinnah et al., 2006). Also reduced are dopamine metabolites, the enzymes responsible for dopamine synthesis, and dopamine transporters (DAT). The loss of dopamine also occurs in HPRT− knockout mice (Jinnah et al., 1994, 1999). In both HPRT− humans and mice, these abnormalities are neurochemically selective, with little change in most other neurotransmitters. The abnormalities are also neuroanatomically selective. Midbrain dopaminergic pathways are affected while other dopaminergic pathways are not. These findings imply midbrain dopamine neurons are unusually dependent upon HPRT-mediated purine recycling.
The mechanisms responsible for dopamine loss in HPRT deficiency are unknown. A popular hypothesis to explain the relationship between dopamine and HPRT is that the absence of purine salvage results in failed development of nigrostriatal axonal arborizations or early degeneration of these axons or neurons (Lloyd et al., 1981; Ernst et al., 1996; Wong et al., 1996; Saito et al., 1999; Visser et al., 2000). This hypothesis has been called into question by recent anatomical studies of the HPRT− mouse brain, which revealed no anatomical defects despite significant loss of striatal dopamine (Egami et al., 2007). Alternative intrinsic biochemical mechanisms must be considered. Because ATP is a downstream product of HPRT, HPRT deficiency may result in energy limitation (McKeran, 1977; McCreanor and Harkness, 1995; Jinnah and Friedmann, 2000). HPRT deficiency also causes secondary metabolic changes that may increase oxidative stress (Visser et al., 2002; Smith and Jinnah, 2007). Because proteasomes are ATP-dependent and affected by oxidative stress, HPRT deficiency also may impair proteasomal function. To determine if dopaminergic neurons are susceptible to the loss of purine recycling via these mechanisms, several HPRT− subclones were prepared from the MN9D cell line. This line expresses many characteristics of immature dopamine neurons, including all enzymes responsible for the synthesis and catabolism of dopamine (Choi et al., 1991; Hermanson et al., 2003).
EXPERIMENTAL PROCEDURES
Establishment of MN9D subclones
The MN9D and N18TG2 cell lines were generously provided by Alfred Heller (Chicago, IL, USA) and cultured at 37 °C under an atmosphere of 5% CO2 and 95% air in DMEM (Sigma, St. Louis, MO, USA) supplemented with 15% fetal bovine serum (FBS), 100 U/mL penicillin, and 50 mg/mL streptomycin. The MN9D cell line is a hybrid that was derived through the somatic fusion of primary midbrain dopaminergic neurons from an embryonic day 14 mouse with the mouse neuroblastoma line N18TG2 (Choi et al., 1991). All experiments conformed to international guidelines on the ethical use of animals. All efforts were made to minimize the number of animals used and their suffering.
To establish HPRT− sublines, cells were plated at a density of 2×104 cells/well in 96-well plates in culture medium supplemented with 30 µM 6-thioguanine (6TG) or 130 µM 8-azaguanine (8AG) according to well-established methods (Nelson et al., 1975). The medium containing these agents was replaced every 3 days for 3–4 weeks until resistant colonies emerged. Colonies were isolated and expanded separately in the same culture medium without 6TG or 8AG, and all subsequent studies were performed in the absence of either agent.
HPRT enzyme activity
HPRT enzyme activity was assessed in live cells by adapting methods previously established for other cells (Wood et al., 1973). In brief, each cell line was grown to ~90% confluency in a T75 flask. Cells were dislodged by trypsinization and pelleted by centrifugation at 1000×g for 5 min. The cells then were resuspended at a concentration of 5×106 in 500 µL in six replicates with fresh medium supplemented with 25 µM [14C]-hypoxanthine (40 µCi/mL, Sigma). Cells were incubated for 60 min at 37 °C with continuous rotation to maintain suspension and then pelleted by centrifugation. The supernatant was discarded and the pellet rinsed by resuspension in cold Dulbecco’s phosphate-buffered saline. After the rinse, the pelleted cells were disrupted by addition of 50 µL of cold 0.1 M perchloric acid and frozen at −80 °C. After thawing, insoluble materials were pelleted by centrifugation at 10,000×g for 10 min, and 20 µL of the supernatant spotted onto microplates with diethylaminoethyl anion exchange paper (Millipore Multiscreen, Millipore, Bedford, MA, USA). The sample was allowed to adsorb for 60 min and each well washed once with 300 µL H2O and three times with 50% methanol in H2O by vacuum filtration. The filters were counted using a LS 6500 Multi-Purpose Scintillation Counter (Beckman Coulter, Fullerton, CA, USA). Protein pellets were dissolved in 2% sodium dodecyl sulfate (SDS) and quantified by the Pierce BCA protein assay (Rockford, IL, USA).
Cell growth
Population doubling times were estimated according to established procedures (Freshney, 2000). Briefly, cells were plated in 24-well plates at concentrations of 1×105, 3×105, and 1×104 cells/mL. Cell numbers were determined at regular intervals by direct counting with a hemocytometer after Trypan Blue staining. The numbers of cells were plotted against time in culture, and the population doubling time estimated during the exponential growth phase.
Cell morphology
For morphometric analyses, confluent cultures of the parent MN9D cells and each of the 10 HPRT− sublines were subcultured in separate six-well plates with a 1:10 split. Within 2–4 days while the cultures were still sparse, a technician blinded to HPRT status obtained 10 random phase-contrast digital (TIFF) photomicrographs from each cell line at 20× magnification. The TIFF images were imported into NeuroLucida (MicroBrightfield, Williston, VT, USA) and a technician blinded to cell line digitally traced at least 50 cells in which all elements could be clearly separated from neighboring elements using Wacom Intuos 2 digitizing tablet. The digital images were imported into NeuroExplorer (MicroBrightField) for determination of several morphometric measures including soma cross-sectional area, number of neurites, and lengths of neurites.
Cell viability
Viability after toxin exposure was evaluated by monitoring the conversion of 3(4,5-dimethyltiazol-2-yl)2,5-diphenyltetrazolium bromide (MTT) into a blue formazan dye (Hansen et al., 1989). The parent and each subline were plated in quadruplicate and assessed after timed exposure to varying doses of the toxin. Cells were exposed continuously for 3 days to rotenone, oligomycin, or 2-deoxyglucose. They were exposed for 36 h to epoxomicin, lactacystin, or MG115. They were exposed for 24 h to dopamine. For H2O2 and menadione, viability was assessed 24 h after a transient 30 min exposure. These exposure times and doses were based on prior studies of each toxin in other cell lines (Fenteany et al., 1994; Desagher et al., 1996; Ding and Keller, 2001; Kweon et al., 2004). To estimate viability after toxin exposure, 0.5 mg/mL MTT was added in fresh medium and the cells incubated at 37 °C for 3 h. Cells were lysed by adding 100 µL per well of 20% SDS, 50% N,N-dimethylformamide at pH 4.7. Samples were mixed gently on an orbital platform for 24 h prior to reading at 570 nm with a spectrophotometer.
Purines
Each cell line was plated separately in quadruplicate on six-well plates and allowed to grow to confluency. One day after reaching confluency, the medium was replaced with 1.5 mL DMEM containing 15% dialyzed FBS. After 24 h, 0.9 mL of conditioned medium was removed and mixed with 0.1 mL of 1 M perchloric acid. The cells were collected by trypsinization, centrifuged at 1000×g for 5 min at 4 °C, the supernatant decanted, 0.5 mL of 0.1 M perchloric acid was added to the pellet and the sample mixed. Samples were stored frozen at −80 °C prior to biochemical analyses.
Cells and conditioned medium were thawed rapidly and centrifuged at 10,000×g for 10 min at 4 °C to sediment acid-precipitated material. Samples were then pH neutralized using 2.5% of 3.5 M K2CO3 and the potassium perchlorate precipitate removed by a second centrifugation step. The final supernatant was filtered through 0.45 µm PVDF microcentrifuge spin filters (Alltech, Deerfield, IL, USA) prior to HPLC analysis. Purines were determined by reverse-phase ion-pair HPLC with an Atlantis dC18 column (Waters, Milford, MA, USA) as previously described (Shirley et al., 2007). Elution was conducted at 1 mL/min with a stepped gradient of buffer A (10 mM ammonium acetate and 2 mM tetrabutylammonium phosphate, pH 5.0) and buffer B (10 mM ammonium phosphate, 2 mM tetrabutylammonium phosphate, 25% acetonitrile, pH 7.0). The gradient consisted of the following sequence; 100% buffer A for 10 min, a linear gradient to 75% buffer B over 15 min, 10 min at 75% buffer B, a linear gradient to 100% buffer B over 5 min, and 100% buffer B for 10 min. The column was then re-equilibrated with 100% buffer A for 15 min prior to the next run. Adenine nucleotides (ATP, ADP, AMP) were identified by comparing their retention times and spectral profiles to known standards with a photodiode array detector, and they were quantified at 254 nm. The adenylate energy charge (AEC) was calculated as an estimate of energy status as previously described (Atkinson, 1968):
Monoamines
Cells and conditioned medium were prepared as described for purines, except that they were grown on 100 mm culture plates. Monoamines were determined by reverse-phase HPLC using a C18 reverse-phase MD-150 column (ESA Inc., Chelmsford, MA, USA) as previously described (Jinnah et al., 1999). Elution was conducted at 0.6 mL/min with a mobile phase consisting of 1.7 mM 1-octanesulfonic acid sodium, 25 mM EDTA, 0.01% triethylamine, and 8% acetonitrile in 75 mM sodium phosphate buffer pH 2.9. Monoamines were identified by comparing their retention times to known standards with an array of electrodes set at 150, 250, 450, and 550 mV.
Quantitative PCR (qPCR)
Total RNA was extracted from a pellet containing approximately 2×107 cells and treated with DNase using Qiagen’s RNeasy® Midi Kit (Valencia, CA, USA). The amount of RNA and the quality of each sample was verified using an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Palo Alto, CA, USA). For reverse transcription of mRNA, 1 µL of RNA at a concentration of 1 µg/µL was incubated overnight at 37 °C with 0.5 µL of Reverse-iT RTase Blend from ABgene (50 U/µL), 4 µL of 5× First Strand Synthesis Buffer, 1 µL of DTT 0.1 M, 1 µL of dNTPs 10 mM, 2 µL of random hexamer oligonucleotides 50 µM (GE Healthcare Biosciences, Uppsala) and 10.5 µL of RNase-free water to a final volume of 20 µL.
qPCR was performed using a LightCycler and the FastStart DNA MasterPLUS SYBR Green I kit (Roche Applied Science, Mannheim, Germany). One microliter of each cDNA was incubated with 0.1 µL of each primer at 0.5 µM designed via Primerbank (pga.mgh.harvard.edu/primerbank), and with 2 µL of 5× FastStart DNA Master SYBR Green containing the FastStart TaqDNA polymerase, MgCl2, the dNTP mix, and the fluorescent dye SYBR Green I. RNase-free water was added to reach the final volume of 10 µL. The pre-incubation and denaturation of the samples were performed at 95 °C for 10 min. This was followed by amplification of the target cDNA through 45 cycles at 95 °C for 10 s, an annealing temperature adapted according to the Tm of primers (Table 1) for 7 s, and elongation at 72 °C for 12 s. Finally, samples were cooled to 35 °C. Each reaction was performed in quadruplicate for each clone.
Table 1.
Primers and conditions for PCR
| ID | Gene | Product | Primer | Sequence | Primer length | Tm (°C) | Amplicon length |
|---|---|---|---|---|---|---|---|
| NM_007744 | Comt | COMT | Fwd | TGACGCAAAAGGCCAAATCAT | 21 | 68.4 | 101 |
| Rev | CCATTCGCACGGCTGAGTAG | 20 | 67.9 | ||||
| NM_016672 | Ddc | DDC | Fwd | TAGCTGACTATCTGGATGGCAT | 22 | 62.5 | 116 |
| Rev | GTCCTCGTATGTTTCTGGCTC | 21 | 62.5 | ||||
| NM_008102 | Gch1 | GCH1 | Fwd | TGTGCATGGTAATGCGAGG | 19 | 65.8 | 120 |
| Rev | AGCTCCTGATTAGTGTGAGGAA | 22 | 61.7 | ||||
| NM_013556 | Hprt | HPRT | Fwd | GTTAAGCAGTACAGCCCCAAA | 21 | 62.8 | 131 |
| Rev | AGGGCATATCCAACAACAAACTT | 23 | 64.3 | ||||
| NM_025730 | Lrrk2 | Leucine-rich receptor kinase 2 | Fwd | CAAGACCTTGGTATCTCCACTCA | 23 | 64.1 | 71 |
| Rev | AAAAAGCAGCACATTGTGGGG | 21 | 67.9 | ||||
| NM_173740 | MaoA | MAO A | Fwd | TGTTGGTGGAGCTTATGTGGG | 21 | 66.9 | 101 |
| Rev | CTAGACGCTCATTGACATTCACT | 23 | 62.1 | ||||
| NM_172778 | MaoB | MAO B | Fwd | ATATGTGGACCTTGGAGGATCTT | 23 | 63.5 | 110 |
| Rev | TCAGCCGCTCAACTTCATTAAC | 22 | 65.2 | ||||
| NM_010860 | Myl6 nm | Myosin light polypeptide 6 | Fwd | CCAGTGTGGGGATGTGATGC | 20 | 68.9 | 226 |
| Rev | ATGACGGATTTCAGCACCCAT | 21 | 67.5 | ||||
| NM_008172 | Nos1 | Neuronal nitric oxide synthase | Fwd | AATGATCGGCCCCTGGTAGA | 20 | 67.6 | 170 |
| Rev | TGGGTCACACGGATGGTCT | 19 | 66.7 | ||||
| NM_016694 | Park2 | Parkin | Fwd | TCTTCCAGTGTAACCACCGTC | 21 | 63.9 | 115 |
| Rev | GGCAGGGAGTAGCCAAGTT | 19 | 63.1 | ||||
| NM_010020 | Slc6a3 | DAT | Fwd | CCTGCTCTCAGTCATCGGC | 19 | 66.3 | 127 |
| Rev | CATCCCGGCAATAACCATGAA | 21 | 68.5 | ||||
| NM_172523 | Slc18a2 | VMAT2 | Fwd | TCACCAACCCATTCATAGGACT | 22 | 64.2 | 163 |
| Rev | CAAGAGGAGCCGATTCCCTG | 20 | 68.1 | ||||
| NM_009221 | Snca | α-Synuclein | Fwd | GCAAGGGTGAGGAGGGGTA | 19 | 66.3 | 90 |
| Rev | CCTCTGAAGGCATTTCATAAGCC | 23 | 66.6 | ||||
| NM_013671 | Sod2 | Mn-superoxide dismutase 2 | Fwd | ACAAACCTGAGCCCTAAGGGT | 21 | 64.7 | 128 |
| Rev | GAACCTTGGACTCCCACAGAC | 21 | 64.8 | ||||
| NM_009377 | Th | TH | Fwd | CTGTCTCGGGCTTTGAAAGTG | 21 | 65.9 | 163 |
| Rev | GACGCACAGAACTGAGGAGG | 20 | 65.1 |
Expression of the Myl6 nm gene, where results showed no significant difference between normal and HPRT− cells, was used to normalize raw data. The qPCR for the target gene and Myl6 nm was performed using the same PCR conditions in each run. Each qPCR assay was designed and verified to amplify a single amplicon of the correct size with uniform PCR efficiency and without co-amplification of non-specific products. The specificity of each amplicon was tested via a melting curve analysis for each run showing a single peak for each amplicon. For all amplicons melting temperatures were always above 80 °C, minimizing the occurrence of primer dimers with a melting temperature at 55–60 °C. Quantification was achieved via the crossing point method. In order to detect differences in PCR efficiency, standard curves for each cDNA were constructed in quadruplicate employing reverse transcribed control RNA from parental cells as calibrator.
Data analysis
Two different methods that addressed different questions were used to compare the parent MN9D line and the 10 HPRT− sublines. In experiments involving only one independent variable, a traditional one-way analysis of variance (ANOVA) was used with post hoc Tukey t-tests where appropriate. This method was useful for detecting variability among all the cell lines, but was not appropriate for determining if the control line differed significantly from the HPRT− sublines as a group. To evaluate group differences, a single sample t-test was employed, comparing the parent line against all of the HPRT− sublines combined (Egami et al., 2007). In experiments involving two independent variables, two-way ANOVA was used.
RESULTS
Mutation frequency
The frequency at which 6TG or 8AG subclones spontaneously arose in the parental MN9D line was determined by comparing the number of sublines isolated to the total number of cells plated in at least three separate experiments. Mutant frequencies per million cells averaged 4.5±1.4 for 6TG and 2.7±0.5 for 8AG. These values are in the same range as those reported in prior studies with other cell lines (Graf et al., 1976; Colgin et al., 2002). The first five sublines isolated after 6TG or 8AG exposure were propagated for further studies.
HPRT enzyme activity
HPRT enzyme activity was determined by monitoring the incorporation of [14C]-hypoxanthine into IMP and its nucleotide derivatives in live cell cultures (Table 2). In accordance with prior studies, the parent MN9D line had readily detectable enzyme activity while the N18TG2 line had none (Choi et al., 1991). All of the HPRT− sublines exhibited enzyme activities that were at or below detectable limits, consistent with prior studies showing a near-complete loss of enzyme function in cells isolated by their resistance to 6TG or 8AG (Fujimoto et al., 1971).
Table 2.
HPRT enzyme activity
| Cell line | Enzyme activity | Percent of control |
|---|---|---|
| MN9D parent | 565.0±43.0 | 100±0.0 |
| N18TG2 | 2.9±1.5 | 0.5±0.2 |
| 6TG-resistant clones | ||
| TG1 | 2.0±0.5 | 0.4±0.1 |
| TG2 | 3.6±0.3 | 0.6±0.1 |
| TG3 | 3.6±0.3 | 0.6±0.1 |
| TG4 | 3.8±0.9 | 0.7±0.2 |
| TG5 | 2.1±0.1 | 0.4±0.1 |
| 8AG-resistant clones | ||
| AG2 | 4.3±1.2 | 0.8±0.2 |
| AG5 | 2.3±1.2 | 0.4±0.2 |
| AG6 | 0.8±0.8 | 0.1±0.1 |
| AG8 | 4.5±1.3 | 0.8±0.2 |
| AG10 | 0.9±0.5 | 0.2±0.1 |
Results of enzyme activity are expressed as average (±S.E.M.) pmol/mg protein per h from four determinations in each line. The limit of detection, defined as 2 S.D. above background, was approximately 4 pmol/mg protein/h, less than 0.5% of the value in the parent MN9D line. ANOVA revealed significant differences among the lines (F11,24=190.0, P<0.001). Post hoc Tukey t-tests confirmed the MN9D parent line to be significantly different from all others (P<0.001).
Cellular growth and morphology
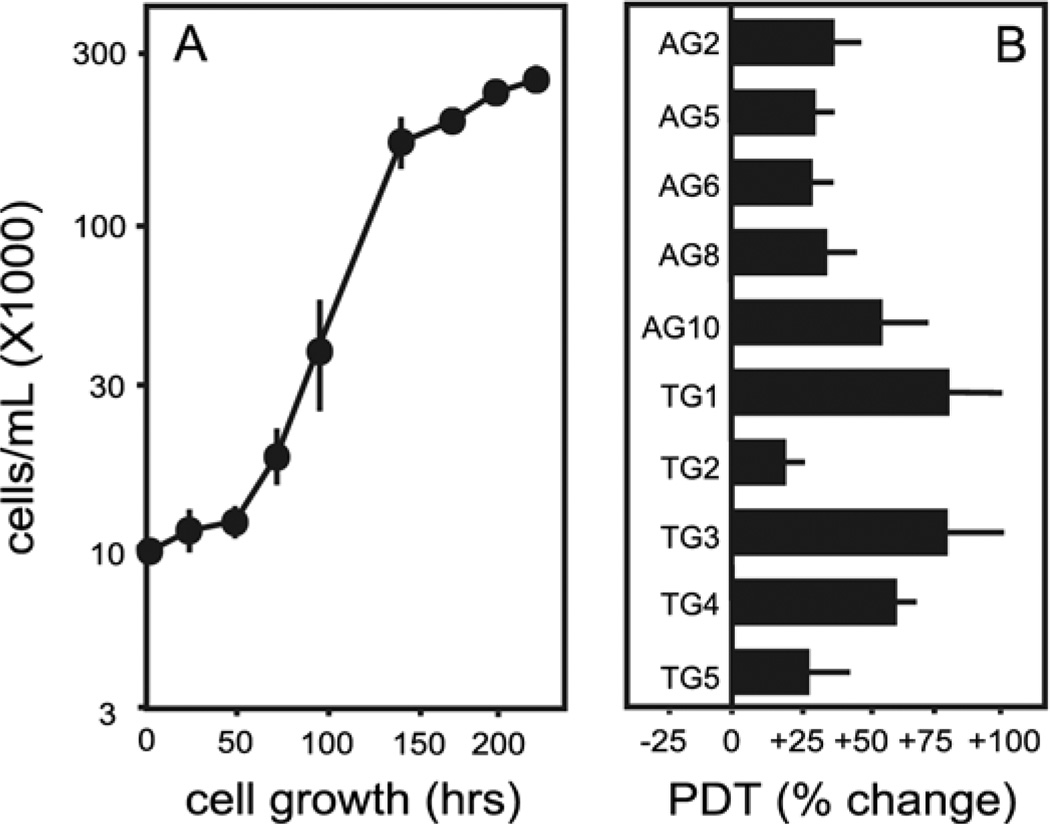
The growth rates for the HPRT− sublines were compared with the parent line by calculating the population doubling times during the exponential growth phase of individual cultures plated at three different densities. The average population doubling time for the parent MN9D cells was 19.7±0.8 h, in accordance with prior estimates (Choi et al., 1991). There was significant variation among the population doubling times for the HPRT− sublines (Fig. 1). Despite this variation, two-way ANOVA revealed a significant group effect for the HPRT− sublines. The population doubling time averaged 23.8±2.6 h for all of the HPRT− sublines as a group, suggesting slower growth than the HPRT+ parent line.
Fig. 1.
Cellular growth rates. (A) Sample growth curve data of the parent MN9D line plated at 104 cells/mL. (B) Population doubling times at each of the three plating densities were averaged for each line and expressed as a percent change from the control line, where the average population doubling time was 19.7±0.8 h. A two-way ANOVA of average population doubling times with HPRT status and plating density as main factors revealed a significant effect for HPRT (F1,27=7.1, P<0.02). There was no significant effect for plating density (F2,27=0.2, P=0.81) and no significant interaction between HPRT status and plating density (F2,27=0.4, P=0.69).
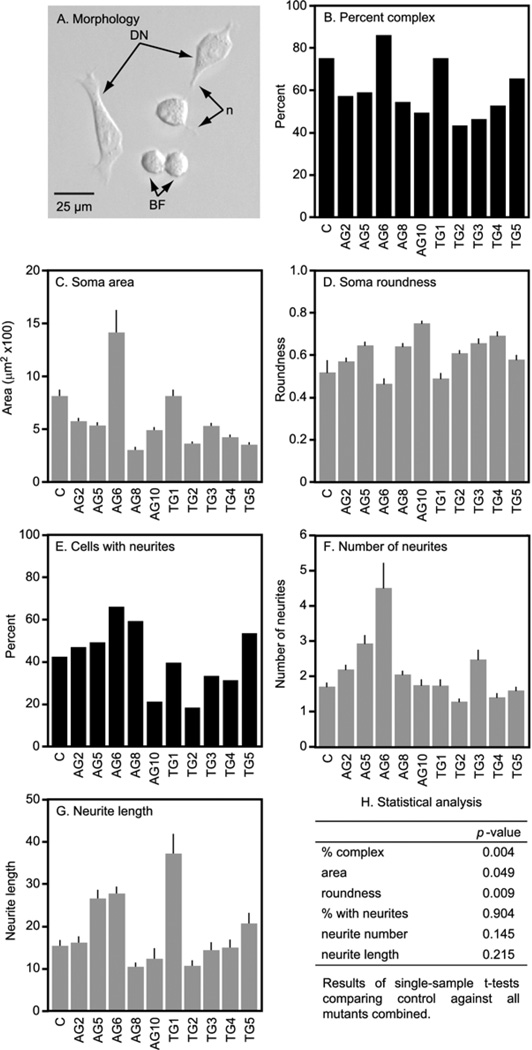
The parent MN9D line had cells with heterogeneous morphologies as previously reported (Choi et al., 1991). Some cells were small round blast-like forms (Fig. 2A). Others were larger and flatter, with thin processes suggesting a partially differentiated form. The HPRT− sublines appeared healthy with no signs of toxicity such as a high frequency of non-viable cells or debris, or morphological signs of necrosis or apoptosis. Each displayed several morphological differences from the parent line, a phenomenon often found when subcloning established cell lines (Biedler et al., 1978; Ciccarone et al., 1989). Some of the lines had a high proportion of complex cells with features suggestive of a more differentiated state such as relatively large and polygonal soma with greater numbers of longer neurites. Others exhibited the opposite trend with a greater proportion of simpler small rounded blast-like forms with fewer and shorter neurites.
Fig. 2.
Morphology of MN9D cells and their HPRT− sublines. A representative photomicrograph of the MN9D parent line (A) shows a mixed population of large complex forms with one or more neurites (n) suggesting a more differentiated neuron (DN) along with simpler rounded cells with few neurites suggestive of blast forms (BF). There were slight but significant decreases in the percentage of complex to simple cells (B) and soma cross-sectional area (C) with a small increase in roundness (D). There were no significant changes in the proportion of cells bearing neurites, the number of neurites per cell, or average neurite lengths. Results reflect averages±S.E.M. (gray bars, n>60 for each line) or population proportions without S.E.M. (black bars). A single sample t-test was used to identify significant differences between the parent (control=c) line and the HPRT− sublines as a group (H).
Quantitative morphometric comparisons revealed few consistent abnormalities in the HPRT− sublines as a group (Fig. 2B–H). There was a decrease in the proportion of complex to simple cell profiles among the HPRT− sublines. This change was paralleled by findings of decreased soma areas and increased roundness. These findings raised the possibility that the HPRT− sublines might comprise a less differentiated population. However, this explanation was not supported by normal results for the proportion of cells with neurites, the numbers of neurites per cell, and average neurite lengths (Fig. 2B–H). These results demonstrate relative minor and probably random variation in the morphological phenotype of the HPRT− MN9D sublines.
Monoamines
Consistent with prior studies (Choi et al., 1991; Hermanson et al., 2003; Kweon et al., 2004), the parent MN9D cells contained dopamine, with only small amounts being released into the tissue culture medium (Table 3). The cells also made the dopamine metabolites dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA). Although not previously described, the MN9D cells released large amounts of DOPA and 3-methoxytyramine (3MT) into the tissue culture medium. This metabolite profile implies the MN9D cells express tyrosine hydroxylase (TH), dopa-decarboxylase (DDC), monoamine oxidase (MAO), and catechol-O-methyltransferase (COMT).
Table 3.
Monoamines
| Sample | Intracellular | Extracellular | ||||||
|---|---|---|---|---|---|---|---|---|
| DA | DOPAC | HVA | 3MT | DA | DOPAC | HVA | 3MT | |
| AG2 | 32.6±1.5 | 11.6±0.6 | 10.0±0.8 | 2.6±0.17 | 27.3±1.6 | 7.7±0.8 | 34.2±3.5 | 17.7±8.4 |
| AG5 | 14.0±0.6 | 0.8±0.0 | ND | 2.4±0.1 | 6.7±0.4 | 1.4±0.3 | 2.8±0.1 | 77.9±3.3 |
| AG6 | 64.8±3.4 | 4.1±3.3 | 3.3±0.4 | 3.2±0.6 | 1.1±0.1 | 4.3±0.2 | 14.0±0.6 | 59.6±2.3 |
| AG8 | 79.0±2.0 | 6.8±0.6 | 6.1±2.8 | 2.8±0.4 | 2.2±0.1 | 8.0±0.4 | 31.6±2.2 | 94.6±8.4 |
| AG10 | 22.2±0.9 | 2.7±0.1 | 3.1±0.2 | 3.1±0.2 | 1.4±0.1 | 6.9±0.3 | 21.6±0.7 | 42.4±0.7 |
| TG1 | 10.3±0.4 | 0.6±0.0 | 0.9±0.1 | 1.5±0.1 | 2.8±0.2 | 0.5±0.0 | 4.2±0.3 | 43.9±2.8 |
| TG2 | 20.8±1.0 | 0.3±0.0 | ND | ND | 1.5±0.3 | 0.3±0.1 | 3.1±0.1 | 17.6±0.7 |
| TG3 | 3.4±0.2 | ND | ND | 3.7±0.3 | 1.8±0.1 | ND | 1.4±0.1 | 78.0±4.4 |
| TG4 | 35.5±2.7 | ND | ND | 3.6±0.4 | 2.5±0.1 | ND | 1.8±0.1 | 87.4±4.8 |
| TG5 | 19.6±1.5 | 0.6±0.0 | 0.9±0.1 | 1.7±0.1 | 4.4±0.4 | 0.7±0.1 | 3.6±0.5 | 35.7±3.6 |
| AVG HPRT− | 30.2±3.7 | 2.8±0.6 | 2.4±0.5 | 2.5±0.2 | 5.2±1.2 | 3.0±0.5 | 11.8±2.0 | 65.3±4.8 |
| AVG Control | 66.4±2.1 | 4.2±0.5 | 3.4±1.1 | 2.6±0.2 | 5.0±0.3 | 8.4±1.6 | 31.4±6.2 | 99.4±6.7 |
| Change | −54.4% | −34.9% | −29.4% | −5.6% | +3.3 | −64.4 | −62.4 | −34.3 |
Monoamines are expressed as average ng/mg protein ±S.E.M. from a total of at least four independent measurements. Each HPRT− subline is shown separately, along with an overall average of all HPRT− sublines combined. To identify significant differences between the control and HPRT− sublines as a group, the results were analyzed by two-way ANOVA, with HPRT status and cell compartment (intracellular vs. extracellular) as the main factors. There were significant effects of HPRT status for dopamine (F=43.5, P<0.0001), DOPAC (F=18.3, P<0.0001), HVA (F=14.6, P<0.0002), and 3MT (F=17.9, P<0.0001). The effect of cellular compartment was significant for dopamine (F=254.2, P<0.0001), HVA (F=44.0, P<0.0001), and 3MT (F=39.0, P<0.0001). The effect of compartment was borderline for DOPAC (F=4.3, P=0.04).
ND=not detectable.
The HPRT− MN9D sublines exhibited neurochemical profiles with significant variability, similar to prior reports for subcloning PC12 cells (Koike and Takashima, 1984; Bitler and Howard, 1986; Clementi et al., 1992). Despite this variation, the HPRT− sublines displayed a significant reduction of intracellular dopamine stores when compared as a group against the parent line (Table 3). The degree of dopamine loss was similar whether the values were normalized to total cellular protein content or to total number of cells in culture. There were also significant reductions in the metabolites DOPAC, HVA, and 3MT.
The ratio of dopamine to its metabolites can provide insight into changes in its metabolism, and the ratio of intracellular to extracellular dopamine can provide insight into relative release (Bitler and Howard, 1986). To explore changes in metabolism or release, the raw data for the entire monoamine profile for each subline were entered into a single database and subjected to several secondary analyses. The loss of dopamine in the HPRT− sublines correlated with a loss of its metabolites (DOPAC, HVA, 3MT). There were no consistent changes in the ratios of dopamine to its various metabolites, or in the ratio of intracellular versus extracellular dopamine (not shown). These results provide indirect evidence that the altered dopamine content of the HPRT− sublines cannot be attributed solely to changes in dopamine catabolism or release.
Correlation between dopamine and cellular growth or morphology
The observation that the HPRT− sublines grew slower than the parent line (Fig. 1) raised the possibility that the low dopamine might be an indirect consequence of lower cell densities at the time of harvest. The morphometric studies raised the additional possibility that low dopamine might result from smaller soma sizes. Neither explanation is adequate to account for the dopamine loss because all cultures were harvested 24 h after reaching confluency, and results were normalized to cellular protein content.
To confirm that the cultures were equivalent at the time of harvest, the total numbers of cells and total cellular protein contents were examined. The results showed no significant differences for cell protein content or cell numbers when comparing the parent line with all of HPRT− sublines. In addition, there was no significant correlation between dopamine and growth rate, total numbers of cells at harvest, or total cell protein at harvest (not shown). These results indicate that changes in morphology or growth rates cannot explain the loss of dopamine.
Energy failure
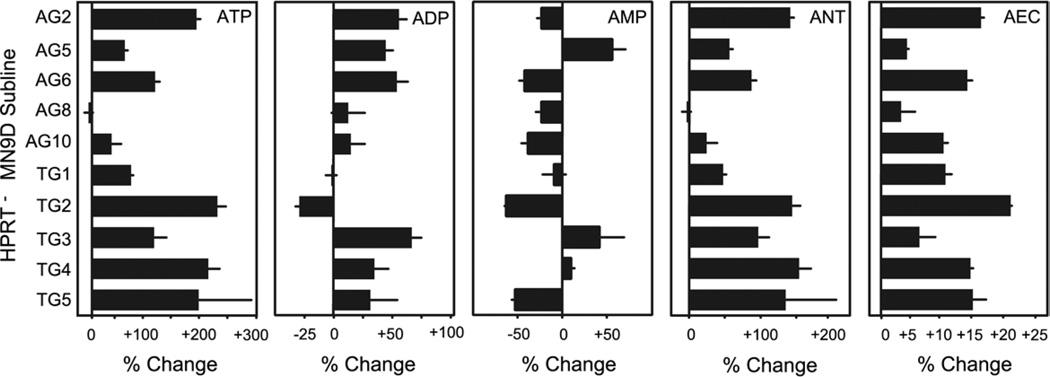
Prior studies have shown dopaminergic neurons to be especially vulnerable to ATP loss due to energy failure, and HPRT-mediated purine recycling is postulated to play a role in maintaining cellular purines, including ATP (McKeran, 1977; McCreanor and Harkness, 1995; Jinnah and Friedmann, 2000). To determine if the loss of HPRT might be associated with reduced ATP, adenine nucleotide levels were measured. The AEC was calculated as a measure of energy status (Atkinson, 1968). ATP and AEC varied among the HPRT− subclones, with no evidence for consistent reduction of either in comparison with the parent line (Fig. 3). Instead, six of 10 HPRT− sublines had two- to threefold increases in ATP and the single sample t-test revealed a significant increase for both ATP and AEC for the mutant sublines as a group.
Fig. 3.
Adenine nucleotide levels in the HPRT− MN9D sublines. Each variable was expressed as a percent change relative to a simultaneously evaluated control line. Results show averages±S.E.M. (n=3). A value of zero means no change. A value of +100 means 100% above the control, or twofold the control. A value of −50 means 50% lower than control or ½ control values. Abbreviations: summary of all adenine nucleotides (ANT). AEC defined as ([ATP]+0.5×[ADP])/([ATP]+[ADP]+[AMP]) was used as an estimate for energy status. The significance of the difference relative to controls was determined for each measure via single sample t-test: ATP (P<0.003), ADP (P<0.03), AMP (P=0.3), ANT (P<0.007), AEC (P<0.0003). A Bonferroni correction for five measures was required to define P<0.01 as a conservative estimate of statistical significance. A result of 0.01<P<0.05 was considered borderline.
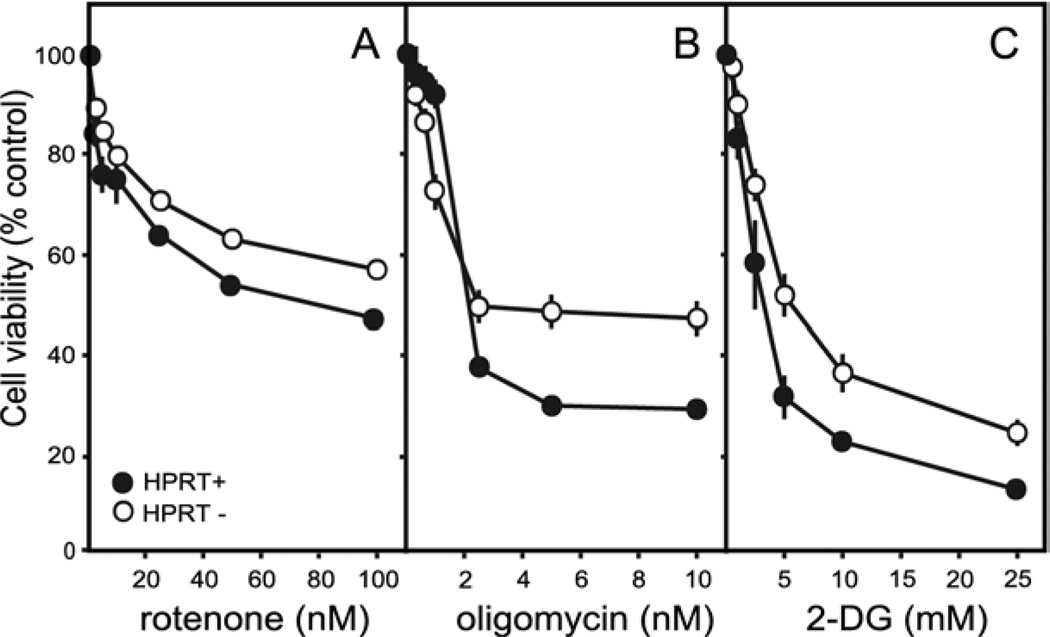
To determine the relative susceptibility of the HPRT− sublines to limitation of ATP production, dose-toxicity profiles were established for the parent line and each HPRT− subline after inhibition of ATP production from mitochondria (rotenone or oligomycin) or glycolysis (2-deoxyglucose). There was no evidence for an increase in sensitivity of HPRT− sublines to any of these toxins (Fig. 4). Instead, the HPRT− sublines as a group appeared less sensitive than the control line to each of the toxins, especially at the highest toxin doses. These results are consistent with the finding of significantly increased ATP levels in these sublines at baseline (Fig. 3). They do not support the hypothesis that the loss of dopamine in the HPRT− MN9D sublines results from an impaired energy status.
Fig. 4.
Dose-toxicity profiles for inhibitors of energy production. Viability was determined for the MN9D line and each of the 10 HPRT− sublines. The results for the control line show averages±S.E.M. for at least six measures (closed symbols). The results for all 10 HPRT− sublines were combined to yield a single average (open symbols). The parent MN9D line was compared against the 10 HPRT− sublines via two-way ANOVA with toxin dose and HPRT as main factors. There was a highly significant effect for dose for each toxin (P<0.0001). The effect of HPRT status varied according to toxin: rotenone (F1,252=24.7, P<0.001), oligomycin (F1,252=1.5, P=0.22), 2-deoxyglucose (2-DG, F1,252=23.6, P<0.001). The interaction between toxin dose and HPRT status also varied according to toxin: rotenone (F5,252=0.4, P=0.9), oligomycin (F5,252=8.0, P<0.001), 2-DG (F5,252=1.6, P=0.2).
Oxidative stress
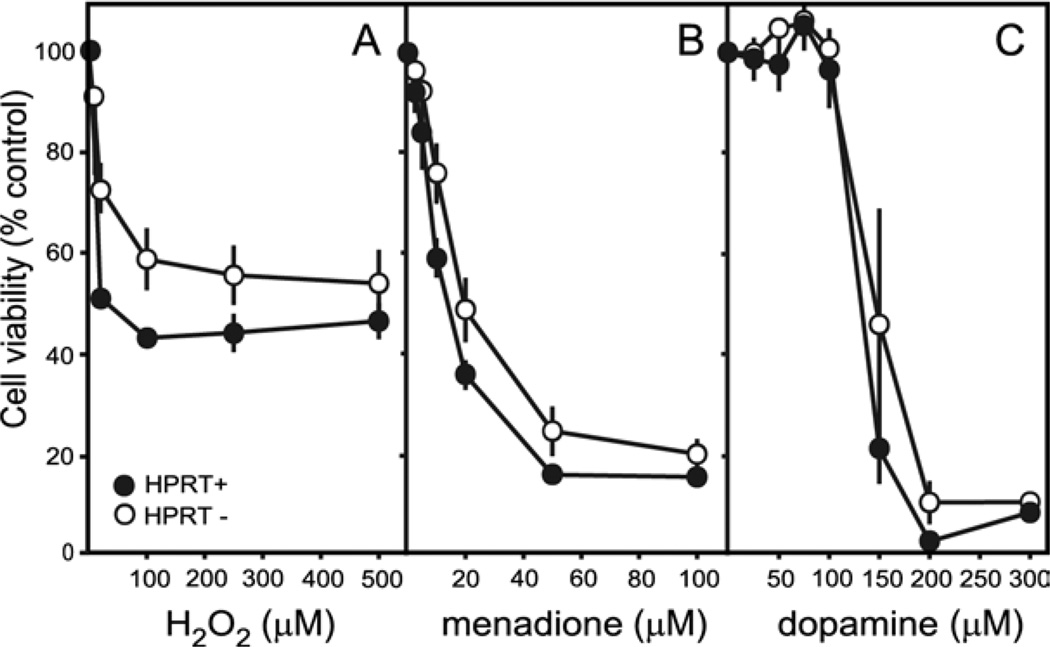
Prior studies have shown dopaminergic neurons to exhibit enhanced vulnerability to oxidative stress, and the loss of HPRT has been postulated to increase the production of oxidant molecules (Visser et al., 2002; Smith and Jinnah, 2007). To determine if the HPRT− sublines might be associated with enhanced vulnerability to oxidative stress, dose-toxicity profiles were established for H2O2, menadione, and exogenously added dopamine. There was no evidence for increased sensitivity among the HPRT− sublines to any of these agents (Fig. 5). Instead, compared with the control line, the HPRT− sublines appeared significantly less vulnerable to H2O2 and menadione, and they exhibited a non-significant trend for reduced vulnerability to dopamine. These results do not support the hypothesis that the loss of dopamine in the HPRT− MN9D sublines derives from a negative impact on the redox potential of the cells.
Fig. 5.
Dose-toxicity profiles for oxidative stressors. Viability was determined for the MN9D line and each of the 10 HPRT− sublines. The results for the control line show averages±S.E.M. for at least six measures (closed symbols). The results for all 10 HPRT− sublines were combined to yield a single average (open symbols). The parent MN9D line was compared against the 10 HPRT− sublines via two-way ANOVA with toxin dose and HPRT as main factors. There was a highly significant effect for dose for each toxin (P<0.0001). The effect of HPRT status varied according to toxin: H2O2 (F1,252=8.3, P<0.005), menadione (F1,252=1.5, P=0.22), and dopamine (F1,98=3.2, P=0.07). The interaction between toxin dose and HPRT status also varied according to toxin: H2O2 (F5,252=0.4, P=0.8), menadione (F5,252=8.0, P<0.001), and dopamine (F6,98=1.4, P=0.2).
Proteasome dysfunction
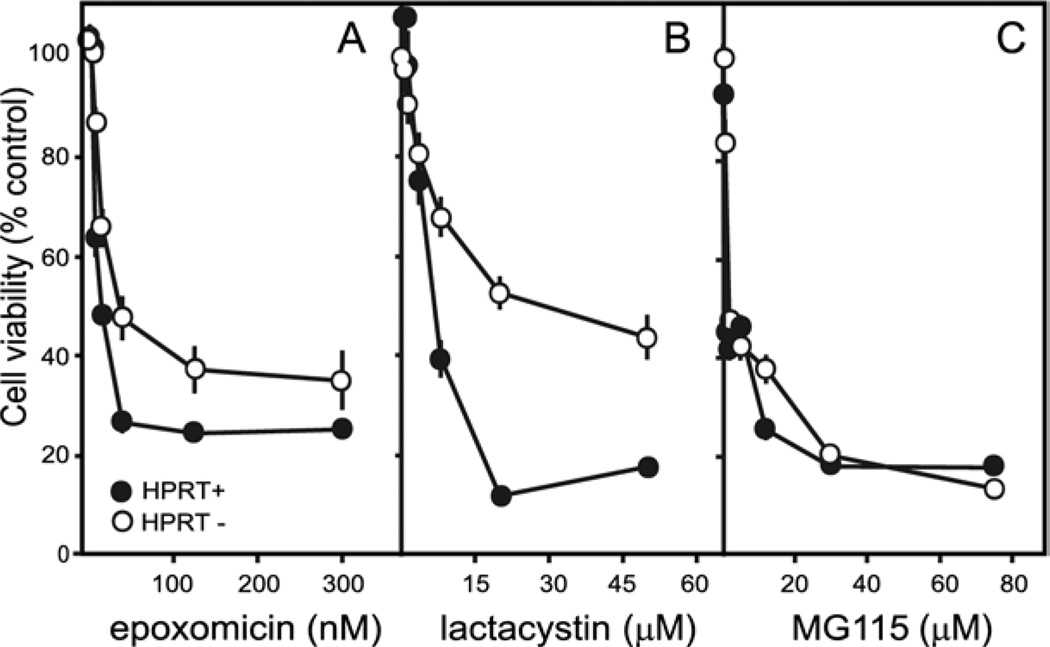
Prior studies have shown dopaminergic neurons to be sensitive to accumulation of misfolded proteins or impairment of proteasome function (Rideout et al., 2005; McNaught et al., 2006). To determine if the HPRT− sublines might be associated with enhanced vulnerability to proteasome dysfunction, dose-toxicity profiles were established for epoxomicin, lactacystin, and MG115. There was no evidence for increased sensitivity of the HPRT− sublines to any of these drugs (Fig. 6). For two of these drugs, the HPRT− sublines appeared less sensitive, particularly at high doses. These results argue against impaired proteasome function in the HPRT− sublines.
Fig. 6.
Dose-toxicity profiles for proteasome inhibitors. Viability was determined for the MN9D line and each of the 10 HPRT− sublines. The results for the control line show averages±S.E.M. for at least six measures (closed symbols). The results for all 10 HPRT− sublines were combined to yield a single average (open symbols). The parent MN9D line was compared against the 10 HPRT− sublines via two-way ANOVA with toxin dose and HPRT as main factors. There was a highly significant effect for dose for each toxin (P<0.0001). The effect of HPRT was also significant for each toxin: epoxomicin (F1,322=38.4, P<0.0001), lactacystin (F1,322=29.7, P<0.0001), and MG115 (F1,322=33.2, P<0.0001). The interaction between toxin dose and HPRT was significant for each toxin as well: epoxomicin (F6,322=3.6, P<0.002), lactacystin (F6,322=18.3, P<0.0001), and MG115 (F6,322=14.1, P<0.001).
Dopamine-related molecular markers
To obtain further insights into the molecular phenotype of these cells, the expression of mRNA transcripts encoding several proteins relevant to dopaminergic neurons was studied by qPCR in the parent line and each of the HPRT− sublines. The parent MN9D cells expressed all tested markers of the dopamine neuron phenotype, though levels of DDC and the DAT were very low.
Consistent with the results of enzyme studies, all of the mutant sublines exhibited a significant reduction in HPRT mRNA (Table 4). As a group, the HPRT− sublines also exhibited significant decreases in the expression of TH mRNA. Immunohistochemical studies revealed a diffuse reduction of TH staining across the entire cell population, rather than a subpopulation of cells that lost staining (not shown). These studies reveal that the reductions in mRNA were accompanied by reductions in the associated protein.
Table 4.
Quantitative PCR
| Gene | TG1 | TG2 | TG3 | TG4 | TG5 | AG2 | AG5 | AG6 | AG8 | AG10 | Overall change | t-Test |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comt | 118.7±13.0 | 40.1±4.3 | 78.5±7.0 | 65.7±10.7 | 55.9±3.3 | 45.7±8.2 | 79.8±5.3 | 63.9±2.6 | 151.5±15.2 | −1.4±2.9 | +69.9±13.6 | 0.0006 |
| Dat | −97.2±0.8 | −78.7±1.7 | −68.9±3.8 | −53.2±3.1 | −80.5±4.1 | −69.5±0.4 | −98.0±0.2 | −84.9±1.0 | −80.3±1.2 | −52.8±3.6 | −76.4±4.9 | <0.0001 |
| Ddc | 11.4±9.9 | −57.1±4.0 | −5.3±2.4 | 34.3±8.4 | −55.7±8.4 | 8.3±6.1 | −84.2±1.4 | −72.8±2.9 | 78.2±3.3 | −15.3±9.0 | −31.5±13.5 | 0.0339 |
| Gch1 | 269.7±12.4 | 222.0±15.2 | 183.2±10.7 | 166.7±5.4 | 113.0±10.0 | 170.7±4.7 | 94.4±4.9 | 151.4±15.8 | 321.6±10.7 | 182.1±14.8 | +187.5±21.0 | <0.0001 |
| Hprt | −78.3±1.1 | −80.0±0.4 | −82.1±1.0 | −75.5±1.1 | −81.4±0.6 | −64.7±2.2 | −82.2±0.6 | −82.1±1.0 | −80.4±0.8 | −81.1±0.8 | −78.8±1.7 | <0.0001 |
| Lrrk2 | 28.7±4.0 | 13.5±6.2 | 2.2±3.4 | 24.7±4.0 | −12.8±2.1 | −4.5±2.1 | −29.4±5.7 | −38.8±3.4 | −7.0±9.4 | −17.7±2.4 | −4.1±7.2 | 0.8187 |
| MaoA | −59.5±2.3 | 8.8±11.3 | −43.8±0.7 | −93.0±0.8 | −46.8±1.7 | 39.2±4.3 | −96.2±1.9 | −10.0±3.7 | 23.1±5.6 | 54.9±6.8 | −22.3±16.5 | 0.1871 |
| MaoB | −97.9±0.2 | −84.9±2.1 | −85.9±2.7 | −86.0±1.8 | −90.8±2.2 | −92.3±2.6 | −98.9±0.3 | −98.9±0.2 | −94.1±1.8 | −98.0±0.6 | −92.8±2.0 | <0.0001 |
| Nos1 | 362.2±23.3 | 57.9±2.7 | 166.2±4.4 | −36.0±2.2 | 98.2±6.9 | 56.3±2.6 | 176.6±4.7 | 187.6±3.6 | −11.7±2.3 | 5.6±3.2 | +106±36.8 | 0.0192 |
| Parkin | −59.0±4.4 | −46.7±6.6 | −62.3±3.7 | −55.1±2.0 | −75.6±2.9 | −65.1±4.4 | −56.1±4.5 | −71.6±2.1 | −74.8±3.8 | −72.0±0.6 | −63.8±3.6 | <0.0001 |
| Sod2 | 133.1±4.3 | 29.4±1.6 | 61.2±4.7 | 58.0±3.2 | −17.3±2.5 | 10.3±2.3 | 8.3±3.7 | −36.2±12.3 | 14.9±2.1 | 15.1±7.1 | +34.9±13.0 | 0.0318 |
| Th | −57.8±1.6 | −43.8±1.7 | −32.7±1.6 | −22.6±1.9 | −43.8±1.0 | −1.1±3.0 | −53.8±1.1 | −55.2±1.6 | −84.7±0.6 | −66.2±1.0 | −46.2±7.2 | 0.0002 |
| Vmat2 | −36.2±3.7 | 11.0±1.6 | −64.6±0.8 | −98.8±0.5 | −70.4±2.4 | −8.5±2.6 | −29.8±3.7 | −66.5±2.3 | −48.5±3.0 | −73.9±1.8 | −48.6±10.2 | 0.0012 |
| α-syn | −10.0±12.6 | 7.8±16.2 | 195.0±27.9 | 64.1±10.3 | 7.2±4.1 | 33.3±5.0 | 12.0±1.1 | 124.3±6.6 | 53.9±7.1 | 42.9±14.2 | −53.1±20.4 | 0.0214 |
The results for each mRNA in each cell line were determined in quadruplicate and normalized to a simultaneously processed control mRNA, Myl6 (myosin light polypeptide 6), where the raw data showed little variation among the lines. Results from the HPRT− lines are presented as percent changes relative to a simultaneously processed sample of the HPRT+ parent line. The overall change reflects the aggregate results of all the HPRT− sublines combined. The significance of the difference relative to controls was determined for each mRNA via single sample t-test. A Bonferroni correction for 14 measures was used to define P<0.004 as a conservative estimate of statistical significance. A result of 0.004<P<0.05 was considered borderline.
There also were significant reductions in mRNAs encoding the DAT, the vesicular monoamine transporter (VMAT2), MAO-B, and parkin (Table 4). Two mRNAs, COMT and GCH1, showed increases two- to threefold above normal levels. Statistically borderline changes in the HPRT− sublines were measured for DDC, α-synuclein, Mn-superoxide dismutase (SOD2) and neuronal nitric oxide synthase (Nos1). MAO-A and leucine-rich repeat kinase 2 (LRRK2) were not affected. Multiple other control markers that were normal included neuron-specific enolase, glyceraldehyde-3-phosphate dehydrogenase, myosin light polypeptide 6, amyloid peptide precursor, and protoporphyrinogen oxidase (not shown). These studies demonstrate a broad but selective change in the molecular phenotype of the HPRT− sublines.
DISCUSSION
These studies demonstrate that loss of HPRT-mediated purine recycling in MN9D cells is associated with a significant loss of dopamine without gross morphological abnormalities or other indicators of impaired cellular viability. Molecular studies reveal an unexpectedly broad change in many mRNAs relevant to the dopaminergic phenotype including TH, DAT, VMAT2, MAO-B, COMT, GCH1, and parkin. Reductions in one of these markers (TH) were confirmed at the protein level by immunostaining. Quantitatively, the changes in the mRNAs were often considerable, with more than 90% reductions in MAO-B and nearly threefold increases in GCH1. The changes do not reflect a global and non-specific abnormality affecting all mRNAs, since multiple control mRNAs were normal.
Cell lines are used widely to model specific biological phenomena of disease. The broad popularity of cell models derives from a number of properties that make them convenient experimental tools. One strength is the ability to make genetic or other manipulations under rigorously controlled conditions, unencumbered by many complex interactions occurring in vivo. Another strength is the ability to precisely evaluate the consequences on molecular, biochemical, physiological and morphological levels. Cell models also have certain limitations. One limitation is that the phenomena being studied may be restricted to the culture environment and therefore may not be relevant to processes occurring in the intact nervous system. This limitation does not apply to the current studies, since the core findings of reduced dopamine and related biochemical measures replicate those already reported for intact midbrain dopamine neurons of humans with HPRT deficiency and HPRT knockout mice (Lloyd et al., 1981; Jinnah et al., 1994, 1999; Saito et al., 1999). Reductions in dopamine also have been reported for cultured midbrain dopamine neurons from the HPRT knockout mice (Smith and Friedmann, 2000; Boer et al., 2001) and for HPRT− rat PC12 pheochromocytoma cells (Bitler and Howard, 1986; Yeh et al., 1998). The marked reductions in MAO-B mRNA in the HPRT− MN9D sublines similarly parallel the marked reductions in MAO enzyme activity reported in prior studies of HPRT− fibroblasts, glioma, and neuroblastoma cell lines (Breakefield et al., 1976; Skaper and Seegmiller, 1976; Skaper and Schafer, 1978; Singh et al., 1979). The loss of dopamine and related biochemical measures in association with HPRT deficiency is therefore a common finding that is not restricted to the in vitro environment, nor idiosyncratic to the MN9D line.
Another limitation of cell models involves inherent experimental variation. Sublines isolated from an established parent line often display large phenotypic variations in both biochemical and morphological properties (Koike and Takashima, 1984; Ciccarone et al., 1989; Clementi et al., 1992; Shirley et al., 2007). This variation occurs because genetic drift in culture leads to heterogeneity in the parent line, and the subsequent isolation of individual sublines reflects any experimental manipulation superimposed upon the population variation. In the current studies, dopamine levels remained stable in the parent MN9D culture despite more than 2 years of culture. Additionally, isolation of 16 HPRT-competent MN9D cells by limiting dilution revealed no difference in the group average when compared with the parent line (Egami et al., 2007). These observations argue against passive drift of the dopamine phenotype during culture or an artifact of subcloning as an explanation for dopamine loss in the HPRT− MN9D sublines. The loss of the dopamine phenotype also is not likely to reflect spurious experimental variation. The problem of inherent variation was addressed in the current studies by focusing on statistically significant group differences between the parent line and 10 independently isolated HPRT− sublines. As expected, the sublines varied widely in many characteristics. Despite this variation, highly statistically significant differences were found between the parent line and the HPRT− sublines as a group for multiple biochemical and molecular measures relevant to the dopamine phenotype. The group differences were not global, since multiple control markers were not affected. The results from the HPRT− MN9D sublines therefore provide converging support for an important biological relationship between HPRT and the phenotype of dopamine neurons.
The molecular and cellular mechanisms responsible for the simultaneous but selective changes in multiple molecular markers of the dopamine phenotype after the loss of HPRT are unknown. A popular hypothesis to explain the relationship between dopamine and HPRT is that the absence of purine salvage results in failed development of nigrostriatal axonal arborizations or early degeneration of these axons or neurons (Lloyd et al., 1981; Ernst et al., 1996; Wong et al., 1996; Saito et al., 1999; Visser et al., 2000). These hypotheses predict overt morphological correlates of dopamine loss, such as a failure of neuritic outgrowth or dystrophic soma. Such morphological changes were not found in a recent anatomical study of the HPRT− mouse brain, despite a 50% reduction in striatal dopamine (Egami et al., 2007). Similarly, morphological changes of midbrain dopamine neurons have not been apparent in postmortem studies of the Lesch-Nyhan disease brain, despite 60–80% loss of striatal dopamine (Saito et al., 1999; Del Bigio and Halliday, 2007). The current studies with HPRT− MN9D cells, together with results from the HPRT-deficient mice and humans, do not support proposals that the loss of dopamine and associated proteins has a morphological basis. Instead, they argue that the relationship between HPRT deficiency and dopamine loss involves an intrinsic metabolic mechanism.
Several biochemical mechanisms might be considered to account for the loss of dopamine in the HPRT− MN9D subclones. In view of the evidence that dopaminergic neurons exhibit an unusual dependence on energy production, one possibility is that the failure of HPRT-mediated purine recycling results in a deficiency of purines, including ATP. The reduction in ATP could, in turn, result in lower dopamine because of a compromised metabolic state. This mechanism is unlikely. In comparison with the control line, the HPRT− sublines as a group had significantly higher ATP levels, higher AEC, and greater resistance to inhibition of ATP production. These changes in purine content may reflect regulatory changes to the loss of purine recycling with increased purine synthesis, as shown for other HPRT− cell lines and tissues (Rosenbloom et al., 1968; Wood et al., 1973; Jinnah et al., 1993; Zoref-Shani et al., 1993).
Another potential mechanism for the loss of dopamine in the HPRT− subclones involves dopamine toxicity and oxidative stress. High levels of dopamine are known to be toxic to cultured cells, especially when energy metabolism is impaired (McLaughlin et al., 1998; Zeevalk et al., 1998; Kweon et al., 2004). It is therefore possible that the loss of HPRT reduces cellular defenses against dopamine toxicity and oxidative stress (Yeh et al., 1998; Visser et al., 2002). This mechanism also is unlikely. The HPRT− sublines did not display an enhanced vulnerability to exogenously supplied oxidants such as H2O2 or menadione. In addition, the levels of added dopamine required for toxicity were orders of magnitude higher than the levels normally found in the parental MN9D line, making it unlikely that toxicity would occur with ranges of dopamine occurring naturally in this line.
A third relevant biochemical mechanism involves toxicity from the accumulation of misfolded proteins or proteasome dysfunction. Although all neurons appear to be susceptible to this process, dopamine neurons appear to be particularly vulnerable (Rideout et al., 2005; McNaught et al., 2006). However, there is no evidence that this mechanism contributes to the loss of dopamine in the HPRT− MN9D sublines. These cells exhibited normal or enhanced resistance to proteasome inhibitors, and displayed no evidence for proteinaceous inclusions. The reasons for enhanced resistance of the HPRT− sublines to proteasome inhibition or oxidative stress are unknown. Their relatively high ATP and energy charges may provide one explanation, since proteasomes and redox status are both energy dependent.
Aside from neurotoxic mechanisms that reduce cell health or viability, what other mechanisms could explain the broad but selective influence of HPRT deficiency on multiple molecular markers of the dopamine phenotype in the MN9D cells? One possibility involves the processes that direct the early development of these neurons. The early generation and differentiation of midbrain dopamine neurons involves a complex interplay among multiple transcription and signaling factors (Lin and Rosenthal, 2003; Smidt et al., 2003). Some of these factors are expressed transiently during early development, where they serve to trigger downstream events. Other factors appear at later stages and their expression persists throughout adulthood, where they regulate multiple biochemical features of the dopamine neuron phenotype. For example, the transcription factor Nurr1 is first expressed at embryonic day 10 in the mouse, and its expression persists postnatally. Nurr1 controls the expression of multiple biochemical aspects of the dopamine neuron phenotype including TH, DDC, VMAT, and DAT (Sacchetti et al., 2001; Chung et al., 2002; Hermanson et al., 2003; Smits et al., 2003). Though the biochemical and morphological differentiation of midbrain dopamine neurons normally proceeds in parallel, these two processes may be separable; ectopic expression of Nurr1 in embryonic stem cells results in the appearance of several biochemical markers of dopamine neurons (TH, DDC, DAT) without morphological signs of differentiation, and Nurr1 knockout mice are reported to have morphologically identifiable nigrostriatal dopamine neurons lacking most of these same biochemical markers (Witta et al., 2000; Sonntag et al., 2004). The biochemical and morphological differentiation of developing midbrain dopamine neurons and the maintenance of these neurons in adults also depend on a number of other factors such as Pitx3, Lmx1b, and engrailed 1 and 2. The roles of each of these factors and their interactions in specifying and maintaining the dopamine neuron phenotype are just beginning to be understood (Lin and Rosenthal, 2003; Smidt et al., 2003). Preliminary studies showed no abnormalities of Nurr1 expression in the HPRT− sublines, but other transcription factors will need to be examined to determine if a change in the developmental program of these neurons is responsible for the change in the dopamine phenotype.
The ability of individual transcription factors to regulate multiple aspects of the dopamine neuron phenotype provides a precedent for interpreting the broad influence of HPRT deficiency on multiple molecular markers of the MN9D cells via a mechanism that does not involve toxicity or neurodegenerative processes. Furthermore, the potential to dissociate the biochemical and morphological aspects of dopamine neuron differentiation under some circumstances provides a mechanism for interpreting the broad changes in the molecular phenotype in the HPRT− MN9D cells, in the absence of any overt morphological correlate. Although further studies are needed to elucidate the mechanisms involved, the current studies establish purine recycling as a biochemical process of particular importance to expression of the molecular phenotype of the dopamine neuron.
Acknowledgments
We thank Dr. Alfred Heller for supplying the MN9D cell line. This work was supported by the National Institutes of Health NS40470, the Association Lesch-Nyhan Action, Fondation Jérôme Lejeune, Fédération des Maladies Orphelines, and Fondation Louis D. Institut de France.
Abbreviations
- AEC
adenylate energy charge
- ANOVA
analysis of variance
- COMT
catechol-O-methyltransferase
- DAT
dopamine transporter
- DDC
dopa-decarboxylase
- DOPAC
dihydroxyphenylacetic acid
- FBS
fetal bovine serum
- HPRT
hypoxanthine-guanine phosphoribosyltransferase
- HPRT−
hypoxanthine-guanine phosphoribosyltransferase deficient
- HVA
homovanillic acid
- MAO
monoamine oxidase
- MTT
3(4,5-dimethyltiazol-2-yl)2,5-diphenyltetrazolium bromide
- qPCR
quantitative PCR
- SDS
sodium dodecyl sulfate
- TH
tyrosine hydroxylase
- VMAT2
vesicular monoamine transporter
- 3MT
3-methoxytyramine
- 6TG
6-thioguanine
- 8AG
8-azaguanine
REFERENCES
- About-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat Rev Neurosci. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- Atkinson DE. The energy charge of the adenylate pool as a regulatory parameter: interaction with feedback modifiers. Biochem. 1968;7:4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar D, Zuk R, Glinka Y. Dopamine neurotoxicity: inhibition of mitochondrial respiration. J Neurochem. 1995;64:718–723. doi: 10.1046/j.1471-4159.1995.64020718.x. [DOI] [PubMed] [Google Scholar]
- Biedler JL, Roffler-Tarlov S, Schachner M, Freedman LS. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 1978;38:3751–3757. [PubMed] [Google Scholar]
- Bitler CM, Howard BD. Dopamine metabolism in hypoxanthine-guanine phosphoribosyltransferase-deficient variants of PC12 cells. J Neurochem. 1986;47:107–112. doi: 10.1111/j.1471-4159.1986.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Boer P, Brosh S, Wasserman L, Hammel I, Zoref-Shani E, Sperling O. Decelerated rate of dendrite outgrowth from dopaminergic neurons in primary culture from brains of hypoxanthine phosphoribosyltransferase-deficient knockout mice. Neurosci Lett. 2001;303:45–48. doi: 10.1016/s0304-3940(01)01716-5. [DOI] [PubMed] [Google Scholar]
- Breakefield XO, Castiglione CM, Edelstein SB. Monoamine oxidase activity decreased in cells lacking hypoxanthine phosphoribosyltransferase activity. Science. 1976;192:1018–1020. doi: 10.1126/science.1273584. [DOI] [PubMed] [Google Scholar]
- Choi HK, Won LA, Kontur PJ, Hammond DN, Fox AP, Wainer BH, Hoffmann PC, Heller AC. Immortalization of embryonic mesencephalic dopaminergic neurons by somatic cell fusion. Brain Res. 1991;552:67–76. doi: 10.1016/0006-8993(91)90661-e. [DOI] [PubMed] [Google Scholar]
- Chung S, Sonntag KC, Andersson T, Bjorklund LM, Park JJ, Kim DW, Jang UJ, Isacson O, Kim KS. Genetic engineering of mouse embryonic stem cells by Nurr1 enhances differentiation and maturation into dopaminergic neurons. Eur J Neurosci. 2002;16:1829–1838. doi: 10.1046/j.1460-9568.2002.02255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarone V, Spengler BA, Meyers MB, Biedler JL, Ross RA. Phenotypic diversification in human neuroblastoma cells: expression of distinct neural crest lineages. Cancer Res. 1989;49:219–225. [PubMed] [Google Scholar]
- Clementi E, Racchetti G, Zacchetti D, Panzeri MC, Meldolesi J. Differential expression of markers and activities in a group of PC12 nerve cell clones. Eur J Neurosci. 1992;4:944–953. doi: 10.1111/j.1460-9568.1992.tb00121.x. [DOI] [PubMed] [Google Scholar]
- Colgin LM, Hackmann AFM, Emond MJ, Monnat RJ. The unexpected landscape of in vivo somatic mutation in a human epithelial cell lineage. Proc Natl Acad Sci U S A. 2002;99:1437–1442. doi: 10.1073/pnas.032655699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson MR. The biochemistry of Parkinson’s disease. Annu Rev Biochem. 2005;74:29–52. doi: 10.1146/annurev.biochem.74.082803.133400. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR, Halliday WC. Multifocal atrophy of cerebellar internal granular neurons in Lesch-Nyhan disease: case reports and review. J Neuropathol Exp Neurol. 2007;66:346–353. doi: 10.1097/nen.0b013e3180515319. [DOI] [PubMed] [Google Scholar]
- Desagher S, Glowinski J, Premont J. Astrocytes protect neurons from hydrogen peroxide toxicity. J Neurosci. 1996;16:2553–2562. doi: 10.1523/JNEUROSCI.16-08-02553.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Keller JN. Proteasome inhibition in oxidative stress neurotoxicity: implications for heat shock proteins. J Neurochem. 2001;77:1010–1017. doi: 10.1046/j.1471-4159.2001.00302.x. [DOI] [PubMed] [Google Scholar]
- Egami K, Yitta S, Kasim S, Lewers JC, Roberts RC, Lehar M, Jinnah HA. Basal ganglia dopamine loss due to defect in purine recycling. Neurobiol Dis. 2007;26:396–407. doi: 10.1016/j.nbd.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Zametkin AJ, Matochik JA, Pascualvaca D, Jons PH, Hardy K, Hankerson JG, Doudet DJ, Cohen RM. Presynaptic dopaminergic deficits in Lesch-Nyhan disease. N Engl J Med. 1996;334:1568–1572. doi: 10.1056/NEJM199606133342403. [DOI] [PubMed] [Google Scholar]
- Fahn S, Cohen G. The oxidant stress hypothesis in Parkinson’s disease: evidence supporting it. Ann Neurol. 1992;32:804–812. doi: 10.1002/ana.410320616. [DOI] [PubMed] [Google Scholar]
- Fenteany G, Standaert RF, Reichard GA, Corey EJ, Schreiber SL. A beta-lactone related to lactacystin induces neurite outgrowth in a neuroblastoma cell line and inhibits cell cycle progression in an osteosarcoma cell line. Proc Natl Acad Sci U S A. 1994;91:3358–3362. doi: 10.1073/pnas.91.8.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freshney R. Culture of animal cells: a manual of basic technique. New York: John Wiley & Sons; 2000. [Google Scholar]
- Fujimoto WY, Subak-Sharpe JH, Seegmiller JE. Hypoxanthine-guanine phosphoribosyltransferase deficiency: chemical agents selective for mutant or normal cultured fibroblasts in mixed and heterozygote cultures. Proc Natl Acad Sci U S A. 1971;68:1516–1519. doi: 10.1073/pnas.68.7.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck MR, Zeevalk GD. Inhibition of brain mitochondrial respiration by dopamine and its metabolites: implications for Parkinson’s disease and catecholamine-associated diseases. J Neurochem. 2004;91:788–795. doi: 10.1111/j.1471-4159.2004.02747.x. [DOI] [PubMed] [Google Scholar]
- Graf LH, McRoberts JA, Harrison TM, Martin DW. Increased PRPP synthetase activity in cultured rat hepatoma cells containing mutations in the hypoxanthine-guanine phosphoribosyltransferase gene. J Cell Physiol. 1976;88:331–342. doi: 10.1002/jcp.1040880309. [DOI] [PubMed] [Google Scholar]
- Greene JG, Dingledine R, Greenamyre JT. Gene expression profiling of rat midbrain dopamine neurons: implications for selective vulnerability in parkinsonism. Neurobiol Dis. 2004;18:19–31. doi: 10.1016/j.nbd.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Hansen MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- Hermanson E, Joseph B, Castro D, Lindqvist E, Aarnisalo P, Wallen A, Benoit G, Hengerer B, Olson L, Perlmann T. Nurr1 regulates dopamine synthesis and storage in MN9D dopaminergic cells. Exp Cell Res. 2003;288:324–334. doi: 10.1016/s0014-4827(03)00216-7. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53(Suppl 3):S36–S38. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Friedmann T. Lesch-Nyhan disease and its variants. In: Scriver CR, et al., editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2000. pp. 2537–2570. [Google Scholar]
- Jinnah HA, Jones MD, Wojcik BE, Rothstein JD, Hess EJ, Friedmann T, Breese GR. Influence of age and strain on striatal dopamine loss in a genetic mouse model of Lesch-Nyhan disease. J Neurochem. 1999;72:225–229. doi: 10.1046/j.1471-4159.1999.0720225.x. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Page T, Friedmann T. Brain purines in a genetic mouse model of Lesch-Nyhan disease. J Neurochem. 1993;60:2036–2045. doi: 10.1111/j.1471-4159.1993.tb03488.x. [DOI] [PubMed] [Google Scholar]
- Jinnah HA, Visser JE, Harris JC, Verdu A, Larovere L, Ceballos-Picot I, Neychev V, Torres RJ, Dulac O, Desguerre I, Schretlen DJ, Robey KL, Barabas G, Bloem BR, Nyhan WL, Kremer R, Eddey GE, Puig JG, Reich SG. Delineation of the motor disorder of Lesch-Nyhan disease. Brain. 2006;129:1201–1217. doi: 10.1093/brain/awl056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinnah HA, Wojcik BE, Hunt MA, Narang N, Lee KY, Goldstein M, Wamsley JK, Langlais PJ, Friedmann T. Dopamine deficiency in a genetic mouse model of Lesch-Nyhan disease. J Neurosci. 1994;14:1164–1175. doi: 10.1523/JNEUROSCI.14-03-01164.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike T, Takashima A. Clonal variability of PC12 pheochromocytoma cells with respect to catecholamine biosynthesis. J Neurochem. 1984;42:1472–1475. doi: 10.1111/j.1471-4159.1984.tb02812.x. [DOI] [PubMed] [Google Scholar]
- Kweon GR, Marks JD, Krencik R, Leung EH, Schumacker PT, Hyland K, Kang UJ. Distinct mechanisms of neurodegeneration induced by chronic complex I inhibition in dopaminergic and non-dopaminergic cells. J Biol Chem. 2004;279:51783–51792. doi: 10.1074/jbc.M407336200. [DOI] [PubMed] [Google Scholar]
- Lin JC, Rosenthal A. Molecular mechanisms controlling the development of dopaminergic neurons. Semin Cell Dev Biol. 2003;14:175–180. doi: 10.1016/s1084-9521(03)00009-0. [DOI] [PubMed] [Google Scholar]
- Lloyd KG, Hornykiewicz O, Davidson L, Shannak K, Farley I, Goldstein M, Shibuya M, Kelley WN, Fox IH. Biochemical evidence of dysfunction of brain neurotransmitters in the Lesch-Nyhan syndrome. N Engl J Med. 1981;305:1106–1111. doi: 10.1056/NEJM198111053051902. [DOI] [PubMed] [Google Scholar]
- McCreanor GM, Harkness RA. Lesch-Nyhan syndrome and its pathogenesis: normal nicotinamide-adenine dinucleotide but reduced ATP concentrations that correlate with reduced poly(ADP-ribose) synthetase activity in HPRT-deficient lymphoblasts. J Inherit Metab Dis. 1995;18:737–747. doi: 10.1007/BF02436765. [DOI] [PubMed] [Google Scholar]
- McKeran RO. Factors in the pathogenesis of the brain damage and anaemia in the Lesch-Nyhan syndrome. CIBA Found Symp. 1977;48:83–96. doi: 10.1002/9780470720301.ch6. [DOI] [PubMed] [Google Scholar]
- McLaughlin BA, Nelson D, Erecinska M, Chesselet MF. Toxicity of dopamine to striatal neurons in vitro and potentiation of cell death by a mitochondrial inhibitor. J Neurochem. 1998;70:2406–2415. doi: 10.1046/j.1471-4159.1998.70062406.x. [DOI] [PubMed] [Google Scholar]
- McNaught KS, Jackson T, Jnobaptiste R, Kapustin A, Olanow CW. Proteasomal dysfunction in sporadic Parkinson’s disease. Neurol. 2006;23(Suppl 4):S37–S49. doi: 10.1212/wnl.66.10_suppl_4.s37. [DOI] [PubMed] [Google Scholar]
- Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson’s disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- Nelson JA, Carpenter JW, Rose LM, Adamson DJ. Mechanisms of action of 6-thioguanine, 6-mercaptopurine, and 8-azaguanine. Cancer Res. 1975;35:2872–2878. [PubMed] [Google Scholar]
- Rideout HJ, Lang-Rollin ICJ, Savalle M, Stefanis L. Dopaminergic neurons in rat ventral midbrain cultures undergo selective apoptosis and form inclusions, but do not up-regulate iHSP70, following proteasome inhibition. J Neurochem. 2005;93:1304. doi: 10.1111/j.1471-4159.2005.03124.x. [DOI] [PubMed] [Google Scholar]
- Rosenbloom FM, Henderson JF, Caldwell IC, Kelley WN, Seegmiller JE. Biochemical bases of accelerated purine biosynthesis de novo in human fibroblasts lacking hypoxanthine-guanine phosphoribosyltransferase. J Biol Chem. 1968;243:1166–1173. [PubMed] [Google Scholar]
- Sacchetti P, Mitchell TR, Granneman JG, Bannon MJ. Nurr1 enhances transcription of the human dopamine transporter gene through a novel mechanism. J Neurochem. 2001;76:1565–1572. doi: 10.1046/j.1471-4159.2001.00181.x. [DOI] [PubMed] [Google Scholar]
- Saito Y, Ito M, Hanaoka S, Ohama E, Akaboshi S, Takashima S. Dopamine receptor upregulation in Lesch-Nyhan syndrome: a postmortem study. Neuropediatrics. 1999;30:66–71. doi: 10.1055/s-2007-973462. [DOI] [PubMed] [Google Scholar]
- Shirley TL, Lewers JC, Egami K, Majumdar A, Kelly M, Ceballos-Picot I, Seidman MM, Jinnah HA. A human neuronal tissue culture model for Lesch-Nyhan disease. J Neurochem. 2007;101:841–853. doi: 10.1111/j.1471-4159.2007.04472.x. [DOI] [PubMed] [Google Scholar]
- Singh S, Willers I, Kluss EM, Goedde HW. Monoamine oxidase and catechol-O-methyltransferase activity in cultured fibroblasts from patients with maple syrup urine disease, Lesch-Nyhan syndrome and healthy controls. Clin Genet. 1979;15:153–159. doi: 10.1111/j.1399-0004.1979.tb01754.x. [DOI] [PubMed] [Google Scholar]
- Skaper SD, Schafer IA. Monoamino oxidase activity reduced in cultured human fetal cells deficient in hypoxanthine-guanine phosphoribosyltransferase activity. Biochem Genet. 1978;16:1135–1138. doi: 10.1007/BF00484533. [DOI] [PubMed] [Google Scholar]
- Skaper SD, Seegmiller JE. Hypoxanthine-guanine phosphoribosyltransferase mutant glioma cells: diminished monoamine oxidase activity. Science. 1976;194:1171–1173. doi: 10.1126/science.996547. [DOI] [PubMed] [Google Scholar]
- Smidt MP, Smits SM, Burbach JPH. Molecular mechanisms underlying midbrain dopamine neuron development and function. Eur J Pharmacol. 2003;480:75–88. doi: 10.1016/j.ejphar.2003.08.094. [DOI] [PubMed] [Google Scholar]
- Smith D, Friedmann T. Characterization of the dopamine defect in primary cultures of dopaminergic neurons from hypoxanthine phosphoribosyltransferase knockout mice. Mol Ther. 2000;1:486–491. doi: 10.1006/mthe.2000.0057. [DOI] [PubMed] [Google Scholar]
- Smith DW, Jinnah HA. Role of neuronal nitric oxide in the dopamine deficit of HPRT-deficient mice. Metab Brain Dis. 2007;22:39–43. doi: 10.1007/s11011-007-9044-7. [DOI] [PubMed] [Google Scholar]
- Smits SM, Ponnio T, Conneely OM, Burbach JPH, Smidt MP. Involvement of Nurr1 in specifying the neurotransmitter identity of ventral midbrain dopaminergic neurons. Eur J Neurosci. 2003;18:1731–1738. doi: 10.1046/j.1460-9568.2003.02885.x. [DOI] [PubMed] [Google Scholar]
- Sonntag KC, Simantov R, Kim KS, Isacson O. Temporarily induced Nurr1 can induce a non-neuronal dopaminergic cell type in embryonic stem cell differentiation. Eur J Neurosci. 2004;19:1141–1152. doi: 10.1111/j.1460-9568.2004.03204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser JE, Baer PR, Jinnah HA. Lesch-Nyhan syndrome and the basal ganglia. Brain Res Rev. 2000;32:449–475. doi: 10.1016/s0165-0173(99)00094-6. [DOI] [PubMed] [Google Scholar]
- Visser JE, Smith DW, Moy SS, Breese GR, Friedmann T, Rothstein JD, Jinnah HA. Oxidative stress and dopamine deficiency in a genetic mouse model of Lesch-Nyhan disease. Dev Brain Res. 2002;133:127–139. doi: 10.1016/s0165-3806(02)00280-8. [DOI] [PubMed] [Google Scholar]
- Witta J, Baffi J, Palkovits M, Mezey E, Castillo SO, Nikodem VM. Nigrostriatal innervation is preserved in Nurr1-null mice, although dopaminergic neuron precursors are arrested from terminal differentiation. Mol Brain Res. 2000;84:67–78. doi: 10.1016/s0169-328x(00)00211-4. [DOI] [PubMed] [Google Scholar]
- Wong DF, Harris JC, Naidu S, Yokoi F, Marenco S, Dannals RF, Ravert HT, Yaster M, Evans A, Rousset O, Bryan RN, Gjedde A, Kuhar MJ, Breese GR. Dopamine transporters are markedly reduced in Lesch-Nyhan disease in vivo. Proc Natl Acad Sci U S A. 1996;93:5539–5543. doi: 10.1073/pnas.93.11.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AW, Becker MA, Minna JD, Seegmiller JE. Purine metabolism in normal and thioguanine-resistant neuroblastoma. Proc Natl Acad Sci U S A. 1973;70:3880–3883. doi: 10.1073/pnas.70.12.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J, Zheng S, Howard BD. Impaired differentiation of HPRT-deficient dopaminergic neurons: a possible mechanism underlying neuronal dysfunction in Lesch-Nyhan syndrome. J Neurosci Res. 1998;53:78–85. doi: 10.1002/(SICI)1097-4547(19980701)53:1<78::AID-JNR8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Zeevalk GD, Bernard LP, Nicklas WJ. Role of oxidative stress and the glutathione system in loss of dopamine neurons due to impairment of energy metabolism. J Neurochem. 1998;70:1421–1430. doi: 10.1046/j.1471-4159.1998.70041421.x. [DOI] [PubMed] [Google Scholar]
- Zoref-Shani E, Bromberg Y, Brosh S, Sidi Y, Sperling O. Characterization of alterations in purine nucleotide metabolism in hypoxanthine-guanine phosphoribosyltransferase-deficient rat neuroma cell line. J Neurochem. 1993;61:457–463. doi: 10.1111/j.1471-4159.1993.tb02146.x. [DOI] [PubMed] [Google Scholar]