Abstract
Study Objectives:
To evaluate safety and efficacy of phentermine 15 mg plus extended-release topiramate 92 mg for treatment of moderate to severe obstructive sleep apnea (OSA) in obese adults.
Design:
This phase 2, randomized, double-blind, placebo-controlled study included 2-week screening and 28-week treatment periods. Overnight polysomnography was performed at baseline, Week 8, and Week 28.
Setting:
Single-center study conducted from August 2008 to September 2009.
Participants:
Forty-five subjects with moderate to severe OSA not receiving positive airway pressure (PAP) treatment with body mass index of 30-40 kg/m2.
Interventions:
Subjects were randomized to receive placebo (n = 23) or phentermine 15 mg plus extended-release topiramate 92 mg (n = 22). Both groups received lifestyle-modification counseling.
Measurements and Results:
Primary endpoint, change in apnea-hypopnea index (AHI), significantly favored phentermine 15 mg plus extended-release topiramate 92 mg (-31.5 events/h, 95% CI: −40.0, −22.9) over placebo (-16.6 events/h, 95% CI: −25.0, −8.2) at Week 28 (P =0.0084). At Week 28, there was a 10.2% (95% CI: −12.7, −7.6; 10.8 kg, 95% CI: −13.5, −8.0) mean decrease in weight in the phentermine 15 mg plus extended-release topiramate 92 mg group compared with 4.3% (95% CI: −6.6, −2.0; 4.7 kg, 95% CI: −7.2, −2.2) in the placebo group (P = 0.0006) and a positive, significant (P = 0.0003) correlation between percent change in weight and change in AHI. Significant improvements in overnight oxygen saturation and reduction in blood pressure compared with placebo were observed. Phentermine 15 mg plus extended-release topiramate 92 mg was well tolerated with low adverse event rates.
Conclusions:
Phentermine 15 mg plus extended-release topiramate 92 mg induced significant weight reductions and concomitant improvements in OSA and related symptoms vs placebo. This suggests weight loss mediated by phentermine 15 mg plus extended-release topiramate 92 mg may be useful in treatment of moderate to severe OSA in obese subjects unable or unwilling to comply with PAP treatment.
Citation:
Winslow DH; Bowden CH; DiDonato KP; McCullough PA. A randomized, double-blind, placebo-controlled study of an oral, extended-release formulation of phentermine/topiramate for the treatment of obstructive sleep apnea in obese adults. SLEEP 2012;35(11):1529-1539.
Keywords: Obstructive sleep apnea, phentermine, topiramate, obesity, apnea-hypopnea index
INTRODUCTION
Obstructive sleep apnea (OSA) is a condition in which individuals experience multiple nighttime episodes of apnea or hypopnea, during which blood oxygen saturation levels fall and normal sleep architecture is disrupted.1,2 OSA affects more than 15 million adults in the United States and becomes more prevalent with increased age.3,4 The current standard of care for OSA is positive airway pressure (PAP); however, it only provides benefits when used and does not consistently address the pathophysiology of OSA.5 When acceptance and adherence are considered, the success rate of PAP therapy can be as low as 50%, and currently, there are no pharmacologic therapies indicated for the treatment of OSA.5–7
The altered physiology induced by the periodic hypoxia and reoxygenation that characteristically occur in OSA result in oxidative stress, endothelial dysfunction, sympathetic activation, and activation of the inflammatory cascade, all of which favor cardiovascular disease and related comorbidities, including obesity and hypertension.8 Indeed, individuals with OSA are known to be at higher risk for increased overall mortality, cardiovascular disease, type 2 diabetes, and motor vehicle accidents, as well as new-onset hypertension, insulin resistance, and metabolic syndrome.3,8–15
Obesity increases the likelihood of OSA; approximately 70% of individuals with OSA are obese.4 In turn, patients with OSA are predisposed to weight gain, suggesting that there is a bidirectional relationship between obesity and OSA.16 Obesity also has been implicated in anatomic alterations that predispose patients to upper airway obstruction and reduced neuromuscular control of the air passages during sleep.17,18
It has been demonstrated that OSA in overweight/obese patients can be ameliorated with significant weight loss, whether achieved by lifestyle modification, medication, or bariatric surgery. Recent studies have demonstrated improvements in weight and OSA symptoms in this population by treating OSA with intensive follow-up and low-energy diets.19–21 Furthermore, weight loss improves both objective parameters and patient-reported symptoms of OSA.19,22–25 However, few patients are able to achieve adequate weight loss with lifestyle changes alone, the first intervention typically recommended.26 The combination of phentermine 15 mg plus extended-release topiramate 92 mg is an investigational, once-daily oral therapy being developed to enhance weight loss in conjunction with lifestyle changes that could improve weight-related conditions, such as OSA.19,27
Phentermine hydrochloride is a US Food and Drug Administration (FDA)–approved synthetic sympathomimetic amine, approved as monotherapy (37.5 mg/day) for short-term (a few weeks) treatment of obesity.28 The mechanism of action of phentermine is both qualitatively and quantitatively different from other stimulants, which typically increase blood pressure and heart rate, or serotonergic drugs such as fenfluramine that cause valvulopathy/pulmonary hypertension disorders.29–34
Topiramate, a sulfamate-substituted monosaccharide approved by the FDA for the treatment of epilepsy (200-400 mg/day) and for the prophylaxis of migraine headaches (100 mg/day), is known to block voltage-dependent sodium channels, augment the activity of the neurotransmitter γ-aminobutyrate (GABA) at some subtypes of the GABA-A receptor, and antagonize the kainite subtype of the glutamate receptor.35 Although not currently approved for the treatment of obesity, topiramate has been shown to promote weight loss in obese individuals.36–40 In addition, topiramate is a known inhibitor of carbonic anhydrase activity, similar to acetazolamide.
The investigational combination therapy, phentermine 15 mg plus extended-release topiramate 92 mg, has previously demonstrated weight loss in excess of 10% of body weight in obese individuals with weight-related comorbidities after 56 weeks of treatment27 and was sustained through 108 weeks.41 This magnitude of weight loss has been demonstrated to be sufficient to achieve measurable improvement in OSA.19 The objectives of this study were to evaluate the safety and efficacy of phentermine 15 mg plus extended-release topiramate 92 mg compared with placebo for the treatment of OSA in obese adults with moderate to severe OSA and to assess the relative contributions of weight loss at Week 28 to reductions in OSA symptoms.
MATERIALS AND METHODS
Study Design
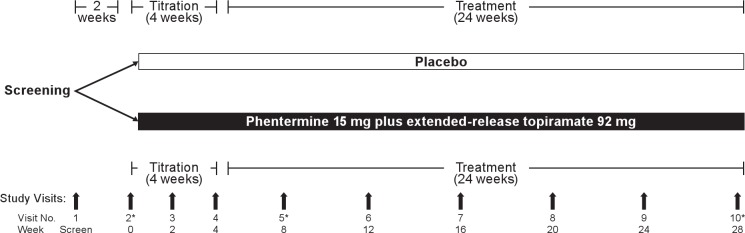
This single-center (Kentucky Research Group), prospective, double-blind, placebo-controlled, parallel-group study randomized eligible subjects to receive placebo or phentermine 15 mg plus extended-release topiramate 92 mg once daily in the morning for 28 weeks (Figure 1). Subjects were randomized to study treatment via a computer-generated table that assigned subjects to treatments arms with equal probability. Both groups also received standardized lifestyle modification counseling (the LEARN program, a clinically proven behavioral weight loss and management program).42
Figure 1.
Study design. *Visits at which overnight polysomnography was performed.
Eligible subjects were 30-65 years of age with a body mass index (BMI) between 30 kg/m2 and 40 kg/m2, a diagnosis of moderate to severe OSA syndrome, and an apnea-hypopnea index (AHI) ≥ 15 at baseline (measured via overnight polysomnography [PSG]) and were unwilling or unable to comply with PAP treatment (defined as > 4 h/night, 70% of the time). Subjects were not eligible if they had a sleep disorder other than OSA syndrome, periodic limb movement arousal index > 10, uncontrolled or poorly controlled blood pressure (systolic > 160 mm Hg or diastolic > 100 mm Hg), or the presence or history of unstable angina, heart failure, cardiac valvulopathy, myocardial infarction, potentially life-threatening cardiac arrhythmia, or clinically significant abnormality on electrocardiogram.
Subjects were screened at Visit 1, and eligible participants were scheduled for baseline overnight PSG (Visit 2). Immediately following the overnight PSG at Visit 2, which provided the baseline AHI value, qualified subjects were randomized 1:1 to receive oral phentermine 15 mg plus extended-release topiramate 92 mg or placebo, both of which were titrated over the first 4 weeks of treatment in increments of phentermine 3.75 mg plus topiramate 23 mg as tolerated, with each step in the titration lasting 1 week until maximum dosage was reached. Subjects then received an additional 24 weeks of treatment for a total treatment period of 28 weeks. Study visits were conducted at Weeks 4, 8, 12, 16, 20, 24, and 28. Overnight assessments of OSA, including PSG, laboratory testing, and quality-of-life surveys, were performed at baseline, Week 8, and Week 28 (or upon early withdrawal).
Prior to randomization, subjects were counseled on reducing their caloric intake by 500 kcal/day and on the importance of light daily exercise. Subjects completed a 24-h dietary recall at their randomization visit, and this information was used for discussions related to recommended dietary modification. Subjects were advised to initiate lifestyle interventions using the LEARN Program.41 Each subject was provided with a LEARN manual and advised to read and implement the material as appropriate to their individual situation. Subjects were also given pedometers and counseled to increase their step count as a form of their light daily exercise. Site personnel were encouraged to discuss these materials with subjects at their regularly scheduled visits; however, no data were collected to document the level of compliance with the program's dietary, lifestyle, and/or exercise recommendations. This study was conducted between August 2008 and September 2009 and was approved by an institutional review board. All subjects provided written informed consent for participation in the study. This trial is registered with ClinicalTrials.gov, number NCT00745251.
Study Assessments
The primary efficacy endpoint was the change in AHI between baseline, Week 8, and Week 28 or early withdrawal. An AHI score of 5-14 is considered mild, 15-29 is considered moderate, and ' 30 is considered severe. Secondary endpoints included changes in additional OSA parameters, such as respiratory disturbance index (RDI), apnea index, hypopnea index, desaturation index, mean overnight oxygen saturation, overnight minimum oxygen saturation, and arousal index (sleep quality defined as the number of arousals from REM and NREM sleep per hour). Subject-reported outcomes were also assessed, including the Pittsburgh Sleep Quality Index (PSQI), Epworth Daytime Sleepiness Scale (ESS), and 36-item Short-Form Health Survey (SF-36) scores. The PSQI is a subjective, self-administered questionnaire used to assess the quality and patterns of sleep in older adults.43 The ESS is a subjective, self-administered, 8-question tool to assess excessive daytime sleepiness and is also used to differentiate between average and significant issues with sleepiness that require intervention.44,45 The SF-36 questionnaire is a 36-item, self-administered health-related quality-of-life questionnaire designed to evaluate functional health and well-being.46
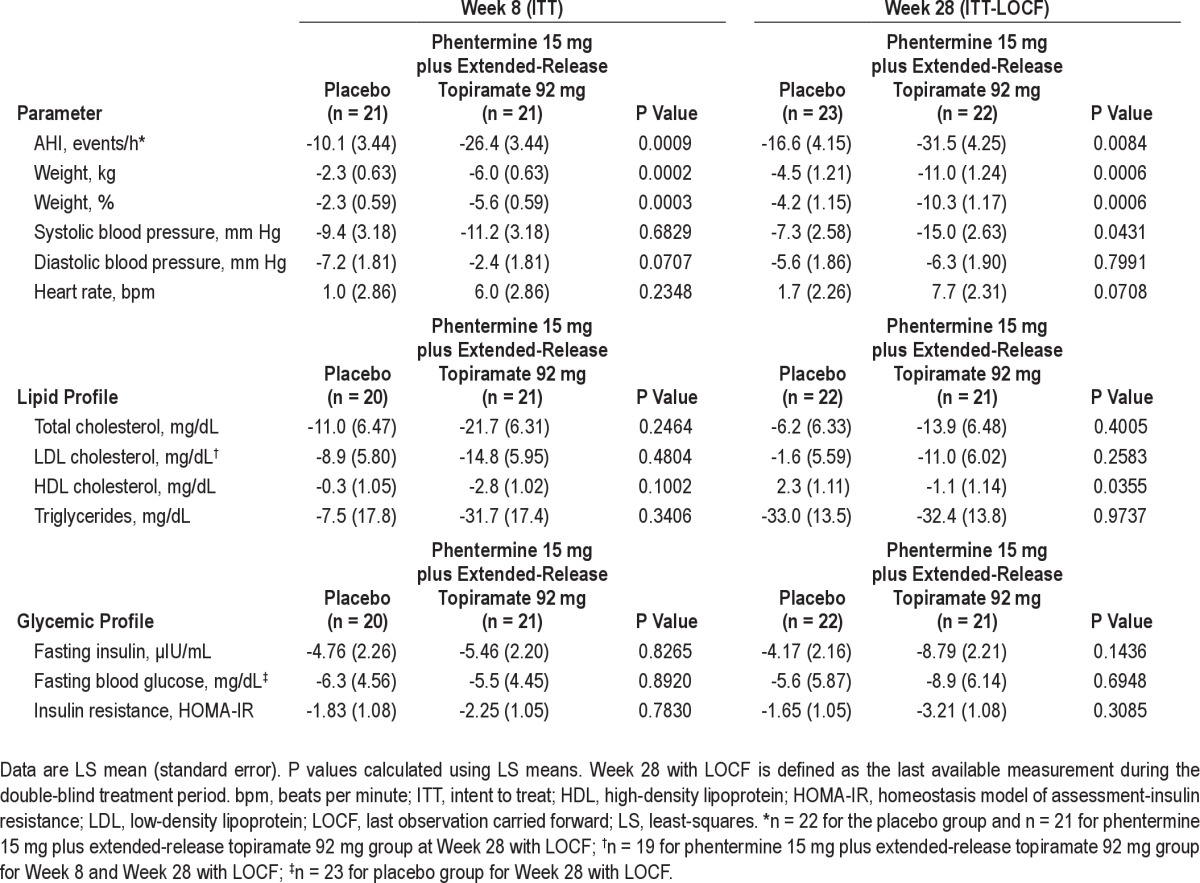
Additional secondary endpoints included changes in cardiometabolic risk factors: blood pressure, heart rate, lipid profile (total cholesterol, low-density lipoprotein [LDL] cholesterol, high-density lipoprotein [HDL] cholesterol, and triglycerides), and glycemic variables (fasting insulin, fasting glucose, and insulin resistance based on homeostasis model of assessment-insulin resistance [HOMA-IR]). Percent weight loss and the percentage of subjects achieving ≥ 5% and 10% weight loss were also assessed as secondary endpoints.
Safety endpoints included laboratory parameters, electrocardiogram, physical examination, and reports of adverse events. Adverse events were assessed throughout the study and were coded using the Medical Dictionary for Regulatory Activities, version 10.1. Treatment-emergent adverse events (TEAE) were defined as adverse events that started on or after the first dose and up to 28 days after the last dose of study drug.
Statistical Methods
The sample size was based on the expected effect on AHI. With 21 subjects per arm, this study had 80% power to detect a mean difference of a 10-point reduction in AHI from baseline (Visit 2) between the 2 treatment groups by a 2-tailed t-test at 5% type I error and an assumed standard deviation of 11.
The randomized set included all subjects randomized to treatment and was used to summarize demographic and baseline variables. The safety set included all randomized subjects who received at least 1 dose of study medication. All safety analyses were conducted based on the safety set. The intent-to-treat (ITT) set included all randomized subjects who received ≥ 1 dose of study medication, had a baseline efficacy measurement, and ≥ 1 post-baseline efficacy assessment. The ITT set was used for the primary and secondary efficacy analysis.
Analysis of the primary efficacy variable, the change in AHI between baseline (Visit 2) and Week 8 (Visit 5) and between baseline and Week 28 or early withdrawal (Visit 10) with last observation carried forward (LOCF), was accomplished using an analysis of covariance (ANCOVA) model, with treatment groups as the main effect and baseline body weight as the covariate. The least-squares (LS) means and corresponding standard errors were presented for the within-group AHI change for each treatment group. For the between-treatment group comparison, the difference in LS means, corresponding standard error, 95% confidence interval, and 1-sided P value were also derived from this ANCOVA model and presented. The normality assumption of the efficacy data was examined prior to fitting the ANCOVA models to ensure that normality was observed. The same statistical methodology described above for the analysis of the primary efficacy variable was performed for secondary variables. Analyses of percentage of categorical weight loss were conducted using a logistic regression model with treatment as the fixed effect and baseline body weight as a covariate. For the between-treatment group comparison, the estimated odds ratio, standard error, 2-sided 95% confidence interval, and 2-sided P value for treatment comparison were to be presented.
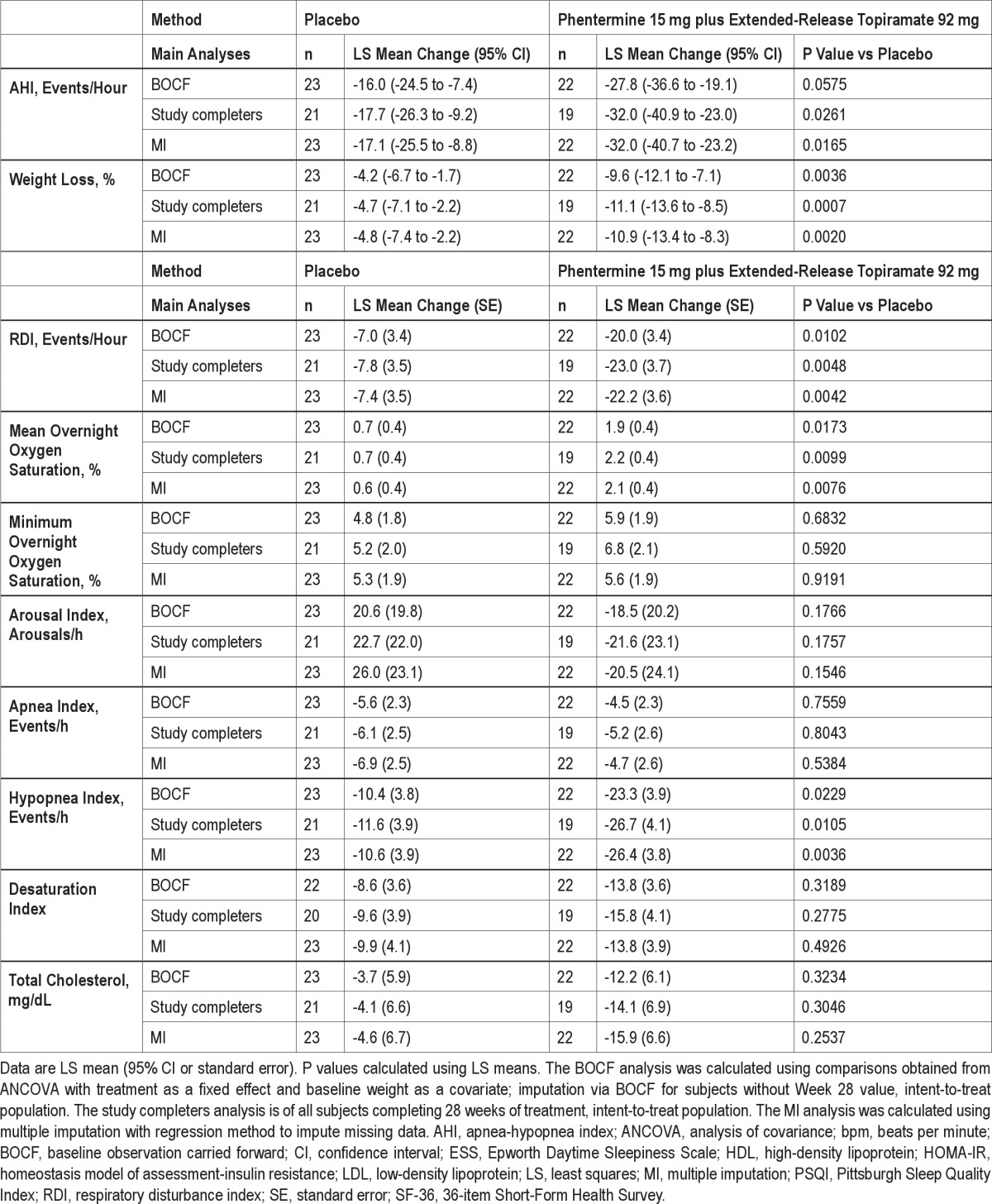
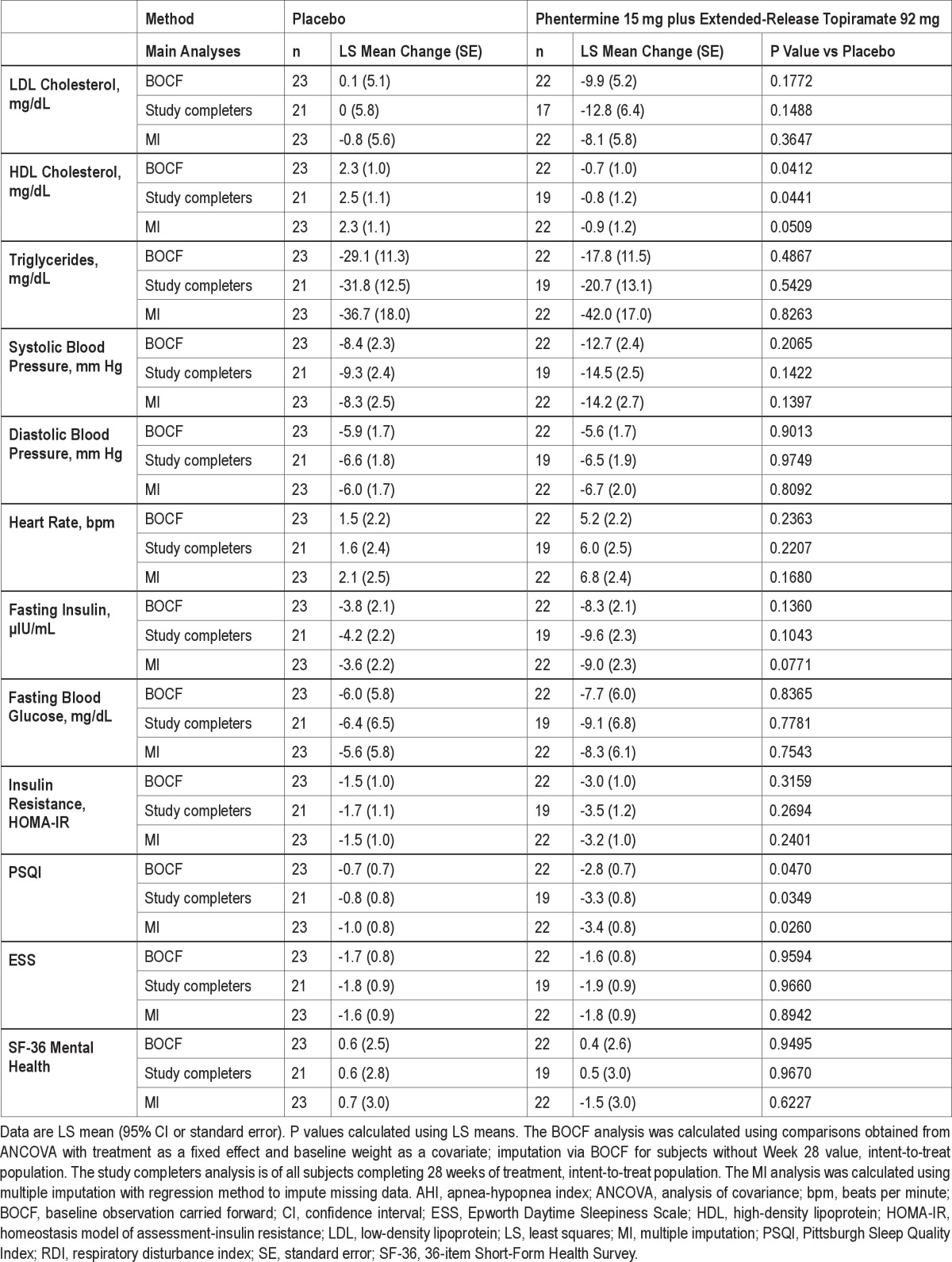
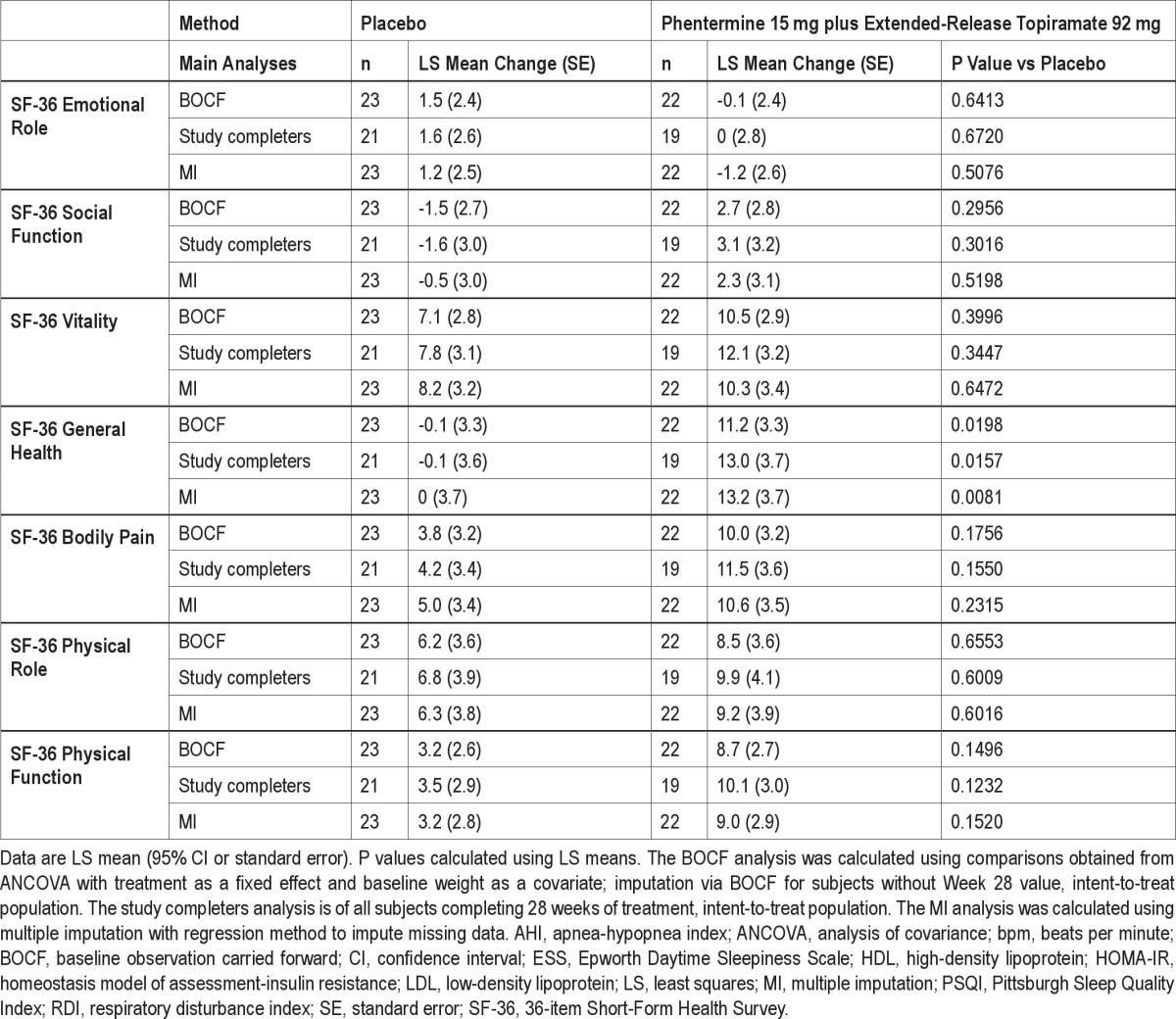
To assess the effect of dropout on the robustness of the conclusions from the prespecified LOCF results, several sensitivity analyses were performed including baseline observation carried forward (BOCF), completers only, and multiple imputation. For BOCF, for subject who did not complete 28 weeks of treatment, the baseline value was carried forward and imputed as the Week 28 result. The completers analysis was performed on subjects who completed the entire 28 weeks of treatment. In addition, the multiple imputation approach used all observed data and treatment assignment to impute missing observations for subjects who discontinued prior to Week 28. For all 3 analyses, the ANCOVA model described above was applied to the imputed datasets (Supplemental Table S1).
RESULTS
Baseline Demographics
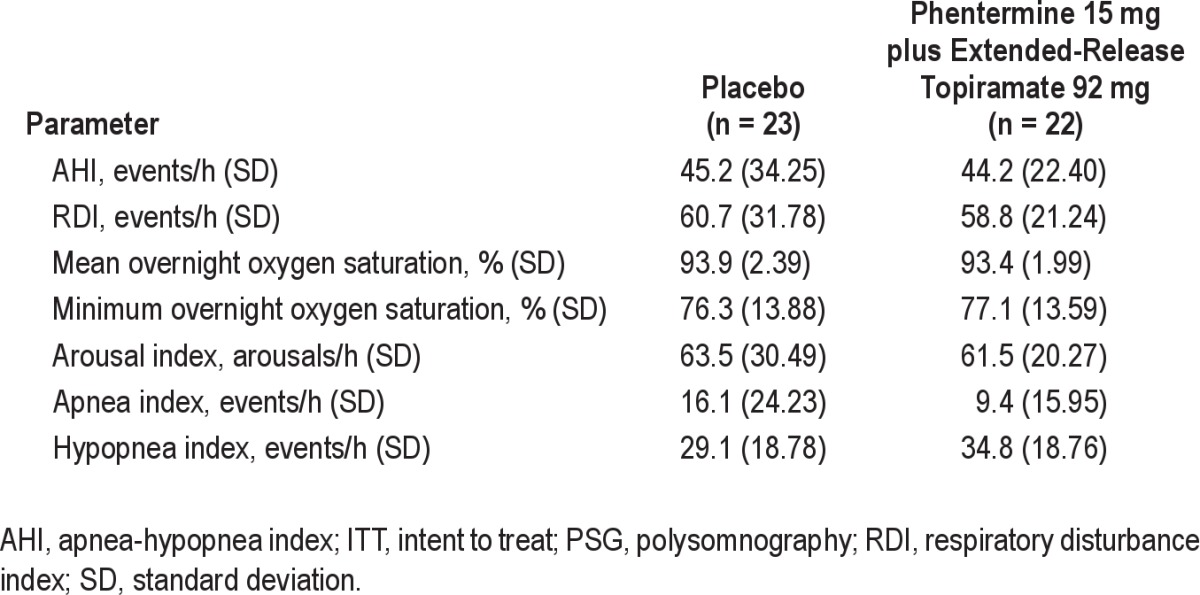
In total, 92 subjects were screened and 45 were randomized. Of the 47 subjects who were not enrolled, 45 subjects did not meet the entry inclusion/exclusion criteria, 1 subject withdrew consent, and 1 subject was lost to follow-up prior to randomization. Five subjects did not complete the study, including 3 subjects who discontinued due to adverse events (2 in the phentermine 15 mg plus extended-release topiramate 92 mg group [nephrolithiasis and irritability] and 1 in the placebo group [cerebrovascular accident]) and 2 subjects who withdrew consent (1 in the phentermine 15 mg plus extended-release topiramate 92 mg group and 1 in the placebo group; Figure 2). Baseline demographics and OSA parameters were well balanced across the treatment arms (Table 1 and Table 2).
Figure 2.
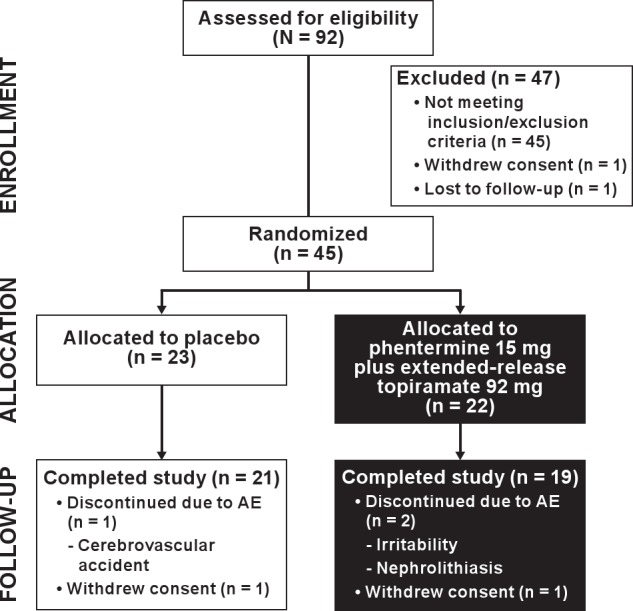
Subject disposition. AE, adverse event.
Table 1.
Baseline demographics (ITT)
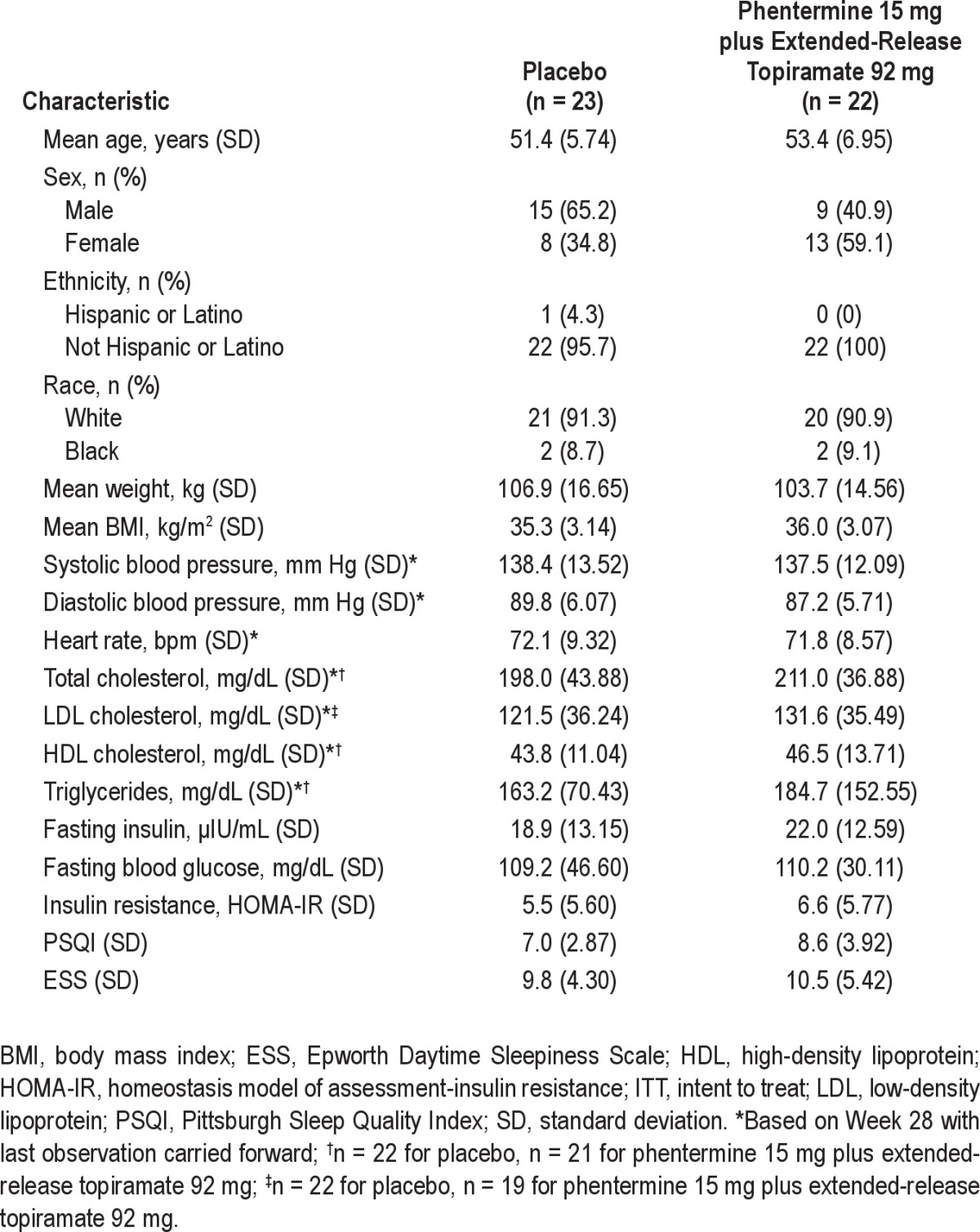
Table 2.
Baseline obstructive sleep apnea parameters (collected via overnight PSG; ITT)
Apnea/Hypopnea Index
The change from baseline in AHI, the primary endpoint, significantly favored the phentermine 15 mg plus extended-release topiramate 92 mg treatment group at Week 8 (P = 0.0009) and Week 28 (LOCF; P = 0.0084; Figure 3; Table 3). The placebo-adjusted LS mean change in AHI at Week 8 was −16.4 (1-sided 95% CI: -∞, −8.14; P = 0.0009) and at Week 28 was −14.9 (LOCF; 1-sided 95% CI: -∞, −4.84; P = 0.0084). The number of apnea-hypopnea events in the phentermine 15 mg plus extended-release topiramate 92 mg group was reduced from a mean of 44 events/h of sleep at baseline (severe) to 14 events/h of sleep at Week 28 (mild), as compared with the placebo group, which experienced a reduction from 45 (severe) to 27 (moderate) events/h of sleep (LOCF). At Week 8, the AHI was decreased to < 15 (mild) in 11 (50.0%) phentermine 15 mg plus extended-release topiramate 92 mg-treated subjects and 7 (30.4%) placebo-treated subjects, and to < 5 (no apnea) in 3 (13.6%) phentermine 15 mg plus extended-release topiramate 92 mg-treated subjects vs 1 (4.3%) placebo-treated subject. In addition, at Week 28, more subjects in the phentermine 15 mg plus extended-release topiramate 92 mg group with a severe AHI (> 45) at baseline achieved an AHI < 5 by Week 28 than those receiving placebo (5 [23.8%] vs 0%, respectively; LOCF).
Figure 3.
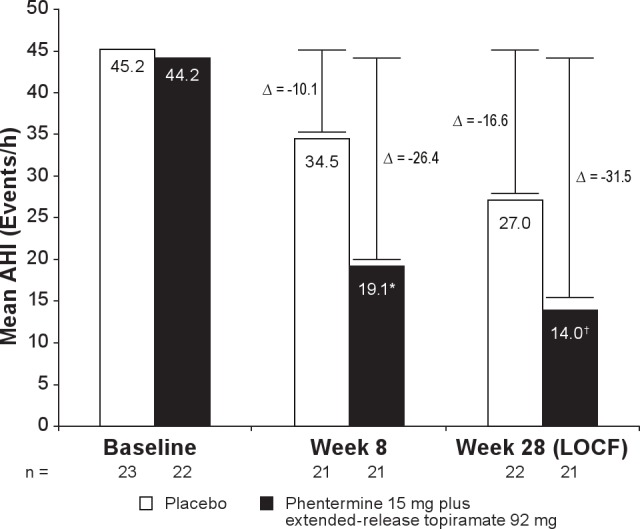
Change from baseline in AHI. Data represent mean values at baseline, Week 8 (ITT), and Week 28 (ITT-LOCF). Change (Δ) represents LS mean change from baseline. Week 28 with LOCF is defined as the last available measurement during the double-blind treatment period. N values represent the number of subjects with values at both time points. AHI, apnea-hypopnea index; CI, confidence interval; ITT, intent to treat; LOCF, last observation carried forward; LS, least-squares.*P = 0.0009 vs placebo (1-sided 95% CI: -∞, −8.1); †P = 0.0084 vs placebo (1-sided 95% CI: -∞, −4.8).
Table 3.
Changes in cardiometabolic risk factors from baseline to Week 8 and Week 28
Weight Loss and Correlation between Improvements in Weight and Apnea/Hypopnea Index
At Week 8, absolute mean weight loss was greater in the phentermine 15 mg plus extended-release topiramate 92 mg group than in the placebo group (-5.9 kg vs −2.4 kg, respectively). This trend continued at Week 28, with the phentermine 15 mg plus extended-release topiramate 92 mg group experiencing a mean weight loss of −10.8 kg, whereas the placebo group experienced a mean weight loss of −4.7 kg (LOCF analysis; Table 3). The placebo-adjusted LS mean change for absolute weight loss at Week 8 was −3.6 kg (95% CI: −5.4, −1.8; P = 0.0002) and at Week 28 with LOCF was −6.5 kg (95% CI: −10.0, −3.0; P = 0.0006). At Week 8, the LS mean percent change in weight from baseline was −5.6% in the phentermine 15 mg plus extended-release topiramate 92 mg group and −2.3% in the placebo group (P = 0.0003). By Week 28, the LS mean percent change in weight from baseline was −10.3% in the phentermine 15 mg plus extended-release topiramate 92 mg group and −4.2% in the placebo group (P = 0.0006; Figure 4, Table 3). The placebo-adjusted LS mean change for percent weight loss at Week 8 was −3.3 (95% CI: −5.0, −1.6; P = 0.0003) and at Week 28 was −6.1 (LOCF; 95% CI: −9.4, −2.7; P = 0.0006). At Week 8, 4.8% (n = 1) of the phentermine 15 mg plus extended-release topiramate 92 mg subjects achieved ≥ 10% weight loss compared with 0% (n = 0) of subjects in the placebo group. This increased to 54.5% (n = 12) at Week 28 (LOCF) in the phentermine 15 mg plus extended-release topiramate 92 mg group compared with 13.0% (n = 3) of the placebo group (P = 0.0044). A significantly higher percentage of subjects achieved ≥ 5% weight loss by Week 8 in the phentermine 15 mg plus extended-release topiramate 92 mg group (52.4% [n = 11]) compared with placebo (19.0% [n = 4]; P = 0.0304). At Week 28, the percent of subjects achieving ≥ 5% weight loss remained higher in the phentermine 15 mg plus extended-release topiramate 92 mg group compared with placebo (72.7% [n = 16] vs 47.8% [n = 11], respectively; P = 0.0846 vs placebo), but this difference was not statistically significant. From baseline to Week 28, a significant positive correlation was found between the percent change in weight and the change in AHI score (n = 43; r = 0.5218; P = 0.0003).
Figure 4.
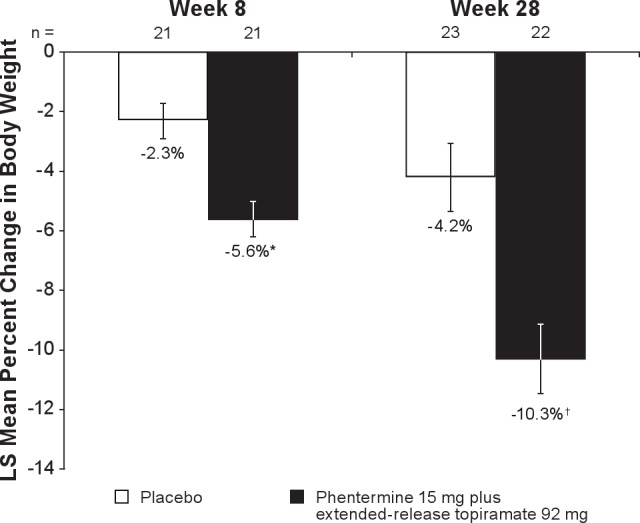
LS mean percent change in body weight from baseline to Week 8 and Week 28 with LOCF. Week 28 with LOCF is defined as the last available measurement during the double-blind treatment period. N values represent the number of subjects with values at both time points. Data represent LS mean change. LOCF, last observation carried forward; LS, least-squares. *P = 0.0003; †P = 0.0006 vs placebo.
Overnight Polysomnography Endpoints
Analysis of the secondary overnight PSG endpoints revealed changes between baseline and Week 28 for additional OSA parameters (Figure 5). Statistically significant changes favored phentermine 15 mg plus extended-release topiramate 92 mg vs placebo for RDI (P = 0.0137), mean overnight oxygen saturation (P = 0.0140), and hypopnea index (P = 0.0064). Both treatment groups had a similar number of arousals and arousal index score at baseline. By Week 8, both groups experienced a reduction in the arousal index, with a greater decrease observed in the phentermine 15 mg plus extended-release topiramate 92 mg group. By Week 28, the difference between the groups had increased, with LS mean decreases in the number of arousals of 19.5 in the phentermine 15 mg plus extended-release topiramate 92 mg group and 21.2 in the placebo group; however, the difference between the groups was not statistically significant.
Figure 5.
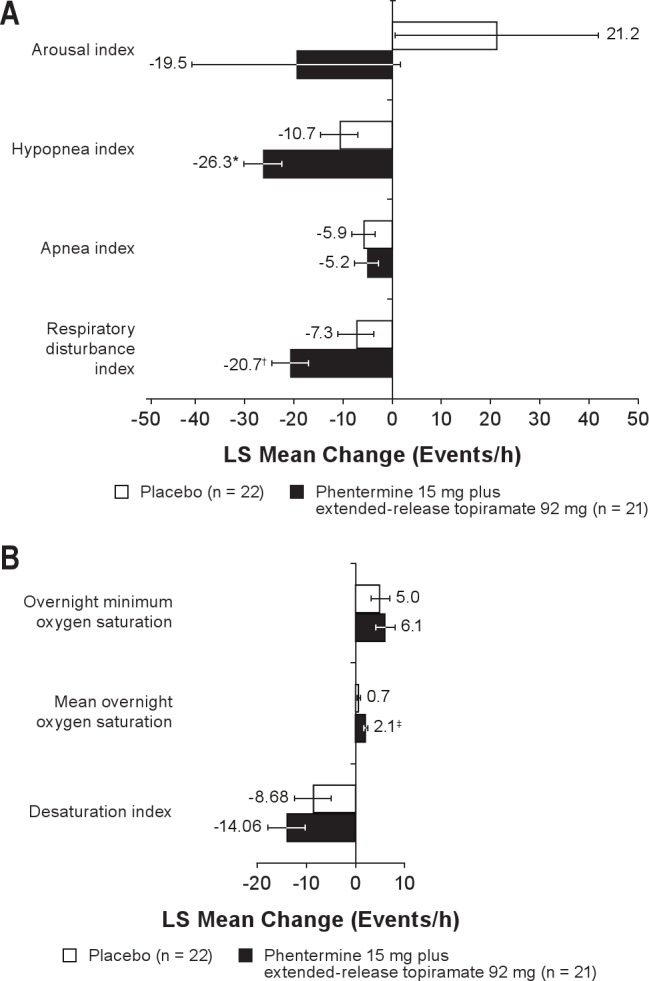
(A) Changes in secondary overnight PSG endpoints from baseline to Week 28.(B) Changes in blood oxygen saturation from baseline to Week 28. LOCF analysis. Arousal index represents sleep architecture and quality of sleep (mean number of all cause arousals per hour of sleep). Hypopnea index represents the average number of episodes (≥ 10 sec during which airflow is reduced ≥ 30% and oxygen saturation drops by ≥ 4%, with ≥ 90% of the event's duration meeting the amplitude criteria for apnea) per hour of sleep. Apnea index represents the average number of episodes of apnea (drop in peak thermal sensor excursion by ≥ 90% of baseline for ≥ 10 sec, with ≥ 90% of the event's duration meeting the amplitude criteria for apnea) per hour of sleep. Respiratory disturbance index represents the mean number of apneas, hypopneas, and respiratory effort-related arousals observed per hour of sleep. Desaturation index represents the mean number of events per hour of sleep during which blood oxygen saturation falls below 90%. Week 28 with LOCF is defined as the last available measurement during the double-blind treatment period. N values represent the number of subjects with values at both time points. LOCF, last observation carried forward; LS, least-squares.*P = 0.0064; †P = 0.0137; ‡P = 0.0140 vs placebo.
Sleep Quality
Secondary analyses of sleep-quality indices (Figure 6) showed a significant difference between phentermine 15 mg plus extended-release topiramate 92 mg and placebo in the mean change in PSQI at Week 28 (P = 0.0419). No significant differences were reported between treatment groups in the ESS evaluation at Week 8 or 28. Overall, there was no significant difference between the treatment groups in SF-36 quality-of-life parameters (Figure 7), with the exception of the general health perceptions score, which favored the phentermine 15 mg plus extended-release topiramate 92 mg group at Week 28 (P = 0.0103).
Figure 6.
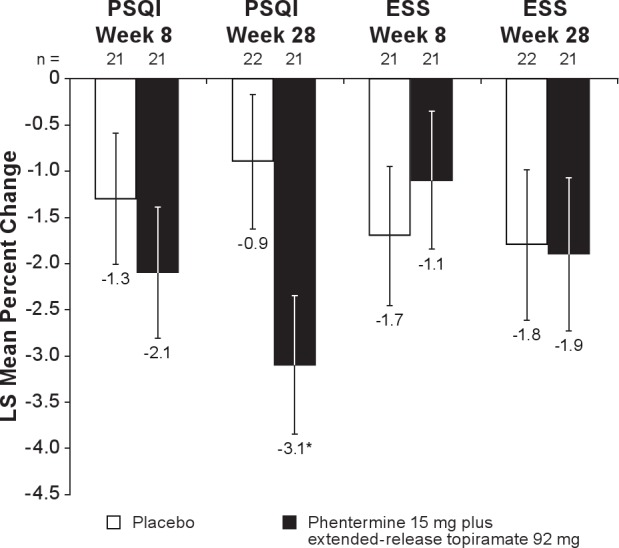
Change in sleep quality indices from baseline to Week 28. Week 28 with LOCF is defined as the last available measurement during the double-blind treatment period. N values represent the number of subjects with values at both time points. Data represent LS mean change. ESS, Epworth Daytime Sleepiness Scale; LOCF, last observation carried forward; LS, least-squares; PSQI, Pittsburgh Sleep Quality Index. *P = 0.0419 vs placebo.
Figure 7.
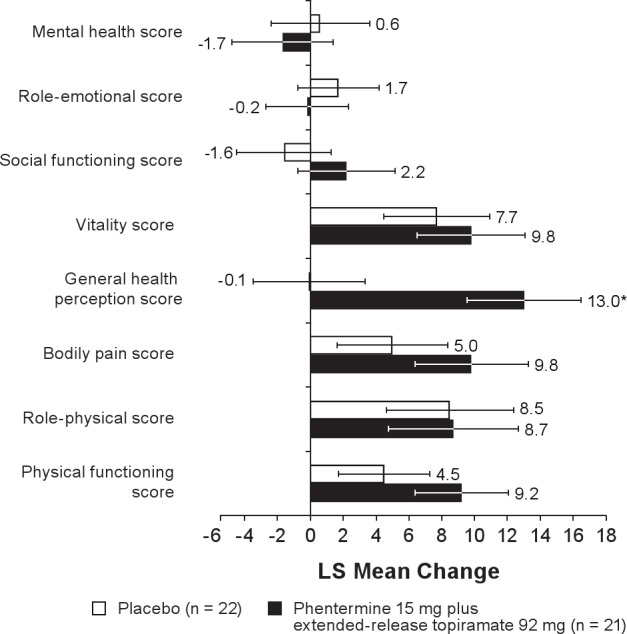
Change in SF-36 (quality of life) scores from baseline to Week 28. Week 28 with LOCF is defined as the last available measurement during the double-blind treatment period. N values represent the number of subjects with values at both time points. Data represent LS mean change. LS, least-squares; SF-36, 36-item Short-Form Health Survey. *P = 0.0103 vs placebo.
Cardiometabolic Risk Factors
Evaluation of cardiometabolic risk factors showed a decrease in blood pressure in both treatment groups, with the difference in systolic blood pressure significantly favoring phentermine 15 mg plus extended-release topiramate 92 mg at Week 28 (P = 0.0431; Table 3). Heart rate increased from baseline in both the placebo and phentermine 15 mg plus extended-release topiramate 92 mg arms. LS mean change in heart rate at Week 8 was 6.0 beats per minute (bpm) and 1.0 bpm for phentermine 15 mg plus extended-release topiramate 92 mg and placebo, respectively (P = 0.2348 vs placebo). At Week 28 (LOCF), LS mean heart rate increased by 7.7 bpm and 1.7 bpm for phentermine 15 mg plus extended-release topiramate 92 mg and placebo, respectively (P = 0.0708 vs placebo). Conversely, mean overnight heart rate decreased from baseline in the phentermine 15 mg plus extended-release topiramate 92 mg and placebo arms at both Weeks 8 and 28. At Week 8, mean overnight heart rate decreased by 1.9 bpm and 2.6 bpm for phentermine 15 mg plus extended-release topiramate 92 mg and placebo, respectively. At Week 28, mean overnight heart rate decreased by 4.8 bpm with phentermine 15 mg plus extended-release topiramate 92 mg and 3.3 bpm with placebo.
Both treatment groups had mean decreases in total cholesterol, LDL cholesterol, triglycerides, fasting insulin, fasting blood glucose, and insulin resistance index (based on HOMA-IR) at Weeks 8 and 28; however, between-group differences were not significant (Table 3).
BOCF, study completers, and multiple imputation analyses for all parameters were also conducted (Supplemental Table S1). In general, conclusions reached on the LOCF analyses were confirmed by these sensitivity analyses.
Safety Outcomes
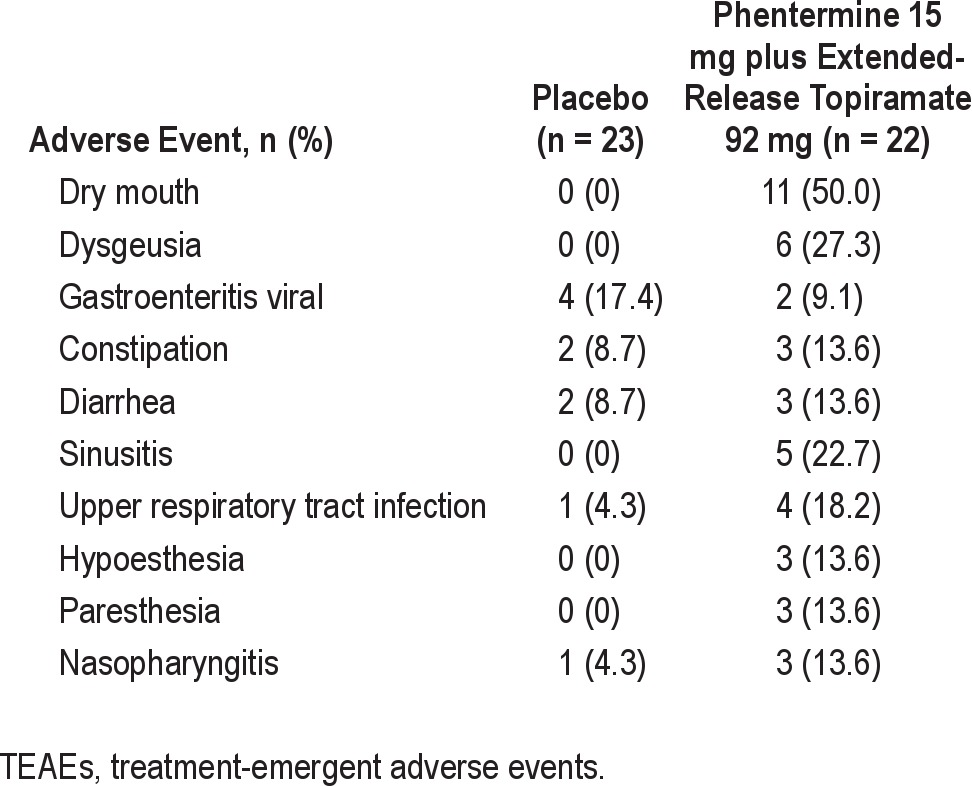
Throughout the study, 37 subjects (82.2%) experienced TEAEs, the most common being dry mouth, dysgeusia, and viral gastroenteritis (Table 4). A higher proportion of subjects in the phentermine 15 mg plus extended-release topiramate 92 mg group experienced TEAEs (n = 20; 90.9%) compared with the placebo group (n = 17; 73.9%). One severe TEAE—severe flank pain, subsequently attributed to nephrolithiasis—occurred in a subject receiving phentermine 15 mg plus extended-release topiramate 92 mg. All other TEAEs were mild or moderate in severity. One serious adverse event, a cerebrovascular accident, occurred in the placebo group. Three subjects discontinued the study drug due to adverse events, including 1 subject receiving placebo (cerebrovascular accident) and 2 subjects receiving phentermine 15 mg plus extended-release topiramate 92 mg (irritability, nephrolithiasis).
Table 4.
TEAEs reported by > 10% in any group
Chemistry panels performed as part of the predetermined safety analysis revealed that, in general, bicarbonate levels were reduced by a greater extent in the phentermine 15 mg plus extended-release topiramate 92 mg group compared with the placebo group at Week 8 (-1.7 mmol/L vs -0.1 mmol/L, respectively). The reductions were not profound, and metabolic acidemia was not reported as an adverse event for any subject.
DISCUSSION
Weight loss, whether achieved by lifestyle modification or medical treatment/surgical intervention, has been shown to improve both objective measures and subjective symptoms of OSA.19,22–25
Phentermine 15 mg plus extended-release topiramate 92 mg, a novel investigational combination therapy, has been shown in large clinical trials to produce significant weight loss as well as improvements in blood pressure, central adiposity, lipid and glycemic profiles, and quality of life.47 In this study, phentermine 15 mg plus extended-release topiramate 92 mg demonstrated significant improvements in RDI, AHI, mean overnight oxygen saturation, PSQI, weight loss, and systolic blood pressure. These beneficial effects on OSA may have multifactorial origins, including induction of weight loss, changes in serum bicarbonate concentration and pH due to topiramate,48,49 and increased daytime wakefulness and improved cognitive performance, potentially due to the activity of phentermine on central arousal.48,49 Fatigue, a commonly reported adverse event of topiramate when used for treatment of epilepsy,36,37 was not seen in this study. In fact, daytime wakefulness was increased, due to either the mitigating effects of phentermine or the lower dose of topiramate used compared with that used for epilepsy treatment.49
The relationship between the degree of weight loss and improvement in AHI is of particular interest. In this trial, subjects treated with phentermine 15 mg plus extended-release topiramate 92 mg demonstrated an LS mean weight loss of 10.3% by Week 28, with more than half of the subjects (54.5%) achieving at least 10% weight loss. A positive, statistically significant correlation was demonstrated in the overall study population between the percent change in weight and reduction in AHI score, regardless of treatment allocation. These results are consistent with longitudinal studies demonstrating approximately a 2.6% decrease in AHI for every 1% decrease in weight.50 Previous studies of phentermine 15 mg plus extended-release topiramate 92 mg in obese adults indicate that a placebo-adjusted weight loss of ≥ 10% of body weight can be achieved after 12 months of treatment and is sustained through 108 weeks.27,41 This magnitude of weight loss alone would be expected to result in measurable improvements in symptoms of OSA as well as other metabolic comorbidities.19,51,52
First-line treatment of OSA has historically been PAP; however, it is only effective while in use and does not consistently affect underlying pathophysiology of OSA in many patients.5 Furthermore, its applicability as a long-term therapy is limited by poor patient acceptance and adherence.5–7 Although other treatments are available, they are also limited by cost, lack of efficacy, and poor patient compliance (adherence).53,54 Surgical procedures, such as mandibular advancement, may offer hope to subjects with an anatomic predisposition to OSA; however, such procedures are significantly more invasive than other available treatments.55 Success with pharmacologic therapy has been limited. Modafinil, a wakefulness promoter, offers some relief from specific symptoms of OSA (residual daytime sleepiness) but does not address the underlying pathogenesis of the disease.56 Acetazolamide has also demonstrated some efficacy in central sleep apnea and OSA but in studies with small populations.57,58
Until recently, there were few randomized studies specifically evaluating the effect of weight loss on OSA; however, several recent studies indicate that that weight loss can improve or eliminate OSA in a significant number of patients.19–21 The Sleep Ahead study in obese patients with diabetes and OSA demonstrated that intensive lifestyle interventions significantly improved AHI over a 1-year period compared with standard of care.19 In addition, Tuomilehto and colleagues demonstrated that in overweight patients with OSA, implementing very-low calorie diets provided sustained improvement in AHI even during a second year of follow-up.21 Furthermore, in a study by Johansson and colleagues, 63 patients received a very-low calorie diet for 7 weeks, followed by 2 weeks of a gradual increase to a 1500-kcal diet and then a weight maintenance period (including exercise and lifestyle changes) from Weeks 9-52. At the end of 1 year, 48% were able to discontinue CPAP use.20 The data in the current analysis further support these findings by demonstrating the effectiveness of phentermine 15 mg plus extended-release topiramate 92 mg for weight loss in obese patients with moderate to severe OSA and improvement in the disease parameters of OSA. The use of phentermine 15 mg plus extended-release topiramate 92 mg may represent a promising alternative to PAP therapy for some obese patients with moderate to severe OSA. In addition, whereas recent studies have used ambulatory sleep monitoring, this study incorporated full overnight polysomnography to measure treatment outcomes and, therefore, allowed for more accurate therapeutic decisions than studies carried out at home.59
Both obesity and OSA progression are believed to contribute to the development of hypertension and cardiovascular disease, with obesity being an independent risk factor for cardiovascular disease and mortality.3,16,60 The mechanisms by which OSA leads to cardiovascular disease are not fully understood, but it has been hypothesized that the hypoxemia, reoxygenation, hypercapnia, intrathoracic pressure changes, and frequent arousals lead to sympathetic activation and metabolic dysregulation (including obesity and insulin resistance) that, in turn, increase the risk of cardiovascular disease.12,16 Although it is unclear where cause and effect lie, reductions in weight and management of OSA both improve cardiovascular disease risk factors.3,27,61 Treatment with phentermine 15 mg plus extended-release topiramate 92 mg combined with lifestyle interventions resulted in greater improvements in cardiometabolic risk factors and significant reductions in weight compared with lifestyle interventions alone, suggesting that, in addition to improving signs and symptoms of OSA, it may be a viable option for addressing the associated cardiovascular risks.
Phentermine 15 mg plus extended-release topiramate 92 mg was well tolerated, as evidenced by the absence of serious adverse events in the phentermine 15 mg plus extended-release topiramate 92 mg group and the low incidence of severe TEAEs and discontinuations due to adverse events. The one severe TEAE of flank pain associated with nephrolithiasis occurred in a subject who had a history of nephrolithiasis and who was also receiving hydrochlorothiazide, a combination that increases the risk of kidney stones. The rates of other TEAEs (including headache and paresthesia) were lower than those observed in other trials of phentermine 15 mg plus extended-release topiramate 92 mg in obese subjects, with or without comorbidities.27 There was an increase seen in mean heart rate with phentermine 15 mg plus extended-release topiramate 92 mg vs placebo, consistent with observations from studies of the combination in obese patients.27 Interestingly, the normal overnight heart rate decrease was observed in both groups, but a greater decrease was seen in the phentermine 15 mg plus extended-release topiramate 92 mg group at Week 28. The reasons for this are unclear, and no adverse clinical sequelae were observed as a result of these changes. No safety concerns were raised by the results of the clinical laboratory tests (serum chemistry and hematology), vital signs, or depression and suicidality questionnaires. The 1 serious adverse event—a stroke that occurred in a subject receiving placebo—emphasizes the importance of the need for treatment for this disease as there are shared risk factors between OSA and stroke that have led many researchers to suspect a cause-and-effect relationship between the two.62
Limitations
The primary weakness of this study was the limited sample size. A larger group of patients receiving additional doses of phentermine plus extended-release topiramate would have been interesting and added valuable data. In addition, extending this study over a longer period of time may have shown greater treatment benefit and statistical differences in this group of subjects. In addition, the lack of significant difference between groups in desaturation index, minimum overnight oxygen saturation, arousal index, AHI, diastolic blood pressure, and glycemic and lipid parameters may be due to subjects receiving placebo experiencing improvements in these parameters because of weight loss relating to the standardized lifestyle modification counseling (LEARN program) that all subjects received. Lastly, compliance with the lifestyle components of this study could have been more objectively documented, or an aggressive lifestyle intervention could have been used for additional analysis of treatment outcome.
CONCLUSIONS
The findings of this randomized controlled trial demonstrate significant improvements in OSA using phentermine 15 mg plus extended-release topiramate 92 mg combined with lifestyle interventions at 28 weeks. Phentermine 15 mg plus extended-release topiramate 92 mg resulted in significantly greater weight loss compared with lifestyle interventions alone. Furthermore, this study showed a correlation between improvements in AHI and weight loss and suggests that phentermine 15 mg plus extended-release topiramate 92 mg combined with lifestyle interventions may be useful as a primary treatment for OSA in patients who are unable to use PAP therapy. Future studies are needed to demonstrate the full potential of this therapy.
DISCLOSURE STATEMENT
This study was funded by VIVUS, Inc. Dr. Winslow has participated in clinical trials for and received research payment from Apnex, Boehringer Ingelheim, Cephalon, Eli Lilly, Forest, GlaxoSmithKline, Merck – Co., Novartis, Pfizer, Philips-Respironics, Sanofi-Aventis, Ventus, and VIVUS. He has served as an advisor and consultant for VIVUS and as a speaker for GlaxoSmithKline. Dr. Bowden and Ms. DiDonato are employees of VIVUS, Inc., the sponsor of the study. Dr. McCullough has participated in clinical trials and received research payment from VIVUS.
ACKNOWLEDGMENTS
The authors acknowledge and thank the research and technical staff of Kentucky Research Group and Sleep Medicine Specialists. The Lockwood Group assisted in the preparation of this manuscript (funded by VIVUS, Inc). Work for this study was performed at Kentucky Research Group, Louisville, KY.
ABBREVIATIONS
- AE
adverse event
- AHI
apnea-hypopnea index
- ANCOVA
analysis of covariance
- BMI
body mass index
- BOCF
baseline observation carried forward
- BPM
beats per minute
- CI
confidence interval
- CPAP
continuous positive airway pressure
- ESS
Epworth Daytime Sleepiness Scale
- FDA
US Food and Drug Administration
- GABA
γ-aminobutyrate
- HDL
high-density lipoprotein
- HOMA-IR
homeostasis model of assessment-insulin resistance
- ITT
intent to treat
- LDL
low-density lipoprotein
- LOCF
last observation carried forward
- LS
least-squares
- OSA
obstructive sleep apnea
- PSG
polysomnography
- PSQI
Pittsburgh Sleep Quality Index
- RDI
respiratory disturbance index
- REM
rapid eye movement
- SD
standard deviation
- SF-36
36-item Short-Form Health Survey
- TEAEs
treatment-emergent adverse events
SUPPLEMENTAL MATERIAL
Table S1.
Change in efficacy parameters from baseline to Week 28 (BOCF, Study Completers, and MI)
REFERENCES
- 1.Caples SM, Gami AS, Somers VK. Obstructive sleep apnea. Ann Intern Med. 2005;142:187–97. doi: 10.7326/0003-4819-142-3-200502010-00010. [DOI] [PubMed] [Google Scholar]
- 2.Flemons WW. Clinical practice. Obstructive sleep apnea. N Engl J Med. 2002;347:498–504. doi: 10.1056/NEJMcp012849. [DOI] [PubMed] [Google Scholar]
- 3.Wolk R, Shamsuzzaman AS, Somers VK. Obesity, sleep apnea, and hypertension. Hypertension. 2003;42:1067–74. doi: 10.1161/01.HYP.0000101686.98973.A3. [DOI] [PubMed] [Google Scholar]
- 4.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013–6. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- 5.Grote L, Hedner J, Grunstein R, Kraiczi H. Therapy with nCPAP: incomplete elimination of sleep related breathing disorder. Eur Respir J. 2000;16:921–7. doi: 10.1183/09031936.00.16592100. [DOI] [PubMed] [Google Scholar]
- 6.Chowdhuri S. Continuous positive airway pressure for the treatment of sleep apnea. Otolaryngol Clin North Am. 2007;40:807–27. doi: 10.1016/j.otc.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Kakkar RK, Berry RB. Positive airway pressure treatment for obstructive sleep apnea. Chest. 2007;132:1057–72. doi: 10.1378/chest.06-2432. [DOI] [PubMed] [Google Scholar]
- 8.George CF. Sleep apnea, alertness, and motor vehicle crashes. Am J Respir Crit Care Med. 2007;176:954–6. doi: 10.1164/rccm.200605-629PP. [DOI] [PubMed] [Google Scholar]
- 9.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–85. [PMC free article] [PubMed] [Google Scholar]
- 10.Meslier N, Gagnadoux F, Giraud P, et al. Impaired glucose-insulin metabolism in males with obstructive sleep apnoea syndrome. Eur Respir J. 2003;22:156–60. doi: 10.1183/09031936.03.00089902. [DOI] [PubMed] [Google Scholar]
- 11.Punjabi NM, Workshop P. Do sleep disorders and associated treatments impact glucose metabolism? Drugs. 2009;69(Suppl 2):13–27. doi: 10.2165/11531150-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290:1906–14. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 13.Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc Am Thorac Soc. 2008;5:207–17. doi: 10.1513/pats.200708-139MG. [DOI] [PubMed] [Google Scholar]
- 14.Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest. 2008;133:496–506. doi: 10.1378/chest.07-0828. [DOI] [PubMed] [Google Scholar]
- 15.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Leinum CJ, Dopp JM, Morgan BJ. Sleep-disordered breathing and obesity: pathophysiology, complications, and treatment. Nutr Clin Pract. 2009;24:675–87. doi: 10.1177/0884533609351532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:144–53. doi: 10.1513/pats.200707-114MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc. 2008;5:185–92. doi: 10.1513/pats.200708-137MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster GD, Borradaile KE, Sanders MH, et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med. 2009;169:1619–26. doi: 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson K, Hemmingsson E, Harlid R, et al. Longer term effects of very low energy diet on obstructive sleep apnoea in cohort derived from randomised controlled trial: prospective observational follow-up study. BMJ. 2011;342:d3017. doi: 10.1136/bmj.d3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuomilehto HP, Seppa JM, Partinen MM, et al. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2009;179:320–7. doi: 10.1164/rccm.200805-669OC. [DOI] [PubMed] [Google Scholar]
- 22.Grunstein RR, Stenlof K, Hedner JA, Peltonen M, Karason K, Sjostrom L. Two year reduction in sleep apnea symptoms and associated diabetes incidence after weight loss in severe obesity. Sleep. 2007;30:703–10. doi: 10.1093/sleep/30.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haines KL, Nelson LG, Gonzalez R, et al. Objective evidence that bariatric surgery improves obesity-related obstructive sleep apnea. Surgery. 2007;141:354–8. doi: 10.1016/j.surg.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–21. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 25.Yee BJ, Phillips CL, Banerjee D, Caterson I, Hedner JA, Grunstein RR. The effect of sibutramine-assisted weight loss in men with obstructive sleep apnoea. Int J Obes. 2007;31:161–8. doi: 10.1038/sj.ijo.0803363. [DOI] [PubMed] [Google Scholar]
- 26.Wadden TA, Berkowitz RI, Womble LG, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353:2111–20. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 27.Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341–52. doi: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]
- 28.Sellersville, PA: Teva Pharmaceuticals USA; 2005. Adipex-P [package insert] [Google Scholar]
- 29.Bonow RO, Carabello BA, Chatterjee K, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118:e523–661. doi: 10.1161/CIRCULATIONAHA.108.190748. [DOI] [PubMed] [Google Scholar]
- 30.Hamidovic A, Childs E, Conrad M, King A, de Wit H. Stress-induced changes in mood and cortisol release predict mood effects of amphetamine. Drug Alcohol Depend. 2010;109:175–80. doi: 10.1016/j.drugalcdep.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jick H, Vasilakis C, Weinrauch LA, Meier CR, Jick SS, Derby LE. A population-based study of appetite-suppressant drugs and the risk of cardiac-valve regurgitation. N Engl J Med. 1998;339:719–24. doi: 10.1056/NEJM199809103391102. [DOI] [PubMed] [Google Scholar]
- 32.Lile JA, Babalonis S, Emurian C, Martin CA, Wermeling DP, Kelly TH. Comparison of the behavioral and cardiovascular effects of intranasal and oral d-amphetamine in healthy human subjects. J Clin Pharmacol. 2011;51:888–98. doi: 10.1177/0091270010375956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothman RB, Ayestas MA, Dersch CM, Baumann MH. Aminorex, fenfluramine, and chlorphentermine are serotonin transporter substrates. Implications for primary pulmonary hypertension. Circulation. 1999;100:869–75. doi: 10.1161/01.cir.100.8.869. [DOI] [PubMed] [Google Scholar]
- 34.Rothman RB, Baumann MH, Dersch CM, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 35.Titusville, NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc; 2009. Topamax [package insert] [Google Scholar]
- 36.Rosenstock J, Hollander P, Gadde KM, et al. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of topiramate controlled release in the treatment of obese type 2 diabetic patients. Diabetes Care. 2007;30:1480–6. doi: 10.2337/dc06-2001. [DOI] [PubMed] [Google Scholar]
- 37.Bray GA, Hollander P, Klein S, et al. A 6-month randomized, placebo-controlled, dose-ranging trial of topiramate for weight loss in obesity. Obes Res. 2003;11:722–33. doi: 10.1038/oby.2003.102. [DOI] [PubMed] [Google Scholar]
- 38.Tonstad S, Tykarski A, Weissgarten J, et al. Efficacy and safety of topiramate in the treatment of obese subjects with essential hypertension. Am J Cardiol. 2005;96:243–51. doi: 10.1016/j.amjcard.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 39.Toplak H, Hamann A, Moore R, et al. Efficacy and safety of topiramate in combination with metformin in the treatment of obese subjects with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Int J Obes. 2007;31:138–46. doi: 10.1038/sj.ijo.0803382. [DOI] [PubMed] [Google Scholar]
- 40.Wilding J, Van Gaal L, Rissanen A, Vercruysse F, Fitchet M. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of topiramate in the treatment of obese subjects. Int J Obes Relat Metab Disord. 2004;28:1399–410. doi: 10.1038/sj.ijo.0802783. [DOI] [PubMed] [Google Scholar]
- 41.Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95:297–308. doi: 10.3945/ajcn.111.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brownell K. The LEARN program for weight management. Dallas: The Life Style Company; 2000. [Google Scholar]
- 43.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 44.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 45.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 46.Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 47.Heymsfield SB, Greenberg AS, Fujioka K, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–75. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 48.Leniger T, Thone J, Wiemann M. Topiramate modulates pH of hippocampal CA3 neurons by combined effects on carbonic anhydrase and Cl-/HCO3- exchange. Br J Pharmacol. 2004;142:831–42. doi: 10.1038/sj.bjp.0705850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Magill RA, Waters WF, Bray GA, et al. Effects of tyrosine, phentermine, caffeine D-amphetamine, and placebo on cognitive and motor performance deficits during sleep deprivation. Nutr Neurosci. 2003;6:237–46. doi: 10.1080/1028415031000120552. [DOI] [PubMed] [Google Scholar]
- 50.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–9. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 51.Case CC, Jones PH, Nelson K, O'Brian Smith E, Ballantyne CM. Impact of weight loss on the metabolic syndrome. Diabetes Obes Metab. 2002;4:407–14. doi: 10.1046/j.1463-1326.2002.00236.x. [DOI] [PubMed] [Google Scholar]
- 52.Espeland L, Hogevold HE, Stenvik A. A 3-year patient-centred follow-up of 516 consecutively treated orthognathic surgery patients. Eur J Orthod. 2008;30:24–30. doi: 10.1093/ejo/cjm081. [DOI] [PubMed] [Google Scholar]
- 53.Eveloff SE. Treatment of obstructive sleep apnea: no longer just a lot of hot air. Chest. 2002;121:674–7. doi: 10.1378/chest.121.3.674. [DOI] [PubMed] [Google Scholar]
- 54.Sadatsafavi M, Marra CA, Ayas NT, Stradling J, Fleetham J. Cost-effectiveness of oral appliances in the treatment of obstructive sleep apnoea-hypopnoea. Sleep Breath. 2009;13:241–52. doi: 10.1007/s11325-009-0248-4. [DOI] [PubMed] [Google Scholar]
- 55.Won CH, Li KK, Guilleminault C. Surgical treatment of obstructive sleep apnea: upper airway and maxillomandibular surgery. Proc Am Thorac Soc. 2008;5:193–9. doi: 10.1513/pats.200708-121MG. [DOI] [PubMed] [Google Scholar]
- 56.Valentino RM, Foldvary-Schaefer N. Modafinil in the treatment of excessive daytime sleepiness. Cleve Clin J Med. 2007;74 doi: 10.3949/ccjm.74.8.561. 561-6, 8-71. [DOI] [PubMed] [Google Scholar]
- 57.Javaheri S. Acetazolamide improves central sleep apnea in heart failure: a double-blind, prospective study. Am J Respir Crit Care Med. 2006;173:234–7. doi: 10.1164/rccm.200507-1035OC. [DOI] [PubMed] [Google Scholar]
- 58.Tojima H, Kunitomo F, Kimura H, Tatsumi K, Kuriyama T, Honda Y. Effects of acetazolamide in patients with the sleep apnoea syndrome. Thorax. 1988;43:113–9. doi: 10.1136/thx.43.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Masa JF, Corral J, Pereira R, et al. Therapeutic decision-making for sleep apnea and hypopnea syndrome using home respiratory polygraphy: a large multicentric study. Am J Respir Crit Care Med. 2011;184:964–71. doi: 10.1164/rccm.201103-0428OC. [DOI] [PubMed] [Google Scholar]
- 60.Logue J, Murray HM, Welsh P, et al. Obesity is associated with fatal coronary heart disease independently of traditional risk factors and deprivation. Heart. 2011;97:564–8. doi: 10.1136/hrt.2010.211201. [DOI] [PubMed] [Google Scholar]
- 61.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mohsenin V. Sleep-disordered breathing: implications in cerebrovascular disease. Prev Cardiol. 2003;6:149–54. doi: 10.1111/j.1520-037x.2003.00986.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Change in efficacy parameters from baseline to Week 28 (BOCF, Study Completers, and MI)