Abstract
Mixed-sequence DNA molecules undergo mechanical overstretching by approximately 70% at 60–70 pN. Since its initial discovery 15 y ago, a debate has arisen as to whether the molecule adopts a new form [Cluzel P, et al. (1996) Science 271:792–794; Smith SB, Cui Y, Bustamante C (1996) Science 271:795–799], or simply denatures under tension [van Mameren J, et al. (2009) Proc Natl Acad Sci USA 106:18231–18236]. Here, we resolve this controversy by using optical tweezers to extend small 60–64 bp single DNA duplex molecules whose base content can be designed at will. We show that when AT content is high (70%), a force-induced denaturation of the DNA helix ensues at 62 pN that is accompanied by an extension of the molecule of approximately 70%. By contrast, GC-rich sequences (60% GC) are found to undergo a reversible overstretching transition into a distinct form that is characterized by a 51% extension and that remains base-paired. For the first time, results proving the existence of a stretched basepaired form of DNA can be presented. The extension observed in the reversible transition coincides with that produced on DNA by binding of bacterial RecA and human Rad51, pointing to its possible relevance in homologous recombination.
Keywords: elongated DNA, mechanical properties of DNA, mechanism of recombination, single molecule, DNA stretching
When a single B-form DNA molecule is pulled mechanically by applying a force to its ends, it undergoes an abrupt elongation by about 70% at 60–70 pN (1, 2). This overstretched form of DNA has been a topic of debate ever since the discovery 15 y ago. Does it represent a structurally defined new form of DNA, or is it just an effect of melting and strands peeling off? An important early observation was that the overstretched form typically survives without the two DNA strands dissociating even if the applied force exceeds 100 pN, suggesting that the overstretched form remains base-paired at least in some parts (1–5).
In the original interpretation (1, 2, 5, 6) the overstretched DNA is a base-paired structure, denoted S-form DNA (1), with a higher rise per base-pair than the B-form. Molecular modeling studies suggest that DNA can indeed form an overstretched base-paired duplex if it unwinds into a ladder-like structure (1, 7–10) and combined measurements of tension and torque have shown that overstretched DNA is indeed underwound relative to the B-form (11, 12). While the putative S-form is still awaiting conclusive experimental evidence, Fu et al. (13, 14) recently noted that overstretching of dsDNA could involve both a slow transition, associated with hysteresis, as one strand peels off from the other strand, as well as a fast, reversible transition to what may be the S-form DNA. Their studies show that the transition pathway is sensitive to the same factors that affect melting: if melting is inhibited then the S-form is favored (13, 14). Also entropy and enthalpy changes suggest that the strands are separated during the hysteretic transition, while they remain in close proximity to each other during the nonhysteretic transition (15).
In a proposed alternative scenario of force-induced melting (3, 4, 16–21) the double stranded DNA gradually denatures during elongation but retains some base-paired regions, explaining why the strands do not separate completely. In yet another melting scenario, transient base-pairing contacts might keep the strands in register. Strand separation is likely to start in AT-rich regions (3, 17, 21) as normally observed during thermal melting (22), either from nicks or from the ends with one strand peeling off from the other (4, 23). Overstretching does, however, also occur in torsionally unconstrained DNA without nicks or free ends (24). The melting interpretation is supported by the fact that the same conditions that lower the melting temperature of DNA also reduce the transition force. Conditions investigated include variation of ionic strength (17), pH (19), temperature and presence of DNA-binding ligands (4, 21, 25, 26).
Here we approach the problem by studying short double-stranded DNA whose base content can be designed at will. The potential occurrence of strand separation is investigated by using inter-strand linkers and by exposing the oligonucleotides to glyoxal during stretching. Glyoxal (21, 26) forms a covalent adduct with guanine bases if they are unpaired and exposed to the solvent. The glyoxal adduct obstructs hydrogen bonding to the complementary cytosine bases and thus inhibits reannealing of the strands (27, 28). The inter-strand linkers are formed using click-chemistry to covalently seal one or both ends of the DNA duplex, a technique previously used to lock DNA constructs (29–32). For the first time, results proving the existence of a stretched base-paired form of DNA can be presented.
Results
Fig. 1A (a) describes the DNA constructs, each consisting of a 60–64 base-pairs double-stranded “kernel” and single-stranded “handles,” the latter formed by terminal transferase extension of the 3′-ends prior to hybridization of the duplex. During the extension either digoxigenin- or biotin-modified nucleotides were incorporated for attachment to polystyrene beads coated with digoxigenin antibodies or streptavidin, respectively. Three kernel sequences were used in this study: OligoGC1 and OligoAT, differing in GC-content (60% and 30%, respectively), and OligoGC2 with the same GC content (60%) as OligoGC1 but differing in nearest-neighbor base composition (Fig. S1). OligoGC1 and OligoAT were designed so that the strands of the kernel could be covalently linked at either or both ends using click-chemistry [Fig. 1A (b–d)]. Here the 5′-clicked OligoAT (5′OligoAT) was studied along with three clicked versions of OligoGC1: 5′-clicked (5′OligoGC1), 3′-clicked (3′OligoGC1), and double-clicked (3′5′OligoGC1). Each inter-strand link connects two bases on opposite strands diagonally from one base to the 5′-neighboring base of its complementary strand. The linker connects the two modified thymine bases at their C5 positions, most likely with the linker residing in the major groove of the helix (Fig. S2). In the experimental setup one bead was held stationary at a pipette tip by suction, while the other was manipulated by an adjustable optical trap (for details on measurement of position and force, see SI Text). Force versus distance curves were recorded by stretching a tethered construct from a lower force limit, Fmin, to an upper force limit, Fmax, thereafter relaxing it to reduce the force from Fmax back to Fmin at the same rate. The force limits were set to ensure that the kernel attained B-form at Fmin and was in the overstretched state at Fmax (for details, see SI Text).
Fig. 1.
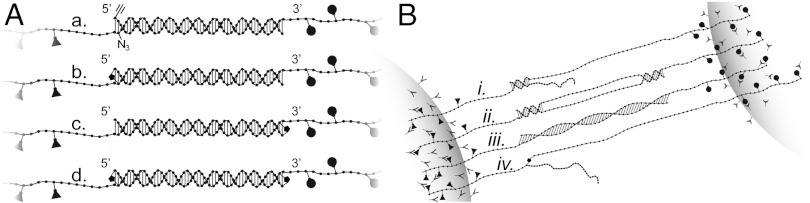
Schemes of DNA constructs, covalent linkage and DNA-bead assembly. (A) DNA constructs consisting of a central double helical part (kernel) and two single-stranded extensions (handles). (a) Kernel strands are complementary 60 nucleotides long (for sequences, see Fig. S1), forming the GC-rich OligoGC1 and OligoGC2 and the AT-rich OligoAT. Each strand is extended at the 3′ end by terminal transferase into a single-strand handle containing biotinylated uracil bases (triangles) or digoxigenin (circles). Clickable (64 bp) kernels contain at the end of one strand an alkyne group (≡) and, on the complementary strand, a matching azide group (N3) prepared for click linking (see Materials and Methods). (b) Kernel covalently sealed at 5′ end of biotinylated strand (e.g., in 5′OligoGC1, click-link marked by small black dot). (c) Kernel sealed at 3′ end (e.g., in 3′OligoGC1). (d) Kernel sealed at both ends (e.g., 3′5′OligoGC1). For synthesis, see SI Text. (B) Bead attachment of DNA constructs and hypothetical overstretched states of kernel. (i) Partially molten kernel with base pairs remaining at one end as one strand peels off at opposite end (peeling model), (ii) Partially molten kernel interior, with base pairs remaining at both ends (internal melting model), (iii) Overstretched base paired duplex. (iv) Fully denatured kernel, with remaining covalent linker.
The thermal stability of the sealed oligonucleotides at zero force was assessed from denaturing polyacrylamide gel-electrophoresis varying the temperature between 22 °C and 74 °C (Fig. S3 and Table S1). As expected, by avoiding complete strand separation the clicked DNA exhibits enhanced thermal stability compared to unmodified duplex (Table 1). Thermal melting was studied in 7 M urea to shift melting to lower temperatures as cross-linking significantly increases melting temperature (29). Sealing either end stabilizes OligoGC1 by at least 20 degrees and sealing both ends by another 10 degrees. Sealing the 5′-end of OligoAT also raises the melting temperature by at least 8 degrees.
Table 1.
Overstretching and thermal melting data
| Species | 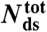 |
n |
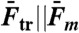 (pN) (pN) |
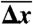 (nm) (nm) |
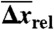 (%) (%) |
Tm(°C) | 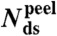 |
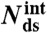 |
| OligoGC1 | 60 | 49 | 63.3 ± 1.11 | 10.4 ± 0.46 | 49.3 ± 2.2 | < 22 | 14 ± 2 | 2 ± 3 |
| OligoGC2 | 60 | 33 | 62.0 ± 1.78 | 11.2 ± 0.57 | 53.1 ± 2.7 | < 22 | 10 ± 2 | −2 ± 3 |
| 3′OligoGC1 | 64 | 12 | 63.4 ± 1.36 | 11.1 ± 0.71 | 49.5 ± 3.2 | 44–48 | 15 ± 3 | 2 ± 4 |
| 5′OligoGC1 | 64 | 34 | 62.1 ± 1.18 | 11.5 ± 0.87 | 51.1 ± 3.2 | 44–48 | 13 ± 4 | 0 ± 5 |
| 3′5′OligoGC1 | 64 | 10 | 62.5 ± 1.21 | 11.3 ± 0.74 | 50.6 ± 3.3 | 54–57 | 14 ± 3 | 1 ± 4 |
| 5′OligoAT | 64 | 17 | 61.5 ± 2.63 | 14.9 ± 1.24 | 66.7 ± 5.5 | ∼30 | −2 ± 5 | −19 ± 6 |
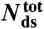 : number of base-pairs in kernel; n: number of studied molecules;
: number of base-pairs in kernel; n: number of studied molecules; 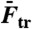 : population average transition force, GC-rich oligonucleotides;
: population average transition force, GC-rich oligonucleotides; 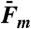 : population average melting force, 5′OligoAT;
: population average melting force, 5′OligoAT; 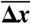 : average extension during transition;
: average extension during transition; 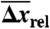 : percentage average extension relative to B-form DNA at Ftr or Fm (see SI Text); Tm: melting temperature (in absence of force) from 7 M urea PAGE;
: percentage average extension relative to B-form DNA at Ftr or Fm (see SI Text); Tm: melting temperature (in absence of force) from 7 M urea PAGE; 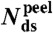 : number of remaining base-pairs in peeling melting model of Fig. 1B (i), with tension in melted region carried by one strand;
: number of remaining base-pairs in peeling melting model of Fig. 1B (i), with tension in melted region carried by one strand; 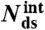 : number of remaining base-pairs for internal melting model of Fig. 1B (ii), with tension in melted region carried by both strands. Statistics reported as mean ± 1 s.d. All force-distance data refer to 1 M NaCl.
: number of remaining base-pairs for internal melting model of Fig. 1B (ii), with tension in melted region carried by both strands. Statistics reported as mean ± 1 s.d. All force-distance data refer to 1 M NaCl.
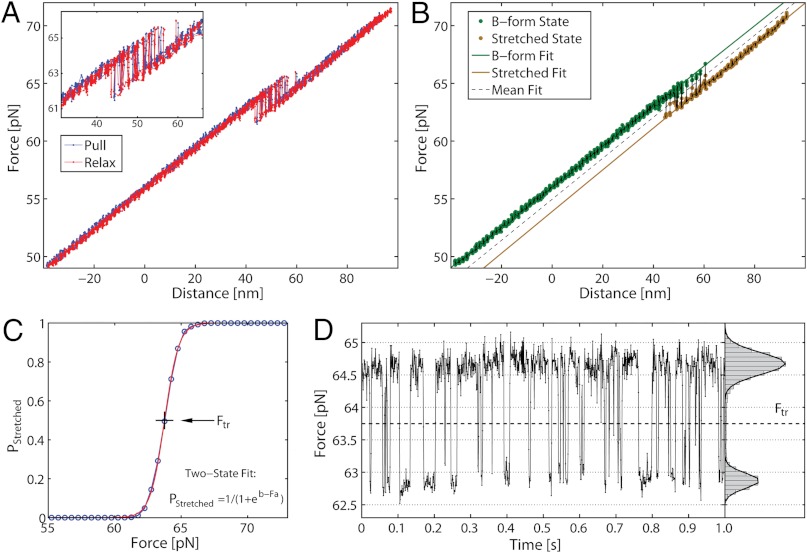
Fig. 2A shows a force-distance curve during a pull-relax cycle for (nonclicked) OligoGC1, illustrating the typical behavior when force is increased up to 70 pN and thereafter reduced. For both processes the optical trap is moved at a constant rate (50 nm/s). OligoGC1 exhibits a force-dependent bistability showing oscillations between the B-form and an overstretched state. Notably, in the trap-position control mode used here, a transition to the extended state is detected as an abrupt decrease in the force as the bead relaxes towards the center of the trap. The reverse transition back to the B-form increases the force as the contracting molecule pulls the bead away from the trap center. As seen from the inset of Fig. 2A, the molecule switches back and forth between the two states during both pulling and relaxation of the construct. The transition appears completely reversible with no detectable hysteresis. Fig. 2D shows a time trace of force when the optical trap is stationary. The OligoGC1-molecule is seen to oscillate between B-form (high force) and overstretched state (lower force) on a millisecond time scale. The histogram of the force data is well described by a double Gaussian fit, justifying the analysis of the mechanical overstretching as a two-state process. The data points are assigned to either of the two states depending on their proximity to fitted lines at low and high force, respectively (Fig. 2B). Fig. 2C shows the probability Pstretched of the overstretched state as a function of force obtained from 16 pull-relax cycles on the same molecule. The transition force Ftr is the force at which Pstretched equals 0.5, and the extension during the transition, Δx, is calculated by dividing the force difference between the two fitted lines at Ftr by the slope of the B-form fitted line (33, 34). Based on data from 49 OligoGC1 molecules, a mean transition force of 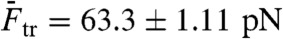 (± 1 s.d.) was obtained, and for OligoGC2 (n = 33)
(± 1 s.d.) was obtained, and for OligoGC2 (n = 33) 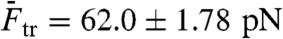 . Corresponding extensions were 10.4 ± 0.46 nm and 11.2 ± 0.58 nm, respectively. Data are summarized in Table 1.
. Corresponding extensions were 10.4 ± 0.46 nm and 11.2 ± 0.58 nm, respectively. Data are summarized in Table 1.
Fig. 2.
Mechanical stretching of single nonclicked 60 base-pair OligoGC1 molecule (sequence in Fig. S1) in 1 M NaCl. (A) Force-distance plot during one cycle of pulling (blue) and relaxation (red). The DNA duplex exhibits a bistability between 61 and 65 pN, with no detectable hysteresis between the pulling and relaxation parts of the cycle. Inset: bistability region in detail. The duplex switches between the overstretched form (low force) and the shorter native B-form (high force) during both pulling and relaxation. (B) Force-distance plot during a single pulling, illustrating method of data analysis. Each data point is assigned to the B-form or the overstretched state depending on its proximity to the lines fitted to the force-distance data at low (green) and high force (brown), respectively. (C) Probability P of finding the molecule in the overstretched state as a function of force. Experimental data (circles) pooled from 16 pull-and-relax cycles from the same OligoGC1 molecule. The curve is a fit to a two-state model P = 1/(1 + eb-Fa) (42) and the transition force Ftr corresponds to P = 0.5 (giving Ftr = 63.75 pN for this particular OligoGC1 molecule). (D) Conversion dynamics between the overstretched and native forms. Time trace of force in the overstretching region for OligoGC1 molecule measured at 1 kHz with trap held stationary. Low force corresponds to overstretched form and high force to shorter B-form. The histogram (to the right) gives the distribution of data points during studied time interval (1 s). The bimodal Gaussian distribution supports the interpretation that the transition is two-state in nature.
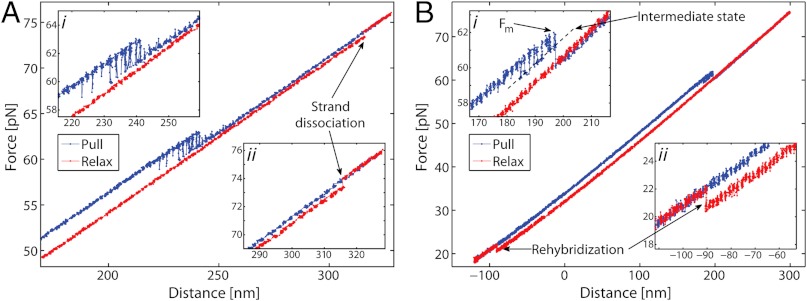
Fig. 3A shows typical force-distance data for 5′-clicked OligoGC1 (Scheme b, Fig. 1A) during one pull-relax cycle. During pulling (blue) the 5′-sealed duplex is seen to undergo the same force-induced oscillation between two states as the unmodified OligoGC1 (Fig. 2A). The 3′- and double-clicked OligoGC1 (c and d in Fig. 1A) exhibit virtually identical behaviors (see Fig. S4). Importantly, compared to nonclicked OligoGC1, our results (Table 1) show that within experimental uncertainty the overstretching transition occurs at the same force whether the ends are sealed or not. The transition lengths are also similar for clicked and nonclicked GC-rich DNA with an extension per base-pair of 0.17–0.19 nm.
Fig. 3.
Force-distance data for GC-rich and AT-rich duplexes covalently sealed at one end (1 M NaCl). Main panel shows one cycle of pulling (blue) and relaxation (red) of single DNA molecule. Inset shows the details of the force-induced transitions for the two different sequences. (A) 5′-clicked OligoGC1. During pulling reversible switching occurs between two states at about 60 pN. At 74 pN an abrupt decrease in force (denoted strand dissociation) occurs during relaxation. Inset i shows switching region in detail, and inset ii that the transition at 74 pN occurs in a single step. (B) 5′-clicked OligoAT. During pulling there is an abrupt transition to an extended state at 62 pN, and hysteresis during relaxation consistent with melting. Rehybridization to B-form is observed as a return to the pulling curve at about 20 pN. Inset i shows the 62 pN transition in detail. Before the abrupt extension, at force Fm, reversible switching to a longer intermediate state is observed. Inset ii shows that the rehybridization at 20 pN occurs in a single step.
In addition to the reversible transition behavior, the 5′-clicked OligoGC1 is also found to undergo a secondary, irreversible extension step at higher force at which the duplex melts. Fig. 3A (insets) shows a force-extension curve for a case when a 5′OligoGC1 molecule is first reversibly overstretched (inset i, blue curve) and then further extended by an additional 3.6 nm at about 74 pN (inset ii, red curve) when it melts during relaxation. The transition is irreversible in the sense that the reverse process, molten strands rehybridizing to form an overstretched state, does not occur. In order to regain the duplex, on the time scale of our experiment, the force had to be lowered to about 20–25 pN before the two strands could reanneal into B-form. Experiments with double-clicked 3′5′OligoGC1 (Fig. S4D) showed no melting behavior even at higher forces (120 pN), supporting the idea that cross-linking of both ends inhibits melting. Control experiments with 3′OligoGC1 revealed no melting (Fig. S4B), and the contrasting behavior compared to 5′OligoGC1 (Fig. 3A) indicates an asymmetry in the OligoGC1 sequence regarding force-induced melting. The nonclicked constructs were frequently observed to dissociate at forces close to or above the overstretching region, but it is hard to discern whether they are falling apart due to strand separation in the kernel or due to loss of a bead anchor, since in both cases the tether between the two beads is lost. These observations illustrate the value of single-clicked constructs in studies of forced-induced melting, since the covalent linker prevents even a fully molten kernel [Fig. 1B (iv)] from undergoing complete disassembly.
Fig. 3B shows AT-rich 5′-clicked OligoAT during a cycle of pulling and relaxation. At 62 pN, the molecule undergoes an irreversible melting transition. The melting process gives rise to a distinct hysteresis during relaxation (red curve) and the construct does not reanneal until force is lowered to about 23 pN (inset ii) when full B-form duplex recovers. Thus, the behavior of AT-rich 5′OligoAT is completely different from that of the GC-rich DNA. Table 1 shows ensemble-averaged forces Fm, at which melting occurs for 5′OligoAT, and increase in length during transition. The average degree of extension is about 67% for the AT-rich duplex; i.e., significantly higher than the 51% for GC-rich duplexes (Table 1), and closer to the 70% reported for long mixed-sequence DNA. Prior to the abrupt transition at Fm, the 5′OligoAT in Fig. 3B exhibits a brief bistability involving an intermediate state (inset i) seen in most, although not all, molecules studied. The intermediate state is on average 7.5 ± 0.7 nm (n = 12) longer than the B-form state, which corresponds to a relative extension of 34 ± 3%. This observation suggests that constructs less stable towards melting also may undergo a more transient reversible conversion to an overstretched state before melting.
Fig. S5 A–C show that when the salt is lowered from 1 M NaCl to 5 mM NaCl, the reversible transition is still observed for 3′OligoGC1 and 3′5′OligoGC1, whereas 5′OligoGC1 melts in a way similar to what was observed for the AT-rich 5′OligoAT in 1 M NaCl. At 5 mM, all measured 3′OligoGC1 melted from the stretched state of the reversible transition, whereas none did at 1 M NaCl. No double-clicked 3′5′OligoGC1 were observed to melt at 5 mM NaCl, confirming that cross-linking inhibits melting. The results indicate that lowering the salt concentration decreases the melting force, but also that high salt concentration is not a requirement for the reversible overstretching transition.
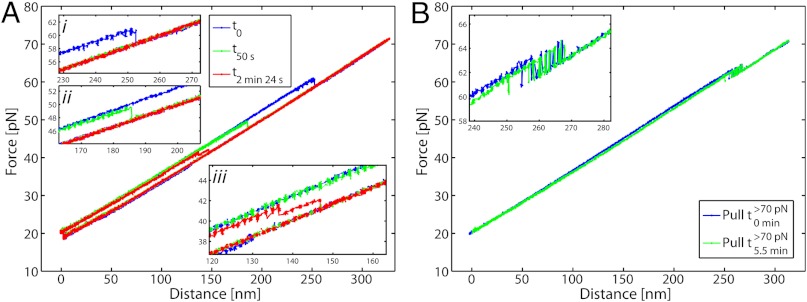
To examine the degree of base-pairing in the two different overstretching transitions, we exposed the 5′-clicked AT-rich oligonucleotide (5′OligoAT, n = 6) as well as double-clicked GC-rich construct (3′5′OligoGC1, n = 7) to 0.5 M glyoxal. Fig. 4A shows a 5′OligoAT molecule before and after the bases are exposed to react with glyoxal present in the medium. The blue curve shows the first pull cycle before glyoxal modification (t0), where the unmodified construct melts at about 60 pN (inset
i) just as it would in the absence of glyoxal. After the molecule melts, the force is reduced to about 40 pN and held at this tension to allow any exposed guanine bases of the molten strands to react with glyoxal. The force is then reduced to 20 pN to allow rehybridization. Within a minute of glyoxal exposure, the melting force is markedly reduced (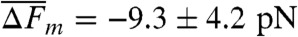 ) due to base modifications in the rehybridized duplex. Additional exposure lowers the melting force even further. The double-clicked GC-rich 3′5′OligoGC1 was exposed to glyoxal in its reversible overstretched state by holding it at 70 pN in presence of the chemical agent. Fig. 4B shows the DNA before and after a long exposure time. The inset indicates that the reversible transition remains unaffected after exposure, indicating that the bases in the reversible overstretched state are indeed not accessible to reaction with glyoxal. Control experiments in which the single-clicked GC-rich 5′OligoGC1 is exposed to glyoxal while the DNA is in its molten state (Fig. S5D) confirm that the same sequence reacts with glyoxal if the strands are separated.
) due to base modifications in the rehybridized duplex. Additional exposure lowers the melting force even further. The double-clicked GC-rich 3′5′OligoGC1 was exposed to glyoxal in its reversible overstretched state by holding it at 70 pN in presence of the chemical agent. Fig. 4B shows the DNA before and after a long exposure time. The inset indicates that the reversible transition remains unaffected after exposure, indicating that the bases in the reversible overstretched state are indeed not accessible to reaction with glyoxal. Control experiments in which the single-clicked GC-rich 5′OligoGC1 is exposed to glyoxal while the DNA is in its molten state (Fig. S5D) confirm that the same sequence reacts with glyoxal if the strands are separated.
Fig. 4.
Probing base-pairing characteristics of overstretched state with glyoxal-reactivity. (A) 5′-clicked AT-rich oligonucleotide, 5′OligoAT. Pull-relax cycles in presence of 0.5 M glyoxal before and after reaction. Blue curve is the first pull cycle before DNA reacts with glyoxal (t0): the unmodified molecule melts at about 60 pN (inset i). After the DNA molecule is melted the force is lowered to approximately 40 pN where it does not reanneal and is held there for some amount of time to allow exposure of the single strands to glyoxal. After this exposure time, the force is lowered to 20 pN, where the molecule rehybridizes within a few seconds. Green curve (t50 s) shows the same molecule after it has been exposed in the melted state to glyoxal a total of 50 s. The molecule is seen to melt at about 49 pN (inset ii), showing that the glyoxal-induced base reaction lowers the melting force of the molecule. The red curve shows the molecule after it is exposed in its melted state to glyoxal for 2.5 min. During pull DNA first melts partially (note absence of bistability) before it melts completely at 41 pN (inset iii). (B) Double-clicked GC-rich oligonucleotide, 3′5′OligoGC1. Blue curve shows first pull in presence of glyoxal; green curve shows pull of the same molecule after 5.5 min of exposure to glyoxal while in the overstretched state (> 70 pN). Curves have been horizontally compensated for instrument drift (< 4 nm/ min) to simplify comparison between different pull cycles.
Discussion
The reversible transition observed for OligoGC1 at 63 pN (Fig. 2, Table 1) proves that a native DNA duplex as short as 60 base-pairs can be mechanically overstretched by 51% compared to its B conformation without strand separation. Four observations in particular prove that the stretched state of the reversible transition is not a molten helix but a structurally and thermodynamically well-defined form of DNA.
(i) While the melting temperature (Tm) at zero force increases when the ends of the duplex are prevented from fraying by click-seals, the four OligoGC1 variants (nonclicked, 5′-clicked, 3′-clicked, and double-clicked) all exhibit the same bistable behavior and approximately the same transition force and extension. The difference in behavior observed here between melting and stretching suggests that the reversible overstretching in the GC-rich duplex is not related to thermal stability.
(ii) A valid melting model must also be able to explain why nonsealed OligoGC1 survives forces above the overstretching region without falling apart. The possibility that the duplex only partially melts during the transition, DNA held together by regions that remain base-paired, can be dismissed based on the following analysis of overstretching length. The number of base-pairs (Nds) that would be left paired upon melting can be estimated as:
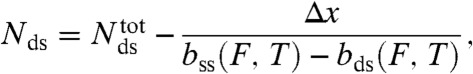 |
[1] |
where 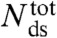 is the total number of base-pairs in the kernel, bss(F,T) the rise per base in the resulting single strand at the transition force Ftr or Fm, respectively, and temperature T; and bds(F,T) is the rise per base-pair of the remaining double-stranded regions at the same force and temperature (see SI Text for details). Table 1 presents the calculated number of remaining base-pairs for the melting models i and ii in Fig. 1B. The main difference between the two models is whether the tension in the molten region is supported by one (i) or both (ii) strands. Assuming that the putative base-paired fraction remains in a B-type structure, numerical estimates in Table 1 indicate that there would be no base-pairs left to hold the strands in register in the internal melting scenario of Fig. 1B (ii). Even if the duplex melts from the ends [peeling scenario Fig. 1B (i)], only 10–15 base-pairs in total would remain, a duplex too short to be stable when exposed to 63 pN at the temperature and ionic conditions of our experiments (35, 36). The same analysis for 5′OligoAT yields -19 remaining base-pairs for an internal melting scenario and -2 remaining base-pairs for a peeling scenario. These small (even negative) numbers contradict a partial melting model, and further support our conclusion that the irreversible transition leading to hysteresis is indeed a consequence of full melting of the double stranded region, as no base-pairs would remain to hold the duplex together. The result for the peeling scenario agrees with the expected response of a fully melted single-clicked structure, Fig. 1B (iv), where only the linker holds the construct together.
is the total number of base-pairs in the kernel, bss(F,T) the rise per base in the resulting single strand at the transition force Ftr or Fm, respectively, and temperature T; and bds(F,T) is the rise per base-pair of the remaining double-stranded regions at the same force and temperature (see SI Text for details). Table 1 presents the calculated number of remaining base-pairs for the melting models i and ii in Fig. 1B. The main difference between the two models is whether the tension in the molten region is supported by one (i) or both (ii) strands. Assuming that the putative base-paired fraction remains in a B-type structure, numerical estimates in Table 1 indicate that there would be no base-pairs left to hold the strands in register in the internal melting scenario of Fig. 1B (ii). Even if the duplex melts from the ends [peeling scenario Fig. 1B (i)], only 10–15 base-pairs in total would remain, a duplex too short to be stable when exposed to 63 pN at the temperature and ionic conditions of our experiments (35, 36). The same analysis for 5′OligoAT yields -19 remaining base-pairs for an internal melting scenario and -2 remaining base-pairs for a peeling scenario. These small (even negative) numbers contradict a partial melting model, and further support our conclusion that the irreversible transition leading to hysteresis is indeed a consequence of full melting of the double stranded region, as no base-pairs would remain to hold the duplex together. The result for the peeling scenario agrees with the expected response of a fully melted single-clicked structure, Fig. 1B (iv), where only the linker holds the construct together.
(iii) The behavior of 5′OligoAT (30% GC) is quite different from that of the GC-rich OligoGC1 and OligoGC2. The hysteresis for 5′OligoAT (Fig. 3B) pulled at the same loading rate as the GC-rich oligonucleotides strongly indicates that the duplex melts under force. This difference in behavior between the GC-rich and AT-rich oligonucleotides at 1 M NaCl demonstrates that sequence is an important parameter controlling the probability of transition from B-form to stretched and/or melted forms. Equally important, this different behavior also implies that the reversible transition observed for GC-rich oligonucleotides is not due to melting. The 5′-clicked OligoGC1 occasionally exhibits hysteresis (Fig. 3A), but then only after a secondary, irreversible transition. This observation provides independent evidence that the reversible overstretching seen in GC-rich duplexes (Fig. 2) and the force-induced melting seen in the AT-rich 5′OligoAT (Fig. 3B) are two completely different processes. They may occur in the same DNA molecule, but are different in origin. Measurements in 5 mM NaCl (Fig. S5 A–C) show that lowering salt concentration lowers stability towards melting, but our results show that the reversible overstretching also occurs at low salt. Note, furthermore, that 5′OligoGC1 in 5 mM NaCl melts without displaying reversible overstretching (Fig. S5C), similar to what was observed for the AT-rich 5′OligoAT in 1 M NaCl. These results imply that it is not the sequence per se that determines whether or not reversible overstretching occurs, but rather the stability of the sequence towards melting. If the stability is low enough, by high AT-content or low salt, for example, the overstretched state will be short-lived and melt.
(iv) Glyoxal probing of nonbase-paired guanines shows that the AT-rich duplex melts but that GC-rich duplexes remain base-paired when overstretched. Refolding of overstretched 5′OligoAT exposed to glyoxal for less than a minute is partially inhibited, and progressively more so as the exposure time is increased to near 3 min (Fig. 4A), showing that a substantial number of guanines have unpaired and reacted. At the same time, the force needed to melt the construct is gradually lowered as more and more glyoxal-modified guanine bases are introduced. By contrast, exposure of overstretched double-clicked 3′5′OligoGC1 to glyoxal for similar or even longer times does not affect the reversible overstretching transition (Fig. 4B). Taken together, these observations strongly support our conclusion that 5′OligoAT melts under force, leaving the guanine bases exposed to glyoxal, whereas the overstretched GC-rich oligonucleotides keep the majority of bases in an overstretched base-paired state, as originally suggested for the S-form DNA (1, 2).
The transition forces that we measure for reversible overstretching of GC-rich duplexes and for the irreversible melting of the AT-rich duplex are similar to forces observed with long natural DNA, indicating that the stretching transitions observed here are also present in those seen with longer molecules. Thus, it is justified to compare the processes despite the several orders of magnitude difference in molecular length. Fu et al. (13, 14) noted that overstretching could involve both a slow transition—associated with hysteresis as one strand peels off from the other strand—and a fast, reversible transition to what may be S-form DNA. The two types of mechanical processes observed in our synthetic oligonucleotides are thus also likely to be represented in the overstretching behavior of naturally occurring, longer DNA molecules. An important distinction, however, is that the two processes are accompanied by a very distinct extension change. The reversible overstretching seen in the GC-rich duplexes extends the molecule by only 50–53% (Table 1), whereas the melting AT-rich duplex at about the same force and for the GC-rich duplex at higher force, extends it by about 67%, much closer to the value typically reported for long natural DNA (70%). In the GC-rich duplexes, the reversible overstretching and force-induced melting are easily distinguishable as separate processes in the same DNA molecule (Fig. 3A): the reversible overstretching transition manifests itself as a simple two-state process (Fig. 2), whereas the force-induced melting is seen as an irreversible process that extends the DNA molecule further.
In the present study, it is shown that the mechanical behavior of short DNA (at 1 M NaCl) is mainly controlled by the overall percentage of GC base-pairs in the sequence. Interestingly, an approximately 50% extension of DNA is also observed upon interaction of recombinases RecA and (human) Rad 51 (37, 38). Based on flow linear dichroism and small-angle neutron scattering data in solution (39), showing that the DNA bases are oriented with their planes perpendicular to the fiber axis of RecA-DNA complexes, some kind of heterogeneous stacking was early proposed and later supported by modeling for these complexes (1, 10). Recently, in crystal it was found that the extension of DNA in the RecA complex is inhomogeneous, three base-pairs stacked together followed by a gap into which an intercalated residue from the protein is inserted (37). Hence, an interesting question is whether the value of 50% extension is a mere coincidence or if the conditions inside the filamentous recombinase complexes may stabilize the same kind of extended base-paired DNA structure that the molecule adopts when subjected to external force applied to its ends. Another question is whether the reversible extension of free double-stranded DNA is homogeneous or could undergo a “disproportionation” (analogous to Peierls instability), yielding a structure in which clusters of stacked nucleobases are surrounded by gaps. The hydrophobic environment of stacked bases is a prerequisite for the hydrogen bonds to exert their full recognition power. One may envisage two ways by which bases can remain stacked in a more extended structure: the bases may either remain orthogonal or tilt. In both cases, the stacking energy (proportional to the contact surface area between the bases) must decrease, thus making the extended DNA less stable, in the orthogonal case due to the occurrence of gaps and in the tilted base arrangement due to a smaller overlap area between adjacent bases (SI Text, Eqs. S5–S8). We note that structures in which the nucleobases are perpendicular to the DNA helix axis, with stacking heterogeneity as found in RecA-DNA with stacked base triplets, are logical for the function involving base recognition as in recombination.
Materials and Methods
Unmodified oligonucleotides were obtained from IDT; US and clickable oligonucleotides from ATDBio, UK (see SI Text, Fig. S6 and Table S2). The terminal transferase (New England Biolabs) reactions were performed using a dUTP-biotin/digoxigenin (Digoxigenin-11-dUTP, Biotin-16-dUTP, Roche) to dATP ratio of 1∶10. The covalent link was formed by the Cu+ catalyzed click-reaction (0.01eq oligonucleotide, 1eq CuSO4·5H2O, 7eq tris-hydroxypropyltriazole, 10eq Na ascorbate, overnight at 22 °C) (29, 40, 41). Streptavidin beads with a nominal diameter of 2.1 μm were used as obtained from Spherotech. AntiDIG-beads of the same size were prepared in-house by cross-linking antidigoxigenin polyclonal antibodies (Roche) onto proteinG-modified polystyrene beads from Spherotech using Dimethyl pimelimidate (Sigma). Measurements were performed in a buffered high (1 M NaCl) or low (5 mM NaCl) salt environment (10 mM Tris pH 7.4, 1 mM EDTA). In the glyoxal exposure experiments, 0.5 M glyoxal (Sigma-Aldrich) was included in the high salt buffer, and the pH was adjusted to 7.9. Force-distance data were sampled at a frequency of 1 kHz. The temperature was measured within the instrument close to the fluidics chamber. The mean temperature for all measurements was 22.3 ± 0.9 °C (± 1 s.d.).
Supplementary Material
ACKNOWLEDGMENTS.
This work was funded by grants to B.N. from the European Research Council and King Abdullah University of Science and Technology.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1213172109/-/DCSupplemental.
References
- 1.Cluzel P, et al. DNA: An extensible molecule. Science. 1996;271:792–794. doi: 10.1126/science.271.5250.792. [DOI] [PubMed] [Google Scholar]
- 2.Smith SB, Cui Y, Bustamante C. Overstretching B-DNA: The elastic response of individual double-stranded and single-stranded DNA molecules. Science. 1996;271:795–799. doi: 10.1126/science.271.5250.795. [DOI] [PubMed] [Google Scholar]
- 3.Rouzina I, Bloomfield VA. Force-induced melting of the DNA double helix 1.Thermodynamic analysis. Biophys J. 2001;80:882–893. doi: 10.1016/S0006-3495(01)76067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Mameren J, et al. Unraveling the structure of DNA during overstretching by using multicolor, single-molecule fluorescence imaging. Proc Natl Acad Sci USA. 2009;106:18231–18236. doi: 10.1073/pnas.0904322106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clausen-Schaumann H, Rief M, Tolksdorf C, Gaub HE. Mechanical stability of single DNA molecules. Biophys J. 2000;78:1997–2007. doi: 10.1016/S0006-3495(00)76747-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cocco S, Yan J, Léger JF, Chatenay D, Marko JF. Overstretching and force-driven strand separation of double-helix DNA. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;70:011910. doi: 10.1103/PhysRevE.70.011910. [DOI] [PubMed] [Google Scholar]
- 7.Konrad MW, Bolonick JI. Molecular dynamics simulation of DNA stretching is consistent with the tension observed for extension and strand separation and predicts a novel ladder structure. J Am Chem Soc. 1996;118:10989–10994. [Google Scholar]
- 8.Lebrun A, Lavery R. Modelling extreme stretching of DNA. Nucleic Acids Res. 1996;24:2260–2267. doi: 10.1093/nar/24.12.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosikov KM, Gorin AA, Zhurkin VB, Olson WK. DNA stretching and compression: Large-scale simulations of double helical structures. J Mol Biol. 1999;289:1301–1326. doi: 10.1006/jmbi.1999.2798. [DOI] [PubMed] [Google Scholar]
- 10.Prévost C, Takahashi M, Lavery R. Deforming DNA: From physics to biology. Chemphyschem. 2009;10:1399–1404. doi: 10.1002/cphc.200900253. [DOI] [PubMed] [Google Scholar]
- 11.Léger JF, et al. Structural transitions of a twisted and stretched DNA molecule. Phys Rev Lett. 1999;83:1066–1069. [Google Scholar]
- 12.Bryant Z, et al. Structural transitions and elasticity from torque measurements on DNA. Nature. 2003;424:338–341. doi: 10.1038/nature01810. [DOI] [PubMed] [Google Scholar]
- 13.Fu H, Chen H, Marko JF, Yan J. Two distinct overstretched DNA states. Nucleic Acids Res. 2010;38:5594–5600. doi: 10.1093/nar/gkq309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu H, et al. Transition dynamics and selection of the distinct S-DNA and strand unpeeling modes of double helix overstretching. Nucleic Acids Res. 2011;39:3473–3481. doi: 10.1093/nar/gkq1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Chen H, Fu H, Doyle PS, Yan J. Two distinct overstretched DNA structures revealed by single-molecule thermodynamics measurements. Proc Natl Acad Sci USA. 2012;109:8103–8108. doi: 10.1073/pnas.1109824109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rouzina I, Bloomfield VA. Force-induced melting of the DNA double helix. 2. effect of solution conditions. Biophys J. 2001;80:894–900. doi: 10.1016/S0006-3495(01)76068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wenner JR, Williams MC, Rouzina I, Bloomfield VA. Salt dependence of the elasticity and overstretching transition of single DNA molecules. Biophys J. 2002;82:3160–3169. doi: 10.1016/S0006-3495(02)75658-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams MC, Rouzina I, Bloomfield VA. Thermodynamics of DNA interactions from single molecule stretching experiments. Acc Chem Res. 2002;35:159–166. doi: 10.1021/ar010045k. [DOI] [PubMed] [Google Scholar]
- 19.Williams MC, Wenner JR, Rouzina I, Bloomfield VA. Effect of pH on the overstretching transition of double-stranded DNA: Evidence of force-induced DNA melting. Biophys J. 2001;80:874–881. doi: 10.1016/S0006-3495(01)76066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams MC, Wenner JR, Rouzina I, Bloomfield VA. Entropy and heat capacity of DNA melting from temperature dependence of single molecule stretching. Biophys J. 2001;80:1932–1939. doi: 10.1016/S0006-3495(01)76163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shokri L, McCauley MJ, Rouzina I, Williams MC. DNA Overstretching in the presence of glyoxal: Structural evidence of force-induced DNA melting. Biophys J. 2008;95:1248–1255. doi: 10.1529/biophysj.108.132688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bloomfield VA, Crothers DM, Ignacio Tinoco J. Nucleic Acids: Structures, Properties, and Functions. Sausalito, CA: University Science Books; 2000. [Google Scholar]
- 23.Gross P, et al. Quantifying how DNA stretches, melts and changes twist under tension. Nat Phys. 2011;7:731–736. [Google Scholar]
- 24.Paik DH, Perkins TT. Overstretching DNA at 65 pN does not require peeling from free ends or nicks. J Am Chem Soc. 2011;133:3219–3221. doi: 10.1021/ja108952v. [DOI] [PubMed] [Google Scholar]
- 25.Murade CU, Subramaniam V, Otto C, Bennink ML. Force spectroscopy and fluorescence microscopy of dsDNA-YOYO-1 complexes: Implications for the structure of dsDNA in the overstretching region. Nucleic Acids Res. 2010;38:3423–3431. doi: 10.1093/nar/gkq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danilowicz C, et al. The structure of DNA overstretched from the 5′5′ ends differs from the structure of DNA overstretched from the 3′3′ ends. Proc Natl Acad Sci USA. 2009;106:13196–13201. doi: 10.1073/pnas.0904729106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broude NE, Budowsky EI. The reaction of glyoxal with nucleic acid components III. Kinetics of the reaction with monomers. Biochim Biophys Acta. 1971;254:380–388. doi: 10.1016/0005-2787(71)90868-9. [DOI] [PubMed] [Google Scholar]
- 28.Hutton JR, Wetmur JG. Effect of chemical modification on the rate of renaturation of deoxyribonucleic acid. Deaminated and glyoxalated deoxyribonucleic acid. Biochemistry. 1973;12:558–563. doi: 10.1021/bi00727a032. [DOI] [PubMed] [Google Scholar]
- 29.Kočalka P, El-Sagheer AH, Brown T. Rapid and efficient DNA strand cross-linking by click chemistry. ChemBioChem. 2008;9:1280–1285. doi: 10.1002/cbic.200800006. [DOI] [PubMed] [Google Scholar]
- 30.El-Sagheer AH, et al. A very stable cyclic DNA miniduplex with just two base pairs. ChemBioChem. 2008;9:50–52. doi: 10.1002/cbic.200700538. [DOI] [PubMed] [Google Scholar]
- 31.Lundberg EP, et al. A new fixation strategy for addressable nano-network building blocks. Chem Commun (Camb) 2010;46:3714–3716. doi: 10.1039/c001513j. [DOI] [PubMed] [Google Scholar]
- 32.Lundberg EP, et al. Nanofabrication yields. Hybridization and click-fixation of polycyclic DNA nanoassemblies. ACS Nano. 2011;5:7565–7575. doi: 10.1021/nn202568q. [DOI] [PubMed] [Google Scholar]
- 33.Forns N, et al. Improving signal/noise resolution in single-molecule experiments using molecular constructs with short handles. Biophys J. 2011;100:1765–1774. doi: 10.1016/j.bpj.2011.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mossa A, et al. Dynamic force spectroscopy of DNA hairpins: I. Force kinetics and free energy landscapes. J Stat Mech Theory Exp. 2009;2009:P02060. [Google Scholar]
- 35.Morfill J, et al. B-S transition in short oligonucleotides. Biophys J. 2007;93:2400–2409. doi: 10.1529/biophysj.107.106112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomac S, et al. Ionic effects on the stability and conformation of peptide nucleic acid complexes. J Am Chem Soc. 1996;118:5544–5552. [Google Scholar]
- 37.Chen Z, Yang H, Pavletich NP. Mechanism of homologous recombination from the RecA-ssDNA/dsDNA structures. Nature. 2008;453:489–494. doi: 10.1038/nature06971. [DOI] [PubMed] [Google Scholar]
- 38.Reymer A, Frykholm K, Morimatsu K, Takahashi M, Nordén B. Structure of human Rad51 protein filament from molecular modeling and site-specific linear dichroism spectroscopy. Proc Natl Acad Sci USA. 2009;106:13248–13253. doi: 10.1073/pnas.0902723106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nordén B, et al. Structure of RecA-DNA complexes studied by combination of linear dichroism and small-angle neutron scattering measurements on flow-oriented samples. J Mol Biol. 1992;226:1175–1191. doi: 10.1016/0022-2836(92)91060-3. [DOI] [PubMed] [Google Scholar]
- 40.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise huisgen cycloaddition process: Copper (i)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed Engl. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 41.Tornøe CW, Christensen C, Meldal M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper (i)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 42.Liphardt J, Onoa B, Smith SB, Tinoco I, Jr, Bustamante C. Reversible unfolding of single RNA molecules by mechanical force. Science. 2001;292:733–737. doi: 10.1126/science.1058498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.