Abstract
Human Cu-ATPases ATP7A and ATP7B maintain copper homeostasis through regulated trafficking between intracellular compartments. Inactivation of these transporters causes Menkes disease and Wilson disease, respectively. In Menkes disease, copper accumulates in kidneys and causes tubular damage, indicating that the renal ATP7B does not compensate for the loss of ATP7A function. We show that this is likely due to a kidney-specific regulation of ATP7B. Unlike ATP7A (or hepatic ATP7B) which traffics from the TGN to export copper, renal ATP7B does not traffic and therefore is unlikely to mediate copper export. The lack of ATP7B trafficking is not due to the loss of a kinase-mediated phosphorylation or simultaneous presence of ATP7A in renal cells. Rather, the renal ATP7B appears 2-3 kDa smaller than hepatic ATP7B. Recombinant ATP7B expressed in renal cells is similar to hepatic protein in size and trafficking. The analysis of ATP7B mRNA revealed a complex behavior of exon 1 upon amplification, suggesting that it could be inefficiently translated. Recombinant ATP7B lacking exon 1 traffics differently in renal and hepatic cells, but does not fully recapitulate the endogenous phenotype. We discuss factors that may contribute to cell-specific behavior of ATP7B and propose a role for renal ATP7B in intracellular copper storage.
Keywords: ATP7B, trafficking, copper, kidney, ATPase
INTRODUCTION
Copper is essential for a wide variety of cellular functions. It is a co-factor for many important enzymes such as cytochrome c oxidase, tyrosinase, superoxide dismutase, ceruloplasmin, peptidylglycine α-amidating monooxygenase and others (1). I n addition, copper plays important yet poorly understood roles in embryonic development, neuronal myelination, angiogenesis, and binds with high affinity to such proteins as amyloid precursor protein and prion protein (2-5). The balance between copper absorption and excretion in humans is tightly regulated. Two copper-transporting ATPases (Cu-ATPases), ATP7A and ATP7B, play a key role in this process by mediating copper transport across cellular membranes in response to changing copper concentrations. Disruptions in the function of ATP7A or ATP7B result in severe metabolic disorders, Menkes disease and Wilson disease, respectively (6-11).
ATP7A and ATP7B are highly homologous proteins that have a similar general function. They both deliver copper to the secretory pathway for incorporation into copper-dependent enzymes (12) and mediate copper efflux out of the cell (13, 14). These two functions are performed in separate cell compartments; the targeting of Cu-ATPases to these compartments is regulated by copper concentration. In low copper, ATP7A is located in the trans-Golgi network (TGN), consistent with its role in delivering copper to copper-requiring enzymes. In high copper, ATP7A relocalizes to vesicles in the vicinity of the plasma membrane, where it sequesters copper for subsequent export via vesicle-mediated fusion (15-20). Current data agree that the trafficking of ATP7A is required for the export of copper from the cell.
The intracellular localization of ATP7B is also thought to be copper-dependent. Studies of recombinant ATP7B in several cell lines and of endogenous ATP7B in hepatocytes demonstrated that in low copper ATP7B has a perinuclear TGN localization (13, 21-23). In elevated copper, ATP7B was found to relocate to vesicles (21-25) and, in polarized hepatic cells, to both the vesicles and the apical plasma membrane (13, 26, 27).
The general similarity of ATP7A and ATP7B functions is further illustrated by the ability of ATP7A to compensate, at least partially, for the lack of functional ATP7B in the cerebellum of Atp7b−/− mice, restoring copper delivery to ceruloplasmin, a copper-dependent ferroxidase (28). Reciprocally, recombinant ATP7B restores copper efflux in the fibroblasts of Menkes disease patients, where ATP7A is defective (29). In contrast to the compensatory effect of recombinant ATP7B in fibroblasts, in tissues such as intestine, brain, or kidney, the isease-induced inactivation of ATP7A is not compensated for by ATP7B, even when co-expressed in the same cells. These observations suggest overlapping yet specific functional roles for two Cu-ATPases and/or distinct mechanisms of regulation. Currently, there is no information available on the relative copper sensitivities of endogenous ATP7A and ATP7B in physiologically relevant cells.
In order to better understand the inability of ATP7B to compensate for the lack of functional ATP7A in tissues, we investigated the localization and trafficking of ATP7A and ATP7B in renal cells. Kidneys express bot h C u-ATPases in their proximal and distal epithelial cells (30) and genetic inactivation of either ATP7A or ATP7B (in Menkes disease and Wilson disease, respectively) results in renal copper imbalance (31-37). Based on the currently existing model of Cu-ATPase regulation we anticipated that both Cu-ATPases would traffic to their respective compartments but may respond to different intracellular levels of copper. Instead, we observed no trafficking of endogenous ATP7B in response to elevated copper in all kidney cell lines that we have tested, in stark contrast to the behavior of ATP7B in hepatocytes. We have investigated the mechanism underlying this new and unexpected behavior of ATP7B in kidney and found that it is determined by differences in ATP7B protein as well as cell environment. The lack of ATP7B trafficking in renal cells suggests a new functional role for this Cu-ATPase in copper storage in intracellular compartments and explains the lack of functional complementation in Menkes disease.
RESULTS
ATP7A and ATP7B are expressed endogenously in Hek293 cells
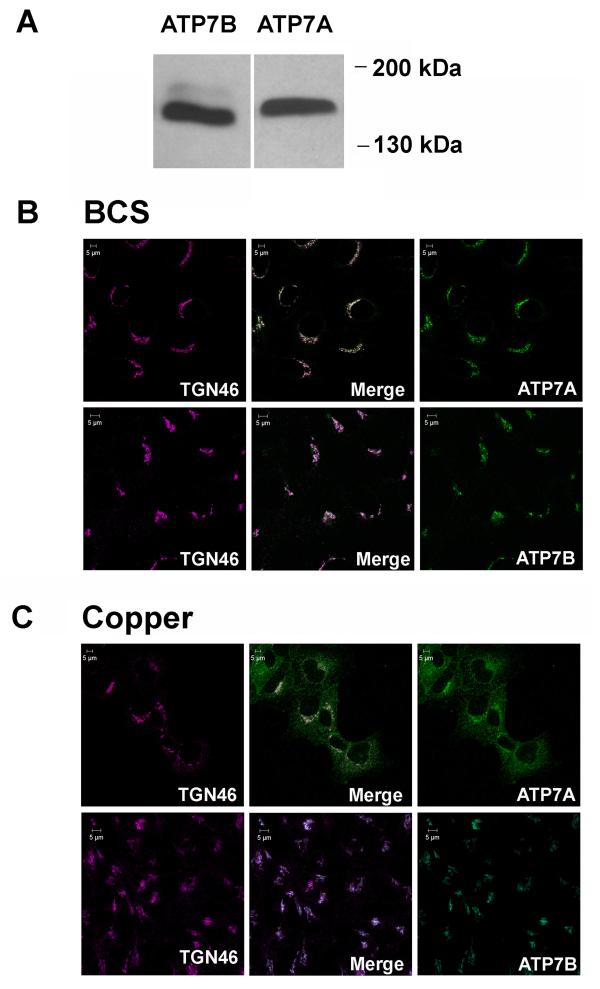
To test our hypothesis that in cells expressing both Cu-ATPases, the intracellular localization and/or trafficking response of ATP7A and ATP7B to elevated copper could be different, we initially utilized Hek293 cells, which are derived from human embryonic kidney. Western blotting of Hek293 membrane fractions revealed that both ATP7A and ATP7B are endogenously expressed in these cells and easily detected (Figure 1A). The antibodies against ATP7A and ATP7B were raised against different epitopes and have different sensitivity; therefore to compare the amounts of endogenous ATP7A and ATP7B, we generated calibration curves using appropriate antigens and carried out quantitative Western blot analysis (Supplementary Figure 1). These studies revealed that in Hek293 cells, ATP7A and ATP7B are present at comparable levels, thus providing an excellent opportunity to directly compare the localization and trafficking of these transporters. Consequently, we first characterized the intracellular localization of each protein under low copper conditions. Immunofluorescent staining of Hek293 cells following treatment with the copper chelator bathocuproine-disulfonate (BCS) demonstrated that ATP7A and ATP7B both display a characteristic perinuclear staining expected for their TGN localization (Figure 1B). Targeting to the TGN was confirmed by co-staining with the antibody against TGN46, a compartment-specific marker (Figure 1B).
Figure 1. ATP7A and ATP7B are co-expressed in Hek293 cells and show different trafficking behavior.
(A) Western blots (merged) of membrane fractions Hek239 cells (60μg protein per lane) probed with anti-ATP7A or anti-ATP7B antibody. (B) Co-Localization of ATP7A (red) with TGN marker TGN46 (green) under basal conditions. (C) Comparison of ATP7A and ATP7B localization following treatment with 200 μM copper. ATP7A (red) is localized primarily at the plasma membrane and vesicles. ATP7B (red) is co-localized with TGN46 (green); no redistribution is detected. Scale bar represent 5 μM length
ATP7A and ATP7B respond differently to elevated copper in Hek293 cells
To compare the ATP7A and ATP7B responses to elevated copper, the intracellular localization of ATP7A and ATP7B was analyzed following treatment with increasing copper concentrations. Marked differences in the trafficking behavior of ATP7A and ATP7B were observed. As expected, ATP7A relocalized from the TGN in response to copper elevation (Figure 1C). In the physiological range of copper concentrations (5-20μM), ATP7A trafficked to vesicles, whereas at markedly elevated copper (50-200 μM), ATP7A relocalized to both vesicles and the plasma membrane (Supplementary Figure 2). In striking contrast, no change in ATP7B localization was observed, even when cells were treated with copper as high as 200μm (Figure 1C). The copper-induced redistribution of ATP7A and the lack of ATP7B re-localization were verified by co-staining with the anti-TGN46 antibody (Figure 1C). The ATP7B pattern was also verified using two other antibodies, targeted to different regions of ATP7B (our unpublished data). Thus, in Hek293 cells, ATP7B does not relocalize in response to elevated copper, whereas redistribution of ATP7A occurs at a wide range of copper concentrations.
The copper-dependent trafficking of endogenous ATP7B is cell-specific
The lack of ATP7B trafficking in response to copper was unexpected and raised concerns that this phenomenon could merely be an unusual property of Hek293 cells. Consequently, we investigated the trafficking behavior of endogenous ATP7B in several other cell lines. In Cos-7 and MDCK cells, which are both kidney-derived, endogenous ATP7B was found in a tight TGN-like compartment in low copper, where it colocalized with TGN46, and no trafficking of ATP7B was detected when copper was elevated (our unpublished data). The surprising and consistent lack of ATP7B trafficking in renal cells led us to re-examine the trafficking of endogenous ATP7B in hepatocytes. HepG2 and Huh7 cells are derived from liver, and have been previously used by several groups to demonstrate trafficking of ATP7B (13, 23). Using the same experimental procedures as described above, we found that in both HepG2 and Huh7 cells, endogenous ATP7B re-localizes from the TGN to vesicles when copper is elevated (Figure 2A, lower panel), in agreement with previous reports (13, 23). The striking difference in ATP7B behavior in hepatic and renal cells suggested that the copper-dependent trafficking of ATP7B is a not a universal, but rather cell-specific phenomenon.
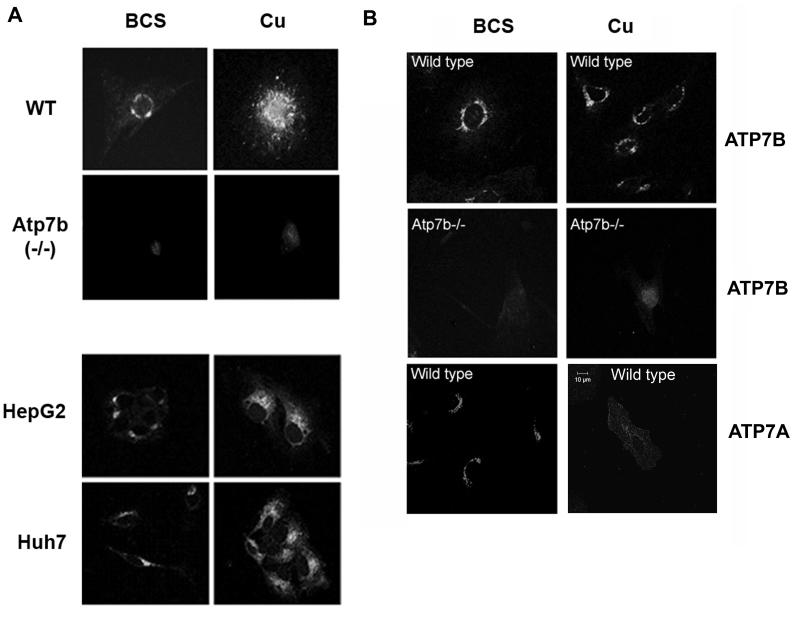
Figure 2. Trafficking response of ATP7B in primary and cultured liver cells differs from its response in primary kidney cells.
(A) Localization of ATP7B in the liver derived cells. Top panel: Primary liver cells isolated from 2 week-old wild-type mice (WT) and ATP7B knock-out (Atp7b−/−) mice were treated with either 200 μM BCS or 200 μM copper. In the wild type mouse, ATP7B displays a perinuclear localization in BCS and traffics to vesicles in response to elevated copper. As expected, ATP7B is not detected in the Atp7b−/− mouse ATP7B. (A light haze represents background staining from the secondary antibody). Lower panel: HepG2 and Huh7 cells were treated with either BCS or copper as above and the localization of ATP7B was detected by immunostaining. In both HepG2 and Huh7 cells ATP7B is located perinuclearly in BCS, but traffics to vesicles upon the addition of 200 μM copper. (B) ATP7B and ATP7A in primary kidney cells respond differently to elevated copper. Primary kidney cells derived from 2 week-old wild-type and Atp7b−/− mice were treated and immuno-stained as in Figure 1. Top panel: in wild-type cells, ATP7B does not relocalize in response to copper; Middle panel: no ATP7B is detected in Atp7b−/− kidney cells, demonstrating specificity of staining; Lower panel: ATP7A displays perinuclear localization in low copper (BCS) and traffics to vesicles and the plasma membrane in response to copper elevation (Cu). All images are at the same magnification; Scale bar represents 10 μ length.
The trafficking behavior of ATP7B in primary cells supports the results from cultured cell lines
To confirm the physiological relevance of cell-specific trafficking of ATP7B, we generated primary cells from mouse liver and kidneys. In primary hepatocytes, ATP7B was localized around the nucleus and relocalized to vesicles when cells were treated with elevated copper (Figure 2A, upper panel). To verify that the immunostaining in primary cells was specific for ATP7B, we generated primary cells from Atp7b−/− mouse liver and repeated the experiments. Only faint background staining was observed in the Atp7b−/− hepatocytes (Figure 2A, upper panel). Thus, in either cultured cells or primary hepatocytes, ATP7B traffics from the TGN to a vesicular compartment in response to elevated copper.
We then generated primary cells using kidneys of 2 week old mice and demonstrated that at this stage, the kidney expresses both ATP7B and ATP7A. ATP7A displayed a perinuclear staining at low copper and redistributed to vesicles and plasma membrane in response to elevated copper (Figure 2B, lower panel). ATP7B also had a perinuclear location under low copper but did not relocalize when treated with copper, consistent with the observations in Hek293 cells (Figure 2B, top panel). The specificity of ATP7B staining was further confirmed by utilizing kidney cells derived from the Atp7b−/− mice (Figure 2B, middle panel); as expected, no signal was detected in these cells. Thus, the lack of trafficking of ATP7B in cultured cells mirrors the behavior of ATP7B in primary renal cells.
Copper uptake in HepG2 cells and Hek293 cells is similar
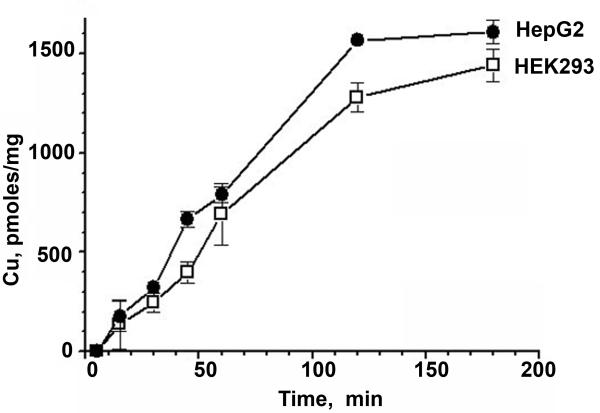
Having established cell specific differences in the trafficking properties of ATP7B, we investigated possible reasons for this phenomenon. Although in our experiments, cells were treated with the same elevated extracellular copper, it seemed possible that copper uptake, and consequently the intracellular copper concentration, could differ greatly in hepatic and renal cells, explaining the difference in response of ATP7B to treatment with copper. To examine this possibility, we compared the rates of 64Cu uptake into Hek293 cells and HepG2 cells. Figure 3 illustrates that the kinetics of copper uptake over a 2-3 hour time period (a time period corresponding to the duration of copper treatment for trafficking studies) are very similar for HepG2 and Hek293 cells, although Hek293 cells do accumulate ~10% less copper than HepG2. These experiments were performed using two different copper concentrations, 5μM and 50 μM, and similar results were obtained for both conditions (50 μM, our unpublished data). Since comparable amounts of copper enter hepatic and renal cells upon copper treatment, it seems unlikely that the lack of trafficking of renal ATP7B is related to variations in copper uptake.
Figure 3. Copper uptake is similar in Hek293 and HepG2 cells.
Radioactive copper was added to live cells and the copper uptake was evaluated by the kinetics of 64Cu accumulation. A representative experiment is shown for 5μM total copper; each point is an average of three independent measurements.
Copper efflux by ATP7A does not contribute to the lack of trafficking response by ATP7B
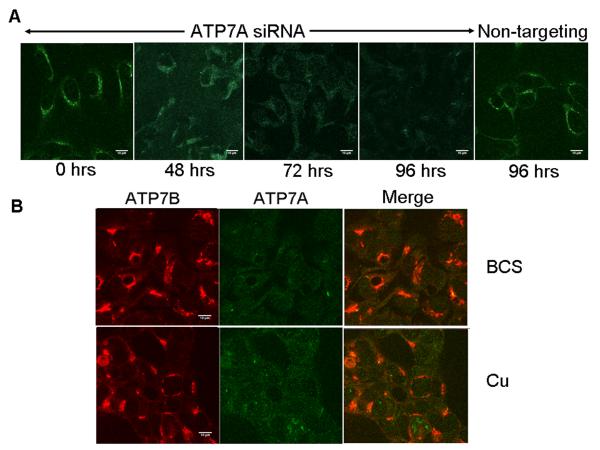
Another possible reason for the different trafficking behavior of ATP7B in renal cells compared to hepatocytes (where ATP7B is the only copper-transporting ATPase) could be the simultaneous expression of ATP7A. ATP7A proceeds faster through catalytic steps and may transport copper more rapidly than ATP7B (28). As a result, the presence of ATP7A in kidney cells may markedly decrease the amount of copper available for ATP7B thus subverting ATP7B trafficking. To test this hypothesis, we decreased the abundance of ATP7A in Hek293 cells using siRNA silencing and examined whether or not ATP7B traffics in response to elevated copper when ATP7A expression is reduced. Maximum depletion of ATP7A was obtained after 96 hours of siRNA transfection (Figure 4A); this time point was used for further analysis of trafficking. The control non-targeting siRNA showed no effect on ATP7A expression (Figure 4A). Down-regulation of ATP7A did not affect either the intensity or distribution of ATP7B in these cells. In either low or elevated copper, ATP7B displayed a TGN-like localization (Figure 4B), which was similar to localization of endogenous ATP7B in Hek293 cells (Figure 1) and in primary kidney cells (Figure 2B). We conclude that ATP7A-mediated export of copper is unlikely to contribute to the lack of ATP7B trafficking in kidney cells.
Figure 4. Decrease of ATP7A abundance using siRNA aimed to increase copper access to ATP7B does not affect localization of ATP7B in Hek293 cells.
(A) Time course of ATP7A down-regulation using specific siRNA; non-targeting siRNA served as a control for specificity of silencing. (B) Down-regulation of ATP7A (evident by the loss of staining, green) has no effect on the localization of ATP7B (red) in the absence (BCS) or presence of copper (Cu). The scale bar represents 10μM length.
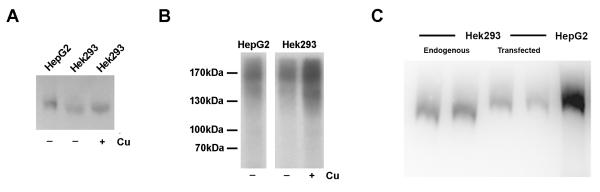
ATP7B from Hek293 and HepG2 cells have different electrophoretic properties
Since copper concentration that induces trafficking of ATP7B in hepatocytes was without an effect in kidney cells, it seemed possible that ATP7B itself might be post-translationally modified in a cell-specific manner or that the modification may differ in elevated copper conditions. To examine this possibility, we isolated membrane fractions from Hek293 and HepG2 cells and analyzed ATP7B electrophoretic mobility following Western blotting. These experiments revealed a small but reproducible difference in electrophoretic mobility of renal and hepatic ATP7B. Upon prolonged electrophoresis, ATP7B from Hek293 cells migrated faster than ATP7B from HepG2 cells (Figure 5A); this difference was independent of copper treatment. Consequently, we concluded that ATP7B protein in the two cell types is different and could be modified.
Figure 5. ATP7B in HepG2 and HEK293 cells differ in size, but not in posttranslational modifications.
(A) Western blot of membrane preparations from Hek293 or HepG2 cells pretreated with either BCS (-) or CuCl2 (+) shows difference in the electrophoretic mobility with ATP7B from Hek293 cells migrating faster that ATP7B from HepG2 cells. (B) Autoradiography of ATP7B immunoprecipitated from HepG2 and Hek293 cells, which were metabolically labeled with 32P. (C) Comparison of electrophoretic mobilities demonstrate that in renal cells endogenous ATP7B differs from the liver-derived recombinant ATP7B (transfected). In contrast, endogenous ATP7B in HepG2 cells is very similar to the liver-derived ATP7B (duplicate HEK293 samples are shown for each condition). The endogenous ATP7B is not visible in the transfected lanes due to much higher levels of recombinant ATP7B and hence lower amount of transfected membranes used; the total membrane protein per lane was adjusted with membranes lacking ATP7B and is the same in all lanes). (To detect phosphorylation of endogenous proteins it is necessary to have a shorter gel run; under these conditions the difference in protein mobility is not apparent).
ATP7B is phosphorylated in Hek293 and HepG2 cells
We have previously shown that in hepatic cells, ATP7B is phosphorylated by a kinase in the absence of copper and be comes hyperphosphorylated when copper levels are increased (25). Phosphorylation changes ATP7B mobility causing a small upward shift in the position of ATP7B on a gel. Since the electrophoretic mobility of ATP7B from HepG2 and Hek293 cells was different (in either the presence or absence of copper), we tested whether the increased mobility of ATP7B from Hek293 cells was due to a lack of phosphorylation. To examine kinase-mediated phosphorylation of ATP7B, Hek293 and HepG2 cells (as a control) were treated with BCS and then metabolically labeled with orthophosphate, 32P. ATP7B was immunoprecipitated from Hek293 and HepG2 cell lysates and incorporation of 32P was evaluated by autoradiography (The immunoprecipitation of ATP7B was confirmed by Western blot analysis, Supplementary Figure 3). Figure 5B illustrates that ATP7B is phosphorylated in both HEK293 cells and HepG2 cells in the absence of copper. Therefore, the loss of phosphorylation is unlikely to be a cause of apparent size difference. (The precision of our current methods, however, is insufficient to exclude partial changes in the stoicheometry of phosphorylation).
Recombinant hepatic ATP7B traffics in HEK293 cells
The observed faster mobility of ATP7B in renal cells (Figure 5A) corresponds to an apparent 3 kDa decrease in molecular weight of ATP7B and could also be a result of regulatory proteolysis or modification of corresponding mRNA. To determine whether modification of ATP7B in Hek293 cells occurs at the protein level (proteolysis), or involves changes in mRNA (alternative splicing, translation), ATP7B cDNA was transfected into Hek293 cells, and the electrophoretic mobilities of recombinant and endogenous proteins were compared on a Western blot. We reasoned that if ATP7B in Hek293 is proteolyzed or otherwise modified at the protein level, the mobilities of the endogenous and recombinant proteins would be similar. However, if changes occur at the mRNA level, then the endogenous and cDNA-encoded ATP7B will differ.
Figure 5C illustrates that the recombinant ATP7B expressed in Hek293 cells has as lower electrophoretic mobility (appears larger) than the endogenous renal ATP7B. Since the ATP7B cDNA, which we utilized to produce recombinant protein, originates from the liver, the difference in endogenous and recombinant ATP7B in Hek293 cells is likely due to tissue-specific differences between the liver and kidney ATP7B mRNA, rather than proteolysis or other posttranslational modification. This conclusion is supported by very similar electrophoretic mobilities of the recombinant hepatic ATP7B expressed in Hek293 cells and endogenous hepatic ATP7B from HepG2 cells (Figure 5C).
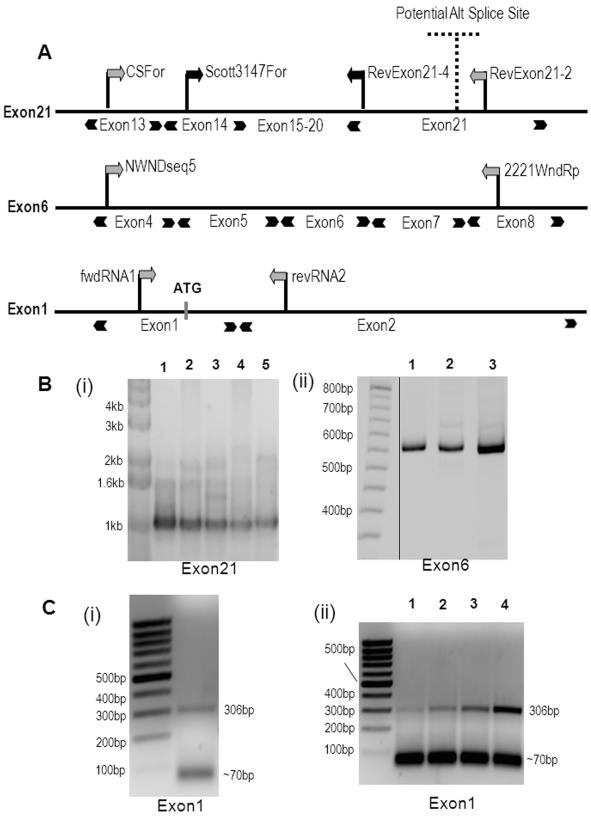
RT-PCR shows no difference in exons 6 and 21 of ATP7B mRNA and a complex behavior of exon 1 upon amplification
The cDNA derived from the liver is generally accepted as encoding the full-length ATP7B. The presence of alternatively spliced mRNA for ATP7B in human tissues has been suggested based on cloning and RT-PCR results (38-40), although the existence of corresponding protein variants has not yet been confirmed. In sheep, alternative splicing of exon 1 was directly demonstrated for the liver ATP7B mRNA (41). To examine whether human kidney and liver cells have distinct ATP7B transcripts, we prepared mRNA from Hek293 and HepG2 cells, generated ATP7B cDNA and PCR-amplified three specific regions (Figure 6A). These regions were selected based on two considerations: (i) their alternative transcripts could account for the shorter ATP7B gene product with the mass difference (about 3 kDa) observed in the Western blots and (ii) their deletion would unlikely to disrupt the activity/structure of ATP7B.
Figure 6. Detection of exons 1, 6 and 21 in ATP7B mRNA from Hek293, HepG2 cells and human kidney tissue.
(A) The position of primers used for the PCR amplification of exons 21, 6, and 1. (B) PCR products generated using cDNA isolated from Hek293 and HepG2 cells. (i) Nested PCR was performed for exon 21 using cDNA generated from Hek293 postnuclear supernatant using random hexamers (1) or polyT primer (2) and from HepG2 generated using random hexamer (3) or polyT primer (4). ATP7B plasmid was used as a positive control (5). All cDNA yielded the product with expected size of ~1kb. (ii) PCR products for exon 6. Lane 1 - Hek293 cDNA, lane 2 - HepG2 cDNA, lane 3 - ATP7B plasmid. All three lanes resulted in similar size products of 530 bp. (C) PCR was performed on total cDNA from mRNA of (i) kidney tissue and (ii) Hek293 (lane1,2) and HepG2 cells (lane 3,4) using primers located within exon1 and exon2 to yield a PCR fragment of 306bp. In addition, a smaller product of size ~70bp of was observed. Samples 1 and 3 were heated to 60oC for 10 min before primers addition; samples 2 and 4 were not heated.
A previous study concluded that kidney ATP7B mRNA has an alternate 3′ exon (designated exon 22), that is generated by the substitution of the part of the exon 21 with the downstream sequence from another gene (38). We did not detect the putative exon 22 using nested PCR primers when either Hek293 or HepG2 cDNAs were used as template (not shown). In contrast, when we used primers designed to amplify exon 21 (Figure 6A), we detected PCR products of expected size for both Hek293 and HepG2 cDNAs (Figure 6B). Therefore, we conclude that exon 21 is intact in ATP7B transcripts from both cell types and cannot be responsible for the observed size difference of the two proteins. Similarly, no difference was observed for exon 6 which codes for the region in the vicinity of transmembrane segment 1, in either Hek293 or HepG2 cDNAs (Figure 6B).
Initial attempts to determine whether exon 1 was alternatively spliced were unsuccessful. RACE analysis of the 5′-region only generated short products, most likely due to an extremely GC-rich sequence in exon 1, which is predicted to form significant secondary structure (not shown). Nested PCR of this region using a forward primer in exon 1 for the first round and a forward primer in exon 2 for the second round of amplification revealed a band corresponding to the expected 395 basepairs for HepG2 cells, but no equivalent band for Hek293 cells (Supplementary Figure 4). This result suggested the loss of exon1 from the renal ATP7B transcript.
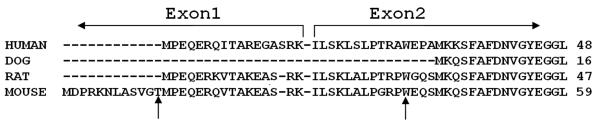
According to the sequence database, the dog ATP7B transcript (accession # DQ016628) does not include the exon 1 sequence (Figure 7). If the exon 1 is critical for the trafficking of ATP7B, as our data suggested, then one would expect canine ATP7B not to traffic in any tissue. This seemed unlikely given the important role of ATP7B relocalization in copper excretion from the liver. Consequently, we chose another approach to establish whether or not the exon 1 was present in renal ATP7B mRNA. RNA was prepared, using another method, from human kidney tissue and yet another set of primers was employed. Two PCR products were detected, a 306 bp band corresponding to the expected size for the exon 1-containing product and another shorter 70 bp band (Figure 6C (i)). Since the 306 bp product could not be cloned (repeatedly) into a Topo-plasmid for further propagation, its identity was verified by the restriction digests and direct sequencing. Cloning followed by sequencing of the 70 bp product revealed that this product had lost portions of the exon 1 and exon 2 and was most likely generated by read-through (without unfolding of the mRNA secondary structure) of RNA polymerase during the reverse transcription step. We reexamined the HEK293 cells mRNA using these new conditions and detected products similar to those found in human tissue, 306 bp and 70 bp, with the latter product being the predominant species (Figure 6C (ii)).
Figure 7. Sequence alignment of ATP7B orthologues.
A sequence alignment of ATP7B orthologues was performed using Clustal W (1.83). The boundary between exon 1 and exon 2 (for human ATP7B, used in this study) is indicated. Canine ATP7B (dog) lacks a corresponding exon 1. Arrows point to the methionines in exon 1 and exon 2, which may serve as initiating Met for hepatic and renal ATP7A, respectively.
Hepatic form of ATP7B expressed in HEK293 cells traffics in response to copper
In the mRNA with the 70 bp sequence, the ATG codons of exon 1 and 2 are deleted, and if such mRNA is translated from the first available ATG, the corresponding protein would be at least 10 kDa shorter than the full-length ATP7B. This difference in protein size is larger than we observed for renal and hepatic ATP7B. Since the PCR reaction can produce artifacts, we decided to employ an alternative strategy to verify that the cell-specific behavior of ATP7B was due to changes in the mRNA, and particularly in the exon 1.
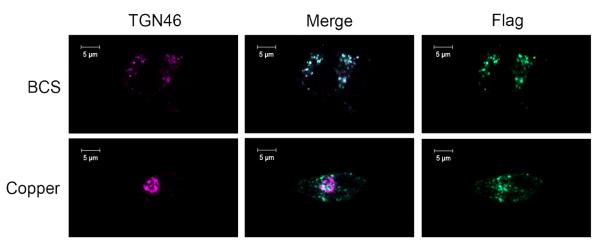
We initially characterized the localization and trafficking of the full-size liver variant of ATP7B (Flag-tagged) in HEK293 cells. We reasoned that if ATP7B is changed at the mRNA level, then the recombinant protein would not only differ from the endogenous in its size (Figure 5) but also in its trafficking behavior. Transient transfection and stable selection experiments do not fully prevent ATP7B overexpression, which can complicate trafficking studies. To overcome this problem, we generated a stable cell line expressing Flag-tagged ATP7B under the control of a tetracycline inducible promoter in FlpIn TREx Hek293 cells (an amino-terminal Flag tag was introduced to distinguish the recombinant ATP7B from endogenous protein). In these cells depleted of copper by treatment with BCS, Flag-ATP7B was found in a perinuclear compartment where it colocalized with the TGN marker, TGN46 (Figure 8, top row), similarly to the endogenous protein. Unlike the endogenous ATP7B, after treatment with 50μM copper chloride, the Flag-ATP7B was targeted to a vesicular compartment (Figure 8, bottom row). This redistribution in response to elevated copper is consistent with the trafficking pattern of hepatic ATP7B, supporting our conclusion that the ATP7B behavior in renal cells is controlled at the mRNA level.
Figure 8. Hepatic ATP7B expressed in FlpIn TREx 293 cells traffics in response to copper.
Expression of Flag-ATP7B was induced with 40ng/mL of tetracycline for 48hrs; cells were treated with either 50 μM BCS or CuCl2 for 1 hour and stained with anti-Flag (green) and anti-TGN46 (purple). In BCS, Flag-ATP7B is localized to the TGN (top row), but traffics to a vesicular compartment when treated with copper (bottom row), as indicated by the loss of co-localization. The scale bar represents 10μM length
ATP7B lacking the exon 1 sequence shows cell-specific trafficking in polarized renal and hepatic cells
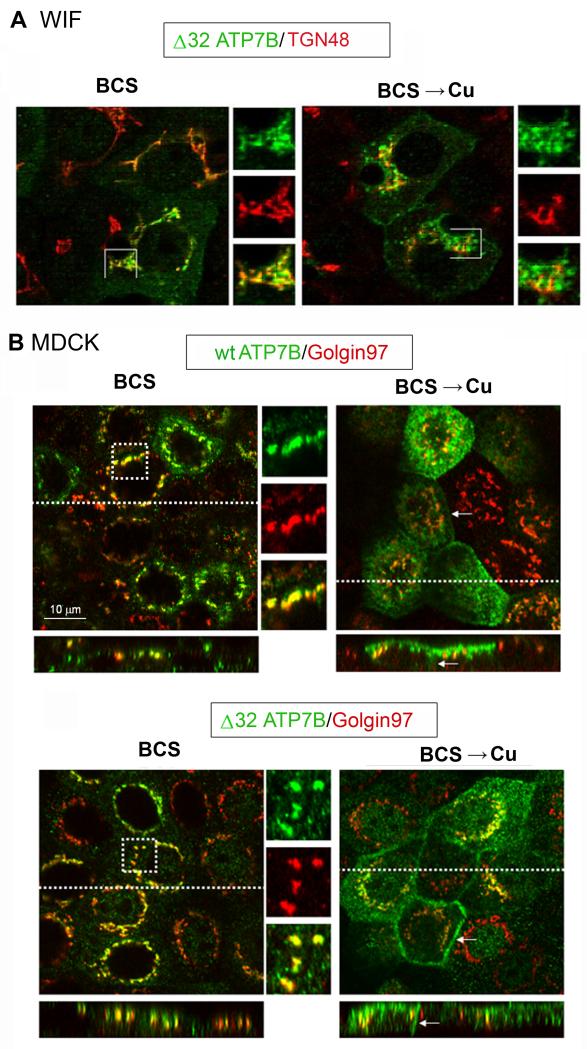
To directly examine the role of the exon 1, we generated an ATP7B construct that allowed translation from the first Met of the exon 2. This was done by deleting the cDNA sequence corresponding to exon 1 as well as sequence prior to the second ATG codon, thus shortening the corresponding protein by 32 amino-acid residues (Δ32ATP7B). The trafficking of the full-length ATP7B and Δ32ATP7B was compared in different cell types. The Tet system is not available for hepatic cells; consequently the full-length ATP7B (used as a control) and Δ32ATP7B were introduced into adenovirus particles and expressed in polarized hepatic (WIF-B) and renal (MDCK) cells. As expected, the full-length ATP7B trafficked to the apical membrane in response to copper in both the WIF-B (26) and MDCK cells (Figure 9), consistent with our data in Hek293 cells. In contrast, the Δ32ATP7B variant showed cell-specific trafficking. Under low copper conditions (BCS treatment), in both cell types Δ32ATP7B had tight TGN localization (Figure 9 A,B). In elevated copper in hepatic WIF-B cells, ATP7B-delta 32 re-localized (almost completely) from the TGN towards the apical membrane (Figure 9A). In the MDCK cells, Δ32ATP7B trafficked to vesicles, basolateral, and apical membrane (Figure 9B), indicative of the loss of precise targeting and/or retention. No basolateral staining was observed for the full-length ATP7B (Figure 9B).
Figure 9. Trafficking of Δ32 ATP7B is cell specific.
WIF-B cells or MDCK were infected with adenovirus expressing either full-length ATP7B or Δ32 ATP7B. Cells were cultured in 10μM BCS (16 hr) then left in BCS or rinsed and incubated in 10μM CuCl2 for an additional 4 hrs. (A) The WIF-B cells were fixed, stained with anti-ATP7B (green) and anti-TGN38 (red) antibodies, then imaged by confocal microscopy (Zeiss 510). In the presence of copper, Δ32 ATP7B relocalized from the TGN to vesicles. (B) MDCK cells were fixed, and stained with anti-ATP7B (green) and anti-Golgin97 (red) antibodies. In the presence of copper, the full-length ATP7B relocalized to vesicles and apical membrane; as expected very little basolateral staining was observed. The Δ32 ATP7B trafficked to the vesicles, apical, and basolateral membranes. Basolateral membrane staining is indicated by an arrow. All images are at the same magnification; the scale bar represents 10μM length.
DISCUSSION
ATP7A and ATP7B differ in their response to elevated copper
Copper homeostasis within cells and tissues is tightly regulated. It is thought that the copper-dependent trafficking of ATP7A and ATP7B is the central mechanism through which these ion-transporting ATPases control intracellular copper (42). We demonstrate that renal cells expressing both endogenous ATP7A and ATP7B have an additional level of regulation in which the two transporters show markedly different responses to elevated copper. In either cultured kidney cells or in primary cells isolated from kidneys of developing mice, ATP7A traffics from the TGN in response to a wide range of copper concentrations, whereas there is no change in the intracellular localization of ATP7B at copper concentrations as high as 200μM. This difference in regulation points to distinct functions of ATP7A and ATP7B in renal cells and helps to explain why in Menkes disease patients, or in the corresponding animal models, copper accumulation due to ATP7A inactivation is not prevented by ATP7B.
In kidney cells, ATP7A traffics mainly to vesicles
Previous studies on the trafficking of ATP7A were mostly carried out using highly elevated copper (typically around 200 μM). Under these conditions, a significant fraction of ATP7A is located at/near the plasma membrane. Our experiments using a wider range of copper concentrations (Supplemental Figure 2) indicate that at lower and more physiologically relevant copper levels (5-10 μM), ATP7A still leaves the TGN, but traffics primarily to vesicles. This situation in kidney cells closely resembles the behavior of endogenous ATP7A in intestinal Caco-2 cells, where ATP7A readily redistributes from the TGN to vesicles in response to changes in copper levels but only approximately 8-10% of the ATP7A is detected at the basolateral cell surface (20). The ATP7A-containing vesicles may rapidly recycle via the plasma membrane, as reported for recombinant ATP7A in MDCK cells (43), and therefore the major function of ATP7A in renal copper homeostasis could be to load vesicles with copper for subsequent export back into circulation. This conclusion is consistent with the recent data showing that in animals injected with high copper, ATP7A is found in the vicinity of the basolateral membrane of renal proximal tubules (30)
Cell-specific control of ATP7B localization
We observed cell-specific regulation of ATP7B trafficking for endogenous protein in both cultured and primary renal cells. The difference between the renal and hepatic cells is not due to different amounts of copper entering cells, since the kinetics of copper transport into Hek293 and HepG2 cells are similar. Also, the ATP7A-mediated copper efflux does not contribute to the lack of ATP7B response in Hek293 cells as the down-regulation of ATP7A by RNA silencing has no effect on ATP7B localization, which remains TGN-like in both low and high copper. One may argue that cell polarization could be necessary for the ability of ATP7B to traffic correctly, as the Hek293 cells are not polarized. However, we have compared the localization of endogenous ATP7B in polarized kidney cells (OK and MDCK cells) in low and high copper, and we observed no effect of copper on the intracellular localization of ATP7B (our unpublished data). Recent studies using mammary gland-derived and neuronal cells suggest that the trafficking of Cu+1-ATPases could also be modulated by hormones or changes in calcium levels (44-46). Whether a similar mechanism may contribute to the regulation of renal ATP7B remains to be determined.
The role of exon 1 in ATP7B trafficking
The lack of copper-induced trafficking for the endogenous ATP7B in all examined cell types, except hepatocytes, was initially surprising given the considerable evidence showing trafficking of transiently transfected ATP7B in CHO cells (18, 47). However, it is now clear that the transfected protein (originating from the liver-derived cDNA) and endogenous protein in renal cells are different. Our data suggest that in kidney, the endogenous ATP7B is modified (compared to hepatic ATP7B), although available data do not yet offer conclusive evidence to the nature of modification. It is apparent that the 5′-end of ATP7B mRNA has an unusual structure. This structure is likely to be responsible for the difficulties with the earlier identification of the initiating Met during cloning of ATP7B (7), (6)), our results with RT-PCR of the exon 1 region, and the presence of at least 3 different 5′ends for human ATP7B mRNA in the Ensemble database (the full-length, one without exon1, and another with an alternative sequence that has no initiating Met). The difficulties with the studies of the ATP7B mRNA 5′-end also raise the question of whether the dog ATP7B transcript truly lacks exon 1, or exon 1 simply has not yet been found.
We demonstrate that the deletion of the protein sequence coded by exon 1 alters the trafficking behavior of recombinant ATP7B in polarized kidney cells: in elevated copper, more ATP7B is retained at the TGN and the polarized re-localization to the apical membrane is lost. This result indicates that the structure of the ATP7B N-terminal region plays a significant role in regulating the ATP7B distribution in kidney cells. This result is particularly interesting in light of recent studies showing the important function of the first 63 amino acids in ATP7B trafficking (26). The deletion of 63 N-terminal amino acids abolishes both the copper responsiveness and the polarized delivery of ATP7B to the apical membrane in hepatocytes (26). The deletion of the first 32 residues (carried out in this work) does not have the same effect. This results points to a critical role of 33-63 region in ATP7B trafficking in hepatic cells.
In contrast in kidney cells, we show that Δ32ATP7B only partially moves out of the TGN when copper is elevated and is unable to distribute in polarized fashion. Thus, the 1-32 region play a role in cell specificity of trafficking. However, the behavior Δ32ATP7B does not fully recapitulate that of endogenous renal ATP7B. We hypothesize that a combination of cell-specific factors might be needed to allow for complete TGN retention or apical trafficking. For example, cell-specific proteins may interact with portions of ATP7B exposed following the loss or modification of the exon1 and thus mediate ATP7B exit from the TGN. If this is the case, then the trafficking behavior of ATP7B in cells may greatly depend not only on ATP7B structure, but also on the precise ratio of ATP7B to cell-specific TGN retention machinery. Expression of ATP7B in excess to such components may yield a phenotype that is neither hepatic nor truly renal. From this point of view it would be interesting to examine the trafficking behavior of the two sheep ATP7B isoforms, for which the alternative splicing of exon 1 has been firmly established (41), but trafficking was investigated using overexpression in CHO cells (cells from different species) and no difference was seen (41).
Loss of copper-dependent trafficking suggests a novel role of ATP7B in vesicular copper storage
It is generally believed that the trafficking of Cu+− ATPases is necessary to facilitate copper excretion. The renal ATP7B does not traffic when copper is elevated. Therefore, in contrast to hepatic ATP7B, it is unlikely to mediate regulated copper export via the apical membrane. This conclusion is consistent with the fact that in the body the liver plays the major role in copper excretion, while copper export into the urine is low. Ceruloplasmin, a protein whose biosynthesis depends on ATP7B activity, is expressed in kidney at a much lower level than liver (48) and is largely undetected in such cells as Hek293 (our unpublished data). These observations raise the question of a specific role for ATP7B in renal cells. One possibility is that ATP7B sustains transport of copper to the TGN when ATP7A leaves this compartment in response to copper elevation. Alternatively, renal ATP7B may have a function that is additional to or distinct from copper delivery to the biosynthetic pathway. The analysis of Hek293 cells shows tight perinuclear staining for both ATP7A and ATP7B (Figure 1) consistent with both of them being in the TGN. However, HEK293 cells are not polarized and therefore their intracellular architecture is not as fully developed as renal epithelial cells. In polarized cells grown on a permeable support, endogenous ATP7B shows less perinuclear staining (our unpublished data) and, in MDCK cells, shows only partial co-localization with golgin 97, a TGN protein involved in the retrograde transport of cargo from the endosome to the TGN (Supplementary Figure 5). It is tempting to speculate that renal ATP7B may be targeted to a specialized TGN/postTGN compartment and mediate copper delivery to this compartment for storage. Such storage function may explain the remarkable ability of kidneys to maintain their copper content under conditions of severe copper deprivation (49).
In conclusion, we have demonstrated that two copper-transporting ATPases, ATP7A and ATP7B, not only have distinct enzymatic properties (28), but also show markedly different trafficking behavior. In kidney cells, ATP7A traffics in response to changes in the copper concentration, while the localization of ATP7B is unaffected by copper. The “non-trafficking” renal ATP7B differs from “trafficking” hepatic ATP7B in apparent molecular mass, which could be generated as a result of modification in ATP7B mRNA. These results illustrate an additional level of regulation that exists in cells in which the two Cu-ATPases are co-expressed and contribute to a better understanding of the disease phenotype caused by ATP7A inactivation.
MATERIALS AND METHODS
Mouse strains
The generation of Atp7b−/− mice has been described previously (50). The Atp7b−/− and the background C57BLx129SV/SvEv mice were housed at the OHSU Department of Comparative Medicine according to the National Institutes of Health guidelines on the use of laboratory and experimental animals.
Cell lines
All cultured cell lines were purchased from ATCC except for FlpIn TREx Hek293 cells (Invitrogen). Hek293, HepG2, and Huh7 cells were maintained in DMEM w/ 10% FBS, and PenStrep (Invitrogen). FlpIn TREx Hek293 cells, which are derived from Hek293 cells, were maintained in MEM + PenStrep + nonessential amino acids + 10% FBS + 15μg/mL blasticidin + 100μg/mL zeocin (Invitrogen). Once under selection, the zeocin was removed and 200μg/mL hygromycin (Invitrogen) was added. For protein expression, the FBS was replaced with Tetracycline-Tested FBS (Invitrogen) and maintained for several days before tetracycline (Fisher) was added to a concentration of 40ng/mL.
Generation of primary kidney and liver cell lines
Kidneys and liver were removed aseptically from 2 weeks-old mice and placed into cold Hibernate-A solution (BrainBits). Tissues were then chopped into 1mm pieces and digested at 37°C in 0.07mg/ml Liberase (Blendzyme 3: Roche Applied Science) in DMEM with no serum for 40 min. 5 mls of DMEM with 15% fetal bovine serum and 100U/ml antibiotic/antimycotic (Invitrogen) was then added to the digested tissue and centrifuged at 850g for 8 min. Pellets were resuspended in 3 ml culture media (as above) and plated in serial dilutions (1/10 and 1/100) for the desired density (50% to 5% confluent). The cells were maintained in media (as above) in 5% CO2 at 37°C for 2 weeks. Primary cultures were re-plated no more than 3 times to avoid cell selection.
Immunofluorescence microscopy
The detection of ATP7B, ATP7A, and trans-Golgi marker TGN46 (GeneTex) in all cell lines except for FlpIn TREx Hek293 cells was performed by culturing cells on glass cover slips. Cells were treated with either 200μm bathocuproinedisulfonic acid disodium salt (BCS), a copper chelator, or with 200μm CuCl2 for 2 hours, fixed by acetone. Cells were blocked in blocking buffer (1% gelatin, 1% BSA, 0.02% sodium azide in PBS) and then incubated with appropriate 1° and 2° antibodies in blocking solution for 1 hour at room temp. (1:500 antibody dilution), Alexa Fluor 488 donkey anti-rat for ATP7B, Alexa Fluor 488 or 555 goat anti-rabbit for ATP7A, or Alexa Fluor 555 donkey anti-sheep for TGN46 (Molecular Probes). Cover slips were mounted onto slides using Vectashield w/DAPI (Vector Laboratories). Images were analyzed using a 630x oil objective with a Zeiss Confocal Scanning microscope (Carl Zeiss).
Copper transport in Hek293 and HepG2 cells
Copper transport by mammalian cells was evaluated by measuring radioactive 64Cu accumulated by cells according to a procedure published earlier (51) with minor modifications. Specifically, cells were placed in 12-well plates (Falcon) (2ml total volume per well) in standard growth media for 24 hours in a CO2-incubator at 370C. Cells were washed using transport buffer (25mM HEPES, 150mM NaCl, 2.5mM MgCl2 pH7.4). Fresh transport buffer (0.9 ml) was added to each well and Cu uptake was initiated by addition of 0.1ml of 50μM or 500 μM copper solution. At specific time points uptake was stopped by three washes in cold stop-buffer (transport buffer,10mM EDTA). The cells were resuspended in 0.1M NaOH and 1ml of each sample was measured in a γ-counter (Cobra Quantum) and protein concentration determined. Uptake experiments were performed at room temperature. As a control for non-specific binding of Cu, cells were pretreated with 5μM or 50 μM copper for 15 minutes on ice, and then underwent the standard experimental procedure. Such pretreatment had no effect on the rate or amount of isotopic uptake.
RNA silencing
Predesigned siRNA of human ATP7A (cat # M-019280-01) and a non-targeting siRNA (D-001210-02-05) was purchased from Dharmacon. For trafficking experiments, Hek293 cells grown on cover-slips in 12-well plates were transfected with 50μl of 2μM stock of siRNA per well using DharmaFECT1 and incubated for 48, 72 and 96h in order to determine time for maximum mRNA knockdown. The non-targeting siRNA was used to verify specificity of silencing. The cells were co-stained with rabbit anti-C-terminal ATP7A and rat anti-N-terminal ATP7B antibodies. Incubation with secondary antibody was done using (for detecting ATP7A) goat anti-rabbit Alexa Fluor 488 labeled Ab (Invitrogen) at 1:500 dilution and (for detecting ATP7B) goat anti rat Alexa Fluor 546 labeled Ab (Invitrogen) at 1:500 dilution. Imaging of the cells was carried out on a Zeiss LSM 5 Pascal confocal microscope with a 100x objective.
Western blot analysis of ATP7B and ATP7A in membrane fractions
60μg of membrane protein was separated on 7.5% Laemmli gel; proteins were then transferred to PDVF membrane (Millipore) and immunostained using either anti-ATP7A (1/5000) or anti-ATP7B (1/5000) antibodies.
In vivo metabolic labeling of Hek293 and HepG2 cells
Cells were grown to confluency on 10cm plates, washed and then incubated for 30min with phosphate free DMEM. The medium was changed to phosphate free DMEM with radioactive orthophosphate (32P) at 1mCi/mL and incubated for 2 hrs at 37°C, 5% CO2.
Subsequently, cells were treated for 1hr with 50μM CuCl2 or 50μM BCS added to the medium. The microsomal membrane fraction was prepared by centrifugation of cell lysates for 30 min at 20,000 g and separated by a 7.5% Laemmli gel. Gel bands above and below the 170kDa protein marker were excised, crushed in a dounce homogenizer, and incubated overnight in immunoprecipitation (IP) buffer (20mM Tris-HCL pH 7.5, 1mM EDTA, 0.1% SDS, 1% Triton X-100, 0.5% Deoxycholate, 1M NaCl) to elute ATP7B from the gel. Gel fragments were pelleted and the supernatant was diluted by 1/4th and transferred to a fresh tube and incubated with IP blocking buffer (1/10th of 10X PBS, 6%BSA, 1% Tween20, and 40ul pre-immune serum) for 1 hr. Protein was immunopurified with anti-ATP7B antibody on Protein G beads (Pierce) and eluted with sample buffer (0.17M Tris pH 6.8, 6.8%SDS, 2.7M Urea, 1%BMe) and run on another 7.5% Laemmli gel. The gel was transferred onto PVDF and placed at -80°C under film (Kodak BioMax MS Film).
PCR of ATP7B cDNA
Cellular RNA was purified from Hek293, HepG2 cells and human kidney using an RNeasy kit (Qiagen). cDNA was generated from 2ug of cellular RNA (postnuclear supernatant) using Superscript III Reverse Transcriptase (Invitrogen) with polydT 48mer (GeneDetect) or random hexamer (Invitrogen) primers (the list of primers is given in the Supplementary Table 1). For human kidney, cDNA was generated from 1ug of total RNA using QuantiTect Reverse Transcription kit (Qiagen). PCR was performed using Platinum Taq DNA Polymerase (Invitrogen). For the primers used for nested PCR, controls were performed showing that single round of PCR amplification using either set of primers did not yield any products (our unpublished data). The PCR product for exon 21 was analyzed on a 1% agarose gel in TAE; the PCR products for exons 6 and exon 1 were analyzed on 8% acrylamide gels in TBE. Gels were stained with SYBR Green (Invitrogen).
Generation of stable Flag-ATP7B cell line in FlpIn TREx 293 cells
An N-terminal FLAG tag was added to ATP7b by PCR using ATP7B pcDNA 3.1(+) (based on liver cDNA) as template Flag-ATP7B was subcloned into pcDNA 5 FRT/ TO (Invitrogen) using BamHI and NotI. FlpIn TREx 293 cells were co-transfected with the pcDNA FRT/TO vectors and pOG44 (Invitrogen) (vector expressing the flipin recombinase) at a DNA ratio of 2:6μg ATP7B pcDNA 5 FRT/TO: pOG44 and 100μl of Lipofectamine 2000 (Invitrogen) for 6hrs at 30°C on a shaker. 48 hours after transfection, cells were placed under selection media. Expression was tested by staining with anti-Flag and visualization by immunofluoresence.
Trafficking of Flag-ATP7B pcDNA 5 FRT/TO
Cells were seeded onto coverslips in 12 well plates and allow to grow for 48hrs, then treated with 50μM BCS or 50μM CuCl2 for 1 hr at 37°C in the presence of 40ng/mL of tetracycline. Cells were fixed in 4% paraformaldehyde, then washed/permeabilized with PBS + 0.02% TritonX-100. The cells were blocked overnight in blocking buffer + 0.02% TritonX-100 at 4°C. Primary antibody incubation with anti-Flag (Sigma) and anti-TGN46 (GeneTex) were performed at room temperature in blocking buffer at a dilution 1:500 for 1 hour. Secondary antibody incubation was done with donkey anti-mouse Alexa488 and donkey anti-sheep Alexa555 (Molecular Probes) for 1 hr at room temperature in the dark. Cells were mounted in Vectashield + DAPI and sealed. Imaging of cells was done on a Zeiss LSM 5 Pascal confocal microscope at 100x objective.
Trafficking of wtATP7B and Δ32 ATP7B in polarized WIF-B and MDCK cells
Untagged Δ32-ATP7B was generated using the QuickChange II XL Site-Directed Mutagenesis Kit and ATP7B cloned into the pAdLOX vector (pYG80 plasmid designation) as the template to generate the plasmid pLB1064. Adenovirus was prepared according to previously published methods (52). WIF-B cells were seeded on glass coverslips, cultured as described (53); (54) and used ~11-12 days later when maximal polarity had been achieved. MDCK cells were seeded on glass coverslips coated with Rat Tail collagen, Type I, according to the manufacturers recomendation (BD Biosciences, Bedford, MA) and used 2-3 days later. Cells were infected (1 hr with ~1-3 ×108 v.p./coverslip), then cultured ~16 hrs in basal medium (0.02μM Cu2+) containing 10μM bathocuproine disulfonic acid (BCS) (Sigma, St. Louis, MO) to chelate copper. For copper treatment, 10μM CuCl2 was added to basal medium for 4 hrs before fixation. Infected cells were fixed and further processed for indirect immunofluorescence according to previously published methods (Ihrke, 1995). Each construct was tested at least two times, and only low expressing cells were evaluated.
Supplementary Material
Supplementary Figure 1 ATP7A and ATP7B are present at similar levels in Hek293 cells. The calibration curves were generated using purified recombinant proteins that earlier served as antigens for preparation of the anti-ATP7A and anti-ATP7B antibodies. WNDP NTD is the N-terminal domain of ATP7B; MNKP CTD is the C-terminal domain of ATP7A. Increasing amounts of each antigen were loaded on the Laemmli gel and the intensity of signals was compared to the intensity of ATP7A or ATP7B bands in the whole cell lysate (CL) using densitometry. The difference in protein size between the full-length Cu-ATPase and the antigens were taken into account. Since current anti-ATP7A and ATP7B antibodies have different sensitivities, different amounts of cell lysates have been used in this experiment (50.58 μg and 6.75 μg, respectively). The arrow indicates the full-size ATP7A. Note: in Figure 1 equal amount of cell lystate was loaded; in this figure more clear (single band) and more sensitive detection of ATP7A was due to the use of different batch of the anti-ATP7A (generated and tested by Dr. Eipper), which was used up by the time this supplementary figure was added.
Supplementary Figure 2 Trafficking of endogenous ATP7A at various copper concentrations in Hek293 cells. Hek293 cells were incubated with increasing concentrations of CuCl2 and then immuno-stained with anti-ATP7A (green) and anti-TGN46 (red) antibodies. Relocalization of ATP7A from TGN to vesicles is observed in as little as 5μM CuCl2. All images are at the same magnification; the scale bar represents 10μM length.
Supplementary Figure 3 Western blot analysis demonstrating immunoprecipitation of ATP7B from metabolically labeled HepG2 and Hek293 cells
Supplementary Figure 4 Efficiency of the exon 1 amplification differs for the mRNA isolated from Hek293 and HepG2 cells. Upper panel: The position of used primers in the PCR and nested PCR reactions for exon1. Lower panel: Lane (1) Hek293 cDNA and (2) HepG2 cDNA. Product of the correct size, 395 bp was only seen in reactions utilizing the HepG2 template.
Supplementary Figure 5 Localization of TGN marker (Golgin97) and ATP7B (WNDP) in the polarized MDCK cells. Cells were grown in Transwells and treated for 3 hours with 50 μM BSC before staining with the anti-golgin97 (left) and anti-ATP7B antibody (middle). Comparison of two patterns (merge, right) shows that patterns are largely non-overlapping. Scale bar represents 5 μM length.
Supplementary Table 1 Sequences of primers used to amplify exons of ATP7B
ACKNOWLEDGMENTS
We thank members of the Dr. Turker laboratory at OHSU for help with preparation of primary cells; Dr. Betty Eipper for the anti-ATP7A antibody, and Dr. Chris Corless and Oregon tissue bank for the kidney tissue sample. This work was funded by the National Institute of Health Grant P01 GM 067166 to SL and JHK, and DK 072084 to ALH
REFERENCES
- 1.Frieden E. Perspectives on copper biochemistry. Clin Physiol Biochem. 1986;4:11–19. [PubMed] [Google Scholar]
- 2.Waggoner DJ, Bartnikas TB, Gitlin JD. The role of copper in neurodegenerative disease. Neurobiol Dis. 1999;6:221–230. doi: 10.1006/nbdi.1999.0250. [DOI] [PubMed] [Google Scholar]
- 3.Linder MC, Wooten L, Cerveza P, Cotton S, Shulze R, Lomeli N. Copper transport. Am J Clin Nutr. 1998;67:965S–971S. doi: 10.1093/ajcn/67.5.965S. [DOI] [PubMed] [Google Scholar]
- 4.Harris ED. A requirement for copper in angiogenesis. Nutr Rev. 2004;62:60–64. doi: 10.1111/j.1753-4887.2004.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 5.Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11:107–116. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bull PC, Cox DW. Long range restriction mapping of 13q14.3 focused on the Wilson disease region. Genomics. 1993;16:593–598. doi: 10.1006/geno.1993.1235. [DOI] [PubMed] [Google Scholar]
- 7.Tanzi RE, Petrukhin K, Chernov I, Pellequer JL, Wasco W, Ross B, Romano DM, Parano E, Pavone L, Brzustowicz LM, Devoto M, Peppercorn J, Bush AI, Sternlieb I, Pirastu M. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet. 1993;5:344–350. doi: 10.1038/ng1293-344. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi Y, Heiny ME, Gitlin JD. Isolation and characterization of a human liver cDNA as a candidate gene for Wilson disease. Biochem Biophys Res Commun. 1993;197:271–277. doi: 10.1006/bbrc.1993.2471. [DOI] [PubMed] [Google Scholar]
- 9.Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat Genet. 1993;3:7–13. doi: 10.1038/ng0193-7. [DOI] [PubMed] [Google Scholar]
- 10.Mercer JF, Livingston J, Hall B, Paynter JA, Begy C, Chandrasekharappa S, Lockhart P, Grimes A, Bhave M, Siemieniak D. Isolation of a partial candidate gene for Menkes disease by positional cloning. Nat Genet. 1993;3:20–25. doi: 10.1038/ng0193-20. [DOI] [PubMed] [Google Scholar]
- 11.Chelly J, Tumer Z, Tonnesen T, Petterson A, Ishikawa-Brush Y, Tommerup N, Horn N, Monaco AP. Isolation of a candidate gene for Menkes disease that encodes a potential heavy metal binding protein. Nat Genet. 1993;3:14–19. doi: 10.1038/ng0193-14. [DOI] [PubMed] [Google Scholar]
- 12.Petris MJ, Strausak D, Mercer JF. The Menkes copper transporter is required for the activation of tyrosinase. Hum Mol Genet. 2000;9:2845–2851. doi: 10.1093/hmg/9.19.2845. [DOI] [PubMed] [Google Scholar]
- 13.Roelofsen H, Wolters H, Van Luyn MJ, Miura N, Kuipers F, Vonk RJ. Copper-induced apical trafficking of ATP7B in polarized hepatoma cells provides a mechanism for biliary copper excretion. Gastroenterology. 2000;119:782–793. doi: 10.1053/gast.2000.17834. [DOI] [PubMed] [Google Scholar]
- 14.Petris MJ, Mercer JF, Culvenor JG, Lockhart P, Gleeson PA, Camakaris J. Ligand-regulated transport of the Menkes copper P-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: a novel mechanism of regulated trafficking. Embo J. 1996;15:6084–6095. [PMC free article] [PubMed] [Google Scholar]
- 15.La Fontaine S, Firth SD, Lockhart P, Brooks H, Camakaris J, Mercer JFB. Activity and intracellular localization of Mnk, the murine homologue of the Menkes protein in cells from blotchy (Moblo) and brindled (Mobr) mouse mutants. Hum Mol Genet. 1999;8:1069–1075. doi: 10.1093/hmg/8.6.1069. [DOI] [PubMed] [Google Scholar]
- 16.La Fontaine S, Firth SD, Lockhart PJ, Brooks H, Parton RG, Camakaris J, Mercer JF. Functional analysis and intracellular localization of the human menkes protein (MNK) stably expressed from a cDNA construct in Chinese hamster ovary cells (CHO-K1) Hum Mol Genet. 1998;7:1293–1300. doi: 10.1093/hmg/7.8.1293. [DOI] [PubMed] [Google Scholar]
- 17.Petris MJ, Mercer JF. The Menkes protein (ATP7A; MNK) cycles via the plasma membrane both in basal and elevated extracellular copper using a C-terminal di-leucine endocytic signal. Hum Mol Genet. 1999;8:2107–2115. doi: 10.1093/hmg/8.11.2107. [DOI] [PubMed] [Google Scholar]
- 18.La Fontaine SL, Firth SD, Camakaris J, Englezou A, Theophilos MB, Petris MJ, Howie M, Lockhart PJ, Greenough M, Brooks H, Reddel RR, Mercer JF. Correction of the copper transport defect of Menkes patient fibroblasts by expression of the Menkes and Wilson ATPases. J Biol Chem. 1998;273:31375–31380. doi: 10.1074/jbc.273.47.31375. [DOI] [PubMed] [Google Scholar]
- 19.Hamza I, Prohaska J, Gitlin JD. Essential role for Atox1 in the copper-mediated intracellular trafficking of the Menkes ATPase. Proc Natl Acad Sci U S A. 2003;100:1215–1220. doi: 10.1073/pnas.0336230100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nyasae L, Bustos R, Braiterman L, Eipper B, Hubbard A. Dynamics of endogenous ATP7A (Menkes protein) in intestinal epithelial cells: copper-dependent redistribution between two intracellular sites. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1181–1194. doi: 10.1152/ajpgi.00472.2006. [DOI] [PubMed] [Google Scholar]
- 21.Forbes JR, Cox DW. Functional characterization of missense mutations in ATP7B: Wilson disease mutation or normal variant? Am J Hum Genet. 1998;63:1663–1674. doi: 10.1086/302163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forbes JR, Cox DW. Copper-dependent trafficking of Wilson disease mutant ATP7B proteins. Hum Mol Genet. 2000;9:1927–1935. doi: 10.1093/hmg/9.13.1927. [DOI] [PubMed] [Google Scholar]
- 23.Huster D, Hoppert M, Lutsenko S, Zinke J, Lehmann C, Mossner J, Berr F, Caca K. Defective cellular localization of mutant ATP7B in Wilson’s disease patients and hepatoma cell lines. Gastroenterology. 2003;124:335–345. doi: 10.1053/gast.2003.50066. [DOI] [PubMed] [Google Scholar]
- 24.Schaefer M, Hopkins RG, Failla ML, Gitlin JD. Hepatocyte-specific localization and copper-dependent trafficking of the Wilson’s disease protein in the liver. Am J Physiol. 1999;276:G639–646. doi: 10.1152/ajpgi.1999.276.3.G639. [DOI] [PubMed] [Google Scholar]
- 25.Vanderwerf SM, Cooper MJ, Stetsenko IV, Lutsenko S. Copper specifically regulates intracellular phosphorylation of the Wilson’s disease protein, a human copper-transporting ATPase. J Biol Chem. 2001;276:36289–36294. doi: 10.1074/jbc.M102055200. [DOI] [PubMed] [Google Scholar]
- 26.Guo Y, Nyasae L, Braiterman LT, Hubbard AL. NH2-terminal signals in ATP7B Cu-ATPase mediate its Cu-dependent anterograde traffic in polarized hepatic cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G904–916. doi: 10.1152/ajpgi.00262.2005. [DOI] [PubMed] [Google Scholar]
- 27.Bartee MY, Lutsenko S. Hepatic copper-transporting ATPase ATP7B: function and inactivation at the molecular and cellular level. Biometals. 2007;20:627–637. doi: 10.1007/s10534-006-9074-3. [DOI] [PubMed] [Google Scholar]
- 28.Barnes N, Tsivkovskii R, Tsivkovskaia N, Lutsenko S. The copper-transporting ATPases, menkes and wilson disease proteins, have distinct roles in adult and developing cerebellum. J Biol Chem. 2005;280:9640–9645. doi: 10.1074/jbc.M413840200. [DOI] [PubMed] [Google Scholar]
- 29.La Fontaine S, Firth SD, Lockhart PJ, Brooks H, Camakaris J, Mercer JF. Intracellular localization and loss of copper responsiveness of Mnk, the murine homologue of the Menkes protein, in cells from blotchy (Mo blo) and brindled (Mo br) mouse mutants. Hum Mol Genet. 1999;8:1069–1075. doi: 10.1093/hmg/8.6.1069. [DOI] [PubMed] [Google Scholar]
- 30.Linz R, Barnes NL, Zimnicka AM, Kaplan JH, Eipper B, Lutsenko S. Intracellular targeting of copper-transporting ATPase ATP7A in a normal and Atp7b-/-kidney. Am J Physiol Renal Physiol. 2008;294:F53–61. doi: 10.1152/ajprenal.00314.2007. [DOI] [PubMed] [Google Scholar]
- 31.Yoshimura N, Kida K, Usutani S, Nishimura M. Histochemical localization of copper in various organs of brindled mice after copper therapy. Pathol Int. 1995;45:10–18. doi: 10.1111/j.1440-1827.1995.tb03374.x. [DOI] [PubMed] [Google Scholar]
- 32.Kodama H, Okabe I, Kihara A, Mori Y, Okaniwa M. Renal tubular function of patients with classical Menkes disease. J Inherit Metab Dis. 1992;15:157–158. doi: 10.1007/BF01800360. [DOI] [PubMed] [Google Scholar]
- 33.Ozawa H, Kodama H, Kawaguchi H, Mochizuki T, Kobayashi M, Igarashi T. Renal function in patients with Menkes disease. Eur J Pediatr. 2003;162:51–52. doi: 10.1007/s00431-002-1082-x. [DOI] [PubMed] [Google Scholar]
- 34.Kirby BJ, Danks DM, Legge GJ, Mercer JF. Analysis of the distribution of Cu, Fe and Zn and other elements in brindled mouse kidney using a scanning proton microprobe. J Inorg Biochem. 1998;71:189–197. doi: 10.1016/s0162-0134(98)10053-3. [DOI] [PubMed] [Google Scholar]
- 35.Yoshimura N. Histochemical localization of copper in various organs of brindled mice. Pathol Int. 1994;44:14–19. doi: 10.1111/j.1440-1827.1994.tb02580.x. [DOI] [PubMed] [Google Scholar]
- 36.Elsas LJ, Hayslett JP, Spargo BH, Durant JL, Rosenberg LE. Wilson’s disease with reversible renal tubular dysfunction. Correlation with proximal tubular ultrastructure. Ann Intern Med. 1971;75:427–433. doi: 10.7326/0003-4819-75-3-427. [DOI] [PubMed] [Google Scholar]
- 37.Linz R, Lutsenko S. Copper-transporting ATPases ATP7A and ATP7B: cousins, not twins. J Bioenerg Biomembr. 2007;39:403–7. doi: 10.1007/s10863-007-9101-2. [DOI] [PubMed] [Google Scholar]
- 38.Thomas GR, Forbes JR, Roberts EA, Walshe JM, Cox DW. The Wilson disease gene: spectrum of mutations and their consequences. Nature Genetics. 1995;9:210–216. doi: 10.1038/ng0295-210. [DOI] [PubMed] [Google Scholar]
- 39.Petrukhin K, Lutsenko S, Chernov I, Ross BM, Kaplan JH, Gilliam TC. Characterization of the Wilson disease gene encoding a P-type copper transporting ATPase: genomic organization, alternative splicing, and structure/function predictions. Hum Mol Genet. 1994;3:1647–1656. doi: 10.1093/hmg/3.9.1647. [DOI] [PubMed] [Google Scholar]
- 40.Borjigin J, Payne AS, Deng J, Li X, Wang MM, Ovodenko B, Gitlin JD, Snyder SH. A novel pineal night-specific ATPase encoded by the Wilson disease gene. J Neurosci. 1999;19:1018–1026. doi: 10.1523/JNEUROSCI.19-03-01018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lockhart PJ, Wilcox SA, Dahl HM, Mercer JF. Cloning, mapping and expression analysis of the sheep Wilson disease gene homologue. Biochim-Biophys-Acta. 2000;1491:229–239. doi: 10.1016/s0167-4781(00)00054-3. [DOI] [PubMed] [Google Scholar]
- 42.Mercer JF. The molecular basis of copper-transport diseases. Trends Mol Med. 2001;7:64–69. doi: 10.1016/s1471-4914(01)01920-7. [DOI] [PubMed] [Google Scholar]
- 43.Pase L, Voskoboinik I, Greenough M, Camakaris J. Copper stimulates trafficking of a distinct pool of the Menkes copper ATPase (ATP7A) to the plasma membrane and diverts it into a rapid recycling pool. Biochem J. 2004;378:1031–1037. doi: 10.1042/BJ20031181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hardman B, Michalczyk A, Greenough M, Camakaris J, Mercer JF, Ackland ML. Hormonal regulation of the Menkes and Wilson copper-transporting ATPases in human placental Jeg-3 cells. Biochem J. 2007;402:241–250. doi: 10.1042/BJ20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schlief ML, West T, Craig AM, Holtzman DM, Gitlin JD. Role of the Menkes copper-transporting ATPase in NMDA receptor-mediated neuronal toxicity. Proc Natl Acad Sci U S A. 2006;103:14919–14924. doi: 10.1073/pnas.0605390103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelleher SL, Lonnerdal BL. Mammary gland copper transport is stimulated by prolactin through alterations in Ctr1 and Atp7A localization. Am J Physiol Regul Integr Comp Physiol. 2006:R1181–1191. doi: 10.1152/ajpregu.00206.2005. [DOI] [PubMed] [Google Scholar]
- 47.La Fontaine S, Theophilos MB, Firth SD, Gould R, Parton RG, Mercer JF. Effect of the toxic milk mutation (tx) on the function and intracellular localization of Wnd, the murine homologue of the Wilson copper ATPase. Hum Mol Genet. 2001;10:361–370. doi: 10.1093/hmg/10.4.361. [DOI] [PubMed] [Google Scholar]
- 48.Klomp LW, Farhangrazi ZS, Dugan LL, Gitlin JD. Ceruloplasmin gene expression in the murine central nervous system. J Clin Invest. 1996;98:207–215. doi: 10.1172/JCI118768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nose Y, Kim BE, Thiele DJ. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab. 2006;4:235–244. doi: 10.1016/j.cmet.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 50.Buiakova OI, Xu J, Lutsenko S, Zeitlin S, Das K, Das S, Ross BM, Mekios C, Scheinberg IH, Gilliam TC. Null mutation of the murine ATP7B (Wilson disease) gene results in intracellular copper accumulation and late-onset hepatic nodular transformation. HumMolGenet. 1999;8:1665–1671. doi: 10.1093/hmg/8.9.1665. [DOI] [PubMed] [Google Scholar]
- 51.Eisses JF, Chi Y, Kaplan JH. Stable plasma membrane levels of hCTR1 mediate cellular copper uptake. J Biol Chem. 2005;280:9635–9639. doi: 10.1074/jbc.M500116200. [DOI] [PubMed] [Google Scholar]
- 52.Bastaki M, Braiterman LT, Johns DC, Chen YH, Hubbard AL. Absence of direct delivery for single transmembrane apical proteins or their “Secretory” forms in polarized hepatic cells. Mol Biol Cell. 2002;13:225–237. doi: 10.1091/mbc.01-07-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cassio D, Hamon-Benais C, Guerin M, Lecoq O. Hybrid cell lines constitute a potential reservoir of polarized cells: isolation and study of highly differentiated hepatoma-derived hybrid cells able to form functional bile canaliculi in vitro. J Cell Biol. 1991;115:1397–1408. doi: 10.1083/jcb.115.5.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ihrke G, Neufeld EB, Meads T, Shanks MR, Cassio D, Laurent M, Schroer TA, Pagano RE, Hubbard AL. WIF-B cells: an in vitro model for studies of hepatocyte polarity. J Cell Biol. 1993;123:1761–1775. doi: 10.1083/jcb.123.6.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 ATP7A and ATP7B are present at similar levels in Hek293 cells. The calibration curves were generated using purified recombinant proteins that earlier served as antigens for preparation of the anti-ATP7A and anti-ATP7B antibodies. WNDP NTD is the N-terminal domain of ATP7B; MNKP CTD is the C-terminal domain of ATP7A. Increasing amounts of each antigen were loaded on the Laemmli gel and the intensity of signals was compared to the intensity of ATP7A or ATP7B bands in the whole cell lysate (CL) using densitometry. The difference in protein size between the full-length Cu-ATPase and the antigens were taken into account. Since current anti-ATP7A and ATP7B antibodies have different sensitivities, different amounts of cell lysates have been used in this experiment (50.58 μg and 6.75 μg, respectively). The arrow indicates the full-size ATP7A. Note: in Figure 1 equal amount of cell lystate was loaded; in this figure more clear (single band) and more sensitive detection of ATP7A was due to the use of different batch of the anti-ATP7A (generated and tested by Dr. Eipper), which was used up by the time this supplementary figure was added.
Supplementary Figure 2 Trafficking of endogenous ATP7A at various copper concentrations in Hek293 cells. Hek293 cells were incubated with increasing concentrations of CuCl2 and then immuno-stained with anti-ATP7A (green) and anti-TGN46 (red) antibodies. Relocalization of ATP7A from TGN to vesicles is observed in as little as 5μM CuCl2. All images are at the same magnification; the scale bar represents 10μM length.
Supplementary Figure 3 Western blot analysis demonstrating immunoprecipitation of ATP7B from metabolically labeled HepG2 and Hek293 cells
Supplementary Figure 4 Efficiency of the exon 1 amplification differs for the mRNA isolated from Hek293 and HepG2 cells. Upper panel: The position of used primers in the PCR and nested PCR reactions for exon1. Lower panel: Lane (1) Hek293 cDNA and (2) HepG2 cDNA. Product of the correct size, 395 bp was only seen in reactions utilizing the HepG2 template.
Supplementary Figure 5 Localization of TGN marker (Golgin97) and ATP7B (WNDP) in the polarized MDCK cells. Cells were grown in Transwells and treated for 3 hours with 50 μM BSC before staining with the anti-golgin97 (left) and anti-ATP7B antibody (middle). Comparison of two patterns (merge, right) shows that patterns are largely non-overlapping. Scale bar represents 5 μM length.
Supplementary Table 1 Sequences of primers used to amplify exons of ATP7B