Abstract
Regulatory T cells (Tregs) are potent immune modulators, but their role in human immunodeficiency virus type 1 (HIV-1) pathogenesis remains poorly understood. We performed a detailed analysis of the frequency and function of Tregs in a large cohort of HIV-1–infected individuals and HIV-1 negative controls. While HIV “elite controllers” and uninfected individuals had similar Treg numbers and frequencies, the absolute numbers of Tregs declined in blood and gut-associated lymphoid tissue in patients with chronic progressive HIV-1 infection. Despite quantitative changes in Tregs, HIV-1 infection was not associated with an impairment of ex vivo suppressive function of flow-sorted Tregs in both HIV controllers and untreated chronic progressors.
(See the editorial commentary by Imamichi and Lane, on pages 1479–82.)
Regulatory T cells (Tregs) are important immunoregulatory cells and have been shown to influence the outcome of various infections [1]. Tregs control excessive immune activation, thus limiting immune-mediated tissue damage, but can also suppress antigen-specific immune responses against pathogens. The impact of Tregs on human immunodeficiency virus type 1 (HIV-1) immune pathogenesis, including potential benefits through protection from HIV-1–associated generalized immune activation and deleterious effects on viral control via suppression of HIV-1–specific immunity, remains poorly understood, and data assessing the fate of Tregs during HIV-1 infection are often conflicting [2]. Recent studies further suggested that Tregs may be able to directly inhibit HIV-1 replication in activated T cells [3], and there is evidence for differential susceptibility of cytotoxic T lymphocytes restricted by protective HLA alleles to suppression by Tregs [4].
In this study we investigated frequencies of Tregs at different stages of HIV-1 infection in blood and gut-associated lymphoid tissue (GALT) and compared ex vivo suppressive activity of flow-sorted Tregs from HIV-1 “elite controllers,” chronic untreated progressors, and HIV-1 negative controls. Our data show that relative Treg frequencies and numbers in elite controllers are not different from those in uninfected controls, but that absolute Treg numbers decline in blood and tissue from chronically infected individuals. Despite the loss of Tregs, the suppressive function of ex vivo flow-sorted Tregs was preserved in chronically infected individuals and not different from that in elite controllers or uninfected controls.
METHODS
Study Population (n = 107)
The study included 26 HIV-1 elite controllers with untreated asymptomatic HIV-1 infection and HIV-1 RNA levels <75 copies/mL, 30 individuals with chronic untreated HIV-1 infection, 20 HIV-1–positive individuals treated with highly active antiretroviral therapy with viral loads <50 copies/mL for >1 year (median, 3.5; range, 2–8), 10 individuals identified during acute HIV-1 infection, and 21 healthy HIV-1 uninfected controls. Individuals with acute HIV-1 infection were defined as HIV-1 RNA positive and HIV-1-p24 enzyme-linked immunosorbent assay (ELISA) negative or p24 ELISA positive with an evolving Western blot (≥3 bands). Clinical and epidemiological data for the study population are summarized in Supplementary Table 1. For the tissue-related studies, colon biopsy specimens from additional 12 HIV-1–negative persons and 10 individuals with chronic untreated HIV-1 infection were investigated (median viral load, 27 633; interquartile range [IQR], 11 238–96 134 copies/mL; median CD4 T-cell count, 185.5; IQR, 5.5–399 cells/μL). The study was approved by the Massachusetts General Hospital Institutional Review Board, and written informed consent was obtained from all study participants.
Flow Cytometry
For quantification of Tregs, cryopreserved peripheral blood mononuclear cells (PBMCs) were thawed and stained with anti-CD3–phycoerythrin (PE)–cyanine 7 (Cy7), anti-CD4–fluorescein isothiocyanate (FITC), anti-CD8–AlexaFluor700, anti-CD25–allophycocyanin (APC), and anti–forkhead box P3 (FOXP3)–PE. Intracellular staining of FOXP3 was performed using the Human Regulatory T Cell Staining Kit (eBioscience). Fluorescence-minus-one controls were applied to assure correct gating. An exclusion channel was used to select for viable cells with the LIVE/DEAD Fixable Violet Dead Cell Staining Kit (Invitrogen) and to exclude CD14+ monocytes and CD19+ B lymphocytes. Flow data were acquired on an LSRII flow cytometer (BD Biosciences) and analyzed with FlowJo software for Apple Macintosh, version 8.8.5, (TreeStar).
Flow-based Cell Sorting and T-Cell Suppression Assays
Fresh blood samples collected in acid citrate dextrose anticoagulated blood collection tubes were enriched for CD4+ T cells using RosetteSep Human CD4+ T Cell Enrichment Cocktail (Stemcell Technologies) and labeled using anti-CD3-PE-Cy7, anti-CD4-FITC, CD25-APC, and CD127-PE. Live CD3+CD4+ CD25+CD127low Tregs and CD3+CD4+CD25−CD127+ responder T-cell subsets were sorted on a FACSAria cell sorter (BD Biosciences). Suppressive Treg function was assessed using T-cell proliferation assays, in which carboxyfluorescein succinimidyl ester (CFSE)–labeled responder T cells were cocultured with sorted Tregs at different ratios in the presence of anti-CD2/anti-CD3/anti-CD28 microbeads (Miltenyi Biotec) at a 1:1 bead to CD4+ T-cell ratio. After 4 days, cells were stained and acquired on an LSR II flow cytometer. Proliferation of responder T cells was quantified using the FlowJo proliferation platform.
Immunofluorescence Staining and Quantification of Colon Tissue Specimens
Paraffin-embedded colon tissue specimens were obtained by endoscopic biopsy and provided through the Brigham and Women's Hospital Pathology Department. Immunofluorescence staining was performed using 4-µm-thick formalin-fixed, paraffin-embedded tissue sections using mouse anti–human CD4 (Vector Burlingame) and anti–human FOXP3 (clone 206D; Biolegend). Slide acquisition was performed using a Zeiss AxioImager Z1 automated multichannel immunofluorescent microscope equipped with TissueFAXS software (Tissuegnostics) to obtain ×40 images, which were sorted for follicular and lamina propria areas. TissueQUEST (Tissuegnostics) image analysis software was used for cell identification, segmentation of cell compartments, and generation of scattergrams based on average fluorescence intensity.
Statistical Analysis
Wilcoxon rank test was used for comparing Tregs between 2 groups. For >2 groups, Kruskal-Wallis test was applied. Rank-based analysis of covariance was performed to adjust for the effect of age. Spearman rank correlation was used for correlation analyses. Longitudinal data analyses using generalized estimating equations was performed to compare the changes in suppression of T-cell proliferation. Differences were considered significant at P < .05.
RESULTS
Untreated Chronic Progressors Distinguished From Elite Controllers by Decrease in Absolute Treg Numbers but Increase in Relative Treg Frequencies
We first investigated the frequency of CD25+FOXP3+ Tregs among peripheral CD4+ T cells in HIV-1–infected study participants at different disease stages and HIV-1–uninfected controls (gating scheme) (Figure 1A). Our data demonstrate that Treg frequencies in elite controllers were low (median, 3.2%; IQR, 2.1%–3.9%) and not significantly different from those in healthy control subjects (median, 2.8%; IQR, 2.3%–4.1%; P = .93) (Figure 1B). In contrast, relative Treg frequencies in individuals with untreated acute and chronic HIV-1 infection were significantly higher than in seronegative individuals, with median Treg frequencies of 4.5% (IQR, 2.3%–6.9%) in untreated chronic progressors (P = .029) and 5.8% (IQR, 3.3%–6.7%) in individuals with acute infection (P = .021). There were no significant differences between relative Treg frequencies in individuals with chronic treated or chronic untreated HIV-1 infection, suggesting that even long-term suppressive highly active antiretroviral therapy does not fully restore Treg numbers to levels found in HIV-1–uninfected individuals [5, 6]. In light of the associations between age and Tregs described elsewhere, we performed multivariate analyses to adjust for the effect of age while comparing Tregs among the patient groups. The results of the adjusted analyses remained the same as the results of the unadjusted analyses.
Figure 1.
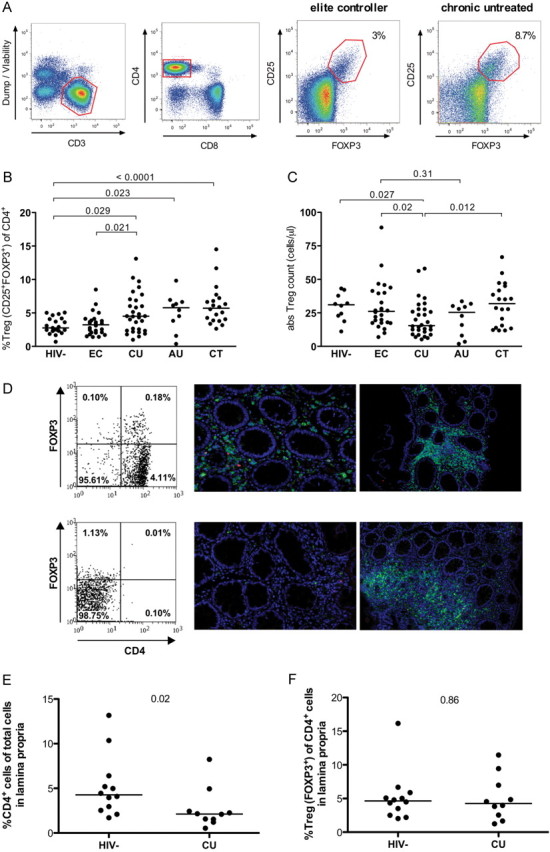
Regulatory T-cell (Treg) frequencies in peripheral blood and colonic lymphoid tissue from individuals infected with human immunodeficiency virus type 1 (HIV-1) at different stages of HIV-minus-one infection. A, Flow cytometry gating strategy: Gates are set to include CD3+ T lymphocytes and exclude dead cells (viability marker+), monocytes (CD14+), and B cells (CD19+). CD4+ Tregs were defined by the coexpression of CD25 and forkhead box P3 (FOXP3) using appropriate fluorescence-minus-one controls. Top right panels show representative examples of CD4+ Tregs in an HIV-1 elite controller and an untreated chronic HIV-1 progressor. B, CD25+FOXP3+ Treg frequencies (%Treg of CD3+CD4+ T cells) are shown. C, Absolute Treg numbers were determined and compared between the study groups: 21 HIV-1–uninfected controls (HIV−), 26 HIV-1 elite controllers (EC), 30 antiretroviral therapy–naive individuals with chronic untreated HIV-1 infection (CU), 10 HIV-1 positive untreated individuals with primary HIV-1 infection (AU), and 20 HIV-1 positive individuals treated with highly active antiretroviral therapy (HAART) (CT). D, Representative TissueQuest scattergrams for a healthy HIV-negative donor (upper left panel) and an untreated chronic progressor (CU) (lower left panel) with examples of lamina propria sections (upper and lower middle panels) and lymphoid follicle sections (upper and lower right panels). Images show CD4+ Treg staining acquired by confocal microscopy (×200 magnification). Blue represents the nucleus (4′, 6-diamidino-2-phenylindole [DAPI]); green, CD4; red, FOXP3. The frequency of FOXP3+ Tregs is measured among DAPI+CD4+ cells using TissueQuest software as represented on the left panels. E, F, The frequencies of CD4+ T cells among total cells (E) and of FOXP3+ Tregs among CD4+ T cells (F) were compared in the lamina propria and lymphoid follicles (not shown) of 12 healthy donors and 10 untreated chronic progressors.
We next calculated absolute Treg numbers in the peripheral blood based on the CD4+ T-cell count of the participants and the relative Treg frequency. Not unexpectedly, HIV-1–negative individuals were found to have the highest absolute numbers of Tregs, which in concordance with the observed relative Treg frequencies were not significantly different from those in elite controllers (medians, 31 [IQR, 21.8–38.9] and 26.1 [IQR, 17.5–40.3] cells/μL, respectively; P = .6) (Figure 1C). In contrast, individuals with chronic untreated HIV-1 infection had the lowest absolute Treg counts (median, 15.5 cells/μL; IQR, 10.4–27.7 cells/μL). Treg frequencies in untreated HIV-1–positive individuals were inversely correlated with CD4 T-cell counts (R = −0.35; P = .008) and positively correlated with HIV loads (R = 0.3; P = .049) and generalized immune activation (R = 0.51; P = .01), in line with published data elsewhere [5, 6].
Chronic HIV-1 Infection Leading to Decrease in Total CD4+ T Cells in the Colonic Lamina Propria Without Preferential Loss of Mucosal Tregs
Having observed these differences in Tregs in peripheral blood, we next examined Tregs in the gut mucosa, a critical reservoir of active viral replication with rapid and profound depletion of CD4+ T cells during primary HIV-1 infection. Using immunofluorescence staining, confocal microscopy, and a novel software-assisted tissue quantification system, we investigated the frequency of CD4+ T cells and CD4+FOXP3+ Treg in colon sections from 10 untreated chronically HIV-1–infected individuals and 12 HIV-seronegative controls. We observed lower frequencies of CD4+ T cells among total cells in the lamina propria of colon biopsy specimens from untreated chronically HIV-1–infected individuals compared with HIV-1–seronegative donors (P = .02), reflecting CD4+ T-cell loss during HIV-1 disease (Figure 1D and 1E). To investigate to what extent CD4+FOXP3+ Tregs were affected by HIV-1–associated CD4+ T-cell depletion, we next studied the frequency of FOXP3+ Treg among CD4+ T cells in lamina propria and lymphoid follicles, and we determined that Treg frequencies were not significantly different in the lamina propria or the follicles of colons from HIV-1–infected individuals compared with healthy donors (Figure 1D and 1F). These findings suggest that despite overall CD4+ T-cell depletion and Treg loss, Tregs were not preferentially depleted in the lamina propria or lymphoid follicles in chronic HIV-1 infection.
Similar Suppressive Capacity in Tregs Isolated From HIV-1 Elite Controllers or Untreated Chronic Progressors
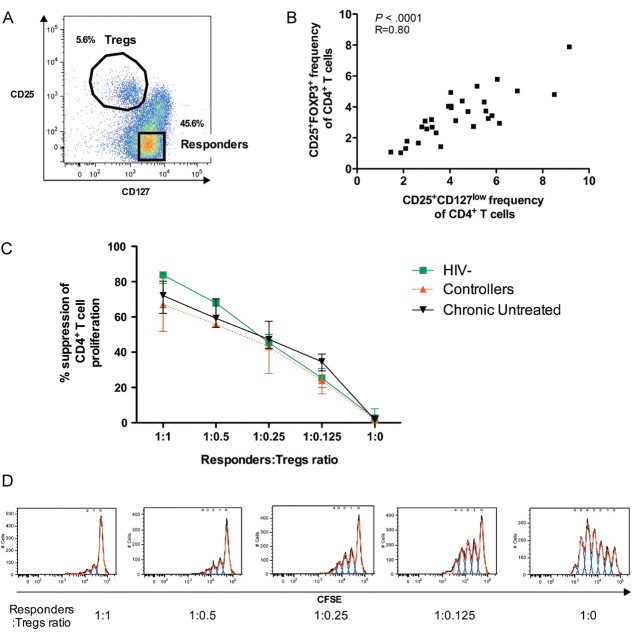
After demonstrating quantitative Treg differences among HIV-1–infected individuals at different stages of disease, we next explored whether Treg functional capacity changed with disease progression. The interleukin 7 (IL-7) receptor-alpha, CD127, has been described as a reliable surface marker for live cell sorting of Tregs [7] (Figure 2A), and we observed a strong correlation between CD4+CD25+CD127low and CD4+CD25+ FOXP3+ T cells (R = 0.80; P < .0001) (Figure 2B). We therefore isolated CD4+CD25+CD127low Tregs by flow-based cell sorting derived from large-volume blood donations in a subset of untreated chronic progressors, HIV-1 controllers, and healthy control subjects. Suppressive activity of the sorted CD4+CD25+CD127−/low Tregs was assessed using coculture of Tregs and CD4+CD25−CD127+ responder cells at different ratios in CFSE proliferation assays. Tregs isolated from HIV-1 controllers, untreated progressors, and HIV-1–negative individuals were equally capable of suppressing T-cell proliferation in a dose-dependent manner (Figure 2C and 2D). These findings show that even though absolute Treg numbers decline during progressive HIV-1 infection, the suppressive function of the remaining Tregs on a per-cell basis remains intact.
Figure 2.
Functional assessment of CD4+ regulatory T cells (Tregs) isolated from individuals infected with human immunodeficiency virus type 1 (HIV-1) and healthy control subjects. A, Representative dot plot showing the sorting strategy for the CD3+CD4+ T-cell subpopulations isolated for suppression assays. Regulatory T cells are defined as CD25+CD127low, and responder T cells as CD25−CD127+. B, Correlation of the frequency of CD4+CD25+CD127low/− and CD4+CD25+FOXP3+ T-cell populations within the same individuals (11 HIV-1–negative individuals, 2 treated with highly active antiretroviral therapy (HAART), 7 untreated chronic progressors, and 10 elite controllers). C, Proliferation of sorted T responder cells stimulated by microbeads in the presence of different ratios of autologous Tregs in HIV-negative (solid green line), HIV-1 elite controllers (dotted red line), and chronic untreated donors (solid black line). Three individuals were studied per group. D, Representative histograms of proliferation of the carboxyfluorescein succinimidyl ester (CFSE)–loaded responder T cells in the presence of different ratios of Tregs as analyzed with the FlowJo software proliferation platform.
DISCUSSION
Chronic immune activation, uncontrolled viral replication, and loss of CD4+ T cells are hallmarks of progressive HIV-1 infection, resulting in disease progression to AIDS. In this context, immune mechanisms with the ability to decrease inflammation and immune activation may play a significant role in maintaining the immunoregulatory equilibrium in peripheral blood and at mucosal sites. Tregs are key immune regulators with established relevance and therapeutic potential in many disease settings such as cancer, autoimmune diseases, and transplantation [8]. However, the role of Tregs in HIV-1 immune pathogenesis remains poorly understood, with data supporting both protective effects through reduction of generalized immune activation [9] and viral replication [3], as well as potential negative effects on viral control through suppression of virus-specific immune responses [10].
Our comprehensive analysis of Tregs in a large cohort of HIV-1–infected individuals at different disease stages revealed that HIV-1 elite controllers maintained systemic Treg numbers and frequencies similar to those in uninfected individuals, suggesting a preserved equilibrium between immune activation and regulation. In contrast, relative Treg frequencies were increased in untreated progressive HIV-1 infection, starting already in primary infection, despite an overall decline in CD4+ T cells. This observed relative enrichment of Tregs among CD4+ T cells, and its association with markers of disease progression, is in line with recent reports [6, 11] and may be a consequence of relative sparing of Tregs from HIV-1–induced apoptosis compared with regular CD4+ effector cells, preferential Treg expansion, peripheral de novo generation or conversion of Tregs, or a combination of these factors [12].
Although mucosal Tregs have been studied in animal models, few studies to date have investigated Tregs in the GALT in the setting of HIV-1 infection [11, 13, 14]. A recent study on Tregs derived from rectal biopsy specimens suggested relative increases in Tregs correlating with HIV-1 viral load and immune activation [11]. Although we found the same associations in PBMCs, we did not observe an enrichment of Tregs in the colon biopsy specimens from the subset of chronic progressors studied, despite an overall CD4+ T-cell decline. The differences observed between the different studies in the GALT of HIV-1–infected individuals may be related to heterogeneity in the tissue types studied (duodenum, colon, or rectal biopsy specimens), limited sample sizes, variable clinical parameters, and heterogeneity in experimental approaches and Treg quantification. Moreover, none of the studies published in the context of human and/or simian immunodeficiency virus to date have definitively distinguished between FOXP3 + CD4+ Tregs and non-Tregs by methods such as epigenetic analysis of DNA demethylation in the FOXP3 locus [15], which is a further limitation of the published data on tissue-based Tregs thus far. Given the importance of this compartment for HIV-1 pathogenesis, further detailed studies addressing these issues are warranted.
Data on the function of Tregs in HIV-1 infection are very limited to date and are often restricted to Treg depletion studies or bead isolation on the basis of suboptimal markers. We were fortunate to have access to flow-sorted Tregs isolated from large blood volumes for functional assays and show the preserved dose-dependent suppressive capacity of Tregs even in chronic progressive HIV-1 infection. Although these data do not exclude impairment of Treg function in an altered tissue microenvironment or impaired cross-talk with other cells such as dendritic cells, our results indicate that the ultimate failure to contain generalized HIV-1–associated immune activation may not be related to an intrinsic impairment of Treg function. Our findings further demonstrate that functional Tregs can be isolated and also expanded (data not shown) from HIV-1–infected individuals, which may be relevant for other disease entities afflicting HIV-1–infected individuals (eg, autoimmune diseases, transplantation), where adoptive transfers of Tregs are being explored as future therapeutic options, albeit currently in experimental stages [16]. Taken together, our data demonstrate that chronic HIV-1 infection is associated with a decline in Treg numbers in blood and GALT but a relative enrichment of Tregs in PBMCs and preserved Treg function across HIV disease states.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://www.oxfordjournals.org/our_journals/jid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank all individuals who participated in this study as well as the Ragon Institute Clinical Platform for critical support with cohort coordination and specimen acquisition. We apologize in advance for not being able to quote all relevant studies in this article owing to space limitations.
Financial support. This study was funded with federal funds from the US National Institutes of Health (NIH) (grants NIAID [National Institute of Allergy and Infectious Diseases] KO8 AI074405 and AI074405-03S1 to M. M. A. and 1K08 AI084546-01 to D. K.). The work also received funding from the Elisabeth Glaser Pediatric AIDS Foundation (Pediatric HIV Vaccine Program award MV-00-9-900-1429-0-00 to M. M. A.), MGH/ECOR (Massachusetts General Hospital/Executive Committee on Research) (Physician Scientist Development Award to M. M. A.), and the Doris Duke Charitable Foundation (M. A.). The work was in part made possible with help from the Harvard University Center for AIDS Research, an NIH-funded program (P30 AI060354) supported by the following NIH cofunding and participating institutes and centers: NIAID, NCI (National Cancer Institute), NICHD (National Institute of Child Health and Human Development), NHLBI (National Heart, Lung, and Blood Institute), NIDA (National Institute on Drug Abuse), NIMH (National Institute of Mental Health), NIA (National Institute on Aging), NCCAM (National Center for Complementary and Alternative Medicine), FIC (Fogarty International Center), and OAR (Office of AIDS Research).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions*. Annu Rev Immunol. 2009;27:551–89. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 2.Fazekas de St Groth B, Landay AL. Regulatory T cells in HIV infection: pathogenic or protective participants in the immune response? AIDS. 2008;22:671–83. doi: 10.1097/QAD.0b013e3282f466da. [DOI] [PubMed] [Google Scholar]
- 3.Moreno-Fernandez ME, Rueda CM, Rusie LK, Chougnet CA. Regulatory T cells control HIV replication in activated T cells through a cAMP-dependent mechanism. Blood. 2011;117:5372–80. doi: 10.1182/blood-2010-12-323162. Epub 2011 Mar 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elahi S, Dinges WL, Lejarcegui N, et al. Protective HIV-specific CD8+ T cells evade Treg cell suppression. Nat Med. 2011;17:989–95. doi: 10.1038/nm.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owen RE, Heitman JW, Hirschkorn DF, et al. HIV+ elite controllers have low HIV-specific T-cell activation yet maintain strong, polyfunctional T-cell responses. AIDS. 2010;24:1095–105. doi: 10.1097/QAD.0b013e3283377a1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulze Zur Wiesch J, Thomssen A, Hartjen P, et al. Comprehensive analysis of frequency and phenotype of T regulatory cells in HIV infection: CD39 expression of FoxP3+ T regulatory cells correlates with progressive disease. J Virol. 2010;85:1287–97. doi: 10.1128/JVI.01758-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 9.Chase AJ, Yang HC, Zhang H, Blankson JN, Siliciano RF. Preservation of FoxP3+ regulatory T cells in the peripheral blood of human immunodeficiency virus type 1-infected elite suppressors correlates with low CD4+ T-cell activation. J Virol. 2008;82:8307–15. doi: 10.1128/JVI.00520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinter A, McNally J, Riggin L, Jackson R, Roby G, Fauci AS. Suppression of HIV-specific T cell activity by lymph node CD25+ regulatory T cells from HIV-infected individuals. Proc Natl Acad Sci USA. 2007;104:3390–5. doi: 10.1073/pnas.0611423104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw JM, Hunt PW, Critchfield JW, et al. Increased frequency of regulatory T-cells accompanies increased T-Cell immune activation in rectal mucosa of HIV+ non-controllers. J Virol. 2011;85:11422–34. doi: 10.1128/JVI.05608-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nilsson J, Boasso A, Velilla PA, et al. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood. 2006;108:3808–17. doi: 10.1182/blood-2006-05-021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epple HJ, Loddenkemper C, Kunkel D, et al. Mucosal but not peripheral FOXP3+ regulatory T cells are highly increased in untreated HIV infection and normalize after suppressive HAART. Blood. 2006;108:3072–8. doi: 10.1182/blood-2006-04-016923. [DOI] [PubMed] [Google Scholar]
- 14.Favre D, Mold J, Hunt PW, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2:32–6. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baron U, Floess S, Wieczorek G, et al. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3+ conventional T cells. Eur J Immunol. 2007;37:2378–89. doi: 10.1002/eji.200737594. [DOI] [PubMed] [Google Scholar]
- 16.Wright GP, Ehrenstein MR, Stauss HJ. Regulatory T-cell adoptive immunotherapy: potential for treatment of autoimmunity. Expert Rev Clin Immunol. 2011;7:213–25. doi: 10.1586/eci.10.96. [DOI] [PubMed] [Google Scholar]