Background: Platelet collagen receptor GPVI likely functions as a dimer rather than a monomer.
Results: Preformed GPVI dimers, but not monomers, in resting platelets bind specific collagen sequences and are essential for platelet adhesion and activation.
Conclusion: Constitutive GPVI dimers on resting platelets support platelet adhesion to collagen and activation.
Significance: Resting platelets bind collagen through GPVI dimers, allowing immediate initiation of thrombus formation.
Keywords: Adhesion, Blood, Collagen, Platelets, Receptors, GPVI, GPVI Dimer, Activation
Abstract
The platelet collagen receptor glycoprotein VI (GPVI) has been suggested to function as a dimer, with increased affinity for collagen. Dissociation constants (Kd) obtained by measuring recombinant GPVI binding to collagenous substrates showed that GPVI dimers bind with high affinity to tandem GPO (Gly-Pro-Hyp) sequences in collagen, whereas the markedly lower affinity of the monomer for all substrates implies that it is not the collagen-binding form of GPVI. Dimer binding required a high density of immobilized triple-helical (GPO)10-containing peptide, suggesting that the dimer binds multiple, discrete peptide helices. Differential inhibition of dimer binding by dimer-specific antibodies, m-Fab-F and 204-11 Fab, suggests that m-Fab-F binds at the collagen-binding site of the dimer, and 204-11 Fab binds to a discrete site. Flow cytometric quantitation indicated that GPVI dimers account for ∼29% of total GPVI in resting platelets, whereas activation by either collagen-related peptide or thrombin increases the number of dimers to ∼39 and ∼44%, respectively. m-Fab-F inhibits both GPVI-dependent static platelet adhesion to collagen and thrombus formation on collagen under low and high shear, indicating that pre-existing dimeric GPVI is required for the initial interaction with collagen because affinity of the monomer is too low to support binding and that interaction through the dimer is essential for platelet activation. These GPVI dimers in resting circulating platelets will enable them to bind injury-exposed subendothelial collagen to initiate platelet activation. The GPVI-specific agonist collagen-related peptide or thrombin further increases the number of dimers, thereby providing a feedback mechanism for reinforcing binding to collagen and platelet activation.
Introduction
GPVI2 was identified as a platelet collagen receptor by analyzing platelets from GPVI-deficient patients (1, 2). GPVI, complexed with the Fc-receptor γ-chain (FcRγ) in the platelet membrane, interacts with subendothelial collagen exposed upon vessel injury to initiate platelet activation. Collagen binding to GPVI induces tyrosine phosphorylation of the immunoreceptor tyrosine-based activation motifs of FcRγ and binding of tyrosine kinase Syk, which generates formation of a signalosome, including LAT, SLP-76, and phospholipase Cγ2 (PLCγ2) (3), leading to a series of downstream pathways that culminate in platelet activation.
GPVI, an immunoglobulin receptor family protein, contains two extracellular immunoglobulin-like domains, D1 and D2 (4). A dimeric structure formed by fusing D1D2 to the IgG Fc domain exhibited high affinity to collagen, although monomeric D1D2 itself showed little collagen affinity (5, 6). Thus, the GPVI dimer was suggested to have a unique conformation that gives rise to high affinity binding to collagen. The crystal structure of the GPVI D1D2 domain (7) indicated that two D1D2 domains can form a dimer with a dimerization interface located on the D2 domain, providing direct evidence for dimer formation, albeit in a nonphysiological setting.
Jung et al. (8) developed antibody Fabs that selectively recognize the GPVI dimer and inhibit GPVI dimer binding to collagen fibers and platelet activation, suggesting a critical role for dimerization in GPVI regulation. Demonstration of direct binding of one of the Fabs, m-Fab-F, to intact platelets indicated that dimers are present in resting platelets, agreeing with previous cross-linking studies (9).
Central to understanding the function of GPVI is determining the following: which collagen sequences are recognized by the dimer; how dimer formation facilitates platelet binding to collagen; whether resting platelets contain dimers in numbers sufficient to allow them to engage collagen; and what effect platelet activation has on dimer number. This study analyzed the effects of two dimer-specific Fabs, m-Fab-F and 204-11 Fab, on binding of recombinant GPVI dimer and monomer to collagen and collagen peptides. Dimeric GPVI accounts for ∼29% of the GPVI on the surface of resting platelets, with the level significantly increasing upon platelet activation. Our present studies demonstrate the physiological role of GPVI dimers by showing that the dimeric form of GPVI is required for both platelet adhesion to collagen and subsequent activation.
EXPERIMENTAL PROCEDURES
Antibodies
Monoclonal mouse anti-GPVI antibodies against the extracellular domain of GPVI, 204-11 (10), 1G5 (11), GPVI-dimer-specific human antibody Fab m-Fab-F (8), and anti-GPVI scFv antibody 1C3 (12) were described before. The human anti-GPVI scFv antibody 10B12 was selected from the Cambridge Antibody Technology (now MedImmune) phage display libraries as described previously (13). 204-11 was cloned and 204-11 Fab was prepared as a recombinant protein by Kaketsuken, Kumamoto, Japan. FITC-conjugated F(ab′)2 goat anti-mouse IgG F(ab′)2 antibody (FITC anti-mouse F(ab′)2) was from Jackson ImmunoResearch. 1G5 Fab was prepared with a Fab preparation kit (Pierce). FITC was conjugated to m-Fab-F by the EasyLink (FITC) antibody conjugation kit (Abcam).
Recombinant GPVI Dimer and Monomer
Recombinant extracellular domain of GPVI (GPVIex, comprising D1D2 (amino acids 1–214) and containing both the N- and O-glycosylation sites; 42 kDa) and the same domain fused with the Fc domain of human IgG (GPVI-Fc2, 150 kDa) was prepared as before (5). Convulxin was biotin-labeled using sulfo-NHS-LC-biotin (Pierce).
Collagen and Collagen Peptides
Collagen Toolkit III peptides and model collagen peptides were synthesized and purified as described previously (14). Pepsin-treated bovine type I and type III collagens were from Koken (Tokyo, Japan).
Platelet Preparation
This study was approved by the Cambridge Human Biology Research Ethics Committee; informed consent was obtained from donors as applicable, according to the Declaration of Helsinki. Blood was drawn from the antecubital vein of healthy volunteers into 0.1 volume of 3.8% sodium citrate. Washed platelets were prepared as described before (15).
ELISA to Determine GPVI Dimer and Monomer Binding to Anti-GPVI Antibodies or Collagen Substrates
Two types of enzyme-linked immunosorbent assays (ELISA) were performed as follows: binding of GPVI-Fc2 (dimer) or GPVIex (monomer) to immobilized antibodies or collagenous substrates and binding of antibody to immobilized dimer or monomer. Substrate was immobilized by reacting the wells of a Nunc Maxisorp 96-well plate with 50 μl of dimer, monomer, antibody, collagen, or collagen peptide (10 μg/ml) in modified HEPES/Tyrodes buffer (HT: 136 mm NaCl, 2.7 mm KCl, 0.42 mm NaH2PO4, 5.5 mm glucose, and 5 mm HEPES, pH 7.4) overnight at 4 °C. The substrate-bound wells were blocked with 2% BSA/HT (1 h). After three washes with HT, ligand (dimer or monomer for antibody- or collagen-peptide-coated wells and antibody for dimer- or monomer-coated wells) was added to the wells and incubated for 1 h. Wells were then washed three times with HT, and one of the following reagents was added to the wells: horseradish peroxidase (HRP)-conjugated goat anti-human IgG Fc (Jackson ImmunoResearch); biotinylated convulxin followed by HRP-extravidin (Sigma); or HRP-conjugated F(ab′)2 goat anti-mouse IgG F(ab′)2 antibody (HRP-anti-mouse F(ab′)2, Jackson ImmunoResearch). After four washes with HT, 0.005% Tween 20, bound ligand was visualized by adding 50 μl of 1-Step Turbo TMB-ELISA (Pierce) to each well, and absorbance at 450 nm was determined after 15–30 min. In some experiments, Nunc Amino ImmobilizerTM plates were used, and the same ELISA procedures were followed.
To determine antibody inhibition of dimer binding, a fixed concentration of GPVI dimer (20 μg/ml, 133 nm) was preincubated with different antibody concentrations, and then GPVI binding to immobilized collagen- or collagen-peptide was determined by ELISA.
Flow Cytometry
To measure GPVI dimer on intact platelets, 10 μl of platelet solution, either 5-fold diluted whole blood or washed platelets (5 × 107 cells/ml), was mixed with 10 μl of HT solution containing FITC-m-Fab-F (200 μg/ml) or 204-11 Fab (40 μg/ml) and incubated for 10 min. For 204-11 Fab, FITC anti-mouse F(ab′)2 antibody (50 μg/ml, final) was added as the secondary antibody and incubated for 10 min. In individual wells of an Eppendorf 96-well (500 μl) deep well plate, each reaction mixture was diluted with 0.15 ml of diluent (Biocytex), and then antibody binding was measured by an Accuri C6 flow cytometer (BD Biosciences). Platelet binding to appropriate controls, unlabeled or FITC-labeled human Fab (Jackson ImmunoResearch) when m-Fab-F was the primary antibody or mouse Fab (Jackson ImmunoResearch) when 204-11 Fab or 1G5 Fab was the primary antibody, was determined. The appropriate isotype control mouse IgG was used for 204 IgG and 1G5 IgG binding. Total GPVI was measured in the same manner except 204-11, 1G5, or 1G5 Fab was used instead of anti-GPVI dimer antibody. The concentration of each primary antibody was optimized in preliminary experiments by determining the antibody concentration versus amount of measured single chain GPVI; a concentration in the plateau region (maximal number of single chain GPVI) was chosen for each antibody for use in the experiments. Similarly the concentration of the secondary antibody was chosen in this manner.
To measure GPVI in activated platelets, CRP (5 μg/ml, final) or bovine thrombin (0.2 unit/ml, final; Sigma) was added to 100 μl of 5-fold diluted whole blood containing 5 mm EDTA; after 1 min, 10 μl of the reaction mixture was mixed with 10 μl of the primary antibody and processed as described above for the resting platelets. Agonist concentration dependence of dimer increase was measured for both CRP and thrombin using washed platelets (1 × 108 cells/ml) and FITC-m-Fab-F (100 μg/ml).
The time course of dimer formation in CRP- or thrombin-induced platelets was measured by adding CRP (5 μg/ml, final) or thrombin (0.2 unit/ml, final) to 1 ml of washed platelets (2.5 × 108 cells/ml). At various times, 100 μl was taken out of the reaction mixture and immediately added to 100 μl of 1% paraformaldehyde in HT. After 30 min, 0.9 ml of HT, 0.2% BSA was added to each mixture. The mixture was centrifuged (7000 rpm in a microcentrifuge, 2 min), and the obtained pellet was suspended in 50 μl of HT; 10 μl was processed for flow cytometry (FITC-m-Fab-F), as described above.
GPVI Quantitation
In resting and agonist-activated platelets, GPVI dimer was quantitated with the dimer-specific antibody 204-11 Fab and total single chain GPVI, with 1G5, 1G5 Fab, and 204-11 IgG, using FITC anti-mouse F(ab′)2 and the Platelet Gp Screen (Biocytex, Marseille, France), which includes beads conjugated with known amounts of mouse IgG to make a standard curve, from which the number of GPVI can be determined.
Effect of Dimer-specific Antibodies on Static Platelet Adhesion to Immobilized Fibrous Collagen
Fibrous type III collagen was prepared as described previously (16). The fibrous collagen suspension (50 μl of 50 μg/ml per each well) was added to the wells of a 96-well Nunc ImmobilizerTM Amino plate (Thermoscientific) and incubated overnight at 4 °C; the plates were allowed to come to room temperature before use. Washed platelets were preincubated with m-Fab-F, 204-11 Fab, 10B12, 1C3, or human Fab (the control) for 10 min prior to initiating the platelet adhesion assay, which was performed as described previously (17), in the presence of 1 mm MgCl2 (total adhesion) or 5 mm EDTA (GPVI-dependent adhesion). The platelets adhering to the immobilized fibrous collagen were lysed, and the alkaline phosphatase in the lysate was assayed with the chromogenic substrate p-nitrophenyl phosphate, which is hydrolyzed by the enzyme to p-nitrophenol, detectable by its absorbance at 405 nm.
Effect of Anti-GPVI Dimer Antibodies on Platelet Adhesion to a Collagen-coated Surface under Flow
Flow adhesion experiments were performed as described previously (18). Glass coverslips were coated with 0.1 mg/ml type III collagen in 10 mm acetic acid (4 °C, overnight) and then washed with HT. Blood, anti-coagulated with 40 μm d-phenylalanyl-l-propyl-l-arginine chloromethyl ketone and fluorescently labeled with 1 μm 3,3′-dihexyloxacarbocyanine iodide, was incubated with 200 μg/ml m-Fab-F, 200 μg/ml control human Fab, or 100 μg/ml 204-11 Fab for 10 min and applied to flow adhesion analysis. Blood was flowed over the collagen-coated glass at the shear rate of 300 or 1000 s−1; after 5 min, Z-stacks of fluorescence images of adhered platelets were collected and analyzed by Image J1.35 (National Institutes of Health). The coverslip plane was defined as the Z-plane with largest platelet area and used to calculate the surface area coverage. Mean thrombus height was calculated as thrombus volume divided by the field area (μm3/μm2), and ZV50 was calculated as the Z-height at which the thrombus volume was half-maximal as described previously (18). Because we had a limited supply of the relatively low affinity m-Fab-F, the results were obtained from two different blood donors, and experiments were performed in duplicate or triplicate.
RESULTS
Throughout this paper, GPVIex and GPVI-Fc2 will be referred to as the monomer and dimer, respectively. The primary sequencers of the collagen model peptides and Toolkit III peptides used in the experiments are shown in Table 1.
TABLE 1.
Primary sequences of collagen model peptides and Toolkit III peptides
The one-letter amino acid abbreviations are as follows: G, glycine; P, proline; O, hydroxyproline; C, cysteine. CRP stands for collagen-related peptide. The peptide (GPO)2(GPP)4(GPO)2 was previously abbreviated as (GPO)2(GPO)4 in previous reports. (GPO)10 and (GPP)12 do not contain cysteine, so cannot form cross-linked polymers. Toolkit III (TK III) peptides with binding affinity to GPVI dimer are shown.
| Peptide | Sequence |
|---|---|
| CRP | GCO–(GPO)10–GCOG-NH2 |
| III-30A | GPC–(GPP)5–GAOGARGGAGPOGPEGGKGAAGPOGPO(GPP)5–GPC–NH2 |
| (GPO)2(GPP)4(GPO)2 | GCP–GPPGPOGPOGPPGPPGPPGPPGPOGPOGPP–GCPG–NH2 |
| GPP–10 | GCP–(GPP)10–GCPG–NH2 |
| (GPO)1 | GCP–GPPGPPGPPGPPGPOGPPGPPGPPGPPGPP–GCPG–NH2 |
| (GPO)2 | GCP–GPPGPPGPPGPPGPOGPOGPPGPPGPPGPP–GCPG–NH2 |
| (GPO)4 | GCP–GPPGPPGPPGPOGPOGPOGPOGPPGPPGPP–GCPG–NH2 |
| (GPO)6 | GCP–GPPGPPGPOGPOGPOGPOGPOGPOGPPGPP–GCPG–NH2 |
| (GPO)10 | (GPO)10G–NH2 |
| (GPP)12 | (GPP)12G–NH2 |
| TK III-1 | GPC–(GPP)5–GLAGYOGPAGPOGPOGPOGTSGHOGSO–(GPP)5–GPC–NH2 |
| TK III-4 | GPC–(GPP)5–GPSGPAGKDGESGROGROGERGLOGPO–(GPP)5–GPC–NH2 |
| TK III-9 | GPC–(GPP)5–GLOGAAGARGNDGARGSDGQOGPOGPO–(GPP)5–GPC–NH2 |
| TK III-22 | GPC–(GPP)5–GSDGKOGPOGSQGESGROGPOGPSGPR–(GPP)5–GPC–NH2 |
| TK III-30 | GPC–(GPP)5–GAOGLRGGAGPOGPEGGKGAAGPOGPO–(GPP)5–GPC–NH2 |
| TK III-40 | GPC–(GPP)5–GAAGFOGARGLOGPOGSNGNOGPOGPS–(GPP)5–GPC–NH2 |
| TK III-49 | GPC–(GPP)5–GDRGENGSOGAOGAOGHOGPOGPVGPA–(GPP)5–GPC–NH2 |
Comparison of Dimer and Monomer Affinities for Immobilized Collagenous Substrates
ELISA (Fig. 1A) was used to assay GPVI dimer and monomer binding to immobilized collagenous substrates (Table 2) to compare their affinities. The GPVI dimer bound to CRP; collagen I; collagen III; (GPO)2, a peptide containing GPVI-binding GPOGPO (19); and (GPO)2(GPP)4(GPO)2, a peptide containing two GPOGPO motifs (19). The dissociation constants (Kd) (Table 2) of the dimer are much lower, indicating that it has 60–900 times higher affinity for the collagenous substrates than the monomer. GPP-10, a peptide with no GPO triplets, shows little binding to dimer (data not shown). The following Hill coefficients (mean ± S.E.) were calculated for dimer binding: collagen type I (1.14 ± 0.07, n = 10); collagen type III (1.16 ± 0.11, n = 12); CRP (1.07 ± 0.08, n = 6); (GPO)2 (1.07 ± 0.21, n = 6); and (GPO)2(GPP)4(GPO)2 (1.50 ± 0.60, n = 4). None of the Hill coefficients were significantly different from unity (p > 0.08), suggesting no cooperative binding of the dimer to any of the peptides.
FIGURE 1.
Comparison of the binding of GPVI dimer and monomer to collagens and collagen-mimetic peptides and effect of peptide density on dimer binding. A, binding of GPVI dimer (closed triangles) and monomer (closed circles), expressed as A450 units, to immobilized collagen and peptides (structures shown in Table 1) were determined by ELISA. These data are representative of two separate experiments giving similar results, with binding data obtained for all the collagen substrates in each experiment; each data point represents the mean ± S.E. of triplicate determinations. Kd values were obtained by nonlinear regression of the data (Table 2). B, effect of density of immobilized (GPO)10 or CRP-XL (cross-linked CRP) on dimer binding. 100% (GPO)10 designates wells (Nunc Amino Immobilizer ELISA plate) reacted with 50 μl of 10 μg/ml (GPO)10; dilutions to lower (GPO)10 were made with the inert peptide (GPP)12, keeping the total peptide concentration the same (i.e. 10 μg/ml, in total), and these were also used to coat the wells. Similarly, undiluted CRP-XL (10 μg/ml) or CRP-XL was diluted with (GPP)12 and used to coat the wells. GPVI dimer binding to the immobilized peptides was determined by ELISA. A high density of (GPO)10 is required to support dimer binding, with affinity rapidly falling off as the peptide is diluted, suggesting that the dimer binds to more than one molecule of (GPO)10 (left graph). In contrast, this is not observed with CRP-XL, which forms cross-linked polymers of CRP molecules in which the component CRP molecules may be close enough so that the dimer can bind across several of them (right graph). Where no error bars are visible, they are equal to or smaller than the size of the symbol.
TABLE 2.
Dissociation constants (Kd) for GPVI dimer and monomer binding to collagenous substrates
| Kd (dimer) | Kd (monomer) | Kd (monomer)/Kd (dimer) | |
|---|---|---|---|
| nm | μm | ||
| CRP | 22.0 ± 2.1 | 2.7 ± 0.3 | 122.7 |
| Collagen type I | 41.7 ± 0.4 | 8.1 ± 1.2 | 194.3 |
| Collagen type III | 58.3 ± 9.4 | 13.8 ± 2.5 | 236.7 |
| III-30A | 11.5 ± 0.6 | 10.2 ± 1.9 | 887.0 |
| (GPO)2 | 75.5 ± 13.8 | a | |
| (GPO)2(GPP)4(GPO)2 | 63.3 ± 17.3 | 3.8 ± 1.5 | 60.0 |
Effect of Peptide Coating Density on Dimer Binding Affinity
To determine whether the dimer may bind to a single triple-helical peptide molecule or across several triple helices, we immobilized (GPO)10, which does not contain cysteine and so cannot spontaneously form disulfide-linked homopolymers, diluted to various densities with the inert peptide (GPP)12. 100% (GPO)10 designates wells reacted with 50 μl of 10 μg/ml (GPO)10; dilutions to lower (GPO)10 were made with (GPP)12, keeping the total peptide concentration the same (i.e. 10 μg/ml in total), and these were also reacted with the wells. Similarly, CRP-XL (a chemically cross-linked, polymeric form of CRP, 10 μg/ml, 100%) was diluted with (GPP)12 and reacted with the wells. By using the Nunc Amino Immobilizer plates, each peptide molecule can be separately immobilized via a covalent linkage to an electrophilic group attached to the plate through a spacer group. As shown in Fig. 1B, a high density of (GPO)10 was required for dimer binding, with as low as a 1:5 dilution markedly increasing the Kd value and further dilution reducing the binding to the level of (GPP)12. This suggests that, presumably, two copies of the (GPO)10 triple helix must be close enough to allow the dimer to bind to both of them. However, CRP-XL showed no marked increase in Kd values until it was diluted to 0.1% because cross-linking multiple individual helices secures them close enough together to enable a dimer to bind across two or more of them.
Effect of Number of GPOs on Dimer Affinity to Peptides
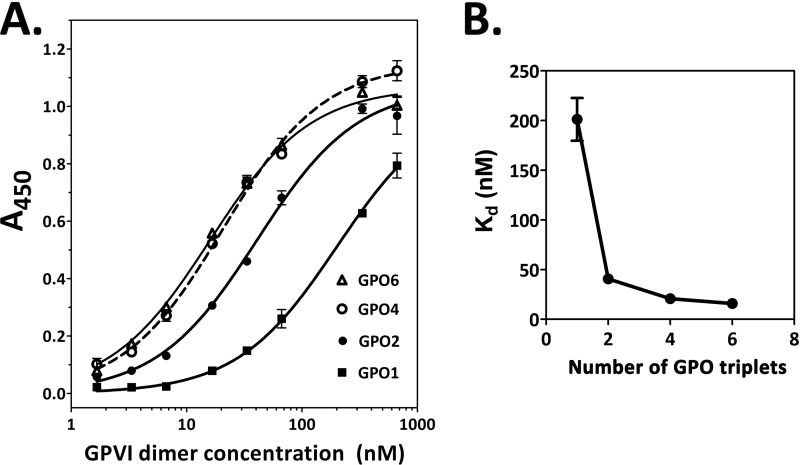
The optimal number of GPO triplets for dimer recognition was determined by measuring binding to peptides containing one to six GPO triplets (Fig. 2A, ELISA): (GPO)1, (GPO)2, (GPO)4, and (GPO)6, respectively (19). Dimer bound to (GPO)1 most weakly (Kd ≅200 nm). The plot of Kd values versus number of GPO triplets (Fig. 2B) shows that affinity increases about 6-fold when a peptide contains two or more contiguous GPO triplets, indicating that at least two contiguous GPO triplets are required for good affinity to GPVI dimer.
FIGURE 2.
Effect of the number of GPO triplets on GPVI dimer binding. ELISA was used to determine dimer binding (expressed as A450 units) to immobilized collagen mimetic peptides with 1, 2, 4, and 6 GPO triplets, corresponding to peptides (GPO)1, (GPO)2, (GPO)4, and (GPO)6, respectively (Table 1). The data presented are representative of three experiments giving similar and consistent results; in each experiment, binding curves for the peptides were determined on the same day and each point is the mean ± S.E. of triplicate determinations. Data were fitted by nonlinear regression to obtain the Kd values. A, binding curves; B, graph of Kd (calculated from the binding curves) versus the number of GPO triplets in the peptides. Peptides with two or more contiguous GPO triplets show about 6-fold higher affinity than (GPO)1 that has only a single GPO triplet, suggesting that GPOGPO is the structure that the dimer binds to with high affinity.
Dimer Binding to Collagen Toolkit III Peptides
We screened the dimer-binding capacity (Fig. 3) of collagen Toolkit III: 57 overlapping homotrimeric peptides comprising the entire primary sequence of the human collagen III COL domain (14). The eight peptides with the highest dimer binding were as follows: high affinity (Kd = 3.2 ± 0.3 nm), III-30; moderate affinity (Kd = 14–48 nm), III-1, -4, -22, -40, and -49; and low affinity (Kd ≥90 nm), III-9 and -39 (Fig. 3, inset, peptides listed in decreasing order of affinity).
FIGURE 3.
Screening for collagen Toolkit III peptides that bind GPVI dimer. The total set of 57 collagen Toolkit III peptides was screened for their ability to bind GPVI dimer. Wells were coated with 10 μg/ml of Toolkit III peptide, collagen type I, collagen type III, GPP-10, or CRP in 10 mm acetic acid, and binding of GPVI dimer (20 μg/ml) to the immobilized substrate was measured by ELISA using HRP-labeled anti-human Fc2 antibody. Binding is expressed as A450, and the data are normalized to the binding of dimer to collagen type III. Each bar shows the mean ± S.E. of three experiments, triplicate determinations for each immobilized substrate. The dimer binding affinities (Kd values) of peptides with the highest affinities for dimer are shown in the inset, in order of decreasing affinities (ND = not determined). The inset also shows the IC50 values for inhibition of dimer binding by the dimer-specific antibody m-Fab-F; in these experiments, dimer was preincubated with different concentrations of m-Fab-F and then reacted with immobilized peptide, using the ELISA method as described under “Experimental Procedures.”
GPVI Dimer and Monomer Specificity of Anti-GPVI Antibodies
Dimer and monomer binding to immobilized antibody (m-Fab-F, 204-11, 204-11 Fab, 1C3, and 1G5, antibodies against the extracellular domain of GPVI) was determined by ELISA (Fig. 4, A and B). Kd is shown in Table 3.
FIGURE 4.
Antibody binding curves for GPVI dimer and monomer. A, binding of GPVI dimer to immobilized antibody: m-Fab-F (black closed diamonds), 204-11 Fab (black closed triangles), 204-11 IgG (gray inverted closed triangles), 1G5 (gray closed circles), and 1C3 (gray closed squares) determined by ELISA using HRP-labeled anti-human-Fc antibody. B, binding of GPVI monomer to immobilized antibody determined by ELISA using biotinylated convulxin/HRP-labeled extravidin; symbols are the same as those for A. C, binding of 204-11 Fab to immobilized GPVI dimer (closed circles) and monomer (closed triangles), as determined by ELISA using HRP-labeled anti-mouse Fab. Binding of GPVI or antibody was expressed as the absorbance at 450 nm. The data presented are representative of three (A), two (B), and two (C) experiments giving similar and consistent results; each point is the mean ± S.E. of triplicate determinations. Data were fitted by nonlinear regression to obtain the binding parameters (Table 3). Where no error bars are visible, they are equal to or smaller than the size of the symbol.
TABLE 3.
Dissociation constants for binding of GPVI dimer and monomer to antibodies against the extracellular domain of GPVI
| Antibody | Form | GPVI dimer |
GPVI monomer |
Kd (monomer)/Kd (dimer) | Specificity | ||
|---|---|---|---|---|---|---|---|
| Kd (dimer) | Bmax, A450/well | Kd(monomer) | Bmax, A450/well | ||||
| nm | nm | ||||||
| m-Fab-F | Fab | 10.83 ± 0.60 | 0.41 ± 0.01 | a | a | Dimer | |
| 204-11 Fab | Fab | 1.05 ± 0.10 | 0.57 ± 0.01 | a | a | Dimer | |
| 204-11 | IgG | 0.16 ± 0.01 | 0.65 ± 0.01 | 4.09 ± 0.44 | 0.54 ± 0.01 | 25.6 | Both |
| 1G5 | IgG | 0.37 ± 0.03 | 0.61 ± 0.01 | 0.20 ± 0.02 | 0.64 ± 0.01 | 0.5 | Both |
| 1C3 | scFv | 0.59 ± 0.08 | 0.43 ± 0.01 | 20.53 ± 2.63 | 3.88 ± 0.01 | 34.8 | Both |
a Because of low (204-11 Fab) or no (m-Fab-F) binding of the antibody, kinetic parameters of acceptable accuracy could not be calculated. The Kd and Bmax values for 204-11 (IgG), 1G5, and 1C3 were calculated from the data shown in Fig. 4 (dimer binding to immobilized antibodies determined by ELISA). To facilitate the comparison of Bmax values, these data were obtained at one time and show the results of one experiment, which is representative of three experiments showing consistent results.
m-Fab-F, designed to bind dimer without binding to either monomer or the Fc of human IgG, bound to dimer only, as reported previously (8). Mouse monoclonal antibody 204-11, raised against the monomer, bound to both dimer and monomer, but it had 25.6-fold higher affinity for the dimer, as shown by the ratio Kd, monomer/Kd, dimer (Table 3). Unexpectedly, 204-11 Fab was found to bind dimer only for all practical purposes, with monomer binding being too low to establish accurate kinetic constants (Fig. 4B). In the reverse ELISA, binding of 204-11 Fab to immobilized GPVI monomer (Fig. 4C), the Fab bound no monomer. The difference between the two ELISAs may be due to monomers being less stable to immobilization or different exposure of binding surfaces.
Although 1C3, an antibody against recombinant GPVI monomer D1D2 (13), binds both dimer and monomer, its Kd value indicates that it is dimer-selective. Interestingly, 1G5, an antibody raised against recombinant GPVI monomer GPVIex, had higher affinity for the monomer than the dimer, but the difference in affinity was insufficient to make it monomer-specific.
Inhibition of Dimer Binding to Collagenous Substrates by Anti-GPVI Antibodies
The epitopes of our GPVI dimer-specific antibodies (m-Fab-F and 204-11 Fab) and those of antibodies binding both forms (1C3 and 1G5) were analyzed by determining their effect on dimer binding to the collagenous substrates (Fig. 5). 1G5 did not inhibit dimer binding to any of the substrates (data not shown). m-Fab-F at concentrations commensurate with its binding affinity nearly completely inhibited dimer binding to immobilized collagens I and III, (GPO)2, (GPO)2(GPP)4(GPO)2, and Toolkit peptides III-39 and III-40, whereas it decreased dimer binding to each of the high affinity substrates, Toolkit peptide III-30 and CRP, by ∼40%. 1C3 almost completely inhibited dimer binding to all substrates, including CRP. 204-11 Fab exerted various degrees of partial inhibition against the collagenous substrates.
FIGURE 5.
Effect of anti-GPVI antibodies on binding of GPVI dimer to collagen, collagen peptides, and representative collagen Toolkit III peptides. ELISA was used to measure GPVI dimer binding to immobilized collagen I and III, CRP, (GPO)2, (GPO)2(GPP)4(GPO)2, and three representative collagen Toolkit III peptides: III-30 (high affinity), III-40 (moderate affinity), and III-39 (low affinity). A fixed concentration of GPVI dimer (20 μg/ml) was preincubated with various concentrations of an antibody before binding to immobilized peptide. Antibodies used were as follows: GPVI dimer-specific antibodies: m-Fab-F (black open circles) and 204-11 Fab (gray closed diamonds); and an antibody binding to both GPVI dimer and monomer, 1C3 (black closed squares). Each point on the curve represents the mean ± S.E. of triplicate determinations. Where no error bars are visible, they are equal to or smaller than the size of the symbol.
We determined the relationship between dimer binding affinity and the ability of m-Fab-F to inhibit dimer binding to immobilized Toolkit III peptides by calculating its IC50 value in ELISAs in which a fixed amount of dimer was preincubated with different concentrations of m-Fab-F and then reacted with an immobilized Toolkit III peptide. The calculated IC50 values for m-Fab-F were inversely related to the Kd value of dimer binding to the peptide (Kd values and IC50 for m-Fab-F shown in Fig. 3, inset).
Relative Amounts of GPVI Dimer in Resting and Agonist-activated Platelets
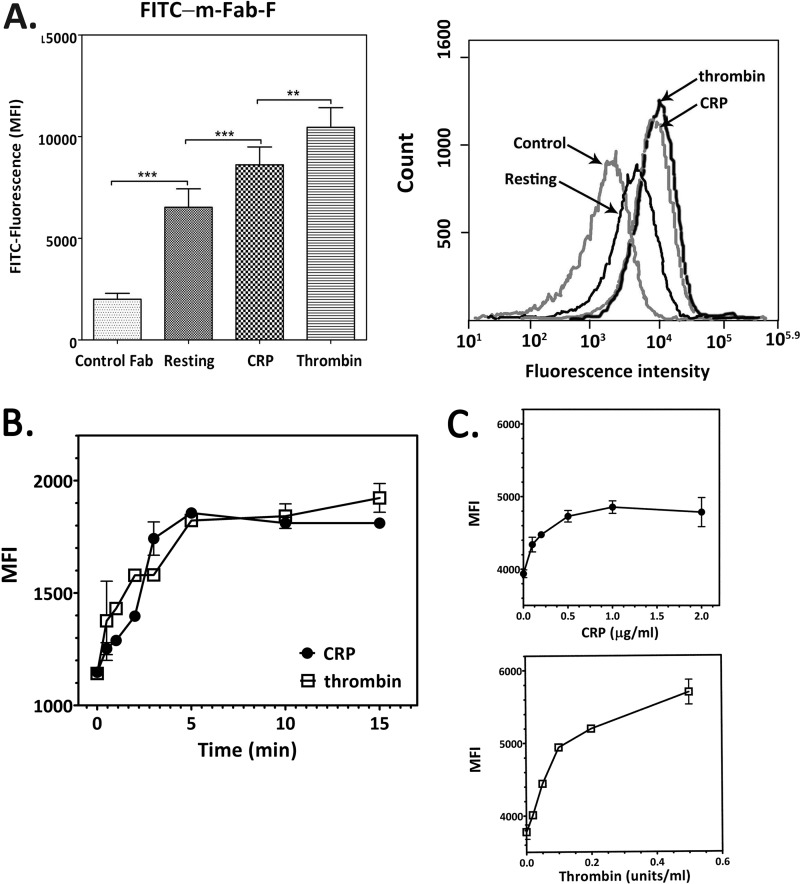
Flow cytometry was used to measure relative amounts of GPVI dimer in resting and activated platelets by direct binding of FITC-m-Fab-F to platelets in whole blood or washed platelets in the presence of 5 mm EDTA. In all samples analyzed using whole blood (n = 16), the median fluorescence intensity of m-Fab-F binding to resting platelets was more than that of FITC-labeled control Fab to resting platelets (6523 ± 859 versus 2001 ± 277, p = 0.0002). In Fig. 6A, the panel shows median fluorescence intensity, and the right panel shows a plot of fluorescence intensity distribution. m-Fab-F binds more to platelets activated by 5 μg/ml CRP (8613 ± 826, p < 0.0001) or 0.2 unit/ml thrombin (10454 ± 921, p < 0.0001) than to resting platelets, with thrombin inducing a greater increase in median fluorescence intensity than CRP (p = 0.0035).
FIGURE 6.
Flow cytometry to determine relative amounts of GPVI dimer on intact resting and activated platelets. A, dimer determination by direct binding of FITC-labeled m-Fab-F to resting, CRP-activated, and thrombin-activated platelets; FITC-labeled control human Fab was used as the control. The left panel compares the median fluorescence intensity, and each bar represents the mean ± S.E. (n = 16), using platelets from different donors; the right panel shows the data from one representative determination. There is a significant increase (p = 0.0002) in median fluorescence intensity (MFI) in resting platelets, compared with resting platelets incubated with the control Fab, demonstrating the existence of dimers. There is an increase in dimer in platelets activated by 5 μg/ml CRP (p < 0.0001) or 0.2 unit/ml thrombin (p < 0.0001), with thrombin inducing a greater increase than CRP (p = 0.0035). **, p = 0.001–0.01; ***, p < 0.001. B, time course of dimer formation in platelets induced by CRP (closed circles) or thrombin (open squares). Washed platelets (2.5 × 108 cells/ml, 1 ml) were added with CRP (5 μg/ml, final concentration) or thrombin (0.2 unit/ml, final concentration); and at various times, a 100-μl aliquot was taken out of the mixture and immediately fixed in 1% paraformaldehyde in HT (30 min). The fixed platelets were processed for flow cytometry using FITC-labeled m-Fab as described under “Experimental Procedures.” C, agonist concentration dependence of dimer increase was measured for CRP at 0.1–2.0 μg/ml (upper panel) and thrombin at 0.1–0.5 units/ml (lower panel) using washed platelets (1 × 108 cells/ml) and FITC-labeled m-Fab-F (100 μg/ml, final concentration).
Similar results were obtained detecting m-Fab-F binding to platelets in whole blood using a FITC-anti-His secondary antibody; resting platelets bind more m-Fab-F (4554 ± 612, n = 11, p = 0.003) than control Fab (2675 ± 366), and CRP- and thrombin-activated platelets bind more m-Fab-F than resting platelets (5673 ± 877, p = 0.0129, and 5882 ± 935, p = 0.0207, respectively, n = 11).
Time and Concentration Dependence of Dimer Increase Induced by CRP and Thrombin
Dimer levels started to increase after 30 s of treatment with either CRP or thrombin, reaching a maximum at 4–5 min (Fig. 6B). Both CRP (Fig. 6C, top panel) and thrombin (Fig. 6C, bottom panel) caused a concentration-dependent increase in dimer level, reaching a maximum at ≥0.5 μg/ml and >0.5 unit/ml, respectively.
Quantitation of GPVI Dimers by Flow Cytometry (Fig. 7 and Table 4)
FIGURE 7.
Determination of the stoichiometry of antibody binding and method for calculation of the number of GPVI dimers and total GPVI in platelets. A, binding of 1G5 IgG (gray closed circles) and Fab (black closed triangles) to immobilized dimer was determined by ELISA using HRP-labeled anti-mouse Fab; these antibodies have the same Bmax. B, binding of 204-11 IgG (gray closed circles) and 204-11 Fab (black closed triangles) to immobilized dimer; the Bmax of 204-11 Fab binding is half that of 204-11 IgG binding. Binding of antibody was expressed as the absorbance at 450 nm. A and B, data presented are representative of two experiments giving similar and consistent results; each point is the mean ± S.E. of triplicate determinations. Data were fitted by nonlinear regression, and the Bmax values were calculated. C, method for calculating the actual number of GPVI dimers and total GPVI, in terms of single chain GPVI molecules, from quantitation data obtained by flow cytometry using 204-11, 1G5, or 1G5Fab as the primary antibody for determining total GPVI (panel i) and 204-11 Fab as the primary antibody for determining the number of GPVI dimers (panel ii). The secondary antibody was FITC anti-mouse F(ab′)2. The binding reaction for the Biocytex Gp quantitation kit calibration beads containing known amounts of mouse IgG is shown in panel iii. A calibration curve was obtained for each experiment; and a representative one is shown in panel iv. Refer to the text for a full description of the calculation method.
TABLE 4.
Flow cytometric quantitation of the number of GPVI dimers in resting and activated platelets
| Platelet preparation | n | No. of dimers per plateleta | % GPVI in dimer formb | % increase after activation |
|---|---|---|---|---|
| Resting platelets | 9 | 861 ± 7.6 | 29 | |
| CRP-activated | 8 | 1174 ± 148 | 40 | 37 |
| Thrombin-activated | 8 | 1326 ± 152 | 44 | 55 |
a The number of dimers/platelet was calculated as described under “Results” and in Fig. 7.
b Percentages calculated with respect to the number of total single chain GPVI molecules; the average of data obtained using 204-11 IgG, 1G5 Fab, and 1G5 IgG is as follows: 5994 single GPVI molecules/platelet; each dimer = two single-chain GPVI molecules.
All determinations were done with platelets in whole blood in the presence of EDTA to prevent activation, and antibodies of mouse origin were used.
The total number of single-chain GPVI molecules was calculated from the measured values (Fig. 7) by the following method. Quantitation of total GPVI using either 204-11, 1G5, or 1G5 Fab as the primary antibody gave a similar number of antibody-binding sites, indicating that each Fab of the FITC anti-mouse F(ab′)2 binds one Fab of the anti-GPVI antibody, whether the latter is intact IgG or Fab (Fig. 7C, panel i). ELISA (Fig. 7A) also shows that 1G5 IgG (Bmax = 0.799 ± 0.015 A450 units/well) and Fab (Bmax = 0.797 ± 0.007 A450 units/well) bound to immobilized dimer to the same extent. These results indicate that bivalent anti-GPVI IgG binds to two copies of the antigen on the platelet surface (Fig. 7C, panel i). Similar conclusions were drawn for the quantitation of platelet integrin αIIbβ3 (20). Thus, the actual number of single-chain GPVI molecules on platelets is twice the number calculated from the number of IgG on the calibration beads. Therefore, the number of mouse IgG equivalents from the calibration curve (Fig. 7C, panel iv) is doubled to obtain the actual number of dimers: 6244 ± 558 (204-11 IgG, n = 9), 5702 ± 294 (1G5, n = 7), and 6034 ± 496 (1G5 Fab, n = 4), average of 5993. Fig. 8 shows that resting and thrombin-activated platelets have similar levels of total GPVI. In CRP-activated platelets, the total GPVI determined with 1G5 Fab is similar to that observed in resting and thrombin-activated platelets, although it is lower when measured using 204-11 IgG or 1G5. We do not know the reason for this difference at present. It should be noted that no matter which of the three antibodies was used, no platelet sample showed an increase in total GPVI upon activation by either CRP or thrombin.
FIGURE 8.
Quantitation of GPVI dimers and total GPVI by flow cytometry. GPVI dimers are quantitated by using 204-11 Fab as the primary antibody, and total GPVI is quantitated by 204-11 IgG, 1G5 Fab, or 1G5 IgG (data for all three primary antibodies shown in the graph); the secondary antibody FITC anti-mouse F(ab′)2 was used for all determinations, including the standard curve with the mouse IgG-bound beads. Each bar represents the mean ± S.E. of the indicated number of platelet samples; see Table 4 for a summary of the quantitation data and “Results” (Quantitation of GPVI Dimers by Flow Cytometry) and Fig. 6 for a detailed description of the calculation method. As observed in the other two methods, compared with the number of GPVI dimers in resting platelets, both CRP (p = 0.0018, n = 8) and thrombin increased the amount of dimer (p = 0.0243, n = 8). Compared with resting platelets, thrombin-activated platelets have a similar amount of total GPVI, although CRP-activated platelets have less. *, p = 0.01–0.05.
The number of GPVI dimers was calculated from the measured values by the following method. The binding of dimer-specific 204-11 Fab to immobilized dimer (Bmax = 0.35 ± 0.01 A450 units/well) is about half that of 204-11 IgG (Bmax = 0.66 ± 0.01 A450 units/well) (Fig. 7B), so one dimer binds one 204-11 Fab molecule (Fig. 7C, panel ii); because one FITC anti-mouse F(ab′)2 would bind to two anti-GPVI Fabs, each on a different dimer, this means that each measured FITC anti-mouse F(ab′)2 is equivalent to two dimers (Fig. 7C, panel ii). Thus the true number of GPVI dimers is twice the value obtained from the calibration curve. So the number of mouse IgG equivalents from the calibration is doubled to obtain the actual number of dimers: 862 ± 96 (n = 9), 1174 ± 148 (n = 8), and 1326 ± 152 (n = 8) in resting, CRP-activated, and thrombin-activated platelets (Fig. 8). Therefore the number of GPVI dimers is 28.6% of the total GPVI in the resting platelets, significantly increasing to 39.2 and 44.2% of the total GPVI in CRP- and thrombin-activated platelets, respectively.
Inhibition of Static Platelet Adhesion by Anti-GPVI Antibodies
All four anti-GPVI antibodies (at concentrations in great excess of their Kd value for dimer) significantly inhibited GPVI-dependent static platelet adhesion to fibrous collagen III (i.e. in the presence of EDTA). Inhibition by dimer-specific m-Fab-F (200 μg/ml, 4.2 μm) is not significantly different from that caused by 10B12 (50 μg/ml, 1.8 μm) or 1C3 (50 μg/ml, 1.8 μm), which bind both dimer and monomer, suggesting that the dimer is the form primarily responsible for static platelet adhesion to fibrous collagen (Fig. 9). The milder effect of 204-11 Fab (100 μg/ml, 2.1 μm) on GPVI-dependent platelet adhesion is consistent with its partial inhibition of dimer binding to collagen III in ELISA. The GPVI-dependent component of adhesion is about 34% of the adhesion due to integrin α2β1 in the presence of Mg2+, which is mainly integrin α2β1-dependent; this is slightly inhibited by m-Fab-F (p < 0.05).
FIGURE 9.
Effect of dimer-specific antibodies on static platelet adhesion to fibrous collagen. We measured static platelet adhesion to fibrous collagen type III immobilized to wells of a 96-well Nunc ImmobilizerTM Amino plate, as described under “Experimental Procedures.” Washed platelets were preincubated with anti-GPVI antibodies, m-Fab-F (200 μg/ml, 4.2 μm), 204-11 Fab (100 μg/ml, 2.1 μm), 10B12 (50 μg/ml, 1.8 μm), 1C3 (50 μg/ml, 1.8 μm), or human Fab as a control (200 μg/ml, 4.2 μm) prior to initiating the platelet adhesion assay in the presence of 1 mm MgCl2 (total adhesion) or 5 mm EDTA (GPVI-dependent adhesion). Adhered platelets were lysed, and alkaline phosphatase in the lysate was assayed by its hydrolysis of p-nitrophenyl phosphate to p-nitrophenol, detectable by its absorbance at 405 nm. In the absence of any antibody, platelet adhesion to fibrous collagen in the presence of EDTA was about 34% that in the presence of Mg2+. All the anti-GPVI antibodies significantly inhibited GPVI-dependent adhesion. The inhibition exerted by dimer-specific m-Fab-F was not significantly different from those by 10B12 and 1C3, antibodies that bind to both GPVI monomer and dimer, suggesting that the monomer contributes little to GPVI-dependent adhesion. Dimer-specific 204-11 Fab exerted less inhibition than m-Fab-F, consistent with its epitope being near but discrete from the collagen-binding site of the dimer. Only m-Fab-F exerted significant, but slight, inhibition of adhesion in the presence of Mg2+. The control Fab had no effect on platelet adhesion.
Effect of Anti-GPVI Dimer Antibodies on Platelet Adhesion under Flow Conditions
At the low shear rate of 300 s−1, m-Fab-F (200 μg/ml, 4.2 μm) inhibited platelet adhesion to immobilized collagen (Fig. 10A), with mainly single and therefore nonactivated platelets adhering to the collagen, in contrast to the patterns for control human Fab and 204-11 Fab (100 μg/ml, 2.1 μm), where most adherent platelets were in aggregates (Fig. 10A); this is reflected in the surface coverage data (Fig. 10B). Inhibition by 10B12 (50 μg/ml, 1.8 μm) is similar to that produced by m-Fab-F (data not shown). These observations are confirmed by the marked decrease in the average thrombus height and ZV50 (Fig. 10B) in m-Fab-treated blood, indicating the decrease of thrombus size and low platelet activation. The decreased surface coverage in the presence of m-Fab-F can be at least partially explained by the decrease of activation through the GPVI dimer and thus of thrombus formation. 204-11 Fab (100 μg/ml) did not inhibit surface coverage and mildly decreased mean thrombus height and ZV50.
FIGURE 10.
Effect of dimer-specific antibodies on the platelet adhesion to a collagen surface under flow conditions. 3,3′-Dihexyloxacarbocyanine iodide-labeled blood in the presence of an anti-GPVI-dimer antibody, m-Fab-F (200 μg/ml, 4.2 μm) or 204-11 Fab (100 μg/ml, 2.1 μm), or control Fab (200 μg/ml, 4.2 μm) was flowed over a surface of immobilized collagen (type III) at 300 or 1000 s−1 for 5 min, and adhered platelets were analyzed. A, fluorescent images of adhered platelets. m-Fab-F-treated blood (right panel) showed decreased platelet adhesion at both shear rates, showing markedly decreased thrombus formation relative to the control blood (left panel, human Fab). 204-11 Fab did not decrease platelet adhesion at 300 s−1 (top row, middle panel), but decreased it at 1000 s−1. B, surface coverage of adhered platelets was calculated from the images in A; mean thrombus height and ZV50, thrombus height at half-maximal thrombus height, were plotted from the data obtained at shear rates of 300 and 1000 s−1. At either shear rate, both 204-11 Fab and m-Fab-F reduced the % surface coverage, a measure of adhesion, and mean thrombus height and ZV50, which show the extent of platelet activation. m-Fab-F, which binds to the collagen-binding site of the dimer, exerts a larger effect than 204-11. The control mouse Fab or human Fab (data not shown) did not affect adhesion or thrombus formation.
At 1000 s−1, there was high surface coverage and thrombus formation in the control sample (mouse Fab or human Fab, data not shown); surface coverage was slightly inhibited by 204-11 Fab and decreased about 50% by m-Fab-F (200 μg/ml). 204-11 Fab and m-Fab both markedly decreased mean thrombus height and ZV50, with m-Fab-F being the more inhibitory (Fig. 10B). Under both shear rates, the anti-GPVI dimer antibodies inhibited the size of thrombi formed much more strongly than surface coverage, indicating that they especially inhibit platelet activation on the collagen surface under flow conditions.
DISCUSSION
What advantage does the presence of GPVI dimers on the platelet surface offer for collagen recognition, and do dimers exist in sufficient numbers to be physiologically meaningful for platelet function? We performed this study to answer these questions, and our results demonstrate that constitutive dimerization enables GPVI to recognize and bind with high affinity to specific sequences in its ligand, collagen. Thus, the GPVI dimer is the form involved in collagen binding, while the low affinity monomer is not and instead provides a pool of dimer components to be called upon when platelets are activated.
Differential Affinity of GPVI Dimer and Monomer for Collagen Model Peptides
CRP, a triple-helical collagen model peptide containing 10 consecutive GPO triplets (21), was the first reagent reported to bind specifically and activate the platelet collagen receptor GPVI (21, 22). Screening the ability of model collagen peptides containing various arrangements of GPO triplets to bind recombinant protein consisting of the GPVI extracellular D1 and D2 domains (D1D2) established that the GPO sequence is a main determinant of GPVI recognition (12, 13, 17). By docking CRP onto D1D2, which forms a dimer in the crystal, Horii et al. (7) proposed the CRP-binding site as a shallow groove defined by charged residues Lys-41, Lys-59, Arg-60, and Arg-166 on the D1 domain. Mutational studies show these residues are able to recognize CRP.
However, the ability of GPVI to recognize the GPO sequence in collagen may not completely explain why GPVI dimer displays such markedly higher affinity, compared with the monomer, for collagen and its model peptides (Fig. 1 and Table 2). GPVI dimer binds with similar Kd values to all of the substrates, but the severe rightward shift of the corresponding binding curves of GPVI monomer indicates its much lower binding affinity. Compared with the other peptides, monomer binds to CRP and (GPO)2(GPP)4(GPO)2 with higher affinity, albeit with much lower affinity than the dimer, suggesting that the closely spaced GPO triplets in these two peptides may allow monomers to bind close enough together to interact, thereby reinforcing their affinity; this is consistent with O'Connor et al. (23) who reported higher binding of D1D2 to peptides containing more GPO triplets.
However, compared with D1D2, dimer binding is less influenced by a high GPO content, with binding to (GPO)2 and (GPO)2(GPP)4(GPO)2 being similar to that for CRP, suggesting that the GPOGPO sequence, the common motif in these peptides, is a structure to which dimers can bind with high affinity. A model peptide with only one GPO (Fig. 2A) has about 6-fold lower affinity than those containing two or more GPO triplets, further supporting this idea. It is interesting that the optimal binding of dimeric GPVI is obtained with peptides containing tandem GPO triplets, with little further increase in affinity obtained with a further increase in GPO content, which contrasts with the stepwise increase in hD1D2 binding reported by Smethurst et al. (19). More GPO triplets may enable a monomeric D1D2 to interact with a second molecule of D1D2, increasing its affinity, whereas dimeric GPVI is already in the conformation with the highest affinity.
Dimer Binding to Toolkit III Peptides
To determine whether our findings using model peptides can be extended to the primary sequence of triple-helical collagen, screening the collagen Toolkit III peptides for their ability to bind to dimer yielded one high affinity peptide (III-30), four of intermediate affinity (III-1, III-4, III-22, and III-40), and three of low affinity (III-9, III-39, and III-49). The Kd values of dimer binding (inset of Fig. 3) showed that they all bound dimer with much higher affinity than monomeric D1D2 (17) or GPVI monomer (data not shown). No obvious close relationship between the number of GPO triplets and dimer binding affinity is suggested by the Kd values, so there may be other determinants of binding. Dimerization vastly improves the interaction with these peptides, compared with the monomer, suggesting that a unique binding site is formed when two chains of GPVI form a dimer.
Does the Dimer Bind to One or Across Several Triple-helical Peptides?
We found that a high density of (GPO)10, a peptide that exists as single triple-helical molecules, is required to support dimer binding, with apparent affinity dropping precipitously as the density decreases, strongly suggesting that the dimer binds to more than one collagen helix. This is further supported by the fact that precross-linked CRP (CRP-XL) does not exhibit this drop-off in affinity, with decreasing density only having a slight effect over a large density range, presumably because cross-linking forms a polymer in which peptide helices are held in close enough proximity for the dimer to bind across several helices. The model proposed by Horii et al. (7) describes the binding site on the GPVI molecule as a shallow groove, large enough to fit three GPO triplets but not multiple helices. Our data therefore suggest that both component molecules of the dimer contribute to binding in a cooperative fashion, forming a composite binding site able to interact with two or more adjacent collagen helices, as might occur in nature, for example within a collagen fiber, shown schematically in the paper by Herr and Farndale (24).
Recognition of GPVI by Dimer-specific Antibodies and Those That Recognize Both Forms
The epitopes of the anti-GPVI antibodies were examined by determining their dimer and monomer binding specificities (Fig. 4 and Table 3) and their effect on dimer binding to collagenous substrates (Fig. 5). m-Fab-F and 204-11 Fab were dimer-specific. 204-11 IgG, 1G5, and 1C3 bound both dimer and monomer; notably, 1G5 showed higher affinity for the monomer than the dimer. 1C3, whose epitope may be near the dimer interface (23), may prevent dimer formation. Dimer-specific m-Fab-F and 204-11 Fab showed different patterns of inhibition against dimer binding. m-Fab-F markedly inhibited the binding to all collagenous substrates, except for binding to CRP, which was decreased about 30% by this Fab, suggesting that its epitope may lie near the collagen recognition site of the dimer (Fig. 5). m-Fab-F recognizes a structure specific for the dimer conformation, previously suggested to be involved in GPVI binding to collagen (8). Interestingly, Val-34 and Leu-36 present at the distal end of D1 were reported to contribute to collagen binding (25), suggesting that a site other than that binding GPO triplets may also interact with collagen. m-Fab-F may also recognize such a site, which is different from the positive groove suggested as a GPO-binding site in the model of Horii et al. (7). The partial inhibition of dimer binding produced by 204-11 Fab against all collagenous substrates other than CRP suggests that it binds to a specific site discrete from the collagen-binding site, yet close enough to hinder ligand binding. 204-11 IgG was observed to increase the binding of m-Fab-F to dimer (8), so it may also influence the conformation of the dimer.
Constitutive GPVI Dimers in Resting Platelets and Increase in Dimer Density upon Platelet Activation
Flow cytometric measurement of the relative amounts of GPVI dimer using FITC-labeled m-Fab-F indicated that resting platelets contain substantial amounts of GPVI dimer, which increased significantly after activation by either CRP or thrombin in concentration- and time-dependent manner. Quantitation of dimer number using the 204-11 Fab/FITC anti-mouse antibody system showed that 29% of the total GPVI in resting platelets is dimeric, increasing to 39 and 44% in CRP- and thrombin-activated platelets, respectively.
Our results contrast with the recent report by Loyau et al. (26) that few resting platelets were positive for dimer (2.25%) and that thrombin receptor-activating peptide activation increased the percent of dimer-positive platelets. It is difficult to compare their results with ours because they reported the percentage of platelets positive for antibody binding, whereas we quantitated the number of dimers per platelet; and they used a different anti-GPVI-dimer antibody, 9E18, which may recognize a different epitope in the dimer or a different form of GPVI dimer.
GPVI Dimeric Homeostasis
The mechanisms that maintain some GPVI in the dimeric state in quiescent platelets remain to be established. We discount the possibility that two GPVI molecules are complexed with one FcRγ to form a GPVI dimer, because an increase of dimer levels upon activation would entail the addition of a free GPVI molecule to a GPVI-FcR-γ complex, yet no free GPVI has been detected in human platelets. Instead, GPVI dimer would be formed from two GPVI monomers, each complexed with a homodimeric FcRγ, supporting the hypothesis of Berlanga et al. (9) and Feng et al. (27). Although the dimer was suggested to be formed from two D1D2, their weak affinity for each other makes spontaneous dimer formation in resting platelets unlikely under physiological conditions. Whether collagen binding stabilizes the dimeric form, as suggested by rapid dimerization of GPVI chains linked near the C terminus, cytoplasmic domains upon platelet activation (28) remain to be determined. Furthermore, because dimer formation is increased by both CRP and thrombin, it is tempting to suggest that there are other yet-unidentified signaling pathways to facilitate formation of more dimers or multimers of dimers, thereby reinforcing activation.
Physiological Role of GPVI Dimers
The physiological function of GPVI dimer was addressed by analyzing the effects of anti-GPVI antibodies on platelet activation by collagen. Jung et al. (8) showed that m-Fab-F inhibits collagen-induced platelet aggregation and GPVI dimer binding to collagen fibers. Here, we show that m-Fab-F inhibits adhesion of platelets to collagen under both static and flow conditions. About 34% of static platelet adhesion to collagen is through GPVI, with the remaining adhesion mainly due to the other platelet collagen receptor integrin α2β1. m-Fab-F markedly inhibits the GPVI-dependent component of adhesion to a level similar to that achieved with either 10B12 or 1C3 that binds both forms of GPVI, suggesting that monomeric GPVI contributes little to this adhesion. The flow adhesion experiment employed conditions similar to those of the vascular system in vivo, low shear (300 s−1) occurring in large arteries and high shear (1000 s−1) in medium-sized arteries (29), and it showed that m-Fab-F specifically inhibited thrombus formation on collagen. Previous flow studies have demonstrated defective thrombus formation on immobilized collagen using blood from GPVI-deficient patients (30, 31) and blood treated with an anti-GPVI antibody also showed a similar defect (32). In our flow experiments, m-Fab-F-treated blood showed defective thrombus formation to an extent similar to that seen in GPVI-deficient platelets, suggesting that GPVI dimer is the form contributing to thrombus formation under physiological conditions. Treatment with m-Fab-F markedly decreased the mean thrombus height and ZV50, parameters reporting platelet activation (18), consistent with the dimer being the form of GPVI that binds to collagen to initiate signaling pathways leading to platelet activation. 204-11 Fab decreased GPVI-dependent static adhesion and decreased both mean thrombus height and ZV50 under flow to a lesser extent than m-Fab-F, consistent with its epitope being dimer-specific but discrete from the collagen-binding site.
Conclusions and Implications of GPVI Dimerization
We have demonstrated that there is constitutive dimerization of GPVI in quiescent platelets; GPVI dimers exist in sufficient numbers to be the form of GPVI that contributes to platelet adhesion to collagen and is the form through which platelet activation is initiated upon collagen engagement. The pre-existence of high affinity GPVI dimers provides a mechanism for early engagement of GPVI, essential given the transient encounter of platelets with blood vessel collagens after arterial damage, and it is consistent with the prominence of GPVI in animal and in vitro models of thrombus deposition under flow. Moreover, the ability of thrombin, and perhaps other stimuli, to up-regulate GPVI dimer expression on the platelet surface offers a mechanism for sensitizing the circulating platelet to exposed subendothelial collagens.
Acknowledgments
We thank Dr. Nicolas Raynal for synthesizing collagen peptides and Dr. David Slatter for helpful discussions about their properties.
This work was supported by Grant PG/10/011/28199 from the British Heart Foundation (to S. M. J.).
- GPVI
- glycoprotein VI
- CRP
- collagen-related peptide.
REFERENCES
- 1. Moroi M., Jung S. M., Okuma M., Shinmyozu K. (1989) A patient with platelets deficient in glycoprotein VI that lack both collagen-induced aggregation and adhesion. J. Clin. Invest. 84, 1440–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sugiyama T., Okuma M., Ushikubi F., Sensaki S., Kanaji K., Uchino H. (1987) A novel platelet aggregating factor found in a patient with defective collagen-induced platelet aggregation and autoimmune thrombocytopenia. Blood 69, 1712–1720 [PubMed] [Google Scholar]
- 3. Watson S. P., Herbert J. M. J., Pollitt A. Y. (2010) GPVI and CLEC-2 in hemostasis and vascular integrity. J. Thromb. Haemost. 8, 1457–1467 [DOI] [PubMed] [Google Scholar]
- 4. Clemetson J. M., Polgar J., Magnenat E., Wells T. N., Clemetson K. J. (1999) The platelet collagen receptor glycoprotein VI is a member of the immunoglobulin superfamily closely related to FcαR and the natural killer receptors. J. Biol. Chem. 274, 29019–29024 [DOI] [PubMed] [Google Scholar]
- 5. Miura Y., Takahashi T., Jung S. M., Moroi M. (2002) Analysis of the interaction of platelet collagen receptor glycoprotein VI (GPVI) with collagen. A dimeric form of GPVI, but not the monomeric form, shows affinity to fibrous collagen. J. Biol. Chem. 277, 46197–46204 [DOI] [PubMed] [Google Scholar]
- 6. Jandrot-Perrus M., Busfield S., Lagrue A. H., Xiong X., Debili N., Chickering T., Le Couedic J. P., Goodearl A., Dussault B., Fraser C., Vainchenker W., Villeval J. L. (2000) Cloning, characterization, and functional studies of human and mouse glycoprotein VI. A platelet-specific collagen receptor from the immunoglobulin superfamily. Blood 96, 1798–1807 [PubMed] [Google Scholar]
- 7. Horii K., Kahn M. L., Herr A. B. (2006) Structural basis for platelet collagen responses by the immune-type receptor glycoprotein VI. Blood 108, 936–942 [DOI] [PubMed] [Google Scholar]
- 8. Jung S. M., Tsuji K., Moroi M. (2009) Glycoprotein (GP) VI dimer as a major collagen-binding site of native platelets. Direct evidence obtained with dimeric GPVI-specific Fabs. J. Thromb. Haemost. 7, 1347–1355 [DOI] [PubMed] [Google Scholar]
- 9. Berlanga O., Bori-Sanz T., James J. R., Frampton J., Davis S. J., Tomlinson M. G., Watson S. P. (2007) Glycoprotein VI oligomerization in cell lines and platelets. J. Thromb. Haemost. 5, 1026–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moroi M., Mizuguchi J., Kawashima S., Nagamatsu M., Miura Y., Nakagaki T., Ito K., Jung S. M. (2003) A new monoclonal antibody, mAb 204-11, that influences the binding of platelet GPVI to fibrous collagen. Thromb. Haemost. 89, 996–1003 [PubMed] [Google Scholar]
- 11. Al-Tamimi M., Mu F. T., Arthur J. F., Shen Y., Moroi M., Berndt M. C., Andrews R. K., Gardiner E. E. (2009) Anti-glycoprotein VI monoclonal antibodies directly aggregate platelets independently of FcγRIIa and induce GPVI ectodomain shedding. Platelets 20, 75–82 [DOI] [PubMed] [Google Scholar]
- 12. O'Connor M. N., Smethurst P. A., Farndale R. W., Ouwehand W. H. (2006) Gain- and loss-of-function mutants confirm the importance of apical residues to the primary interaction of human glycoprotein VI with collagen. J. Thromb. Haemost. 4, 869–873 [DOI] [PubMed] [Google Scholar]
- 13. Smethurst P. A., Joutsi-Korhonen L., O'Connor M. N., Wilson E., Jennings N. S., Garner S. F., Zhang Y., Knight C. G., Dafforn T. R., Buckle A., IJsseldijk M. J., De Groot P. G., Watkins N. A., Farndale R. W., Ouwehand W. H. (2004) Identification of the primary collagen-binding surface on human glycoprotein VI by site-directed mutagenesis and by a blocking phage antibody. Blood 103, 903–911 [DOI] [PubMed] [Google Scholar]
- 14. Raynal N., Hamaia S. W., Siljander P. R., Maddox B., Peachey A. R., Fernandez R., Foley L. J., Slatter D. A., Jarvis G. E., Farndale R. W. (2006) Use of synthetic peptides to locate novel integrin α2β1-binding motifs in human collagen III. J. Biol. Chem. 281, 3821–3831 [DOI] [PubMed] [Google Scholar]
- 15. Jung S. M., Moroi M. (1998) Platelets interact with soluble and insoluble collagens through characteristically different reactions. J. Biol. Chem. 273, 14827–14837 [DOI] [PubMed] [Google Scholar]
- 16. Jung S. M., Takemura Y., Imamura Y., Hayashi T., Adachi E., Moroi M. (2008) Collagen-type specificity of glycoprotein VI as a determinant of platelet adhesion. Platelets 19, 32–42 [DOI] [PubMed] [Google Scholar]
- 17. Jarvis G. E., Raynal N., Langford J. P., Onley D. J., Andrews A., Smethurst P. A., Farndale R. W. (2008) Identification of a major GpVI-binding locus in human type III collagen. Blood 111, 4986–4996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pugh N., Simpson A. M., Smethurst P. A., de Groot P. G., Raynal N., Farndale R. W. (2010) Synergism between platelet collagen receptors defined using receptor-specific collagen-mimetic peptide substrata in flowing blood. Blood 115, 5069–5079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smethurst P. A., Onley D. J., Jarvis G. E., O'Connor M. N., Knight C. G., Herr A. B., Ouwehand W. H., Farndale R. W. (2007) Structural basis for the platelet-collagen interaction. The smallest motif within collagen that recognizes and activates platelet glycoprotein VI contains two glycine-proline-hydroxyproline triplets. J. Biol. Chem. 282, 1296–1304 [DOI] [PubMed] [Google Scholar]
- 20. Wagner C. L., Mascelli M. A., Neblock D. S., Weisman H. F., Coller B. S., Jordan R. E. (1996) Analysis of GPIIb/IIIa receptor number by quantification of 7E3 binding to human platelets. Blood 88, 907–914 [PubMed] [Google Scholar]
- 21. Morton L. F., Hargreaves P. G., Farndale R. W., Young R. D., Barnes M. J. (1995) Integrin α2β1-independent activation of platelets by simple collagen-like peptides. Collagen tertiary (triple-helical) and quaternary (polymeric) structures are sufficient alone for α2β1-independent platelet reactivity. Biochem. J. 306, 337–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kehrel B., Wierwille S., Clemetson K. J., Anders O., Steiner M., Knight C. G., Farndale R. W., Okuma M., Barnes M. J. (1998) Glycoprotein VI is a major collagen receptor for platelet activation. It recognizes the platelet-activating quaternary structure of collagen, whereas CD36, glycoprotein IIb/IIIa, and von Willebrand factor do not. Blood 91, 491–499 [PubMed] [Google Scholar]
- 23. O'Connor M. N., Smethurst P. A., Davies L. W., Joutsi-Korhonen L., Onley D. J., Herr A. B., Farndale R. W., Ouwehand W. H. (2006) Apical transport and folding of prostate-specific membrane antigen occurs independent of glycan processing. J. Biol. Chem. 281, 33505–3351016956881 [Google Scholar]
- 24. Herr A. B., Farndale R. W. (2009) Structural insights into the interactions between platelet receptors and fibrillar collagen. J. Biol. Chem. 284, 19781–19785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lecut C., Arocas V., Ulrichts H., Elbaz A., Villeval J. L., Lacapère J. J., Deckmyn H., Jandrot-Perrus M. (2004) Identification of residues within human glycoprotein VI involved in the binding to collagen. Evidence for the existence of distinct binding sites. J. Biol. Chem. 279, 52293–52299 [DOI] [PubMed] [Google Scholar]
- 26. Loyau S., Dumont B., Ollivier V., Boulaftali Y., Feldman L., Ajzenberg N., Jandrot-Perrus M. (2012) Platelet glycoprotein VI dimerization, an active process inducing receptor competence, is an indicator of platelet reactivity. Arterioscler. Thromb. Vasc. Biol. 32, 778–785 [DOI] [PubMed] [Google Scholar]
- 27. Feng J., Garrity D., Call M. E., Moffett H., Wucherpfennig K. W. (2005) Convergence on a distinctive assembly mechanism by unrelated families of activating immune receptors. Immunity 22, 427–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arthur J. F., Shen Y., Kahn M. L., Berndt M. C., Andrews R. K., Gardiner E. E. (2007) Ligand binding rapidly induces disulfide-dependent dimerization of glycoprotein VI on the platelet plasma membrane. J. Biol. Chem. 282, 30434–30441 [DOI] [PubMed] [Google Scholar]
- 29. De Groot P. G. (2003) in Thrombosis: Fundamental and Clinical Aspects (Arnout J., Hoylaerts A. M., eds) pp. 32–38, Leuven University Press, Leuven, Belgium [Google Scholar]
- 30. Moroi M., Jung S. M., Shinmyozu K., Tomiyama Y., Ordinas A., Diaz-Ricart M. (1996) Analysis of platelet adhesion to a collagen-coated surface under flow conditions. The involvement of glycoprotein VI in the platelet adhesion. Blood 88, 2081–2092 [PubMed] [Google Scholar]
- 31. Hermans C., Wittevrongel C., Thys C., Smethurst P. A., Van Geet C., Freson K. (2009) A compound heterozygous mutation in glycoprotein VI in a patient with a bleeding disorder. J. Thromb. Haemost. 7, 1356–1363 [DOI] [PubMed] [Google Scholar]
- 32. Siljander P. R., Munnix I. C., Smethurst P. A., Deckmyn H., Lindhout T., Ouwehand W. H., Farndale R. W., Heemskerk J. W. (2004) Platelet receptor interplay regulates collagen-induced thrombus formation in flowing human blood. Blood 103, 1333–1341 [DOI] [PubMed] [Google Scholar]