Summary
SUMO modification regulates the activity of numerous transcription factors that have a direct role in cell cycle progression, apoptosis, cellular proliferation, and development, but its role in differentiation processes is less clear. Keratinocyte differentiation requires the coordinated activation of a series of transcription factors, and as several critical keratinocyte transcription factors are known to be SUMO substrates, we investigated the role of sumoylation in keratinocyte differentiation. In a human keratinocyte cell line model (HaCaT cells), calcium-induced differentiation led to the transient and coordinated transcriptional activation of the genes encoding critical sumoylation system components, including SAE1, SAE2, Ubc9, SENP1, Miz-1 (PIASxβ), SUMO2, and SUMO3. The increased gene expression resulted in higher levels of the respective proteins and changes in the pattern of sumoylated substrate proteins during the differentiation process. Similar to the HaCaT results, stratified human foreskin keratinocytes showed an upregulation of Ubc9 in the suprabasal layers. Lastly, abrogation of sumoylation by Gam1 expression severely disrupted normal HaCaT differentiation, consistent with an important role for sumoylation in the proper progression of this biological process.
Keywords: Keratinocyte, differentiation, SUMO, HaCaT, Ubc9
Introduction
Posttranslational modification of proteins is a rapid and efficient mechanism to modulate protein activity in response to a given stimulus. The Small Ubiquitin like MOdifier, SUMO, has emerged as a complex and intriguing posttranslational modifier with a large variety of targets and a wide-range of effects on its substrates. Four SUMO isoforms (SUMOs 1–4) have been characterized in mammalian cells (Bohren et al., 2004; Su and Li, 2002), though SUMO4 expression is restricted to the kidney and other specific tissues suggesting a limited biological role for SUMO4. In contrast to SUMO4, SUMOs 1–3 are all widely expressed in mammalian cells (Xu and Au, 2005), though it is likely that these 3 proteins have at least partially distinct biological roles since SUMO1 shares only 50% identity to the closely related SUMOs 2 and 3 (Saitoh and Hinchey, 2000). Consistent with the sequence divergence, distinct preferences for substrates (Guo et al., 2005; Manza et al., 2004; Rosas-Acosta et al., 2005b) and for specific de-sumoylating proteases (Melchior et al., 2003) have been observed for each SUMO type. Despite the above differences, all SUMOs are covalently attached to proteins via a series of enzymatic reactions and biochemical steps involving the same SUMO specific enzymes (Johnson, 2004). The activating enzyme (E1), a heterodimeric protein composed of two subunits, SAE1 and SAE2 (Azuma et al., 2001), transfers SUMO to Ubc9, the only E2 conjugating enzyme (Desterro et al., 1997). The Ubc9/SUMO complex can directly interact with specific substrates and this interaction leads to the formation of an isopeptide bond between SUMO and a lysine residue on its target (Okuma et al., 1999; Tatham et al., 2003). SUMO ligases (E3 enzymes) such as RanBP2 (Pichler et al., 2002), the PIAS protein family (Kotaja et al., 2002),and the polycomb protein Pc2 (Kagey et al., 2003) are not required for sumoylation in vitro, but when added to a sumoylation reaction they substantially increase the rate of this process and appear to play an essential role in vivo. Lastly, there are specific SUMO proteases (SENP protein family) that are involved in both SUMO processing and de-sumoylation (Mossessova and Lima, 2000). The continuous interplay of the enzymes described above makes SUMO modification an active, reversible, and dynamic process in mammalian cells.
SUMO modification usually exerts significant effects on its targets: it can increase protein stability (Desterro et al., 1998), affect protein-protein interactions (Seeler and Dejean, 2001), alter sub-cellular localization (Morita et al., 2005; Wilson and Rangasamy, 2001), and impact nuclear trafficking (Pichler and Melchior, 2002). To date, transcription factors (TFs) represent the largest group of SUMO substrates identified, and the most common effect associated with TF sumoylation is modulation of their transcriptional activity (Gill, 2003; Verger et al., 2003). In general, sumoylation results in the negative regulation of the activity of most TFs, including widely expressed factors such as AP-1 (Bossis et al., 2005), Sp1/Sp3 (Ross et al., 2002; Spengler and Brattain, 2006), and C/EBP (Kim et al., 2002), though activation has been reported for some TFs, including TCF4 (Ihara et al., 2005) and IKaros (Arco et al., 2005). Recent studies have established multiple mechanisms for negative regulation of TFs by sumoylation, including recruitment of transcriptional co-repressors such as HDACs (Shiio and Eisenman, 2003; Yang and Sharrocks, 2004), sequestration in the cytoplasm (Morita et al., 2005; Salinas et al., 2004), or ubiquitination and subsequent degradation (Ghioni et al., 2005).
Many of the TFs known to be SUMO targets, including pRB (Ledl et al., 2005), the p53/63/73 family (Ghioni et al., 2005; Melchior and Hengst, 2002), AP-2 (Eloranta and Hurst, 2002), and Sp1/Sp3 (Ross et al., 2002; Spengler and Brattain, 2006) are involved in regulating gene expression during cell cycle progression and/or differentiation (Herwig and Strauss, 1997; Li and Kellems, 2003; Santini et al., 2001), suggesting that sumoylation could coordinate complex transcriptional programs in the cell. Several recent studies in metazoan systems support such a role for sumoylation in aspects of development and differentiation. Sumoylation is required for correct vulvar development in C. elegans (Leight et al., 2005; Poulin et al., 2005), is implicated in male germ cell maturation (Vigodner et al., 2006), and promotes differentiation of postsynaptic dendrites (Shalizi et al., 2006). Lastly, using Ubc9−/− blastocyst cells, Nacerddine et al demonstrated that sumoylation is critical for maintaining nuclear structure and chromosomal segregation during blastocyst development, with the absence of Ubc9 leading to an embryonic lethal phenotype (Nacerddine et al., 2005).
Skin is a complex and renewable organ for which little is known about the expression and function of the sumoylation system, though the presence of the SUMO1 protein in a mouse keratinocyte cell line has been reported (Zhong et al., 2000). The epidermis is comprised of multiple layers of differentiated keratinocytes which are continually regenerated from the replicative basal layer. In skin, keratinocytes differentiate vertically due to a calcium gradient established throughout the different epithelial layers, increasing from the basal to the outermost layer of the epithelium (Menon et al., 1985; Vicanova et al., 1998). Calcium signaling drives proliferating keratinocytes out of the cell cycle and into a committed path of terminal differentiation, but the intermediate signaling transduction pathways that lead to this process are still poorly understood (Bikle et al., 2001; Lansdown, 2002; Tu et al., 2004). A network of keratin markers biochemically defines the keratinocyte stage within the epithelium structure (Eichner et al., 1986; Smith, 2003; Sun et al., 1985). Expression of keratin 5 (K5) and keratin 14 (K14) characterizes the basal proliferative phenotype restricted to the stem cells layer where the calcium concentration is low (Schweizer and Winter, 1983). Early during differentiation K5 and K14 are repressed and differentiation markers such as K1, K10, and involucrin begin to be expressed. Finally, keratinocytes complete terminal differentiation by producing the outer cornified layer of the skin characterized by markers such as filaggrin and involucrin (Candi et al., 2005; Eichner et al., 1986; Fuchs and Green, 1980). This pattern of markers permits discrimination between proliferating, differentiating and terminally differentiated keratinocytes. The well-coordinated expression of specific sets of TFs induces and represses these keratinocyte genes as the cells migrate through the epithelium layers, and a number of these critical TFs are already known to be SUMO targets in other tissues (Girdwood et al., 2004; Verger et al., 2003), suggesting that sumoylation could play an important role in regulating epidermal differentiation.
Cultured keratinocytes, such as HaCaT cells, provide well-established differentiation models that can recapitulate many aspects of stratified epithelium (Boukamp et al., 1988; Schoop et al., 1999). HaCaTs are spontaneously immortalized and do not express exogenous transforming genes that could interfere with the natural process of sumoylation and differentiation. Moreover, HaCaT cells have the capacity to revert back and forth between the differentiated and the basal phenotype, and therefore are widely used to model keratinocyte differentiation in culture (Schoop et al., 1999). Here, we examine the sumoylation system in HaCaT cells during calcium-induced differentiation. We show that the sumoylation system is transiently up regulated by calcium signaling in HaCaT cells at both the RNA and protein level, while abrogation of sumoylation leads to abnormal differentiation. These results suggest cross talk between the sumoylation system and the keratinocyte differentiation process that contributes to the normal program of morphological and biochemical changes during differentiation.
Results
HaCaTs, a naturally immortalized human keratinocyte cell line, express the SUMO machinery
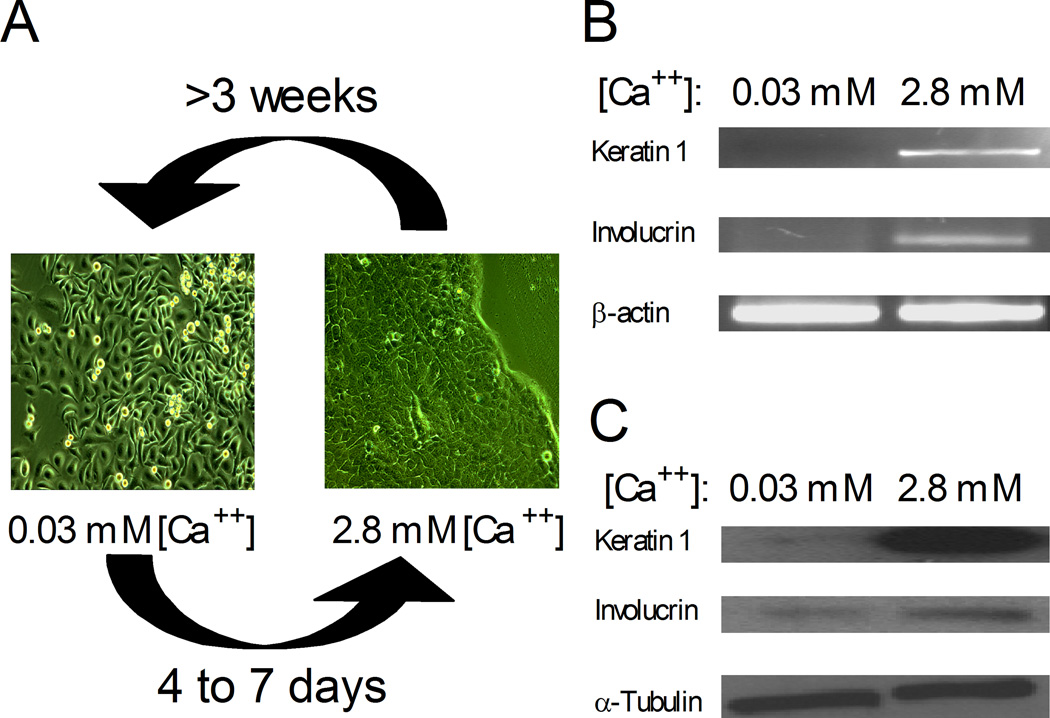
The SUMO system has been extensively studied, and the enzymes required for sumoylation are widely expressed in many tissues (Chen et al., 1998; Johnson, 2004). However, the sumoylation system remains largely unexplored in human keratinocytes, an important model for differentiation. In order to study the functional importance of this modifier in the context of keratinocyte differentiation, we first developed culture conditions to maintain HaCaT cells stably in either a basal or differentiated phenotype. HaCaT cells cultured in 0.03 mM calcium were spindle-shaped and loosely packed (Figure 1A). In addition, there was little or no expression of two differentiation markers, keratin 1 (K1) and involucrin (Inv), at the RNA (Figure 1B) or protein (Figure 1C) level, consistent with these cells remaining in a basal like state. In contrast, HaCaT cells maintained in 2.8 mM calcium became more cuboidal, developed cell-to-cell tight junctions, and expressed both differentiation markers, K1 and Inv, at the RNA and protein level (Figures 1A–C). Under both high and low calcium conditions, the cultures could be maintained for many passages without any subsequent changes in morphology or marker expression pattern. Therefore, these growth conditions allow HaCaT cells to be stably maintained in vitro in two alternative states resembling pre- and post- differentiated skin cells.
Figure 1.
HaCaT cell cultures exhibit phenotypic differences in response to calcium concentration. (A) Phase contrast microscopy of HaCaT cells maintained in low calcium medium (0.03 mM Ca++) or in high calcium medium (2.8 mM Ca++). Upon calcium addition to the medium, basal HaCaT cells need 4 to 7 days to assume a complete differentiated state. Inversely, upon calcium depletion, differentiated HaCaT cells need about 3 weeks to revert to a basal phenotype. (B) RT-PCR analyses of total mRNA harvested from basal (0.03 mM Ca++) and differentiated HaCaT (2.8 mM Ca++) cultures. (c) Immunoblot analyses showing expression of keratin 1, involucrin, and α-tubulin in total cell extracts harvested from basal (0.03 mM Ca++) and differentiated (2.8 mM Ca++) HaCaT cultures.
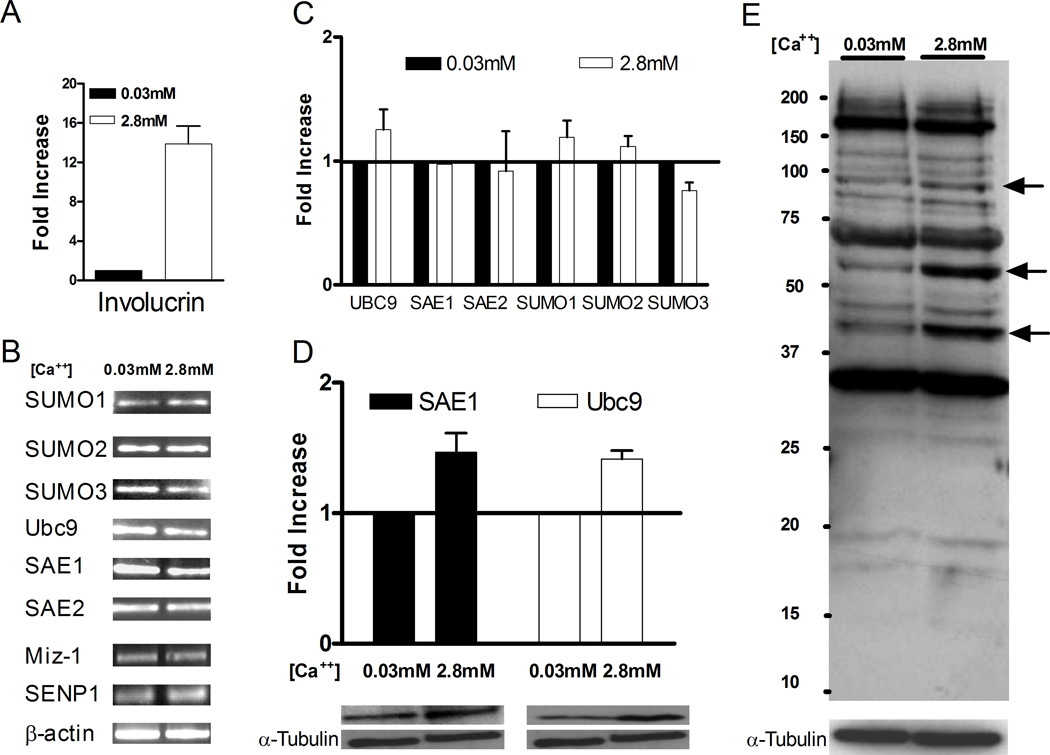
To characterize the sumoylation system in human keratinocytes, basal and stably differentiated HaCaT cultures were evaluated for expression of sumoylation components at both the RNA and protein levels. RT-PCR and quantitative RT-PCR (Q-PCR) analyzes were used to detect transcripts for all the core SUMO components, including SUMO1, SUMO2, SUMO3, SAE1, SAE2, and Ubc9 (Figures 2B and 2C), as well as the differentiation marker involucrin (Figure 2A). Additionally, one SUMO ligase (Miz-1 or PIASxβ) and one SUMO protease (SENP1) were examined by RT-PCR. Consistent with the qualitative results shown in Figure 1B, Q-PCR detected approximately 14-fold higher levels of involucrin mRNA in the high calcium-maintained keratinocytes compared to the low calcium culture, confirming the differentiation state of these cells (Figure 2A). By RT-PCR, transcripts for all of the SUMO component genes were detected in both the basal and differentiated HaCaT cells, indicating that the relevant genes for an active sumoylation system were actively transcribed in both cell populations (Figure 2B). Although the HaCaT cells were maintained in two distinct phenotypic states, the transcript levels for the sumoylation components tested were similar under both culture conditions (Figure 2C). Immunoblot analyses confirmed the expression of Ubc9 and SAE1 proteins, and the levels of each of these proteins were only slightly higher in the differentiated cells compared to the undifferentiated HaCaT cells (Figure 2D). Next, we tested if the sumoylation system was active in HaCaT cells as evidenced by the presence of sumoylated proteins. Immunoblotting of total cell extracts using a polyclonal anti-SUMO antibody showed that sumoylation occurred in both the basal and differentiated cell populations (Figure 2E). Although the differences in expression levels of the sumoylation components were small, the sumoylation patterns in differentiated versus basal cells were slightly different: bands at ~40, 60, and 95 kDa were intensified in high calcium cells compared to basal cells (Figure 2E, arrows). Since the basal and differentiated cell cultures were both equally proliferative and were generated from a single stock of HaCaT cells, we believe that these changes in sumoylation pattern reflect differentiation-related events rather than intrinsic differences in genetic background or growth capacity of the two populations of cells.
Figure 2.
Components of the sumoylation system are expressed in basal and differentiated HaCaT cells. RNA (A–C) or protein (D and E) were harvested from HaCaT cells maintained in either low calcium (0.03 mM) or high calcium (2.8 mM) medium. Harvesting and analysis were performed as described in Materials and Methods. (A) Quantitative RT-PCR of involucrin mRNA levels. (B) RT-PCR analysis of the indicated sumoylation system genes. (C) Quantitative RT-PCR analysis of the relative levels of Ubc9, SAE1, SAE2, SUMO1, SUMO2, and SUMO3 mRNAs. (D) Immunoblots for SAE1, Ubc9, and α-tubulin. SAE1 and Ubc9 blots were quantitated by densitometry and normalized to α-tubulin. The graph shows the relative protein levels for HaCaT cells maintained in low and high calcium medium, and the lower panels show representative SAE1 and Ubc9 immunoblots with their corresponding α-tubulin controls. (E) Anti-SUMO immunoblot of total cell extracts from HaCaT cells maintained in either low or high calcium medium. Protein concentrations in the extracts were normalized to α-tubulin, and the samples were electrophoresed on a 10% SDS-polyacrylamide gel. Arrows indicated sumoylated proteins whose levels appear higher in the high calcium culture. Quantitative results in (A), (C), and (D) are the average of at least 3 independent experiments.
SUMO and Ubc9 are expressed in stratified human foreskin keratinocytes
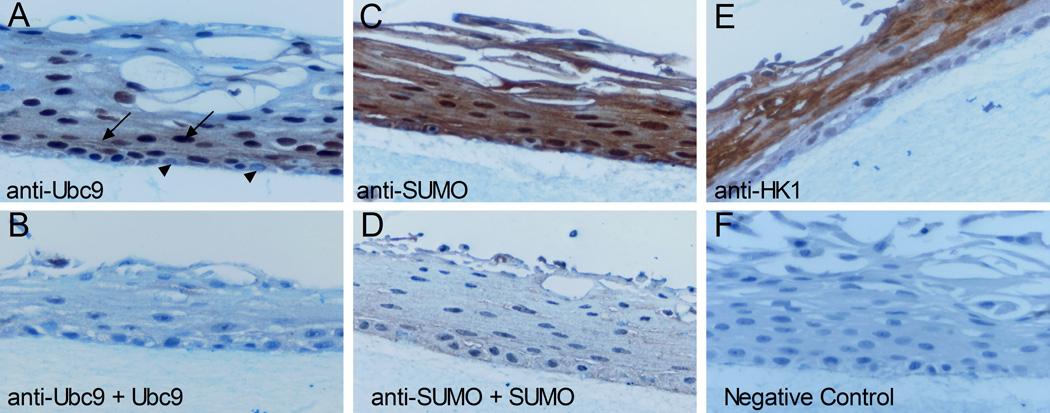
To corroborate the HaCaT observations, the expression of SUMO and Ubc9 was evaluated in primary human foreskin keratinocytes (HFKs) that were stratified in organotypic cultures. Immunohistochemistry analyses were performed with an anti-SUMO polyclonal antiserum and a purified polyclonal antibody against Ubc9, and antibody specificity was confirmed by blocking experiments using purified SUMO1 or Ubc9 (Figure 3). SUMO was detected in the nucleus and cytoplasm of keratinocytes found in all layers of the epithelium (Figure 3C). However, as this antiserum cross reacts with SUMO2/3 (unpublished observations), which SUMO types were present and whether or not there were changes in type expression in different layers is unclear. In contrast, Ubc9 was barely detected in the basal layer of the epithelium where the cells remained relatively unstained (Figure 3A, arrowheads). Strong anti-Ubc9 staining was detected in the intermediate differentiated layers just above the basal layer, and Ubc9 was present in both the nucleus and cytoplasm of the keratinocytes in this region (Figure 3A, black arrows). Although Ubc9 could be detected in nuclei throughout the upper layers (Figure 3A and data not shown), its overall expression faded as keratinocytes moved closer to the outer layer of the epithelium. It appears from these results that Ubc9 expression is low in basal cells, transiently increases with the initiation of differentiation in the suprabasal layers, and declines as terminal differentiation progresses. As Ubc9 is the only E2 enzyme for sumoylation, we speculate that the pool of sumoylated protein might be dramatically altered as well during this process. Interestingly, Ubc9 expression is maximal in the layers just below where keratin 1 expression commences (compare Figures 3A and 3E). Overall, these analyses demonstrate that sumoylation components are present in stratified human skin epithelium and that Ubc9 levels change during differentiation. The similar observations in HaCaT cultures suggest that monolayer HaCaT cells are a good representative model of the sumoylation system in normal human keratinocytes.
Figure 3.
Profile of SUMO and Ubc9 expression in organotypic cultures of human foreskin keratinocytes. Tissues sections were processed as described in Materials and Methods and were evaluated for protein expression using antibodies to Ubc9 (A and B), SUMO (B and D), or human keratin 1 (E). For samples (B) and (D), the respective purified proteins (25 µg of Ubc9 or 7 µg of SUMO1) were included in the primary antibody incubation step to block detection of the corresponding antigens. Sample (F) was a negative control where the primary antibody was absent. All sections were counterstained with hematoxylin. Arrows indicate cells expressing high levels of Ubc9. Arrowheads indicate cells in the basal layer exhibiting low levels of Ubc9 expression.
Calcium induced differentiation in HaCaT cells up regulates the sumoylation system
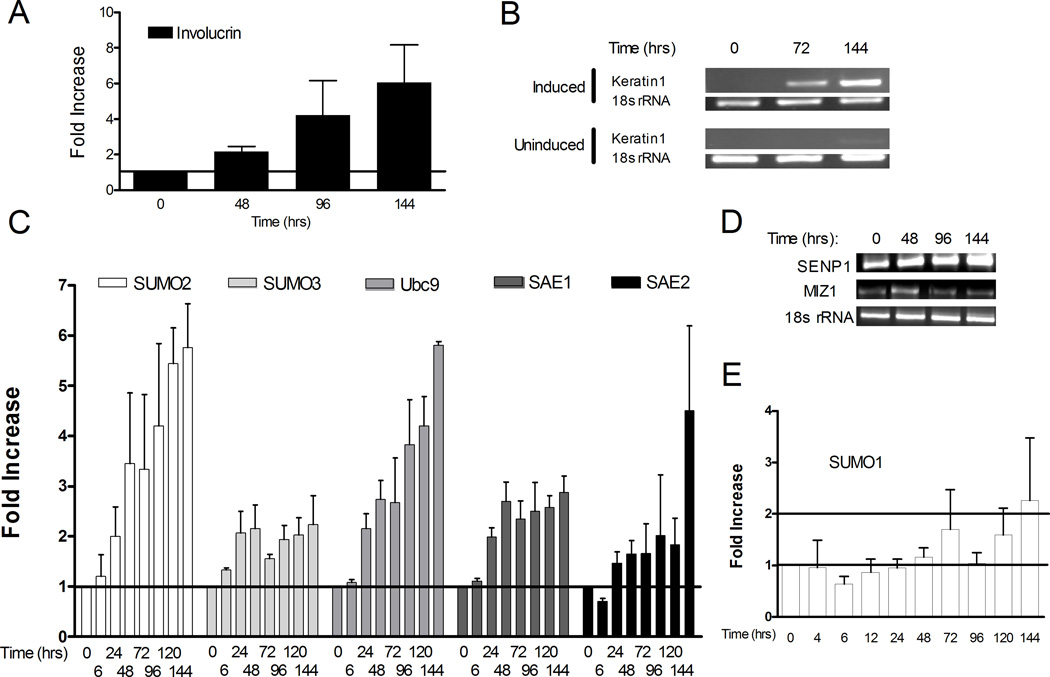
Although only minor differences in expression of the sumoylation components were detected between the two steady-state populations of HaCaT cells, the immunohistochemistry results suggested that more substantial changes in sumoylation might be taking place during the process of differentiation. To investigate this possibility we examined the sumoylation system in HaCaT cells undergoing active calcium-induced differentiation. Addition of calcium to the basal HaCaT cultures triggered a rapid response resulting in repression of the basal keratin markers, morphological changes (data not shown), and mRNA expression of differentiation markers such as K1 and Inv within 3 to 4 days (Figures 4A and 4B). The Inv transcript detected by Q-PCR progressively increased from 48 to 144 hours following calcium induction (Figure 4A). Likewise, the K1 transcript, which was completely repressed in undifferentiated (0 hours) HaCaT cells, was detected by RT-PCR at 72 hours post calcium induction, reaching a maximum expression by 120 to 144 hours (Figure 4B). No K1 expression was detected in uninduced cells for up to 120 hours (Figure 4B). Note, however, that the faint expression of K1 in uninduced cells by 144 hours was the result of the HaCaT cells reaching high confluency at this late time point (Capone et al., 2000). Based on K1 and involucrin expression, complete in vitro differentiation of HaCaT was reached within 144 hours after calcium-induction. Therefore, we chose to limit our study to a six-day period during which we monitored the regulation of the sumoylation system while the HaCaT cells progressively differentiated. RT-PCR and Q-PCR showed rapid changes in the transcript levels for the genes encoding many of the sumoylation components (Figures 4C and 4D). Transcripts for SUMO2, SUMO3, Ubc9, and SAE1 were up regulated 2-fold or more by 24 hours post-induction, with the SUMO2 and Ubc9 transcripts increasing by 5-fold or more at 144 hours post-induction (Figure 4C). The final increase for SUMO3 and SAE1 was more modest, reaching a maximum increase of 2–3 fold. The results for SAE2 were less clear, and the transcript levels did not consistently cross the 2-fold threshold until day 6. SENP1 transcripts detected by RT-PCR also gradually increased up to 144 hours, while Miz-1 (PIASxβ) transcripts were expressed maximally at 48 hours post induction and then declined (Figure 4D). We conclude from these observations that there is a general upregulation of transcription of the genes for the sumoylation components accompanying the early stages of differentiation. Interestingly, the SUMO1 gene responded differently to calcium induction than did the SUMO2 and 3 genes (Figure 4E). SUMO1 transcript levels did not increase significantly during the first 5 days post-induction with calcium, and even at day 6 the transcript levels were barely at the 2-fold threshold. Thus, SUMO1 gene expression during calcium-induced differentiation appears to be regulated differently than the genes for the other SUMO components, though the biological significance of this result is not yet defined.
Figure 4.
Keratinocyte differentiation is accompanied by a transcription upregulation of the sumoylation system. HaCaT cells maintained in low calcium medium (Time 0) were induced to differentiate by replacing the medium with high calcium medium. RNA was extracted at various times post calcium induction as indicated. The extracted RNA was analyzed for expression of differentiation marker genes (A and B) or sumoylation system genes (C–E), and the mRNAs were detected either by quantitative RT-PCR (A, C, and E) or RT-PCR (B and D) as described in Materials and Methods. For the quantitative RT-PCR, the 0 hour time samples were set to a value of 1, and the values at other time points are relative to the 0 hour value. For the RT-PCR, 18s rRNA was used as the internal standard. The quantitative results in parts (A), (C), and (E) were the average of at least three independent experiments.
Transcriptional upregulation of the sumoylation system upon calcium signaling is accompanied by an increase in the corresponding proteins and an increase of overall sumoylation in HaCaT cells
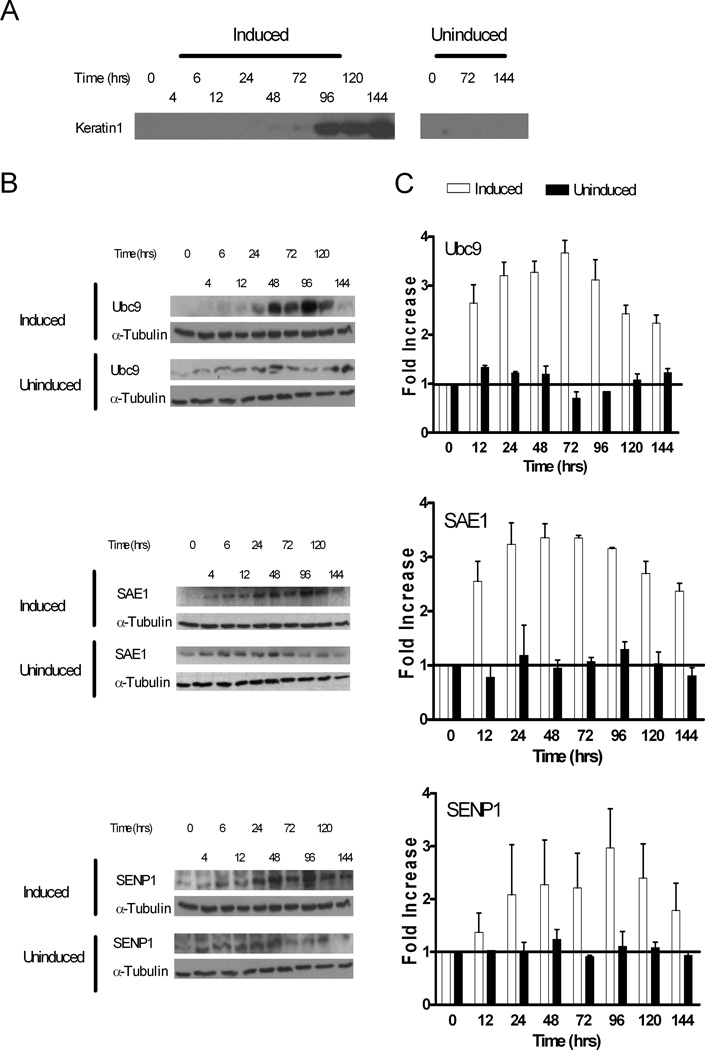
Increase of specific transcripts during a biological process is not necessarily functionally important unless it is followed by a corresponding increase in protein expression. Immunoblot analyses of representative sumoylation components indicated that the Ubc9, SAE1, and SENP1 proteins levels remained relatively constant in uninduced cultures over the 6-day incubation period (Figure 5, B and C). In contrast, all three of these proteins were rapidly, but transiently, upregulated by more than 3-fold during HaCaT cell differentiation (Figure 5, B and C). Maximum protein expression was reached at 48 to 96 hours post induction and then declined. Although the absolute increases for SAE1, Ubc9 and SENP1 were slightly different, the overall expression pattern was similar for all 3 enzymes (Figure 5C). Interestingly, while protein expression levels declined after 96 hours post induction, transcripts were still elevated and had not begun to decline during the time period examined (Figure 4C). The explanation for this discordance is not known, but may reflect the existence of a posttranscriptional regulatory mechanism that controls the level of protein expression for the different components of the sumoylation system during keratinocyte differentiation. Nonetheless, the combined results in figures 2, 4 and 5 indicate that calcium triggers a rapid and coordinated activation of transcription for many SUMO system genes leading to a corresponding accumulation of the SUMO system proteins, eventually followed by a decline to near basal protein levels. The observation in HaCaT cells that there is a transient upregulation of Ubc9 expression strongly correlates with the results from the Ubc9 immunohistochemistry data in the stratified HFKs (Figure 3) which showed a transient increase in Ubc9 protein levels in the suprabasal layers that declined with further differentiation. Importantly, as in the stratified HFKs, the peak expression of Ubc9 in differentiating HaCaT cells occurred just prior to expression of the K1 protein (Figure 5A–C).
Figure 5.
Keratinocyte differentiation is accompanied by a transient upregulation of several sumoylation system proteins. Parallel HaCaT cell cultures were maintained either in low calcium medium (Uninduced samples) or switched from low calcium to high calcium medium (Induced samples). At the indicated times post calcium induction, protein extracts were prepared from the paired cultures as described in Materials and Methods. The extracts were assayed for expression of K1 (A), Ubc9 (B), SAE1 (B), and SENP1 (B) by immunoblotting with specific antibodies. The induced and uninduced cell extracts were also evaluated for α-tubulin expression, and the immunoblots for α-tubulin are duplicated in each panel of (B) for comparative purposes. (C) The immunoblots in (B) were quantified by densitometry, and the values for Ubc9, SAE1, and SENP1 were normalized against α-tubulin. The 0 hour time values for both the induced and uninduced samples were set to 1, and the values for the later time points are relative to the 0 hour time. The quantitative results in part (C) were the average of at least three independent experiments.
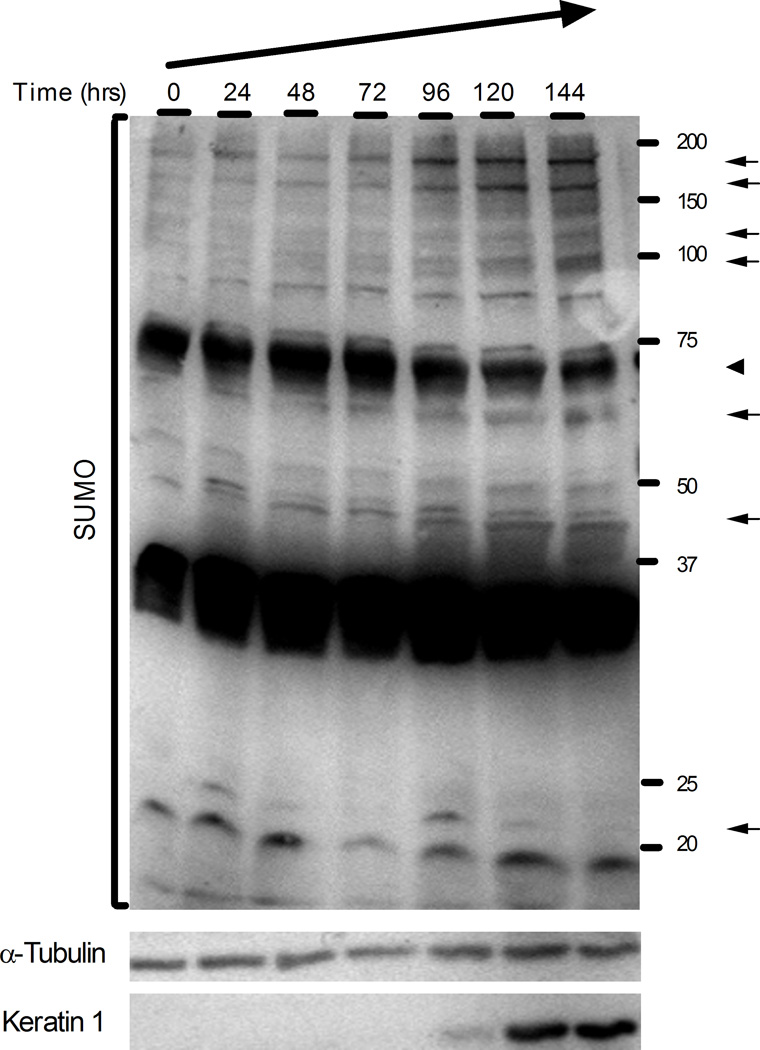
Next, we wanted to test if the accumulation of sumoylation components increased the overall population of sumoylated targets. Total cell extracts of calcium-induced HaCaT cells were collected every 24 hours up to 144 hours post-induction and were analyzed by immunoblotting (Figure 6). Overall sumoylation was clearly increased between 96 and 144 hours and discernible changes in the sumoylation of specific substrates were observed. During the differentiation process, several substrates (indicated by arrows) showed increased sumoylation, while a major sumoylated product at about 70 kDa decreased (arrowhead). In addition, new sumoylated bands appeared at about 45 and 95 kDa (arrows). No changes in substrate sumoylation were observed in parallel cultures maintained in low calcium medium (data not shown). Enhanced sumoylation during HaCaT differentiation was even more pronounced when the samples were evaluated by 2D gel electrophoresis (unpublished observations). These results indicate that the increased expression of the sumoylation components leads to increased sumoylation activity, and that calcium-induced differentiation of keratinocytes is accompanied by dynamic changes in the pattern of sumoylated proteins.
Figure 6.
The pattern of SUMO conjugated substrates changes during HaCaT cell differentiation. Total cell extracts were prepared at the indicated times post calcium induction and analyzed by immunoblotting using antibodies to SUMO1 (upper panel), α-tubulin (middle panel), or keratin 1 (lower panel). Protein concentrations in the samples were equalized based on the α-tubulin levels. The position of molecular weight markers is shown on the right. Arrows and arrowheads indicate bands whose quantity increased or decreased, respectively, during the time period examined.
Inhibition of the sumoylation system perturbs the HaCaT cell differentiation process
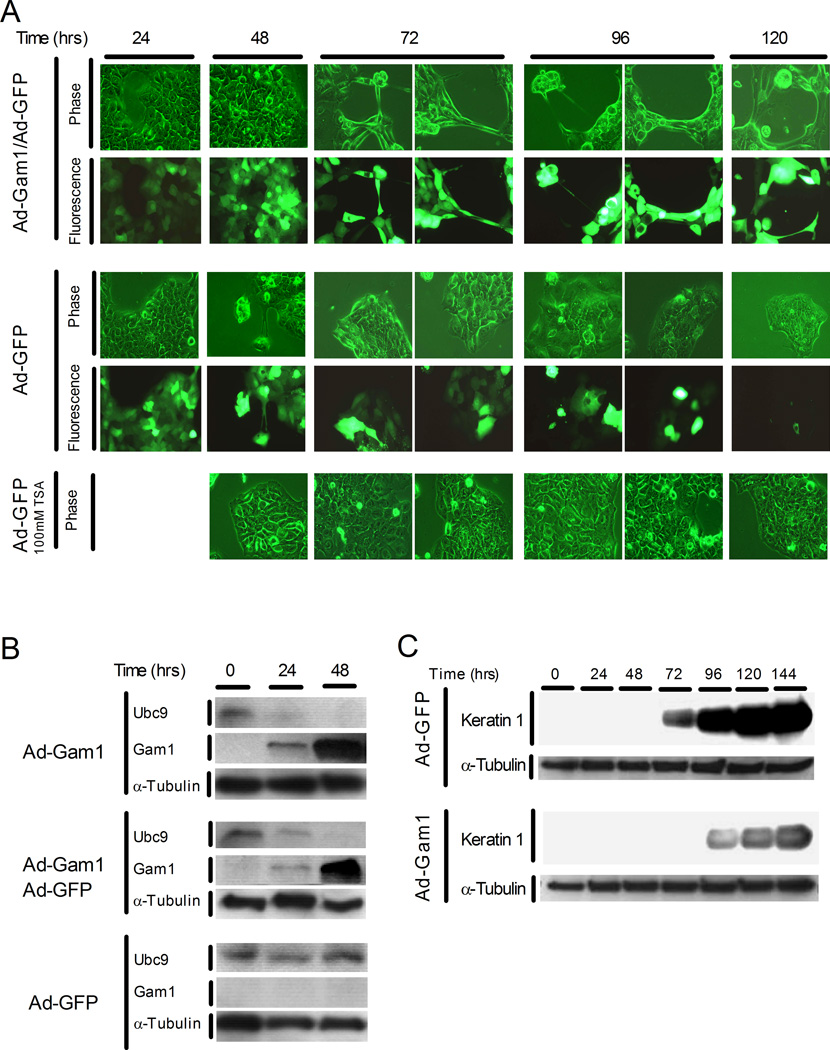
To investigate the functional importance of sumoylation in the process of keratinocyte differentiation, we infected basal HaCaT cells with an adenovirus expressing c-Myc tagged Gam1 (Ad-Gam1) and then exposed the cells to high calcium medium to trigger differentiation. Gam1 is known to inhibit sumoylation by inducing degradation of SAE1/2 and Ubc9 (Boggio et al., 2004). By 24 hours post-infection, Gam1 was detected in HaCaT cells infected with Ad-Gam1, but not in cells infected with Ad-GFP (Figure 7B). In the Ad-GFP infected cells, the Ubc9 initially present increased 3.2-fold by 48 hours post calcium induction, consistent with the response seen in the absence of viral infection (see Fig. 5B). In contrast, cells expressing Gam1 showed diminished Ubc9 levels by 24 hours and no detectable Ubc9 by 48 hours when Gam1 levels had reached maximal expression. A similar effect was seen in co-infections with Ad-Gam1 and Ad-GFP, indicating that Ad-GFP had no stabilizing effect on Ubc9. In contrast to these infection studies where the transgene could be delivered effectively into nearly 100% of the cells, alternative attempts to inhibit sumoylation via transfection approaches were unsuccessful due to the poor transfection efficiency of HaCaT cells in low calcium medium (unpublished results).
Figure 7.
Inhibition of sumoylation perturbs normal HaCaT cell differentiation. (A) HaCaT cells undergoing calcium-induced differentiation exhibit abnormal morphology when expressing Gam1 protein. Shown are phase contrast and fluorescence microscopy images of HaCaT cell cultures at 24-hour intervals. HaCaT cells were infected with Ad-Gam1/Ad-GFP (upper panel; MOI 150/150) or Ad-GFP alone (middle and bottom panels; MOI 300) and induced to differentiate by switching to high calcium medium. For the bottom panel, 100 mM TSA (an HDAC inhibitor) was added 24 hours post-infection/induction and was re-added in fresh medium at 48-hour intervals. (B) Immunoblot analysis for Ubc9, Gam1, and α-tubulin in infected HaCaT cells. Cells were infected with the Ad-Gam1 (MOI 300), Ad-Gam1/Ad-GFP (MOI 150/150), or Ad-GFP (MOI 300) and induced to differentiate as in part (A). Cell extracts were prepared at the indicated times and immunoblotted as described in Materials and Methods. (C) Immunoblot analysis for keratin 1 and α-tubulin. Cells were infected with the indicated adenovirus constructs at an MOI of 300. Cell extracts were prepared and analyzed as in part (B).
When examined microscopically, HaCaT cells expressing Gam1 exhibited a disruption of the normal morphological changes associated with differentiation. By 72 hours after Ad-Gam1/Ad-GFP co-infection and calcium induction, when Ubc9 was absent, the differentiating HaCaT cells showed dramatic cellular rearrangements, including lose of tight cell-cell junctions and typical cuboidal shape (Figure 7A). This abnormal morphology was not observed in cells infected with Ad-GFP alone at any time during the differentiation process (Figure 7A). We also observed in the co-infected cultures that GFP expression increased throughout the experiment, while in cells infected with Ad-GFP alone the GFP expression gradually diminished and was almost undetectable by 120 hours post induction. This observation was consistent with Gam1’s known enhancing effect on transcription (Chiocca et al., 2002), and further demonstrated Gam1’s activity in HaCaT cells. Identical morphological abnormalities were also observed in cultures infected with Ad-Gam1 alone (data not shown). Additionally, when basal cultures were infected with Ad-Gam1 without subsequent calcium induction there was no change in cell morphology, while stably differentiated HaCaT cultures were susceptible to Gam1 (Supplemental Figure 1). The insensitivity of the basal cultures to Gam1 confirms that inhibition of sumoylation is not directly affecting growth or morphology of the keratinocytes in the absence of differentiation. In contrast, the susceptibility of the stably differentiated cells to Gam1 further links differentiation state with a sumoylation requirement.
In addition to its inhibition of sumoylation, Gam1 is also known to inhibit HDAC activity (Chiocca et al., 2002). To exclude the possibility that the observed morphological effects on HaCaT cells could be due to HDAC inhibition, we conducted the calcium induction in the presence of trichostatin A (TSA), a chemical inhibitor of HDACs (Yoshida and Horinouchi, 1999). Basal HaCaT cells were infected with Ad-GFP, induced with calcium, and then treated with 100 mM TSA at 24 hours after induction/infection. GFP expression was prolonged in the TSA-treated cultures compared to the untreated cells, consistent with TSA mediated enhancement of transcription through HDAC inhibition (data not shown). However, no abnormal morphology was observed during differentiation in TSA, indicating that HDAC inhibition does not contribute to the Gam1 effect on differentiating keratinocytes (Figure 7A).
In addition to keratinocyte morphology, the effect of Gam1 expression on the K1 differentiation marker was also evaluated. Gam1 caused a delay in and reduction of K1 expression in calcium induced cells (Figure 7C). While we cannot exclude that an unknown, off-target effect of Gam1 may also contribute to both the defects in morphology and marker expression, the simplest explanation is that these defects are related to the dramatic inhibition of sumoylation. Altogether, the results presented here suggest that modification with SUMO may be a key event for the proper timing of the steps leading to keratinocyte differentiation, and that sumoylation may be an important regulator for this biological process.
Discussion
Recent studies on myoblast differentiation (Riquelme et al., 2006), embryonic development (Yamaguchi et al., 2005), genital tissue maturation (Vigodner et al., 2006), and synaptic formation (Shalizi et al., 2006) highlight sumoylation not only as a modifier of individual proteins, but also as a global regulator of cellular development. In the present study, we investigated the role of sumoylation in another complex process, keratinocyte differentiation. Using an immortalized human keratinocyte line, HaCaT cells, we demonstrated that there was a coordinated transcriptional upregulation of many sumoylation system genes upon exposure to a high calcium environment, a well established signal for induction of keratinocyte differentiation (Eckert et al., 1997b). In particular, gene expression for Ubc9, the sole conjugating enzyme for the sumoylation process, was strongly activated. This transcriptional upregulation of Ubc9 and several other components of the sumoylation pathway led to increased expression of their respective proteins and to changes in the overall pattern of sumoylated substrates. Furthermore, in the absence of normal sumoylation, expression of a differentiation marker, K1 protein, was delayed, and normal keratinocyte morphogenesis was severely perturbed. In agreement with the HaCaT studies, both Ubc9 and SUMO proteins were expressed in organotypic cultures of normal human foreskin keratinocytes, consistent with an active sumoylation process in this stratified tissue. We also noted that Ubc9 immunostaining was most prominent in the intermediate layers of the epithelium just above the basal layer, suggesting increased accumulation of Ubc9 in early differentiation similar to that seen in calcium-induced HaCaT cells. These combined results strongly suggest that sumoylation helps to orchestrate proper steps of keratinocyte differentiation and is therefore a key regulator in this biological event.
One of the salient observations of our studies was the coordinate transcriptional activation of multiple sumoylation genes following exposure of basal HaCaT cells to high calcium culture conditions. Mechanistically, such coordination could be achieved through common promoter elements responding to calcium-induced TFs. High calcium concentration, both in vitro and in vivo, is known to trigger keratinocyte differentiation through multiple signaling pathways that activate a set of effector TFs (Fuchs, 1990; Sharpe et al., 1989), which includes NFAT, Sp1, C/EBP, and AP1 (Eckert et al., 1997a; Santini et al., 2001). While the promoters for the sumoylation component genes have not been experimentally characterized, bioinformatics analysis (www.genomatrix.de) of the putative promoter regions for the sumoylation genes tested in this study revealed the presence of binding sites for various combinations of the above TFs. There were single NFAT and AP1 binding sites and three Sp1 binding sites on the SUMO2 promoter; five Sp1 binding sites on the Ubc9 promoter; three C/EBP binding sites on the SAE1 promoter; and one Sp1 binding site on the predicted SAE2 promoter, consistent with all of these promoters being stimulated concurrently by calcium-responsive TFs. Concordantly, SUMO1 transcription exhibited little or no change upon calcium stimulation in HaCaT cells, and analysis of the SUMO1 putative promoter region did not reveal any binding sites for TFs directly activated by calcium. Lastly, the diversity of TF binding sites on the SUMO system promoters could also account for the quantitatively different transcriptional responses among the SUMO components that did respond to calcium signaling. Detailed promoter studies will be needed to determine which, if any, of these predicted TF binding sites are directly influencing transcriptional expression of the sumoylation genes.
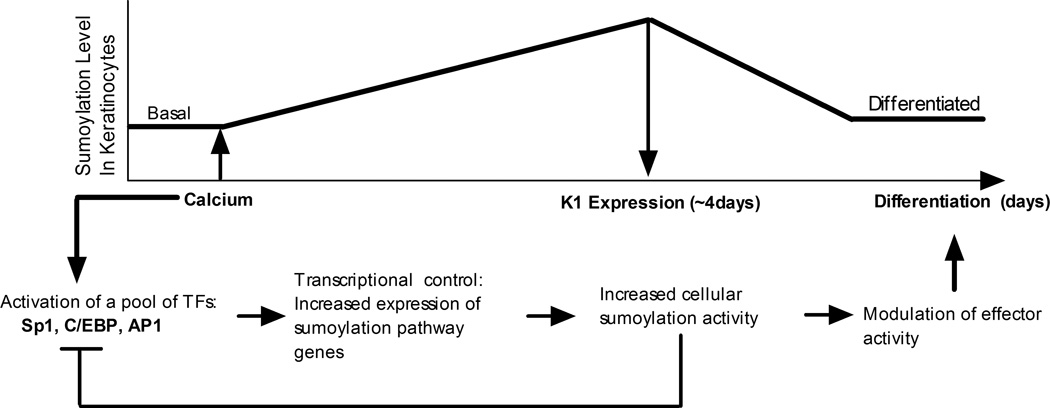
The observed upregulation of the sumoylation system appears to be a transient process associated with active differentiation, as overall transcript and protein levels for the sumoylation components vary only slightly between cells stably maintained in basal versus differentiated conditions (Figure 2). Since the differentiating HaCaT cells remain normally proliferative, it is likely that the sumoylation changes reflect differentiation-dependent events and not changes related to growth state. We propose a simple model (Figure 8) for downregulating the sumoylation response based on the known repressive effect that sumoylation exerts on the activity of most TFs (Gill, 2003). The relevant keratinocyte early differentiation transcription factors, SP1, C/EBP, and AP1, are all known to be SUMO targets that are negatively regulated by sumoylation (Bossis et al., 2005; Eaton and Sealy, 2003; Spengler and Brattain, 2006; Terui et al., 2004). As sumoylation activity increases following transcriptional upregulation of the SUMO pathway genes, it is likely that the above TFs would become SUMO modified and repressed, leading to a decline in transcription of the sumoylation genes. This negative feedback system would dampen the sumoylation response as differentiation progresses resulting in a return to a basal level of sumoylation activity in terminally differentiated cells.
Figure 8.
Model for differentiation-dependent changes in the sumoylation system. The model proposes a negative feedback mechanism to explain the transient increase in sumoylation observed during HaCaT cell differentiation. Initially, a pool of TFs, including Sp1, C/EBP, and AP1, which are known to be directly stimulated by calcium induced-differentiation, cause upregulation of the sumoylation system. As sumoylation activity increases, these TFs are in turn modified by SUMO conjugation which decreases their transcriptional activity and leads to a decline in expression of the sumoylation pathway genes. A second feature of the model is that increased sumoylation is an active contributor to the differentiation process, through SUMO conjugation to downstream effectors of the differentiation signals. Additional details are provided in the text.
Our transcriptional results also suggest that there are different roles for SUMO2/3 versus SUMO1 in keratinocyte differentiation, since we observed activation of the SUMO2 and 3 promoters, but not of the SUMO1 promoter. Additionally, we observed an increase in SENP1 protein levels during differentiation. SENP1 cleaves the precursor forms of all three SUMOs to produce their mature forms, but also exhibits preferential desumoylating activity for SUMO1-modified substrates compared to SUMO2/3-conjugates (Xu and Au, 2005). The net result of the transcriptional and proteolytic changes may be an overall decrease in SUMO1 conjugates with a corresponding increase in SUMO2/3 modified proteins. While the biological significance of such a change in SUMO type utilization is not known, growing evidence supports distinct functions for SUMO2/3 versus SUMO1. It is well established that SUMO1 has only 50% identity to SUMO2/3, while SUMO2 and SUMO3 are closely related (Saitoh and Hinchey, 2000). In a recent proteomics study, we demonstrated significant differences between the array of targets modified by SUMO1 versus SUMO3, indicating that these two modifications are not equivalent with regard to substrate preference (Rosas-Acosta et al., 2005b). Other studies have shown SUMO type-specific differences in cellular localization (Ayaydin and Dasso, 2004), response to external stimuli (Manza et al., 2004), and preference for SUMO ligases (Rosas-Acosta et al., 2005a; Tatham et al., 2005) and proteases (Gong and Yeh, 2006; Reverter and Lima, 2004), re-enforcing the concept that the individual SUMOs have both common and unique biological activities. Intriguingly, there is evidence that SUMO1 is more specific for nuclear substrates while SUMO2/3 targets are both nuclear and cytoplasmic proteins (Manza et al., 2004). Cytoplasmic events, such as formation of desmosomal junctions and specific cytoskeletal rearrangements, mark important aspects of keratinocytes differentiation (Hennings and Holbrook, 1983; Yin and Green, 2004). Preventing sumoylation by Gam1 expression during HaCaT cell differentiation led to a severe disruption of cell shape, implicating sumoylation as requisite for these morphological changes. A direct cytoplasmic role for SUMO2/3 modification may contribute to the dramatic cytoskeletal changes that accompany keratinocyte differentiation.
In conjunction with a possible direct modification of cytoplasmic proteins, the observed increase in sumoylation activity following calcium signaling likely contributes broadly to the keratinocyte differentiation process through effects on nuclear TFs (Figure 8). A recent publication by Riquelme et al also demonstrated a sumoylation requirement during myogenesis (Riquelme et al., 2006): when the sumoylation system was inhibited, myoblast fusion-deficient phenotypes were observed. Although in their system they demonstrated an overall decrease of sumoylation during myogenesis, they showed that further decreasing sumoylation using a siRNA to Ubc9 dramatically affected overall myogenic differentiation and cellular fusion. Additionally, Shaliz et al demonstrated the importance of sumoylation in a neuronal differentiation system. Using granule neurons, they showed that the MEF2 TF is activated through a calcium signaling pathway in these cells, and that sumoylation of MEF2 is necessary to promote differentiation of dendritic claw structures (Shalizi et al., 2006). Thus, both these studies and our own strongly support an important role for sumoylation in cellular differentiation. Because inactivation of proliferation-enhancing TFs, such as p63 (Ghioni et al., 2005), has already been shown to be a prerequisite for keratinocyte differentiation, we propose that enhanced sumoylation facilitates this inactivation through direct modification of these or other targets. Ongoing proteomic studies in our laboratory are cataloging and identifying substrates whose sumoylation status changes during HaCaT differentiation (data not shown).
In conclusion, this study identifies sumoylation as a potential regulator in skin biology. Calcium signaling stimulates expression of the sumoylation system leading to changes in the sumoylation state of target proteins and allowing proper keratinocyte differentiation. Calcium signaling is also an important mediator of lymphocyte activation and differentiation (Freedman, 2006), oesteoblastic activity and bone formation (Henriksen et al., 2006), neuronal stem cell development (D'Ascenzo et al., 2006), and smooth muscle formation (Wamhoff et al., 2006). While the intermediate signals are largely unknown for these processes, our work suggests that sumoylation may be modulated in each of these systems through calcium-responsive transcription factors. Therefore, the interaction between calcium signaling and the sumoylation system may also be playing a critical role for proper development of these and other tissues. Lastly, many skin diseases, such as psoriasis, skin cancer (Eckert et al., 2004), or infections due to microbial pathogens (Alfandari et al., 1999), hijack the normal process of keratinocyte differentiation and lead to abnormal skin formation. If dysregulation of sumoylation is contributing to an aberrant differentiation process, then modulating sumoylation may have therapeutic benefits for the treatment for those diseases.
Materials and Methods
Cell Culture
HaCaT cells (kindly provided by Dr. Bokoch, The Scripps Research Institute, La Jolla, CA) were cultured in calcium-free DMEM (HyClone), with calcium-depleted 10% FBS (Gemini Bioproducts), 4 mM L-Glutamine (HyClone), and supplemented with calcium chloride to a final concentration of 0.03 mM (low calcium medium) or 2.8 mM final concentration (high calcium medium). FBS was calcium-depleted by incubation with Chelex 100 resin (Bio-Rad Laboratories, Inc.) for 1 hour at 4°C according to the manufacturer’s protocol. The Chelex was subsequently removed using a 50 ml Millipore 0.22 µm filter unit system (Millipore Corp). To obtain cells exhibiting a basal keratinocyte phenotype, HaCaT cells were cultured in low calcium medium for at least 3 weeks and were maintained in the same culture conditions thereafter. Low calcium HaCaT cultures were never allowed to exceed 85% confluency. For stably differentiated HaCaT cells, the cultures were maintained in high calcium medium for at least 3 weeks prior to analysis. HaCaT cultures in either medium remained fully proliferative with similar doubling times (unpublished observations). Human embryonic kidney 293A (HEK 293A) cells (Invitrogen Corp.) were maintained in DMEM supplemented with 10% FBS (Gemini Bioproducts). For the trichostatin A (TSA) experiment, HaCaT cells were treated with 100 mM TSA (Tocris Bioscience) 24 hours after infection with an adenoviral vector expressing GFP (Ad-GFP) and calcium induction. HaCaT cells were observed up to 144 hours post calcium induction.
RT-PCR and quantitative RT-PCR
All RNAs were extracted using the RNAqueous kit (Ambion). RNA concentration was measured using a spectrophotometer and 5 ng/µl aliquots were stored at −80°C until use. All primers were designed to overlap exon-exon junctions, therefore only amplifying the cDNAs targets and not the genomic sequences (primers sequences are provided in the supplemental Tables 1 and 2). The one-step RT-PCR mixture contained 50 ng of RNA in a final reaction volume of 25 µl. The mixture also included 2.5 µl of 10X Taq DNA Polymerase buffer (Promega), 50 units of MMLV reverse transcriptase, 1 unit of Platinum Taq (Invitrogen Corp.), 4 mM dNTPs, 20 units of RNAse OUT (Invitrogen Corp.), 6 mM of MgCl2, and 0.4 µM of each primer. The one step RT-PCR was performed for 20 minutes at 42°C for the reverse transcription step, followed by 90 seconds at 94°C, and 40 cycles of amplification (94°C for 30 seconds; 60°C for 30 seconds; 72°C for 60 seconds). Amplifications were performed in a PTC-200 Peltier Thermal Cycler machine (MJ Research, Inc.). Amplified products were analyzed on 2.5% agarose gels and visualized with an Innotech Alphaimager system (Alpha Innotech Corp.). For the quantitative RT- PCR (Q-PCR), one step RT reactions were performed in a single well using 50 ng of harvested RNA in a 50 µl final volume. In addition to the RT components described above, reactions for Q-PCR contained 0.2 µM of LUX primers (designed with the Invitrogen custom primer software), 20 units of RNAse OUT, 2 µl of Super Script III enzyme solution (which includes MMLV-RT, Platinum Taq, and dNTPs), and 1 µl of Rox dye , all from Invitrogen Corp. The LUX β-actin primer set (Invitrogen) was used to detect the internal control gene. The PCR reaction conditions for Q-PCR were the same as the RT-PCR described above. The Q-PCR plates were read with an ABI 7500 real time PCR instrument (Applied Biosystems), and detection of the FAM or JOE label was recorded during the 72°C step. Results were graphed as the fold increase of the relative quantitative RQ values where RQ =2(−ΔΔCt). ΔCt was calculated as the average Ct for β-actin minus the average Ct for the gene of interest, with each sample being run in duplicate. Using the ΔCt values from each time point, the ΔΔCt for each mRNA examined was calculated as the ΔCt of the time 0 sample minus the ΔCt of time X (in hours) after induction. The data shown are the average from at least 3 independent RNA preparations collected in separate experiments.
Affinity purified polyclonal antibodies against Ubc9
Rabbit polyclonal serum #12741 was produced in-house using affinity purified Ubc9 as the immunogen. A four immunization regime was followed, and 2 weeks after the final boost the rabbit was exsanguinated. To affinity purify anti-Ubc9 antibodies, 750 µg of affinity-purified GST-Ubc9 were diluted in a final volume of 500 µl using 1× PBS, and the resulting dilution was dispensed on an 82 mm diameter, 0.45 µm pore size, Protran Nitrocellulose filter (Schleicher & Schuell Inc.). The membrane was dried for 30 minutes at room temperature, then re-wetted and blocked by incubation at room temperature for 30 minutes in 15 ml of 1× PBS supplemented with 1% BSA. The blocked membrane was incubated for 2 hours at room temperature with 10 ml of a solution containing 2.5 ml of rabbit polyclonal serum #12741, and 7.5 ml of 1× PBS supplemented with 10 mg/ml BSA and 0.05% Tween 20. The membrane was subsequently washed four times each with 15 ml of 1× PBS supplemented with 0.05% Tween 20, and four additional times each with 15 ml of 1× PBS alone. The bound antibodies were eluted by incubation with 2 ml of elution buffer (100 mM glycine, pH 2.5, 0.02% NaN3) for 5 minutes at room temperature. The eluted antibodies were neutralized with 200 µl of 1.0 M Tris-Cl (pH 8.0), aliquoted, and stored at −70°C. The purity of the affinity-purified antibodies was determined by gel electrophoresis and immunoblotting, and its reactivity against Ubc9 was determined by immunoblotting using purified Ubc9 and unfractionated mammalian cell extracts.
Immunohistochemistry
Human foreskin keratinocytes were grown in organotypic raft cultures to form stratified epithelium as previously described (Lambert et al., 2005). All chemicals used for immunohistochemistry were from Biocare Medical (Concord, CA). Slides were deparafinized in xylene and re-hydrated according to the manufacturer’s protocols. The following antibodies and dilutions were used: anti-SUMO serum #12783 (Rosas-Acosta et al., 2005a), 1:750; affinity purified polyclonal antibodies against Ubc9, 1:20; and polyclonal anti-human keratin 1 (anti-HK1, Covance, Berkeley, CA), 1:5000. Specificity control blocking experiments were performed by adding the corresponding purified proteins (25 µg of Ubc9 or 7 µg of SUMO1) to the cognate antibody dilution. The SUMO and Ubc9 proteins used in the experiments were purified as previously described (Rosas-Acosta et al., 2005a). After immunostaining, the slides were counterstained with hematoxylin.
Immunoblots and densitometry
Total cell extracts were prepared by adding a 1:1 (V/V) mixture of RIPA buffer (50 mM Tris-Cl, pH8, 150 mM NaCl, 5 mM EDTA, 15 mM MgCl2, 1% NP40, 0.1% SDS, 1 mM DTT, 1:200 protease inhibitor cocktail and 10 mM N-ethylmaleimide) and 4 × sample buffer (100 mM Tris-Cl, pH 6.8, 20% glycerol, 8% SDS, 0.02% bromophenol blue, 4% β-mercaptoethanol) directly to the cells. The cells were shaken gently for 5–10 seconds, and the resulting lysate was collected by pipeting. Samples were heated at 95°C for 5 minutes and sonicated for 30 seconds using a Misonix sonicator 3000 (Misonix, Inc.). Samples were electrophoresed on 10% or 12.5% polyacrylamide gels and then transferred onto 0.45 µm Immobilon-P membranes (Millipore). The membranes were blocked for at least 15 minutes with 3% non-fat milk in TTBS (150 mM NaCl, 50 mM Tris-Cl, pH 7.4, 0.005% Tween 20), and incubated for 1 hour or over night with the primary antibodies listed below at the indicated dilution: rabbit anti α-tubulin (Santa Cruz Biotechnology Inc.), 1:15,000; anti-c-Myc monoclonal antibody (Santa Cruz Biotechnology Inc.), 1:500; anti-RanGAP monoclonal antibody (Zymed/Invitrogen Corp.), 1:2,500; rabbit serum #12783 against SUMO (Rosas-Acosta et al., 2005b), 1:1,000; affinity-purified polyclonal antibodies against Ubc9, 1:500; anti-human K1 rabbit serum (Covance), 1:1000; anti-SAE1 sheep serum (Axxora), 1:2,000; anti-SENP1 rabbit serum (Imgenex Corp.), 1:2,000; and anti-involucrin rabbit serum (LabVision Corp.), 1:1,000. After reaction with the primary antibodies, the membranes were incubated with Horseradish Peroxidase-conjugated antibodies (Santa Cruz Biotechnology) at 1:10,000 for one hour. The membranes were subsequently rinsed in TTBS, treated with the Western Lightning Chemiluminescence reagent (PerkinElmer Life And Analytical Sciences, Inc.), and then visualized with X-ray film. Quantitative differences were determined by densitometry using an Innotech Alphaimager (Alpha Innotech Corp.) and were normalized to the α-tubulin signal. Quantitative results are the average of at least 3 separate experiments.
Virus production and infection
C-Myc tagged Gam1 adenoviral DNA (Ad-Gam1) was kindly provided by Dr. Matt Cotten (GPC-biotech, Munich, Germany). The Ad-Gam1 DNA was transfected into HEK 293A cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s recommendation. Cells were lysed by freeze-thaw and the supernatant was collected. The initial viral stock was subsequently amplified with two rounds of infection on HEK 293A cells and the final freeze-thaw supernatant collected as the high-titer stock. Adenovirus expressing GFP (Ad-GFP) was kindly provided by Dr. G. Davis (Texas A&M Health Science Center, College Station, TX) and amplification was performed as above. Titer was assessed by the limiting dilution method (Qbiogene Adenovirus Manual, version 1.4) using HEK 293A cells plated at 1 × 106 cells/well on 6-well plates. For adenovirus experiments, HaCaT cells maintained in low calcium medium were plated at 5 × 106 in T75 flasks 20 hours prior infection. Each culture was infected at an MOI of 300 in 3 ml of calcium-free medium supplemented with 8 µg/ml polybrene (Fisher Scientific). Three hours after infection the medium was removed, cells were trypsinized, and released cells were resuspended in 10 ml of high calcium medium to induce differentiation. The resulting cells suspension was split among the wells of a 6 well plate at a ratio such that the cells in each well would achieve approximately 80% confluency by the time of collection. Cells were maintained in high calcium medium and harvested at various times post plating by direct lysis in the wells using the 1X RIPA:4X sample buffer mixture described above. Proteins were analyzed by immumoblotting as described in the previous section. Cells were visualized by phase contrast and fluorescence microscopy at a magnification of 200X using an Olympus IX70 microscope. Images were captured digitally using a Qcolor3 camera (Olympus).
Supplementary Material
Inhibition of sumoylation by Gam1 perturbs cell morphology of stably differentiated HaCaT cultures, but not of basal HaCaT cultures. HaCaT cultures were maintained in low (BASAL) or high calcium (DIFFERENTIATED) medium for at least 3 weeks prior to infection with Ad-Gam1 or Ad-GFP as indicated. Infections were at an MOI of 300 for each virus. The DIFFERENTIATED HaCaT culture infected with Ad-GFP was also treated with 100mM TSA as in Figure 7. Phase contrast (black and white) and fluorescence microscopy (green) images were taken every 24 hours for 3 days.
Acknowledgements
We thank Dr. Gary Bokoch for the providing us with the HaCaT cell line, Dr. Matthew Cotten for providing us with the Gam1 adenoviral DNA, Dr. George Davis for the Ad-GFP vector, and Dr. Wayne Sampson for assistance with the immunohistochemistry. This research was supported by a grant from the National Institutes of Health (CA089298).
References
- Alfandari J, Magal SS, Jackman A, Schlegel R, Gonen P, Sherman L. HPV16 E6 oncoprotein inhibits apoptosis induced during serum-calcium differentiation of foreskin human keratinocytes. Virology. 1999;257:383–396. doi: 10.1006/viro.1999.9675. [DOI] [PubMed] [Google Scholar]
- Arco PGD, Koipally J, Georgopoulos K. Ikaros SUMOylation: Switching out of repression. Mol. Cell. Biol. 2005;25:2688–2697. doi: 10.1128/MCB.25.7.2688-2697.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaydin F, Dasso M. Distinct in vivo dynamics of vertebrate SUMO paralogues. Mol. Biol. Cell. 2004;15:5208–5218. doi: 10.1091/mbc.E04-07-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma Y, Tan SH, Cavenagh MM, Ainsztein AM, Saitoh H, Dasso M. Expression and regulation of the mammalian SUMO-1 E1 enzyme. FASEB J. 2001;15:1825–1827. doi: 10.1096/fj.00-0818fje. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Ng D, Tu CL, Oda Y, Xie Z. Calcium- and vitamin D-regulated keratinocyte differentiation. Mol. Cell. Endocrinol. 2001;177:161–171. doi: 10.1016/s0303-7207(01)00452-x. [DOI] [PubMed] [Google Scholar]
- Boggio R, Colombo R, Hay RT, Draetta GF, Chiocca S. A mechanism for inhibiting the SUMO pathway. Mol. Cell. 2004;16:549–561. doi: 10.1016/j.molcel.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Bohren KM, Nadkarni V, Song JH, Gabbay KH, Owerbach D. A M55V polymorphism in a novel SUMO gene (SUMO-4) differentially activates heat shock transcription factors and is associated with susceptibility to type I diabetes mellitus. J. Biol. Chem. 2004;279:27233–27238. doi: 10.1074/jbc.M402273200. [DOI] [PubMed] [Google Scholar]
- Bossis G, Malnou CE, Farras R, Andermarcher E, Hipskind R, Rodriguez M, Schmidt D, Muller S, Jariel-Encontre I, Piechaczyk M. Down-regulation of c-Fos/c-Jun AP-1 Dimer activity by sumoylation. Mol. Cell. Biol. 2005;25:6964–6979. doi: 10.1128/MCB.25.16.6964-6979.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat. Rev. Mol. Cell. Biol. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- Capone A, Visco V, Belleudi F, Marchese C, Carinali G, Bellocci M, Picardo M, Frati L, Torrisi MR. Up-modulation of the expression of functional keratinocyte growth factor receptor induced by high cell density in the human keratinocyte HaCaT cell line. Cell Growth Diff. 2000;11:607–614. [PubMed] [Google Scholar]
- Chen A, Mannen H, Li SS. Characterization of mouse ubiquitin-like SMT3A and SMT3B cDNAs and gene/pseudogenes. Bioch. Mol. Biol. Intern. 1998;46:1161–1174. doi: 10.1080/15216549800204722. [DOI] [PubMed] [Google Scholar]
- Chiocca S, Kurtev V, Colombo R, Boggio R, Sciurpi MT, Brosch G, Seiser C, Draetta GF, Cotten M. Histone deacetylase 1 inactivation by an adenovirus early gene product. Curr.Biol. 2002;12:594–598. doi: 10.1016/s0960-9822(02)00720-0. [DOI] [PubMed] [Google Scholar]
- D'Ascenzo M, Piacentini R, Casalbore P, Budoni M, Pallini R, Azzena GB, Grassi C. Role of L-type Ca2+ channels in neural stem/progenitor cell differentiation. Eur. J. Neurosci. 2006;23:935–944. doi: 10.1111/j.1460-9568.2006.04628.x. [DOI] [PubMed] [Google Scholar]
- Desterro JM, Rodriguez MS, Hay RT. SUMO-1 modification of IkappaBalpha inhibits NF-kappaB activation. Mol.Cell. 1998;2:233–239. doi: 10.1016/s1097-2765(00)80133-1. [DOI] [PubMed] [Google Scholar]
- Desterro JM, Thomson J, Hay RT. Ubch9 conjugates SUMO but not ubiquitin. FEBS Lett. 1997;417:297–300. doi: 10.1016/s0014-5793(97)01305-7. [DOI] [PubMed] [Google Scholar]
- Eaton EM, Sealy L. Modification of CCAAT/enhancer-binding protein-beta by the small ubiquitin-like modifier (SUMO) family members, SUMO-2 and SUMO-3. J. Biol. Chem. 2003;278:33416–33421. doi: 10.1074/jbc.M305680200. [DOI] [PubMed] [Google Scholar]
- Eckert RL, Crish JF, Banks EB, Welter JF. The epidermis: genes on - genes off. J. Invest. Dermatol. 1997a;109:501–509. doi: 10.1111/1523-1747.ep12336477. [DOI] [PubMed] [Google Scholar]
- Eckert RL, Crish JF, Efimova T, Balasubramanian S. Antioxidants regulate normal human keratinocyte differentiation. Biochem. Pharmacol. 2004;68:1125–1131. doi: 10.1016/j.bcp.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Eckert RL, Crish JF, Robinson NA. The epidermal keratinocyte as a model for the study of gene regulation and cell differentiation. Physiol. Rev. 1997b;77:397–424. doi: 10.1152/physrev.1997.77.2.397. [DOI] [PubMed] [Google Scholar]
- Eichner R, Sun TT, Aebi U. The role of keratin subfamilies and keratin pairs in the formation of human epidermal intermediate filaments. J. Cell Biol. 1986;102:1767–1777. doi: 10.1083/jcb.102.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eloranta JJ, Hurst HC. Transcription factor AP-2 interacts with the SUMO-conjugating enzyme UBC9 and is sumolated in vivo. J. Biol. Chem. 2002;277:30798–30804. doi: 10.1074/jbc.M202780200. [DOI] [PubMed] [Google Scholar]
- Freedman BD. Mechanisms of calcium signaling and function in lymphocytes. Crit. Rev. Immunol. 2006;26:97–111. doi: 10.1615/critrevimmunol.v26.i2.10. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Epidermal differentiation: the bare essentials. J Cell Biol. 1990;111:2807–2814. doi: 10.1083/jcb.111.6.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980;19:1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Ghioni P, D'Alessandra Y, Mansueto G, Jaffray E, Hay RT, La Mantia G, Guerrini L. The protein stability and transcriptional activity of p63 alpha are regulated by SUMO-1 conjugation. Cell Cycle. 2005;4:183–190. doi: 10.4161/cc.4.1.1359. [DOI] [PubMed] [Google Scholar]
- Gill G. Post-translational modification by the small ubiquitin-related modifier SUMO has big effects on transcription factor activity. Curr.Opin. Gen. Develop. 2003;13:108–113. doi: 10.1016/s0959-437x(03)00021-2. [DOI] [PubMed] [Google Scholar]
- Girdwood DWH, Tatham MH, Hay RT. SUMO and transcriptional regulation. Sem. Cell Develop.Biol. 2004;15:201–210. doi: 10.1016/j.semcdb.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Gong L, Yeh ET. Characterization of a Family of Nucleolar SUMO-specific Proteases with Preference for SUMO-2 or SUMO-3. J. Biol. Chem. 2006;281:15869–15877. doi: 10.1074/jbc.M511658200. [DOI] [PubMed] [Google Scholar]
- Guo DH, Han JY, Adam BL, Colburn NH, Wang MH, Dong Z, Eizirik DL, She JX, Wang CY. Proteomic analysis of SUM04 substrates in HEK293 cells under serum starvation-induced stress. Biochem. Biophys. Res. Comm. 2005;337:1308–1318. doi: 10.1016/j.bbrc.2005.09.191. [DOI] [PubMed] [Google Scholar]
- Hennings H, Holbrook KA. Calcium regulation of cell-cell contact and differentiation of epidermal cells in culture. An ultrastructural study. Exp. Cell Res. 1983;143:127–142. doi: 10.1016/0014-4827(83)90115-5. [DOI] [PubMed] [Google Scholar]
- Henriksen Z, Hiken JF, Steinberg TH, Jorgensen NR. The predominant mechanism of intercellular calcium wave propagation changes during long-term culture of human osteoblast-like cells. Cell Calcium. 2006;39:435–444. doi: 10.1016/j.ceca.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Herwig S, Strauss M. The retinoblastoma protein: a master regulator of cell cycle, differentiation and apoptosis. Eur. J. Biochem. 1997;246:581–601. doi: 10.1111/j.1432-1033.1997.t01-2-00581.x. [DOI] [PubMed] [Google Scholar]
- Ihara M, Yamamoto H, Kikuchi A. SUMO-1 modification of PIASy, an E3 ligase, is necessary for PIASy-dependent activation of Tcf-4. Mol. Cell. Biol. 2005;25:3506–3518. doi: 10.1128/MCB.25.9.3506-3518.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ES. Protein modification by SUMO. Ann. Review Biochem. 2004;73:355–382. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- Kagey MH, Melhuish TA, Wotton D. The polycomb protein Pc2 is a SUMO E3. Cell. 2003;113:127–137. doi: 10.1016/s0092-8674(03)00159-4. [DOI] [PubMed] [Google Scholar]
- Kim J, Cantwell CA, Johnson PF, Pfarr CM, Williams SC. Transcriptional activity of CCAAT/enhancer-binding proteins is controlled by a conserved inhibitory domain that is a target for sumoylation. J. Biol.Chem. 2002;277:38037–38044. doi: 10.1074/jbc.M207235200. [DOI] [PubMed] [Google Scholar]
- Kotaja N, Karvonen U, Janne OA, Palvimo JJ. PIAS proteins modulate transcription factors by functioning as SUMO-1 ligases. Mol. Cell. Biol. 2002;22:5222–5234. doi: 10.1128/MCB.22.14.5222-5234.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert PF, Ozbun MA, Collins A, Holmgren S, Lee D, Nakahara T. Using an immortalized cell line to study the HPV life cycle in organotypic "raft" cultures. Human Papillomaviruses: Methods and Protocols. 2005;vol. 119:141–155. doi: 10.1385/1-59259-982-6:141. [DOI] [PubMed] [Google Scholar]
- Lansdown AB. Calcium: a potential central regulator in wound healing in the skin. Wound Repair Regen. 2002;10:271–285. doi: 10.1046/j.1524-475x.2002.10502.x. [DOI] [PubMed] [Google Scholar]
- Ledl A, Schmidt D, Muller S. Viral oncoproteins E1A and E7 and cellular LxCxE proteins repress SUMO modification of the retinoblastoma tumor suppressor. Oncogene. 2005;24:3810–3818. doi: 10.1038/sj.onc.1208539. [DOI] [PubMed] [Google Scholar]
- Leight ER, Glossip D, Kornfeld K. Sumoylation of LIN-1 promotes transcriptional repression and inhibition of vulval cell fates. Development. 2005;132:1047–1056. doi: 10.1242/dev.01664. [DOI] [PubMed] [Google Scholar]
- Li M, Kellems RE. Sp1 and Sp3 Are important regulators of AP-2gamma gene transcription. Biol. Reprod. 2003;69:1220–1230. doi: 10.1095/biolreprod.103.015545. [DOI] [PubMed] [Google Scholar]
- Manza LL, Codreanu SG, Stamer SL, Smith DL, Wells KS, Roberts RL, Liebler DC. Global shifts in protein sumoylation in response to electrophile and oxidative stress. Chem.Res. Toxicol. 2004;17:1706–1715. doi: 10.1021/tx049767l. [DOI] [PubMed] [Google Scholar]
- Melchior F, Hengst L. SUMO-1 and p53. Cell Cycle. 2002;1:245–249. [PubMed] [Google Scholar]
- Melchior F, Schergaut M, Pichler A. SUMO: ligases, isopeptidases and nuclear pores. Trends Biochem. Sci. 2003;28:612–618. doi: 10.1016/j.tibs.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Menon GK, Grayson S, Elias PM. Ionic calcium reservoirs in mammalian epidermis: ultrastructural localization by ion-capture cytochemistry. J. Invest. Dermatol. 1985;84:508–512. doi: 10.1111/1523-1747.ep12273485. [DOI] [PubMed] [Google Scholar]
- Morita Y, Kanei-Ishii C, Nomura T, Ishii S. TRAF7 sequesters c-Myb to the cytoplasm by stimulating its sumoylation. Mol. Biol. Cell. 2005;16:5433–5444. doi: 10.1091/mbc.E05-08-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossessova E, Lima CD. Ulp1-SUMO crystal structure and genetic analysis reveal conserved interactions and a regulatory element essential for cell growth in yeast. Mol. Cell. 2000;5:865–876. doi: 10.1016/s1097-2765(00)80326-3. [DOI] [PubMed] [Google Scholar]
- Nacerddine K, Lehembre F, Bhaumik M, Artus J, Cohen-Tannoudji M, Babinet C, Pandolfi PP, Dejean A. The SUMO pathway is essential for nuclear integrity and chromosome segregation in mice. Develop. Cell. 2005;9:769–779. doi: 10.1016/j.devcel.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Okuma T, Honda R, Ichikawa G, Tsumagari N, Yasuda H. In vitro SUMO-1 modification requires two enzymatic steps, E1 and E2. Biochem. Biophys. Res. Comm. 1999;254:693–698. doi: 10.1006/bbrc.1998.9995. [DOI] [PubMed] [Google Scholar]
- Pichler A, Gast A, Seeler JS, Dejean A, Melchior F. The nucleoporin RanBP2 has SUMO1 E3 ligase activity. Cell. 2002;108:109–120. doi: 10.1016/s0092-8674(01)00633-x. [DOI] [PubMed] [Google Scholar]
- Pichler A, Melchior F. Ubiquitin-related modifier SUMO1 and nucleocytoplasmic transport. Traffic. 2002;3:381–387. doi: 10.1034/j.1600-0854.2002.30601.x. [DOI] [PubMed] [Google Scholar]
- Poulin G, Dong Y, Fraser AG, Hopper NA, Ahringer J. Chromatin regulation and sumoylation in the inhibition of Ras-induced vulval development in Caenorhabditis elegans. EMBO J. 2005;24:2613–2623. doi: 10.1038/sj.emboj.7600726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reverter D, Lima CD. A basis for SUMO protease specificity provided by analysis of human Senp2 and a Senp2-SUMO complex. Structure. 2004;12:1519–1531. doi: 10.1016/j.str.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Riquelme C, Barthel KK, Qin XF, Liu X. Ubc9 expression is essential for myotube formation in C2C12. Exp. Cell Res. 2006;12:2132–2141. doi: 10.1016/j.yexcr.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Rosas-Acosta G, Langereis MA, Deyrieux A, Wilson VG. Proteins of the PIAS family enhance the sumoylation of the papillomavirus E1 protein. Virology. 2005a;331:190–203. doi: 10.1016/j.virol.2004.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Acosta G, Russell WK, Deyrieux A, Russell DH, Wilson VG. A universal strategy for proteomic studies of SUMO and other ubiquitin-like modifiers. Mol.Cell. Proteom. 2005b;4:56–72. doi: 10.1074/mcp.M400149-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S, Best JL, Zon LI, Gill G. SUMO-1 modification represses Sp3 transcriptional activation and modulates its subnuclear localization. Mol. Cell. 2002;10:831–842. doi: 10.1016/s1097-2765(02)00682-2. [DOI] [PubMed] [Google Scholar]
- Saitoh H, Hinchey J. Functional heterogeneity of small ubiquitin-related protein modifiers SUMO-1 versus SUMO-2/3. J. Biol. Chem. 2000;275:6252–6258. doi: 10.1074/jbc.275.9.6252. [DOI] [PubMed] [Google Scholar]
- Salinas S, Briancon-Marjollet A, Bossis G, Lopez MA, Piechaczyk M, Jariel-Encontre I, Debant A, Hipskind RA. SUMOylation regulates nucleo-cytoplasmic shuttling of Elk-1. J. Cell Biol. 2004;165:767–773. doi: 10.1083/jcb.200310136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini MP, Talora C, Seki T, Bolgan L, Dotto GP. Cross talk among calcineurin, Sp1/Sp3, and NFAT in control of p21(WAF1/CIP1) expression in keratinocyte differentiation. Proc. Natl. Acad. Sci. USA. 2001;98:9575–9580. doi: 10.1073/pnas.161299698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoop VM, Mirancea N, Fusenig NE. Epidermal organization and differentiation of HaCaT keratinocytes in organotypic coculture with human dermal fibroblasts. J. Invest. Dermatol. 1999;112:343–353. doi: 10.1046/j.1523-1747.1999.00524.x. [DOI] [PubMed] [Google Scholar]
- Schweizer J, Winter H. Keratin biosynthesis in normal mouse epithelia and in squamous cell carcinomas. mRNA-dependent alterations of the primary structure of distinct keratin subunits in tumors. J. Biol. Chem. 1983;258:13268–13272. [PubMed] [Google Scholar]
- Seeler JS, Dejean A. SUMO: of branched proteins and nuclear bodies. Oncogene. 2001;20:7243–7249. doi: 10.1038/sj.onc.1204758. [DOI] [PubMed] [Google Scholar]
- Shalizi A, Gaudilliere B, Yuan ZQ, Stegmuller J, Shirogane T, Ge QY, Tan Y, Schulman B, Harper JW, Bonni A. A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science. 2006;311:1012–1017. doi: 10.1126/science.1122513. [DOI] [PubMed] [Google Scholar]
- Sharpe GR, Gillespie JI, Greenwell JR. An increase in intracellular free calcium is an early event during differentiation of cultured human keratinocytes. FEBS Lett. 1989;254:25–28. doi: 10.1016/0014-5793(89)81002-6. [DOI] [PubMed] [Google Scholar]
- Shiio Y, Eisenman RN. Histone sumoylation is associated with transcriptional repression. Proc. Natl. Acad. Sci. USA. 2003;100:13225–13230. doi: 10.1073/pnas.1735528100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith F. The molecular genetics of keratin disorders. Am. J. Clin. Dermatol. 2003;4:347–364. doi: 10.2165/00128071-200304050-00005. [DOI] [PubMed] [Google Scholar]
- Spengler ML, Brattain MG. Sumoylation inhibits cleavage of Sp1 N-terminal negative regulatory domain and inhibits Sp1-dependent transcription. J. Biol. Chem. 2006;281:5567–5574. doi: 10.1074/jbc.M600035200. [DOI] [PubMed] [Google Scholar]
- Su HL, Li SSL. Molecular features of human ubiquitin-like SUMO genes and their encoded proteins. Gene. 2002;296:65–73. doi: 10.1016/s0378-1119(02)00843-0. [DOI] [PubMed] [Google Scholar]
- Sun TT, Tseng SC, Huang AJ, Cooper D, Schermer A, Lynch MH, Weiss R, Eichner R. Monoclonal antibody studies of mammalian epithelial keratins: a review. Ann. N.Y. Acad. Sci. 1985;455:307–329. doi: 10.1111/j.1749-6632.1985.tb50419.x. [DOI] [PubMed] [Google Scholar]
- Tatham MH, Chen Y, Hay RT. Role of two residues proximal to the active site of Ubc9 in substrate recognition by the Ubc9 center dot SUMO-1 thiolester complex. Biochem. 2003;42:3168–3179. doi: 10.1021/bi026861x. [DOI] [PubMed] [Google Scholar]
- Tatham MH, Kim S, Jaffray E, Song J, Chen Y, Hay RT. Unique binding interactions among Ubc9, SUMO and RanBP2 reveal a mechanism for SUMO paralog selection. Nature Struct. Mol. Biol. 2005;12:67–74. doi: 10.1038/nsmb878. [DOI] [PubMed] [Google Scholar]
- Terui Y, Saad N, Jia S, McKeon F, Yuan JY. Dual role of sumoylation in the nuclear localization and transcriptional activation of NFAT1. J. Biol. Chem. 2004;279:28257–28265. doi: 10.1074/jbc.M403153200. [DOI] [PubMed] [Google Scholar]
- Tu CL, Oda Y, Komuves L, Bikle DD. The role of the calcium-sensing receptor in epidermal differentiation. Cell Calcium. 2004;35:265–273. doi: 10.1016/j.ceca.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Verger A, Perdomo J, Crossley M. Modification with SUMO - A role in transcriptional regulation. EMBO Reports. 2003;4:137–142. doi: 10.1038/sj.embor.embor738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicanova J, Boelsma E, Mommaas AM, Kempenaar JA, Forslind B, Pallon J, Egelrud T, Koerten HK, Ponec M. Normalization of epidermal calcium distribution profile in reconstructed human epidermis is related to improvement of terminal differentiation and stratum corneum barrier formation. J. Invest. Dermatol. 1998;111:97–106. doi: 10.1046/j.1523-1747.1998.00251.x. [DOI] [PubMed] [Google Scholar]
- Vigodner M, Ishikawa T, Schlegel PN, Morris PL. SUMO-1, human male germ cell development, and the androgen receptor in the testis of men with normal and abnormal spermatogenesis. Amer. J. Physiol. Endocrinol. Metabol. 2006;290:E1022–E1033. doi: 10.1152/ajpendo.00527.2005. [DOI] [PubMed] [Google Scholar]
- Wamhoff BR, Bowles DK, Owens GK. Excitation-transcription coupling in arterial smooth muscle. Circ. Res. 2006;98:868–878. doi: 10.1161/01.RES.0000216596.73005.3c. [DOI] [PubMed] [Google Scholar]
- Wilson VG, Rangasamy D. Intracellular targeting of proteins by sumoylation. Exp. Cell Res. 2001;271:57–65. doi: 10.1006/excr.2001.5366. [DOI] [PubMed] [Google Scholar]
- Xu Z, Au SWN. Mapping residues of SUMO precursors essential in differential maturation by SUMO-specific protease, SENP1. Biochem. J. 2005;386:325–330. doi: 10.1042/BJ20041210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Sharma P, Athanasiou M, Kumar A, Yamada S, Kuehn MR. Mutation of SENP1/SuPr-2 reveals an essential role for desumoylation in mouse development. Mol. Cell. Biol. 2005;25:5171–5182. doi: 10.1128/MCB.25.12.5171-5182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Sharrocks AD. SUMO promotes HDAC-mediated transcriptional repression. Mol. Cell. 2004;13:611–617. doi: 10.1016/s1097-2765(04)00060-7. [DOI] [PubMed] [Google Scholar]
- Yin T, Green KJ. Regulation of desmosome assembly and adhesion. Semin. Cell. Dev. Biol. 2004;15:665–677. doi: 10.1016/j.semcdb.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Horinouchi S. Trichostatin and leptomycin. Inhibition of histone deacetylation and signal-dependent nuclear export. Ann. N.Y. Acad. Sci. 1999;886:23–36. doi: 10.1111/j.1749-6632.1999.tb09397.x. [DOI] [PubMed] [Google Scholar]
- Zhong S, Muller S, Ronchetti S, Freemont PS, Dejean A, Pandolfi PP. Role of SUMO-1-modified PML in nuclear body formation. Blood. 2000;95:2748–2753. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Inhibition of sumoylation by Gam1 perturbs cell morphology of stably differentiated HaCaT cultures, but not of basal HaCaT cultures. HaCaT cultures were maintained in low (BASAL) or high calcium (DIFFERENTIATED) medium for at least 3 weeks prior to infection with Ad-Gam1 or Ad-GFP as indicated. Infections were at an MOI of 300 for each virus. The DIFFERENTIATED HaCaT culture infected with Ad-GFP was also treated with 100mM TSA as in Figure 7. Phase contrast (black and white) and fluorescence microscopy (green) images were taken every 24 hours for 3 days.