Abstract
The shortage of transplantable organs provides an impetus to develop tissue-engineered alternatives. Producing tissues similar to immature kidneys from simple suspensions of fully dissociated embryonic renal cells is possible in vitro, but glomeruli do not form in the avascular environment. Here, we constructed renal organoids from single-cell suspensions derived from E11.5 kidneys and then implanted these organoids below the kidney capsule of a living rat host. This implantation resulted in further maturation of kidney tissue, formation of vascularized glomeruli with fully differentiated capillary walls, including the slit diaphragm, and appearance of erythropoietin-producing cells. The implanted tissue exhibited physiologic functions, including tubular reabsorption of macromolecules, that gained access to the tubular lumen on glomerular filtration. The ability to generate vascularized nephrons from single-cell suspensions marks a significant step to the long-term goal of replacing renal function by a tissue-engineered kidney.
CKD affects 5%–7% of people worldwide. ESRD can be treated by dialysis, which is imperfect and has a major impact on the quality of life, or renal transplantation. Worldwide, there is a shortage of organs available for transplanting, and this shortage has driven a strong research interest in developing methods for engineering new kidney tissue de novo from a patient’s own cells or another source. This enterprise requires the development of three successful technologies: (1) a method to isolate or generate stem cells capable of generating renal tissue,1–3 (2) a method to engineer a simple culture or suspension of these cells into a tissue similar to immature kidney,4,5 and (3) a method to transplant this culture or suspension in a host to ensure integration and maturation into functional renal tissue.
In normal renal development, cells of the ureteric bud (UB), an outgrowth of the Wolffian duct, invade and reciprocally interact with cells of the metanephric mesenchyme (MM)6–10 to initiate nephron formation. The MM induces UB branching morphogenesis and is, in turn, induced to form neural cell adhesion molecule (NCAM) -positive and paired box gene 2 (Pax-2) -positive caps (four to five cells deep) around the advancing tips of the UB.11 These caps give rise to cells that commit to making nephrons: first by making tight aggregates that express Wilm’s Tumor 1 (WT-1) strongly and then by making epithelial vesicles. The renal vesicles are polarized, and different zones of them become visceral and parietal cells of the glomerulus, proximal tubule cells, loop of Henle cells, and distal tubule cells. In subsequent developmental stages, WT-1 is maintained by podocytes, which also start expressing the key stimulatory molecule vascular endothelial growth factor (VEGF),12–15 whereas differentiated parietal cells express claudin-1. Endothelial progenitors (angioblasts) within the stroma express VEGF receptor FLK-1 and migrate into the cleft of the developing glomerulus in response to podocyte secretion of VEGF, driving the formation of capillary loops.8 At later stages, podocytes express a number of proteins such as synaptopodin, CD2-associated protein (CD2AP), and nephrin, which interacts to organize a specialized capillary filtering barrier.16 In this way, a functional glomerulus is formed.
Novel approaches to grow new kidney tissue into animal hosts have shown that intact fetal kidneys or fragments become vascularized, undergo maturation, and may exhibit functional properties.17–23 Cultured fetal kidneys24 or cultured recombined component tissues25 also become vascularized and form mature glomeruli on in vivo transplantation. The vascular environment is not dispensable for the tissue to integrate into the host and fully proceed through the normal developmental program of the kidney. Vessels, however, do not form to any meaningful extent in vitro. This finding poses a serious limit to the maturation of tissue obtained from a simple culture or suspension of precursor cells.4,5 Attempts to drive vascular formation with low oxygen26,27 and VEGF14 resulted in rudimentary capillaries from endothelial precursors in metanephric culture systems.
Here, we developed a novel approach to renal tissue engineering by building on previous in vitro attempts4 and established an in vivo transplantation method to assess how well the single cell-derived tissue can become integrated into a host recipient and continue its developmental program. Specifically, we have addressed the question of whether single-cell suspensions can generate both glomeruli capable of filtering blood and tubuli capable of reabsorbing filtered macromolecules from the lumen in vivo. Transplanting single cell-derived murine kidney tissue into the rat host allowed us to assess maturation and functional properties of the neonephrons. The system described in this study introduces a novel direction for generating donor tissue and the means to validate the ability of kidney stem cells established from various sources to become differentiated specialized cells.
Results
Renal Organoids Generated In Vitro Produce Tissues Typical of Normal Fetal Kidney
Each renal organoid was constructed from a suspension of single cells made by enzymatic dissociation of embryonic day (E) 11.5 mouse kidneys (Figure 1).4 The average cell viability immediately after complete dissociation into single cells was 92%. Suspensions containing 105 cells were subjected to mild centrifugation, and the pellets were cultured on a filter in advanced DMEM supplemented with 2% embryonic serum. Two hours after starting the culture, ureteric bud (UB) cells reformed bud epithelia, expressing the UB marker calbindin D28k (Figure 2A). At 3 days, UB structures and nephrons were identified by staining for calbindin D28k and the general basement membrane marker, laminin, respectively (Figure 2B). The nephrons showed morphologies typical of nephron development in vivo (renal vesicles, comma-shaped bodies, and S-shaped bodies) in their correct temporal order. The distal poles of some nephrons connected to adjacent UB (Figure 2B), which would occur in normal kidney from about E13.5. No evidence of either nephron–nephron or bud–bud connection (which would be abnormal) was observed. At 5 days of culture, numerous UB/collecting duct branching events were observed (Figure 2C), and elongating tubular profiles expressed calbindin D28k (Figure 2D). At this time, developing nephrons displayed a pattern of protein expression associated with specific zones along the proximodistal axis, including E-cadherin for distal tubule and UBs (Figure 2E) and cytokeratin for UBs28 (Figure 2F).
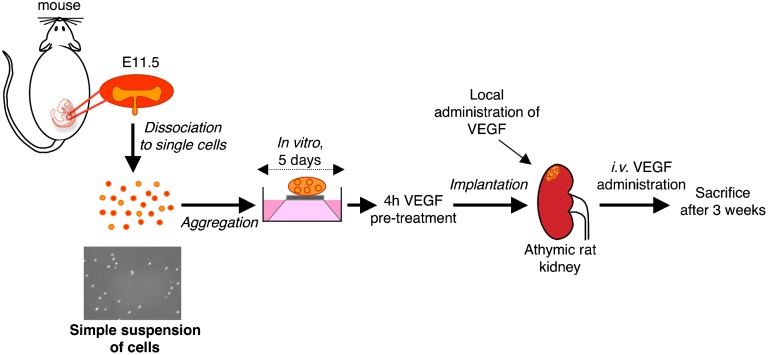
Figure 1.
Experimental design. E11.5 mouse embryonic kidneys were fully dissociated into single cells. On suspension, cells were recollected by centrifugation, and the pellets were cultured in vitro on a filter on a metal grid. After 5 days, the aggregates (organoids) were pretreated with 2 µg VEGF for 4 hours and finally implanted beneath the renal capsule of athymic rats subjected to right nephrectomy. Rat kidneys were treated locally with 1 µg VEGF immediately after renal organoid implantation. Recipients were also treated intravenously with 1 µg VEGF every 3 days for 3 weeks, and then, the kidney was processed for analysis.
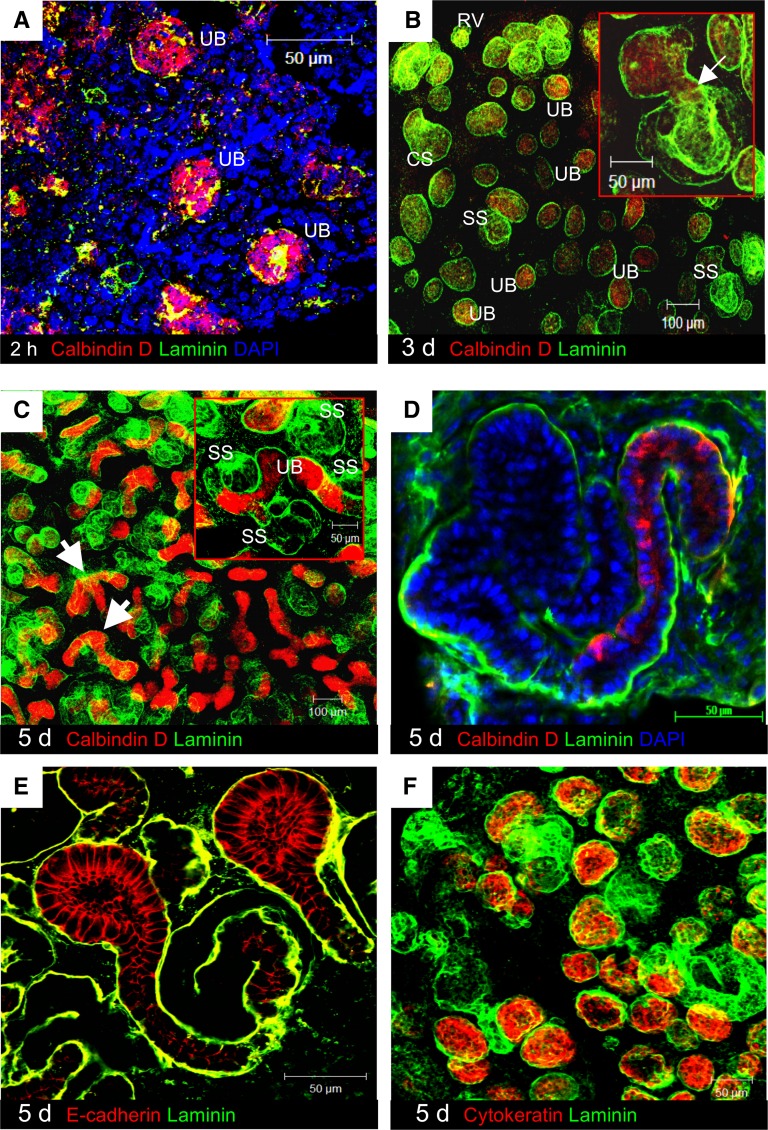
Figure 2.
Morphology of a renal organoid and expression of epithelial molecules. (A) Two hours after cell aggregation, renal organoids consisted of a mixture of embryonic renal cells. Bright calbindin D28k staining (red) was concentrated in reforming UB. Cells positive for the general basement membrane marker laminin (green) were sparsely distributed. (B) After 3 days, the organoid developed laminin-positive epithelial renal structures (green), including renal vesicles (RVs), comma-shaped bodies (CSs), and S-shaped bodies (SSs). (B, inset) The developing nephron showed connection to UB (arrow). (C) After 5 days, calbindin D28k-positive UB branching was seen (arrows). (D) Elongating tubular profiles also became visible in continuity with calbindin D28k-positive epithelia. (E) E-cadherin (red) was expressed by UB and distal domain. (F) Cytokeratin (red) was detected in UB.
We went on to characterize the differentiation process in cellular aggregates in more detail than has been done before.4 In normal kidney development, NCAM and Pax-2 are transiently expressed in a spatiotemporally specific manner.11,29,30 Organoids at 1 day revealed strong NCAM expression by a cap of three to five cell layers of condensed MM around the reformed ureteric buds, and no staining of NCAM was detectable in the UB epithelium itself (Figure 3A). At 5 days, NCAM expression was found in renal vesicles, comma-shaped bodies, and S-shaped bodies in a linear pattern at the periphery of the cells (Figure 3B). Some nephrons exhibited a more developed phenotype, in which NCAM was partly downregulated and confined to cells of distal portions and cells right at the glomerular pole (Figure 3B). However, no staining was found for claudin-1, a well established marker of differentiated parietal cells.31 As observed during nephrogenesis in vivo, the transcription factor Pax-232 was abundantly expressed in all the derivatives of the mesenchyme and UBs at 1 day (Figure 3C). Pax-2 colocalized with NCAM to caps of condensed mesenchyme (Figure 3C) and was maintained there at 5 days (Figure 3D). Brush borders of proximal tubuli appeared as wheat germ agglutinin (WGA)-lectin–positive fuzzy condensates inside a narrow tubule, phenotypically mimicking an in vivo nephrogenic program33 (Figure 3D).
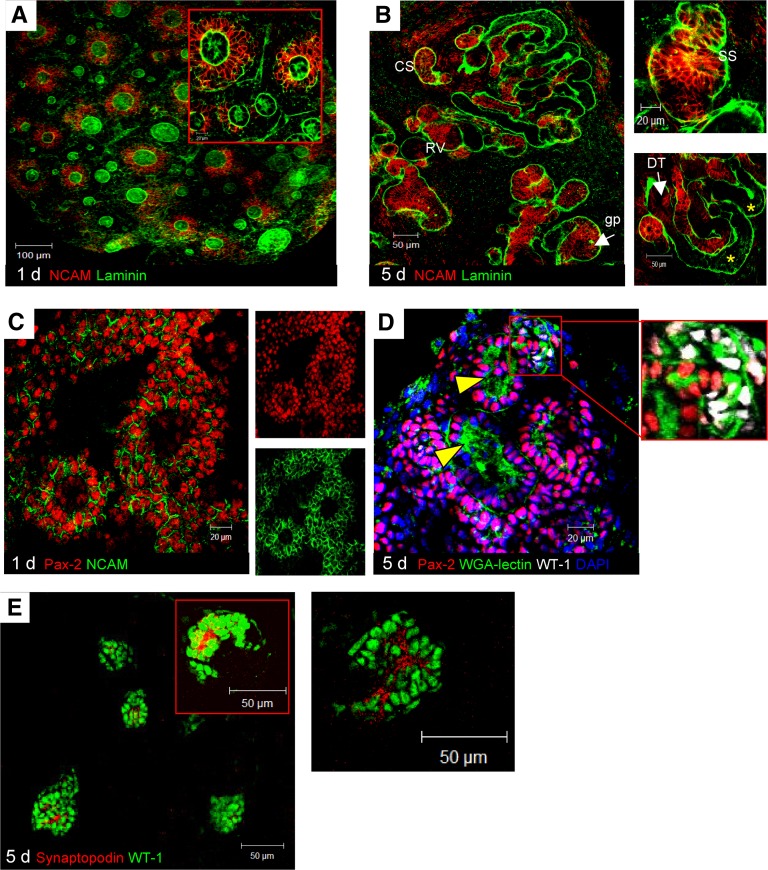
Figure 3.
Expression of NCAM and Pax-2 and epithelial differentiation in organoid in vitro. (A) At 1 day, NCAM (red) was strongly expressed by condensed MM around UB tips. (B) After 5 days, NCAM persisted in RVs, CSs, and SSs and focally in surrounding MM. In some nephrons, NCAM was confined to cells at glomerular pole (gp); it was lost by adjacent tubule cells (asterisks) and partly persisted in distal portions (DT). (C) At 1 day, Pax-2 (red) was expressed by condensed MM and at variance with NCAM (green), by UB. (D) After 5 days, Pax-2 persisted in all epithelial derivatives. Tubular brush borders were visualized as condensates labeled by lectin-WGA (arrowheads). WT-1–positive nuclei were found in gp (white in D, inset), at which lectin-WGA bound to cytoplasm of presumptive glomerular cells. (E) Podocytes were identified as synaptopodin-positive cells.
Colocalization experiments detected cells positive for Pax-2 and strongly expressing the early podocyte-associated molecule WT-1.34 Pax-2/WT-1 costained in cells at the developing glomerular pole (Figure 3D). The presence of presumptive podocytes was supported by evidence of more mature cells devoid of Pax-2 and coexpressing WT-1 and WGA-lectin binding sites35 (Figure 3D). Scattered cells stained for synaptopodin, which was taken as a marker of phenotypic maturity (Figure 3E). The slit diaphragm protein, nephrin, was undetectable. Collectively, these results suggested the potential for embryonic cell-derived organoids to generate nephrons through normal developmental patterns up to a reasonably advanced stage of nephron development for engraftment into living recipients.
Renal Organoids Survive and Grow under the Kidney Capsule
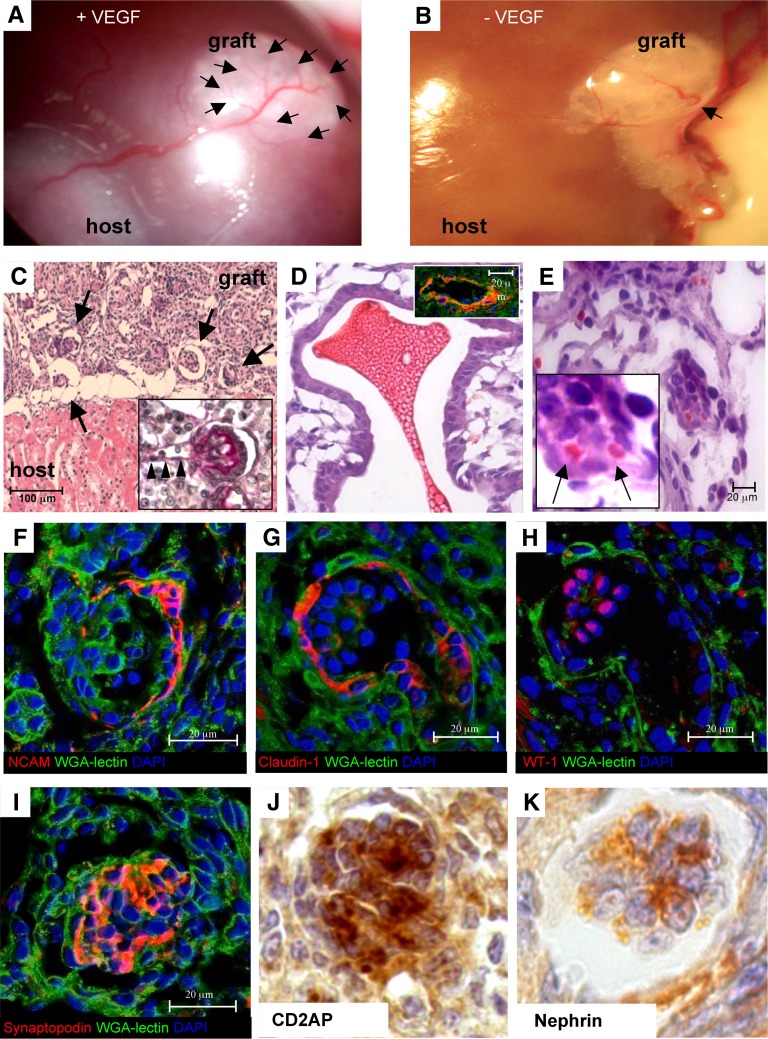
To examine whether the self-organizing tissues could be able to sustain development in vivo, organoids made in vitro from suspensions of different numbers of single cells (105 to 4×105) and cultured for 1 or 5 days were implanted through a catheter beneath the renal capsule of unilaterally nephrectomized athymic rats (Figures 1 and 4A). The rats were euthanized 3 weeks later. Five-day organoids that were constructed from just 105 cells failed to survive. By contrast, when larger 5-day organoids derived from 3.5×105 to 4×105 cells were used, more than 90% of the implants survived and increased in size. Histologic examination revealed that these renal organoids within recipient rat kidneys contained mainly tubuli (Figure 4, B and C, asterisks) and rudimentary glomerular-like structures. Under these conditions, however, the glomeruli were few and scattered (Figure 4C, arrow).
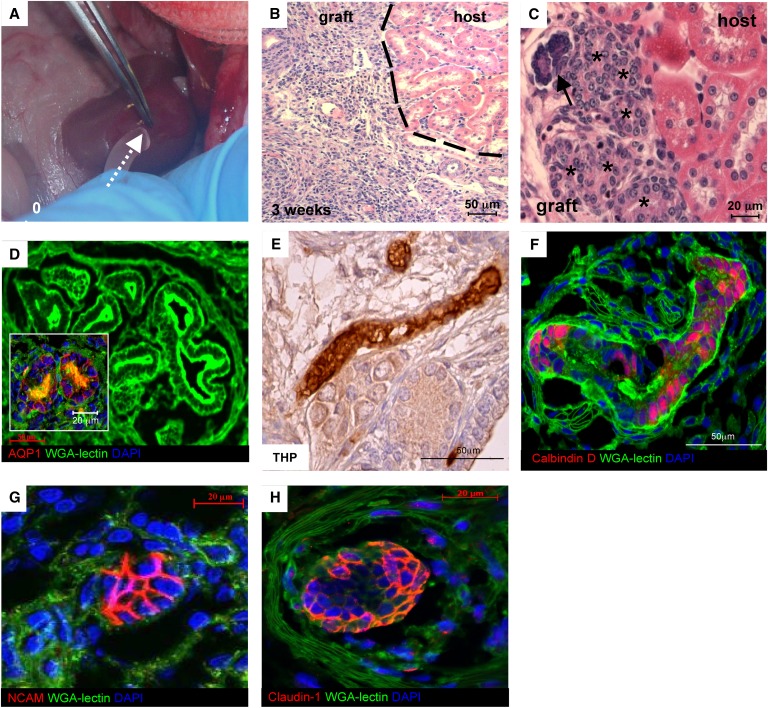
Figure 4.
Implantation and maturation of renal organoid in vivo. (A) Organoid was constructed from 4×105 cells (LCA culture, 5 days) and implanted beneath the kidney capsule (arrow). (B and C) Histology of organoid (graft) at 3 weeks shows tubuli (asterisks) and a rudimentary glomerular-like structure (arrow; B, H&E; C, periodic acid-Schiff). (D) Confocal microscopy images. Maturing proximal tubuli showed enlarged lumens and were able to bind FITC-lectin-WGA on brush borders. The epithelium also expressed AQP-1 (red). (E) Immunoperoxidase analysis of THP as a marker of thick ascending limb of the loop of Henle into the graft. (F) Distal tubuli were identified by parallel lack of brush border and specific staining for calbindin D28k (red). (G) NCAM staining (red) is associated with a cell cluster reminiscent of nephrogenic aggregate. (H) Claudin-1 (red) is seen in peripheral cells of a rudimentary glomerular-like structure.
This starting condition (5-day organoid obtained from 4×105 cells or large cell aggregation culture [LCA]) was used throughout the rest of this study.
Implanted Renal Organoids Show Tubular Maturation and Imperfect Glomerulogenesis
Most of the tubuli seen in the cross-section were of the proximal type, which was shown by binding of WGA to brush borders (Figure 4D) and expression of aquaporin-1 (AQP-1) (Figure 4D, inset). The maturation of thick ascending limbs and distal tubular portions was assessed by expression of Tamm–Horsfall glycoprotein (THP) and calbindin D28k, respectively (Figure 4 E and F). We performed immunostaining for NCAM, claudin-1, a marker of glomerular parietal epithelial cells,31 and podocyte-associated proteins. NCAM was expressed by cell clusters, reminiscent of previously described nephrogenic aggregates36 (Figure 4G). Most of the claudin-1–positive cells were found in peripheral clusters (Figure 4H). No staining was found for the podocyte markers, WT-1, synaptopodin, CD2AP, or nephrin. Overall, these patterns suggested an imperfect glomerulogenesis, with cell loss and/or dedifferentiation of the WT-1–positive presumptive podocytes, which were, instead, detectable in preimplantation organoids.
VEGF Treatment Promotes Glomerulogenesis and Vascularization of Implanted Organoids
Based on the hypothesis that a relative lack of VEGF-dependent signaling and cytoprotection might have prevented the continuation of glomerulogenesis,13,14 we soaked renal organoids in VEGF in vitro, which was followed by local VEGF injection into the area of organoid implantation in recipient kidney and subsequent intravenous VEGF injections for 3 weeks until euthanasia (Figure 1). The pre- and postimplant administration of VEGF had profound positive effects on the morphology of the developing tissue and its interaction with the recipient kidney. Systemic injections of VEGF increased the density of both small and large vessels in the host kidney, which was shown by immunostaining with rat endothelial cell antigen-1 (RECA-1) and α-smooth muscle actin (α-SMA), respectively (Supplemental Figure 1). Macroscopically, implanted tissues seemed to be supplied by branched vessels originating from the hylar region of the host kidney (Figure 5A), which is in contrast with the smaller, minimally branching vessels seen in VEGF-untreated organoid recipients (Figure 5B). Histologic analysis showed several maturing glomeruli (Figure 5C) with glomerular arterioles (Figure 5C, inset) and larger vessels (Figure 5D, inset) (positive for α-SMA staining) in contrast with the few glomerular-like structures that were found in VEGF-untreated organoids (Figure 4C). Red cells were found in vascular and glomerular structures of maturing tissue (Figure 5, D and E), indicating efficient connection with the vascular system of the recipient. Glomerular NCAM staining was closely restricted to cells lining the Bowman’s capsule (Figure 5F) in a distribution similar to the distribution of adult mouse kidney (Supplemental Figure 2A). Pax-2 staining was distributed focally in glomerular parietal layers and tubular cells (Supplemental Figure 2, B and C), also resembling the adult mouse kidney (Supplemental Figure 2D).
Figure 5.
Effect of VEGF treatment on growth and maturation of implanted organoid. (A and B) Macroscopic examination shows greatly enhanced vascularization of the graft (arrows) by (A) VEGF treatment (arrows) compared with (B) VEGF-untreated recipient 3 weeks postimplantation. (C) Histology shows several glomerular profiles (arrows; H&E) with glomerular arterioles (arrowheads in C, inset). (D and E) Vascular and glomerular structures contain red cells (arrows; H&E). (D, inset) Intragraft vessel is stained by α-SMA (red), WGA-lectin (green), and DAPI (blue). (F) NCAM is expressed by peripheral cells lining the Bowman’s capsule. (G and H) Claudin-1 and WT-1 staining confirms maturation of glomerular parietal and visceral epithelium (serial sections). (I–K) Glomerular podocytes express differentiation molecules.
Confocal microscopy analysis of sequential sections of glomeruli of implanted organoids treated with VEGF showed both claudin-1–positive parietal cells clearly lining the capsule and WT-1–positive podocytes within glomeruli (Figure 5, G and H). Glomeruli displayed robust staining for synaptopodin (Figure 5I), CD2AP (Figure 5J), and nephrin (Figure 5K), reflecting podocyte differentiation.
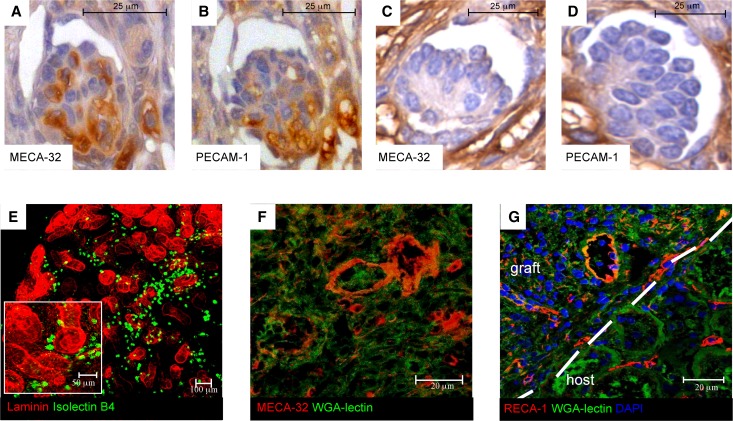
Staining for the mouse endothelial cell antigen-32 (MECA-32) revealed MECA-32–positive cells diffusely distributed in glomerular tufts of the implanted tissue (Figure 6A). Staining of sequential glomerular sections for platelet endothelial cell adhesion molecule-1 (PECAM-1), a marker for endothelial cells, showed codistribution with MECA-32, further suggesting an ongoing process of endogenous glomerular capillary formation (Figure 6B). Neither MECA-32– nor PECAM-1–positive cells were observed in the glomerular-like structures of untreated graft tissue (Figure 6, C and D). Glomeruli showed no staining for RECA-1, although abundant staining by RECA-1 in the host tissues verified that this antibody was working. These findings (summarized in Supplemental Figure 3) implied the mouse origin of glomerular endothelium and thus, the preexistence of endothelial progenitors in preimplantation organoids accounting for their vasculogenic competence.23 The latter was confirmed directly on cultured 5-day organoids by evidence of binding of FITC-conjugated Bandeiraea semplicifolia isolectin B4, which specifically recognizes embryonic but not adult endothelial cells24 (Figure 6E). Intragraft MECA-32–positive cells were also found in both the endothelial lining of neoforming extraglomerular vessels and the peritubular parenchyma (Figure 6F). These data showed that grafted organoids generate their own glomerular capillaries and intrinsic endothelium. We also found abundant RECA-1–positive vessels and capillaries at the organoid–host interface (Figure 6G), consistent with the ability of the VEGF-treated recipient to better support intragraft trophism and angiogenesis. No MECA-32–positive capillaries were present in rat kidneys, and similarly, no RECA-1–positive cells were present in mouse kidney tissue (Supplemental Figure 2, E–H).
Figure 6.
Implanted organoids develop vascularized nephrons and endogenous vessels on VEGF treatment. (A and B) Immunoperoxidase analysis of mouse endothelial antigens in glomerulus of implanted organoid tissue on VEGF treatment. A glomerular tuft shows both capillary MECA-32 and PECAM-1 staining in serial sections of the organoid taken after engraftment and VEGF treatment (3 weeks). (C and D) Imperfect glomerular-like structure of untreated engrafted tissue shows no staining. (E) Embryonic endothelial cells, identified by Bandeiraea semplicifolia isolectin B4 staining (green), were present in organoid in vitro. (F) MECA-32 was also expressed in both small capillaries and large blood vessels. (G) RECA-1–positive vessels and capillaries are seen at the organoid–host interface.
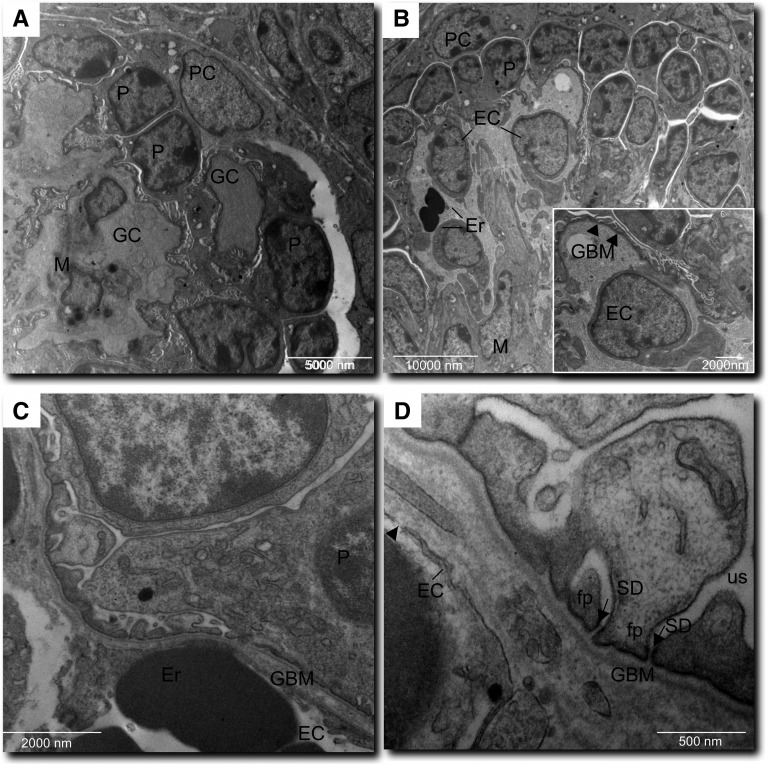
To establish whether the maturing glomeruli developed capillary structures that sustain filtering function, we analyzed VEGF-treated grafts by electron microscopy. Glomeruli showed distinct capillaries that were organized around mesangial-like regions, covered by podocytes at various maturation stages, and contained erythrocytes. (Figure 7, A and B). Capillary walls consisted of endothelial cells or cytoplasmic extensions, the glomerular basement membrane, and interdigitating processes of podocytes (Figure 7B, inset). A continuous endothelial lining and images of fenestration were visible (Figure 7, C and D). The podocyte component, in addition to showing immature cellular junctions, exhibited highly differentiated morphology with well formed foot processes and remarkably, filtration slit diaphragms (Figure 7D). Cells in mesangial-like areas appeared less densely arranged and contained smaller or elongated nuclei, few cytoplasmic organelles, and in some cells, osmiophilic granular structures (Figure 7A).
Figure 7.
Electronmicrographs of intragraft glomerular structures. (A) Glomerular capillaries (GC) are arranged around a mesangial-like area (M). Podocyte (P) and parietal cells (PC) are clearly recognizable. (B) A capillary structure containing erythrocytes (Er) is lined by endothelium (EC). (B, inset) GC wall with glomerular basement membrane (GBM; arrowheads) and foot process layer. (C) GC wall with a continuous EC layer and maturing GBM. (D) Complete foot processes (fp) and slit diaphragms (SD; arrows) tightly separate GBM from urinary space (us). The endothelium shows fenestration (arrowhead).
Evidence for Physiologic Function
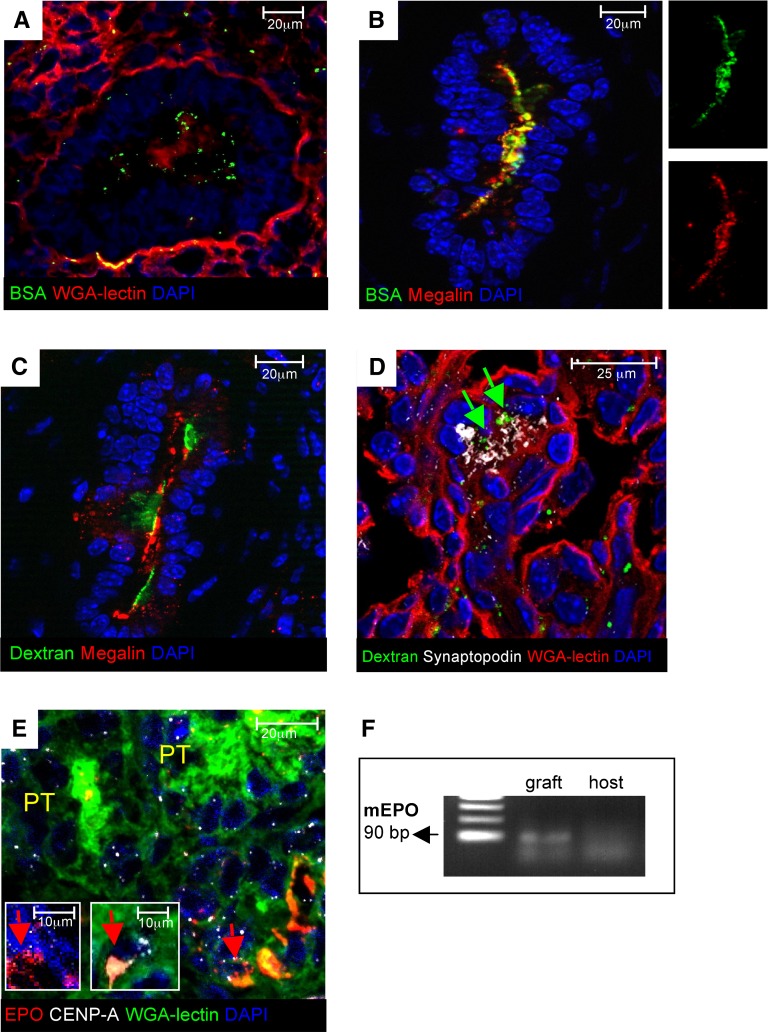
When we compared the proximal tubuli of transplanted organoids (Figure 4D) with the proximal tubuli of nontransplanted organoids (maintained in vitro) (Figure 3D), it was apparent that their lumens were enlarged and that the apical region of the epithelium was intensely stained by WGA-lectin. We tested the basic physiologic function of both glomeruli and proximal tubuli by injecting FITC-conjugated BSA into the host blood system 1 hour before euthanasia. In our grafts, proximal tubular cells showed the ability to concentrate FITC-BSA endocytosed from the tubular lumen (Figure 8A), which was further documented by colocalization in a punctate apical pattern with megalin receptor for endocytosis (Figure 8B). Megalin-positive proximal tubuli also showed staining of injected FITC-labeled dextran, an in vivo tracer for renal tubular endosomal function that is necessary for reabsorption of low-molecular weight proteins (Figure 8C). These data imply reabsorption capacity by the neoformed tubular epithelium for systemically injected macromolecules that reach the tubular lumen on glomerular ultrafiltration. Consistently, labeling by injected FITC-dextran was detected within glomeruli in synaptopodin-positive areas (Figure 8D). We next assessed the potential of the engineered tissue to generate cells endowed with ability to produce erythropoietin (EPO). EPO was not expressed in the renal organoid in vitro. EPO staining was detected in cells within graft interstitium of hosts that were made anemic. The mouse origin of most of these cells was confirmed by double staining with centromere protein A (CENP-A) (Figure 8E, insets). Consistently, PCR of RNA extracted from grafted tissue 3 weeks after implantation showed expression of mouse EPO mRNA (Figure 8F).
Figure 8.
Implanted organoids exhibit functional activities. (A and B) FITC-conjugated BSA (green) within proximal tubuli of the graft. FITC-BSA was injected intravenously into host animals 1 hour before euthanasia. (A) Punctate fluorescent signal is seen in the subapical region (red; positive for WGA-lectin). (B) FITC-BSA colocalized with endocytic receptor megalin (red). (C) FITC-labeled dextran (green) within megalin-positive proximal tubuli (red) of the graft. FITC-dextran was injected intravenously into host animals 30 minutes before euthanasia. (D) FITC-dextran (green, marked by arrows) was also found in glomeruli stained with synaptopodin (white). (E) EPO (red) expression in the graft (arrows) by cells of mouse origin specifically identified by CENP-A (white). (F) RT-PCR analysis of mRNA isolated from grafts 3 weeks after implantation. Mouse EPO transcript (90 bp) was detected into the graft tissue but not the host.
Discussion
This report presents a significant advance in development of engineered functional nephrons. Here, we show, for the first time, that organoids constructed in vitro from suspensions of fully dissociated cells can be integrated into a living recipient and exert relevant functions. Many successful attempts have been made to rebuild kidney tissue in experimental animals.17,20–22,37 Advances using kidney culture systems allowed propagation of subdivided UB and subsequent recombination of these UBs with fresh intact metanephric mesenchyme to yield morphologically normal glomeruli on in vivo implantation.25,38
From the first set of experiments, we established a more advanced protocol of organoid construction4 starting from a large cell aggregation (LCA) culture that seems to be of utmost methodological importance to achieve the formation of a complete nephron. The newly formed kidney tissue survived on transplantation into the host and developed glomerular-like structures and well formed tubuli, paving the way for additional manipulation of the system to obtain vascularized nephron epithelia. Glomeruli showed partial differentiation, including the appearance of presumptive parietal cells expressing claudin-1 in peripheral clusters. Partly akin to our system but with major differences, suspensions of murine metanephric mesenchymal E11.5 progenitor cells selected for high expression of the zinc finger nuclear factor, Sall-1, have been shown to generate in vitro renal epithelia containing rudimental glomerular-like structures.39 This cell preparation required coculture with an exogenous spinal cord cell layer, which has been used with traditional canonical developmental studies,39,40 and for clinical use, this feeder would have to come from human fetus, which would be ethically problematic. In contrast, the growth of the LCA organoid here only required an adult recipient’s kidney, and it was much more promising: it was sustained by the recipient for at least 3 weeks, a period that represents postnatal nephron development in rodents. This window of time offers a tool to better unravel the early- and long-term developmental effects of reciprocal single-cell interactions among metanephric mesenchymal cells and other precursor component cells within a totally self-organizing tissue.
Efficient self-organization of a functional organ has been recently obtained, for the first time, for the adenohypophysis using an LCA culture of embryonic stem cells. A completely unprecedented finding of the present report is the ability of the reforming kidney to acquire basic function, including tubular endocytosis of systemically injected macromolecules that reach the tubular lumen on ultrafiltration across extremely well differentiated glomerular capillary walls. The appearance of EPO-producing cells is another functionally important feature of the engineered tissue applicable to renal transplantation. Our data showing generation of vascularized nephrons from patternless aggregates on VEGF treatment document the importance of using LCA culture to reconstruct the functional unit of the kidney. Here, the only requirements were the successful implantation of the culture aggregate and the exposure to appropriate stimuli that, in turn, greatly improved fetal tissue–host interactions, allowing for efficient vascularization. We assessed the effects of combined VEGF stimulation of fetal tissue and host site because of the unique ability of VEGF to mediate podocyte–endothelial cell communication and foster the formation of glomerular capillary loops.12–15 VEGF of podocyte origin drives capillary formation by interacting with receptors on the surface of endothelial progenitors.8 Indeed, VEGF as a single factor seems sufficient to compensate pharmacologically for a state of relative podocyte paucity inherent in the kidney culture and create cellular interactions and microenvironments that overcome the tendency of embryonic renal precursors to die.41,42 Thus, murine embryonic precursor cells found in the LCA culture were able to generate MECA-32–expressing endothelium of glomeruli and vessels within the implanted tissue through processes that recapitulate the insufficiently understood programs of glomerulogenesis and vasculogenesis.24,43 Differentiation of presumptive podocytes and parietal cells was shown by survival and/or the new generation of synaptopodin-expressing cells, de novo appearance of CD2AP and nephrin, and appearance of claudin-1 and selective maintenance of NCAM in parietal cell layers. Key ultrastructural findings of interdigitating foot processes and slit diaphragms indicate a phenotype that would conceivably support glomerular filtration as typical of terminally differentiated podocytes. The degree of maturation of the glomerular capillary wall seems high enough to even serve sieving function, where the passage of circulating macromolecules can be selectively restricted at least by some portions of the glomerular barrier. Finally, the enhanced vascularization from the host by VEGF treatment plays at least a permissive role or amplifies the beneficial action of VEGF itself and trophic factors on reforming kidney. The stimulatory effect of VEGF treatment on the growth and invasion of host vessels is reminiscent of the direct in vitro effects previously shown on microvessel formation in embryonic aortic explants and aorta.44,45
Overall, data shown here may anticipate multiple avenues to building efficient kidney tissue and testing the potential of kidney stem cell systems established from pluripotent stem cells, such as embryonic stem and induced pluripotent stem cells. Organoids have already been shown to easily incorporate cells of another source, such as human amniotic fluid stem cells,46 to build a chimeric tissue in vitro: in such chimera, the effects of normal or disease genes can be studied in the context of wild-type tissue.47 Reforming chimeric nephrons within LCA culture might be used as bioscaffolds, where murine cells are selectively induced to die and then replaced with human stem cells before implantation. The single-cell stage allows the use of transfection-type methods to humanize cells, improve compatibility, and reduce risks of rejection. Moreover, this system can be exploited combined with advanced approaches to construct chimeric kidney from human stem cells37 and selectively eliminate animal cells after transplantation. Finally, another major challenge in constructing fully efficient kidney tissue relates to the need for a draining collection system. To address this problem, it may be possible to combine the LCA implantation protocol with the recently developed in vitro technique for making cultured aggregates arranged around and connected to a single collecting duct system5: this combination of techniques would allow supply and vascularization of nephron structures that can drain like a normal kidney.
In summary, the unique features of this study compared with previously published work in the field of organ reconstruction can be summarized as follows: (1) generation of vascularized renal tissue starting from a suspension of single cells, (2) in vivo viability for at least 3 weeks and phenotypic maturation of the engineered tissue, including the formation of glomerular capillary walls with fenestrated endothelium and fully differentiated foot processes and slit diaphragms, (3) glomerular filtering and tubular functions, and (4) maturation of EPO-producing cells. Future steps will require combined approaches with other techniques to obtain connection with the collecting system and excretory function.
Concise Methods
Dissociation of Embryonic Kidneys and Pellet Cultures
E11.5 kidney rudiments were dissected, dissociated, and then aggregated as described before.4 Briefly, E11.5 CD1 mouse (Charles River Italia S.p.A., Calco, Italy) embryonic kidneys were dissociated into single-cell suspensions that were filtered through a 40-μm cell strainer (BD Falcon, Oxford, United Kingdom). The presence of cellular clumps or tissue remnants in each cell preparation after filtration was excluded by microscopic analysis (Figure 1). A total of 1×105 to 4×105 freshly dissociated renal cells were centrifuged at 900×g for 4 minutes, and each pellet was placed on top of a 5-μm pore polycarbonate filter (Millipore, Watford, United Kingdom) in a humidified atmosphere with 5% CO2 at 37°C and allowed to grow. At different time points (2 hours, 1 day, or 3 or 5 days), cultures were fixed and processed for immunofluorescence analysis. Additional details are available in Supplemental Materials and Methods.
Implantation of Renal Organoids
Renal organoids of 1 or 5 days were implanted beneath the renal capsule of male athymic nude rats 6–8 weeks old (Harlan Laboratories, Inc., Indianapolis, IN). Athymic rats were subjected to right nephrectomy just before implantation to accelerate the development of transplanted tissues, and they were euthanized 3 weeks later. Selected animals were injected intravenously with FITC-labeled BSA (10 mg; Sigma) or FITC-labeled dextran (35 mg; Sigma) 1 hour or 30 minutes, respectively, before euthanasia. In the second set of experiments, rats received organoids preconditioned with 2 µg recombinant rat VEGF (Invitrogen) for 4 hours and local VEGF injection (1 µg) into the area of implantation and intravenous VEGF injections (1 µg three times per week) into the tail vein for 3 weeks until euthanasia. Three weeks after the day of implantation, rats were euthanized by CO2 inhalation, and the kidneys were removed and processed for renal histology, immunohistochemistry, and immunofluorescence analysis. One additional subgroup of recipients was euthanized 6 weeks after transplantation. No additional studies were done in this subgroup, because grafts showed involution after the prolonged observation period.
Renal Histology, Immunohistochemistry, and Immunofluorescence Analysis
After euthanasia, the kidneys containing the implanted organoids were fixed in Duboscq-Brazil (DiaPath, Bergamo, Italy) and embedded in paraffin; 3-μm sections were stained with hematoxylin-eosin (H&E; Bio-Optica, Milan, Italy) and periodic acid-Schiff reagent (BioOptica) as previously described30 and observed by light microscopy (BH2-RFCA; Olympus, Melville, NY). THP, nephrin, and CD2AP expressions were detected by immunoperoxidase analysis after incubation with rabbit anti-THP (1:20), goat antinephrin (1:100), or rabbit anti-CD2AP antibody (1:100; Santa Cruz Biotechnology). For erythrocyte detection, a modified protocol of H&E staining was applied in periodate-lysine-paraformaldehyde–fixed cryosections. For immunofluorescence staining, 3-μm periodate-lysine-paraformaldehyde–fixed cryosections were incubated with the following antibodies: rabbit anti–AQP-1 (1:50; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anticlaudin-1 (undiluted; Thermo Scientific, Rockford, IL), rabbit anti–WT-1 (1:50; Santa Cruz Biotechnology), mouse antisynaptopodin (undiluted; Progen Biotechnik GmbH, Heidelberg, Germany), mouse anti-NCAM (1:2; Developmental Studies Hybridoma Bank, University of Iowa), rabbit anti–Pax-2 (1:50; Zymed Laboratories, San Francisco, CA), Cy3-conjugated anti–α-SMA (1:100; Sigma-Aldrich), rat anti–PECAM-1 (1:50; BD Biosciences), rat anti–MECA-32 (1:100; University of Iowa), mouse anti–RECA-1 (1:50; AbD Serotec, Kidlingdon, Oxford, United Kingdom), goat antimegalin (1:100; Santa Cruz Biotechnology), and goat anti-EPO (1:25; Santa Cruz Biotechnology) followed by the specific FITC or Cy3- or Cy5-conjugated secondary antibodies (Jackson Laboratories, ME). To enhance EPO production, anemia was induced by a rapid withdrawal of blood from the tail vein at a volume of 1.5% vol/wt body weight using a previously described method with minor modifications.48 To confirm the origin of graft tissue integrated into the host (Supplemental Figure 2) a mouse-specific anti–CENP-A (1:40; Cell Signaling Technology, Inc.) was used and followed by Cy5-conjugated goat anti-rabbit (1:50; Jackson Laboratories). Details are available in Supplemental Materials and Methods.
Electron Microscopy Analysis of Glomerular Structures
Specimens of organoids obtained from VEGF-treated rats were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) overnight at 4°C and washed in cacodylate buffer. After postfixation in 1% osmium tetroxide for 1 hour and one additional wash in cacodylate buffer, the fragments were dehydrated through ascending grades of alcohol and embedded in Epon resin. Semithin sections were stained with toluidine blue in borax and examined by light microscopy. Thin sections (60–100 nm) were cut on an EM UC7 ultramicrotome (Leica Microsystems, Mannheim, Germany), collected on copper grids, and stained with uranyl acetate and lead citrate for analysis by transmission electron microscopy (Morgagni 268D; Philips, Brno, Czech Republic).
RT-PCR (RT-PCR Endpoint)
RNA was extracted from grafts 3 weeks after implantation and subjected to RT-PCR. Primers and PCR conditions are shown in Supplemental Materials and Methods.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Lorena Longaretti, Daniela Cavallotti, and Manuela Passera for excellent technical assistance.
V.B. and P.R. are recipients of fellowships from Fondazione Aiuti per la Ricerca sulle Malattie Rare (ARMR), Bergamo, Italy. This study was supported by the Juvenile Diabetes Research Foundation (JDRF) and KIDSTEM European Research Training Network “Developing a stem cell-based therapy to replace nephrons lost through reflux nephropathy” (http://www.kidstem.org) funded by the European Community as part of the Framework program 6 (FP6 036097-2). The study was also partially supported by the ERC-2010-AdG-268632 RESET Grant. The authors are also grateful for the support of Ing. Stefano Rimondi from Bellco, Italy.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Growing New Kidneys from Embryonic Cell Suspensions: Fantasy or Reality?,” on pages 1763–1766.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012050505/-/DCSupplemental.
References
- 1.Yokoo T, Ohashi T, Shen JS, Sakurai K, Miyazaki Y, Utsunomiya Y, Takahashi M, Terada Y, Eto Y, Kawamura T, Osumi N, Hosoya T: Human mesenchymal stem cells in rodent whole-embryo culture are reprogrammed to contribute to kidney tissues. Proc Natl Acad Sci U S A 102: 3296–3300, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song B, Niclis JC, Alikhan MA, Sakkal S, Sylvain A, Kerr PG, Laslett AL, Bernard CA, Ricardo SD: Generation of induced pluripotent stem cells from human kidney mesangial cells. J Am Soc Nephrol 22: 1213–1220, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thatava T, Armstrong AS, De Lamo JG, Edukulla R, Khan YK, Sakuma T, Ohmine S, Sundsbak JL, Harris PC, Kudva YC, Ikeda Y: Successful disease-specific induced pluripotent stem cell generation from patients with kidney transplantation. Stem Cell Res Ther 2: 48, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unbekandt M, Davies JA: Dissociation of embryonic kidneys followed by reaggregation allows the formation of renal tissues. Kidney Int 77: 407–416, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Ganeva V, Unbekandt M, Davies JA: An improved kidney dissociation and reaggregation culture system results in nephrons arranged organotypically around a single collecting duct system. Organogenesis 7: 83–87, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saxén L, Sariola H: Early organogenesis of the kidney. Pediatr Nephrol 1: 385–392, 1987 [DOI] [PubMed] [Google Scholar]
- 7.Vainio S, Lin Y: Coordinating early kidney development: Lessons from gene targeting. Nat Rev Genet 3: 533–543, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Dressler GR: The cellular basis of kidney development. Annu Rev Cell Dev Biol 22: 509–529, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Nigam SK, Shah MM: How does the ureteric bud branch? J Am Soc Nephrol 20: 1465–1469, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Velagapudi C, Nilsson RP, Lee MJ, Burns HS, Ricono JM, Arar M, Barnes VL, Abboud HE, Barnes JL: Reciprocal induction of simple organogenesis by mouse kidney progenitor cells in three-dimensional co-culture. Am J Pathol 180: 819–830, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bard JB, Gordon A, Sharp L, Sellers WI: Early nephron formation in the developing mouse kidney. J Anat 199: 385–392, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitamoto Y, Tokunaga H, Miyamoto K, Tomita K: VEGF is an essential molecule for glomerular structuring. Nephrol Dial Transplant 17[Suppl 9]: 25–27, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Kitamoto Y, Tokunaga H, Tomita K: Vascular endothelial growth factor is an essential molecule for mouse kidney development: Glomerulogenesis and nephrogenesis. J Clin Invest 99: 2351–2357, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tufro A, Norwood VF, Carey RM, Gomez RA: Vascular endothelial growth factor induces nephrogenesis and vasculogenesis. J Am Soc Nephrol 10: 2125–2134, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Guan F, Villegas G, Teichman J, Mundel P, Tufro A: Autocrine VEGF-A system in podocytes regulates podocin and its interaction with CD2AP. Am J Physiol Renal Physiol 291: F422–F428, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Quaggin SE, Kreidberg JA: Development of the renal glomerulus: Good neighbors and good fences. Development 135: 609–620, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Rogers SA, Lowell JA, Hammerman NA, Hammerman MR: Transplantation of developing metanephroi into adult rats. Kidney Int 54: 27–37, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Yokote S, Yokoo T, Matsumoto K, Utsunomiya Y, Kawamura T, Hosoya T: The effect of metanephros transplantation on blood pressure in anephric rats with induced acute hypotension [published online ahead of print April 18, 2012]. Nephrol Dial Transplant doi:10.1093/ndt/gfs006 [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto K, Yokoo T, Yokote S, Utsunomiya Y, Ohashi T, Hosoya T: Functional development of a transplanted embryonic kidney: Effect of transplantation site. J Nephrol 25: 50–55, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Dilworth MR, Clancy MJ, Marshall D, Bravery CA, Brenchley PE, Ashton N: Development and functional capacity of transplanted rat metanephroi. Nephrol Dial Transplant 23: 871–879, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Woolf AS, Palmer SJ, Snow ML, Fine LG: Creation of a functioning chimeric mammalian kidney. Kidney Int 38: 991–997, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Dekel B, Burakova T, Arditti FD, Reich-Zeliger S, Milstein O, Aviel-Ronen S, Rechavi G, Friedman N, Kaminski N, Passwell JH, Reisner Y: Human and porcine early kidney precursors as a new source for transplantation. Nat Med 9: 53–60, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Loughna S, Hardman P, Landels E, Jussila L, Alitalo K, Woolf AS: A molecular and genetic analysis of renalglomerular capillary development. Angiogenesis 1: 84–101, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Robert B, St John PL, Hyink DP, Abrahamson DR: Evidence that embryonic kidney cells expressing flk-1 are intrinsic, vasculogenic angioblasts. Am J Physiol 271: F744–F753, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Rosines E, Sampogna RV, Johkura K, Vaughn DA, Choi Y, Sakurai H, Shah MM, Nigam SK: Staged in vitro reconstitution and implantation of engineered rat kidney tissue. Proc Natl Acad Sci U S A 104: 20938–20943, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tufro-McReddie A, Norwood VF, Aylor KW, Botkin SJ, Carey RM, Gomez RA: Oxygen regulates vascular endothelial growth factor-mediated vasculogenesis and tubulogenesis. Dev Biol 183: 139–149, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Loughna S, Yuan HT, Woolf AS: Effects of oxygen on vascular patterning in Tie1/LacZ metanephric kidneys in vitro. Biochem Biophys Res Commun 247: 361–366, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Cho EA, Patterson LT, Brookhiser WT, Mah S, Kintner C, Dressler GR: Differential expression and function of cadherin-6 during renal epithelium development. Development 125: 803–812, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Abbate M, Brown D, Bonventre JV: Expression of NCAM recapitulates tubulogenic development in kidneys recovering from acute ischemia. Am J Physiol 277: F454–F463, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Benigni A, Morigi M, Rizzo P, Gagliardini E, Rota C, Abbate M, Ghezzi S, Remuzzi A, Remuzzi G: Inhibiting angiotensin-converting enzyme promotes renal repair by limiting progenitor cell proliferation and restoring the glomerular architecture. Am J Pathol 179: 628–638, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohse T, Pippin JW, Vaughan MR, Brinkkoetter PT, Krofft RD, Shankland SJ: Establishment of conditionally immortalized mouse glomerular parietal epithelial cells in culture. J Am Soc Nephrol 19: 1879–1890, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dressler GR, Deutsch U, Chowdhury K, Nornes HO, Gruss P: Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development 109: 787–795, 1990 [DOI] [PubMed] [Google Scholar]
- 33.Laitinen L, Virtanen I, Saxén L: Changes in the glycosylation pattern during embryonic development of mouse kidney as revealed with lectin conjugates. J Histochem Cytochem 35: 55–65, 1987 [DOI] [PubMed] [Google Scholar]
- 34.Armstrong JF, Pritchard-Jones K, Bickmore WA, Hastie ND, Bard JB: The expression of the Wilms’ tumour gene, WT1, in the developing mammalian embryo. Mech Dev 40: 85–97, 1993 [DOI] [PubMed] [Google Scholar]
- 35.Saxen L: Organogenesis of the Kidney, New York, Cambridge University Press, 1987 [Google Scholar]
- 36.Davies JA, Garrod DR: Induction of early stages of kidney tubule differentiation by lithium ions. Dev Biol 167: 50–60, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Yokoo T, Fukui A, Ohashi T, Miyazaki Y, Utsunomiya Y, Kawamura T, Hosoya T, Okabe M, Kobayashi E: Xenobiotic kidney organogenesis from human mesenchymal stem cells using a growing rodent embryo. J Am Soc Nephrol 17: 1026–1034, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Steer DL, Bush KT, Meyer TN, Schwesinger C, Nigam SK: A strategy for in vitro propagation of rat nephrons. Kidney Int 62: 1958–1965, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Osafune K, Takasato M, Kispert A, Asashima M, Nishinakamura R: Identification of multipotent progenitors in the embryonic mouse kidney by a novel colony-forming assay. Development 133: 151–161, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Auerbach R, Grobstein C: Inductive interaction of embryonic tissues after dissociation and reaggregation. Exp Cell Res 15: 384–397, 1958 [DOI] [PubMed] [Google Scholar]
- 41.Coles HS, Burne JF, Raff MC: Large-scale normal cell death in the developing rat kidney and its reduction by epidermal growth factor. Development 118: 777–784, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Giuliani S, Perin L, Sedrakyan S, Kokorowski P, Jin D, De Filippo R: Ex vivo whole embryonic kidney culture: A novel method for research in development, regeneration and transplantation. J Urol 179: 365–370, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Ekblom P, Sariola H, Karkinen-Jääskeläinen M, Saxén L: The origin of the glomerular endothelium. Cell Differ 11: 35–39, 1982 [DOI] [PubMed] [Google Scholar]
- 44.Akimoto T, Hammerman MR: Microvessel formation from mouse aorta is stimulated in vitro by secreted VEGF and extracts from metanephroi. Am J Physiol Cell Physiol 284: C1625–C1632, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Akimoto T, Liapis H, Hammerman MR: Microvessel formation from mouse embryonic aortic explants is oxygen and VEGF dependent. Am J Physiol Regul Integr Comp Physiol 283: R487–R495, 2002 [DOI] [PubMed] [Google Scholar]
- 46.De Coppi P, Bartsch G, Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A: Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 25: 100–106, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Siegel N, Rosner M, Unbekandt M, Fuchs C, Slabina N, Dolznig H, Davies JA, Lubec G, Hengstschläger M: Contribution of human amniotic fluid stem cells to renal tissue formation depends on mTOR. Hum Mol Genet 19: 3320–3331, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Yokoo T, Fukui A, Matsumoto K, Ohashi T, Sado Y, Suzuki H, Kawamura T, Okabe M, Hosoya T, Kobayashi E: Generation of a transplantable erythropoietin-producer derived from human mesenchymal stem cells. Transplantation 85: 1654–1658, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.