Abstract
Recent technical advances have re-invigorated the study of sphingolipid metabolism in general, and helped to highlight the varied and important roles that sphingolipids play in pancreatic β-cells. Sphingolipid metabolites such as ceramide, glycosphingolipids, sphingosine 1-phosphate and gangliosides modulate many β-cell signaling pathways and processes implicated in β-cell diabetic disease such as apoptosis, β-cell cytokine secretion, ER-to-golgi vesicular trafficking, islet autoimmunity and insulin gene expression. They are particularly relevant to lipotoxicity. Moreover, the de novo synthesis of sphingolipids occurs on many subcellular membranes, in parallel to secretory vesicle formation, traffic and granule maturation events. Indeed, the composition of the plasma membrane, determined by the activity of neutral sphingomyelinases, affects β-cell excitability and potentially insulin exocytosis while another glycosphingolipid, sulfatide, determines the stability of insulin crystals in granules. Most importantly, sphingolipid metabolism on internal membranes is also strongly implicated in regulating β-cell apoptosis.
Keywords: apoptosis, ceramide, endoplasmic reticulum stress, islet, lipidomics, lipotoxicity, palmitate, pancreatic β-cell, trafficking, type 1 diabetes, type 2 diabetes
Together with glycerophospholipids and neutral lipids, sphingolipids constitute one of the major classes within the mammalian lipidome. Sphingolipids are structurally diverse, synthesized and modified in a wide variety of cellular localizations, and play multiple roles acting as both signaling molecules and structural components of membranes.1-3 Perhaps to an even greater extent than for the other lipid classes, recent methodological advances have provided great insights into biological roles of particular sphingolipid metabolites, helped resolve older controversies in this field, and generated unexpected avenues for future investigation.1,3 As with other biological systems, there is a disparate and long-standing literature that implicates sphingolipids in β-cell dysfunction (predominately). Until recently, however, a coherent picture has been difficult to establish, primarily due to methodological limitations. A handful of newer studies, using the latest genetic and lipidomic approaches, have provided a more comprehensive analyses of sphingolipid metabolism in β-cell biology, thereby generating new insights that both aid in the re-interpretation of the earlier work, and raise new experimental questions to be addressed. Our aim here is to review the current understanding of sphingolipid biology in β-cells and highlight some areas where further knowledge is needed.
Sphingolipid Metabolism
The central sphingolipid metabolite is ceramide, comprising a sphingoid base as a backbone to which is attached a single fatty acid (FA) side-chain of varying length and degree of saturation1-3 (see Figure 1). The sphingoid backbone can either be synthesized de novo (sphinganine) or salvaged (as sphingosine) via the breakdown of more complex sphingolipids.4 Although the latter pathway is thought to pre-dominate in most differentiated cells under normal conditions,4 de novo synthesis is the ultimate precursor of cellular ceramide and may be especially important under certain pathophysiological conditions such as lipotoxicity (see below).† The first and rate-limiting step of de novo synthesis is catalyzed by serine palmitoyltransferase (SPT) and condenses two abundant nutrient metabolites, serine and palmitoyl-CoA.5 Sphinganine is generated after several further modifications and serves (as does sphingosine from the salvage pathway) as a substrate for ceramide synthase (CerS formerly known as Lass). This is now known to comprise a family of 6 enzymes, which display differing selectivity for the type of FA chain they attach to the sphingoid base.6 As such, this reaction accounts for the diversity of sphingolipid isoforms in different tissues and under different conditions.6,7 For example, C18 ceramide (containing a stearic acid side-chain) is abundant in many tissues such as brain and muscle, but much less so in (at least murine) β-cells (see below).1 The product of CerS in the de novo pathway, dihydroceramide, must be desaturated to generate ceramide, but this enzyme produces ceramide directly when the salvaged base sphingosine is used as a substrate.1-3
Figure 1. Ceramide synthesis. The first step of de novo ceramide synthesis involves the condensation of palmitoyl-CoA and serine by serine palmitoyltransferase. Other long-chain fatty acyl-CoAs, commonly 14 to 26 carbons long can also be incorporated as a side-chain following sphinganine synthesis by 3-ketosphinganine reductase (not shown, see Figure 2) by the action of ceramide synthases 1–6. These synthases, also known as longevity assurance homologs (Lass) 1–6, have different chain length specificities in regard to their fatty acyl-CoA substrates. Following ceramide breakdown to its sphingosine backbone by neutral or acidic ceramidases (CDase) within different subcellular compartments, the sphingosine is transported out of the lysosome and can be reincorporated back into ceramide via ceramide synthase. This salvage pathway of ceramide synthesis utilizes the same ceramide synthases involved in the de novo pathway.
Ceramide serves as the precursor for a series of more complex sphingolipids (Fig. 2) of which the most abundant in mass terms is sphingomyelin (SM). This is generated (along with diacylglycerol) from the condensation of ceramide with the phospholipid phosphatidylcholine, or in some cases phosphatidylethanolamine.8 This reaction is catalyzed by one of two SM synthase enzymes (SMS1 or 2). The other major route of ceramide metabolism is via glycosylation, which gives rise to a range of products (glycosphingolipids) that vary depending on the type and number of sugar residues attached. The most abundant mono-hexosyl derivates are glucosylceramide (GluCer) and galactosylceramide (GalCer),1-3 which are formed by the actions of GluCer synthase and ceramide galactosyltransferase respectively. GluCer serves as the precursor for a plethora of compounds of the ganglioside family, whereas GalCer can be sulfated to produce sulfatide or sialosylated to GM4 ganglioside.5 Finally, ceramide can also be phosphorylated by the enzyme ceramide kinase to generate ceramide 1-phosphate.9 All of these compounds can in turn be catabolized, thereby regenerating ceramide which can be further catabolised to sphingosine, via the action of a variety of ceramidase enzymes8 (see Figure 2). This occurs in a number of cellular compartments as part of the salvage pathway. Sphingosine can be reconverted to ceramide, as discussed above, or phosphorylated by sphingosine kinases to form sphingosine 1-phosphate (S1P).9
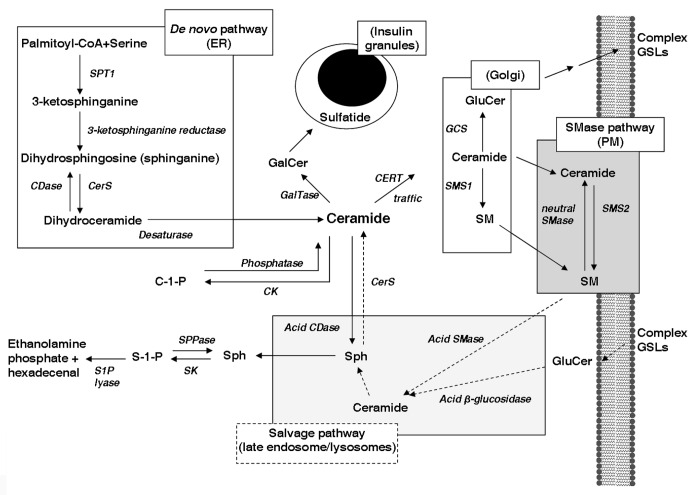
Figure 2. Sphingolipid synthesis and its subcellular topology. The synthesis of the central sphingolipid, ceramide, begins with the de novo pathway at the surface of the endoplasmic reticulum (ER) and the condensation of palmitoyl-CoA and serine by serine palmitoyl transferase 1 (SPT). Ceramide is transported to the golgi by ceramide transport protein (CERT) where glucosylceramide (GluCer) and sphingomyelin (SM) are synthesized. Ceramide is a precursor for subspecies ceramide-1-phosphate (C-1-P) and galactosylceramide (GalCer) within neutral compartments. GalCer may be sulphated forming sulfatide which associates with insulin crystals within granules. Higher glycosphingolipids (GSLs) such as gangliosides are formed at the trans-golgi then delivered to the plasma membrane (PM) along with ceramide and SM. SM can produce localized increases in ceramide via activation of the sphingomyelinase (SMase) pathway which causes association of ceramide with cholesterol and GSLs in subpools of the PM termed detergent resistant membranes. GSLs, SM and ceramide are degraded to sphingosine (Sph) in the late endosomes and lysosomes. Sphingosine exits the lysosome and may either be salvaged back into ceramide or form sphingosine-1-phosphate (S-1-P), a key lipid signaling intermediate, which can be terminally degraded to ethanolamine phosphate and hexadecenal. Dotted lines indicate salvage/recycling pathways, whereas solid lines signify de novo synthesis. SMase, sphingomyelinase; SMS1/2, sphingomyelin synthase 1/2; GCS, glucosylceramide synthase; CerS, ceramide synthase; GalTase, galactosyltransferase; SK, sphingosine kinase; CK, ceramide kinase; CDase, ceramidase; S1P lyase, sphingosine-1-phosphate lyase; SPPase, sphingosine-1-phosphate phosphatase.
A major determinant of the fate of ceramide is the cellular compartmentalization of many of the metabolic pathways described above.10,11 All of the steps of de novo synthesis, and the regeneration of ceramide from sphingosine, take place on the external leaflet of the endoplasmic reticulum (ER). For generation of GluCer and SM this ceramide must be transported to the Golgi compartment.10,11 This process is best defined for synthesis of SM with FA side-chains ≤ C20 where the ceramide substrate makes use of a ceramide transport protein (CERT), whereas longer chain ceramides (> C20) are translocated as part of the normal process of ER-to-golgi vesicular trafficking.12 Unlike SM, which is produced on the surface of the golgi, GluCer is found in the lumen, implying a requirement for transmembrane transport. Whether this occurs at the golgi itself via a flippase, or whether the newly formed GluCer is transported back to the ER, internalized and then re-exported is unclear.11,13 The evidence for the site of GalCer synthesis favors the ER, which is also the site of action of ceramide kinase.11,13 In terms of sphingolipid degradation, the lysosome plays a key role in breaking down both SM and GluCer via acid SMase and β-glucosidase respectively.14 The ceramide generated from these reactions is further metabolized in the lysosome by acid ceramidase, thereby making sphingosine available into the salvage pathway.8 There are also neutral SMases localized to the plasma membrane and ER, responsible for controlling SM and ceramide levels in those compartments.10,11 A feature of this regulation is the sequestration of SM and ceramide, together with cholesterol, into detergent resistant membranes (DRMs) that serve as platforms for specialized signaling functions, and to this end SM and cholesterol concentrations are tightly and coordinately regulated at multiple levels.15,16 Activation of (especially neutral) SMases also occurs in specific signaling cascades especially those downstream of receptors for cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor α (TNFα).11
Sphingolipids and Type 1 Diabetes
β-cell cytokine signaling
Early investigations of ceramide generation in β-cells focused in the area of Type 1 diabetes (T1D), prompted by studies of inflammatory signaling cascades in other cell systems. It was first shown that exogenous ceramide provided either directly or generated following addition of SMase was sufficient to reproduce some of the effects of IL-1β in repressing insulin production and promoting β-cell death in fetal islets and β-cell lines.17-19 These treatments did not activate the NFκB pathway that mediates induction of iNOS and generation of nitric oxide.19,20 Direct evidence for activation of SMase by cytokines, however, has been harder to demonstrate using β-cells than in other systems. IL-1β reportedly promoted a transient (5min) decrease in SM in clonal RINm5F cells,19 but this was not consistent with other studies using rat islets20 or β-TC3 cells17 possibly explained by technical limitations of the tracer methodologies that had been employed in all three reports. Moreover, increases in ceramide mass under these conditions were only observed in RINm5F cells,19 but not β-TC3 cells.17 These modest or non-significant effects are perhaps consistent with observations that cytokines can activate both neutral and acidic ceramidases in β-cells,21,22 which would tend to counteract any ceramide accumulation. Pharmacological inhibitors of ceramide synthesis or SMase activity also failed to alter IL-1β-stimulated responses in a rat β-cell line,23 although the potentiation of IL-1β effects on cell death by TNFα might involve an enhanced sensitivity to basal acid SMase.24 Finally, Il-1β and TNFα augment S1P in β-cells,25 which might represent a protective response against cell death via S1P receptor mediated pathways.24 The overall evidence would thus tend to argue against alterations in sphingolipid metabolism playing major roles in cytokine-mediated β-cell destruction in the context of T1D. However, this conclusion is perhaps not definitive and this topic might benefit from further investigation focusing on longer-term effects of cytokines, employing more sensitive assay methods, and extending measurements to metabolites in addition to ceramide and SM.
Immune cell infiltration in Type 1 diabetes
The in vitro studies discussed above focused on the downstream responses of β-cells to the extracellular milieu of cytotoxic cytokines encountered during autoimmune cellular destruction of the islets in T1D. These cytokines come from the many different immune cell types that migrate to the islet in response to islet self-antigens. Fewer studies have investigated the effect of altering sphingolipid metabolism from the reverse perspective; whether alterations in the sphingolipid content of β-cells, immune cells or serum might modulate immune cell infiltration, or otherwise act upstream of cytokine release. This seems feasible given known effects of sphingolipid modulators in other inflammatory diseases,26 and some recent indications that downregulation of certain SM species in the circulation is associated with decreased progression to T1D in both humans and non-obese diabetic (NOD) mice.27 However, SM was only one of several lipids that were altered and this observation was not pursued mechanistically. Other studies have focused on glycosphingolipids that can serve as ligands for cell surface receptors. One such is the macrophage scavenger receptor, CD14, which is primarily expressed on monocytes, macrophages and other immune cell types with lipopolysaccharide (LPS) as its natural ligand. CD14 is also expressed on a subset of β-cells from human islets.28 In addition to LPS, GalCer, acting via the CD14 receptor and presumably in an auto/paracrine manner, was also found to also stimulate β-cells to secrete TNF-α, IL-1β and IL-8. Conversely sulfatide, another endogenous glycolipid found in insulin secretory granules, inhibited LPS effects mediated via the CD14 receptor.28 However, the real physiological role of CD14 on the β-cell surface and whether it contributes to β-cell death seen in diabetes is as yet unclear.
There is some evidence that S1P may be critical in the protection of β-cell destruction by cytokines. Recent studies of islet allografts in diabetic mice demonstrated that the S1P receptor agonist, FTY720, enabled long-term survival of these grafts.29,30 This S1P analog was found to prevent lymphoangiogenesis that was key to subsequent T cell infiltration, inflammation and β-cell destruction of these allografts.30 Long-term FTY720 treatment also prevented the onset of diabetes in rodent models of T1D by halting islet immune cell infiltration and pro-inflammatory cytokine-mediated destruction.31,32 Modulation of S1P signaling may therefore be an attractive candidate for future studies focused on the preservation of islet architecture for the prevention of T1D.
There is a longer-standing literature suggesting that various sphingolipids in β-cells act as auto-antigens. Dotta and colleagues identified GM2–1 as a pancreatic islet specific species of ganglioside33 that was a target of IgG islet cell autoantibodies. The presence of these antibodies not only correlated strongly with progession to diabetes in relatives of subjects with T1D, but the content of this glycolipid was hyperexpressed in the NOD mouse.33 Furthermore, this ganglioside is present in β-cell secretory granules, similar to other autoantigens in Type 1 diabetes such as insulin and carboxypeptidase H. More recently, cell surface patches of SM were shown to be the epitope for IC2 a monoclonal antibody that specifically recognizes the surface of β-cells.34 Since this antibody was developed from the diabetic BB rat, which possesses circulating auto-antigens to β-cells, these findings at least raise the possibility that cell surface SM might be involved in β-cell autoimmunity.
Sphingolipids and Type 2 Diabetes
Ceramide and β-cell apoptosis
Although ceramide had long been implicated in apoptosis, its involvement in lipotoxicity was first suggested by studies in hemopoietic cells showing that saturated but not unsaturated FAs induced cell death, which was associated with enhanced de novo synthesis of ceramide.35 This concept was extended to β-cells in pioneering work from the Unger laboratory using the Zucker diabetic fatty (ZDF) rat model of T2D.36,37 Key evidence included observations that ceramide content was augmented in islets of the diabetic animals and that the accompanying enhancement of apoptosis was reduced by fumonisin B1, an inhibitor of SPT.37 Tracer analyses implicated an enhancement of de novo ceramide synthesis, which was consistent with observed increases in SPT expression in islets of ZDF rats, and islets of control rats treated with elevated FAs.36 In vivo treatment of diabetic animals with an SPT inhibitor also reduced apoptosis of islets assayed ex vivo.36 This work proved seminal for the future development of the lipotoxicity field, not only in β-cells, but also in the broader context of the whole metabolic syndrome. In retrospect, however, it could be argued that the ZDF model represented an extreme case in that these islets accumulate massive amounts of lipid. Indeed, the partitioning of palmitate tracer into ceramide in islets of ZDF rats greatly outweighs the amount that is oxidized.38 More recent work using another animal model of the metabolic syndrome has shown only modest increases in ceramide late in the progression of β-cell failure,39 and in fact β-cells appear relatively resistant to lipid overload as compared with other tissues in this model.40
A milder and much more commonly employed model of lipotoxicity comprises the chronic exposure of β-cells to elevated concentrations of FAs in vitro. A caveat of this approach is that the actual concentration of free FA depends on the ratio of total FA to albumin in the medium, and so can vary markedly between protocols and in a manner that is not always easy to evaluate.41 The discussion below is focused on reports that are less likely to be confounded by the use of very high levels of free FAs. The general conclusion from these studies is that whereas both saturated and unsaturated FAs promote defects in glucose-stimulated insulin secretion,42,43 β-cell apoptosis is much more restricted to saturated FAs, and indeed unsaturated FAs appear to be protective in this case.44-46 Islets from ZDF rats appear to be an exception as these were sensitive to mixtures (2:1 unsaturated to saturated) of FA36 but this might reflect the high overall concentrations employed or be a special feature of the ZDF model as discussed above. Although a variety of mechanisms have been proposed to account for β-cell lipo-apoptosis one of the best substantiated relates to ER stress.47,48 According to this hypothesis, apoptosis ensues from a failure of the adaptive mechanisms serving to protect cells from ER stress due to an accumulation of misfolded proteins in the lumen of ER. Secretory cells such as β-cells are particularly susceptible to ER stress due to the high rate of protein traffic through the ER.49,50 Evidence in support of this hypothesis includes the observation that ER stress is preferentially triggered by saturated FAs,47,48,51 and that (at least mild) lipo-apotosis is reduced by inhibition of ER stress signaling.47,48,52
A separate and longer standing body of work has pointed to involvement of ceramide synthesis in β-cell apoptosis due to saturated FAs, consistent with the substrate preference of SPT for palmitate.6 This has been supported by recent observations showing that 3H-serine incorporation into ceramide (also catalyzed by SPT) was augmented by pre-exposure to palmitate.51 Enzymatic measurements of ceramide mass, however, have generally reported only modest increases with FA pretreatment unless glucose is concomitantly elevated.53-55 And yet inhibitors of de novo ceramide synthesis, acting at the level of either SPT (cycloserine or myriocin) or CerS (fumonisin B1), generally reduce lipo-apoptosis in β-cells44-46,54,56-58 although this is not universally observed, particularly with higher concentrations of FAs or when combined with elevated glucose.59,60 A possible explanation for these somewhat disparate observations might be that apoptosis is triggered by increases in ceramide that are restricted to particular species or cellular locations, and which are less apparent when the total cellular content is assayed. Alternatively, ceramide generated via different pathways (de novo synthesis vs. salvage pathway or SMase activity) might differ in its apoptotic potential. Finally, the toxic intermediate might not be ceramide itself, but a metabolite of it.
To some extent these possibilities have been resolved by two recent studies using β-cell lines that combine genetic modulation of sphingolipid metabolism with accurate quantification of ceramide mass using mass spectroscopy.56,58 Our work, aimed at identifying the lipid metabolite responsible for lipotoxic ER stress, comprised a comprehensive and unbiased lipidomic screen and largely excluded involvement of phospholipids or neutral lipids.56 Following pretreatment of the MIN6 β-cells with palmitate for 48h we were unable to detect increases in major ceramide species at the whole cell level, but hexosylceramide (predominately GluCer) was augmented, as was flux through the de novo synthetic pathway as determined by incorporation of 3H-serine. However, overexpression of GluCer synthase to enhance conversion of ceramide to GluCer did not exacerbate lipotoxicity, but partially protected against apoptosis and induction of ER stress markers. This intervention also overcame the defects in ER-to-golgi protein trafficking due to palmitate pretreatment56 that we had previously postulated contributes to lipotoxic ER stress through protein overload.61 These results suggested that chronic palmitate induces ER stress via increases in a subpool of ceramide, without altering total ceramide mass. Broadly consistent with our findings Veret et al. were able to show increases in ceramide using INS-1 cells treated with higher concentrations of palmitate, but that this effect was maximal at 12 h and was no longer apparent at 48 h, except in the presence of elevated glucose concentrations.58 The increased ceramide was observed with most side-chain species, not just C16, and appeared to arise from the de novo synthetic pathway because there were accompanying increases in di-hydroceramide and sphinganine, but not sphingosine. In this model, however, there was minimal lipo-apoptosis unless glucose was also augmented and this correlated best with accumulation of C18:0, C22:0 and C24:1 ceramide species, also consistent with increased expression of CerS4 under these conditions. Finally, up- or downregulation of CerS4 expression enhanced or reduced glucolipotoxicity respectively.58 The conclusion that C18:0 and C22:0 Cer are the toxic metabolites would be consistent with unpublished data that these species are also selectively increased in our model of lipotoxicity. One caveat, however, is that C18:0 and C22:0 ceramide are very minor metabolites in MIN6 cells56 and mouse islets (EB, PJM, TJB unpublished). Indeed there may be pronounced species differences since C16:0 greatly abounds over C24:0 ceramide in mouse β-cells56 (EB, PJM, TJB unpublished), but the opposite is true in rat INS-1 cells.58,62 It will thus be important to investigate key aspects of sphingolipid metabolism in rat, and ultimately human islets, to confirm findings obtained in the cell lines, and to determine which of the rodent species is the better model for human islets.
Although de novo synthesis appears to be the major mechanism for lipotoxic ceramide generation there are other possibilities. Ramanadham and co-authors have demonstrated that strong ER stress, triggered either pharmacologically in vitro62-64 or using a genetic model of pro-insulin misfolding64 itself promotes ceramide accumulation secondary to the activation of neutral SMase 2. In this instance ceramide accumulation in the mitochondria would be one mechanism whereby ER stress might trigger apoptosis.62 Whether ceramide generated via de novo synthesis during lipotoxicity could also act in this manner (perhaps independently of ER stress) is yet to be determined. In contrast, using our milder lipotoxic model, ER stress was neither necessary nor sufficient for any of the observed alterations in lipid metabolites, including ceramide, SM and GluCer.51 Nevertheless it cannot be excluded that small, perhaps localized, increases in ceramide and decreases in SM might contribute to lipo-apoptosis, either up or downstream of ER stress. This will require detailed analyses of sphingolipid metabolites in different subcellular compartments, and testing the functional effects of manipulating those metabolites in selected compartments.
Mechanisms of ceramide-induced apoptosis in β-cells
Our studies based on the protection afforded by GluCer synthase overexpression clearly implicate ceramide as acting upstream of defective protein trafficking and the activation of lipotoxic ER stress.56,61 This mechanism does not appear to apply in liver,65,66 but ceramide can promote ER stress in some other cell types.67,68 Ceramide has also been reported to disrupt ER-to-golgi protein trafficking, although this seemed to involve the salvage rather than de novo synthetic pathway.69 In principle, ceramide might also promote ER stress independently of effects on trafficking, by depleting Ca2+ from the ER and thereby causing protein misfolding.70 This has not been assessed in β-cells.
As intimated above, ceramide could promote apoptosis by mechanisms other than ER stress, especially during glucolipotoxicity or very strong lipotoxicity. One such possibility would be disruption of mitochondrial function as mentioned previously62 and which has been implicated as causative in some aspects of defective β-cell function using SMS1 knockout mice.70 This is discussed further below. Although exogenous ceramide is sufficient to disrupt mitochondrial function71 there is currently no evidence linking ceramide accumulation in mitochondria to lipo-apoptosis in β-cells. Ceramide also has the ability to form large stable channels in membranes which directly affects membrane conductance and hence may have biological relevance at the mitochondria or plasma membrane, particularly in regard to cytochrome c release and apoptotic signaling.7 Another potential apoptotic mechanism would be alterations in plasma membrane DRMs. Indirect evidence for this comes from studies of β-cell apoptosis due to islet amyloid polypeptide (IAPP),72 accumulation of which is linked to the pathogenesis of T2D. In this instance IAPP appeared to augment ceramide, and apoptosis was not observed in islets of mice deficient in acid SMase.73 The latter enzyme was also required for IAPP-mediated inhibition of a K+ current that had previously been implicated in apoptosis and whose function might depend on clustering in DRMs.73 If confirmed such a mechanism would potentially apply to many signaling pathways in addition to the gating of K+ channels. A further means whereby ceramide might mediate β-cell lipotoxicity is via inhibition of the anti-apoptotic kinase, Akt, as best described in muscle.74,75 In β-cells, however, Akt is inhibited by both oleate and palmitate,76,77 which might argue against involvement of ceramide because of the substrate specificity of SPT. Moreover, several labs have shown that chronic exposure to moderately elevated palmitate activates rather than inhibits Akt55,78,79 and high FA concentrations stimulate Akt phophorylation at early time points, and only inhibit at 24h or more.76 In muscle activation of the protein phosphatase PP2A is implicated in mediating the palmitate/ceramide effects on Akt.80 It is therefore conceivable that PP2A might contribute to β-cell lipotoxicity via substrates other than Akt,81 although this remains to be investigated.
Ceramide and insulin gene expression
Co-treatment with palmitate inhibits the augmentation of pro-insulin mRNA levels due to chronically elevated glucose.80,81 The fact that this did not seem to occur with oleate82 and that ceramide was sufficient to repress insulin content18 potentially implicated de novo ceramide synthesis in this context. This was directly confirmed by Kelpe et al., who demonstrated that inhibitors of the de novo synthetic pathway overcame both the increases in ceramide due to glucolipotoxicity, as well as the accompanying reduction in pro-insulin gene expression.55 Mechanistically there appears to be a key role for the transcription factor C/EBPβ, whose expresssion is enhanced by palmitate82-84 but not oleate83 and which competes with Pdx1 and MafA on the proinsulin gene promoter.85,86 Administration of exogenous ceramide or blockade of further ceramide metabolism in the presence of palmitate is sufficient to activate ERK87 or modulate proinsulin and C/EBPβ gene expression respectively.57,84 Whether ERK activation occurs upstream82 or independently87 of C/EBPβ, remains to be resolved. To date, however, there is no direct evidence that ceramide accumulation is actually necessary for the enhancement of C/EBPβ function due to palmitate, although this would seem to be likely and could easily be tested by genetic or pharmacological intervention. With the use of lipidomic tools such as mass spectrometry, the contribution of endogenous ceramides to transcriptional regulation will be easier to elucidate, obviating the reliance on short-chain length exogenous ceramides (such as C2-ceramide), whose physiological relevance within the cell has often been questioned.
Ceramide and insulin secretory defects
In contrast to the strong evidence favoring ceramide as a mediator for lipo-apoptosis and reduced insulin gene expression, a corresponding role in defective insulin secretion appears less probable. Ceramide analogs have only marginal effects on glucose-stimulated insulin secretion,17 and inhibitors of de novo ceramide synthesis do not overcome the secretory defects due to lipotoxicity.60,79 This is probably not surprising since unsaturated FAs (as well as saturated FAs) inhibit glucose-stimulated insulin secretion43 and these are not substrates for SPT. It is possible that chronic exposure to oleate might augment ceramide through other mechanisms, perhaps via side-chain incorporation. This seems unlikely, however, since we observed no evidence for accumulation of sphingolipid metabolites in a palmitate-resistant subline of MIN6 cells,56 which display an augmented ratio of unsaturated to saturated FAs due to enhanced expression of stearoyl CoA desaturase.88 Even so, it is conceivable that localized, or more subtle, alterations in sphingolipid metabolism might still impact on stimulus-secretion coupling. Indeed a whole body knockout of SMS1 resulted in the expected decreases in SM and increases in ceramide in β-cells and was accompanied by defects in insulin secretion and mitochondrial function and increased ROS production.70 However, because these mice also display lipodystrophy it is possible that there is disruptive lipid accumulation in β-cells secondary to increases in circulating FAs. Confirming whether this interesting secretory phenotype is truly intrinsic to the β-cell would require further investigation using tissue-specific knockout mice. However, knockdown or pharmacological inhibition both SMS1 and SMS2 in INS-1 has also been recently shown to inhibit insulin secretion in vitro.89 The mechanism in this instance appears to involve defective export of secretory proteins from the golgi to the plasma membrane. Whether this is explained by increased ceramide, or decreases in either SM or DAG, which are both products of SMS activity, was not addressed. Although taken together these studies suggest that SMS function is essential for the maintenance insulin secretion, it remains to be determined whether loss of SMS activity contributes to the secretory defects observed in the setting of diabetes.
The sequestration of SM and/or ceramide within DRMs post-stimulus, such as in the case of IAPP accumulation described above,73 promotes clustering of receptors and other plasma membrane proteins into these microdomains to augment downstream signaling. Recent work characterizing the expression, gating and association of potassium and calcium channels at the plasma membrane of β-cells has revealed a regulated compartmentalization of these ion channels into DRMs during the time-frame of insulin release.90 Voltage-gated Ca2+ channels (Cav1.2) and K+ channels (Kv2.1), two critical determinants of β-cell electrical excitability, are directly associated within these DRMs with exocytotic proteins such syntaxin 1A, SNAP-25 and VAMP2. Dissolution or disordering of those lipid microdomains with β-cyclodextrin (which removes cholesterol from the membrane), inhibited Kv2.1 activity, but not Cav1.2 channel activity and this actually enhanced single-cell exocytotic events and insulin secretion.90 It is therefore possible that alterations in sphingolipid metabolism that impact on DRMs could influence insulin secretion, but that this has yet to be tested directly.
Although it is unclear whether ceramide itself mediates defective insulin secretion, the minor yet highly physiologically active ceramide metabolite, S1P, has recently been shown to accumulate in response to glucose in both MIN6 and pancreatic islets.91 Furthermore, the activity of SK2 was responsible for this S1P accrual and its inhibition reduced GSIS in vitro and impaired insulin availability and glucose tolerance in vivo. The downstream signaling of S1P from its five G-protein coupled receptors may therefore play an essential and as yet to be defined role in GSIS.
Glycosphingolipids in Type 2 Diabetes
Glycosphingolipids such as GM3 have been implicated in T2D, most specifically in the development of insulin resistance.92 GM3 ganglioside synthase levels were found to be upregulated in Zucker fa/fa rat and ob/ob mouse models of T2D.92 Recently, studies in ZDF rats93,94 have observed cytoprotective effects within the pancreatic islets of these animals following dietary supplementation with small molecule glycosphingolipid synthase inhibitors Genz-12334694 and AMP-DNM.93 Both these studies reported a partial protection of β-cell content and islet architecture following chronic (10 week94 or 60 d93) glycosphingolipid synthase inhibition. Basal93 and glucose-stimulated insulin serum content94 was diminished, corresponding to a lower serum glucose levels in both the fasted and non-fasted rats,93 indicating an increase in whole body insulin sensitivity. The possibility of gangliosides being directly involved in lipo-apoptotic signaling pathways was entertained as a mechanism behind this protection from β-cell destruction,94 but this was not assessed directly. In contrast, subcellular remodelling of β-cell glycosphingolipid species in response to saturated lipid oversupply was seen to be cytoprotective in our recent publication,56 in which enhanced synthesis of GluCer prevented β-cell lipopapotosis, ER stress and a previously reported protein trafficking defect.61 This discrepancy between the in vitro56 and in vivo93,94 effects of GluCer synthase inhibitors on β-cell function possibly reflects differences between responses intrinsic to the β-cell vs. those mediated indirectly via whole body effects.
As discussed above, there is a limited literature addressing the role of glycosphingolipids in the inflammation of T1D. However, the growing realization that inflammation also contributes to β-cell dysfunction in T2D95 raises the possibility of a broader role. There is only very limited data addressing this using the Cohen diabetes sensitive rat, which under environmental pressure (high sucrose diet), develops diabetes characterized by blunted glucose stimulated insulin secretion, glucose intolerance and various pancreatic lesions including exocrine steatosis and IL-1β positive macrophage infiltration.96,97 In a study where these animals were co-treated for one month with daily IP injections of β-glucosyl and β-lactosylceramides, which are known stimulators of natural killer T and CD8 lymphocytes (via dendritic cells), pancreatic steatosis was markedly decreased and glucose stimulated insulin secretion was restored.97 The beneficial effects of these glycosphingolipids upon the islet were therefore deemed to be mediated by immunomodulation of T cells.
Sulfatide
A glycosphingolipid derivative of GalCer, sulfatide, appears to be particularly important to secretory cells such as the pancreatic β-cell and neuronal cells. This lipid, GalCer-3-O-sulfate, is sulfated by sulfate transferase and present in β-cells but not exocrine tissue of the islets in humans and other species including rat, mouse, pig and monkey.98 Sulfatide is synthesized in the golgi and is packaged into insulin secretory granules with insulin in the trans-golgi network.99,100 It binds to insulin crystals to preserve the crystal structure at pH 5.5 as well as aiding the conversion of insulin hexamers to monomers at pH 7.4 at the cell surface.101 It also promotes proinsulin folding and oxidation within the secretory pathway.101 Patch clamp studies show that sulfatide negatively regulates glucose stimulated insulin secretion potentially via its action on K+ATP channels.101,102 Furthermore, loss of the C16:0 isoform of sulfatide in β-cells has been implicated in the pathogenesis of T2D. This specific isoform is lacking in islets from ob/ob and db/db mouse models of T2D as compared with normal human pancreatic tissue, BALB/c mice and the non-diabetic Lewis rat.99 Moreover, C16:0 sulfatide significantly improves insulin crystal preservation.99 As discussed earlier, the co-secretion of sulfatide with insulin also appears to negatively regulate CD14 signaling to prevent excessive secretion of pro-inflammatory cytokines from the β-cell that may precipitate β-cell destruction.28 Collectively these studies make a strong case for sulfatide playing an important role in β-cell biology, and this is another topic for reinvestigation using newer genetic and analytical tools.
Concluding Remarks
It is becoming increasing apparent that sphingolipids have varied roles in many critical aspects of β-cell biology; these are summarized in Table 1. The composition of DRMs determined by the activity of neutral SMases at the plasma membrane facilitate the proper complexing and activation of the ion channels and cell surface receptors that can impact on insulin or cytokine secretion as well as responses to other extracellular stimuli. The glycosphingolipids within the plasma membrane also function as receptors that signal to β-cell cytokine secretion, which might in turn modulate recruitment and activation of immune cells. The pancreatic specific ganglioside GM2–1 also elicits autoantibody production, although the relevance of these glycosphingolipids within both T1D and T2D are as yet unknown. This and other glycosphingolipids such as sulfatide are found within insulin secretory granules, where they might regulate other β-cell processes including the stabilization of mature insulin crystals.
Table 1. Sphingolipid metabolites in the β-cell and their biological functions.
| Sphingolipid metabolite | References | Biological process (Enzymes involved) |
|---|---|---|
| Ceramide |
35-38 |
Proapoptotic; accumulates in the ZDF rat model of lipotoxicity |
| |
56,58 |
Proapoptotic; induces ER stress in mild β-cell models of lipotoxicity, longer chain ceramide species implicated in toxicity |
| |
62-64 |
Proapoptotic; accumulates at the ER and mitochondria in response to strong ER stressors (nSMase 2, iPLA2β) |
| |
56,69 |
Disrupts ER-to-golgi traffic |
| |
72 |
Proapoptotic; intraislet IAPP deposits induce ceramide accumulation (aSMase) |
| |
55,84 |
Represses transcription of proinsulin mRNA |
| Sphingomyelin |
27 |
SM serum content in NOD mice and humans with T1D associated with T1D progression |
| |
34 |
Pancreatic β-cell specific surface SM possesses the epitope for IC2 monoclonal antibody found in the diabetic BB rat |
| |
70 |
Regulates GSIS and ROS homeostasis in mouse pancreatic islets (SMS1) |
| |
89 |
Regulates GSIS from β-cell line, INS-1 (SMS1) |
| Sphingosine 1-phosphate |
91 |
Regulates GSIS in β-cell line MIN6 and pancreatic islets (SK) |
| |
24,25 |
Antiapoptotic; stimulates cytokine-induced S1P signaling (SK) |
| |
29,30 |
Anti-inflammatory; inhibits lymphangiogenesis and immune cell infiltration of islet allografts (SK) |
| |
31,32 |
Anti-inflammatory; prevents autoimmune mediated islet destruction in a rat model of T1D (SK) |
| Galactosylceramide |
28 |
Activates β-cell CD14 receptor to secrete TNF-α, IL-1β and IL-8 |
| Glucosylceramide |
93,94 |
Contributes to insulin sensitivity in ZDF rats |
| β-Glucosylceramide, β-lactosylceramide |
97 |
Exerts immunomodulatory effects on NKT and CD8 T cells to prevent pancreatic steatosis in the Cohen diabetic rat |
| Sufatide |
28 |
Modulates β-cell CD14 receptor signaling |
| |
101,102 |
Binds to and stabilizes insulin crystals within granules, modulates β-cell K+-channel activity and exocytosis |
| GM2–1 | 33 | Pancreatic islet specific ganglioside that is the target of IgG autoantibodies correlating strongly with T1D progression in patients with diabetic relatives |
Abbreviations: aSMase, acid sphingomyelinase; BB, biobreeding; ER, endoplasmic reticulum; GSIS, glucose stimulated insulin secretion; iPLA2β, calcium-independent phospholipase A2 β; IL-1β, interleukin 1-β; NOD, non-obese diabetic; nSMase, neutral sphingomyelinase; IL-8, interleukin-8; NKT, natural killer T; ROS, reactive oxygen species; S1P, sphingosine 1-phosphate; SK, sphingosine kinase; SM, sphingomyelin; SMS1, sphingomyelin synthase 1; T1D, Type 1 diabetes; TNF-α, tumor necrosis factor α; ZDF, Zucker diabetic fatty
Ceramide is postulated to be the lipid ‘second messenger’ responsible for β-cell death due to saturated FA exposure yet how ceramide accumulation mediates this end remains elusive. It can impact on known protein signaling intermediates such as Akt and C/EBPβ, which are thought to contribute to apoptosis induction and blunted insulin gene expression. The accumulation of certain species of ceramide in membranes of organelles such as the ER and mitochondria might in theory modulate the shape and function of these membranes. This itself may impact on vesicle formation, protein trafficking, and processing of secretory cargo, as well as mitochondrial membrane potential. These responses could exert diverse effects on ER stress, apoptosis and even autophagy. In our opinion future studies should focus on modulating sphingolipids in different cellular compartments, using genetic or pharmacological intervention, as a means of addressing the actions of ceramide, SM or glycosphingolipid on β-cell secretion, insulin expression, apoptosis and ER function. Despite the biochemical and biophysical challenges that such studies will entail, we believe that this approach will not only expand our understanding of the roles of sphingolipids in β-cell biology, and but also shed light on the fundamental mechanisms underlying β-cell failure in both forms of diabetes.
Glossary
Abbreviations:
- CerS
ceramide synthase
- DRM
detergent resistant membrane
- ER
endoplasmic reticulum
- FA
fatty acid
- GalCer
galactosylceramide
- GluCer
glucosylceramide
- IAPP
islet amyloid polypeptide
- IL-1β
interleukin-1β
- LPS
lipopolysaccharide
- NOD
non-obese diabetic
- SMS
sphingomyelin synthase
- S1P
sphingosine 1-phosphate
- SM
sphingomyelin
- SPT
serine palmitoyltransferase
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- TNFα
tumor necrosis factor α
- ZDF
Zucker diabetic fatty
Footnotes
Recent work demonstrates that ceramide is present in the circulation, so its uptake might potentially contribute significantly to total ceramide in a small well-vascularized tissue bed such as pancreatic islets. To our knowledge, however, this possibility has not been addressed specifically.
Previously published online: www.landesbioscience.com/journals/islets/article/20102
References
- 1.Chen Y, Liu Y, Sullards MC, Merrill AH., Jr. An introduction to sphingolipid metabolism and analysis by new technologies. Neuromolecular Med. 2010;12:306–19. doi: 10.1007/s12017-010-8132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Futerman AH. Intracellular trafficking of sphingolipids: relationship to biosynthesis. Biochim Biophys Acta. 2006;1758:1885–92. doi: 10.1016/j.bbamem.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Hannun YA, Obeid LM. Many ceramides. J Biol Chem. 2011;286:27855–62. doi: 10.1074/jbc.R111.254359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tettamanti G. Ganglioside/glycosphingolipid turnover: new concepts. Glycoconj J. 2004;20:301–17. doi: 10.1023/B:GLYC.0000033627.02765.cc. [DOI] [PubMed] [Google Scholar]
- 5.Hanada K. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim Biophys Acta. 2003;1632:16–30. doi: 10.1016/s1388-1981(03)00059-3. [DOI] [PubMed] [Google Scholar]
- 6.Stiban J, Tidhar R, Futerman AH. Ceramide synthases: roles in cell physiology and signaling. Adv Exp Med Biol. 2010;688:60–71. doi: 10.1007/978-1-4419-6741-1_4. [DOI] [PubMed] [Google Scholar]
- 7.Grösch S, Schiffmann S, Geisslinger G. Chain length-specific properties of ceramides. Prog Lipid Res. 2012;51:50–62. doi: 10.1016/j.plipres.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Kitatani K, Idkowiak-Baldys J, Hannun YA. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2008;20:1010–8. doi: 10.1016/j.cellsig.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chalfant CE, Spiegel S. Sphingosine 1-phosphate and ceramide 1-phosphate: expanding roles in cell signaling. J Cell Sci. 2005;118:4605–12. doi: 10.1242/jcs.02637. [DOI] [PubMed] [Google Scholar]
- 10.Futerman AH, Riezman H. The ins and outs of sphingolipid synthesis. Trends Cell Biol. 2005;15:312–8. doi: 10.1016/j.tcb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–50. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 12.Hanada K, Kumagai K, Tomishige N, Kawano M. CERT and intracellular trafficking of ceramide. Biochim Biophys Acta. 2007;1771:644–53. doi: 10.1016/j.bbalip.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 13.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–24. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolter T, Sandhoff K. Lysosomal degradation of membrane lipids. FEBS Lett. 2010;584:1700–12. doi: 10.1016/j.febslet.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 15.Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol. 2008;9:125–38. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- 16.Lippincott-Schwartz J, Phair RD. Lipids and cholesterol as regulators of traffic in the endomembrane system. Annu Rev Biophys. 2010;39:559–78. doi: 10.1146/annurev.biophys.093008.131357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Major CD, Gao ZY, Wolf BA. Activation of the sphingomyelinase/ceramide signal transduction pathway in insulin-secreting β-cells: role in cytokine-induced β-cell death. Diabetes. 1999;48:1372–80. doi: 10.2337/diabetes.48.7.1372. [DOI] [PubMed] [Google Scholar]
- 18.Sjöholm A. Ceramide inhibits pancreatic β-cell insulin production and mitogenesis and mimics the actions of interleukin-1 β. FEBS Lett. 1995;367:283–6. doi: 10.1016/0014-5793(95)00470-T. [DOI] [PubMed] [Google Scholar]
- 19.Welsh N. Interleukin-1 β-induced ceramide and diacylglycerol generation may lead to activation of the c-Jun NH2-terminal kinase and the transcription factor ATF2 in the insulin-producing cell line RINm5F. J Biol Chem. 1996;271:8307–12. doi: 10.1074/jbc.271.14.8307. [DOI] [PubMed] [Google Scholar]
- 20.Kwon G, Bohrer A, Han X, Corbett JA, Ma Z, Gross RW, et al. Characterization of the sphingomyelin content of isolated pancreatic islets. Evaluation of the role of sphingomyelin hydrolysis in the action of interleukin-1 to induce islet overproduction of nitric oxide. Biochim Biophys Acta. 1996;1300:63–72. doi: 10.1016/0005-2760(95)00223-5. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Q, Jin JF, Shan XH, Liu CP, Mao XD, Xu KF, et al. Chronic activation of neutral ceramidase protects beta-cells against cytokine-induced apoptosis. Acta Pharmacol Sin. 2008;29:593–9. doi: 10.1111/j.1745-7254.2008.00781.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Q, Shan X, Miao H, Lu Y, Xu J, You N, et al. Acute activation of acid ceramidase affects cytokine-induced cytotoxicity in rat islet beta-cells. FEBS Lett. 2009;583:2136–41. doi: 10.1016/j.febslet.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 23.Veluthakal R, Jangati GR, Kowluru A. IL-1beta-induced iNOS expression, NO release and loss in metabolic cell viability are resistant to inhibitors of ceramide synthase and sphingomyelinase in INS 832/13 cells. JOP. 2006;7:593–601. [PubMed] [Google Scholar]
- 24.Laychock SG, Sessanna SM, Lin MH, Mastrandrea LD. Sphingosine 1-phosphate affects cytokine-induced apoptosis in rat pancreatic islet β-cells. Endocrinology. 2006;147:4705–12. doi: 10.1210/en.2006-0456. [DOI] [PubMed] [Google Scholar]
- 25.Mastrandrea LD, Sessanna SM, Laychock SG. Sphingosine kinase activity and sphingosine-1 phosphate production in rat pancreatic islets and INS-1 cells: response to cytokines. Diabetes. 2005;54:1429–36. doi: 10.2337/diabetes.54.5.1429. [DOI] [PubMed] [Google Scholar]
- 26.Wu D, Fujio M, Wong CH. Glycolipids as immunostimulating agents. Bioorg Med Chem. 2008;16:1073–83. doi: 10.1016/j.bmc.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sysi-Aho M, Ermolov A, Gopalacharyulu PV, Tripathi A, Seppänen-Laakso T, Maukonen J, et al. Metabolic regulation in progression to autoimmune diabetes. PLOS Comput Biol. 2011;7:e1002257. doi: 10.1371/journal.pcbi.1002257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osterbye T, Funda DP, Fundová P, Månsson JE, Tlaskalová-Hogenová H, Buschard K. A subset of human pancreatic beta cells express functional CD14 receptors: a signaling pathway for beta cell-related glycolipids, sulfatide and β-galactosylceramide. Diabetes Metab Res Rev. 2010;26:656–67. doi: 10.1002/dmrr.1134. [DOI] [PubMed] [Google Scholar]
- 29.Liu L, Wang C, He X, Shang W, Bi Y, Wang D. Long-term effect of FTY720 on lymphocyte count and islet allograft survival in mice. Microsurgery. 2007;27:300–4. doi: 10.1002/micr.20360. [DOI] [PubMed] [Google Scholar]
- 30.Yin N, Zhang N, Xu J, Shi Q, Ding Y, Bromberg JS. Targeting lymphangiogenesis after islet transplantation prolongs islet allograft survival. Transplantation. 2011;92:25–30. doi: 10.1097/TP.0b013e31821d2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jörns A, Rath KJ, Terbish T, Arndt T, Meyer Zu Vilsendorf A, Wedekind D, et al. Diabetes prevention by immunomodulatory FTY720 treatment in the LEW.1AR1-iddm rat despite immune cell activation. Endocrinology. 2010;151:3555–65. doi: 10.1210/en.2010-0202. [DOI] [PubMed] [Google Scholar]
- 32.Penaranda C, Tang Q, Ruddle NH, Bluestone JA. Prevention of diabetes by FTY720-mediated stabilization of peri-islet tertiary lymphoid organs. Diabetes. 2010;59:1461–8. doi: 10.2337/db09-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dotta F, Previti M, Lenti L, Dionisi S, Casetta B, D’Erme M, et al. GM2-1 pancreatic islet ganglioside: identification and characterization of a novel islet-specific molecule. Diabetologia. 1995;38:1117–21. doi: 10.1007/BF00402184. [DOI] [PubMed] [Google Scholar]
- 34.Kavishwar A, Medarova Z, Moore A. Unique sphingomyelin patches are targets of a beta-cell-specific antibody. J Lipid Res. 2011;52:1660–71. doi: 10.1194/jlr.M017582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paumen MB, Ishida Y, Muramatsu M, Yamamoto M, Honjo T. Inhibition of carnitine palmitoyltransferase I augments sphingolipid synthesis and palmitate-induced apoptosis. J Biol Chem. 1997;272:3324–9. doi: 10.1074/jbc.272.6.3324. [DOI] [PubMed] [Google Scholar]
- 36.Shimabukuro M, Higa M, Zhou YT, Wang MY, Newgard CB, Unger RH. Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J Biol Chem. 1998;273:32487–90. doi: 10.1074/jbc.273.49.32487. [DOI] [PubMed] [Google Scholar]
- 37.Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced β cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci USA. 1998;95:2498–502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimabukuro M, Wang MY, Zhou YT, Newgard CB, Unger RH. Protection against lipoapoptosis of β cells through leptin-dependent maintenance of Bcl-2 expression. Proc Natl Acad Sci USA. 1998;95:9558–61. doi: 10.1073/pnas.95.16.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medina-Gomez G, Gray SL, Yetukuri L, Shimomura K, Virtue S, Campbell M, et al. PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet. 2007;3:e64. doi: 10.1371/journal.pgen.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medina-Gomez G, Yetukuri L, Velagapudi V, Campbell M, Blount M, Jimenez-Linan M, et al. Adaptation and failure of pancreatic β cells in murine models with different degrees of metabolic syndrome. Dis Model Mech. 2009;2:582–92. doi: 10.1242/dmm.003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poitout V, Amyot J, Semache M, Zarrouki B, Hagman D, Fontés G. Glucolipotoxicity of the pancreatic beta cell. Biochim Biophys Acta. 2010;1801:289–98. doi: 10.1016/j.bbalip.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, Unger RH. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc Natl Acad Sci USA. 1994;91:10878–82. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou YP, Grill VE. Long-term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose fatty acid cycle. J Clin Invest. 1994;93:870–6. doi: 10.1172/JCI117042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lupi R, Dotta F, Marselli L, Del Guerra S, Masini M, Santangelo C, et al. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that β-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes. 2002;51:1437–42. doi: 10.2337/diabetes.51.5.1437. [DOI] [PubMed] [Google Scholar]
- 45.Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic β-cell turnover and function. Diabetes. 2003;52:726–33. doi: 10.2337/diabetes.52.3.726. [DOI] [PubMed] [Google Scholar]
- 46.Maedler K, Spinas GA, Dyntar D, Moritz W, Kaiser N, Donath MY. Distinct effects of saturated and monounsaturated fatty acids on β-cell turnover and function. Diabetes. 2001;50:69–76. doi: 10.2337/diabetes.50.1.69. [DOI] [PubMed] [Google Scholar]
- 47.Cunha DA, Hekerman P, Ladrière L, Bazarra-Castro A, Ortis F, Wakeham MC, et al. Initiation and execution of lipotoxic ER stress in pancreatic β-cells. J Cell Sci. 2008;121:2308–18. doi: 10.1242/jcs.026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, et al. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007;50:752–63. doi: 10.1007/s00125-006-0590-z. [DOI] [PubMed] [Google Scholar]
- 49.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 50.Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with β-cell failure and diabetes. Endocr Rev. 2008;29:317–33. doi: 10.1210/er.2007-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karaskov E, Scott C, Zhang L, Teodoro T, Ravazzola M, Volchuk A. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic β-cell apoptosis. Endocrinology. 2006;147:3398–407. doi: 10.1210/en.2005-1494. [DOI] [PubMed] [Google Scholar]
- 52.Akerfeldt MC, Howes J, Chan JY, Stevens VA, Boubenna N, McGuire HM, et al. Cytokine-induced β-cell death is independent of endoplasmic reticulum stress signaling. Diabetes. 2008;57:3034–44. doi: 10.2337/db07-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El-Assaad W, Joly E, Barbeau A, Sladek R, Buteau J, Maestre I, et al. Glucolipotoxicity alters lipid partitioning and causes mitochondrial dysfunction, cholesterol, and ceramide deposition and reactive oxygen species production in INS832/13 ss-cells. Endocrinology. 2010;151:3061–73. doi: 10.1210/en.2009-1238. [DOI] [PubMed] [Google Scholar]
- 54.González-Pertusa JA, Dubé J, Valle SR, Rosa TC, Takane KK, Mellado-Gil JM, et al. Novel proapoptotic effect of hepatocyte growth factor: synergy with palmitate to cause pancreatic β-cell apoptosis. Endocrinology. 2010;151:1487–98. doi: 10.1210/en.2009-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kelpe CL, Moore PC, Parazzoli SD, Wicksteed B, Rhodes CJ, Poitout V. Palmitate inhibition of insulin gene expression is mediated at the transcriptional level via ceramide synthesis. J Biol Chem. 2003;278:30015–21. doi: 10.1074/jbc.M302548200. [DOI] [PubMed] [Google Scholar]
- 56.Boslem E, MacIntosh G, Preston AM, Bartley C, Busch AK, Fuller M, et al. A lipidomic screen of palmitate-treated MIN6 β-cells links sphingolipid metabolites with endoplasmic reticulum (ER) stress and impaired protein trafficking. Biochem J. 2011;435:267–76. doi: 10.1042/BJ20101867. [DOI] [PubMed] [Google Scholar]
- 57.Guo J, Qian Y, Xi X, Hu X, Zhu J, Han X. Blockage of ceramide metabolism exacerbates palmitate inhibition of pro-insulin gene expression in pancreatic β-cells. Mol Cell Biochem. 2010;338:283–90. doi: 10.1007/s11010-009-0362-4. [DOI] [PubMed] [Google Scholar]
- 58.Véret J, Coant N, Berdyshev EV, Skobeleva A, Therville N, Bailbé D, et al. Ceramide synthase 4 and de novo production of ceramides with specific N-acyl chain lengths are involved in glucolipotoxicity-induced apoptosis of INS-1 β-cells. Biochem J. 2011;438:177–89. doi: 10.1042/BJ20101386. [DOI] [PubMed] [Google Scholar]
- 59.Beeharry N, Chambers JA, Green IC. Fatty acid protection from palmitic acid-induced apoptosis is lost following PI3-kinase inhibition. Apoptosis. 2004;9:599–607. doi: 10.1023/B:APPT.0000038039.82506.0c. [DOI] [PubMed] [Google Scholar]
- 60.Thörn K, Bergsten P. Fatty acid-induced oxidation and triglyceride formation is higher in insulin-producing MIN6 cells exposed to oleate compared to palmitate. J Cell Biochem. 2010;111:497–507. doi: 10.1002/jcb.22734. [DOI] [PubMed] [Google Scholar]
- 61.Preston AM, Gurisik E, Bartley C, Laybutt DR, Biden TJ. Reduced endoplasmic reticulum (ER)-to-Golgi protein trafficking contributes to ER stress in lipotoxic mouse beta cells by promoting protein overload. Diabetologia. 2009;52:2369–73. doi: 10.1007/s00125-009-1506-5. [DOI] [PubMed] [Google Scholar]
- 62.Lei X, Zhang S, Bohrer A, Ramanadham S. Calcium-independent phospholipase A2 (iPLA2 β)-mediated ceramide generation plays a key role in the cross-talk between the endoplasmic reticulum (ER) and mitochondria during ER stress-induced insulin-secreting cell apoptosis. J Biol Chem. 2008;283:34819–32. doi: 10.1074/jbc.M807409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lei X, Zhang S, Bohrer A, Bao S, Song H, Ramanadham S. The group VIA calcium-independent phospholipase A2 participates in ER stress-induced INS-1 insulinoma cell apoptosis by promoting ceramide generation via hydrolysis of sphingomyelins by neutral sphingomyelinase. Biochemistry. 2007;46:10170–85. doi: 10.1021/bi700017z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramanadham S, Hsu FF, Zhang S, Jin C, Bohrer A, Song H, et al. Apoptosis of insulin-secreting cells induced by endoplasmic reticulum stress is amplified by overexpression of group VIA calcium-independent phospholipase A2 (iPLA2 beta) and suppressed by inhibition of iPLA2 beta. Biochemistry. 2004;43:918–30. doi: 10.1021/bi035536m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fu S, Yang L, Li P, Hofmann O, Dicker L, Hide W, et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473:528–31. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291:E275–81. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 67.Sauane M, Su ZZ, Dash R, Liu X, Norris JS, Sarkar D, et al. Ceramide plays a prominent role in MDA-7/IL-24-induced cancer-specific apoptosis. J Cell Physiol. 2010;222:546–55. doi: 10.1002/jcp.21969. [DOI] [PubMed] [Google Scholar]
- 68.Swanton C, Marani M, Pardo O, Warne PH, Kelly G, Sahai E, et al. Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell. 2007;11:498–512. doi: 10.1016/j.ccr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 69.Giussani P, Maceyka M, Le Stunff H, Mikami A, Lépine S, Wang E, et al. Sphingosine-1-phosphate phosphohydrolase regulates endoplasmic reticulum-to-golgi trafficking of ceramide. Mol Cell Biol. 2006;26:5055–69. doi: 10.1128/MCB.02107-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yano M, Watanabe K, Yamamoto T, Ikeda K, Senokuchi T, Lu M, et al. Mitochondrial dysfunction and increased reactive oxygen species impair insulin secretion in sphingomyelin synthase 1-null mice. J Biol Chem. 2011;286:3992–4002. doi: 10.1074/jbc.M110.179176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Veluthakal R, Palanivel R, Zhao Y, McDonald P, Gruber S, Kowluru A. Ceramide induces mitochondrial abnormalities in insulin-secreting INS-1 cells: potential mechanisms underlying ceramide-mediated metabolic dysfunction of the beta cell. Apoptosis. 2005;10:841–50. doi: 10.1007/s10495-005-0431-4. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Y, Ranta F, Tang C, Shumilina E, Mahmud H, Föller M, et al. Sphingomyelinase dependent apoptosis following treatment of pancreatic beta-cells with amyloid peptides Abeta(1-42) or IAPP. Apoptosis. 2009;14:878–89. doi: 10.1007/s10495-009-0364-4. [DOI] [PubMed] [Google Scholar]
- 73.Lang F, Ullrich S, Gulbins E. Ceramide formation as a target in beta-cell survival and function. Expert Opin Ther Targets. 2011;15:1061–71. doi: 10.1517/14728222.2011.588209. [DOI] [PubMed] [Google Scholar]
- 74.Chavez JA, Knotts TA, Wang LP, Li G, Dobrowsky RT, Florant GL, et al. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem. 2003;278:10297–303. doi: 10.1074/jbc.M212307200. [DOI] [PubMed] [Google Scholar]
- 75.Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem. 1999;274:24202–10. doi: 10.1074/jbc.274.34.24202. [DOI] [PubMed] [Google Scholar]
- 76.Martinez SC, Tanabe K, Cras-Méneur C, Abumrad NA, Bernal-Mizrachi E, Permutt MA. Inhibition of Foxo1 protects pancreatic islet β-cells against fatty acid and endoplasmic reticulum stress-induced apoptosis. Diabetes. 2008;57:846–59. doi: 10.2337/db07-0595. [DOI] [PubMed] [Google Scholar]
- 77.Wrede CE, Dickson LM, Lingohr MK, Briaud I, Rhodes CJ. Protein kinase B/Akt prevents fatty acid-induced apoptosis in pancreatic β-cells (INS-1) J Biol Chem. 2002;277:49676–84. doi: 10.1074/jbc.M208756200. [DOI] [PubMed] [Google Scholar]
- 78.Higa M, Shimabukuro M, Shimajiri Y, Takasu N, Shinjyo T, Inaba T. Protein kinase B/Akt signalling is required for palmitate-induced β-cell lipotoxicity. Diabetes Obes Metab. 2006;8:228–33. doi: 10.1111/j.1463-1326.2005.00488.x. [DOI] [PubMed] [Google Scholar]
- 79.Watson ML, Macrae K, Marley AE, Hundal HS. Chronic effects of palmitate overload on nutrient-induced insulin secretion and autocrine signalling in pancreatic MIN6 beta cells. PLoS ONE. 2011;6:e25975. doi: 10.1371/journal.pone.0025975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gremlich S, Bonny C, Waeber G, Thorens B. Fatty acids decrease IDX-1 expression in rat pancreatic islets and reduce GLUT2, glucokinase, insulin, and somatostatin levels. J Biol Chem. 1997;272:30261–9. doi: 10.1074/jbc.272.48.30261. [DOI] [PubMed] [Google Scholar]
- 81.Jacqueminet S, Briaud I, Rouault C, Reach G, Poitout V. Inhibition of insulin gene expression by long-term exposure of pancreatic β cells to palmitate is dependent on the presence of a stimulatory glucose concentration. Metabolism. 2000;49:532–6. doi: 10.1016/S0026-0495(00)80021-9. [DOI] [PubMed] [Google Scholar]
- 82.Plaisance V, Perret V, Favre D, Abderrahmani A, Yang JY, Widmann C, et al. Role of the transcriptional factor C/EBPbeta in free fatty acid-elicited β-cell failure. Mol Cell Endocrinol. 2009;305:47–55. doi: 10.1016/j.mce.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 83.Busch AK, Cordery D, Denyer GS, Biden TJ. Expression profiling of palmitate- and oleate-regulated genes provides novel insights into the effects of chronic lipid exposure on pancreatic β-cell function. Diabetes. 2002;51:977–87. doi: 10.2337/diabetes.51.4.977. [DOI] [PubMed] [Google Scholar]
- 84.Guo J, Zhu JX, Deng XH, Hu XH, Zhao J, Sun YJ, et al. Palmitate-induced inhibition of insulin gene expression in rat islet β-cells involves the ceramide transport protein. Cell Physiol Biochem. 2010;26:717–28. doi: 10.1159/000322339. [DOI] [PubMed] [Google Scholar]
- 85.Lu M, Seufert J, Habener JF. Pancreatic β-cell-specific repression of insulin gene transcription by CCAAT/enhancer-binding protein β. Inhibitory interactions with basic helix-loop-helix transcription factor E47. J Biol Chem. 1997;272:28349–59. doi: 10.1074/jbc.272.45.28349. [DOI] [PubMed] [Google Scholar]
- 86.Seufert J, Weir GC, Habener JF. Differential expression of the insulin gene transcriptional repressor CCAAT/enhancer-binding protein β and transactivator islet duodenum homeobox-1 in rat pancreatic β cells during the development of diabetes mellitus. J Clin Invest. 1998;101:2528–39. doi: 10.1172/JCI2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fontés G, Semache M, Hagman DK, Tremblay C, Shah R, Rhodes CJ, et al. Involvement of Per-Arnt-Sim Kinase and extracellular-regulated kinases-1/2 in palmitate inhibition of insulin gene expression in pancreatic β-cells. Diabetes. 2009;58:2048–58. doi: 10.2337/db08-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Busch AK, Gurisik E, Cordery DV, Sudlow M, Denyer GS, Laybutt DR, et al. Increased fatty acid desaturation and enhanced expression of stearoyl coenzyme A desaturase protects pancreatic β-cells from lipoapoptosis. Diabetes. 2005;54:2917–24. doi: 10.2337/diabetes.54.10.2917. [DOI] [PubMed] [Google Scholar]
- 89.Subathra M, Qureshi A, Luberto C. Sphingomyelin synthases regulate protein trafficking and secretion. PLoS ONE. 2011;6:e23644. doi: 10.1371/journal.pone.0023644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xia F, Gao X, Kwan E, Lam PP, Chan L, Sy K, et al. Disruption of pancreatic β-cell lipid rafts modifies Kv2.1 channel gating and insulin exocytosis. J Biol Chem. 2004;279:24685–91. doi: 10.1074/jbc.M314314200. [DOI] [PubMed] [Google Scholar]
- 91.Stanford JC, Morris AJ, Sunkara M, Popa GJ, Larson KL, Ozcan S. Sphingosine-1 phosphate (S1P) regulates glucose-stimulated insulin secretion in pancreatic beta cells. J Biol Chem. 2012 doi: 10.1074/jbc.M111.268185. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tagami S, Inokuchi Ji J, Kabayama K, Yoshimura H, Kitamura F, Uemura S, et al. Ganglioside GM3 participates in the pathological conditions of insulin resistance. J Biol Chem. 2002;277:3085–92. doi: 10.1074/jbc.M103705200. [DOI] [PubMed] [Google Scholar]
- 93.Aerts JM, Ottenhoff R, Powlson AS, Grefhorst A, van Eijk M, Dubbelhuis PF, et al. Pharmacological inhibition of glucosylceramide synthase enhances insulin sensitivity. Diabetes. 2007;56:1341–9. doi: 10.2337/db06-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao H, Przybylska M, Wu IH, Zhang J, Siegel C, Komarnitsky S, et al. Inhibiting glycosphingolipid synthesis improves glycemic control and insulin sensitivity in animal models of type 2 diabetes. Diabetes. 2007;56:1210–8. doi: 10.2337/db06-0719. [DOI] [PubMed] [Google Scholar]
- 95.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 96.Weksler-Zangen S, Yagil C, Zangen DH, Ornoy A, Jacob HJ, Yagil Y. The newly inbred cohen diabetic rat: a nonobese normolipidemic genetic model of diet-induced type 2 diabetes expressing sex differences. Diabetes. 2001;50:2521–9. doi: 10.2337/diabetes.50.11.2521. [DOI] [PubMed] [Google Scholar]
- 97.Zigmond E, Zangen SW, Pappo O, Sklair-Levy M, Lalazar G, Zolotaryova L, et al. Beta-glycosphingolipids improve glucose intolerance and hepatic steatosis of the Cohen diabetic rat. Am J Physiol Endocrinol Metab. 2009;296:E72–8. doi: 10.1152/ajpendo.90634.2008. [DOI] [PubMed] [Google Scholar]
- 98.Buschard K, Blomqvist M, Osterbye T, Fredman P. Involvement of sulfatide in beta cells and type 1 and type 2 diabetes. Diabetologia. 2005;48:1957–62. doi: 10.1007/s00125-005-1926-9. [DOI] [PubMed] [Google Scholar]
- 99.Blomqvist M, Osterbye T, Månsson JE, Horn T, Buschard K, Fredman P. Selective lack of the C16:0 fatty acid isoform of sulfatide in pancreas of type II diabetic animal models. APMIS. 2003;111:867–77. doi: 10.1034/j.1600-0463.2003.1110905.x. [DOI] [PubMed] [Google Scholar]
- 100.Fredman P, Mânsson JE, Rynmark BM, Josefsen K, Ekblond A, Halldner L, et al. The glycosphingolipid sulfatide in the islets of Langerhans in rat pancreas is processed through recycling: possible involvement in insulin trafficking. Glycobiology. 2000;10:39–50. doi: 10.1093/glycob/10.1.39. [DOI] [PubMed] [Google Scholar]
- 101.Osterbye T, Jørgensen KH, Fredman P, Tranum-Jensen J, Kaas A, Brange J, et al. Sulfatide promotes the folding of proinsulin, preserves insulin crystals, and mediates its monomerization. Glycobiology. 2001;11:473–9. doi: 10.1093/glycob/11.6.473. [DOI] [PubMed] [Google Scholar]
- 102.Buschard K, Høy M, Bokvist K, Olsen HL, Madsbad S, Fredman P, et al. Sulfatide controls insulin secretion by modulation of ATP-sensitive K(+)-channel activity and Ca(2+)-dependent exocytosis in rat pancreatic beta-cells. Diabetes. 2002;51:2514–21. doi: 10.2337/diabetes.51.8.2514. [DOI] [PubMed] [Google Scholar]