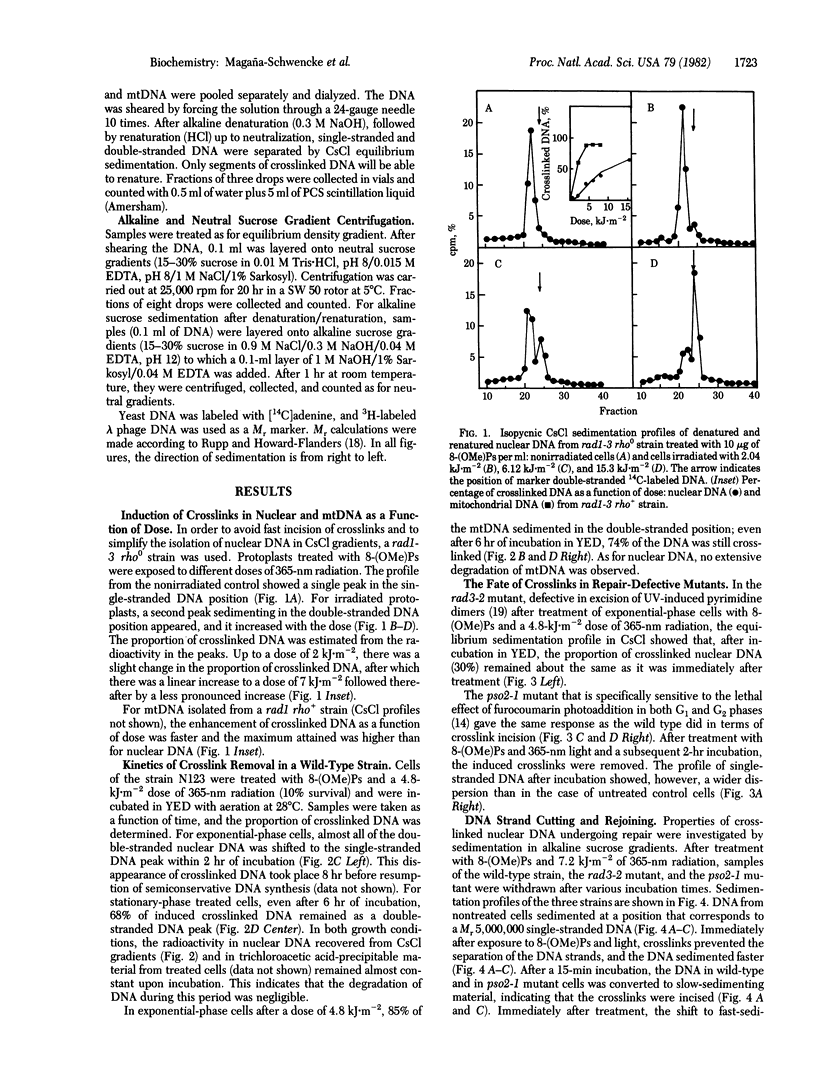
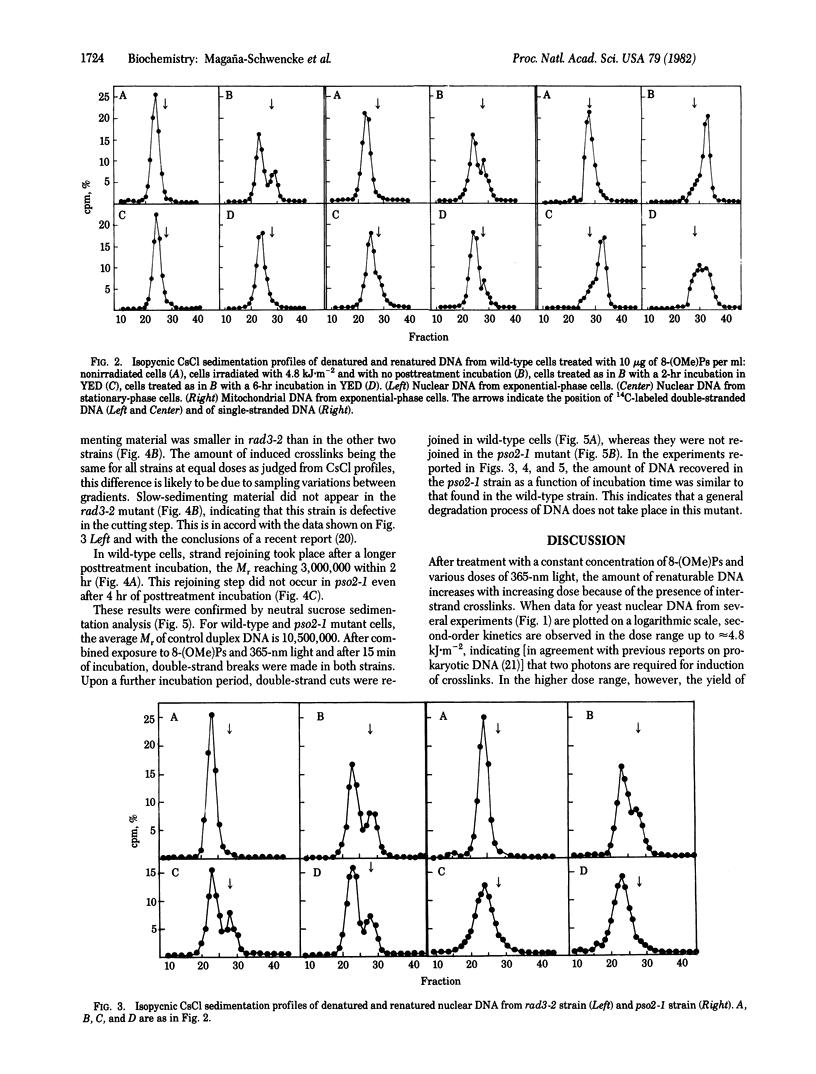
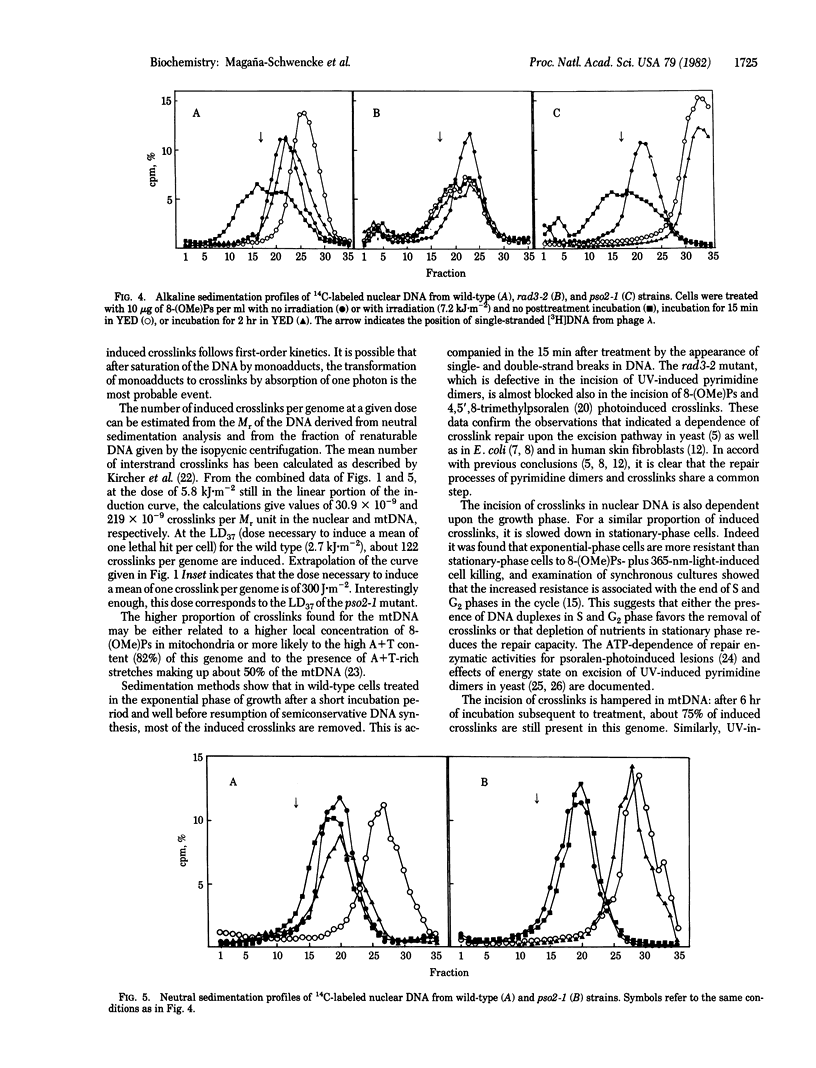
Abstract
In Saccharomyces cerevisiae, after 8-methoxypsoralen [8-(OMe)Ps] photoaddition, more crosslinks are induced per unit dose in mitochondrial DNA than in nuclear DNA. In wild-type cells treated in the exponential phase of growth, single- and double-strand breaks are produced during crosslink removal and then are rejoined upon postexposure incubation. The incision step is almost blocked in the rad 3-2 mutant, which is also defective in excision-repair of UV-induced (254 nm) pyrimidine dimers. The cutting of crosslinks from nuclear DNA is depressed in wild-type stationary-phase cells. This is correlated with a higher sensitivity of such cells to 8-(OMe)Ps photoinduced cell killing. The incision of crosslinks is dramatically reduced in mitochondrial DNA. The rejoining of single- and double-strand breaks is not only dependent on the product of the RAD51 gene (as shown by others) but also of the PSO2 gene. A correlation was found between the ability to recombine and strand rejoining. Therefore, as in bacteria, both the excision and the recombinational repair systems are involved in crosslink repair in yeast. However, double-strand breaks in yeast constitute repair intermediates which are not detected in Escherichia coli. The LD37 (dose necessary to induce a mean of one lethal hit per cell) corresponds to about 120 crosslinks per genome in exponential-phase cells of the wild type and to 1-2 crosslinks in the pso2-1 mutant.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Averbeck D., Moustacchi E. 8-Methoxypsoralen plus 365 nm light effects and repair in yeast. Biochim Biophys Acta. 1975 Jul 23;395(4):393–404. doi: 10.1016/0005-2787(75)90063-5. [DOI] [PubMed] [Google Scholar]
- Averbeck D., Moustacchi E. Genetic effect of 3-carbethoxypsoralen, angelicin, psoralen and 8-methoxypsoralen plus 365-nm irradiation in Saccharomyces cerevisiae: induction of reversions, mitotic crossing-over, gene conversion and cytoplasmic "petite" mutations. Mutat Res. 1979 Oct;68(2):133–148. doi: 10.1016/0165-1218(79)90141-1. [DOI] [PubMed] [Google Scholar]
- Ben-Hur E., Elkind M. M. DNA cross-linking in Chinese hamster cells exposed to near ultraviolet light in the presence of 4,5',8-trimethylpsoralen. Biochim Biophys Acta. 1973 Dec 7;331(2):181–193. doi: 10.1016/0005-2787(73)90431-0. [DOI] [PubMed] [Google Scholar]
- Cassier C., Chanet R., Henriques J. A., Moustacchi E. The effects of three PSO genes on induced mutagenesis : a novel class of mutationally defective yeast. Genetics. 1980 Dec;96(4):841–857. doi: 10.1093/genetics/96.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassuto E., Gross N., Bardwell E., Howard-Flanders P. Genetic effects of photoadducts and photocross-links in the DNA of phage lambda exposed to 360 nm light and tri-methylpsoralen or khellin. Biochim Biophys Acta. 1977 Apr 19;475(4):589–600. doi: 10.1016/0005-2787(77)90319-7. [DOI] [PubMed] [Google Scholar]
- Cole R. S., Levitan D., Sinden R. R. Removal of psoralen interstrand cross-links from DNA of Escherichia coli: mechanism and genetic control. J Mol Biol. 1976 May 5;103(1):39–59. doi: 10.1016/0022-2836(76)90051-6. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Repair of DNA containing interstrand crosslinks in Escherichia coli: sequential excision and recombination. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1064–1068. doi: 10.1073/pnas.70.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson L. R., Cox B. S. The role of dimer excision in liquid-holding recovery of UV-irradiated haploid yeast. Mutat Res. 1980 Jan;69(1):19–41. doi: 10.1016/0027-5107(80)90173-6. [DOI] [PubMed] [Google Scholar]
- Game J. C., Mortimer R. K. A genetic study of x-ray sensitive mutants in yeast. Mutat Res. 1974 Sep;24(3):281–292. doi: 10.1016/0027-5107(74)90176-6. [DOI] [PubMed] [Google Scholar]
- Henriques J. A., Chanet R., Averbeck D., Moustacchi E. Lethality and "petite" mutation induced by the photoaddition of 8-methoxypsoralen in yeast: influence of ploidy, growth phases and stages in the cell cycle. Mol Gen Genet. 1977 Dec 14;158(1):63–72. doi: 10.1007/BF00455120. [DOI] [PubMed] [Google Scholar]
- Henriques J. A., Moustacchi E. Isolation and characterization of pso mutants sensitive to photo-addition of psoralen derivatives in Saccharomyces cerevisiae. Genetics. 1980 Jun;95(2):273–288. doi: 10.1093/genetics/95.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho K. S. Induction of DNA double-strand breaks by X-rays in a radiosensitive strain of the yeast Saccharomyces cerevisiae. Mutat Res. 1975 Dec;30(3):327–334. [PubMed] [Google Scholar]
- Jachymczyk W. J., von Borstel R. C., Mowat M. R., Hastings P. J. Repair of interstrand cross-links in DNA of Saccharomyces cerevisiae requires two systems for DNA repair: the RAD3 system and the RAD51 system. Mol Gen Genet. 1981;182(2):196–205. doi: 10.1007/BF00269658. [DOI] [PubMed] [Google Scholar]
- Kaye J., Smith C. A., Hanawalt P. C. DNA repair in human cells containing photoadducts of 8-methoxypsoralen or angelicin. Cancer Res. 1980 Mar;40(3):696–702. [PubMed] [Google Scholar]
- Kircher M., Fleer R., Ruhland A., Brendel M. Biological and chemical effects of mustard gas in yeast. Mutat Res. 1979 Dec;63(2):273–289. doi: 10.1016/0027-5107(79)90059-9. [DOI] [PubMed] [Google Scholar]
- Krasin F., Hutchinson F. Repair of DNA double-strand breaks in Escherichia coli cells requires synthesis of proteins that can be induced by UV light. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3450–3453. doi: 10.1073/pnas.78.6.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C. W., Christensen R. UV mutagenesis in radiation-sensitive strains of yeast. Genetics. 1976 Feb;82(2):207–232. doi: 10.1093/genetics/82.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magaña-Schwencke N., Averbeck D., Pegas-Henriques J. A., Moustacchi E. Absence de pontages inter-chaînes dan l'ADN traité par le 3-carbéthoxypsoralène et une irradiation à 365 nm. C R Seances Acad Sci D. 1980 Sep 15;291(2):207–210. [PubMed] [Google Scholar]
- McKee R. H., Lawrence C. W. Genetic analysis of gamma-ray mutagenesis in yeast. I. Reversion in radiation-sensitive strains. Genetics. 1979 Oct;93(2):361–373. doi: 10.1093/genetics/93.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons B. J. Psoralen photochemistry. Photochem Photobiol. 1980 Dec;32(6):813–821. doi: 10.1111/j.1751-1097.1980.tb04061.x. [DOI] [PubMed] [Google Scholar]
- Petes T. D., Fangman W. L. Sedimentation properties of yeast chromosomal DNA. Proc Natl Acad Sci U S A. 1972 May;69(5):1188–1191. doi: 10.1073/pnas.69.5.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash L. Repair of pyrimidine dimers in nuclear and mitochondrial DNA of yeast irradiated with low doses of ultraviolet light. J Mol Biol. 1975 Nov 15;98(4):781–795. doi: 10.1016/s0022-2836(75)80010-6. [DOI] [PubMed] [Google Scholar]
- Prakash L. Repair of pyrimidine dimers in radiation-sensitive mutants rad3, rad4, rad6 and rad9 of Saccharomyces cerevisiae. Mutat Res. 1977 Oct;45(1):13–20. doi: 10.1016/0027-5107(77)90038-0. [DOI] [PubMed] [Google Scholar]
- Prunell A., Bernardi G. The mitochondrial genome of wild-type yeast cells. IV. Genes and spacers. J Mol Biol. 1974 Jul 15;86(4):825–841. doi: 10.1016/0022-2836(74)90356-8. [DOI] [PubMed] [Google Scholar]
- Resnick M. A., Martin P. The repair of double-strand breaks in the nuclear DNA of Saccharomyces cerevisiae and its genetic control. Mol Gen Genet. 1976 Jan 16;143(2):119–129. doi: 10.1007/BF00266917. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- Saeki T., Machida I., Nakai S. Genetic control of diploid recovery after gamma-irradiation in the yeast Saccharomyces cerevisiae. Mutat Res. 1980 Dec;73(2):251–265. doi: 10.1016/0027-5107(80)90192-x. [DOI] [PubMed] [Google Scholar]
- Schwencke J., Magaña-Schwencke N., Laporte J. Yeast protoplasts from stationary and starved cells: preparation, ultrastructure and vacuolar development. Ann Microbiol (Paris) 1977 Jan;128A(1):3–18. [PubMed] [Google Scholar]
- Scott B. R., Pathak M. A., Mohn G. R. Molecular and genetic basis of furocoumarin reactions. Mutat Res. 1976;39(1):29–74. doi: 10.1016/0165-1110(76)90012-9. [DOI] [PubMed] [Google Scholar]
- Sinden R. R., Cole R. S. Repair of cross-linked DNA and survival of Escherichia coli treated with psoralen and light: effects of mutations influencing genetic recombination and DNA metabolism. J Bacteriol. 1978 Nov;136(2):538–547. doi: 10.1128/jb.136.2.538-547.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden R. R., Cole R. S. Topography and kinetics of genetic recombination in Escherichia coli treated with psoralen and light. Proc Natl Acad Sci U S A. 1978 May;75(5):2373–2377. doi: 10.1073/pnas.75.5.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P. S., Tapley K. J., Jr Photochemistry and photobiology of psoralens. Photochem Photobiol. 1979 Jun;29(6):1177–1197. doi: 10.1111/j.1751-1097.1979.tb07838.x. [DOI] [PubMed] [Google Scholar]
- Swanbeck G., Thyresson M. Induction of respiration-deficient mutants in yeast by psoralen and light. J Invest Dermatol. 1974 Aug;63(2):242–244. doi: 10.1111/1523-1747.ep12680024. [DOI] [PubMed] [Google Scholar]
- Waters R., Moustacchi E. The fate of ultraviolet-induced pyrimidine dimers in the mitochondrial DNA of Saccharomyces cerevisiae following various post-irradiation cell treatments. Biochim Biophys Acta. 1974 Oct 28;366(3):241–250. doi: 10.1016/0005-2787(74)90282-2. [DOI] [PubMed] [Google Scholar]
- Yoakum G. H., Cole R. S. Role of ATP in removal of psoralen cross-links from DNA of Escherichia coli permeabilized by treatment with toluene. J Biol Chem. 1977 Oct 25;252(20):7023–7030. [PubMed] [Google Scholar]


