Summary
As humans evolved, perhaps the two strongest selection determinants of survival were a robust immune response able to clear bacterial, viral, and parasitic infection and an ability to efficiently store nutrients to survive times when food sources were scarce. These traits are not mutually exclusive. It is now apparent that critical proteins necessary for regulating energy metabolism such as peroxisome proliferator-activated receptors (PPARs), Toll-like receptors (TLRs), and fatty acid-binding proteins (FABPs) also act as links between nutrient metabolism and inflammatory pathway activation in immune cells. Obesity in humans is a symptom of energy imbalance: the scale has been tipped such that energy intake exceeds energy output and may be a result, in part, of evolutionary selection toward a phenotype characterized by efficient energy storage. As discussed in this review, obesity is a state of low-grade, chronic inflammation that promotes the development of insulin resistance and diabetes. Ironically, the formation of systemic and/or local, tissue-specific insulin resistance upon inflammatory cell activation may actually be a protective mechanism that co-evolved to repartition energy sources within the body during times of stress during infection. However, the point has been reached where a once beneficial adaptive trait has become detrimental to the health of the individual and an immense public health and economic burden. This article reviews the complex relationship between obesity, insulin resistance/diabetes, and inflammation, and while the liver, brain, pancreas, muscle, and other tissues are relevant, we focus specifically on how the obese adipose microenvironment can promote immune cell influx and sustain damaging inflammation that can lead to the onset of insulin resistance and diabetes. Finally, we address how substrate metabolism may regulate the immune response and discuss how fuel uptake and metabolism may be a targetable approach to limit or abrogate obesity-induced inflammation.
Keywords: obesity, diabetes, insulin resistance, macrophage, plasticity, metabolism
Obesity and Inflammation, an Introduction
Over the past 20 years, the prevalence of obesity in the United States, and worldwide, has reached epidemic proportions (1). In the United States, the most recent National Health and Nutrition Examination Survey (NHANES) reported that 68.3% of individuals studied were overweight, as defined by having a Body Mass Index (BMI, kilograms/meter2) of at least 25. Over a third of the people surveyed were obese (BMI of at least 30) (1). Nineteen percent of U.S. children ages 6–11 years of age are overweight, as defined by being over the 95th percentile for their age in BMI (2). Globally, the World Health Organization estimates 500 million adults and almost 43 million children under the age of 5 years are obese (3). The pathogenesis of obesity is influenced by the elaborate interactions among a complex web of factors including inherited genetic traits, behavioral, physical, or psychological factors, environmental characteristics such as access to and availability of food sources, cultural identity, education level, and socioeconomic status (4).
Obesity is associated with a constellation of pathologies that together are defined clinically as the Metabolic Syndrome. The salient features of Metabolic Syndrome include insulin resistance, hyperinsulinemia, impaired glucose tolerance, dyslipidemia, hyper-coagulatablility, hypertension, and, of course, obesity, particularly central adiposity defined as a waist:hip ratio of greater than 0.90 for men and 0.85 for women (5). The concurrence of any three of these conditions will result in a diagnosis of Metabolic Syndrome. An in-depth discussion of insulin resistance is beyond the scope of this review; however, in the pre-diabetic state, insulin resistance occurs when the biological function of the insulin hormone signaling cascade is blunted in metabolically sensitive tissues. Physiologically, insulin resistance manifests as decreased insulin-stimulated blood glucose uptake by adipose and skeletal muscle and a failure to inhibit glucose production by the liver and triacylglyceride lipolysis and non-esterified free fatty acid (NEFA) release from adipose stores (6). The impaired insulin function may be temporarily alleviated through compensatory increases in islet β cell number and size aimed at boosting insulin production in an effort to overcome the insulin signaling impairment. Hyperinsulinemia can compensate for the lack of insulin sensitivity for a time; however, overtaxing β cells is not a permanent solution. Overworked β cells will eventually fail, ultimately resulting in insulin insufficiency in susceptible patients or mouse models (6).
The combined effects of insulin resistance can lead to the development of hyperglycemia and hyperlipidemia that may progress to the point at which an individual is diagnosed with Type 2 diabetes mellitus (referred to as ‘Type 2 diabetes’ throughout this review), a condition tightly associated with obesity. This is in contrast to Type 1 diabetes mellitus, an autoimmune disease that typically afflicts children and is characterized by a lack of pancreatic insulin production due to immune-mediated ablation of β cells (7, 8). About 90–95% of diabetes cases are individuals with Type 2 diabetes and an estimated 25.8 million people have been diagnosed with Type 2 diabetes in the United States. Epidemiologists predict that Type 2 diabetes may affect as much as 33% of the United States population by 2050 (9). Diabetes is also a worldwide epidemic with the WHO predicting a doubling of diabetes-related deaths from 2005 to 2030, with poorer countries suffering the greatest burden (3).
Obesity, characterized as a state of low-level inflammation, is a powerful determinant influencing the development of insulin resistance and progression to Type 2 diabetes (10, 11). Two seminal manuscripts published in the Journal of Clinical Investigation in 2003 (12, 13) brought to light a new understanding of the role of adipose inflammation, particularly macrophage-mediated inflammation, in obesity. The results of the experiments described in these papers demonstrated that macrophages infiltrate adipose tissue at the onset of weight gain and directly contribute to and perpetuate the inflammatory state of fat, eventually leading to systemic insulin resistance and the development of obesity in both mouse models and humans (12, 13). Indeed, there is a 4-5-fold increase in adipose tissue macrophage content from the lean to the obese state with macrophages constituting up to 50% of the cells present in obese adipose (14). Although this process is still unclear, macrophages likely infiltrate adipose in response to a ‘stress’ signal emanating from the adipocyte, with larger, more insulin resistant adipocytes in obesity suffering the most stress (15, 16). Macrophage quantities are highly correlated with adipocyte size, as well as age, female gender, and the expression of numerous inflammatory markers (14, 17–23). Obesity and Metabolic Syndrome are currently and will continue to be significant public health concerns worldwide; therefore, there is a pressing need for the development of a deep understanding of the relationships between obesity, inflammation, and metabolism.
Obesity-associated inflammation contributes to the development of insulin resistance
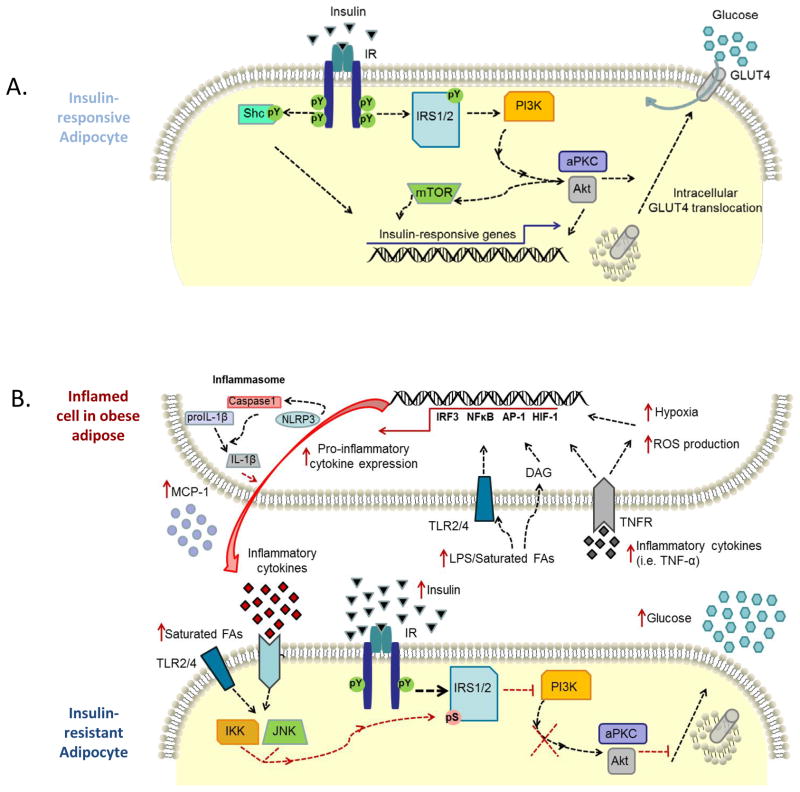
Work over the past decade has increasingly linked obesity and inflammation. Contrary to an acute response to an injury such as a laceration that we normally associate with inflammation, which would typically induce pain and swelling, to be soothed with a bandage and antibiotics to ease the insult, obesity-associated inflammation is a chronic, unmitigated, inflammation with insidious results (20, 24–27). Cytokines, reactive oxygen species (ROS), and other agents produced by immune cells and adipocytes are released that can activate important stress pathways and disrupt critical metabolic processes implicated in obesity such as the insulin signaling cascade (Fig. 1A).
Fig. 1. Mechanisms of inflammation-induced insulin resistance.
(A). Insulin signaling is initiated via binding of insulin to the insulin receptor (IR) inducing autophosphorylation of tyrosine residues on intracellular domains, as shown on an insulin-responsive adipocyte. The activated IR subsequently phosphorylates tyrosine residues on a variety of substrates including the insulin receptor substrate (IRS) family of proteins and Shc isoforms. IRS interacts with various effector molecules such as phosphatidylinositol 3-kinase (PI3K). Simplified, PI3K phosphorylation of membrane phospholipids ultimately leads to recruitment and activation of several kinases such as protein kinase B/Akt and atypical protein kinase C (aPKC). Akt and aPCK are serine/threonine kinases that stimulate membrane translocation of GLUT4 from intracellular vesicles. GLUT4 accumulation at the plasma membrane allows insulin responsive uptake of glucose and reduces circulating glucose levels in the fed state. (B). As the adipose depot expands in size, a variety of cell populations begin to exhibit an inflamed or stressed state through various mechanisms including hypoxia, release of pro-inflammatory non-esterified fatty acids, elevated reactive oxygen species (ROS) production, and cytokines, among others. Increased adipose mass and adipocyte diameter can lead to increases (red arrow) in hypoxia. In addition, elevated levels of circulating saturated fatty acids (FAs) in the obese states can activate Toll-like receptor signaling (TLR) or become hydrolyzed into inflammatory bioactive lipid mediators such as diacylglyceride (DAG), which ultimately lead to activation of cellular stress signaling pathways. Additionally, inflammatory cytokines, such as TNF-α, are secreted into the microenvironment of the obese adipose, further propagating the immune response. Accelerated energy metabolism in the face of enhanced nutrient availability (glucose and FAs) can increase the production of ROS. The culmination of stress in an inflamed adipose cell induced by hypoxia, fatty acids, glucose, ROS, and inflammatory cytokines results in the transcription of inflammatory cytokines and enzymes via activation of transcription factors, such as interferon regulatory factor 3 (IRF-3), NFκB, hypoxia-inducible factor 1 (HIF-1), and AP-1. Furthermore, the NLRP3 (NLR family, pyrin domain containing 3) inflammasome is activated by hypoxia and is glucose-dependent. NLRP3 regulates secretion of the inflammatory cytokine, IL-1β following cleavage of pro-IL-1β via caspase1. Taken together, through direct effects on adipocytes or through paracrine release of mediators, such as saturated fatty acids and cytokines, stress kinases are activated to blunt the insulin signaling cascade. Activated IKK and JNK prevent PI3K activation by phosphorylating IRS on inhibitory serine residues. Further, inflammatory cytokines increase SOCS3 expression, which can interfere with IR activity. Ultimately, insulin resistance leads to impaired insulin-dependent GLUT4 trafficking, and thus elevated levels of circulating glucose, compensatory secretion of insulin by pancreatic beta cells and ultimately type 2 diabetes.
Almost 20 years ago, Hotamisligil et al. (28) reported the first evidence that adipose tumor necrosis factor-α (TNFα), a pro-inflammatory cytokine, has the ability to disrupt the insulin signaling cascade (Fig. 1B). More recently, Weisberg et al. (12) used a macrophage-depleted bone marrow transplant model system to demonstrate that adipose macrophages are the source of the majority of the TNFα expressed in this tissue and that bone marrow-derived macrophages are necessary for supporting an obese phenotype. Furthermore, animals lacking macrophage chemotactic protein-1 (MCP-1, Ccl2) or its receptor Ccr2, the ligand and receptor classically associated with macrophage trafficking, are characterized by decreased macrophage infiltration into adipose, decreased susceptibility to systemic insulin resistance and obesity (14, 15, 29).
Since then, it has become accepted that obesity-associated inflammation contributes to insulin resistance and the inflammatory nature of the adipose microenvironment continues to be an active area of research. Inflammatory mediators including IL1-β, IL-6, IL-8, IL-10, transforming growth factor-β (TGFβ), TNFα, MCP-1 (CCL2), plasminogen activating inhibitor-1 (PAI-1/Serpine), macrophage migratory inhibitory factor (MIF), metallothionine, osteopontin, chemerin, and prostaglandin E2 (PGE2) are just a few bioactive mediators secreted from obese adipose (10, 30–34). Interestingly, the adipose stromal vascular fraction, which contains primarily nonfat cells including macrophages, may play a more prominent role in the development of obesity-associated inflammation and insulin resistance, as cells in this fraction tend to secrete levels of inflammatory mediators exceeding those excreted by adipocytes (35).
Adipose microenvironment
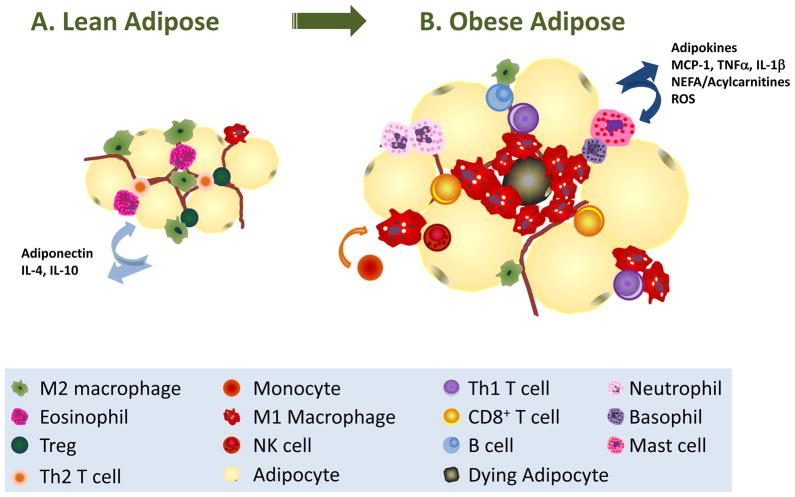
What role are adipocytes, macrophages and other immune cells playing in the progression of obesity and insulin resistance? Monocyte trafficking and activation are well-characterized aspects of atherosclerosis that may offer some clues as to the function of immune cells in obese adipose (36). Extensive research focused on monocyte trafficking in atherosclerotic lesions demonstrates that macrophage infiltration of the blood vessel wall, subsequent uptake of oxidized lipoprotein, and the resultant pro-inflammatory response are central components involved in the formation of cholesterol-laden foam cells and expanding atherosclerotic plaques (37–43). Similarly, as obesity progresses, immune cells infiltrate either transiently (neutrophils) or continuously over time (innate and adaptive cells) while other beneficial immune cells decrease in number or are functionally overshadowed (Fig. 2). As macrophages disperse into adipose tissue and accumulate triglycerides, they become activated and subsequently cause tissue damage in a fashion reminiscent to the formation of an atherosclerotic lesion (12, 13, 44). Therefore, lessons may be learned from considering other immune-linked metabolic diseases like atherosclerosis, which can be applied to the obese adipose microenvironment: the prime lesson being to focus on the complexity of cell-cell interactions rather than the tissue as a whole.
Fig. 2. Immune cell trafficking in obesity.
(A). In lean adipose tissue, adipocytes store triglycerides in a large unilocular droplet. Insulin-mediated repression of lipolysis is present along with little hypoxia, absence of inflammation, and physiologic levels of NEFA, glucose, and ROS. In lean adipose, alternatively activated ‘M2’ macrophages are resident and their phenotype is maintained by the presence of T-regulatory (Treg) cells, Th2 cells, and eosinophils. Lean adipose secretes adipokines such as adiponectin, IL-4, and IL-10, which act to maintain insulin sensitivity. (B). As obesity progresses and adipose tissue expands, hypertrophy and hyperplasia ensues: adipocytes accumulate triglycerides and grow large, while pre-adipocytes are differentiated to mature adipocytes. Alterations in adipokines are prognostic: leptin rises and adiponectin falls with increasing obesity. Chemokines are released, such as MCP1, which recruit monocytes that polarize to pro-inflammatory M1 macrophages. M1 macrophages surround dying adipocytes in classic ‘crown-like structures’ and release many pro-inflammatory mediators. The loss of eosinophils, Tregs, and Th2 T cells as obesity progresses is paired with the infiltration of CD4+ Th1 cells, CD8+ T cells, NK cells, and other granulocytes such as neutrophils, mast cells, and basophils. Elevated cytokines, such as TNFα and IL-1β, levels of NEFA, acylcarnitines, and ROS release contribute to the pro-inflammatory microenvironment.
Adipocytes
The adipocyte is obviously the cell best studied in adipose biology with much work focused on adipocyte differentiation and triglyceride droplet formation. Once thought to be a static storage depot for calories as triglycerides, adipose tissue is now considered to be an endocrine organ with an important role in local and systemic homeostasis. It has been demonstrated that adipose has the ability to secrete many hormones derived from adipocytes. These hormones include cytokines and chemokines called ‘adipokines’ or lipid mediators recently termed ‘lipokines’. These molecules function as either systemic, paracrine, or autocrine hormones. Leptin, the classic adipokine first discovered by Friedman’s group (45), is primarily secreted by adipocytes. As adipocytes grow and accumulate triglyceride, circulating leptin concentrations increase proportionally; therefore, leptin concentration is an indicator of adipose mass. In an effort to maintain metabolic homeostasis, leptin negatively regulates appetite, drives physical activity, and promotes insulin sensitivity. However, chronically elevated levels of leptin in the obese results in a state of central leptin resistance, ultimately limiting satiety cues (reviewed in 46). Adiponectin is another well-known adipokine that is regulated inversely to leptin. In contrast to leptin’s expression, adiponectin production becomes blunted with increasing adiposity (47). Adiponectin promotes insulin sensitivity (48, 49) and is anti-inflammatory (50–52). Indeed, low adiponectin level is associated with Metabolic Syndrome (53).
In contrast to leptin and adiponectin, several adipokines have been shown to promote insulin resistance. Resistin, which is secreted mainly from adipocytes in rodents but primarily by macrophages in humans, has been demonstrated to induce insulin resistance (54–56). Retinol-binding protein 4 (RBP4) (57) and angiotensinogen (58, 59) are examples of other adipokines that have been linked to the development of insulin resistance and inflammation. Hyper-coagulation is another hallmark of Metabolic Syndrome and there is evidence that adipose tissue is responsible for the secretion of several acute phase proteins involved in the inflammation and thrombosis linked to Metabolic Syndrome including serum amyloid A (SAA), PAI-1, and CRP (60–64). Adipose tissue is also a source of angiogenic mediators such as vascular endothelial growth factor (VEGF), matrix metalloproteinase-2 (MMP-2), matrix metalloproteinase-9 (MMP-9), and hepatocyte growth factor (HGF) (35). Together, these adipokines, chemokines, and cytokines constitute a complex network of pro- and anti-inflammatory regulatory mediators.
In addition to secreted proteins, lipids are also released that act locally and systemically. In a lean state, insulin signaling inhibits lipolysis and NEFA release from adipocytes. However, limited insulin responsiveness in obese adipocytes results in reduced attenuation of lipolysis and subsequently elevated levels of circulating NEFAs. NEFAs play a large role in the lipotoxic effect associated with inflammation (discussed below). Additionally, certain oxidized NEFA species function as bioactive lipid mediators, or lipokines, such as the eicosanoids prostaglandin D2 (PGD2) and its derivative prostaglandin J2 (PGJ2), have been shown to be relevant mediators of leptin and adiponectin release (65, 66). In addition, a recent publication reported that palmitoleic acid drives improvements in insulin sensitivity systemically and several epidemiologic studies support this notion (67–69). Why do we see these drastic changes in adipokine and lipokine secretion from obese adipose tissues in humans and animal models? Various mechanisms including hypoxia and oxidative stress are addressed in this review, but one must first comprehend the entirety of the adipose microenvironment, which includes considering the dynamics of stromal constituents in obesity, especially the migration of immune cells such as macrophages, lymphocytes, and other granulocytes.
Macrophages
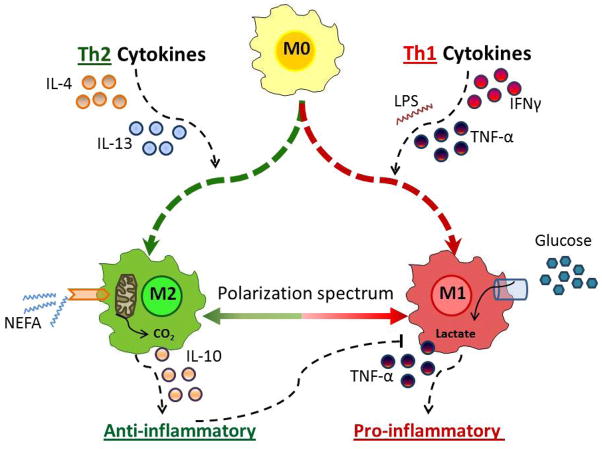
In addition to the adipocyte, the microenvironment of adipose consists of many other cells central to the health of the tissue that are involved in pathogenesis of obesity. These cell types include pre-adipocytes, endothelial cells, fibroblasts, and immune cells; while this review is focusing on the latter, it does not preclude the importance of other stromal constituents (reviewed in 70). As mentioned above, the bone marrow-derived macrophage was the first immune cell shown to be highly relevant to adipose biology and is the most investigated (12, 13, 71, 72). However, the adipose stromal microenvironment is far more complex; macrophages are not just one simple cell type but rather can exist displaying a range of phenotypes or subtypes, can be resident or infiltrating, and perform different functions depending on tissue localization (73). Broadly speaking, ‘M1’ macrophages are pro-inflammatory, activated via the classical pathway wherein type 1 T-helper (Th1) inflammatory cytokines interferon γ (IFNγ) and TNFα are induced by agents such as cytokines, infections, or saturated fatty acid stimulation (74–77) (Fig. 3). In contrast, ‘M2’ macrophages are activated by type 2 T-helper (Th2) cytokines IL-4 and IL-13 (78, 79). M2 or alternatively activated macrophages are found to be resident cells in adipose tissue from lean individuals and are thought to be involved in remodeling and tissue repair through the production and expression of IL-10, IL-1 receptor antagonist, and arginase-1 (75, 80–83). Different macrophage subtypes have been shown to modulate inflammation-associated insulin resistance in metabolic tissues such as adipose and liver. Indeed, manipulation of the macrophage population can ameliorate or worsen pathology (75, 80, 84).
Fig. 3. Metabolism drives macrophage polarization.
Macrophages subtypes exist and can be broadly categorized as pro-inflammatory M1 or alternatively activated M2, although in vivo studies reveal that macrophage plasticity results in a spectrum of macrophage phenotypes. M1 macrophages are polarized from precursor M0 macrophages via the classical pathway, wherein components of bacteria such as lipopolysaccharide (LPS) and type 1 T-helper (Th1) inflammatory cytokines interferon γ (IFNγ) and TNFα drive expression of pro-inflammatory cytokines such as TNFα. In contrast, M2 macrophages are activated by type 2 (Th2) cytokines IL-4 and IL-13. M2 macrophages are resident in lean adipose and are thought to be involved in remodeling, tissue repair, and maintenance of insulin sensitivity through the production and expression of IL-10, IL-1 receptor antagonist, and arginase-1. Plasticity along the polarization spectrum is an intensely investigated topic. M1 macrophages tend to utilize the glycolytic pathway for energy and metabolite generation, while M2 macrophages are reliant upon β-oxidation of fatty acids. Regulating macrophage substrate metabolism is one potential means for manipulation of the inflammatory response.
As an individual becomes increasingly obese, there is a dramatic increase in M1 macrophages into adipose tissue. As lipid accumulation continues, both in adipocytes and macrophages within the adipose tissue, there is a shift in macrophage subtype to a pro-inflammatory, M1 polarization (75, 76, 84–86). In obese adipose, chronic inflammation ensues with pro-inflammatory release of TNF-α, IL-1β, PAI-1, and ROS (12, 13, 15, 35). Lumeng et al. (75, 84) demonstrated that M2 macrophages are dispersed in adipose tissue, whereas infiltrating M1 macrophages form ‘crown-like structures’ around dying adipocytes (Fig. 2B). Through the use of genetic alteration and bone marrow transplant models, M2 ‘anti-inflammatory’ macrophages are demonstrated to maintain insulin sensitivity in liver and adipose, while M1 macrophages have been shown to inhibit insulin sensitivity, which can lead to systemic insulin resistance and the promotion of an ‘obesogenic’ inflammatory microenvironment (80, 87, 88). In addition to subtype, location of adipose tissue is highly relevant to the inflammatory response with visceral (fat including gonadal fat pads, omentum, and other fat surrounding the viscera) being more inflammatory than subcutaneous adipose underlying skin (89). Interestingly, obesity-induced macrophage infiltration also occurs in the normal breast mammary gland, a large fat pad (90), suggesting that immune cell-mediated inflammation is not restricted to subcutaneous and visceral depots.
The role of fuel substrate metabolism in macrophage subtype polarization
While categorizing M1 vs. M2 subtypes is convenient for in vitro purposes, in vivo studies demonstrate that there is, in actuality, a vast spectrum of macrophage subtypes with some plasticity rather than solely M1- or M2-polarized (91) (Fig. 3). Indeed, the macrophage subtype may be molded by the microenvironment in which the cell is present or systemic characteristics of the organism. To date, the mechanisms influencing plasticity of M1 and M2 macrophages remains a hotly debated topic.
One potential modifier of plasticity is the availability of fuel substrates to the macrophages in the microenvironment. For example, macrophage phenotype may be shaped by the availability of excess glucose characteristic of hyperglycemia in diabetes or elevated fatty acids as is the case with hypertriglyceridemia or uncontrolled lipolysis in the obese state. Vats et al. demonstrated that M1 macrophages tend to utilize the glycolytic pathway for energy generation, while M2 macrophages may prefer β-oxidation of fatty acids as fuel (80, 92, 93). M2 macrophage polarization is dependent upon the transcription factors PPARδ and γ as well as the co-activator PGC1β, known to promote mitochondrial biogenesis and fatty acid β-oxidation (92). In monocytes/macrophages lacking the nuclear hormone receptors PPARδ or γ, PGC1β, or when treated with etomoxir, the pharmacological inhibitor of carnitine palmitoyltransferase 1 (CPT1) which blunts long chain fatty acid uptake into mitochondria and therefore β-oxidation, cells cannot be polarized to the M2 phenotype and display M1 characteristics (92). Although there are high levels of M2-polarizing lipids released from the adipose in an obese state, the coordinated changes of cytokine production and immune cell populations as adiposity increases results in an inflammatory microenvironment favoring an M1 phenotype. Furthermore, when one considers the metabolism of a single amino acid, it becomes evident how important metabolites are to the immune response. IL-4 activation of the stat6/PPARγ pathway acts to increase transcription of arginase 1, an enzyme characteristic of M2-polarized macrophages. Arginase 1 metabolizes L-arginine to L-ornithine (and urea) as the last step in the urea cycle. The end product ornithine can be modified through a glutamic semialdehyde intermediate to proline, a necessary amino acid in the synthesis of collagen (94), supporting the role for M2 macrophage in tissue remodeling. In contrast, in M1 macrophages arginine is used for the generation of a completely different end product – the reactive oxygen species nitric oxide via the catalytic activity of nitric oxide synthase. The expression of nitric oxide synthase is a hallmark of pro-inflammatory M1 macrophage (76). As the inflammatory state of macrophages may be inherently linked to a preference for a single energy-producing pathway or relevant metabolite over another in these cells, regulating the fuel substrate usage by macrophages is one potential means for manipulation of the inflammatory response, to be discussed further below.
B and T-lymphocytes in adipose inflammation
Most work on obesity and inflammation has focused on macrophage infiltration and activation in the adipose. However, recent reports have demonstrated unique roles for a variety of immune cells in the adipose, including cells from both innate and adaptive arms of the immune system. Similar to macrophages, B cells and T cells play dynamic roles in the transition from metabolic homeostasis in lean adipose to a state of chronic inflammation and insulin resistance in the obese (95–97) (Fig. 2).
B and T lymphocytes can influence obesity and insulin resistance. B lymphocytes have been shown to infiltrate adipose with onset of weight gain (98) and contribute to insulin resistance (99) but have been studied to a much lesser extent then adipose-resident T cells. T cells were first identified in visceral fat, and secretion of Th1 cytokines was shown to regulate adipose inflammation. Importantly, appearance of T cells was proximal to induction of insulin resistance (98, 100–104). T cells infiltrate adipose early in the development of obesity, prior to a major influx of macrophages (98). Interestingly, in Rag2−/− mice, which lack mature B and T cells, there is an increase in natural killer (NK) cells and macrophages in adipose suggesting that early infiltration of B and T cells may be necessary for inflammatory suppression in this tissue (98). However, one publication (105) suggested that T cells were not involved in insulin resistance despite elevation of some cytokines.
Contradictory findings highlight the complexity of T-cell biology, again underscoring that populations and subtypes must be considered when studying obesity and inflammation. For instance, it is well known that CD4+ Th1 cytokines, such as regulated on activation, normal T cell expressed and secreted (RANTES) and IFNγ, drive inflammation and polarization of macrophages towards the M1 phenotype in fat and liver in obesity (103, 104, 106). Light was shed upon adipose T-cell complexity through the study of CD4+ T-regulatory cells (Tregs) subtype. Tregs are anti-inflammatory tolerogenic lymphocytes, typically associated with allergic reaction, that secrete Th2 cytokines such as IL-4 and IL-13. Indeed, Tregs play an integral role in preventing adipose inflammation and insulin resistance (107). Tregs are found in lean adipose and decrease with increasing obesity, as do CD4+ Th2 cells (99, 106). In contrast, CD8+ and CD4+ Th1 cells increase with obesity, and CD8+ T cells were shown to contribute to macrophage recruitment in obesity (108). Immunotherapy aimed at blunting Th1 cells using CD3-specific antibody or its F(ab′)(2) fragment or acutely depleting adipose of T cells was shown to normalize adipose inflammation and insulin sensitivity in mice (99, 109, 110). Finally, there are also depot-specific differences in lymphocyte populations, which support other immune cell-mediated findings in terms of inflammation, diabetes, and obesity (111). O’Rourke et al. (112) demonstrated the relevance of lymphocytes in visceral adipose, relative to subcutaneous adipose, which was characterized by elevated T and natural killer (NK) cells, elevated IFN-γ, and greater activation of TNFα secretion from macrophages.
In sum, the accumulation of inflammatory cells, such as CD8+ T cells and Th1 cells, and the relative decline in anti-inflammatory Th2 cells and Tregs account for skewing the adipose towards an inflammatory state and drive macrophage polarization towards an M1 phenotype (Fig. 2). Much remains unknown about T-cell biology in adipose tissue, including timing and localization of interactions among cell types with regard to cytokine, hormone, and other substrate exposures within the adipose. Indeed, similar to macrophage fuel metabolism, T-cell subtype skewing occurs through modulation of substrate metabolism regulated by cytokines and hormones, such as leptin and intracellular nutrient sensing kinases such as protein kinase B (PKB/AKT), liver kinase B1 (LKB1), AMP-activated protein kinase (AMPK), and mammalian target of rapamycin (mTOR), among others (113–119). However, little is known about how the obese state modulates T-cell metabolism. Interestingly, obese mice and humans have altered memory T-cell responsiveness (120, 121). The question arises: is there a link between obesity altering T-cell metabolism and thus function? Taken together, B and T-cell populations in adipose tissue must not be overlooked when considering obesity-induced inflammation.
Other immune cells
The factors driving adipose inflammation are likely multiple and involve complicated relationships among varied signaling molecules. Indeed, essentially all other hematopoietic cells have been identified in obesity-associated inflammation in addition to macrophages and lymphocytes (122). Natural killer T (NKT) cells have been shown to be necessary and sufficient to drive obesity-induced inflammation through invariant NKT deficiency and agonist studies (123–125). In contrast, a recent publication has suggested that NKT cells behave in the opposite manner, as a protective mechanism through secretion of Th2 M2-polarizing cytokines, thus improving insulin sensitivity. This group further showed that NKT cells decrease with increasing adiposity and insulin resistance (126). In addition, a subset of NK cells (not NKT cells) was also recently identified by Moro et al. (127) that express c-Kit and Sca-1 and are located in what they term ‘fat-associated lymphoid cluster’ (FALC). This subset of NK cells also secretes Th2 cytokines and maintains insulin sensitivity. Clearly, further characterization of NKT and NK cell subsets, relevant signaling molecules, and biologic activity of these cells need to be further clarified in a manner similar to understanding the role of differentially polarized macrophages in obesity.
Mast cells, primary mediators of allergic reactions, have been implicated in obesity and insulin resistance through the use of animal models of mast cell activation and dysfunction (128). Furthermore, it has been demonstrated that mast cells can modulate lipogenesis via paracrine action of the eicosanoid 15-deoxy-delta-12, 14-PGJ2 (129). In fact, obese humans and mice have an overall increased burden of mast cells (128) and adipose tissue has been identified as a source of mast cell progenitor cells (130), suggesting a synergistic relationship between adipose tissue and mast cell propagation. Therefore, it is reasonable to expect that obesity-induced mast cell propagation may be a driver of allergic diseases such as asthma (131).
Other granulocytes have also been shown to be relevant to the immune response in adipose. Elgazar-Carmon et al. (132) demonstrated that after just three days of high fat feeding, neutrophils infiltrate adipose tissue. In humans, neutrophils were positively associated with Metabolic Syndrome (133). Basophils are the rarest granulocyte and the relationship between these cells and obesity-associated outcomes is less clear. Epidemiologic data are conflicting: while two human studies found non-statistically significant elevations in serum basophils with obesity, two studies found a positive relationship between circulating basophils and either BMI or markers of Metabolic Syndrome (134–137). Although leptin has been shown to regulate basophil migratory response and survival (134), to date, there are no reports of investigations examining basophil infiltration in obese adipose or direct relationship between basophils and insulin resistance.
Unlike other granulocytes, eosinophils have been shown to play a protective role against the formation of obesity and insulin resistance through blunting adipose inflammation. Eosinophil numbers decrease with obesity and have been shown to be essential to maintaining the macrophage M2 phenotype and insulin sensitivity in vivo. In fact, an interesting experiment by Wu et al. (138, 139) utilized parasitic infection to induce eosinophil activation and improved insulin resistance in mice. Clearly, the role of these lesser studied granulocytes should be further investigated. Taken together, adipose inflammation is a complex interaction of acute innate responses, adaptive immunity, and the loss or maintenance of chronic adaptive and innate cell populations that together act to perpetuate and exacerbate adipose inflammation.
The challenge of characterizing the adipose microenvironment
As discussed above, adipose tissue is not simply composed of adipocytes. Therefore, to thoroughly study chronic inflammation in this tissue, one must consider the complexity of the adipose microenvironment. As one of the largest endocrine organs in the body, adipose is responsible for the production and release of many potent signaling molecules including adipokines, lipokines, and inflammatory mediators that may elicit effects both locally and systemically. The inflammatory state of the adipose tissue regulates the identity and concentration of these released molecules. It has taken some time to clarify the relative contributions of as well as the interactions among the various cell types contained within the microenvironment to the development of obesity-induced insulin resistance. To achieve this, several technological hurdles needed to be overcome.
Immunohistochemistry, a standard technique used to determine the expression level and localization of proteins in tissues, is a challenge in adipose tissue, because the immense triglyceride droplet contained within the adipocyte forces the nucleus and other organelles to be pushed to the perimeter of the cell. Therefore, it is difficult to distinguish the boundaries of neighboring adipocytes. Partitioning of adipose-enriched versus stromal vascular fractions for flow cytometry, quantitative polymerase chain reaction (PCR) or Western blot analysis is fraught with technical difficulties. Digestion with enzymes such as collagenase must be sufficiently rigorous to dissociate immune cells from adipocytes to avoid cross-fraction contamination, as the stromal vascular fraction contains many cells types (including pre-adipocytes, macrophages, T cells, endothelial cells, among others), while at the same time be gentle enough so as not to destroy or damage the adipocyte. Flow cytometry of adipocytes from dissociated tissue is difficult due to the delicate nature of the adipocyte; on the other hand, the stromal vascular fraction can be readily characterized by separation of its cell types through the use of fluorescence-activated cell sorting (FACS) or cell isolation kits (84). Finally, in vivo adoptive bone marrow transplant or cell transfer studies are techniques commonly used to study obesity-induced inflammation. Therefore studies in vivo, in combination with ex vivo culture of primary cells or, alternatively, co-culture techniques using primary or established cell lines, are necessary to attempt to model and characterize the complex microenvironment in adipose.
Genetic loss-of-function studies are often used to study the adipose microenvironment using Cre-flox technologies to achieve tissue or cell type-specific gene knockdown in animals. In the past, many studies employed the use of what was thought to be an adipocyte-specific promoter for the fatty acid binding protein aP2/FABP4 gene; however, work by our group and others demonstrated that aP2 is present in activated macrophages as well as other cells, and this protein is relevant for many immune-mediated responses including insulin resistance, atherosclerosis, experimental allergic encephalomyelitis (EAE), and asthma (26, 140–143). An alternative to the aP2 promoter is the promoter for adiponectin that allows for adipocyte specific expression of Cre recombinase (144). To study immune cells, lysosomal-M (LysM)-Cre is an excellent tool for targeted gene deletion in cells of the monocyte/macrophage lineage and is used often in studying obesity-induced inflammation. One caveat is that LysM-Cre is also expressed in microglia (brain macrophages) (145). The role of neural alterations impacting appetite, energy expenditure, and feeding behavior regulation stemming from gene deletion in microglia is a critical, often overlooked aspect of the development of obesity and insulin resistance (146). While the heterogeneous nature of adipose is complex, the variety of experimental approaches makes the study of macrophage and other immune cell biology in adipose inflammation an attractive field.
Mechanisms of inflammatory activation
As is the case with other aspects pertaining to the pathogenesis of obesity, the mechanism regulating the activation of obesity-associated inflammation is multifactorial. As alluded to above, we posit that nutrient metabolism plays an immense role in shaping the immune response in adipose tissue. With a focus on the macrophage, the role of glucose and glucotoxicity, fatty acids and lipotoxicity, as well as stress kinases, ER stress, and inflammasome activation on macrophage polarization will be addressed.
Immune cells express pattern recognition receptors (PRRs) specific for pathogen-associated molecular patterns (PAMPs), which allows for induction of a rapid inflammatory response. Bacterial component lipopolysaccharide (LPS) is a ligand for Toll-like receptor 4 (TLR). TLR4 activation through LPS results in activation of cell-signaling pathways that ultimately result in the production of pro-inflammatory cytokines, such as TNFα. Interestingly, TLR4 can be activated through saturated fatty acids, as there is a degree of similarity in structure among LPS and saturated fatty acids (147) (Fig. 1B). In addition, binding of cytokines to plasma membrane receptors or intracellular lipid mediators such as diacylglyceride (DAG) can initiate inflammatory signaling pathways. Regardless of the inflammatory stimulus, a variety of kinases link extracellular stress signals with intracellular production of inflammatory molecules. Over 500 different kinases have been identified (148), some of which are directly involved in mediating inflammatory activation. Stress kinases associated with inflammatory activation in adipose include the DAG-activated protein kinase C (PKC) (149–152), mitogen-activated protein kinase (MAPK) pathway members including c-Jun N-terminal kinase (JNK) (153–156), extracellular signal-regulated kinase (ERK) (157, 158), p38 MAPK (153, 159), as well as inhibitor of κB (IκB) kinase (IKK) (17, 160, 161). JNK and IKK activate transcription factors integral to inflammation, such as activator protein 1 (AP1), c-Jun/Fos, and nuclear factor-κB (NFκB). Importantly, in addition to activating typical inflammatory responses, activation of these kinases has been linked to inhibition of insulin signaling. For instance, JNK and IKK are serine kinases that have been shown to phosphorylate and inhibit the insulin receptor substrate 1 (IRS-1) via phosphorylation of serine residues, thus blunting the insulin signaling cascade (155) (Fig. 1B). Further, JNK and IKK signaling also results in activation of transcription factors integral to inflammation, such as AP1, c-Jun/Fos, and NFκB.
Through the use of genetic knockout models, pharmacologic treatments and studies in humans, it has become clear that the actions of inflammatory kinases such as JNK and IKK play central roles in both obesity and insulin resistance (17, 77, 154–156, 160–167). However, decreased inflammatory kinase activity achieved through genetic manipulation in mouse models does not necessarily result in decreased obesity. Despite the fact that many models with JNK or IKK inhibition display increased lipid accumulation in liver and skeletal muscle, animals with absent or blunted stress kinase activation remain insulin sensitive (17, 166), demonstrating that while adiposity and triglyceride accumulation are a harbingers of inflammation and insulin resistance, lipid accumulation per se is not always linked to insulin resistance: activation of inflammatory response is paramount. There are several links between substrate metabolism, kinase activation, and the pro-inflammatory response included in states of glucotoxicity and lipotoxicity.
Glucotoxicity and obesity-induced inflammation
High glucose exposure is known to drive the pro-inflammatory activation of macrophages potentially through multiple major pathways including the production of reactive oxygen and nitrogen species (namely, oxidative burst and endogenous production), alterations in cellular redox potential, activation of TGFβ-stimulated release of pro-inflammatory cytokines, and glucose-induced lipotoxicity.
Upon activation, macrophages classically demonstrate a ‘respiratory burst’ when attacking an offending bacterium (reviewed in 168) and significantly increase glucose uptake to allow for production of metabolites necessary to kill the pathogen through generation of reactive oxygen and nitrogen species. The respiratory burst is initiated by activation of the phospholipase C-mediated release of DAG and inositol triphosphate (IP3) following extracellular macrophage stimulation. Next, Ca2+ is released from the endoplasmic reticulum, which leads to activation of PKC, the kinase responsible for the phosphorylation of NADPH oxidase subunits, particularly p47phox (149). NADPH oxidase is fully assembled and subsequently translocated to the cellular plasma membrane. NADPH oxidase catalyzes the conversion of extracellular oxygen to superoxide (O2·−) which can be utilized by macrophages to kill invading pathogens or may be dismuted into hydrogen peroxide (H2O2) and oxygen (169). The respiratory burst is often called a ‘glycolytic burst’ due to the increase in glucose uptake. Macrophages express glucose transporters GLUT1, 3 and 5, with GLUT1 being the primary transporter. GLUT1 expression is increased with macrophage activation (170–173, authors’ unpublished observations).
Similar to a pathogenic response to an invading bacterium, excess nutrients found in the obese adipose microenvironment can lead to the pro-inflammatory activation of macrophages. Chait and colleagues (174–177) have extensively studied the links between glucose and ROS production in obesity and have published data demonstrating that elevated glucose exposure drives inflammation and adipose tissue macrophage infiltration. Activation of the main enzyme involved in the respiratory burst, NAPDH oxidase (also known as NOX4), ROS production, and pro-inflammatory chemokine MCP-1 release were shown to be dependent upon glycolysis and the pentose phosphate pathway of glucose metabolism (178, 179). In addition, excessive glucose availability, in conjunction with high LDL exposure also resulted in elevated NADPH oxidase activity, H2O2 production, and chemotaxis in monocytes (180). Therefore, glucose uptake and metabolism drives ROS production and inflammation.
Second, endogenous production of ROS is a physiologic aspect of metabolism. The mitochondrial electron transport chain is the primary site for the production of ROS. ROS production is accelerated when the flow of electrons through the electron transport chain is impaired or when the constituents of the electron transport chain remain in a reduced state (181). In cells with elevated exposure to and uptake of glucose, the electron transport chain is hyperpolarized and produces excessive ROS (182). ROS, specifically H2O2, can also signal intracellularly in a physiologic manner (183) within macrophages, leading to enhanced activation of well-known pro-inflammatory signaling cascades. For example, treatment of macrophage cell lines with non-toxic concentrations of H2O2 resulted in increased accumulation of nuclear NFκB and AP-1, presumably by acting as a secondary messenger in the NFκB and MAPK signaling pathways (184, 185). Therefore, glucose-mediated ROS production can occur as part of the typical burst associated with activation as well as in response to metabolism in the face of excess glucose availability.
Cellular redox potential is a third mechanism that can be modified with excessive glucose exposure, as in the case of Type 2 diabetes. When intracellular glucose concentrations overwhelm the glycolytic pathway, glucose is instead reduced to sorbitol via the polyol pathway (also known as the sorbitol-aldose reductase pathway) resulting in decreased concentrations of NADPH (balanced by an increase in NADP+) and a blunting of the anti-oxidant glutathione levels (186). The decreased NADPH availability will systemically affect redox reactions since NADPH is a required cofactor for many of these reactions. A fourth mechanism is one linked to TGFβ. Exposure to high concentrations of glucose result in increased secretion of TGFβ-1 which, following receptor binding, causes the phosphorylation of small mothers against decapentaplegic proteins (SMADS-2 and -3) that subsequently translocate to nucleus, interact with SMAD-4 and ultimately stimulate transcription of pro-inflammatory proteins such as PAI-1, discussed above (187, 188). Furthermore, NADPH oxidase subunits NOX1-5, p22phox, p47phox, p67phox, and Rac1/Rac2 are all SMAD target genes displaying increased transcription rates stemming from TGFβ-1 signaling, underscoring the mechanism for the increase in NADPH oxidase activity with increased glucose exposure. Last but certainly not least, glucose-mediated modification of lipid metabolism and synthesis is well-characterized and should be considered as a potential glucose-derived source of inflammatory activators. Elevated glucose uptake has been shown to increase the pro-inflammatory eicosanoid-generating COX-2 pathway (189). In addition, glucose can regulate lipid synthesis which contributes to production of pro-inflammatory fatty acid, as well as obesity, through triglyceride accumulation. Glucose signals through carbohydrate response element binding protein (ChREBP) to drive transcription of fatty acid synthase, which produces palmitate, a saturated fatty acid that can stimulate pro-inflammatory pathways, as detailed below (147, 190). Taken together, elevated glucose uptake and metabolism activates multiple pathways that are linked to the inflammatory response, including kinase and transcriptional activation, ROS production, a shift in redox potential, TGFβ-mediated cytokine production, and synthesis of pro-inflammatory lipids (Fig. 3).
Adipocyte hypoxia, glucose uptake, and endoplasmic reticulum (ER) stress
While glucose may be elevated in diabetes, it is often not high in pre-diabetic or insulin resistant patients due to compensatory β-cell proliferation and elevated secretion of insulin leading to hyperinsulinemia. So, how are elevated glucose uptake and metabolism linked to obesity-induced inflammation in adipose tissue? One potential mechanism is through hypoxia-induced glucose uptake. Cellular hypoxia occurs when oxygen tension decreases to the point that oxygen availability is not adequate to support normal cellular processes. Obese adipose is hypoxic, in part, because of rapid adipocyte expansion, such that the diffusion distance of oxygen is exceeded, which forces an increase in angiogenesis and tissue remodeling in an effort to increase blood flow to the adipose tissue (191–195). To survive a hypoxic episode, cells must adapt to the low-oxygen environment and this adaptation includes altering gene expression patterns (193). Hypoxia-inducible factor-1 (HIF-1) is a well-characterized transcription factor that accumulates during hypoxia (196). Increased HIF-1 results in increased transcription of target genes involved in erythropoiesis and angiogenesis (197). Importantly, hypoxia has been implicated in the development of adipose inflammation and increased macrophage infiltration as well as Th17 and Treg differentiation (194, 198). During hypoxia, immune cells, found in the stromal vascular fraction, predominately macrophages, have been shown to increase pro-inflammatory cytokine production (191) (Fig. 1B). Of particular interest for this review, it has been reported that adipose hypoxia causes an induction of macrophage GLUT1 expression through up-regulation of HIF-1-mediated gene transcription (199). Importantly, GLUT1 expression was not increased in muscle, indicating that the hypoxic effect was local and adipose-specific rather than systemic.
In addition, another mechanism up-regulating glucose metabolism is one linked to the endoplasmic reticulum (ER). Secreted and membrane proteins are generated and folded in the ER and membrane lipid synthesis also occurs here. The ER is sensitive to shifts in cellular homeostasis brought about in response to hypoxia, obesity, and altered nutrient availability including glucose and lipids such as palmitate and cholesterol (200). Hypoxia is linked to ER stress because it results in an accumulation of misfolded proteins within the ER, thus initiating the unfolded protein response (UPR) in an effort to cope and, at the same time, increasing HIF-1 accumulation and driving increased GLUT1 expression (201, 202). Importantly, ER stress is activated in the adipose of obese mice, and Ozcan et al. (200, 203, 204) demonstrated that chronic ER stress drives obesity-induced inflammation and that blunting ER stress with genetic means or pharmacologically blunts insulin resistance.
Lipotoxicity and obesity-induced inflammation
Lipotoxicity is the state of lipid stress associated with elevated lipid content and ectopic lipid storage in tissues, such as liver and muscle, which can be chronic or acute. Obesity, persistent post-prandial lipoprotein circulation, weight loss, and fasting are conditions which result in an elevation of circulating levels of NEFA and, subsequently, an increase in inflammation (205–208). Macrophages take up VLDL and LDL, and Hasty et al. (209) demonstrated that VLDL loading of macrophages induces lipid storage and consequent inflammatory response. In addition to lipoprotein delivery, adipose is a likely source of fatty acids and, in fact, adipocytes may be the primary lipid source for macrophages found in this tissue (210, 211). Fatty acids can be derived from several sources, all known to be elevated in the obese state: (i) NEFA in serum; (ii) lipoprotein lipase (LPL) action on very low-density lipoproteins (VLDL) and chylomicron particles (212, 213); (iii) lipase action on triglyceride droplets in adipose (214); (iv) de novo lipogenesis, and/or (v) phagocytosis of apoptotic adipocytes (15, 23).
Fatty acids likely enter the macrophage through plasma membrane fatty acid transporters such as CD36, also known as fatty acid translocase (FAT) (215), which has been shown to play a role in the pro-inflammatory response in adipose (216). CD36 expression is elevated in macrophages isolated from obese animals and is induced when insulin signaling is dysregulated by insulin receptor deficiency (217, 218). Cytosolic lipid transporters, namely fatty acid-binding proteins, are also central to obesity and inflammation (26, 37, 140, 219, 220). Fatty acid-binding proteins are expressed in macrophages and play a major role in fatty acid and cholesterol metabolism (26, 37, 140, 219–221). Consequently, fatty acid transporters and binding proteins in adipose macrophages play critical roles in the immune response, serving as a link between fatty acid metabolism and inflammation in the context of obesity. Once inside the macrophage, however, little is known about the partitioning of NEFA either toward oxidation, triacylglyceride synthesis, or conversion into signaling lipid mediators and this remains an area of intense investigation.
Fatty acid signaling, metabolism, and ER stress
Increased circulating or intracellular NEFA possess the ability to modulate inflammation through several fatty acid-sensing mechanisms including cellular membrane receptors such as TLRs and G-protein coupled receptors (GPRs), and by serving as ligands for transcription factors such as the peroxisome proliferator activated receptor family (PPARsα, γ, δ). In addition, metabolism of fatty acids can result in release of oxidation intermediates called acylcarnitines or promote the generation of ROS through mitochondrial electron transport chain activity. Finally, fatty acids can be converted into bioactive lipid mediators such as eicosanoids, DAG, and ceramides, well-known modulators of inflammation through subsequent activation of signaling cascades including IKK, PKC, or the inflammasome, detailed in the following discussion.
The specific fatty acid species and the relative ratio of particular fatty acids found within the microenvironment are two highly relevant mediators of the inflammatory response. Saturated fatty acids, particularly stearic, lauric, and palmitic acid, activate TLR2 and TLR4 and drive the pro-inflammatory response by activation of the NFκB pathway, leading to increased expression of pro-inflammatory target genes (147, 222) and prostaglandins via an increase of COX-2 expression and activity in monocytes (190). In contrast, unsaturated fatty acids blunt induction of COX-2 and NFκB target genes (190, 222, 223). Second, GPRs such as GPR40, 41, 43, 84, 119, and 120 are receptors for some fatty acids (224, 225). For example, iGPR120, an omega-3 fatty acid receptor on expressed on macrophages, plays a role in blunting M1 macrophage activation and enhances gene expression patterns typical of M2 macrophages (226, 227). Therefore, not only is the fatty acid milieu relevant to the inflammatory response, but also the pattern of TLRs and GPRs that fatty acids can signal through are highly significant. In summary, the inflammatory response to fatty acids suggests that modifying consumption of different types of dietary fat may help modulate several of the inflammatory pathways discussed here.
An additional mechanism by which lipotoxicity leads to inflammation is through defective β-oxidation. Excessive levels of NEFA, as characteristic of obesity, will force an increase in mitochondrial β-oxidation – the process by which fatty acids are hydrolyzed to generate ATP – simply by increased delivery of substrate. Excess accumulation of lipids in tissues, i.e. lipotoxicity, however, can overwhelm the capacity of mitochondria to fully catabolize fatty acid substrate, resulting in incomplete β-oxidation. This phenomenon occurs when mitochondrial uptake of fatty acids exceeds the need for ATP production (228). With fatty acid overload, β-oxidation is highly elevated, as is gene expression for enzymes in this pathway; however, genes for the tricarboxcylic acid cycle (TCA) remain unaltered and are therefore hindered in their capacity to accept the acetyl-coA generated via β-oxidation. This typically occurs when there is limited drive for increased production of ATP, as in the obese state (228). Apparent overload of the β-oxidative pathway leads to production and accumulation of partially oxidized lipid byproducts including acylcarnitines, eventually leak from tissues (229). Importantly, increased serum and muscle acylcarnitines are detected during insulin resistant episodes, including fasting, persistent ingestion of a high fat diet, and obesity (228–233). Conversely, the inverse correlation is observed in models of enhanced insulin sensitivity, including during times of exercise training and in models employing genetically mediated reversal of diet-induced insulin resistance (228, 230, 233). We and others have shown that acylcarnitines are not only markers of defective oxidation but also mediate the pro-inflammatory response by blunting AMPK expression similar to M1 activation and through activation of NFκB transcription (229, 234, 235). Indeed, several epidemiological studies have linked acylcarnitine profiles with insulin resistance and other pathologies (236). Furthermore, similar to elevated glucose metabolism, elevated NEFA levels result in increased production of endogenous ROS through complete metabolism of fatty acids in the electron transport chain (237, 238), which has been linked to altered insulin responsiveness in both mice and humans (239–241). Interestingly, the saturated fatty acid palmitate also activates NADPH oxidase and ROS production, similar to glucose and the oxidative burst detailed above. Therefore, ROS and acylcarnitines serve as important intermediaries between incomplete and complete β-oxidation of NEFA and elevation of inflammatory kinase activity in macrophages.
The NLR family, pyrin domain containing 3 (NLRP3) inflammasome is another signaling cascade that has been shown to play a critical role in insulin resistance (242–244). The inflammasome is a multimeric complex constructed of an intracellular stress signal receptor, typically a Nod-like receptor such as NLRP3, the cysteine protease precursor procaspase-1, and an adapter protein apoptosis-associated speck-like protein containing a CARD (ASC) (reviewed in detail in 243). Inflammasomes are found in cells involved in the innate immune system and mediate the maturation and secretion of the pro-inflammatory cytokine IL-1β and IL-18. Upon activation, procaspase-1 assembles into its catalytically active form and cleaves pro-IL-1 β and pro-IL-18, which is followed by secretion of the cytokines by macrophages. The binding of IL-1β to the IL-1 receptor on target cells triggers the production and secretion of many pro-inflammatory cytokines, which, in turn, promote further inflammation, thus IL-1β secretion initiates and sustains a progressively amplified cytokine network (245). How is the inflammasome linked to obesity-induced inflammation?
In adipose, elevated levels of saturated fatty acids trigger inflammasome activation in macrophages residing within this tissue, resulting in decreased AMPK activity and mitochondrial autophagy (246). Reduced mitochondrial turnover results in an accumulation of damaged and malfunctioning mitochondria, which directly contributes to increased ROS production in these cells. Elevated ROS triggers the inflammasome formation culminating in enhanced IL-1β and IL-18 secretion into the adipose microenvironment. IL-1β interaction with adipocyte IL-1 receptor promotes disruption of the adipocyte insulin signaling pathway, thus contributing to decreased insulin sensitivity (246). Similar to TLR activation, saturated fatty acids, such as palmitate, but not unsaturated fatty acids have been linked to inflammasome activation in macrophages. Ceramide, generated from sphingomyelin degradation or the combination of long-chain fatty acids and sphingosine, and saturated fatty acids also activate procaspase-1 and subsequent IL-1β release. In macrophages, activation of the NLRP3 inflammasome has been shown to play a critical role in the development of insulin resistance. Furthermore, IL-1β secretion is blunted in NLRP−/− mice, and genetic deletion of either NLRP3, ASC, or caspase-1 leads to increased glucose tolerance and insulin sensitivity in mice (244–249). Of note, less B and T cells are detected in adipose of NLRP3−/− mice (245). Finally, glucose metabolism has also been demonstrated to be essential to NLRP3-mediated inflammasome activation (250, 251). In sum, ROS, acylcarnitine accumulation, ER stress, and the inflammasome are pathways integral to obesity-induced inflammation and are activated by nutrient levels, namely glucose and fatty acids.
Links between obesity, inflammation, and human health
Chronic diseases
The overlap of obesity and chronic disease incidence is well-documented. Perhaps the best known example of this is the link between metabolic disorders, macrophage-mediated inflammation and atherosclerosis, which has been reviewed extensively (252, 253). Inflammation also primes premalignant cells for cancerous transformation and plays a role in immune surveillance during tumorigenesis. An inflamed tissue microenvironment, perhaps similar to that described in obese individuals, offers an ideal setting for tumor onset and progression. Indeed, the development of many cancers including esophageal, pancreatic, colon and rectal, breast, uterine, kidney, thyroid, and gallbladder are associated with obesity (http://www.cancer.gov/cancertopics/factsheet/Risk/obesity). Tumor initiation is supported and promoted by increased oxidative stress stemming from activation of pro-inflammatory immune cells (reviewed in 254). Continuous secretion of pro-inflammatory cytokines by activated immune cells not only sustains ROS production but also can elicit epigenetic changes in pre-malignant cells stemming from aberrant DNA methylation-driven and microRNA-mediated gene silencing, particularly in tumor suppressor genes (255). In addition, the cytokines produced by activated immune cells can promote the reversion of premalignant cells to a progenitor cell-like phenotype, thus serving to expand the pool of stem cells within tissues that may be more susceptible to damaging inflammation (254).
Once premalignant cells have been transformed and tumor formation has been initiated, immune cells that infiltrate the tumor produce cytokines that activate transcription factors involved in cell cycling regulation, proliferation, and survival. Interestingly, NFκB is among the group of transcription factors upregulated during tumor promotion processes (256), suggesting that the low-grade inflammation and cytokine production associated with obesity can not only self-propagate but can also lead to transformation of pre-cancerous cells by modulating expression of tumor suppressor and cell cycling genes. In addition, the obese microenvironment provides nutrients such as glucose for fuel and building blocks necessary for a metabolic advantage associated with cell proliferation and evasion of apoptosis (257). Interestingly, in contrast to obese adipose, the proportion of M1 and M2 macrophages is reverse: more M2 macrophages are present in tumors and it is thought that M2 cytokines blunt the tumoricidal activity of M1 macrophages (258). Although recent work by our group (91) has revealed that similar to adipose, macrophages in the tumor microenvironment are more complex that simply M1 or M2 macrophages. The role of macrophages and other immune cells in cancer is reviewed in detail (259, 260).
Liver disease
Non-alcoholic and alcoholic fatty liver diseases are two states also affected by obesity-induced inflammation. Although beyond the scope of this review, the liver and resident macrophages, called Kupffer cells, display phenotypic changes similar to adipose with increasing triglyceride storage, lipotoxicity, glucotoxicity, and immune cell influx (reviewed in 261). Given the extent of fatty liver disease, the role of alcohol as an additional stressor should be considered. While some studies suggest that moderate alcohol use may be associated with a decrease in cardiovascular events and overall mortality (262, 263), heavy use is associated development of chronic liver disease and liver-related mortality (264, 265). In addition, alcohol consumption was recently reported to increase the risk of breast cancer (266). We and others have shown that alcohol is a modulator of the inflammatory response, causing dysregulation of both innate and adaptive immunity in the liver, lung, and adipose (267–272, authors’ unpublished observations). Like obesity, alcohol intake results in increased ROS production and overall levels of oxidative stress, thus promoting systemic inflammation and tissue pathology (273, 274). Alcohol exposure also elevates circulating concentrations of lipids, a hallmark of multiple sclerosis (MS) caused by unregulated adipose triglyceride lipolysis (271, 275). Therefore, the interaction between the immune response, obesity, and insults such as alcohol intake are relationships to be further identified.
Obesity and infection susceptibility
It is well-known that obese individuals have impaired immune defenses and are more susceptible to infections (11, 276). Despite heightened inflammatory responses in obesity, immune surveillance is compromised (120, 121). During the 2009 influenza A/H1N1 pandemic, obesity was identified as an independent risk factor for increased influenza-related morbidity and mortality (277, 278). A recent study in obese mice demonstrated that lung pathology and decreased survival following influenza infection is, in part, due to impaired M2 macrophage function (279), but little else is known about the effect of macrophage polarization on the severity of influenza infection. Because of the significant global burden of obesity and influenza, and the high probability that both obesity and influenza will occur simultaneously within an individual, it is important to clarify the underlying mechanisms linking a worse outcome in obese individuals who contract influenza in future studies.
Summary and concluding remarks
Obesity is an insidious disease correlated with multi-organ damage and increased susceptibility to a host of diseases including metabolic disorders, cardiovascular disease, cancer onset and progression, and infections such as influenza. Obesity is a state of low-grade, chronic inflammation associated with alterations in immune cell populations, including dynamic fluxes in the number and types of cells found within the inflamed tissue. Immune cells have been shown to infiltrate adipose at the onset of weight gain; these cells directly contribute to continued weight gain, persistent adipose inflammation, and systemic insulin resistance.
A spectrum of macrophage phenotypes exist ranging from the pro-inflammatory, classically activated M1 subtype to the anti-inflammatory, alternatively activated M2 subtype. In lean individuals, M2 macrophages are dispersed throughout adipose tissue, where they produce IL-10, IL-1 receptor antagonist, and express arginase-1 for collagen synthesis, which is necessary for tissue remodeling and repair. At the onset of obesity and as weight gain progresses, a shift in macrophage subtype occurs such that the M1 subtype becomes dominant resulting in propagation of inflammation by continued production of mediators such as TNFα, IL-1β, MCP-1, PAI-1, and ROS. M1 macrophages form crown-like structures surrounding dying adipocytes and M2 macrophages support, while M1 macrophages disrupt insulin sensitivity in adipose and liver. Similar alterations occur with other immune cells including influx of neutrophils, mast, CD4+ Th1, and NK cells and a concomitant loss of insulin sensitizing cells, Th2 cells, Tregs, and eosinophils.
It is well-established that losing 10% of body weight causes dramatic improvements in MS (280). While the most effective means for ameliorating metabolic abnormalities associated with obesity is weight loss, treatment with anti-inflammatory medications may also be beneficial. Stanley et al. (281) reported that administration of the TNFα signaling disruptor etanercept (Enbrel®) improved insulin sensitivity and plasma inflammation markers in a randomized controlled trial involving 40 obese individuals suffering from MS. These results were corroborated by other studies (282, 283). In addition, blockade of IL-1β signaling in individuals with Type 2 diabetes through the use of anakinra, a recombinant IL-1 receptor antagonist, resulted in decreased blood glucose concentrations, improved pancreatic β cell function, and reduced circulating inflammatory markers (284). Hence, developing therapeutic interventions aimed at converting M1-polarized macrophages to the protective M2 subtype may be beneficial to millions of individuals worldwide who already suffer from overweight and obesity. We suggest that targeting substrate metabolism uniquely activated in pro-inflammatory immune cells or by shifting availability of substrate or receptors, manipulation of the inflammatory response is an attractive approach to control obesity-induced inflammation and insulin resistance.
Acknowledgments
The project was funded by AA017376, ES019472, and University of North Carolina University Cancer Research Fund (UCRF) grants to L.M. This project was also supported in part by National Institutes of Health NIDDK grants P30DK056350 to the UNC-CH Nutrition Obesity Research Center and P30DK034987 to the Center for Gastrointestinal Biology and Disease to L.M. We thank Dr. Melinda Beck for assistance with manuscript review.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. Jama. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Organization WH. Obesity and Overweight. 2012 [Google Scholar]
- 4.Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med. 2005;56:45–62. doi: 10.1146/annurev.med.56.082103.104751. [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM. What is the contribution of obesity to the metabolic syndrome? Endocrinol Metab Clin North Am. 2004;33:267–282. doi: 10.1016/j.ecl.2004.03.001. table of contents. [DOI] [PubMed] [Google Scholar]
- 6.Reaven GM. Why Syndrome X? From Harold Himsworth to the insulin resistance syndrome. Cell Metab. 2005;1:9–14. doi: 10.1016/j.cmet.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Polonsky KS, Sturis J, Bell GI. Seminars in Medicine of the Beth Israel Hospital, Boston. Non-insulin-dependent diabetes mellitus -a genetically programmed failure of the beta cell to compensate for insulin resistance. N Engl J Med. 1996;334:777–783. doi: 10.1056/NEJM199603213341207. [DOI] [PubMed] [Google Scholar]
- 8.Odegaard JI, Chawla A. Connecting Type 1 and Type 2 Diabetes through Innate Immunity. Cold Spring Harbor perspectives in medicine. 2012;2:a007724. doi: 10.1101/cshperspect.a007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Population health metrics. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milner JJ, Beck MA. The impact of obesity on the immune response to infection. Proc Nutr Soc. 2012:1–9. doi: 10.1017/S0029665112000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weisberg SP, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neels JG, Olefsky JM. Inflamed fat: what starts the fire? J Clin Invest. 2006;116:33–35. doi: 10.1172/JCI27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arkan MC, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 18.Cancello R, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 19.Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab. 2006;290:E961–967. doi: 10.1152/ajpendo.00506.2005. [DOI] [PubMed] [Google Scholar]
- 20.Bruun JM, Lihn AS, Pedersen SB, Richelsen B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab. 2005;90:2282–2289. doi: 10.1210/jc.2004-1696. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto Y, et al. Comparison of mitochondrial and macrophage content between subcutaneous and visceral fat in db/db mice. Exp Mol Pathol. 2007;83:73–83. doi: 10.1016/j.yexmp.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Murano I, et al. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res. 2008;49:1562–1568. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Cinti S, et al. Adipocyte death defines macrophage localization and functionin adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Christiansen T, Richelsen B, Bruun JM. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int J Obes (Lond) 2005;29:146–150. doi: 10.1038/sj.ijo.0802839. [DOI] [PubMed] [Google Scholar]
- 25.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–934. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makowski L, Hotamisligil GS. Fatty acid binding proteins--the evolutionary crossroads of inflammatory and metabolic responses. J Nutr. 2004;134:2464S–2468S. doi: 10.1093/jn/134.9.2464S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrante AW., Jr Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262:408–414. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 28.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 29.Kanda H, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006 doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Gregorio GB, et al. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes. 2005;54:2305–2313. doi: 10.2337/diabetes.54.8.2305. [DOI] [PubMed] [Google Scholar]
- 31.Rasouli N, Kern PA. Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93:S64–73. doi: 10.1210/jc.2008-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Unal R, et al. Matrix metalloproteinase-9 is increased in obese subjects and decreases in response to pioglitazone. J Clin Endocrinol Metab. 2010;95:2993–3001. doi: 10.1210/jc.2009-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 34.Liang X, et al. Plasminogen activator inhibitor-1 modulates adipocyte differentiation. Am J Physiol Endocrinol Metab. 2006;290:E103–E113. doi: 10.1152/ajpendo.00605.2004. [DOI] [PubMed] [Google Scholar]
- 35.Fain JN. Release of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfat cells: a review. Mediators Inflamm. 2010:513948. doi: 10.1155/2010/513948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in Atherosclerosis. Circulation journal: official journal of the Japanese Circulation Society. doi: 10.1253/circj.cj-09-0706. [DOI] [PubMed] [Google Scholar]
- 37.Makowski L, Hotamisligil GS. The role of fatty acid binding proteins in metabolic syndrome and atherosclerosis. Curr Opin Lipidol. 2005;16:543–548. doi: 10.1097/01.mol.0000180166.08196.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Z, et al. Troglitazone inhibits atherosclerosis in apolipoprotein E-knockout mice: pleiotropic effects on CD36 expression and HDL. Arterioscler Thromb Vasc Biol. 2001;21:372–377. doi: 10.1161/01.atv.21.3.372. [DOI] [PubMed] [Google Scholar]
- 39.Chawla A, et al. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 40.Linton MF, Fazio S. Macrophages, inflammation, and atherosclerosis. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S35–40. doi: 10.1038/sj.ijo.0802498. [DOI] [PubMed] [Google Scholar]
- 41.Bursill CA, Channon KM, Greaves DR. The role of chemokines in atherosclerosis: recent evidence from experimental models and population genetics. Curr Opin Lipidol. 2004;15:145–149. doi: 10.1097/00041433-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Aiello RJ, et al. Monocyte chemoattractant protein-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:1518–1525. doi: 10.1161/01.atv.19.6.1518. [DOI] [PubMed] [Google Scholar]
- 43.Dawson TC, Kuziel WA, Osahar TA, Maeda N. Absence of CC chemokine receptor-2 reduces atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 1999;143:205–211. doi: 10.1016/s0021-9150(98)00318-9. [DOI] [PubMed] [Google Scholar]
- 44.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 46.Scarpace PJ, Zhang Y. Leptin resistance: a prediposing factor for diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2009;296:R493–500. doi: 10.1152/ajpregu.90669.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arita Y, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 48.Yamauchi T, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 49.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 50.Ouchi N, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 51.Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clinica chimica acta; international journal of clinical chemistry. 2007;380:24–30. doi: 10.1016/j.cca.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kadowaki T, Yamauchi T, Kubota N. The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett. 2008;582:74–80. doi: 10.1016/j.febslet.2007.11.070. [DOI] [PubMed] [Google Scholar]
- 53.Vasseur F, Meyre D, Froguel P. Adiponectin, type 2 diabetes and the metabolic syndrome: lessons from human genetic studies. Expert Rev Mol Med. 2006;8:1–12. doi: 10.1017/S1462399406000147. [DOI] [PubMed] [Google Scholar]
- 54.Banerjee RR, et al. Regulation of fasted blood glucose by resistin. Science. 2004;303:1195–1198. doi: 10.1126/science.1092341. [DOI] [PubMed] [Google Scholar]
- 55.Steppan CM, Lazar MA. The current biology of resistin. J Intern Med. 2004;255:439–447. doi: 10.1111/j.1365-2796.2004.01306.x. [DOI] [PubMed] [Google Scholar]
- 56.Patel L, et al. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun. 2003;300:472–476. doi: 10.1016/s0006-291x(02)02841-3. [DOI] [PubMed] [Google Scholar]
- 57.Yang Q, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 58.Kalupahana NS, et al. Overproduction of angiotensinogen from adipose tissue induces adipose inflammation, glucose intolerance, and insulin resistance. Obesity (Silver Spring) 2012;20:48–56. doi: 10.1038/oby.2011.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siriwardhana N, et al. n-3 and n-6 polyunsaturated fatty acids differentially regulate adipose angiotensinogen and other inflammatory adipokines in part via NF-kappaB-dependent mechanisms. J Nutr Biochem. 2012 doi: 10.1016/j.jnutbio.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 60.Oda E. CRP may be superior to anthropometric markers of obesity. Circulation journal: official journal of the Japanese Circulation Society. 2007;71:1332. doi: 10.1253/circj.71.1332. author reply 1332–1333. [DOI] [PubMed] [Google Scholar]
- 61.Memoli B, et al. Inflammation may modulate IL-6 and C-reactive protein gene expression in the adipose tissue: the role of IL-6 cell membrane receptor. Am J Physiol Endocrinol Metab. 2007;293:E1030–1035. doi: 10.1152/ajpendo.00697.2006. [DOI] [PubMed] [Google Scholar]
- 62.Calabro P, Chang DW, Willerson JT, Yeh ET. Release of C-reactive protein in response to inflammatory cytokines by human adipocytes: linking obesity to vascular inflammation. J Am Coll Cardiol. 2005;46:1112–1113. doi: 10.1016/j.jacc.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 63.Han CY, et al. Adipocyte-derived serum amyloid A3 and hyaluronan play a role in monocyte recruitment and adhesion. Diabetes. 2007;56:2260–2273. doi: 10.2337/db07-0218. [DOI] [PubMed] [Google Scholar]
- 64.Mertens I, Verrijken A, Michiels JJ, Van der Planken M, Ruige JB, Van Gaal LF. Among inflammation and coagulation markers, PAI-1 is a true component of the metabolic syndrome. Int J Obes (Lond) 2006;30:1308–1314. doi: 10.1038/sj.ijo.0803189. [DOI] [PubMed] [Google Scholar]
- 65.Peeraully MR, Sievert H, Bullo M, Wang B, Trayhurn P. Prostaglandin D2 and J2-series (PGJ2, Delta12-PGJ2) prostaglandins stimulate IL-6 and MCP-1, but inhibit leptin, expression and secretion by 3T3-L1 adipocytes. Pflugers Arch. 2006;453:177–187. doi: 10.1007/s00424-006-0118-x. [DOI] [PubMed] [Google Scholar]
- 66.Bullo M, Peeraully MR, Trayhurn P. Stimulation of NGF expression and secretion in 3T3-L1 adipocytes by prostaglandins PGD2, PGJ2, and Delta12-PGJ2. Am J Physiol Endocrinol Metab. 2005;289:E62–67. doi: 10.1152/ajpendo.00008.2005. [DOI] [PubMed] [Google Scholar]
- 67.Mozaffarian D, et al. Trans-palmitoleic acid, metabolic risk factors, and new-onset diabetes in U.S. adults: a cohort study. Ann Intern Med. 2010;153:790–799. doi: 10.1059/0003-4819-153-12-201012210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mozaffarian D, et al. Circulating palmitoleic acid and risk of metabolic abnormalities and new-onset diabetes. Am J Clin Nutr. 2010;92:1350–1358. doi: 10.3945/ajcn.110.003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cancello R, Clement K. Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. BJOG: an international journal of obstetrics and gynaecology. 2006;113:1141–1147. doi: 10.1111/j.1471-0528.2006.01004.x. [DOI] [PubMed] [Google Scholar]
- 71.Oh DY, Morinaga H, Talukdar S, Bae EJ, Olefsky JM. Increased macrophage migration into adipose tissue in obese mice. Diabetes. 2012;61:346–354. doi: 10.2337/db11-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gordon S. The macrophage: past, present and future. European journal of immunology. 2007;37 (Suppl 1):S9–17. doi: 10.1002/eji.200737638. [DOI] [PubMed] [Google Scholar]
- 74.Suganami T, et al. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- 75.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 77.Nguyen MT, et al. JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. J Biol Chem. 2005;280:35361–35371. doi: 10.1074/jbc.M504611200. [DOI] [PubMed] [Google Scholar]
- 78.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 79.Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annu Rev Pathol. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Odegaard JI, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Odegaard JI, Chawla A. Mechanisms of macrophage activation in obesity-induced insulin resistance. Nat Clin Pract Endocrinol Metab. 2008;4:619–626. doi: 10.1038/ncpendmet0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee CH, et al. PPARdelta regulates glucose metabolism and insulin sensitivity. Proc Natl Acad Sci U S A. 2006;103:3444–3449. doi: 10.1073/pnas.0511253103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stienstra R, Duval C, Keshtkar S, van der Laak J, Kersten S, Muller M. Peroxisome proliferator-activated receptor gamma activation promotes infiltration of alternatively activated macrophages into adipose tissue. J Biol Chem. 2008;283:22620–22627. doi: 10.1074/jbc.M710314200. [DOI] [PubMed] [Google Scholar]
- 84.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lumeng CN, Deyoung SM, Saltiel AR. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am J Physiol Endocrinol Metab. 2007;292:E166–174. doi: 10.1152/ajpendo.00284.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prieur X, et al. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes. 2011;60:797–809. doi: 10.2337/db10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Odegaard JI, et al. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kang K, et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: the paradox between visceral and subcutaneous fat. Current diabetes reviews. 2006;2:367–373. doi: 10.2174/1573399810602040367. [DOI] [PubMed] [Google Scholar]
- 90.Sun X, et al. Normal breast tissue of obese women is enriched for macrophage markers and macrophage-associated gene expression. Breast Cancer Res Treat. 2012;131:1003–1012. doi: 10.1007/s10549-011-1789-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stewart DA, Yang Y, Makowski L, Troester MA. Basal-like breast cancer cells induce phenotypic and genomic changes in macrophages. Molecular cancer research: MCR. 2012 doi: 10.1158/1541-7786.MCR-11-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vats D, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lacy-Hulbert A, Moore KJ. Designer macrophages: oxidative metabolism fuels inflammation repair. Cell Metab. 2006;4:7–8. doi: 10.1016/j.cmet.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 94.Barbul A. Proline precursors to sustain Mammalian collagen synthesis. J Nutr. 2008;138:2021S–2024S. doi: 10.1093/jn/138.10.2021S. [DOI] [PubMed] [Google Scholar]
- 95.Caspar-Bauguil S, Cousin B, Bour S, Casteilla L, Penicaud L, Carpene C. Adipose tissue lymphocytes: types and roles. J Physiol Biochem. 2009;65:423–436. doi: 10.1007/BF03185938. [DOI] [PubMed] [Google Scholar]
- 96.Caspar-Bauguil S, et al. Adipose tissues as an ancestral immune organ: site-specific change in obesity. FEBS Lett. 2005;579:3487–3492. doi: 10.1016/j.febslet.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 97.Winer S, Winer DA. The adaptive immune system as a fundamental regulator of adipose tissue inflammation and insulin resistance. Immunology and cell biology. 2012 doi: 10.1038/icb.2011.110. [DOI] [PubMed] [Google Scholar]
- 98.Duffaut C, Galitzky J, Lafontan M, Bouloumie A. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem Biophys Res Commun. 2009;384:482–485. doi: 10.1016/j.bbrc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 99.Winer DA, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kintscher U, et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1304–1310. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- 101.Wu H, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 102.Duffaut C, et al. Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arterioscler Thromb Vasc Biol. 2009;29:1608–1614. doi: 10.1161/ATVBAHA.109.192583. [DOI] [PubMed] [Google Scholar]
- 103.Rocha VZ, et al. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res. 2008;103:467–476. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Matter CM, Handschin C. RANTES (regulated on activation, normal T cell expressed and secreted), inflammation, obesity, and the metabolic syndrome. Circulation. 2007;115:946–948. doi: 10.1161/CIRCULATIONAHA.106.685230. [DOI] [PubMed] [Google Scholar]
- 105.Sultan A, et al. T cell-mediated inflammation in adipose tissue does not cause insulin resistance in hyperlipidemic mice. Circ Res. 2009;104:961–968. doi: 10.1161/CIRCRESAHA.108.190280. [DOI] [PubMed] [Google Scholar]
- 106.Feuerer M, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ilan Y, et al. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/obmice. Proc Natl Acad Sci U S A. 2010;107:9765–9770. doi: 10.1073/pnas.0908771107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nishimura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 109.Winer S, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang H, et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185:1836–1845. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ortega Martinez de Victoria E, et al. Macrophage content in subcutaneous adipose tissue: associations with adiposity, age, inflammatory markers, and whole-body insulin action in healthy Pima Indians. Diabetes. 2009;58:385–393. doi: 10.2337/db08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.O’Rourke RW, et al. Depot-specific differences in inflammatory mediators and a role for NK cells and IFN-gamma in inflammation in human adipose tissue. Int J Obes (Lond) 2009;33:978–990. doi: 10.1038/ijo.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Galic S, et al. Hematopoietic AMPK beta1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. J Clin Invest. 2011;121:4903–4915. doi: 10.1172/JCI58577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Michalek RD, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jacobs SR, et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell. 2007;18:1437–1446. doi: 10.1091/mbc.E06-07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.MacIver NJ, et al. The liver kinase B1 is a central regulator of T cell development, activation, and metabolism. J Immunol. 2011;187:4187–4198. doi: 10.4049/jimmunol.1100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maya-Monteiro CM, Bozza PT. Leptin and mTOR: partners in metabolism and inflammation. Cell Cycle. 2008;7:1713–1717. doi: 10.4161/cc.7.12.6157. [DOI] [PubMed] [Google Scholar]
- 119.Finlay D, Cantrell DA. Metabolism, migration and memory in cytotoxic T cells. Nat Rev Immunol. 2011;11:109–117. doi: 10.1038/nri2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sheridan PA, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes (Lond) 2011 doi: 10.1038/ijo.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Karlsson EA, Sheridan PA, Beck MA. Diet-induced obesity in mice reduces the maintenance of influenza-specific CD8+ memory T cells. J Nutr. 2010;140:1691–1697. doi: 10.3945/jn.110.123653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Surmi BK, Hasty AH. Macrophage infiltration into adipose tissue: initiation, propagation and remodeling. Future Lipidol. 2008;3:545–556. doi: 10.2217/17460875.3.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu L, et al. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1200498109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Satoh M, et al. Type II NKT cells stimulate diet-induced obesity by mediating adipose tissue inflammation, steatohepatitis and insulin resistance. PLoS One. 2012;7:e30568. doi: 10.1371/journal.pone.0030568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ohmura K, et al. Natural killer T cells are involved in adipose tissues inflammation and glucose intolerance in diet-induced obese mice. Arterioscler Thromb Vasc Biol. 2010;30:193–199. doi: 10.1161/ATVBAHA.109.198614. [DOI] [PubMed] [Google Scholar]
- 126.Ji Y, et al. Activation of Natural Killer T Cells Promotes M2 Macrophage Polarization in Adipose Tissue and Improves Systemic Glucose Tolerance via Interleukin-4 (IL-4)/STAT6 Protein Signaling Axis in Obesity. J Biol Chem. 2012;287:13561–13571. doi: 10.1074/jbc.M112.350066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moro K, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 128.Liu J, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tanaka A, Nomura Y, Matsuda A, Ohmori K, Matsuda H. Mast cells function as an alternative modulator of adipogenesis through 15-deoxy-delta-12, 14-prostaglandin J2. Am J Physiol Cell Physiol. 2011;301:C1360–1367. doi: 10.1152/ajpcell.00514.2010. [DOI] [PubMed] [Google Scholar]
- 130.Poglio S, et al. Adipose tissue as a dedicated reservoir of functional mast cell progenitors. Stem Cells. 2010;28:2065–2072. doi: 10.1002/stem.523. [DOI] [PubMed] [Google Scholar]
- 131.Zhang J, Shi GP. Mast cells and metabolic syndrome. Biochim Biophys Acta. 2012;1822:14–20. doi: 10.1016/j.bbadis.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49:1894–1903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- 133.Kim DJ, et al. The associations of total and differential white blood cell counts with obesity, hypertension, dyslipidemia and glucose intolerance in a Korean population. Journal of Korean medical science. 2008;23:193–198. doi: 10.3346/jkms.2008.23.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Suzukawa M, et al. Leptin enhances survival and induces migration, degranulation, and cytokine synthesis of human basophils. J Immunol. 2011;186:5254–5260. doi: 10.4049/jimmunol.1004054. [DOI] [PubMed] [Google Scholar]
- 135.Kim JA, Park HS. White blood cell count and abdominal fat distribution in female obese adolescents. Metabolism. 2008;57:1375–1379. doi: 10.1016/j.metabol.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 136.Laurson KR, McCann DA, Senchina DS. Age, sex, and ethnicity may modify the influence of obesity on inflammation. Journal of investigative medicine: the official publication of the American Federation for Clinical Research. 2011;59:27–31. doi: 10.231/JIM.0b013e318200151a. [DOI] [PubMed] [Google Scholar]
- 137.Johannsen NM, Priest EL, Dixit VD, Earnest CP, Blair SN, Church TS. Association of white blood cell subfraction concentration with fitness and fatness. British journal of sports medicine. 2010;44:588–593. doi: 10.1136/bjsm.2008.050682. [DOI] [PubMed] [Google Scholar]
- 138.Maizels RM, Allen JE. Immunology. Eosinophils forestall obesity. Science. 2011;332:186–187. doi: 10.1126/science.1205313. [DOI] [PubMed] [Google Scholar]
- 139.Wu D, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Makowski L, et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med. 2001;7:699–705. doi: 10.1038/89076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Reynolds JM, et al. Deficiency of fatty acid-binding proteins in mice confers protection from development of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:313–321. doi: 10.4049/jimmunol.179.1.313. [DOI] [PubMed] [Google Scholar]
- 142.Shum BO, et al. The adipocyte fatty acid-binding protein aP2 is required in allergic airway inflammation. J Clin Invest. 2006;116:2183–2192. doi: 10.1172/JCI24767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Furuhashi M, et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 2007;447:959–965. doi: 10.1038/nature05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Owen C, et al. Adipocyte-specific protein tyrosine phosphatase 1B deletion increases lipogenesis, adipocyte cell size and is a minor regulator of glucose homeostasis. PLoS One. 2012;7:e32700. doi: 10.1371/journal.pone.0032700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cho IH, et al. Role of microglial IKKbeta in kainic acid-induced hippocampal neuronal cell death. Brain: a journal of neurology. 2008;131:3019–3033. doi: 10.1093/brain/awn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Thaler JP, Choi SJ, Schwartz MW, Wisse BE. Hypothalamic inflammation and energy homeostasis: resolving the paradox. Frontiers in neuroendocrinology. 2010;31:79–84. doi: 10.1016/j.yfrne.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 147.Lee JY, et al. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem. 2004;279:16971–16979. doi: 10.1074/jbc.M312990200. [DOI] [PubMed] [Google Scholar]
- 148.Sopko R, Andrews BJ. Linking the kinome and phosphorylome--a comprehensive review of approaches to find kinase targets. Molecular bioSystems. 2008;4:920–933. doi: 10.1039/b801724g. [DOI] [PubMed] [Google Scholar]
- 149.Talior I, Tennenbaum T, Kuroki T, Eldar-Finkelman H. PKC-delta-dependent activation of oxidative stress in adipocytes of obese and insulin-resistant mice: role for NADPH oxidase. Am J Physiol Endocrinol Metab. 2005;288:E405–411. doi: 10.1152/ajpendo.00378.2004. [DOI] [PubMed] [Google Scholar]
- 150.Talior I, Yarkoni M, Bashan N, Eldar-Finkelman H. Increased glucose uptake promotes oxidative stress and PKC-delta activation in adipocytes of obese, insulin-resistant mice. Am J Physiol Endocrinol Metab. 2003;285:E295–302. doi: 10.1152/ajpendo.00044.2003. [DOI] [PubMed] [Google Scholar]
- 151.Haasch D, et al. PKCtheta is a key player in the development of insulin resistance. Biochem Biophys Res Commun. 2006;343:361–368. doi: 10.1016/j.bbrc.2006.02.177. [DOI] [PubMed] [Google Scholar]
- 152.Samuel VT, et al. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 2007;117:739–745. doi: 10.1172/JCI30400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bluher M, et al. Activated Ask1-MKK4-p38MAPK/JNK stress signaling pathway in human omental fat tissue may link macrophage infiltration to whole-body Insulin sensitivity. J Clin Endocrinol Metab. 2009;94:2507–2515. doi: 10.1210/jc.2009-0002. [DOI] [PubMed] [Google Scholar]
- 154.Tuncman G, Hirosumi J, Solinas G, Chang L, Karin M, Hotamisligil GS. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2006;103:10741–10746. doi: 10.1073/pnas.0603509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vallerie SN, Hotamisligil GS. The role of JNK proteins in metabolism. Sci Transl Med. 2010;2:60rv65. doi: 10.1126/scitranslmed.3001007. [DOI] [PubMed] [Google Scholar]
- 156.Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 157.Lii CK, et al. Diallyl trisulfide suppresses the adipogenesis of 3T3-L1 preadipocytes through ERK activation. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2012;50:478–484. doi: 10.1016/j.fct.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 158.Jager J, et al. Deficiency in the extracellular signal-regulated kinase 1 (ERK1) protects leptin-deficient mice from insulin resistance without affecting obesity. Diabetologia. 2011;54:180–189. doi: 10.1007/s00125-010-1944-0. [DOI] [PubMed] [Google Scholar]
- 159.Crunkhorn S, et al. Peroxisome proliferator activator receptor gamma coactivator-1 expression is reduced in obesity: potential pathogenic role of saturated fatty acids and p38 mitogen-activated protein kinase activation. J Biol Chem. 2007;282:15439–15450. doi: 10.1074/jbc.M611214200. [DOI] [PubMed] [Google Scholar]
- 160.Kim JK, et al. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001;108:437–446. doi: 10.1172/JCI11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hotamisligil GS. Role of Endoplasmic Reticulum Stress and c-Jun NH2-Terminal Kinase Pathways in Inflammation and Origin of Obesity and Diabetes. Diabetes. 2005;54(Suppl 2):S73–78. doi: 10.2337/diabetes.54.suppl_2.s73. [DOI] [PubMed] [Google Scholar]
- 163.Vallerie SN, Furuhashi M, Fucho R, Hotamisligil GS. A predominant role for parenchymal c-Jun amino terminal kinase (JNK) in the regulation of systemic insulin sensitivity. PLoS One. 2008;3:e3151. doi: 10.1371/journal.pone.0003151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Holzer RG, et al. Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation. Cell. 2011;147:173–184. doi: 10.1016/j.cell.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Solinas G, Naugler W, Galimi F, Lee MS, Karin M. Saturated fatty acids inhibit induction of insulin gene transcription by JNK-mediated phosphorylation of insulin-receptor substrates. Proc Natl Acad Sci U S A. 2006;103:16454–16459. doi: 10.1073/pnas.0607626103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Solinas G, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 167.Hundal RS, et al. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109:1321–1326. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Iles KE, Forman HJ. Macrophage signaling and respiratory burst. Immunologic research. 2002;26:95–105. doi: 10.1385/IR:26:1-3:095. [DOI] [PubMed] [Google Scholar]
- 169.Sheppard FR, Kelher MR, Moore EE, McLaughlin NJ, Banerjee A, Silliman CC. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. J Leukoc Biol. 2005;78:1025–1042. doi: 10.1189/jlb.0804442. [DOI] [PubMed] [Google Scholar]
- 170.Ahmed N, Kansara M, Berridge MV. Acute regulation of glucose transport in a monocyte-macrophage cell line: Glut-3 affinity for glucose is enhanced during the respiratory burst. Biochem J. 1997;327 (Pt 2):369–375. doi: 10.1042/bj3270369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fu Y, Maianu L, Melbert BR, Garvey WT. Facilitative glucose transporter gene expression in human lymphocytes, monocytes, and macrophages: a role for GLUT isoforms 1, 3, and 5 in the immune response and foam cell formation. Blood Cells Mol Dis. 2004;32:182–190. doi: 10.1016/j.bcmd.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 172.Elsegood CL, Chang M, Jessup W, Scholz GM, Hamilton JA. Glucose metabolism is required for oxidized LDL-induced macrophage survival: role of PI3K and Bcl-2 family proteins. Arterioscler Thromb Vasc Biol. 2009;29:1283–1289. doi: 10.1161/ATVBAHA.108.180778. [DOI] [PubMed] [Google Scholar]
- 173.Chang M, Hamilton JA, Scholz GM, Elsegood CL. Glycolytic control of adjuvant-induced macrophage survival: role of PI3K, MEK1/2, and Bcl-2. J Leukoc Biol. 2009;85:947–956. doi: 10.1189/jlb.0908522. [DOI] [PubMed] [Google Scholar]
- 174.Yeop Han C, et al. Differential effect of saturated and unsaturated free fatty acids on the generation of monocyte adhesion and chemotactic factors by adipocytes: dissociation of adipocyte hypertrophy from inflammation. Diabetes. 2010;59:386–396. doi: 10.2337/db09-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Subramanian V, Ferrante AW., Jr Obesity, inflammation, and macrophages. Nestle Nutr Workshop Ser Pediatr Program. 2009;63:151–159. doi: 10.1159/000209979. discussion 159–162, 259-168. [DOI] [PubMed] [Google Scholar]
- 176.Subramanian S, Chait A. The effect of dietary cholesterol on macrophage accumulation in adipose tissue: implications for systemic inflammation and atherosclerosis. Curr Opin Lipidol. 2009;20:39–44. doi: 10.1097/mol.0b013e32831bef8b. [DOI] [PubMed] [Google Scholar]
- 177.Subramanian S, et al. Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:685–691. doi: 10.1161/ATVBAHA.107.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Han CY, et al. NADPH Oxidase-derived Reactive Oxygen Species Increases Expression of Monocyte Chemotactic Factor Genes in Cultured Adipocytes. J Biol Chem. 2012;287:10379–10393. doi: 10.1074/jbc.M111.304998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Musset B, Cherny VV, DeCoursey TE. Strong glucose dependence of electron current in human monocytes. Am J Physiol Cell Physiol. 2012;302:C286–295. doi: 10.1152/ajpcell.00335.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ullevig S, Zhao Q, Lee CF, Seok Kim H, Zamora D, Asmis R. NADPH oxidase 4 mediates monocyte priming and accelerated chemotaxis induced by metabolic stress. Arterioscler Thromb Vasc Biol. 2012;32:415–426. doi: 10.1161/ATVBAHA.111.238899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bonnefont-Rousselot D. Glucose and reactive oxygen species. Current opinion in clinical nutrition and metabolic care. 2002;5:561–568. doi: 10.1097/00075197-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 183.Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am J Respir Crit Care Med. 2002;166:S4–8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- 184.Iles KE, Dickinson DA, Watanabe N, Iwamoto T, Forman HJ. AP-1 activation through endogenous H(2)O(2) generation by alveolar macrophages. Free radical biology & medicine. 2002;32:1304–1313. doi: 10.1016/s0891-5849(02)00840-7. [DOI] [PubMed] [Google Scholar]
- 185.Kaul N, Forman HJ. Activation of NF kappa B by the respiratory burst of macrophages. Free radical biology & medicine. 1996;21:401–405. doi: 10.1016/0891-5849(96)00178-5. [DOI] [PubMed] [Google Scholar]
- 186.Bravi MC, et al. Polyol pathway activation and glutathione redox status in non-insulin-dependent diabetic patients. Metabolism. 1997;46:1194–1198. doi: 10.1016/s0026-0495(97)90216-x. [DOI] [PubMed] [Google Scholar]
- 187.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 188.Koya D, Jirousek MR, Lin YW, Ishii H, Kuboki K, King GL. Characterization of protein kinase C beta isoform activation on the gene expression of transforming growth factor-beta, extracellular matrix components, and prostanoids in the glomeruli of diabetic rats. J Clin Invest. 1997;100:115–126. doi: 10.1172/JCI119503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shanmugam N, Gaw Gonzalo IT, Natarajan R. Molecular mechanisms of high glucose-induced cyclooxygenase-2 expression in monocytes. Diabetes. 2004;53:795–802. doi: 10.2337/diabetes.53.3.795. [DOI] [PubMed] [Google Scholar]
- 190.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 191.O’Rourke RW, et al. Hypoxia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells. Diabetologia. 2011;54:1480–1490. doi: 10.1007/s00125-011-2103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lolmede K, Durand de Saint Front V, Galitzky J, Lafontan M, Bouloumie A. Effects of hypoxia on the expression of proangiogenic factors in differentiated 3T3-F442A adipocytes. Int J Obes Relat Metab Disord. 2003;27:1187–1195. doi: 10.1038/sj.ijo.0802407. [DOI] [PubMed] [Google Scholar]
- 193.Hosogai N, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 194.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 2008;32:451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 195.Skurk T, Mack I, Kempf K, Kolb H, Hauner H, Herder C. Expression and secretion of RANTES (CCL5) in human adipocytes in response to immunological stimuli and hypoxia. Horm Metab Res. 2009;41:183–189. doi: 10.1055/s-0028-1093345. [DOI] [PubMed] [Google Scholar]
- 196.Semenza GL. HIF-1 and human disease: one highly involved factor. Genes & development. 2000;14:1983–1991. [PubMed] [Google Scholar]
- 197.Semenza GL, Shimoda LA, Prabhakar NR. Regulation of gene expression by HIF-1. Novartis Found Symp. 2006;272:2–8. discussion 8–14, 33–16. [PubMed] [Google Scholar]
- 198.Shi LZ, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Trayhurn P, Wang B, Wood IS. HIF-1alpha protein rather than mRNA as a marker of hypoxia in adipose tissue in obesity: focus on “inflammation is associated with a decrease of lipogenic factors in omental fat in women,” by Poulain-Godefroy et al. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1097. doi: 10.1152/ajpregu.90633.2008. author reply R1098. [DOI] [PubMed] [Google Scholar]
- 200.Hotamisligil GS. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int J Obes (Lond) 2008;32(Suppl 7):S52–54. doi: 10.1038/ijo.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.van den Beucken T, Koritzinsky M, Wouters BG. Translational control of gene expression during hypoxia. Cancer biology & therapy. 2006;5:749–755. doi: 10.4161/cbt.5.7.2972. [DOI] [PubMed] [Google Scholar]
- 202.Pfafflin A, Brodbeck K, Heilig CW, Haring HU, Schleicher ED, Weigert C. Increased glucose uptake and metabolism in mesangial cells overexpressing glucose transporter 1 increases interleukin-6 and vascular endothelial growth factor production:role of AP-1 and HIF-1alpha. Cell Physiol Biochem. 2006;18:199–210. doi: 10.1159/000097667. [DOI] [PubMed] [Google Scholar]
- 203.Ozcan U, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 204.Ozcan U, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gordon S. Macrophage heterogeneity and tissue lipids. J Clin Invest. 2007;117:89–93. doi: 10.1172/JCI30992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kosteli A, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Alipour A, Elte JW, van Zaanen HC, Rietveld AP, Cabezas MC. Postprandial inflammation and endothelial dysfuction. Biochem Soc Trans. 2007;35:466–469. doi: 10.1042/BST0350466. [DOI] [PubMed] [Google Scholar]
- 208.Blackburn P, et al. Postprandial variations of plasma inflammatory markers in abdominally obese men. Obesity (Silver Spring) 2006;14:1747–1754. doi: 10.1038/oby.2006.201. [DOI] [PubMed] [Google Scholar]
- 209.Saraswathi V, Hasty AH. The role of lipolysis in mediating the pro-inflammatory effects of very low density lipoproteins in mouse peritoneal macrophages. J Lipid Res. 2006 doi: 10.1194/jlr.M600159-JLR200. [DOI] [PubMed] [Google Scholar]
- 210.Rader DJ, Pure E. Lipoproteins, macrophage function, and atherosclerosis: beyond the foam cell? Cell Metab. 2005;1:223–230. doi: 10.1016/j.cmet.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 211.Takahashi S, Sakai J, Fujino T, Miyamori I, Yamamoto TT. The very low density lipoprotein (VLDL) receptor--a peripheral lipoprotein receptor for remnant lipoproteins into fatty acid active tissues. Mol Cell Biochem. 2003;248:121–127. doi: 10.1023/a:1024184201941. [DOI] [PubMed] [Google Scholar]
- 212.Chawla A, et al. PPARdelta is a very low-density lipoprotein sensor in macrophages. Proc Natl Acad Sci U S A. 2003;100:1268–1273. doi: 10.1073/pnas.0337331100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lee CH, et al. Peroxisome proliferator-activated receptor delta promotes very low-density lipoprotein-derived fatty acid catabolism in the macrophage. Proc Natl Acad Sci U S A. 2006;103:2434–2439. doi: 10.1073/pnas.0510815103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Milosavljevic D, Kontush A, Griglio S, Le Naour G, Thillet J, Chapman MJ. VLDL-induced triglyceride accumulation in human macrophages is mediated by modulation of LPL lipolytic activity in the absence of change in LPL mass. Biochim Biophys Acta. 2003;1631:51–60. doi: 10.1016/s1388-1981(02)00355-4. [DOI] [PubMed] [Google Scholar]
- 215.Ibrahimi A, Abumrad NA. Role of CD36 in membrane transport of long-chain fatty acids. Current opinion in clinical nutrition and metabolic care. 2002;5:139–145. doi: 10.1097/00075197-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 216.Nicholls HT, et al. Hematopoietic cell-restricted deletion of CD36 reduces high-fat diet-induced macrophage infiltration and improves insulin signaling in adipose tissue. Diabetes. 2011;60:1100–1110. doi: 10.2337/db10-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Liang CP, et al. Increased CD36 protein as a response to defective insulin signaling in macrophages. J Clin Invest. 2004;113:764–773. doi: 10.1172/JCI19528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Han S, et al. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 2006;3:257–266. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 219.Erbay E, et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15:1383–1391. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Makowski L, Brittingham KC, Reynolds JM, Suttles J, Hotamisligil GS. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor gamma and IkappaB kinase activities. J Biol Chem. 2005;280:12888–12895. doi: 10.1074/jbc.M413788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Fu Y, Luo N, Lopes-Virella MF. Oxidized LDL induces the expression of ALBP/aP2 mRNA and protein in human THP-1 macrophages. J Lipid Res. 2000;41:2017–2023. [PubMed] [Google Scholar]
- 222.Dasu MR, Jialal I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. Am J Physiol Endocrinol Metab. 2011;300:E145–154. doi: 10.1152/ajpendo.00490.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Dasu MR, Ramirez S, Isseroff RR. Toll-like receptors and diabetes: a therapeutic perspective. Clin Sci (Lond) 2012;122:203–214. doi: 10.1042/CS20110357. [DOI] [PubMed] [Google Scholar]
- 224.Vinolo MA, Hirabara SM, Curi R. G-protein-coupled receptors as fat sensors. Current opinion in clinical nutrition and metabolic care. 2012;15:112–116. doi: 10.1097/MCO.0b013e32834f4598. [DOI] [PubMed] [Google Scholar]
- 225.Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3:858–876. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Oh DY, Lagakos WS. The role of G-protein-coupled receptors in mediating the effect of fatty acids on inflammation and insulin sensitivity. Current opinion in clinical nutrition and metabolic care. 2011;14:322–327. doi: 10.1097/MCO.0b013e3283479230. [DOI] [PubMed] [Google Scholar]
- 227.Oh DY, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Koves TR, et al. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem. 2005;280:33588–33598. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- 229.Sampey BP, Freemerman AJ, Zhang J, Kuan P-F, Galanaki JA, O’Connell TM, Ilkayeva OR, Muehlbauer MJ, Stevens RD, Newgard CB, Brauer HA, Troester MA, Makowski L. Metabolomic Profiling Reveals Mitochondrial-Derived Lipid Biomarkers that Drive Obesity-Associated Inflammation. PloS One. 2012 doi: 10.1371/journal.pone.0038812. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.An J, et al. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med. 2004;10:268–274. doi: 10.1038/nm995. [DOI] [PubMed] [Google Scholar]
- 231.Koves TR, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 232.Makowski L, et al. Metabolic profiling of PPARalpha−/− mice reveals defects in carnitine and amino acid homeostasis that are partially reversed by oral carnitine supplementation. Faseb J. 2009;23:586–604. doi: 10.1096/fj.08-119420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Noland RC, et al. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J Biol Chem. 2009;284:22840–22852. doi: 10.1074/jbc.M109.032888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Adams SH, et al. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr. 2009;139:1073–1081. doi: 10.3945/jn.108.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sag D, Carling D, Stout RD, Suttles J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181:8633–8641. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012 doi: 10.1016/j.cmet.2012.01.024. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Bloch-Damti A, Bashan N. Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxidants & redox signaling. 2005;7:1553–1567. doi: 10.1089/ars.2005.7.1553. [DOI] [PubMed] [Google Scholar]
- 238.Toborek M, Hennig B. Fatty acid-mediated effectson the glutathione redox cycle in cultured endothelial cells. Am J Clin Nutr. 1994;59:60–65. doi: 10.1093/ajcn/59.1.60. [DOI] [PubMed] [Google Scholar]
- 239.Rudich A, Tirosh A, Potashnik R, Hemi R, Kanety H, Bashan N. Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Diabetes. 1998;47:1562–1569. doi: 10.2337/diabetes.47.10.1562. [DOI] [PubMed] [Google Scholar]
- 240.Furukawa S, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 242.Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010;327:296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- 243.Wen H, Ting JP, O’Neill LA. A role for the NLRP3 inflammasome in metabolic diseases--did Warburg miss inflammation? Nature immunology. 2012;13:352–357. doi: 10.1038/ni.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Stienstra R, Tack CJ, Kanneganti TD, Joosten LA, Netea MG. The inflammasome puts obesity in the danger zone. Cell Metab. 2012;15:10–18. doi: 10.1016/j.cmet.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 245.Vandanmagsar B, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Wen H, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nature immunology. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Stienstra R, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12:593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Stienstra R, et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A. 2011;108:15324–15329. doi: 10.1073/pnas.1100255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 250.Maedler K, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Masters SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nature immunology. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 254.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Cooper CS, Foster CS. Concepts of epigenetics in prostate cancer development. British journal of cancer. 2009;100:240–245. doi: 10.1038/sj.bjc.6604771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 257.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Mantovani A, Germano G, Marchesi F, Locatelli M, Biswas SK. Cancer-promoting tumor-associated macrophages: new vistas and open questions. European journal of immunology. 2011;41:2522–2525. doi: 10.1002/eji.201141894. [DOI] [PubMed] [Google Scholar]
- 259.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 261.Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol. 2009;51:212–223. doi: 10.1016/j.jhep.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Muntwyler J, Hennekens CH, Buring JE, Gaziano JM. Mortality and light to moderate alcohol consumption after myocardial infarction. Lancet. 1998;352:1882–1885. doi: 10.1016/S0140-6736(98)06351-X. [DOI] [PubMed] [Google Scholar]
- 263.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166:2437–2445. doi: 10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- 264.Kamper-Jorgensen M, Gronbaek M, Tolstrup J, Becker U. Alcohol and cirrhosis: dose--response or threshold effect? J Hepatol. 2004;41:25–30. doi: 10.1016/j.jhep.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 265.Malyutina S, et al. Relation between heavy and binge drinking and all-cause and cardiovascular mortality in Novosibirsk, Russia: a prospective cohort study. Lancet. 2002;360:1448–1454. doi: 10.1016/S0140-6736(02)11470-X. [DOI] [PubMed] [Google Scholar]
- 266.Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA. 2011;306:1884–1890. doi: 10.1001/jama.2011.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kang L, Sebastian BM, Pritchard MT, Pratt BT, Previs SF, Nagy LE. Chronic ethanol-induced insulin resistance is associated with macrophage infiltration into adipose tissue and altered expression of adipocytokines. Alcoholism, clinical and experimental research. 2007;31:1581–1588. doi: 10.1111/j.1530-0277.2007.00452.x. [DOI] [PubMed] [Google Scholar]
- 268.Bird MD, Zahs A, Deburghgraeve C, Ramirez L, Choudhry MA, Kovacs EJ. Decreased pulmonary inflammation following ethanol and burn injury in mice deficient in TLR4 but not TLR2 signaling. Alcoholism, clinical and experimental research. 2010;34:1733–1741. doi: 10.1111/j.1530-0277.2010.01260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Bird MD, Morgan MO, Ramirez L, Yong S, Kovacs EJ. Decreased pulmonary inflammation after ethanol exposure and burn injury in intercellular adhesion molecule-1 knockout mice. Journal of burn care & research: official publication of the American Burn Association. 2010;31:652–660. doi: 10.1097/BCR.0b013e3181e4c58c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Karavitis J, Murdoch EL, Gomez CR, Ramirez L, Kovacs EJ. Acute ethanol exposure attenuates pattern recognition receptor activated macrophage functions. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2008;28:413–422. doi: 10.1089/jir.2007.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zhong W, et al. Chronic Alcohol Exposure Stimulates Adipose Tissue Lipolysis in Mice Role of Reverse Triglyceride Transport in the Pathogenesis of Alcoholic Steatosis. Am J Pathol. 2012 doi: 10.1016/j.ajpath.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Zhang P, Bagby GJ, Happel KI, Raasch CE, Nelson S. Alcohol abuse, immunosuppression, and pulmonary infection. Current drug abuse reviews. 2008;1:56–67. doi: 10.2174/1874473710801010056. [DOI] [PubMed] [Google Scholar]
- 273.Nordmann R, Ribiere C, Rouach H. Implication of free radical mechanisms in ethanol-induced cellular injury. Free radical biology & medicine. 1992;12:219–240. doi: 10.1016/0891-5849(92)90030-k. [DOI] [PubMed] [Google Scholar]
- 274.Loomba R, Yang HI, Su J, Brenner D, Iloeje U, Chen CJ. Obesity and alcohol synergize to increase the risk of incident hepatocellular carcinoma in men. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2010;8:891–898. 898 e891–892. doi: 10.1016/j.cgh.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 275.McGuinness OP. Defective glucose homeostasis during infection. Annu Rev Nutr. 2005;25:9–35. doi: 10.1146/annurev.nutr.24.012003.132159. [DOI] [PubMed] [Google Scholar]
- 276.Beck MA. Influenza and obesity: will vaccines and antivirals protect? The Journal of infectious diseases. 2012;205:172–173. doi: 10.1093/infdis/jir740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.CDC. H1N1 flu. 2009 [Google Scholar]
- 278.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 279.O’Brien KB, et al. Impaired wound healing predisposes obese mice to severe influenza virus infection. The Journal of infectious diseases. 2012;205:252–261. doi: 10.1093/infdis/jir729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Reaven GM. Insulin resistance: the link between obesity and cardiovascular disease. The Medical clinics of North America. 2011;95:875–892. doi: 10.1016/j.mcna.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 281.Stanley TL, et al. TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjectswith features of the metabolic syndrome. J Clin Endocrinol Metab. 2011;96:E146–150. doi: 10.1210/jc.2010-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Gonzalez-Gay MA, Gonzalez-Juanatey C, Vazquez-Rodriguez TR, Miranda-Filloy JA, Llorca J. Insulin resistance in rheumatoid arthritis: the impact of the anti-TNF-alpha therapy. Ann N Y Acad Sci. 2010;1193:153–159. doi: 10.1111/j.1749-6632.2009.05287.x. [DOI] [PubMed] [Google Scholar]
- 283.Solomon DH, Massarotti E, Garg R, Liu J, Canning C, Schneeweiss S. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305:2525–2531. doi: 10.1001/jama.2011.878. [DOI] [PubMed] [Google Scholar]
- 284.Larsen CM, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]