Abstract
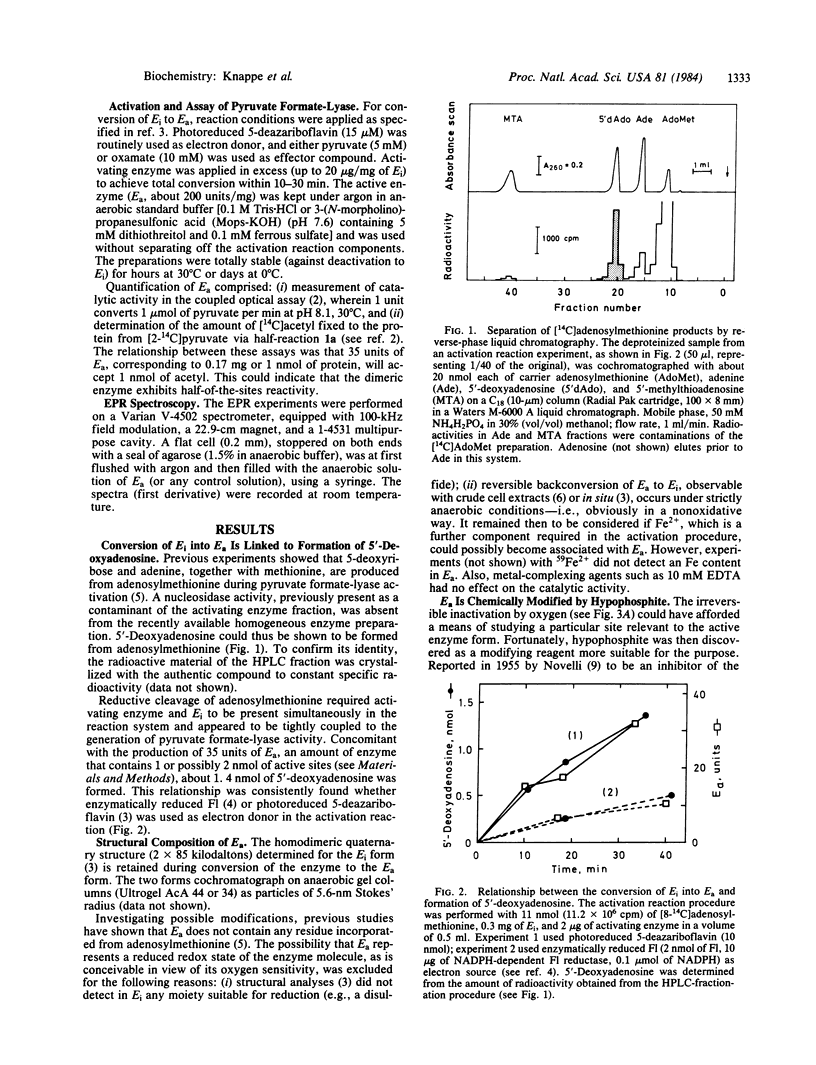
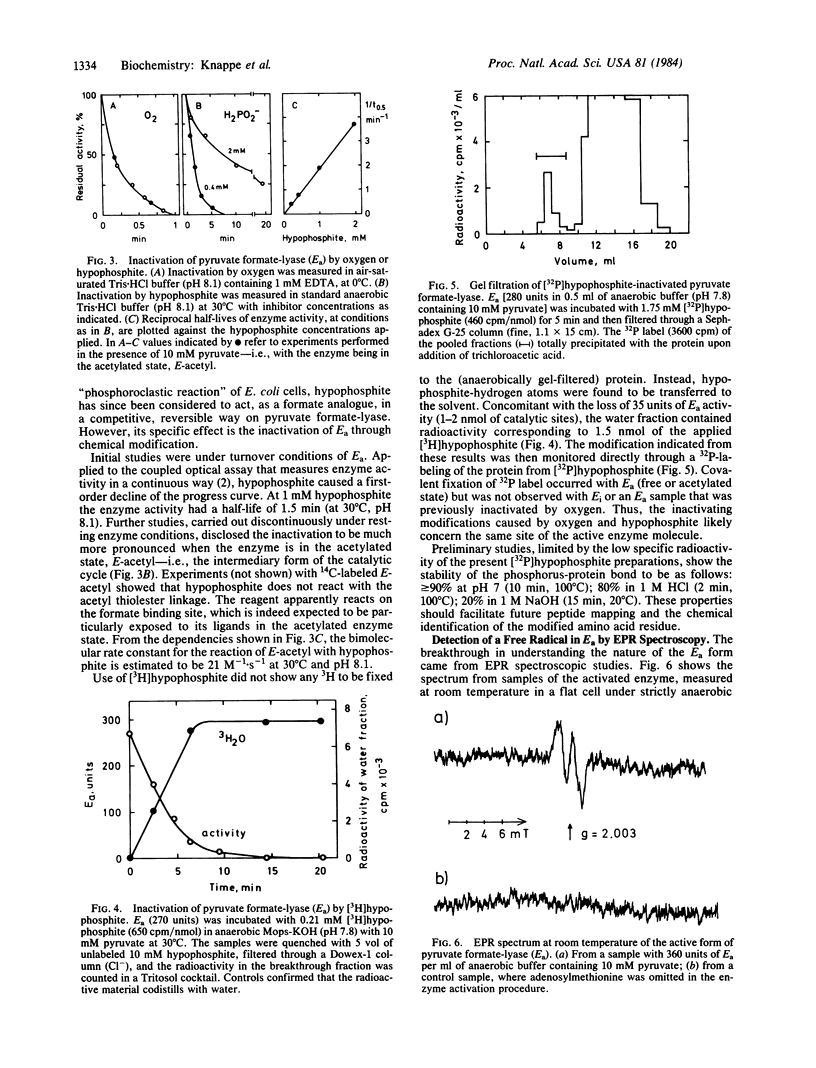
Pyruvate formate-lyase (formate acetyltransferase; EC 2.3.1.54) of Escherichia coli cells is post-translationally interconverted between inactive and active forms. Conversion of the inactive to the active form is catalyzed by an Fe2+-dependent activating enzyme and requires adenosylmethionine and dihydroflavodoxin. This process is shown here to introduce a paramagnetic moiety into the structure of pyruvate formate-lyase. It displays an EPR signal at g = 2 with a doublet splitting of 1.5 mT and could comprise an organic free radical located on an amino acid residue of the polypeptide chain. Hypophosphite was discovered as a specific reagent that destroys both the enzyme radical and the enzyme activity; it becomes covalently bound to the protein. The enzymatic generation of the radical, which is linked to adenosylmethionine cleavage into 5'-deoxyadenosine and methionine, possibly occurs through an Fe-adenosyl complex. These results suggest a radical mechanism for the catalytic cycle of pyruvate formate-lyase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow T., Eliasson R., Platz A., Reichard P., Sjöberg B. M. Enzymic modification of a tyrosine residue to a stable free radical in ribonucleotide reductase. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1492–1495. doi: 10.1073/pnas.80.6.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschkowski H. P., Neuer G., Ludwig-Festl M., Knappe J. Routes of flavodoxin and ferredoxin reduction in Escherichia coli. CoA-acylating pyruvate: flavodoxin and NADPH: flavodoxin oxidoreductases participating in the activation of pyruvate formate-lyase. Eur J Biochem. 1982 Apr;123(3):563–569. [PubMed] [Google Scholar]
- Conradt H., Hohmann-Berger M., Hohmann H. P., Blaschkowski H. P., Knappe J. Pyruvate formate-lyase (inactive form) and pyruvate formate-lyase activating enzyme of Escherichia coli: isolation and structural properties. Arch Biochem Biophys. 1984 Jan;228(1):133–142. doi: 10.1016/0003-9861(84)90054-7. [DOI] [PubMed] [Google Scholar]
- Knappe J., Blaschkowski H. P., Gröbner P., Schmitt T. Pyruvate formate-lyase of Escherichia coli: the acetyl-enzyme intermediate. Eur J Biochem. 1974 Dec 16;50(1):253–263. doi: 10.1111/j.1432-1033.1974.tb03894.x. [DOI] [PubMed] [Google Scholar]
- Knappe J., Schacht J., Möckel W., Höpner T., Vetter H., Jr, Edenharder R. Pyruvate formate-lyase reaction in Escherichia coli. The enzymatic system converting an inactive form of the lyase into the catalytically active enzyme. Eur J Biochem. 1969 Dec;11(2):316–327. doi: 10.1111/j.1432-1033.1969.tb00775.x. [DOI] [PubMed] [Google Scholar]
- Knappe J., Schmitt T. A novel reaction of S-adenosyl-L-methionine correlated with the activation of pyruvate formate-lyase. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1110–1117. doi: 10.1016/0006-291x(76)90768-3. [DOI] [PubMed] [Google Scholar]
- NOVELLI G. D. The exchange of H14COOH with the carboxyl group of pyruvate by Clostridium butylicum and Micrococcus lactilyticus. Biochim Biophys Acta. 1955 Dec;18(4):594–596. doi: 10.1016/0006-3002(55)90170-0. [DOI] [PubMed] [Google Scholar]
- Pecher A., Blaschkowski H. P., Knappe K., Böck A. Expression of pyruvate formate-lyase of Escherichia coli from the cloned structural gene. Arch Microbiol. 1982 Oct;132(4):365–371. doi: 10.1007/BF00413390. [DOI] [PubMed] [Google Scholar]
- Reichard P., Ehrenberg A. Ribonucleotide reductase--a radical enzyme. Science. 1983 Aug 5;221(4610):514–519. doi: 10.1126/science.6306767. [DOI] [PubMed] [Google Scholar]


