Abstract
We examined the myogenic response to infarction in neonatal and adult mice to determine the role of c-kit+ cardiovascular precursor cells (CPC) that are known to be present in early heart development. Infarction of postnatal day 1–3 c-kitBAC-EGFP mouse hearts induced the localized expansion of (c-kit)EGFP+ cells within the infarct, expression of the c-kit and Nkx2.5 mRNA, myogenesis, and partial regeneration of the infarction, with (c-kit)EGFP+ cells adopting myogenic and vascular fates. Conversely, infarction of adult mice resulted in a modest induction of (c-kit)EGFP+ cells within the infarct, which did not express Nkx2.5 or undergo myogenic differentiation, but adopted a vascular fate within the infarction, indicating a lack of authentic CPC. Explantation of infarcted neonatal and adult heart tissue to scid mice, and adoptive transfer of labeled bone marrow, confirmed the cardiac source of myogenic (neonate) and angiogenic (neonate and adult) cells. FACS-purified (c-kit)EGFP+/(αMHC)mCherry− (noncardiac) cells from microdissected infarcts within 6 h of infarction underwent cardiac differentiation, forming spontaneously beating myocytes in vitro; cre/LoxP fate mapping identified a noncardiac population of (c-kit)EGFP+ myocytes within infarctions, indicating that the induction of undifferentiated precursors contributes to localized myogenesis. Thus, adult postinfarct myogenic failure is likely not due to a context-dependent restriction of precursor differentiation, and c-kit induction following injury of the adult heart does not define precursor status.
Keywords: heart repair, stem cell, vasculogenesis, angiogenesis
Cardiac infarction results in permanent heart dysfunction due to insufficient myogenesis. This myogenic failure has been attributed to the absence of multipotent cardiovascular progenitor cells (CPC) in the heart, or alternatively to a context-related failure of extant CPC to adopt a cardiac fate within the infarct. Although the presence of authentic progenitors in the adult heart is controversial (1–8), CPC in the developing heart are capable of forming all three cardiovascular lineages (9–15), and recent data indicate that substantial cardiac regeneration occurs in the injured neonatal heart, although this regeneration has been ascribed to the expansion of differentiated myocytes (16). Here we directly address the question of whether CPC adopt cardiac fates and contribute to heart regeneration following infarction of the neonatal mouse heart, and examine the role of c-kit+ cells that are induced following injury of the adult heart. Our finding of robust myogenesis following infarction of the neonate, but no myogenesis in the identically injured adult heart, connects myogenic potential to cells that exist at this early stage of cardiac developmental. Moreover, we show that CPC partially underlie myogenic infarct repair in neonates, but that c-kit+ cells that are locally induced following infarction of the adult mouse heart, do not adopt a cardiac fate. These results highlight the heterogeneity of c-kit–expressing cells, and the distinction between cardiovascular and vascular progenitors.
Results and Discussion
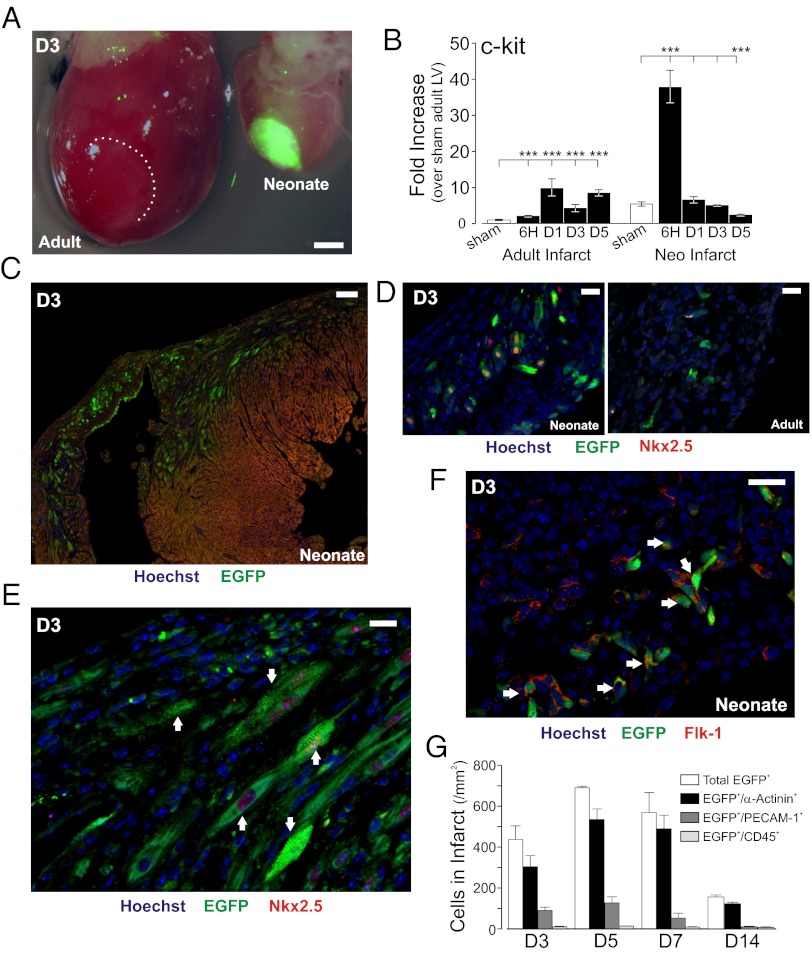
To determine whether neonatal hearts are capable of regenerative cardiac myogenesis, cryoinfarctions were performed in postnatal day (PN) 1–3 mice harboring a transcription marker for c-kit (c-kitBAC-EGFP; ref. 14), a gene product linked to CPC status in the heart (10, 14, 15, 17–19). Infarction resulted in the localization of (c-kit)EGFP+ cells and a marked induction of c-kit and EGFP mRNA within the area of injury, far greater than observed in identically infarcted adult hearts (14, 19, 20)(Fig. 1 A–C and Fig. S1A). Individual (c-kit)EGFP+ cells of varying morphology were observed throughout the neonatal infarct, but not in areas remote from the injury. The early cardiac transcription factor Nkx2.5 was identified in numerous Nkx2.5+ and (c-kit)EGFP+/Nkx2.5+ immature cells, and Nkx2.5 mRNA was induced within the neonatal infarct, but neither c-kit+/Nkx2.5+ cells nor Nkx2.5 message induction were observed in time matched adult infarcts (Fig. 1D and Fig. S1 B and C), excluding its expression as part of the revascularization process (10). Consistent with the (c-kit)EGFP marker identifying cells that include multipotent cardiovascular precursors (9–11), (c-kit)EGFP+ cells were observed at various stages of endothelial cell (EC, Flk-1+; PECAM-1+) or cardiomyocyte (α-actinin+; Nkx2.5+) differentiation (Fig. 1 D–G). Mast cells (tryptase+ or toluidine blue+) expressing (c-kit)EGFP were only observed at the epicardial border and were not consistent with the marked expansion of c-kit(EGFP)+ cells at the neonatal infarct border (Fig. S1D). Quantitative morphometry indicated that, at day 3 after infarction (D3), 67.2 ± 2.2% of (c-kit)EGFP+ cells within the infarct and border zone were of cardiac phenotype, increasing to 87.7 ± 4.9% at D7; the great majority of the remaining (c-kit)EGFP+ cells were identified as endothelial cells (EC) (Fig. 1G).
Fig. 1.
Cardiac myogenesis following infarction of the neonatal heart. (A) Induction of (c-kit)EGFP+ cells in neonatal and adult heart at 3 d after cryoinfarction (D3). Merged fluorescent/bright-field images show localized fluorescence within the neonatal infarct. Adult fluorescent cells not visible at this camera gain. (B) Quantitative PCR (qPCR) of c-kit mRNA in microdissected infarcts. Note higher message in neonatal vs. adult sham, and higher induction in neonatal infarct. Statistical comparisons to group sham. (C) Expression of (c-kit)EGFP in infarcted neonatal heart. (D) Nuclear Nkx2.5 expression in undifferentiated (c-kit)EGFP+ cells in neonatal, but not adult, heart. (E) Striated (c-kit)EGFP+ myocytes within neonatal infarct (arrows) by D3; some cells are also Nkx2.5+. (F) Flk-1+ cells in infarct include (c-kit)EGFP+ cells (arrows). (G) Time course and phenotype of (c-kit)EGFP+ cells following infarction of the neonatal heart. Note predominant cardiac phenotype. Error bars show SEM throughout. (Scale bars: 2,000 μm in A; 100 μm in C; 20 μm in D and E; and 50 μm in F.)
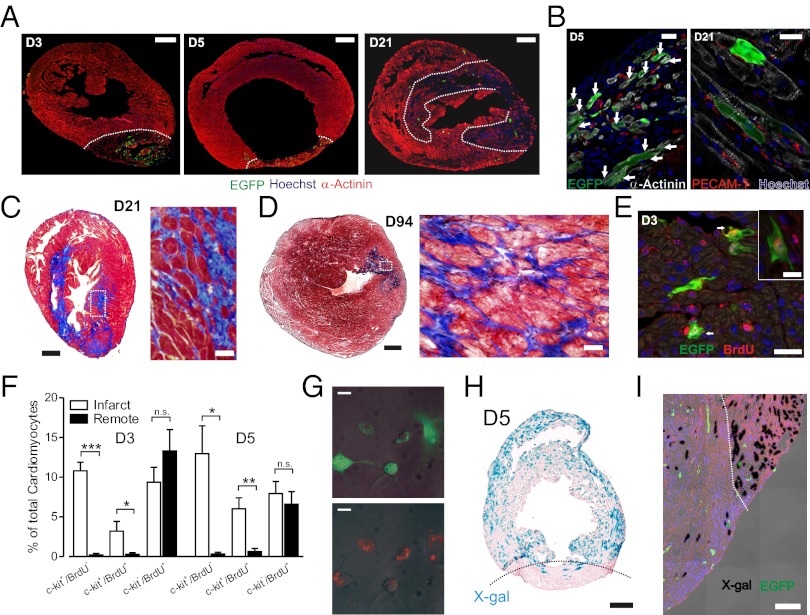
Most importantly, neonatal cryoinfarction lesions underwent marked neomyogenesis and regeneration following injury (Fig. 2 A and B); by D5, many striated cardiomyocytes were intermixed within areas of fibrosis. By the first week following injury, cardiac myocytes were observed within clusters of (c-kit)EGFP+ cells, and by D21, the infarcted region was infiltrated with numerous α-actinin positive myocytes, some of which were (c-kit)EGFP+, and fibrotic areas were progressively remodeled by 3 mo (Fig. 2 B–D). Quantitative analysis indicated an increase in cellular content within the scar area to 20.4 ± 4.1% at 3 wk and 43.2 ± 3.0% at 3 mo (P < 0.001; Fig. S2A). Thus, following an ablative infarction, c-kit transcriptionally active CPC migrate to the site of injury and progressively differentiate into heart cells, resulting in a regenerative response to the injury.
Fig. 2.
Regeneration of infarcted myocardium through expansion of cardiovascular precursor pool. (A) α-actinin staining at D3, D5, and D21 shows initial fibrosis and clusters of (c-kit)EGFP+ cells, followed by progressive muscle regeneration. (B) Myocyte clusters with many α-actinin+/(c-kit)EGFP+ cells (arrows) at D5 (Left) and fewer (c-kit)EGFP+ myocytes at D21 (Right). (C) Regression of fibrosis and myocyte clusters interspersed within fibrotic regions (trichrome). (D) Marked regeneration at D94, with further regression of fibrotic areas. (E) BrdU incorporation in infarcted neonatal heart. (Inset) BrdU incorporation in a (c-kit)EGFP+ striated myocyte. (F) Quantitative morphometry from BrdU incorporation in all myocytes. (G) Conversion of green to red fluorescence in vitro demonstrating myogenic capacity of (c-kit)EGFP+ cells. Cells shown are at day 14 from an initial plating devoid of red cells. Same field shown as overlay of light and green fluorescence (Upper), and light and red fluoresence (Lower) images. Separation scheme and purity are shown in Fig. S2. (H and I) Fate mapping of myocytes (c-kitBAC-EGFP/αMHC-CreER/R26-floxed STOP-βGal) at D5 shows limited recombination in infarct region at low (H) and high (I) magnification (red is autofluorescence). (Scale bars: 400 μm in A Left and Center; 250 μm in A Right; 20 μm in B; 200 μm in C Left; 30 μm in C Right; 1,000 μm in D Left; 25 μm in D Right; 20 μm in E; 10 μm in E Inset; 25 μm in G, 200 μm in H; and 100 μm in I.)
In vivo pulse-chase labeling with BrdU indicated that cardiac myogenesis was accompanied by a marked expansion of the (c-kit)EGFP+ pool that was localized to the infarct (Fig. 2 E and F). At day 3 postinfarction (PI), the number of (c-kit)EGFP+ cardiomyocytes within the infarct was 6.7-fold greater than in remote areas; overall, 20.5 ± 4.7 and 32.1 ± 2.9% of (c-kit)EGFP+ myocytes incorporated BrdU at D3 and D5, respectively. Moreover, (c-kit)EGFP+ cells were a major component of all myocytes showing evidence of DNA replication within the infarct, accounting for 25.4 ± 8.5 and 42.0 ± 6.9% of all BrdU+ cardiomyocytes at D3 and D5, respectively, compared with 2.2 ± 1.3 and 7.0 ± 4.1% in remote regions (Fig. 2F). By contrast, determination of histone H3 phosphorylation (pHH3) in myocytes, which reflects the pool of currently expanding myocytes, indicated a similar stimulation of mitosis in existing myocytes in remote and infarcted regions. pHH3+ cardiomyocytes increased from a control of 0.07 ± 0.01 to 0.22 ± 0.03% and 0.23 ± 0.08%, in remote and infarcted regions, respectively (Fig. S2 B and C). Taken together, these data are consistent with a generalized expansion of existing myocytes throughout the heart, but a localized expansion of (c-kit)EGFP+ cells and cardiac differentiation within the infarct. Quantitatively, by D5, ∼32% of (c-kit)EGFP+ cardiomyocytes localized to the infarct have passed through S phase, and more than 75% of (c-kit)EGFP+ have adopted a cardiac fate (Fig. 1G), indicating that postinfarct cardiac myogenesis arises at least partially from a local expansion of the (c-kit)EGFP+ CPC pool. The pancardiac expansion of myocytes following neonatal infarction is not associated with c-kit expression as evidenced by minimal c-kit+/BrdU+ cells outside of the infarct zone (Fig. 2F).
The (c-kit)EGFP marker reports current c-kit transcriptional activity and thus cannot definitively identify the developmental origin of myocytes observed within the infarction. To unequivocally establish the ability of undifferentiated (c-kit)EGFP+ cells to adopt cardiac fates, we infarcted neonatal c-kitBAC-EGFP/αMHC-mCherry mice and FACS purified cells for in vitro differentiation experiments within 6 h of infarction, to prevent dedifferentiation of myocytes. Pure populations of EGFP+/mCherry− (noncardiac) cells began to express mCherry after incubation in cardiac differentiation medium (14) (Fig. 2G). By 12–13 d, 12.0 ± 1.4% of cells expressed mCherry (Fig. S2 F and G), whereas no EGFP−/mCherry+ cells were observed to express EGFP in parallel experiments. Moreover, purified EGFP+/mCherry− cells did not simply express mCherry, but formed a contractile cardiac phenotype with spontaneous contractions (Movies S1 and S2). FACS-purified neonate (c-kit)EGFP+ cells also adopted cardiac fates when injected into adult infarcts (SI Methods and Fig. S2H), whereas FACS-purified adult (c-kit)EGFP+ cells do not undergo cardiac differentiation in vitro or when transplanted into adult infarcts (14, 15). Thus, although our data do not exclude expansion of existing myocytes (16), they provide evidence of a pool of precursor cells that are capable of supporting myogenesis postinfarction in neonatal mice.
We next sought to further discriminate between progenitor and cardiomyocyte contributions to the expansion of (c-kit)EGFP+ cells, and attendant postinfarction myogenesis. We infarcted triallelic (c-kitBAC-EGFP/ αMHC-CreER/R26-floxed STOP-βGal) neonatal mice, in which myocytes were marked with β-gal by tamoxifen-induced recombination before birth, resulting in a minimum of 3 d between tamoxifen exposure and infarction. Infarcted regions contained a markedly lower percentage of recombined cells than remote areas at D5, the time point of maximum (c-kit)EGFP+ cells within the infarct, and differing distinctly from the localized expansion of (c-kit)EGFP+ cells (Fig. 2 H and I and Fig. S2D). To exclude (c-kit)EGFP+ cells that adopt an EC fate, we compared the percentage of recombined (c-kit)EGFP+ myocytes to the overall cardiomyocyte recombination rate. In three experiments, the recombination rate of (c-kit)EGFP+ myocytes was 68.8 ± 4.2% that of EGFP− cardiomyocytes (P < 0.01; Fig. S2E), consistent with a nonmyocyte source for at least one-third of these cells, particularly when viewed in light of the induction of c-kit in differentiated myocytes following injury (14).
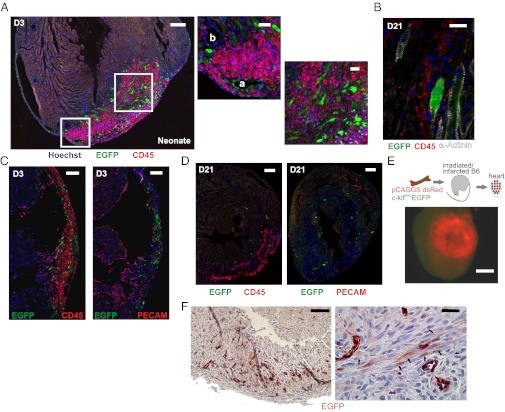
Our data indicate a major expansion of the (c-kit)EGFP+ cells in response to cardiac injury; however, myogenesis associated with these cells could reflect an influx of bone-marrow-derived c-kit+ cells following injury, as ∼50% of c-kit+ cells have been reported to be copositive for the hematopoietic lineage marker CD45 in adult mice (20). We observed that less than 3% of (c-kit)EGFP+ cells were CD45+ during the first week following infarction of the neonatal mouse (Fig. 1G). CD45+/(c-kit)EGFP− cells migrated to the infarct in large numbers, but did not participate in the formation of new myocytes or vascular structures. Rather, these cells surrounded vessels and myocytes, persisting for weeks following the injury (Fig. 3 A–D). Unlike (c-kit)EGFP+ cells, CD45+ cells also did not coexpress cardiac α-actinin (Fig. 3B) or spatially overlap with PECAM-1+ cells (Fig. 3 C and D), further establishing (c-kit)EGFP+/CD45− cells as the precursor pool.
Fig. 3.
Heart–derived (c-kit)EGFP+ cells adopt myogenic and EC fates following neonatal infarction. (A) CD45+ cells surround (c-kit)EGFP+ cells within infarct and do not participate in angiogenesis/myogenesis. (Left) New vessel within infarct (a) formed by (c-kit)EGFP+ endothelial cells; vessel outside of infarct (b) has no incorporation. (Right) Myocyte clusters formed by (c-kit)EGFP+ cells, surrounded by EGFP−/CD45+ mononuclear cells. (B) At D21, some CD45+ cells persist, but are α-actinin negative. (C and D) CD45 and PECAM stained sections from D3 and D21 hearts show distinct, nonvascular location of CD45+ cells within infarcts. (E) Infarction of reconstituted (PN2 c-kitBAC-EGFP/(pCAGGS)dsRed marrow) B6 adults results in homing of only dsRed cells to the infarct (green/red image). (F) (c-kit)EGFP induction and myogenic/endothelial differentiation in neonatal c-kitBAC-EGFP infarcted tissue in SCID mouse. (Left) Low magnification of infarcted area. (Right) Higher magnification shows clusters of striated (c-kit)EGFP+ myocytes (arrows) as well as endothelial cells. (Scale bars: 200 μm in A Left; 50 μm in A Center and Right; 20 μm in B; 200 μm in C; 200 μm in D Left; 50 μm in D Right; 1,000 μm in E; 100 μm in F Left; and 25 μm in F Right.)
Although controversial in the adult heart (21, 22), neonatal c-kit+/CD45− bone-marrow-derived stem cells could contribute to the formation of new heart cells, as neonatal cells might possess enhanced differentiation potential compared with adult hematopoietic stem cells. To test this possibility, we transplanted pooled marrow from 5-d-old c-kitBAC-EGFP/pCAGGS-dsRed (23) mice into irradiated adult B6 recipients, and infarcted these mice following reconstitution. Virtually no (c-kit)EGFP+ cells were observed in infarcts in these experiments, although dsRed cells did home to the site of injury as expected (Fig. 3E), and microscopic evaluation failed to reveal (c-kit)EGFP+ or dsRed+ myocytes or vascular endothelial cells, excluding hematopoietic stem cells as the myogenic/vascular precursors. This experiment is limited by the potential loss of transdifferentiation capacity of neonatal bone marrow during reconstitution (14–21 d), but reconstitution with PN2-3 neonatal marrow for 2–4 d also failed to result in the migration of any (c-kit)EGFP+ cells to the heart (SI Methods). To further confirm the cardiac origin of (c-kit)EGFP+ cells, we transplanted infarcted c-kitBAC-EGFP neonatal ventricular tissue into SCID mice. Three to five days after transplantation, the infarcted tissue showed induction of (c-kit)EGFP transcriptional activity in cells within the transplant, many of which adopted cardiac fates (Fig. 3F). By contrast, striated myocytes were not observed in adult transplanted tissue (see below), excluding the induction of EGFP in differentiated myocytes. Thus, cardiac CPC migrate to the region of cardiac injury, undergo cell division, and initiate a wave of cardiovascular differentiation.
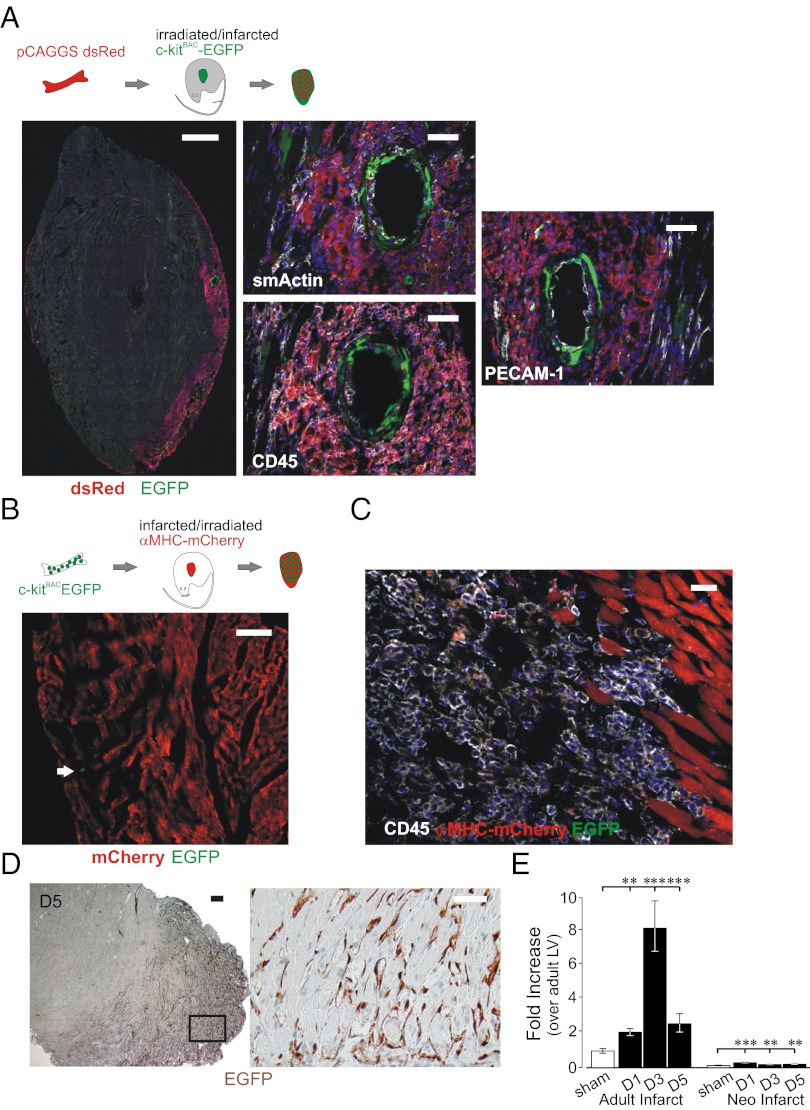
As c-kit+ cells participate in the formation of new vessels within adult infarcts, which are devoid of CPCs (14, 15), our results indicate that at least two distinct pools of (c-kit)EGFP+ cells participate in cardiac repair. Furthermore, because marrow-derived cells appeared to play at most a very minor role in revascularization of the neonatal infarct (Fig. 3 A–D), our results might reflect a previously unappreciated endogenous source for such cells in the adult heart. To further define the pool of c-kit–expressing cells participating in adult cardiac repair, we performed adoptive transfer experiments using adult dsRed marrow transplanted to c-kitBAC-EGFP recipients, as well as c-kitBAC-EGFP donor marrow transplanted to dsRed recipients, and infarcted the mice following reconstitution. As shown in Fig. 4A, dsRed-labeled marrow cells prominently occupy the infarct, but these cells play little or no role in the formation of vascular structures, whereas host (c-kit)EGFP+ cells markedly participate in new vessel formation. Conversely, despite complete reconstitution, (c-kit)EGFP+ marrow cells were rarely detected in the infarcted hearts of cardiac mCherry (αMHC-mCherry) mice and never adopted cardiac or vascular lineages, although numerous CD45+ cells were observed (Fig. 4 B and C). Thus, as in the neonate, there is little evidence of traffic of c-kit+ hematopoietic stem cells to the infarcted adult heart. As (c-kit)EGFP+ cells are not found in adult mice (14), these results indicate activation of the reporter in resident cells that migrate to the infarct and adopt strictly vascular fates, a process similar to recent reports of perivascular precursors that adopt EC and smooth muscle fates in vitro, and underlie atherosclerotic injury responses (24–26). To definitively confirm that adult c-kit+ cells derive from the surrounding, nonablated cardiac tissue, we transplanted the left ventricular freewall of adult c-kitBAC-EGFP/(pCAGGS)dsRed mice into SCID mice s.c. immediately following cryoinfarction. As in neonatal mice, (c-kit)EGFP+ cells were observed 5 d following infarction/transplantation of adult heart tissue; however, (c-kit)EGFP+ cells contributed only to the formation of vascular structures (Fig. 4D). Highlighting the distinct molecular features of the neonatal and adult processes, the expression of sca-1, which has been implicated in angiogenic responses mediated by adventitial vascular precursors (24, 26), was significantly up-regulated in adult infarcts, but is not a feature of the neonatal response (Fig. 4E). Thus, in adult infarcts, resident vascular precursors, rather than authentic CPC, migrate from the surrounding heart tissue and differentiate to form vascular structures.
Fig. 4.
Heart–derived (c-kit)EGFP+ cells adopt vascular fates following adult infarction. (A) Infarction of reconstituted ((pCAGGS)dsRed marrow) c-kitBAC-EGFP mice indicates (c-kit)EGFP+ cells adopt endothelial (PECAM-1) and smooth muscle (α-smActin) fates; bone marrow-derived cells are mainly CD45+. (B) Infarction of reconstituted (c-kitBAC-EGFP marrow) αMHC-mCherry mice reveals minimal (c-kit)EGFP+ cells homing to the heart (arrow indicates rare EGFP+ cell). (C) CD45+ cells from c-kitBAC-EGFP marrow home to the infarct, but do not form myocytes; mCherry+ myocytes at border zone. (D) Adult c-kitBAC-EGFP infarcted tissue in SCID mouse undergoes (c-kit)EGFP induction, but adopt strictly vascular fates (Right). (E) Induction of sca-1 mRNA in microdissected infarcts. (Scale bars: 500 μm in A Left; 100 μm in A Center and Right; 100 μm in B; 25 μm in C; 200 μm in D Left; and 50 μm in D Right.)
Taken together, our results suggest that the failure of myogenesis following adult infarction is not a result of a context-dependent limit to differentiation associated with the infarct per se, but depends critically on the developmental state of the heart, consistent with data indicating that the early human heart possesses significant regenerative potential (8). Recent data indicate that early cardiac regeneration involves activation of myocyte cell cycle reentry in the entire neonatal heart, resulting in widespread dedifferentiation and proliferation, without formation of a fibrotic scar (16). Our data do not exclude the participation of non-terminally differentiated cardiomyocytes in neonatal infarct repair, but the local expansion of undifferentiated, (c-kit)EGFP+ cells at the area of infarction, the induction of the early cardiac transcription factor Nkx2.5 in these cells, and their adoption of multiple developmental fates, coupled with a limited expansion of previously differentiated heart cells at the site of injury, clearly establishes the role of CPC in this process. Strikingly, our explant studies demonstrate the intrinsic capacity of the neonatal heart for myogenesis, a capacity that temporally correlates with the existence of authentic CPC (14, 15, 19). We further demonstrate that the neonatal and adult heart walls contain a reservoir of angiogenic precursors, and that activation of these cells results in c-kit expression. Finally, our data highlight the critical distinction in developmental potential of adult heart cells that express the stem cell factor receptor, c-kit. Mobilization of c-kit+ cells in the infarcted adult heart from surrounding heart tissue results in extensive angiogenesis/vasculogenesis. Although incapable of cardiac myogenesis, these c-kit+ vascular progenitors may be attractive targets for directed cardiac differentiation in vivo.
Methods
Neonatal and Adult Infarction.
Tg(RP24-330G11-EGFP)1Mik Mice or combined lines were anesthetized to a surgical plane using hypothermia (27), a ventrolateral thoracotomy performed, and a 1-mm-diameter copper probe equilibrated in liquid nitrogen applied to the left ventricle near the apex for 5–10 s and removed. Adult mice were infarcted as described (28). For neonatal mice, the incision was at left intercostal space 7–8. Following application of the cryoprobe, the pleural cavity was filled with sterile saline, the musculature and s.c. tissue was reconstructed over the intercostal incision with 8-0 vicryl, and the saline was removed from the pleural space. Skin closure was with subcuticular sutures of 8-0 vicryl to prevent maternal rejection. Mice were recovered by rewarming, administered 10 μg of morphine s.c., and placed with foster dams when strong purposeful movement was present. Infarct regions described in this manuscript comprise the infarct and border zone areas. All procedures were approved by the Cornell Institutional Animal Care and Use Committee and adhered to the Guide for the Care and Use of Laboratory Animals.
Explantation of Heart Tissue into SCID Hosts.
Mice were euthanized immediately following infarction reperfusion and cryoablated tissue transplanted to the dorsal s.c. space of anesthetized adult B6 SCID recipients.
BrdU Labeling.
Neonates were given i.p. injections of BrdU (2.5 mg/mL, 50 μg/g body weight; BD 550891) at days 0, 1, and 2 after the cryoablation. The hearts were harvested either 3 or 5 d postcryoablation and Stefanini fixed overnight before sectioning and analysis.
Adoptive Bone Marrow Transfer.
Bone marrow transplantation was as described (29). See SI Methods.
Induced Recombination and Fate Mapping.
Pregnant females carrying triallelic (c-kitBAC-EGFP/αMHC-CreER/R26-floxed STOP-βGal) pups were injected intraperitoneally with 75 mg/kg tamoxifen at ED 17 or 18. Injected mice commonly gave birth at ED 18–20, the pups were then infarcted as described above. Quantitative analysis of recombination frequency was performed at 5 d postinfarction (PN7) by staining for galactosidase activity with X-Gal using standard methods.
Immunohistochemistry and Quantitative Morphometry.
See SI Methods.
Quantitative PCR.
See SI Methods.
Statistical Analysis.
Data were analyzed by one-way ANOVA, using Dunnett’s comparison with control, Tukey–Kramer multiple comparisons, or unpaired t tests. Summary values are mean ± SEM. Significant differences are reported at the P < 0.05 (*), P < 0.01 (**), and P < 0.001 (***) levels.
Supplementary Material
Acknowledgments
We thank Dr. Andrew Yen and Lavanya Sayam for FACS separation procedures; Ms. Pat Fisher and Dr. Longying Dong for immunohistochemistry support; Dr. Jinhyang Choi for pilot bone marrow transplantation experiments and teaching S.A.J. and S.R. this procedure; Babu Singh for help construction of the α-MHC-mCherry transgene; and Ruizhe Ren for technical support. The cytometry core was supported in part by the Empire State Stem Cell Board, New York State Department of Health, Contract 123456. This work is supported by National Institutes of Health Grants DK065992 and DK072277 (to M.I.K.), and NYSTEM Grant C023050 (to A.Y.N), and European Union's Seventh Framework Programme consortium CardioCell Grant 223372 (to B.F.K).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1208114109/-/DCSupplemental.
References
- 1.Sussman MA, Anversa P. Myocardial aging and senescence: Where have the stem cells gone? Annu Rev Physiol. 2004;66:29–48. doi: 10.1146/annurev.physiol.66.032102.140723. [DOI] [PubMed] [Google Scholar]
- 2.Anversa P, Kajstura J, Leri A, Bolli R. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation. 2006;113:1451–1463. doi: 10.1161/CIRCULATIONAHA.105.595181. [DOI] [PubMed] [Google Scholar]
- 3.Rubart M, Field LJ. Cardiac regeneration: Repopulating the heart. Annu Rev Physiol. 2006;68:29–49. doi: 10.1146/annurev.physiol.68.040104.124530. [DOI] [PubMed] [Google Scholar]
- 4.Murry CE, Reinecke H, Pabon LM. Regeneration gaps: Observations on stem cells and cardiac repair. J Am Coll Cardiol. 2006;47:1777–1785. doi: 10.1016/j.jacc.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh PC, et al. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pouly J, et al. Cardiac stem cells in the real world. J Thorac Cardiovasc Surg. 2008;135:673–678. doi: 10.1016/j.jtcvs.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Wu SM, Chien KR, Mummery C. Origins and fates of cardiovascular progenitor cells. Cell. 2008;132:537–543. doi: 10.1016/j.cell.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergmann O, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moretti A, et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Wu SM, et al. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–1150. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 11.Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11:723–732. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Yang L, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 13.Christoforou N, et al. Mouse ES cell-derived cardiac precursor cells are multipotent and facilitate identification of novel cardiac genes. J Clin Invest. 2008;118:894–903. doi: 10.1172/JCI33942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tallini YN, et al. c-kit expression identifies cardiovascular precursors in the neonatal heart. Proc Natl Acad Sci USA. 2009;106:1808–1813. doi: 10.1073/pnas.0808920106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaruba MM, Soonpaa M, Reuter S, Field LJ. Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts. Circulation. 2010;121:1992–2000. doi: 10.1161/CIRCULATIONAHA.109.909093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porrello ER, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beltrami AP, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 18.Kattman SJ, Adler ED, Keller GM. Specification of multipotential cardiovascular progenitor cells during embryonic stem cell differentiation and embryonic development. Trends Cardiovasc Med. 2007;17:240–246. doi: 10.1016/j.tcm.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Fransioli J, et al. Evolution of the c-kit-positive cell response to pathological challenge in the myocardium. Stem Cells. 2008;26:1315–1324. doi: 10.1634/stemcells.2007-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fazel S, et al. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murry CE, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 22.Nygren JM, et al. Myeloid and lymphoid contribution to non-haematopoietic lineages through irradiation-induced heterotypic cell fusion. Nat Cell Biol. 2008;10:584–592. doi: 10.1038/ncb1721. [DOI] [PubMed] [Google Scholar]
- 23.Vintersten K, et al. Mouse in red: Red fluorescent protein expression in mouse ES cells, embryos, and adult animals. Genesis. 2004;40:241–246. doi: 10.1002/gene.20095. [DOI] [PubMed] [Google Scholar]
- 24.Hu Y, et al. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasquinelli G, et al. Thoracic aortas from multiorgan donors are suitable for obtaining resident angiogenic mesenchymal stromal cells. Stem Cells. 2007;25:1627–1634. doi: 10.1634/stemcells.2006-0731. [DOI] [PubMed] [Google Scholar]
- 26.Passman JN, et al. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci USA. 2008;105:9349–9354. doi: 10.1073/pnas.0711382105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phifer CB, Terry LM. Use of hypothermia for general anesthesia in preweanling rodents. Physiol Behav. 1986;38:887–890. doi: 10.1016/0031-9384(86)90058-2. [DOI] [PubMed] [Google Scholar]
- 28.Roell W, et al. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature. 2007;450:819–824. doi: 10.1038/nature06321. [DOI] [PubMed] [Google Scholar]
- 29.Choi J, Curtis SJ, Roy DM, Flesken-Nikitin A, Nikitin AY. Local mesenchymal stem/progenitor cells are a preferential target for initiation of adult soft tissue sarcomas associated with p53 and Rb deficiency. Am J Pathol. 2010;177:2645–2658. doi: 10.2353/ajpath.2010.100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.