Abstract
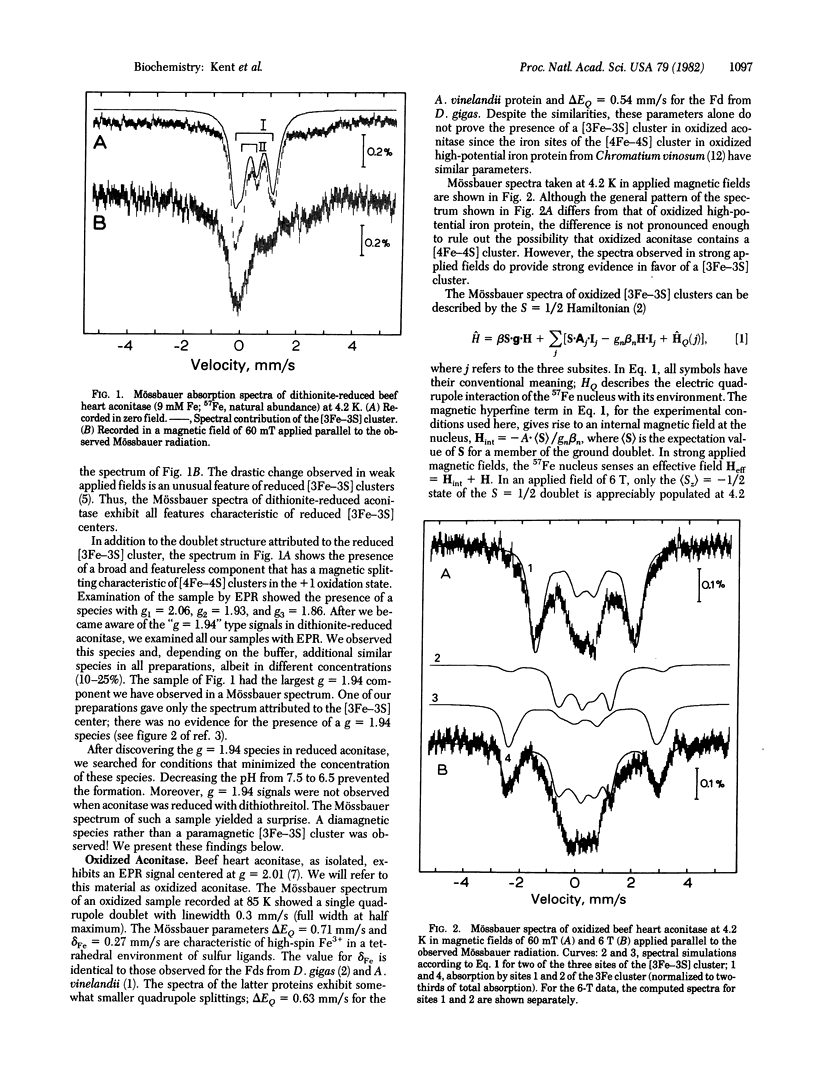
Beef heart aconitase, isolated under aerobic conditions, has been studied with Mössbauer and EPR spectroscopy. In the oxidized state, the enzyme exhibits an EPR signal at g = 2.01. The Mössbauer data show that this signal is associated with a 3Fe cluster. In dithionite-reduced aconitase, the 3Fe cluster, probably of the [3Fe-3S] type, is in a paramagnetic state of interger electronic spin (S = 2); the Mössbauer spectra exhibit al the unique features reported for proteins with 3Fe clusters. On activation of aconitase with ferrous ion, the paramagnetic 3Fe cluster of dithionite-reduced enzyme is converted into a diamagnetic (S = 0) form. Activation studies with iron enriched in either 27 Fe or 56 Fe suggest that activation transforms the 3Fe cluster into a center that has a [4Fe-4S] core. This conclusion is supported by the observation that EPR signals characteristic of reduced [4Fe-4S] clusters can be elicited under appropriate conditions. It has frequently been assumed that the activation of aconitase with Fe2+ produces an active site containing a single ferrous ion. The data reported here suggest that a ferrous ion is used to rebuild a [4Fe-4S] cluster.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Emptage M. H., Kent T. A., Huynh B. H., Rawlings J., Orme-Johnson W. H., Münck E. On the nature of the iron-sulfur centers in a ferredoxin from Azotobacter vinelandii. Mössbauer studies and cluster displacement experiments. J Biol Chem. 1980 Mar 10;255(5):1793–1796. [PubMed] [Google Scholar]
- Ghosh D., Furey W., Jr, O'Donnell S., Stout C. D. Structure of a 7Fe ferredoxin from Azotobacter vinelandii. J Biol Chem. 1981 May 10;256(9):4185–4192. [PubMed] [Google Scholar]
- Huynh B. H., Moura J. J., Moura I., Kent T. A., LeGall J., Xavier A. V., Münck E. Evidence for a three-iron center in a ferredoxin from Desulfovibrio gigas. Mössbauer and EPR studies. J Biol Chem. 1980 Apr 25;255(8):3242–3244. [PubMed] [Google Scholar]
- Kent T. A., Huynh B. H., Münck E. Iron-sulfur proteins: spin-coupling model for three-iron clusters. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6574–6576. doi: 10.1073/pnas.77.11.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton P., Dickson D. P., Johnson C. E., Rush J. D. Interpretation of the Mössbauer spectra of the high-potential iron protein from Chromatium. Eur J Biochem. 1980 Feb;104(1):289–296. doi: 10.1111/j.1432-1033.1980.tb04427.x. [DOI] [PubMed] [Google Scholar]
- Rose I. A., O'Connell E. L. Mechanism of aconitase action. I. The hydrogen transfer reaction. J Biol Chem. 1967 Apr 25;242(8):1870–1879. [PubMed] [Google Scholar]
- Ruzicka F. J., Beinert H. A mitochondrial iron protein with properties of a high-potential iron-sulfur protein. Biochem Biophys Res Commun. 1974 Jun 4;58(3):556–563. doi: 10.1016/s0006-291x(74)80456-0. [DOI] [PubMed] [Google Scholar]
- Ruzicka F. J., Beinert H. A new iron-sulfur flavoprotein of the respiratory chain. A component of the fatty acid beta oxidation pathway. J Biol Chem. 1977 Dec 10;252(23):8440–8445. [PubMed] [Google Scholar]
- Ruzicka F. J., Beinert H. The soluble "high potential" type iron-sulfur protein from mitochondria is aconitase. J Biol Chem. 1978 Apr 25;253(8):2514–2517. [PubMed] [Google Scholar]


