Abstract
Background
CD154-blockade-based immunosuppression successfully prevents both humoral and cellular adaptive immune responses in baboons receiving α1,3-galactosyltransferase gene-knockout (GTKO) pig organs. Using a GTKO pig artery transplantation model in baboons, we evaluated the efficacy of CD28/B7 costimulatory pathway blockade in comparison to CD154-blockade.
Methods
Baboons received artery patch grafts from GTKO pigs, with either no (Group1), anti-CD154mAb-based (Group2), or CTLA4-Ig-based (Group3) immunosuppressive therapy. Anti-pig IgM and IgG antibody and cellular responses were monitored. Xenografts were immunohistologically evaluated for antibody and complement deposition, and cellular infiltration.
Results
Group1 baboons developed increased IgM and IgG antibody and cellular responses against GTKO antigens. In Group2, anti-CD154mAb alone prevented the development of both IgM and IgG antibody and cellular responses, but not cellular infiltration of the graft. In the single baboon that received ATG+MMF+anti-CD154mAb, cellular infiltration of the graft was not seen. In Group3, CTLA4-Ig with ATG+MMF inhibited the cellular proliferative response to pig antigens, but did not prevent the IgG response or cellular infiltration.
Conclusions
(i) Artery patch transplantation is a simple model to monitor the adaptive immune response to xenografts; (ii) anti-CD154mAb prevents sensitization, but not cellular infiltration (but, without anticoagulation, may result in early thrombosis of a pig xenograft); (iii) although in only one baboon, the addition of ATG and MMF prevents cellular infiltration, and (iv) replacement of anti-CD154mAb by CTLA4-Ig (at the doses used), even in combination with ATG and MMF, prevents the cellular proliferative response to GTKO pig antigens, but is insufficient to prevent the development of anti-pig antibodies.
Keywords: α1,3-galactosyltransferase gene-knockout; Anti-CD154 monoclonal antibody; Artery patch; Costimulation blockade; CTLA4-Ig; Pig; Xenotransplantation
INTRODUCTION
The availability of pigs homozygous for α1,3-galactosyltransferase gene-knockout (GTKO) (1, 2) has prolonged survival of pig organs after transplantation (Tx) into nonhuman primates. CD154 blockade-based immunosuppression has been shown to be successful in blocking the adaptive immune response in GTKO organ xenograft recipients. Initial studies by Kuwaki et al indicated that, when the recipient baboon received an effective immunosuppressive regimen based on costimulation blockade with an anti-CD154 monoclonal antibody (mAb) that prevented sensitization to pig antigens, GTKO pig heart grafts survived for 2 to 6 months (3, 4); graft failure was from a thrombotic microangiopathy (5) rather than from classical acute humoral xenograft rejection (AHXR).
Subsequently, however, Chen et al reported both hyperacute rejection and AHXR in renal grafts from GTKO pigs in baboons that received an immunosuppressive regimen that did not prevent an elicited antibody response (6). We have also seen early graft failure from AHXR when immunosuppressive therapy has been inadequate, particularly when an adaptive immune response developed (7). From this experience, we would conclude that an immunosuppressive regimen that successfully prevents the adaptive immune response is essential for prolonged GTKO graft survival.
In the studies of Kuwaki (3) and Tseng (4) the CD154 costimulation blockade-based immunosuppressive regimen consisted of induction with anti-thymocyte globulin (ATG), and maintenance with an anti-CD154mAb, mycophenolate mofetil (MMF), and methylprednisolone. It was uncertain whether all of the agents in the immunosuppressive regimen were essential to prevent an adaptive immune response. Yamada and his colleagues, using a GTKO pig-to-baboon thymokidney Tx model and the same costimulation blockade-based regimen, with additional therapy aimed at inducing tolerance, demonstrated that maintenance methylprednisolone may not be essential (8, 9). In addition, it is not known whether CD28/B7 costimulatory pathway blockade can replace CD154 blockade in that regimen, and efficiently prevent the adaptive immune response.
We developed a simple artery patch Tx procedure in the GTKO pig-to-baboon model, which successfully exposed the baboon to sufficient GTKO (nonGal) antigens to induce an adaptive immune response. In this model, we were able to assess the humoral and cellular immune responses to xenografts in nonhuman primates, with or without costimulation-based immunosuppression.
CD28/B7 costimulatory pathway blockade has been used in clinical trials in organ alloTx with encouraging results, e.g., by administering clinically-approved belatacept (10). As the currently-available anti-CD154mAbs are unlikely to be available for clinical use in the near future, we assessed the efficacy of CD28/B7 costimulatory pathway blockade versus CD154 blockade in preventing the adaptive immune response in xenograft recipients.
METHODS
Animals
Baboons (Papio species, n=14; Division of Animal Resources Oklahoma University Health Sciences Center, Oklahoma City, OK) weighing 6-10kg and of known ABO blood type, were recipients of GTKO pig artery grafts. GTKO pigs (n=7) of blood group O (nonA) weighing 7-20kg (Revivicor, Inc., Blacksburg, VA), generated by nuclear transfer/embryo transfer from modified fibroblasts from Large White/Landrace/Duroc cross-breed pigs (1, 11), served as sources of carotid artery patches.
All animal care was in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH publication No. 86-23, revised 1985). Protocols were approved by the University of Pittsburgh or the University of Maryland Institutional Animal Care and Use Committee.
Surgical procedures
Anesthesia in pigs and baboons, and intravascular catheter placements in baboons have been described previously (12-14). In the source GTKO pig, a length of carotid artery was excised, and stored in University of Wisconsin solution in ice (at approximately 4°C) until transplanted into the baboons. The ischemic period was approximately 6h (n=10) or 20h (n=4). (Some grafts were transported to us by colleagues at the University of Maryland. The ischemic period did not correlate with transplant outcome). The artery was divided longitudinally to form a patch, which was cut into two for insertion into two baboons. Each patch was approximately 2×1cm. In the recipient baboon, the abdomen was opened through a midline incision, and the abdominal aorta was exposed. After partial heparinization (100U/kg i.v.), the aorta was clamped immediately distal to the renal vessels and at the bifurcation, and opened longitudinally. The artery patch was sutured in place as an onlay graft using 5.0 polypropylene sutures (Figure 1). The clamps were then released, and, after determining there was good flow to both legs, the abdomen was closed.
Figure 1. GTKO pig artery patch xenograft.
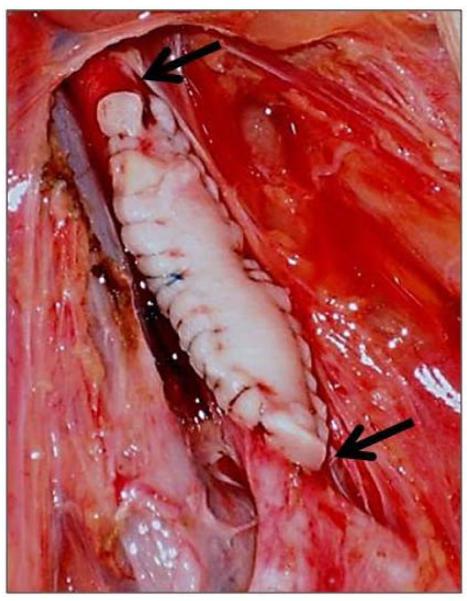
After exposure of the recipient abdominal aorta (below the renal arteries and above the bifurcation), the pig artery patch (2×1cm) was sutured in place as an onlay graft.
Arrows indicate the extent of the graft.
Immunosuppressive regimens
Recipients received either no immunosuppressive therapy (Group 1), anti-CD154mAb-based (Group 2), or CTLA4-Ig-based (Group 3) immunosuppression (Tables 1 and 2). However, baboons in all three groups received induction cobra venom factor (CVF; Complement Technology, Inc., Tyler, Texas) and methylprednisolone (on days −1, 0, and 1), which may prevent the occasional incidence of hyperacute rejection that has been reported after GTKO organ xenotransplantation; it has been our policy to administer CVF to all baboon recipients of GTKO pig heart grafts. The decision not to include corticosteroids for maintenance in the regimen was based on the observations of Yamada and his colleagues mentioned above (8,9). Anti-CD154mAb (ABI193) was generously provided by Novartis Pharma, Basel, Switzerland). Numerous previous measurements in other baboons have demonstrated that, at the dose given, the serum level is maintained at >200μg/mL throughout the course of the experiment. ATG was obtained from Genzyme, Cambridge, MA, and mycophenolate mofetil (MMF) was obtained from Roche, Nutley, NJ. CTLA4-Ig (Abatacept) was obtained, from BMS, Princeton, NJ. (Belatacept [LEA 29Y] was not available to us). CTLA4-Ig was also not measured during the course of the experiments, but was measured in retrospect. At the dose given, the serum level was >150μg/mL. In contrast to most of our studies involving pig organ Tx in baboons, maintenance corticosteroid therapy was not included in the regimen (based on the observations by Yamada and colleagues mentioned above [8,9])
TABLE 1.
INDUCTION AND MAINTENANCE IMMUNOSUPPRESSIVE THERAPY, AND SUPPORTIVE THERAPY
| Dose | Duration | |
|---|---|---|
| Induction Therapy | ||
| Thymoglobulin (ATG) | 1-10mg/kg i.v. | days −3, −1 * |
| Cobra venom factor | 100 units/day i.v. | days −1, 0, 1 |
| Methylprednisolone | 5mg/kg | before each dose of ATG, and on days −1, 0, 1 |
| Maintenance Therapy | ||
| Anti-human CD154mAb | 25mg/kg i.v | days −1, 0, 4, 7, 10, 14 and then every 5 days (to maintain a blood trough level >400μg/mL) |
| OR | ||
| CTLA4-Ig | 25mg/kg i.v | days −1, 0, 2, 4, 7, 10, 14, and then every 5 days (to maintain a blood trough level of >200μg/mL) |
| PLUS | ||
| MMF | 25-110mg/kg/day | ontinuous i.v. infusion from day −2 (to maintain a constant blood level of 3-5μg/mL) |
| Heparin# | 5-50U/kg/h | from day 0 (to maintain PTT at 100-150sec from day 3) |
| Ketorolac# | 5mg/kg | before each dose of anti-CD154mAb, beginning on day −1 |
| Ganciclovir | 5mg/kg/day | from day −3 |
Day −1 dose of ATG was given only if needed to reduce lymphocyte count <500×103/mm3.
Heparin and ketorolac were given in selected cases.
TABLE 2.
GROUPS, IMMUNOSUPPRESSIVE REGIMENS, SURVIVAL AND COMPLICATIONS AFTER GTKO PIG ARTERY TRANSPLANTATION IN BABOONS*
| Group | Sub- group |
Immunosuppressive Therapy (a) |
Survival (days) |
Complications |
|---|---|---|---|---|
| 1 | None (n=3) | 14 (n=1) >28 (n=2) |
Ruptured patch graft; euthanized | |
|
| ||||
| 2 | 2A | Anti-CD154mAb alone (n=5) | <2 (n=1) >28 (n=4) |
Aortic thrombosis; euthanized |
| 2B | Anti-CD154mAb-based full regimen (Anti-CD154mAb + ATG + MMF; n=1) |
>28 (n=1) | ||
|
| ||||
| 3 | 3A | CTLA4-Ig alone (n=1) | >28 (n=1) | |
| 3B | CTLA4-Ig-based full regimen (CTLA4-Ig + ATG + MMF; n=4) |
14 (n=1) >28 (n=3) (b) |
Disconnected catheter, euthanized | |
Anticoagulation
Despite the importance of anticoagulation when using anti-CD154mAb in the pig-to-baboon xenoTx model (15, 16), it was initially felt that this would be unnecessary in the artery patch model (Group 2). However, when anti-CD154mAb was administered alone (without anticoagulation), the abdominal aorta thrombosed at the site of the graft within 48h (Table 2), suggesting that, in the presence of anti-CD154mAb, anticoagulation was required. In all subsequent experiments in Group 2, either continuous heparin (n=3) or intermittent ketorolac (n=3) (17) was administered (Table 1). Heparin and ketorolac were equally successful in preventing artery thrombosis. The single baboon in Group 3 that received 3 doses of anti-CD154mAb received heparin throughout the course of study.
Measurement of anti-pig antibodies
Anti-pig (nonGal) antibody levels were measured by flow cytometry using GTKO porcine aortic endothelial cells (PAEC) as target cells. Baboon sera (20μL) were heat-inactivated for 30min at 56°C, and then incubated with 0.1-0.2×106 PAEC for 30min at 4°C. PAEC were washed and incubated for 30min at 4°C with secondary antibodies (1:20) - anti-human FITC-IgM (μ-chain-specific) and FITC-IgG (γ-chain-specific) (Invitrogen, Carlsbad, CA). Negative controls were obtained by incubating the target cells with secondary anti-human antibodies only (and no serum). Relative mean fluorescence intensity (MFI) was calculated by dividing the MFI value for each sample by the negative control. Samples were analysed using LSR II flow. Data were analyzed using Flowjo or WinMDI software.
Mixed leukocyte reaction (MLR)
MLR were carried out before Tx and between days 21-28 after Tx. Baboon peripheral blood mononuclear cells (PBMC) and irradiated GTKO pig PBMC were isolated from heparinized blood by centrifugation at 700g for 30min over Ficoll-Paque PLUS (GE Healthcare, Piscataway, NJ). The total mononuclear cell fraction was washed twice with PBS (Invitrogen, Carlsbad, CA) and contaminating red cells were lysed with ammonium chloride/potassium solution, when necessary. PBMC were then resuspended in AIM V medium (Invitrogen). Baboon PBMC were used as responders (0.4×106 cells/well) and irradiated GTKO pig PBMC as stimulators (0.4×106 cells/well), with a responder:stimulator ratio of 1:1. 3H-thymidine (1μCi/well) was added during the last 16h of incubation. Cells were harvested and analyzed by beta-scintillation counter (PerkinElmer, Waltham, MA). Samples were tested in quadruplicate and the mean of 3H-thymidine uptake was calculated. Stimulation index (SI) was calculated by dividing the value of proliferation in response to GTKO PBMC by the value of proliferation in response to autologous PBMC.
Monitoring of CD4+ and CD8+ T cells
Whole blood samples were collected before any therapy, and 2-3 weeks after Tx. FITC-conjugated mouse anti-human CD3ε (Cat# 556611), PEcy7-conjugated anti-CD4 (Cat# 557852), and PE-conjugated anti-CD8 (Cat# 555367) antibodies were obtained from BD Pharmingen (San Diego, CA). Percentages of CD3+CD4+ and CD3+CD8+ T cells were determined by flow cytometry. Whole blood samples were analysed using LSR II flow. All data were analyzed using Flowjo or WinMDI software.
Histopathology and immunohistopathology of porcine artery grafts
Pig artery patch xenografts were isolated at euthanasia. For conventional histology, tissues were fixed in 10% formalin and embedded in paraffin. Sections (4μm) were stained with hematoxylin and eosin (H&E) for light microscopy. Staining for IgM, IgG, complement (C3), CD3+, CD4+, and CD8+ T cells, CD20+ B cells, neutrophils (myeloperoxidase), macrophages (CD68+), and platelets (CD42+) was performed on paraffin sections, as previously described (6, 7)
RESULTS
Recipient baboon survival and clinical complications
In Group 1, one graft ruptured into the lumen of adherent small intestine on day 14 (presenting as acute anemia); the baboon was euthanized (Table 2). One Group 2 baboon was euthanized following thrombosis of the abdominal aorta within 48h at the site of the graft; this complication presented with paresis of the lower limbs. Postmortem examination of the abdominal aorta demonstrated no technical reason for the development of thrombosis. One Group 3 baboon was euthanized on day 14 following a catheter complication. All other grafts in all groups remained in situ without complication for 28-33 days, at which time the baboons were electively euthanized.
Antibody responses to GTKO pig artery grafts
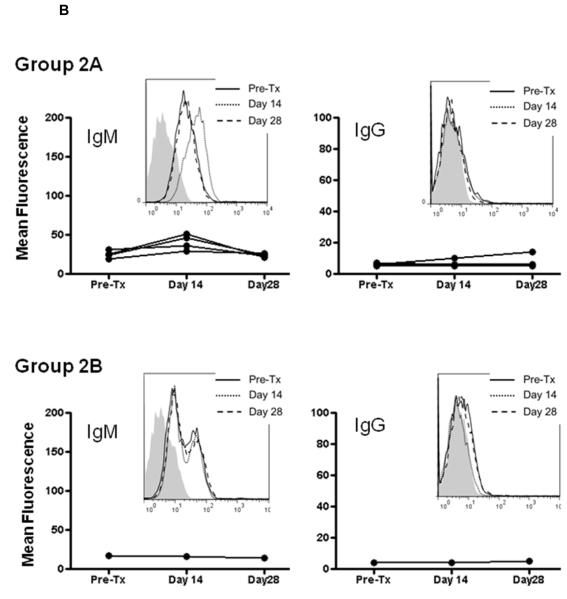
Baboon IgM antibodies directed to pig nonGal antigens were present in all baboons before artery Tx (Figure 2). Low titers of anti-nonGal IgG were also detectable in all recipients. In Group 1, there was an increase in both anti-nonGal IgM and IgG titers within 14 days, indicating sensitization to nonGal antigens (Figure 2A). While IgM was declining by day 28, IgG continued to rise (probably due to antibody Isotype class switch). When anti-CD154mAb was administered alone (Group 2A), a very slight increase in IgM was observed in all four recipients on day 14, which decreased to pre-transplant levels by day 28. There was no detectable increase in IgG on days 14 or 28, except in one recipient, where a possible very slight increase was measured (Figure 2B). In the one recipient that received anti-CD154mAb combined with induction ATG and MMF maintenance (Group 2B), there was no increase in IgM or IgG (Figure 2B).
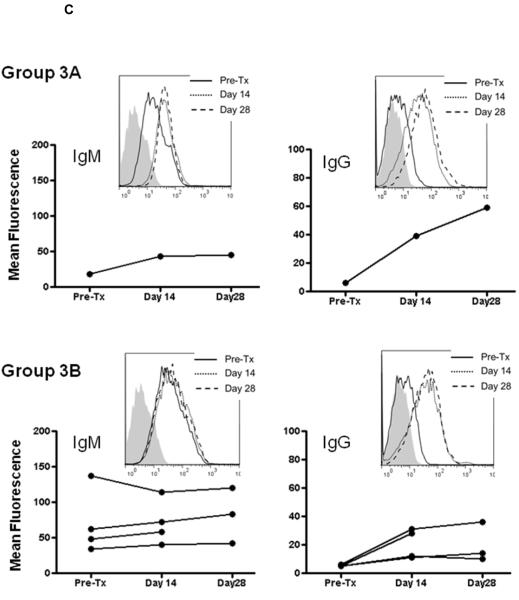
Figure 2. IgM and IgG antibody levels in baboon recipients of GTKO pig artery patches.
IgM (left) and IgG (right) antibodies were measured by flow cytometry. Graphs represent relative mean fluorescence values (as described in Methods). Insets represent histograms for one representative recipient from each group (before Tx [solid line], 14 days after Tx [dotted line], and 28 days after Tx [dashed line]; isotype controls are in grey). (A) Group 1 (no immunosuppression) showing initial increase in IgM followed by decline, and rise im IgG. (B) Group 2A (anti-CD154mAb alone) – slight transient increase in IgM, and no or possible minimal increase in IgG, and Group 2B (anti-CD154mAb+ATG+MMF) - no significant increase in IgM or IgG. (C) Group 3A (CTLA4-Ig alone) - increased IgM and IgG, and Group 3B (CTLA4-Ig+ATG+MMF) – a very slight increase in IgM in 3 baboons, and a clear increase in IgG in 2 baboons.
Although only in one baboon, when CTLA4-Ig was given alone (Group 3A), there were increases in both IgM and IgG on days 14 and 28 (Figure 2C). When CTLA4-Ig was administered with ATG+MMF (Group 3B), there was a slight increase in IgM on days 14 and 28 in three recipients (Figure 2C), and a moderate increase in IgG by day 14 in at least two recipients (one of which had received three doses of anti-CD154mAb in the induction phase [Table 2]), maintained to day 28. In the remaining two, there was a slight rise in IgG.
Antibody and complement deposition in GTKO pig artery grafts
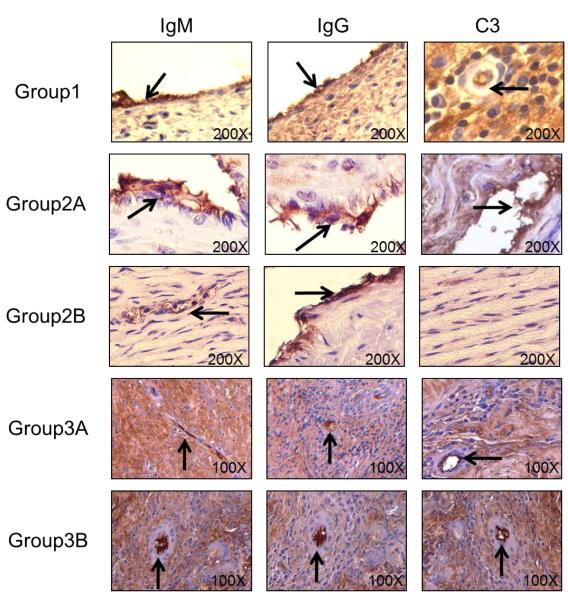
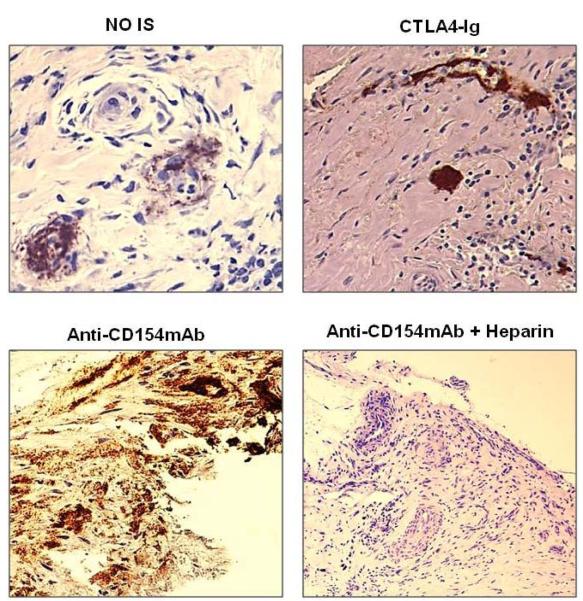
With no immunosuppression (Group 1), antibody (IgM and IgG) and complement (C3) deposition were detected in all three aortic patches (Figure 3). When either anti-CD154mAb or CTLA4-Ig was given alone (Groups 2A and 3A), or when CTLA4-Ig was administered with ATG+MMF (Group 3B), antibody and complement deposition were still detected in the grafts (Figure 3). When anti-CD154mAb was administered with ATG+MMF (Group 2B), IgG deposition was detected, with minimal IgM and no complement deposition.
Figure 3. Antibody and complement deposition in GTKO pig artery patch xenograft recipients.
By immunohistochemistry, deposition of antibodies (IgM and IgG) and complement (C3) were determined in aortic patch xenografts. Magnification is indicated in each figure. Brown indicates positive staining. In Group 1 (no immunosuppression), and in Groups 2A, 3A and 3B antibody and complement deposition were detected in all grafts. With an anti-CD154mAb-based full regimen (Group 2B), minimal IgM, strong IgG, and no complement deposition were detected. (Arrows indicate positive staining)
Baboon PBMC proliferation in response to pig nonGal antigens
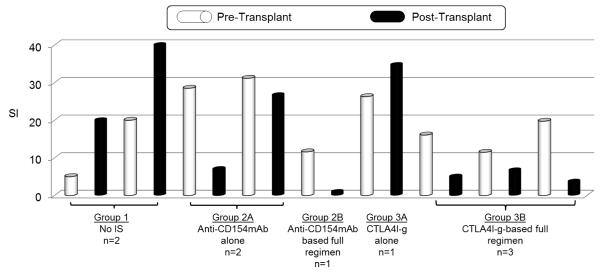
PBMC were not obtained in every baboon, either due to complications (Table 2) or impaired cell viability (probably related to high levels of anti-CD154mAb). On MLR, with no immunosuppression (Group 1), two recipients developed increased PBMC proliferation in response to GTKO PBMC after Tx, indicating sensitization (Figure 4). Recipients that received anti-CD154mAb alone (Group 2A) showed reduced PBMC proliferation, while in the one recipient that received CTLA4-Ig alone (Group 3A), PBMC proliferation was not reduced. In recipients that received either the full anti-CD154 mAb (Group 2B) or CTLA4-Ig (Group 3B)-based regimen, PBMC proliferation was inhibited.
Figure 4. Cellular response in GTKO pig artery patch recipients.
Baboon PBMC were collected before (white) and one month after (black) artery patch Tx, and were tested for proliferation in response to irradiated GTKO PBMC in MLR. Proliferation is presented as stimulation index (SI). PBMC proliferation was increased with either no immunosuppression (Group 1) or when CTLA4-Ig alone was administered (Group 3A). There was minimal or no increase in PBMC proliferation when anti-CD154mAb alone was administered (Group 2A), and no increase when an anti-CD154mAb-based full regimen (Group 2B) or a CTLA4-Ig-based full regimen (Group 3B) was administered.
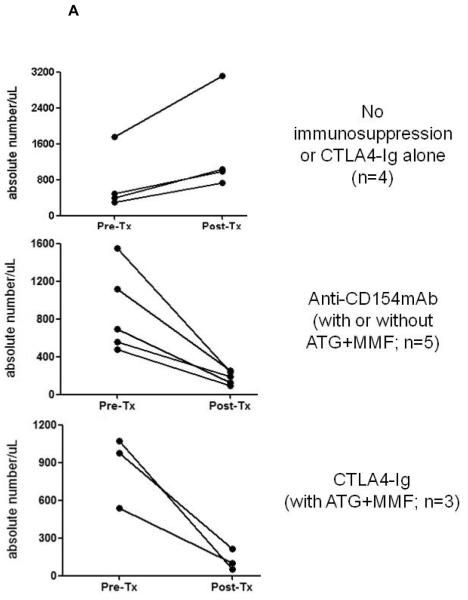
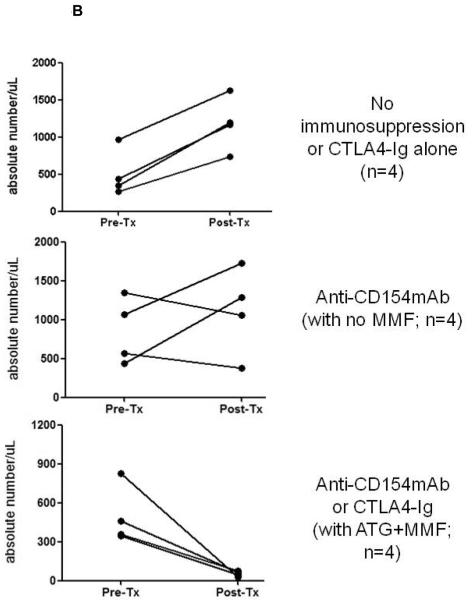
CD4+ and CD8+ T cell kinetics in artery patch recipients
The numbers of CD4+ (Figure 5A) and CD8+ (Figure 5B) T cells were monitored before and 21-28 days after Tx. With no immunosuppression (Group 1) or when CTLA4-Ig alone was administered, both CD4+ and CD8+ T cells increased after Tx. When anti-CD154mAb was given alone, low levels of CD4+ T cells were maintained after Tx, while low levels of CD8+ T cells were maintained in 2 of 4 baboons. When anti-CD154mAb or CTLA4-Ig was combined with ATG+MMF, low numbers of CD4+ and CD8+ T cells were maintained. There was a tendency towards low numbers of B cells after Tx when either of the full regimens was administered (not shown).
Figure 5. CD4+ and CD8+ T cell kinetics in artery patch recipients.
Absolute numbers of (A) CD4+ and (B) CD8+ T cells were monitored in the blood, before and 21-28 days after Tx. Whole blood samples were analyzed by flow cytometry to obtain the percentages of CD4+ (A) and CD8+ (B) T cells, and absolute numbers were calculated based on the total white blood cell count. (Note that the results are not presented by Group, as in the other figures.) In (A), data are presented from recipients with no immunosuppressive therapy or CTLA4-Ig alone (top), anti-CD154mAb +/− ATG+MMF (middle), or with the CTLA4-Ig-based full regimen (bottom). In (B), data in are presented from recipients with no immunosuppressive therapy or CTLA4-Ig alone (top), anti-CD154mAb alone (middle), or with the anti-CD154mAb- or CTLA4-Ig-based full regimens (bottom).
Cellular infiltration in GTKO pig artery grafts
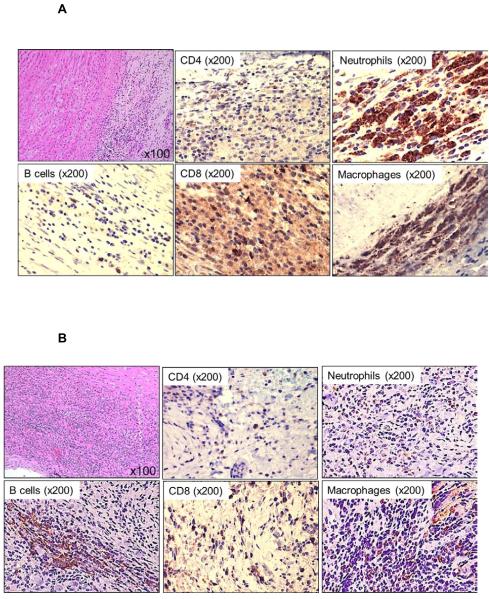
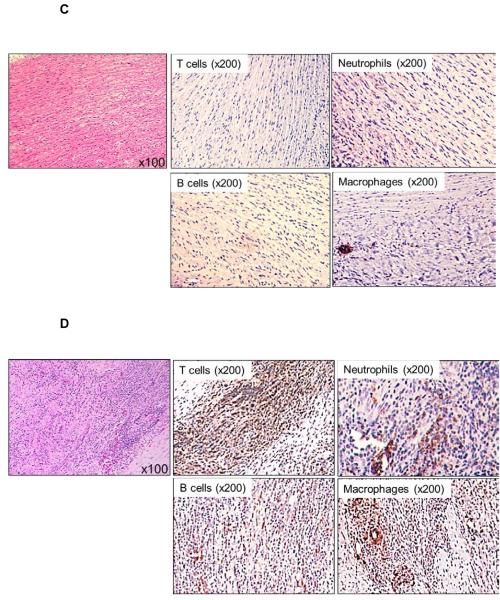
With no immunosuppression (Group 1), there was intense cellular infiltration in all layers of the artery patch (intima, media, and adventitia), consisting mainly of T cells (CD4+ and CD8+), some B cells, with abundant macrophages and neutrophils (Figure 6A). When anti-CD154mAb (Group 2A) was given alone, moderate T cell (with fewer CD4+T than CD8+T cells) and B cell infiltration was seen, with stronger infiltration of neutrophils and macrophages (Figure 6B). Although only in one recipient, when the anti-CD154mAb-based full regimen was used (Group 2B), there was no cellular infiltration, except for minimal macrophage infiltration (Figure 6C). When CTLA4-Ig was used alone (Group 3A) there was intense T cell and moderate B cell infiltration, with moderate neutrophil and macrophage infiltration (Figure 6D). When the full CTLA4-Ig-based regimen was administered (Group 3B), there were fewer CD4+ and CD8+ T cell and no B cell infiltrates, but extensive neutrophil and macrophage infiltrates were present (Figure 6E).
Figure 6. Cellular infiltration of innate and adaptive immune cells in GTKO pig artery patches.
Histology of pig artery patches (H&E), and, by immunohistochemistry, infiltration with T cells (CD3+, CD4+, CD8+), B cells (CD20), neutrophils and macrophages (CD68) was assessed. Magnification is indicated in each figure. Positive cellular staining is in brown. (A) With no immunosuppression (Group1), there was strong cellular infiltration, including CD4+ and CD8+ T cells, B cells, neutrophils, and macrophages. (B) When anti-CD154mAb was administered alone (Group 2A), cellular infiltration consisted mainly of B cells, macrophages and neutrophils, with fewer CD4+ and CD8+ T cells. (C) With the anti-CD154mAb-based full regimen (Group 2B), there were no T cell, B cell, or neutrophil infiltrates, and very few macrophages. (D) When CTLA4-Ig was administered alone (Group 3A), there was strong T and B cellular infiltration, with fewer macrophages. (E) With the CTLA4-Ig-based full regimen (Group 3B), cell infiltration consisted more of macrophages and neutrophils, with fewer CD4+ and CD8+ T cells, and no B cells.
Platelet deposition in GTKO pig artery patch grafts
Platelet deposition was detected in all artery patch grafts in Groups 1 and 3 (except the single baboon in Group 3 that received a heparin infusion) (Figure 7). Platelet deposition was prevented only when heparin was administered Group 2); when ketorolac replaced heparin, platelet deposition could still be detected.
Figure 7. Platelet deposition in GTKO pig artery patches.
Platelet deposition in the aortic patches was assessed by immunohistochemistry. Platelet deposition (CD42+) was detected in the patches in Group 1 (top left) and Group 3 (top right) and in the Group 2 patches when ketorolac was administered (bottom left). Only when heparin was administered with the anti-CD154mAb-based full regimen was platelet deposition absent (bottom right).
DISCUSSION
Our data indicate that, in the GTKO pig-to-baboon artery patch Tx model, in the absence of immunosuppressive therapy (Group 1) baboons developed humoral and cellular adaptive immune responses. Rejection was associated with deposition of IgM, IgG, and complement, and with cellular infiltrates (although some of these features were also seen in non-rejecting grafts in other Groups). The nature of the cellular infiltrate suggested the importance of the innate immune response, as reported previously (7), in addition to T and B cell infiltrates. We concluded that the GTKO pig artery patch graft is a simple model to monitor innate and adaptive immune responses to pig xenografts.
The survival of two of the three pig artery patch xenografts in non-immunosuppressed baboons was perhaps surprising. Whereas one patch eroded and ruptured, leading to death from hemorrhage, two did not. We presume this was due to bolstering the patches externally with adequate host tissue (e.g., omentum) and replacement of donor endothelial cells with fibrous tissue. The primary purpose of the study, however, was to expose the recipient to sufficient pig antigen for an essential period of time to induce an adaptive immune response.
The administration of anti-CD154mAb alone (Group 2A) prevented an elicited IgG antibody response and a proliferative response on MLR, but did not completely prevent cellular infiltration in the graft. Although tested in only one recipient, the addition of ATG and MMF (Group 2B) prevented both the elicited antibody and cellular responses and cellular infiltration of the graft. These data correlate well with those from a GTKO heart Tx model (3, 4, 16). These observations indicate that methylprednisolone used in previously reported xenoTx studies (3, 4, 16, 18, 19) may not be absolutely essential in the prevention of the adaptive immune response, as reported by Yamada and his colleagues (8, 9). It should be noted that anti-CD154mAb does not act only by blocking the CD40/CD154 pathway, but also by depleting activated T cells (that express CD154) (3,4,16), and thus has a significant impact on the MLR.
At the dosage used, CTLA4-Ig (Group 3) did not seem to be a potent alternative for anti-CD154mAb. Although no proliferative response was documented on MLR, CTLA4-Ig (even in combination with ATG and MMF) did not completely prevent either an elicited antibody response or graft infiltration, although the increase in anti-pig IgG was only modest and the cellular infiltrate reduced when compared to that in Group 1. CTLA4-Ig, therefore, did not replicate the results obtained with anti-CD154mAb. The addition to CTLA4-Ig of three doses of anti-CD154mAb in the induction phase did not modify the outcome. Although, on the basis of studies of alloTx, CTLA4-Ig-based therapy might not have been expected to be as efficient as anti-CD154mAb-based therapy, we are not aware of any xenoTx study in nonhuman primates in which this observation has been documented previously.
Our in vivo data are in contrast to published in vitro studies indicating that the human anti-porcine cellular response was blocked more effectively by CTLA4-Ig than by anti-CD154mAb (20). Alternative CD28/B7 costimulatory pathway blockade agents, e.g., LEA29Y (belatacept), might prove more effective, though this remains uncertain (21); however, LEA 29Y was not available to us at the time of this study. CD40-specific antibody (e.g., Chi220) has been used as an alternative strategy to block the CD40/CD154 pathway; graft survival of neonatal porcine islets in Rhesus macaques was maintained up to a mean of 90 days (22).
One point that should be considered is that baboons in Group 2 received either continuous heparin or intermittent ketorolac, whereas those in Group 3 did not. These agents may inhibit chemotaxis, alter lymphocyte activity, and inhibit neutrophil activation and aggregation, and therefore may have affected the outcome of the experiments, in particular in relation to cellular infiltration of the grafts. In retrospect, these agents should have been administered to the baboons in all groups. Nevertheless, we believe that the results of our study do indicate a difference in potency in relation to suppressing the immune response between anti-CD154 mAb and CTLA4-Ig.
Although Group 2B (full anti-CD154mAb-based regimen) consisted of only one experiment, we have previously demonstrated that the full anti-CD154mAb-based regimen prevents sensitization after GTKO pig heart Tx in baboons, and therefore did not feel it essential to carry out further experiments in the present study. Furthermore, although Group 3A (CTLA4-Ig alone) also consisted of only one experiment, the fact that the full CTLA4-Ig-based regimen (Group 3B) failed to prevent sensitization indicated that further experiments in Group 3A were probably unwarranted.
The greatly increased antigen load of an organ xenograft may prove a greater ‘challenge’ to the immune system of the host than an artery patch. However, in that we have demonstrated that a CTLA4-Ig-based immunosuppressive regimen does not prevent sensitization to a GTKO pig cardiac xenograft, whereas an anti-CD154mAb-based regimen does (23; Ekser B, manuscript in progress), a close correlation exists between the results in the current study and those seen in genetically-engineered pig heart transplants at our center. In this respect, we would suggest that artery patch Tx is an adequate model to evaluate the efficacy of immunosuppression on prevention of the adaptive immune response in xenograft recipients.
In view of the significant role coagulation dysfunction plays in the failure of pig organ xenografts (7, 13, 24-27), our observation that thrombosis led to occlusion of the abdominal aorta at the site of the GTKO pig patch graft within the first 48h when an anti-CD154mAb was administered (Group 2), yet was not seen in the absence of this agent (Groups 1 and 3), suggests that anti-CD154mAb is thrombogenic in the presence of even a small pig xenograft. The association of thrombotic complications with anti-CD154mAb therapy has been demonstrated in alloTx (17, 28) and xenoTx (16, 19). Our study also confirms the report by Kawai et al in a kidney alloTx model that the anti-inflammatory agent, ketorolac, successfully prevents the thrombosis associated with anti-CD154mAb therapy (17). However, whether ketorolac would prove sufficient after pig organ Tx, when the surface of graft vascular endothelium exposed would be immeasurably greater, remains uncertain.
Although this study was not aimed at monitoring parameters of coagulation dysfunction, the artery patch model might provide relevant data. In the present study, platelet adhesion to the grafts was seen in several cases. Furthermore, during the one month period of follow-up, a rise in thrombin-antithrombin (TAT) was documented, although platelet counts, fibrinogen and fibrin degradation products did not change significantly (data not shown). We suggest that coagulation dysfunction occurs, but develops at a reduced speed compared to that seen after pig organ xenoTx.
We conclude that (i) artery patch Tx is a simple model to monitor the innate and adaptive immune responses to a xenograft; (ii) without anticoagulation, anti-CD154mAb may result in early thrombosis of a pig xenograft; (iii) anti-CD154mAb alone prevents sensitization, but not cellular infiltration; (iv) the addition of ATG and MMF prevents cellular infiltration, and (v) replacement of anti-CD154mAb by CTLA4-Ig (at the doses used), even in combination with ATG and MMF, is insufficient to prevent an adaptive humoral immune response.
ACKNOWLEDGEMENTS
We thank Hiroshi Watanabe MD for assistance with the surgical procedures, and Robin Pierson MD for providing GTKO pig arteries to us on two occasions. We thank Walter Schuler, PhD, and his colleagues at the Novartis Institutes for Biomedical Research (Basel, Switzerland) for making ABI793 available to us, and Roche Pharmaceuticals (Nutley, NJ, USA) for generously providing mycophenolate mofetil. Work in our laboratories is supported in part by NIH grants #U01 AI068642, #R21 A1074844, and #U19 A1090959, and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc. Burcin Ekser, MD is a recipient of NIH NIAID T32 AI 074490 Training Grant. Most of the baboons used in the study were from the Oklahoma University Health Sciences Center Division of Animal Resources, which is supported by NIH P40 sponsored grant RR012317-09.
ABBREVIATIONS
- AHXR
acute humoral xenograft rejection
- ATG
anti-thymocyte globulin
- CVF
cobra venom factor
- GTKO
α1,3-galactosyltransferase gene-knockout
- mAb
monoclonal antibody
- MMF
mycophenolate mofetil
- pAEC
porcine aortic endothelial cells
- PBMC
peripheral blood mononuclear cells
- Tx
transplantation
Footnotes
DISCLOSURE David Ayares is shareholder in Revivicor,. Inc. The other authors declare they have no conflict of interest.
REFERENCES
- 1.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299(5605):411–4. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolber-Simonds D, Lai L, Watt SR, Denaro M, Arn S, Augenstein ML, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci U S A. 2004;101(19):7335–40. doi: 10.1073/pnas.0307819101. PMCID: 409919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11(1):29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 4.Tseng YL, Kuwaki K, Dor FJ, Shimizu A, Houser S, Hisashi Y, et al. alpha1,3-Galactosyltransferase gene-knockout pig heart transplantation in baboons with survival approaching 6 months. Transplantation. 2005;80(10):1493–500. doi: 10.1097/01.tp.0000181397.41143.fa. [DOI] [PubMed] [Google Scholar]
- 5.Houser SL, Kuwaki K, Knosalla C, Dor FJ, Gollackner B, Cheng J, et al. Thrombotic microangiopathy and graft arteriopathy in pig hearts following transplantation into baboons. Xenotransplantation. 2004;11(5):416–25. doi: 10.1111/j.1399-3089.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen G, Qian H, Starzl T, Sun H, Garcia B, Wang X, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med. 2005;11(12):1295–8. doi: 10.1038/nm1330. PMCID: 3018862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ezzelarab M, Garcia B, Azimzadeh A, Sun H, Lin CC, Hara H, et al. The innate immune response and activation of coagulation in alpha1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87(6):805–12. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11(1):32–4. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 9.Griesemer AD, Hirakata A, Shimizu A, Moran S, Tena A, Iwaki H, et al. Results of gal-knockout porcine thymokidney xenografts. Am J Transplant. 2009;9(12):2669–78. doi: 10.1111/j.1600-6143.2009.02849.x. PMCID: 2801602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study) Am J Transplant. 2010;10(3):535–46. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 11.Dai Y, Vaught TD, Boone J, Chen SH, Phelps CJ, Ball S, et al. Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat Biotechnol. 2002;20(3):251–5. doi: 10.1038/nbt0302-251. [DOI] [PubMed] [Google Scholar]
- 12.Cooper DKC, Ye Y, Niekrasz M. Heart transplantation in primates. In: Cramer DVPL, Makowka L, editors. Handbook of Animal Models in Transplantation Research Boca Raton. CRC Press; 1994. pp. 173–200. [Google Scholar]
- 13.Kozlowski T, Shimizu A, Lambrigts D, Yamada K, Fuchimoto Y, Glaser R, et al. Porcine kidney and heart transplantation in baboons undergoing a tolerance induction regimen and antibody adsorption. Transplantation. 1999;67(1):18–30. doi: 10.1097/00007890-199901150-00004. [DOI] [PubMed] [Google Scholar]
- 14.Buhler L, Yamada K, Kitamura H, Alwayn IP, Basker M, Appel JZ, 3rd, et al. Pig kidney transplantation in baboons: anti-Gal(alpha)1-3Gal IgM alone is associated with acute humoral xenograft rejection and disseminated intravascular coagulation. Transplantation. 2001;72(11):1743–52. doi: 10.1097/00007890-200112150-00007. [DOI] [PubMed] [Google Scholar]
- 15.Knosalla C, Gollackner B, Cooper DK. Anti-CD154 monoclonal antibody and thromboembolism revisted. Transplantation. 2002;74(3):416–7. doi: 10.1097/00007890-200208150-00024. [DOI] [PubMed] [Google Scholar]
- 16.Kuwaki K, Knosalla C, Dor FJ, Gollackner B, Tseng YL, Houser S, et al. Suppression of natural and elicited antibodies in pig-to-baboon heart transplantation using a human anti-human CD154 mAb-based regimen. Am J Transplant. 2004;4(3):363–72. doi: 10.1111/j.1600-6143.2004.00353.x. [DOI] [PubMed] [Google Scholar]
- 17.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6(2):114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 18.Buhler L, Awwad M, Basker M, Gojo S, Watts A, Treter S, et al. High-dose porcine hematopoietic cell transplantation combined with CD40 ligand blockade in baboons prevents an induced anti-pig humoral response. Transplantation. 2000;69(11):2296–304. doi: 10.1097/00007890-200006150-00013. [DOI] [PubMed] [Google Scholar]
- 19.Knosalla C, Gollackner B, Buhler L, Mueller NJ, Houser S, Mauiyyedi S, et al. Correlation of biochemical and hematological changes with graft failure following pig heart and kidney transplantation in baboons. Am J Transplant. 2003;3(12):1510–9. doi: 10.1046/j.1600-6135.2003.00258.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee RS, Yamada K, Womer KL, Pillsbury EP, Allison KS, Marolewski AE, et al. Blockade of CD28-B7, but not CD40-CD154, prevents costimulation of allogeneic porcine and xenogeneic human anti-porcine T cell responses. J Immunol. 2000;164(6):3434–44. doi: 10.4049/jimmunol.164.6.3434. [DOI] [PubMed] [Google Scholar]
- 21.Larsen CP, Pearson TC, Adams AB, Tso P, Shirasugi N, Strobert E, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5(3):443–53. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 22.Thompson P, Cardona K, Russell M, Badell IR, Shaffer V, Korbutt G, et al. CD40-specific costimulation blockade enhances neonatal porcine islet survival in nonhuman primates. Am J Transplant. 2011;11(5):947–57. doi: 10.1111/j.1600-6143.2011.03509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ekser B, Long C, Kumar G, et al. Heart transplantation using genetically-engineered pigs: can CTLA4-Ig replace anti-CD154mAb in the immunosuppressive regimen? (Abstract) Xenotransplantation. 2011;18(5):284. [Google Scholar]
- 24.Ierino FL, Kozlowski T, Siegel JB, Shimizu A, Colvin RB, Banerjee PT, et al. Disseminated intravascular coagulation in association with the delayed rejection of pig-to-baboon renal xenografts. Transplantation. 1998;66(11):1439–50. doi: 10.1097/00007890-199812150-00006. [DOI] [PubMed] [Google Scholar]
- 25.Buhler L, Basker M, Alwayn IP, Goepfert C, Kitamura H, Kawai T, et al. Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates. Transplantation. 2000;70(9):1323–31. doi: 10.1097/00007890-200011150-00010. [DOI] [PubMed] [Google Scholar]
- 26.Robson SC, Cooper DK, d’Apice AJ. Disordered regulation of coagulation and platelet activation in xenotransplantation. Xenotransplantation. 2000;7(3):166–76. doi: 10.1034/j.1399-3089.2000.00067.x. [DOI] [PubMed] [Google Scholar]
- 27.Lin CC, Cooper DK, Dorling A. Coagulation dysregulation as a barrier to xenotransplantation in the primate. Transpl Immunol. 2009;21(2):75–80. doi: 10.1016/j.trim.2008.10.008. PMCID: 2757551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirk AD, Harlan DM, Armstrong NN, Davis TA, Dong Y, Gray GS, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci U S A. 1997;94(16):8789–94. doi: 10.1073/pnas.94.16.8789. PMCID: 23132. [DOI] [PMC free article] [PubMed] [Google Scholar]