Abstract
Previous studies have shown that animal cells contain isoprenoid-modified proteins and that one of these proteins, lamin B, contains a thioether-linked farnesyl group that is attached to cysteine. In the present study, a novel isoprenoid-modification was identified by labeling HeLa cells with [3H]mevalonic acid and analyzing proteolytic digests of the total cell protein. Radioactive fragments were purified from these digests and treated with Raney nickel. The released, labeled material was analyzed by gas-liquid chromatography (GC) and mass spectrometry (MS). This approach revealed that an all-trans geranylgeranyl group was a major isoprenoid modification.
Studies of swiss 3T3 cells that were labeled with [3H]mevalonic acid provided the first evidence that animal cells contain proteins that are posttranslationally modified by isoprenoid groups. Several radioactive proteins were observed, and proteolytic hydrolysates of the total cell protein were shown to contain labeled fragments that had apparent molecular weights of 1000 and 500 daltons (1). This result suggested that different proteins in animal cells may be modified by different isoprenoid groups. Following these early observations, isoprenoid-modified proteins were detected in many other cell types (2). Furthermore, proteolytic fragments of HeLa cell proteins were prepared that resembled those derived from Swiss 3T3 cell proteins; one of the labeled proteins from HeLa cells was identified as lamin B (3) and was shown to contain a cysteinyl thioether-linked famesyl group (4). Proteolytic digests of lamin B only yielded radioactive fragments that corresponded to the 500-dalton fragments of HeLa cell total proteins. Therefore, the identity of the isoprenoid modification in the 1000-dalton fragments was left unresolved.
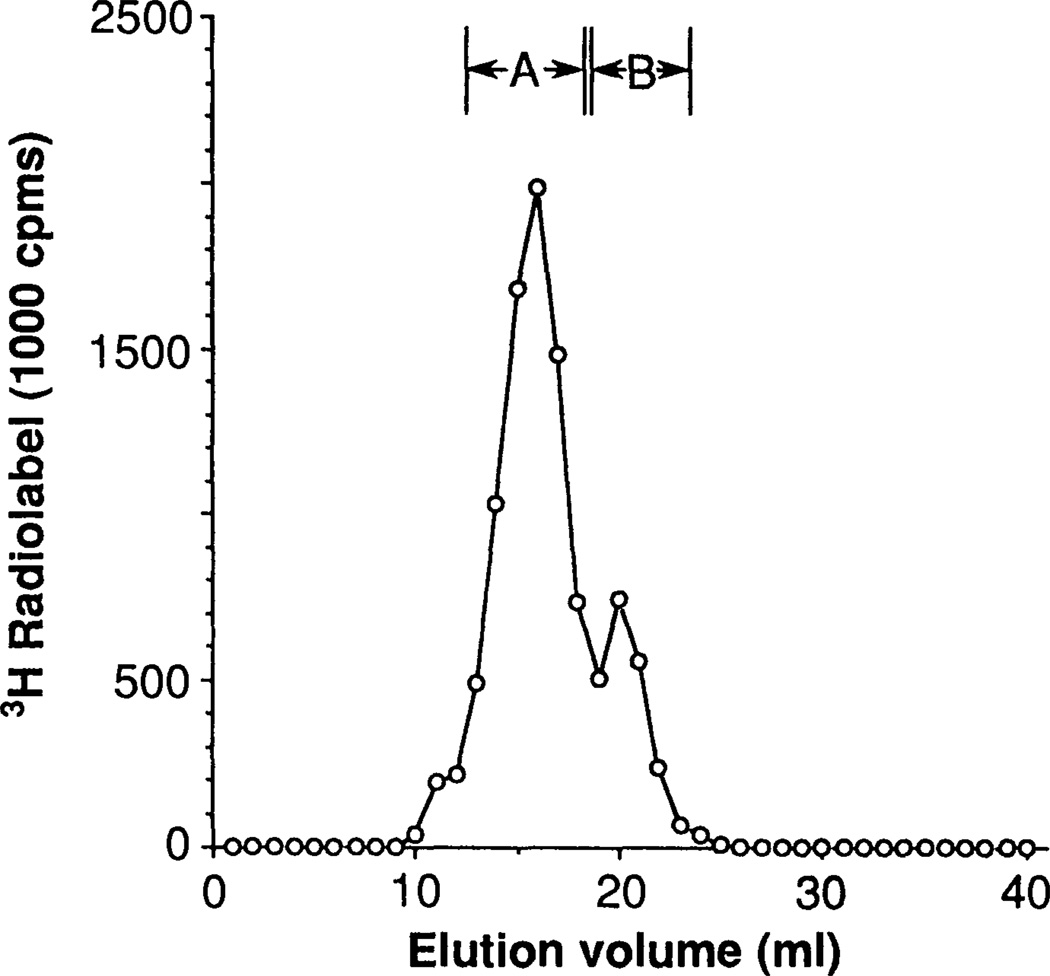
To address this question we labeled HeLa cells with [3H]mevalonic acid, extensively digested the total cell protein with proteases, and subfractioned the digests by anion exchange chromatography and gel filtration on Sephadex LH-20. Two peaks of radioactive material, termed peaks A and B, were obtained, and their contents were pooled into separate fractions (Fig. 1). The radioactive material was initially only slightly soluble in pentane, but became pentane-extractable following treatment of the fractions with Raney nickel (Table 1). In contrast, little or no additional radioactive material became pentane-soluble following treatment with methanolic KOH. This suggested that the radioactive material was linked to the protein fragments through thioether bonds.
Fig. 1.
Size-exclusion chromatography of proteolytic hydrolysates of HeLa cell total proteins on Sephadex LH-20. Cells were labeled for 36 hours with [5-3H]mevalonic acid in the presence of 30 µM mevinolin, harvested, washed with phosphate-buffered saline, and extracted with lipid solvents. Cell pellets were then successively digested with proteases, and labeled digestion products were concentrated and purified by step elution from DEAE Sephacel. The eluted material was then passed through Sephadex LH-20 in 20% formic acid in ethanol at a flow rate of 0.25 nl/mmin, and 1.0-ml fractions were collected (4). Peak A, which corresponded to 1000-dalton material, contained 74% of the recovered label. Peak B, which corresponded to 500-dalton material, contained 22% of the recovered label. Recovery of the total applied label was 78%. Comparable chromatograms were obtained in six separate experiments.
Table 1.
Release of 3H label from proteolytic fragments of HeLa cell proteins. Material corresponding to peak A in Fig. 1 was solubilized in 200 µl of 8M guanidinium chloride, 50 mg of Raney nickel and 1.0 ml of pentane were added, and the samples were incubated for 15 hours at 10°C. Values are percentages of total peak A radioactivity. Control samples were treated similarly, but in the absence of Raney nickel. Additional peak A material was treated for 1 hour with 0.1M methanolic KOH at 23°C and then acidified and extracted with pentane (4). Values in parentheses represent numbers of experiments done.
| Sample treatment |
Relative radioactivity (percent) |
|---|---|
| + Raney nickel (2) | 88 ± 2 |
| − Raney nickel (2) | 14 ± 4 |
| + Methanolic KOH (3) | 11 ± 0.5 |
| − Methanolic KOH (3) | 5 ± 0.1 |
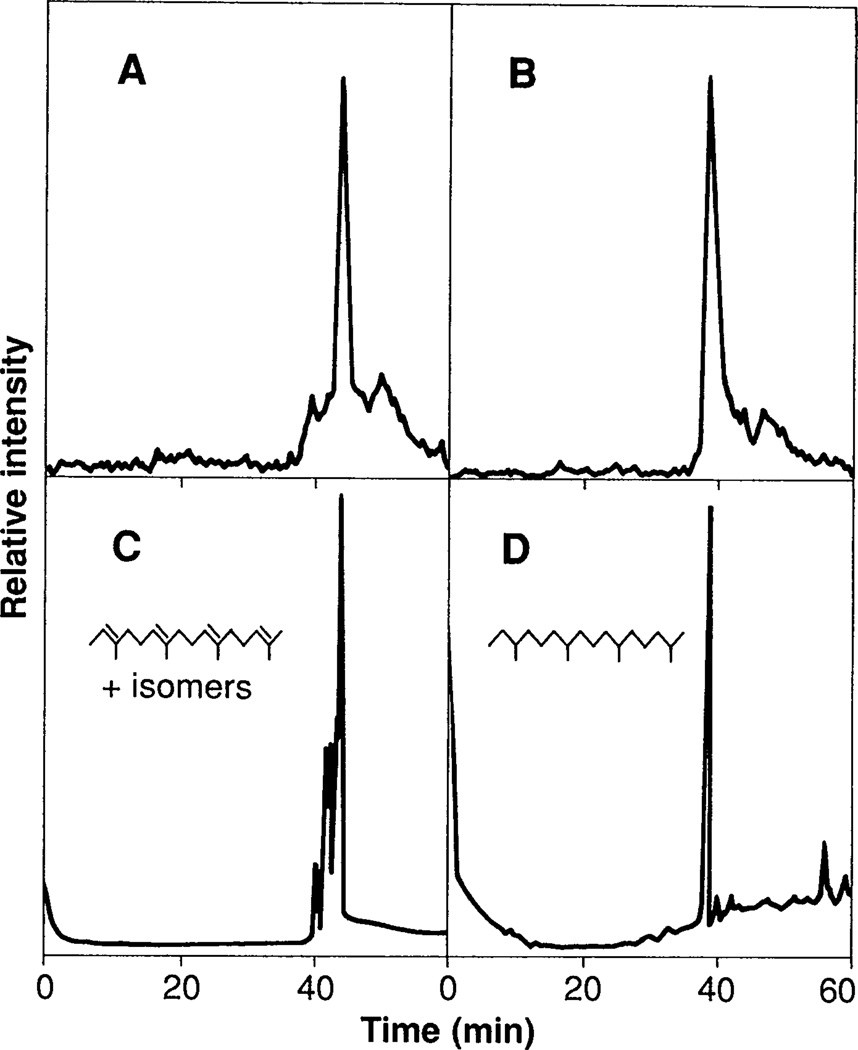
The radioactive material that was released into pentane was analyzed by radiometric GC. A major peak of radioactive material derived from peak A eluted with a retention time of 44.0 min, which was identical to that of all-trans 2,6,10,14-tetramethyl-2,6,10,14-hexadecatetraene (compare A and C in Fig. 2). The major radioactive peak derived from peak B eluted significantly earlier, in the position of all-trans 2,6,10-trimethyl-2,6,10-dodecatriene (4). Parallel samples of the peak A–derived material that had been hydrogenated over platinum yielded a single peak of radioactivity that eluted in the position of phytane (2,6,10,14-tetramethylhexadecane) (compare B and D in Fig. 2).
Fig. 2.
Gas chromatographic (GC) analyses of labeled, Raney nickel-released material from proteolytic fragments of HeLa cell proteins. Tritium-labeled material corresponding to peak A (Fig. 1) was treated with Raney nickel and extracted with pentane as described in Table 1. (A) Pentane-soluble label was then concentrated, and one-half of it was analyzed. (B) The remainder was hydrogenated over platinum and analyzed. (C) A mixture of all eight possible cis-trans isomers of 2,6,10, 14-tetramethyl-2,6,10,14-hexadecatetraene, which yielded eight distinct peaks when analyzed by capillary GC, was cochromatographed with the all-trans isomer (major peak). (D) Authentic phytane was also analyzed. Analyses were performed on a 15-m DB-5 megabore column with a temperature program that started at 80°C for 2 min, then increased 2°C per minute up to 220°C. Peak mass was detected by flame ionization, and radio-activity was detected with a Flow-One Beta Detector (Radiomatic Instrument Co., Tampa, Florida) (4). In this system the all-trans and nearest cis-containing tetraene isomers were separated sufficiently (0.4 min) to ensure unambiguous identification of the protein-derived material as the all-trans tetraene. The all-trans tetraene was synthesized from all-trans geranylgeraniol by conversion to the bromide followed by treatment with superhydride (9). The isomeric mixture of tetraenes was synthesized by a Wittig reaction between commercially available famesyl acetone (mixture of all possible cis-trans isomers) and the ylide derived from (ethyl)triphenylphosphonium bromide (4). The structures of all of the synthetic compounds were verified by high-resolution proton nuclear magnetic resonance.
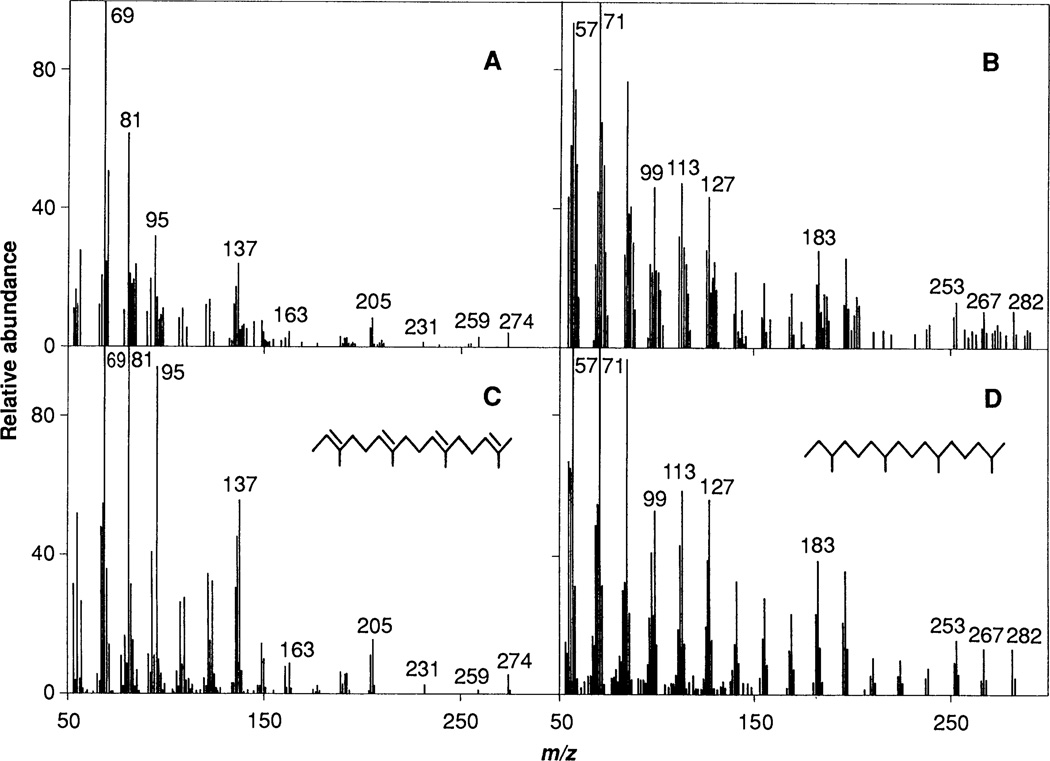
The structure of the isoprenoid compound derived from peak A was established by combined GC-MS analysis. A parent ion peak with a mass-to-charge (m/z) value of 274 and a MS fragmentation pattern identical to that of all-trans 2,6,10,14-tetramethyl-2,6,10,14-hexadecatriene were observed (compare A and C in Fig. 3). Corresponding material from peak A that had been hydrogenated over platinum showed a parent ion peak with an m/z value of 282 (high-resolution MS analysis gave 282.3305; calculated for C20H42, 282.3325). The MS fragmentation pattern is identical to that of authentic phytane (compare B and D in Fig. 3). Raney nickel cleavage of the carbon-sulfur bond is predicted to generate 2,6,10,14-tetramethyl-2,6,10,14-hexadeca-tetraene (3), and further treatment with hydrogen and platinum would give phytane (C20H42) as shown below:
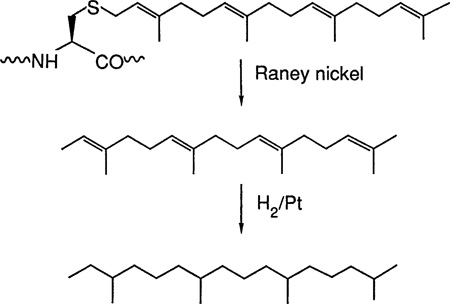
Fig. 3.
Electron ionization spectra of Raney nickel—released material from proteolytic fragments of HeLa cell proteins. Samples were prepared from 3 liters of cultured cells as described in Figs. 1 and 2, then analyzed by GC-MS on a 30-m DB-5 column, before or after hydrogenation (3). (A) Nonhydrogenated, peak A–derived material corresponding to the major peak of radioactivity in Fig. 2A. (B) Hydrogenated, peak A–derived material corresponding to the peak of radioactivity in Fig. 2B. (C) All-trans 2,6,10,14-tetramethyl-2,6,10,14-hexadecatriene. (D) Phytane. All spectra have been background corrected, and the spectra in (A) and (C) have been enhanced. Minor differences between spectra of protein-derived samples and standards are likely due to the much smaller amounts of naturally derived materials analyzed.
These results unequivocally demonstrate that the proteolytic fragments in peak A contained a covalently attached all-trans geranylgeranyl group and that geranylgeranyl groups can be attached to proteins of natural origin. The mode of attachment of this group to proteins remains to be established, but its release by treatment with Raney nickel strongly suggests that it is attached to cysteine through a thioether bond. Although the proteins that are modified with a geranylgeranyl group and the prenyltransferase that attaches the isoprenoid group to proteins remain to be identified, geranylgeranyl pyrophosphate is probably a substrate in the reaction. An enzyme that synthesizes this isoprenoid polyphosphate from isopentenyl pyrophosphate and farnesyl pyrophosphate has been purified from pig liver (5).
It will be important to identify the geranylgeranyl-modified proteins and study their structures in view of current information regarding farnesylated proteins. The cDNA-derived amino acid sequences of those farnesylated proteins that have been identified contain a conserved CAAX motif at the COOH-terminus (where C is cysteine and A and X refer to aliphatic and any amino acid, respectively). Furthermore, prenylation occurs on the cysteine in this motif (4, 6), and ras proteins may be similarly modified (6–8).
Several of the farnesylated proteins also have been shown to be further modified. The last three amino acids (AAX) are removed from the COOH-terminus, and the newly exposed cysteine α-carboxyl group is converted to the methyl ester (7, 8). The CAAX motif may be the critical recognition element in this modification pathway because engineering of this sequence onto the COOH-terminus of a heterologous, cytosolic protein is sufficient to direct prenylation of the protein (7). In the cases so far examined, the isoprenoid group seems to be required for membrane localization and biological activity (6–8).
Similar possibilities warrant consideration in the case of geranylgeranyl-modified proteins. Therefore, a major task is to determine whether these proteins contain a directing structural motif such as the CAAX sequence, whether further posttranslational modifications occur, and whether these modifications play an important biological role.
Contributor Information
Christopher C. Farnsworth, Howard Hughes Medical Institute Laboratory, University of Washington, Seattle, WA 98195
Michael H. Gelb, Departments of Biochemistry and Chemistry, University of Washington, Seattle, WA 98195
John A. Glomset, Howard Hughes Medical Institute Laboratory, Department of Medicinal Chemistry, University of Washington, Seattle, WA 98195
REFERENCES AND NOTES
- 1.Schmidt RA, Schneider CJ, Glomset JA. J. Biol. Chem. 1984;259:10175. [PubMed] [Google Scholar]
- 2.Sinensky M, Logel J. Proc. Natl. Acad. Sci. U.S.A. 1985;82:3257. doi: 10.1073/pnas.82.10.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]; Maltese WA, Sheridan KM. J. Cell Physiol. 1987;133:471. doi: 10.1002/jcp.1041330307. [DOI] [PubMed] [Google Scholar]; J. Biol. Chem. 1988;263:10104. [PubMed] [Google Scholar]; Azrolan Sopp-Lorenzino N, Coleman PS. FEBS Lett. 1989;245:110. doi: 10.1016/0014-5793(89)80202-9. [DOI] [PubMed] [Google Scholar]; Bruenger E, Rifling HC. Biochem. Biophys. Res. Commun. 1986;139:209. doi: 10.1016/s0006-291x(86)80100-0. [DOI] [PubMed] [Google Scholar]; Beck LA, et al. J. Cell Biol. 1988;107:1307. doi: 10.1083/jcb.107.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolda SL, Glomset JA. J. Biol. Chem. 1988;263:5997. [PubMed] [Google Scholar]
- 4.Farnsworth CC, Wolda SL, Gelb MH, Glomset JA. ibid. 1989;264:20422. [PMC free article] [PubMed] [Google Scholar]
- 5.Sagami H, et al. Biochem. Int. 1981;3:669. [Google Scholar]
- 6.Clark S, Vogel JP, Deschenes RJ, Stock J. Proc. Natl. Acad. Sci. U.S.A. 1988;85:4643. doi: 10.1073/pnas.85.13.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fujino M, et al. Naturwissenschaften. 1980;67:406. [Google Scholar]; Kamiya Y, et al. Agric. Biol. Chem. 1979;43:363. [Google Scholar]; Anderegg R, Betz R, Carr SA, Crabb JW, Duntze W. J. Biol. Chem. 1988;263:18236. [PubMed] [Google Scholar]
- 7.Hancock JF, et al. Cell. 1989;57:1167. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- 8.Schafer WR, et al. Science. 1989;245:379. doi: 10.1126/science.2569235. [DOI] [PubMed] [Google Scholar]
- 9.Corey EJ, Kim CU, Takeda M. Tetrahedron Lett. 1972;42:4339. [Google Scholar]; Brown HC, Krisnamurthy S. J. Am. Chem. Soc. 1973;95:1669. [Google Scholar]
- 10.We thank W. Howald for assistance with mass spectrometry, H.-K. Lin for synthesis of alkene standards, and R. Coates for a gift of all-trans geranylgeraniol.