Abstract
Objective
CD4+ T cells from patients with active lupus have impaired ERK pathway signaling that decreases DNA methyltransferase expression, resulting in DNA demethylation, overexpression of immune genes and autoimmunity. The ERK pathway defect is due to impaired phosphorylation of T505 in the PKCδ activation loop. However, the mechanisms preventing PKCδ T505 phosphorylation in lupus T cells are unknown. Others have reported that oxidative modifications, and nitration in particular, of T cells as well as serum proteins correlate with lupus disease activity. We hypothesized that nitration inactivates PKCδ, contributing to impaired ERK pathway signaling in lupus T cells.
Methods
CD4+ T cells were purified from lupus patients and controls then stimulated with PMA. Signaling protein levels, nitration and phosphorylation were quantitated by immunoprecipitation and immunoblotting of T cell lysates. Transfections were performed by electroporation.
Results
Treating CD4+ T cells with peroxynitrite nitrated PKCδ, preventing PKCδ T505 phosphorylation and inhibiting ERK pathway signaling similar to that observed in lupus T cells. Patients with active lupus had higher nitrated T cell PKCδ levels than controls which correlated directly with disease activity, and anti-nitrotyrosine immunoprecipitations demonstrated that nitrated PKCδ, but not unmodified PKCδ, was refractory to PMA stimulated T505 phosphorylation, similar to PKCδ in peroxynitrite treated cells.
Conclusions
Oxidative stress causes PKCδ nitration, which prevents its phosphorylation and contributes to the decreased ERK signaling in lupus T cells. These results identify PKCδ as a link between oxidative stress and the T cell epigenetic modifications in lupus.
Keywords: Systemic lupus erythematosus, PKCδ, T cells, Signal transduction, Oxidative stress
Systemic lupus erythematosus (SLE) is an incompletely understood chronic, relapsing autoimmune disease. Persuasive evidence indicates that genetic factors contribute to disease development. However, a lack of complete disease concordance in identical twins and the relapsing nature of the disease indicate that environmental factors are important as well (1). The full repertoire of the environmental factors, and the mechanism(s) by which the environmental agents contribute to the onset of lupus and lupus flares, remain unknown. Our group has reported that T cell DNA demethylation contributes to the pathogenesis of human lupus by causing overexpression of immune genes, leading to T cell autoreactivity and the development of lupus in genetically predisposed hosts (2). This suggests that the environment may trigger lupus flares by inhibiting T cell DNA methylation. The mechanisms by which environmental agents cause T cell DNA demethylation in lupus are also unknown.
DNA methylation patterns are replicated each time a cell divides by DNA methyltransferase 1 (Dnmt1) (3), whose levels are regulated in part by the ERK signaling pathway (4–6). We reported that treating proliferating T cells with ERK pathway inhibitors like hydralazine and U0126, or Dnmt inhibitors like procainamide, is sufficient to demethylate the DNA, and that the demethylated T cells are sufficient to cause a lupus-like disease in murine models (7, 8). Further, transgenic mice with an inducible T cell ERK pathway signaling defect develop a lupus-like disease (9). We also reported that T cells from patients with active lupus have impaired ERK pathway signaling, low Dnmt1 levels and overexpress methylation sensitive genes (10), suggesting that impaired ERK pathway signaling contributes to DNA demethylation in human lupus. Interestingly, CD4+ lupus T cells demethylate the same genetic regulatory elements demethylated by ERK pathway inhibitors and Dnmt inhibitors (11), and the level of lupus disease activity is directly related to the degree of signaling impairment and DNA demethylation (10). These studies thus suggest an important role for impaired ERK pathway signaling in triggering lupus flares.
More recent studies traced the lupus ERK pathway signaling defect to impaired protein kinase C δ (PKCδ) phosphorylation (12). PKCδ is a key molecule that is phosphorylated in response to a variety of signals, and thus activates other signaling pathways including the ERK pathway (13–15). PKCδ in T cells from patients with active lupus or hydralazine-treated T cells is not properly phosphorylated in response to PMA or other stimuli, and the defect correlates with the lupus ERK pathway defect. Transfecting human T cells with a kinase inactive PKCδ causes decreased ERK phosphorylation, indicating that PKCδ is critically required for ERK pathway activation. Additionally, the transfected cells exhibited demethylation and overexpression of the TNFSF7 (CD70) gene, similar to lupus patients (12). However, the mechanisms inhibiting PKCδ phosphorylation in lupus T cells are unknown.
Inflammation is associated with the generation of reactive oxygen species (ROS), and patients with active lupus have increased levels of ROS and reactive nitrogen intermediates (RNI), as well as decreased levels of oxidant scavengers (16–18). This imbalance causes increased levels of superoxide (O2−), hydrogen peroxide (H2O2) and peroxynitrite (ONOO−), all highly reactive metabolites that cause direct toxicity by inducing chemical modifications in lipids, proteins and DNA (19). ONOO− nitrates Tyr residues to prevent phosphorylation (20) and may thus affect signaling pathways. However, the consequences of oxidative stress on T cell signaling are also poorly understood.
Since T cell PKCδ kinase activity is decreased in patients with active lupus, and active lupus is characterized by the generation of ROS/RNI and oxidative protein damage, we hypothesized that PKCδ may be covalently modified by ROS/RNI in lupus T cells, preventing its activation. The goal of this work was to determine if oxidative modifications produced by ONOO− contribute to impaired T cell PKCδ phosphorylation, causing the decreased ERK pathway signaling observed in patients with active lupus.
MATERIALS AND METHODS
Reagents
Hydralazine was purchased from VWR (West Chester, PA), and peroxynitrite from Calbiochem (Gibbstown, NJ). All other chemicals were from Sigma.
Antibodies
The following primary antibodies were used: polyclonal rabbit anti-phospho-PKCα (T638/641), anti-phospho-PKCθ (T538), anti-phospho-PKCδ (T505), anti-phospho-PKCδ (Y311) and anti-phospho-PDK1 (Ser241), at 1:1000 dilution (Cell Signaling Tech., Beverly, MA). For immunoprecipitation, anti-nitrotyrosine, clone 1A6 agarose conjugate, was used (Upstate-Millipore, Billerica, MA). Rabbit polyclonal anti-active MAPK (1:5000) was from Promega (Madison WI), and anti-total PKCδ was from Santa Cruz Biotechnology (Santa Cruz, CA). Secondary antibodies included: anti-rabbit IgG horseradish peroxidase (1:2000, Cell Signaling Technology, Danvers, MA) and anti-mouse IgG horseradish peroxidase (1:4000, Amersham, Piscataway, NJ).
Subjects
Lupus patients (n=16, average age 42 range 27–64 years) with active and inactive disease were recruited from the outpatient rheumatology clinics and inpatient services at the University of Michigan, and healthy controls (n=21) were recruited by advertising. All lupus patients met the revised American College of Rheumatology criteria for SLE (21). Lupus disease activity was quantitated using the systemic lupus erythematosus disease activity index (SLEDAI) (22), and the range was 4–10 (mean 6.2) for the patients with active lupus, and 0–2 (mean 0.5) for patients with inactive disease. Controls were matched to the lupus patients for age, race and sex. These protocols were reviewed and approved by the University of Michigan Institutional Review Board for Human Subject Research. The demographics and medications received by the patients are summarized in Table 1.
Table 1.
Demographics, disease activity, and treatment in the SLE patients. SLEDAI: Systemic Lupus Erythematosus Disease Activity Index.
| Patients | |
|---|---|
| SLEDAI (mean ± S.D.) | |
| Active | 6.2 ± 2.3 |
| Inactive | 0.5 ± 1.0 |
| Age, years (mean ± S.D.) | 41.8 ± 11.1 |
| Gender, ratio (F:M) | 14:2 |
| Medications | (%) |
| Prednisona | 62 |
| Antimalarials | 62 |
| Azathioprine | 25 |
| Mycophenolato mofetil | 44 |
| Methotrexate | 0 |
T cell isolation
Peripheral blood mononuclear cells were isolated from healthy donors or SLE patients by Ficoll-Hypaque density gradient centrifugation. CD4+ T cells were then purified by negative selection using magnetic beads (CD4+ T cell isolation kit; Miltenyi Biotec, Auburn, CA) as previously reported (12).
T cell stimulation and protein isolation
CD4+ T cells were suspended in RPMI 1640 supplemented with 10% fetal calf serum, 2 mM glutamine and penicillin/streptomycin then left unstimulated or stimulated with 50 ng/ml PMA for 15 min at 37°C. Treatment with peroxynitrite was performed at the concentrations and time specified in each experiment and before PMA stimulation. Following stimulation, whole cell lysates were obtained according to previous protocols (12) and protein content quantified using the BCA Protein Assay (Pierce, Rockford, IL).
Transient transfections
T cells from normal donors were immediately transfected with siRNA or the indicated mutants using Amaxa nucleofection technology (Gaithersburg, MD) according to previous protocols (12). After 24 h the cells were treated as indicated, harvested and cell lysates obtained as above. siRNA PP2Ac and PP2Ac mutants H118N and L199P were kindly provided by Dr. G. Tsokos and used as described by Katsiari et al. (23). Transfection efficiency was 63 ± 6% of total cell number and verified by fluorescence microscopy of cells transfected with the positive control vector pmaxGFP encoding a green fluorescence protein and provided with the kit.
Immunoblot Analyses
Immunoblots were performed as previously described (12). Briefly, 20 μg of whole cell protein were fractionated by SDS-PAGE, transferred to nitrocellulose membranes (Schleicher and Schuell, Keene, N.H.) then stained with Ponceau S (Sigma) to verify equal protein loading between lanes. After incubation with the kinase specific antibody then the secondary antibody, protein bands were visualized by chemiluminescence (Amersham, Piscataway, NJ). The bands on X-ray films were scanned and analyzed with ImageQuant 5.2 software (Amersham) for quantification. Where indicated, blots were stripped and re-probed with the corresponding antibody. Values were normalized to β-actin or the corresponding kinase as indicated.
Immunoprecipitations
Immunoprecipitations were performed according to the manufacturer’s instructions. Briefly, lysates from normal or lupus T cells were incubated with the agarose-conjugated anti-nitrotyrosine antibody overnight at 4°C. Following centrifugation an aliquot of the supernatant (containing non-nitrated proteins) and the beads were resuspended in Laemmli sample buffer and boiled for 5 min. Proteins from each fraction were then analyzed in parallel by SDS-PAGE and immunoblot analysis.
Statistical Analyses
The significance of the difference between means was determined using Student’s t-test, regression analysis, or analysis of variance (ANOVA) as appropriate. p values ≤ 0.05 were considered significant.
RESULTS
PDK-1 does not contribute to the PKCδ signaling defect in lupus T cells
We examined the mechanisms regulating PKCδ phosphorylation to determine the causes of lower phospho-T505 PKCδ levels in PMA-stimulated lupus T cells.
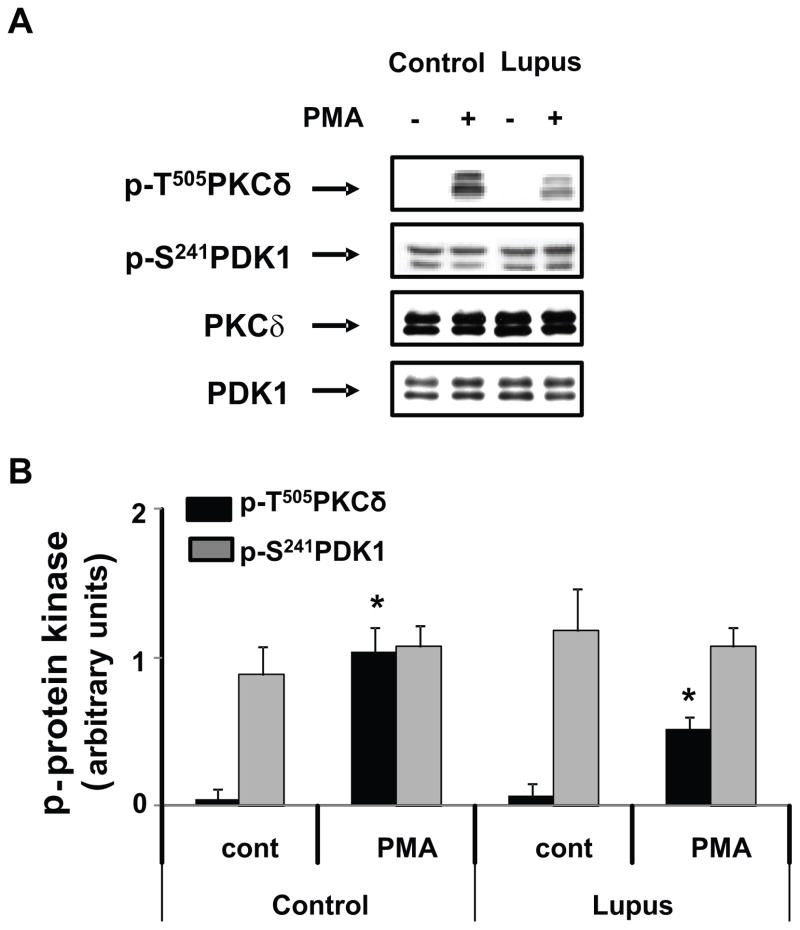
3'-Phosphoinositide-dependent protein kinase-1 (PDK-1) controls phosphorylation of T505 in the PKCδ catalytic loop (24). We therefore examined PDK-1 activation. PDK-1 has five sites (Ser-25, Ser-393, Ser-396, Ser-410 and Ser-241) that are phosphorylated, but only Ser-241 is located in the activation loop and required for PDK-1 activity (25). PDK-1 activation was therefore studied using antibodies to PDK-1 p-Ser241 and immunoblotting. There was no significant difference in PDK-1 phosphorylation between control and lupus T cells, although PKCδ p-T505 was decreased in PMA-stimulated CD4+ T cells from lupus patients with active disease (Figure 1A). The densitometric analysis of four similar experiments confirmed no significant difference in PDK-1 activation between lupus patients and controls (p>0.05) (Figure 1B). Our observation that p-PDK-1 is expressed at similar levels in resting and PMA-stimulated T cells agrees with the observation that PDK-1 is constitutively active due to autophosphorylation of its activation loop (26). These results indicate that a PDK-1 activity defect is unlikely causing the decreased PKCδ T505 phosphorylation in CD4+ lupus T cells.
Fig. 1.
PDK-1 is not affected in lupus T cells. CD4+ T cells from healthy donors (control) or from lupus patients were stimulated or not with PMA for 15 min. 20 μg of whole cell lysates were subjected to SDS-PAGE fractionation, transferred to nitrocellulose membranes and probed with a polyclonal antibody against PKC δ p-T505, as described in Materials and Methods. Membranes were stripped and reprobed with anti-phospho-PDK1. PKCδ and PDK-1 were used as controls for the corresponding total protein expression. Panel A shows a representative blot comparing phosphorylation of PDK1 in a lupus patient with a normal donor. Panel B represents the mean ± S.E of four similar experiments performed in different cell preparations from normal and SLE patients. * p≤0.02 lupus vs normal
ONOO− inhibits PKCδ T505 phosphorylation
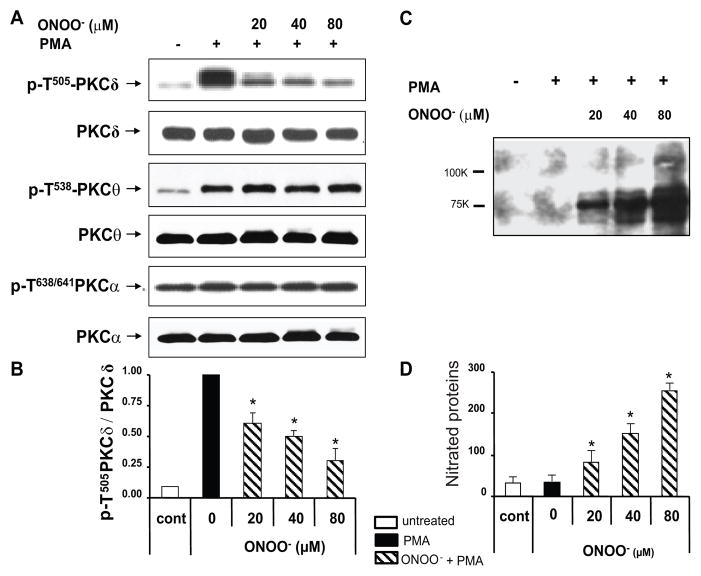
Oxidative stress plays an important role in triggering lupus flares, and others have reported that serum protein oxidation correlates with disease activity in lupus patients, and that serum proteins are abnormally nitrated in patients with active lupus (16, 27). Since ONOO− is a potent oxidizing agent that nitrates proteins, causing functional loss (20), we asked if ONOO− causes the PKCδ T505 phosphorylation defect observed in lupus T cells. CD4+ T cells from healthy subjects were treated with increasing concentrations of ONOO− then stimulated with PMA. Treatment with ONOO− caused a concentration–dependent decrease in PKCδ T505 phosphorylation while PKC α and θ activation loop phosphorylation was not appreciably affected (Fig 2A). Figure 2B shows the quantitative densitometric analysis of four serial repeat experiments similarly measuring the effects of ONOO− on PKCδ p-T505. These results indicate that the inhibitory effects of ONOO− on PKCδ are isoform-specific since phosphorylation of other isoforms was unaffected.
Fig. 2.
Peroxynitrite decreases PKCδ T505 phosphorylation while increasing protein nitration in T cells. CD4+ T cells from healthy donors were untreated or treated with 20 – 80 μM peroxynitrite for 15 min and then PMA stimulated as indicated. Whole cell proteins were obtained and subjected to Western blot analysis. A. Representative immunoblot showing phosphorylation at the activation loops of PKCδ, PKCθ and PKCα using antibodies specific to the phosphorylated PKC isoforms as indicated. B. Quantitative analysis of PKCδ T505 from 4 healthy donors in four different experiments (mean ± S.E.M). Values were normalized to total PKCδ. PMA-stimulated CD4+ T cells but without ONOO− treatment (solid bar) were considered as 100%. C. Representative experiment using anti 3-nitrotyrosine antibody to probe PMA stimulated T cell lysates. D. Relative quantitation of 3-nitrotyrosine proteins from four similar experiments (mean ± S.E.M.). Untreated cells and PMA-unstimulated cells (□) were used as controls (*p < 0.01 ONOO− treated vs untreated).
Since ONOO− nitrates Tyr residues to form 3-nitrotyrosine (20), we confirmed the effect of ONOO− by measuring 3NO2-tyrosine protein derivative formation. CD4+ T cells from a healthy donor were treated with increasing ONOO− concentrations then stimulated with PMA. 3NO2-tyrosine modified proteins were studied by immunoblotting. We observed a concentration-dependent increase in 3-nitrotyrosine derivative formation (Fig 2C). Figure 2D shows the mean ± SD of densitometric analyses from four similar experiments, confirming that T cell proteins are nitrated by ONOO− and their nitration levels correlate with the ONOO− induced inhibition of p-T505 PKCδ shown in Fig 2B.
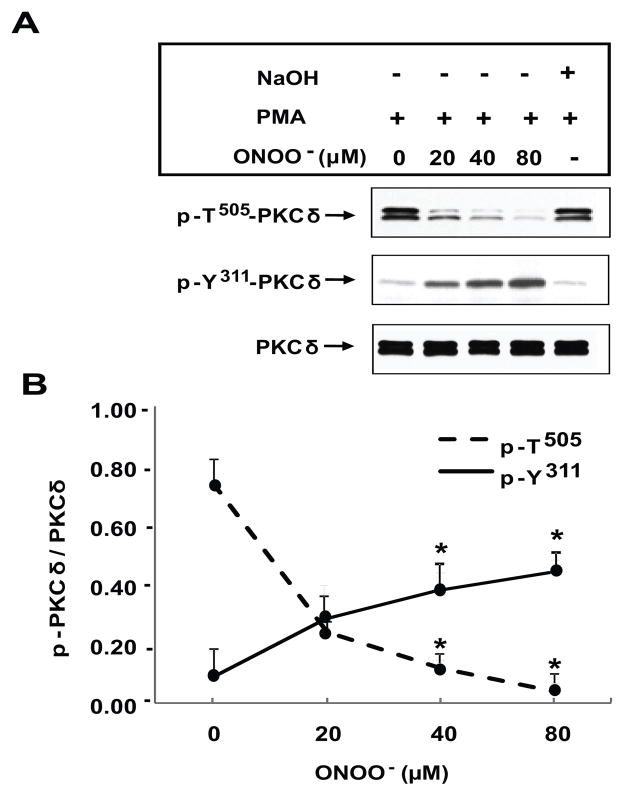
We also studied the phosphorylation of Y311 in the PKCδ molecule, because this amino acid is phosphorylated in cells undergoing oxidative stress (28, 29). Figure 3A shows a representative immunoblot comparing effects of ONOO− on PMA stimulated PKCδ T505 and Y311 phosphorylation. In untreated (0 μM ONOO−) CD4+ T cells, PMA causes a substantial increase in T505 phosphorylation, but has no appreciable effect on Y311 phosphorylation. It is important to note that p-Y311 is almost undetectable in resting CD4+ T cells (not shown) similar to p-T505. However, ONOO− induces a concentration-dependent increase in Y311 phosphorylation in PMA stimulated cells that is inversely related to T505 phosphorylation (ONOO− vs non-ONOO− p≤0.01, n=4). Fig 3B clearly demonstrates a different pattern of PKCδ phosphorylation that is stimulus-dependent. While PMA promotes phosphorylation on T505, Y311 is almost insensitive. In contrast, ONOO− increases Y311 phosphorylation with a concomitant decrease in p-T505.
Fig. 3.
Differential PKCδ phosphorylation induced by peroxynitrite. A. Representative experiment showing the effect of different ONOO− concentrations on PKCδ phosphorylation. CD4+ T cells were isolated from healthy controls and treated with ONOO− at the concentrations specified. Following treatment the cells were stimulated with 50 ng/ml PMA for 15 min and PMA-stimulated peroxynitrite-untreated cells were used as control. Protein lysates were then subjected to electrophoresis, transferred to nitrocellulose and the membranes probed with anti-p-T505-PKCδ. The blot was then stripped and reprobed with anti-p-Y311-PKC δ. NaOH was used as vehicle control and added to the culture for 15 min before PMA stimulation at the same final concentration as in the ONOO− solution. B. Differential dose-response curves of p-PKCδ following ONOO- treatment. The graph represents the mean ± SEM of p-PKCδ expression relative to PKCδ of four independent experiments (*p≤0.04; ONOO-treated vs ONOO-untreated cells).
The lack of additional effects caused by NaOH excludes nonspecific effects due to pH changes caused by the ONOO− solution (Fig 3A).
Protein phosphatase 2Ac (PP2Ac) does not participate in the ONOO−-induced PKCδ defect
ONOO− increases PP2Ac activity in some cell types (30), and PP2Ac has the highest specific phosphatase activity towards PKCδ (31). Further, PP2Ac expression and activity is increased in T cells from lupus patients (23). We tested if increased PP2Ac activity could be responsible for the PKCδ dephosphorylation caused by ONOO−. T cells from healthy donors were transfected with 5 μg of plasmids encoding the dominant negative PP2Ac mutants H118N or L199P (32), or 100nM PP2Ac-specific siRNA according to Katsiari et al. (23). Cells transfected with an irrelevant siRNA were used as control and 2 μg of the empty GFP vector were transfected as an internal control for transfection efficiency in each experiment. Twenty-four h later the cells were treated with ONOO− for 15 min followed by PMA-stimulation. In three serial repeats, PKCδ T505 phosphorylation was not increased in ONOO− treated cells overexpressing the catalytically inactive PP2Ac mutants (mean ± SEM: H118N 23.39 ± 4.2%; L199P 31.58 ± 4.5%) or the siRNA (mean ± SEM: 28.36 ± 5.2%) relative to non-transfected cells (mean ± S.D.: 21.01 ± 4.8%; non-transfected vs transfected cells: p=0.62, n=3) relative to PMA-stimulated T cells (100%), confirming that PP2Ac is not causing the p-PKCδ T505 defect in this system.
PKCδ nitration correlates with decreased ERK phosphorylation
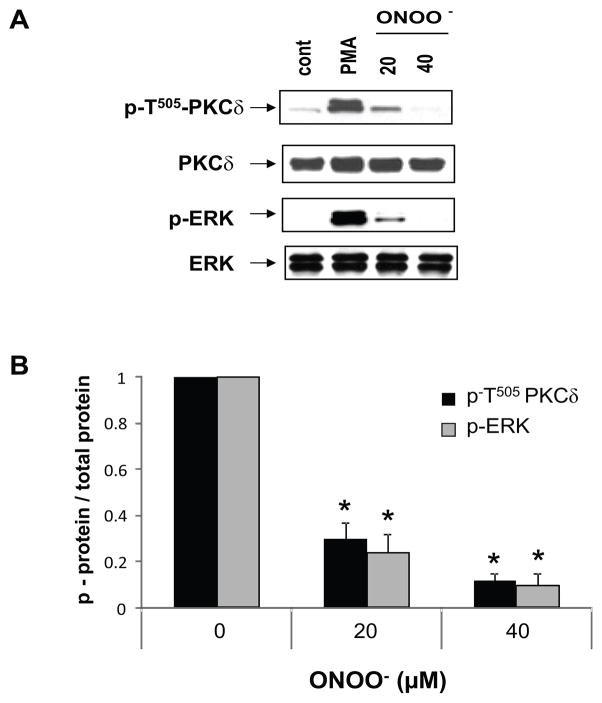
We previously reported that PKCδ T505 phosphorylation is decreased in T cells from patients with active lupus (12). The decreased PKCδ T505 phosphorylation correlates with decreased ERK phosphorylation in CD4+ lupus T cells, and in CD4+ T cells treated with pharmacologic PKCδ inhibitors, or transfected with PKCδ siRNA or a dnPKCδ. To determine if PKCδ nitration also inhibits ERK phosphorylation, CD4+ T cells from healthy controls were treated with different concentrations of ONOO− for varying times then stimulated with PMA. Figure 4A shows that as expected, PMA-stimulated ERK phosphorylation declines with increasing ONOO− concentrations, and parallels the decrease in PKCδ T505 phosphorylation. This agrees with our previous results showing that PKCδ T505 phosphorylation is upstream of ERK (12) and its impairment decreases ERK pathway signaling. Figure 4B shows the quantification of 4 serial repeats confirming this observation.
Fig. 4.
Peroxynitrite decreases ERK phosphorylation. A. CD4+ T cells from normal donors were treated or not for 15 min with peroxynitrite at the indicated concentrations then stimulated with PMA. Cells left untreated were used as control (cont). Whole cell extracts were fractionated by SDS-PAGE and followed by immunoblot using anti p-T505 PKC δ. After stripping the blot was re-probed with anti p- T202/Y204-ERK. No variation in total PKCδ or ERK protein expression is observed. This blot is representative of 4 independent experiments. B. Quantitative immunoblot analysis of 4 different experiments. PMA-stimulated non-peroxynitrite treated cells were considered as 1. Values are the mean ± SEM. * p ≤ 0.001 peroxynitrite vs non-peroxynitrite.
PKCδ nitration in lupus
Lupus is characterized by increased oxidative stress (16, 33, 34). T cells from patients with active lupus generate large amounts of reactive oxygen species (35), and serum proteins are nitrated in these patients (36). We therefore hypothesized that PKCδ is inactivated in T cells from patients with active lupus through nitration, similar to ONOO−treated T cells.
To address this hypothesis CD4+ T cells from 6 lupus patients with active disease (SLEDAI ≥ 4) were stimulated with PMA and nitrated proteins immunoprecipitated from the lysates with anti-3NO2-Tyr antibodies. Controls included similarly treated CD4+ T cells from 4 lupus patients with inactive disease, and 3 control subjects. Total and T505 phosphorylated PKCδ content were then compared by immunoblotting.
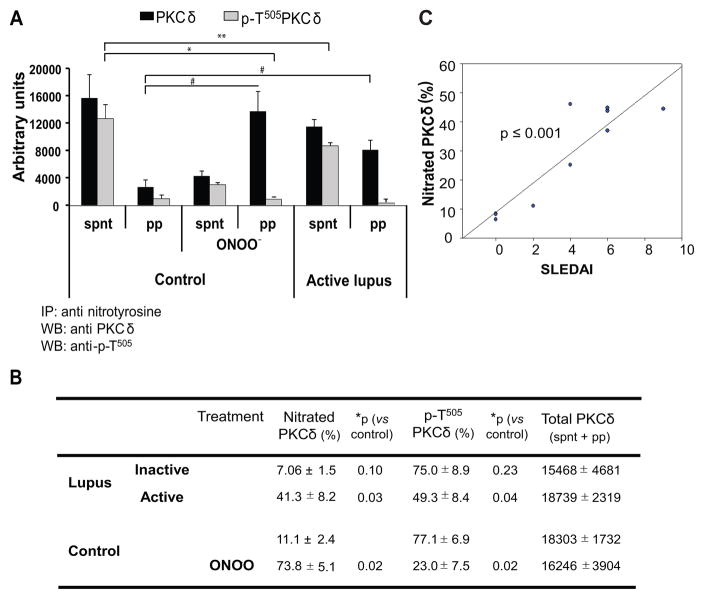
Nitrated and non-nitrated PKCδ were analyzed as the total PKCδ content in the precipitate and supernatant respectively. The levels of total PKCδ (supernatant + immunoprecipitate) were similar in untreated or ONOO− treated T cells from control donors and lupus patients (Fig. 5 A and B ). These results confirm previous data showing no differences in PKCδ protein expression in control and lupus patients (12). As anticipated, the amount of nitrated PKCδ (precipitated) was higher in T cells from patients with active lupus relative to controls (p<0.03) and with a pattern similar to ONOO− treated control T cells (Fig 5A). In contrast, Fig 5B shows that the pattern of nitration and phosphorylation of PKCδ was not significantly different in CD4+ T cells from patients with inactive lupus when compared to healthy controls. Importantly, the level of T cell PKCδ nitration in lupus patients correlated directly with the SLEDAI scores (Fig. 5C).
Fig. 5.
PKCδ nitration in lupus T cells. CD4+ T cells from three healthy donors (control) were untreated or treated with peroxynitrite followed by PMA stimulation. In parallel CD4+ T cells from six active and four inactive lupus patients were PMA-stimulated. Total lysates were immunoprecipitated then the supernatants and precipitates were immunoblotted with anti-p-T505 PKC δ and membranes reprobed with anti-total PKCδ. A: The bar graph shows the quantitative densitometric analysis of total PKC δ (■) and p-T505 PKCδ (
 ) in the supernatant (spnt) and the precipitate (pp) using CD4+ T cells from healthy donors and patients with active disease. * p≤0.01, ** p≤0.04, #≤0.02. Values are the mean ± SEM of six independent experiments. B: The table shows nitrated PKCδ (total PKCδ content in pp) and p-T505PKC δ (p-PKCδ in spnt + pp) as percent of total PKCδ (expressed in arbitrary units) in spnt and pp in each experimental condition. Values are the mean ± SEM of six experiments. * p values vs control. C: The graph shows the correlation between the nitrated PKCδ levels in lupus patients and the SLEDAI scores. p value was determined by analysis of variance (ANOVA).
) in the supernatant (spnt) and the precipitate (pp) using CD4+ T cells from healthy donors and patients with active disease. * p≤0.01, ** p≤0.04, #≤0.02. Values are the mean ± SEM of six independent experiments. B: The table shows nitrated PKCδ (total PKCδ content in pp) and p-T505PKC δ (p-PKCδ in spnt + pp) as percent of total PKCδ (expressed in arbitrary units) in spnt and pp in each experimental condition. Values are the mean ± SEM of six experiments. * p values vs control. C: The graph shows the correlation between the nitrated PKCδ levels in lupus patients and the SLEDAI scores. p value was determined by analysis of variance (ANOVA).
However, overall PKCδ T505 phosphorylation was decreased in stimulated T cells from patients with active lupus relative to healthy controls (Fig 5B, p=0.04), and the nitrated PKCδ fraction from the active lupus patients was almost completely refractory to PMA-stimulated phosphorylation (4 ± 2% of nitrated PKCδ, p≤0.04, active lupus vs control, Fig 5A). As predicted, 74% of the PKCδ in T cells treated with ONOO− was nitrated but only 6 % of this fraction was T505 phosphorylated after PMA stimulation (Fig. 5A). In contrast, non-nitrated PKCδ was phosphorylated in both control and active or inactive lupus T cells (supernatant) (Fig 5A). These experiments thus demonstrate more extensive PKCδ nitration, and less T505 phosphorylation, in T cells from patients with active lupus relative to both inactive lupus and control, suggesting that T505 phosphorylation decreases in proportion to the extent of nitration and that the degree of nitration is directly related to the disease activity.
DISCUSSION
The goal of this work was to investigate mechanisms causing the PKCδ phosphorylation defect responsible for decreased ERK pathway signaling in lupus T cells.
PKCδ belongs to the PKC family of related serine/threonine kinases with active roles in growth regulation and apoptosis. PKC isoforms contain a highly conserved C-terminal catalytic domain. However, they are subdivided into three subfamilies according to their N-terminal regulatory domains. Conventional isoforms comprise PKC α, β and γ, bind diacylglycerol (DAG)/PMA in their C1 domain, and bind anionic phospholipids in a calcium-dependent manner in their C2 domain. Novel isoforms include PKC δ, ε, η, μ and θ and are activated by DAG/PMA without a calcium requirement. Atypical isoforms, ζ and λ/ι, are DAG/PMA and calcium-independent (37).
PKCδ is ubiquitously expressed among cells and tissues, and is the only isoform that can be activated by three different mechanisms: a. through Ser/Thr phosphorylation, b. through tyrosine phosphorylation and, c. by caspase 3-dependent proteolytic cleavage. These are independent mechanisms that regulate PKCδ activity, substrates and cellular localization and play critical roles during cell growth, differentiation, programmed cell death as well as the cellular response to oxidative stress (38).
Our previous work identified decreased PKCδ p-T505 levels in stimulated lupus T cells, which correlated with the decreased ERK pathway signaling previously reported in lupus T cells. We also demonstrated that PKCδ is upstream of ERK, and that impaired PKCδ-ERK pathway signaling in T cells causes demethylation and overexpression of methylation sensitive genes (12). Importantly, transgenic mice lacking T cell PKCδ activity develop a lupus-like disease with decreased ERK signaling, overexpression of methylation sensitive genes and production of anti-dsDNA antibodies similar to those observed in lupus patients (Gorelik et al., Lupus 19:7, 2010 and manuscript in preparation), strongly supporting the hypothesis that defective PKCδ signaling is sufficient to cause lupus.
PDK1 phosphorylates PKCδ on T505 in the activation loop, promoting alignment of these residues with the catalytic pocket and controlling catalytic activity of the enzyme (24). We demonstrated that phosphorylation of Ser241, required for PDK-1 kinase activity, is not appreciably affected in lupus T cells relative to T cells from healthy donors and under the same assay conditions in which PKC δ p-T505 was decreased. This implies that another mechanism inhibits PKC δ T505 phosphorylation in lupus T cells.
Chronic inflammation is associated with oxidative stress, and a pro-oxidant state has been described in patients with lupus (27, 34). In this pro-oxidative state, higher levels of ROS/RNI are present and can directly cause toxicity through post-translational modifications of proteins and alterations of lipids and DNA. NO is overproduced in lupus (39), and can combine with O2− to form ONOO−, a highly reactive and potentially pathogenic molecule. Multiple markers of protein oxidation have been found in SLE patient sera, including protein carbonyls, protein-bound methionine sulfoxide, decreased levels of protein thiols (27) and increases in protein 3-nitro-tyrosines that correlate with worsening disease status (16, 33, 40) indicating that protein oxidation and in particular nitrating pathways may play an important role in the pathogenesis of SLE (33, 34, 36). However, while it is recognized that overproduction of ROS/RNI alters and modifies T cell signaling, their target molecules and the intracellular mechanisms that they affect are not well understood.
Based on these observations, we investigated whether the defective PKCδ activation in lupus T cells was caused by oxidative damage. ONOO− was used as the oxidizing agent, and caused PKCδ nitration that resulted in decreased phosphorylation of T505. The fact that PKCδ was the only PKC isoform catalytically affected indicates that the ONOO− inhibitory effect is selective and specific to PMA-stimulated PKCδ p-T505.
PKCδ is not phosphorylated at the activation loop (T505) in resting T cells, but phosphorylation increases following PMA stimulation and translocation to the cytoplasmic membrane. In our studies, oxidation modified the phosphorylation pattern, increasing tyrosine phosphorylation while decreasing threonine phosphorylation, indicating specific effects on PKCδ phosphorylation regulation. This differential PKCδ phosphorylation pattern also modifies its intracellular translocation, resulting in changes to the interaction of PKCδ with downstream targets (41). Recent studies also indicate that reactive oxygen species can also modulate PKCδ activity in other cell types. In keratinocytes, oxidation increases PKCδ-Y phosphorylation resulting in reduced enzymatic activity (42), whereas in other cells oxidation enhances the enzymatic activity (43) or even modifies the enzymatic specificity (44), indicating that oxidative modifications of PKCδ activity may be cell type-dependent and/or phosphorylated residue-dependent (28). The precise effects of oxidation on T cell PKCδ are unexplored, but it is possible that oxidation of certain residues in the molecule causes distortions in the secondary and tertiary structure, resulting in a decreased accessibility of T505 to phosphorylation. It is also possible that the rate of dephosphorylation increases by oxidation. The phospho-serine/threonine phosphatases PP1c, PP2Ac and PP2Cα dephosphorylate PKCδ, with PP2Ac demonstrating the highest specific activity towards PKCδ (31). However, our results show that T cells transfected with catalytically inactive PP2Ac mutants, or the corresponding siRNA, did not restore PKCδ phosphorylation following ONOO− treatment, suggesting that modifications of PP2Ac activity are unlikely to be responsible for the decreased T505 phosphorylation observed in oxidized PKCδ. This observation agrees with a report demonstrating that even though the mRNA, protein, and enzymatic activity of the PP2A catalytic subunit (PP2Ac) is increased in patients with lupus, the defect is independent of disease activity (23), while the PKCδ phosphorylation impairment is directly related to disease activity (12). The present work shows that oxidation of PKCδ results in a selective decrease in PKCδ p-T505 and directly correlates with decreased p-ERK in T cells, consistent with our previous results.
The consequences of an oxidative environment on ERK activity are not clearly defined, and vary depending on the cell system and stimulus. Decreased phosphorylation of ERK induced by peroxynitrite in kidneys of βs sickle cell mice has been reported (45), and similarly an alteration of the cellular redox state leads to increased MAPK phosphatase-1 (MKP-1) expression in fibroblasts, resulting in ERK inactivation (46). This suggests that additional mechanisms may contribute to the decreased ERK phosphorylation caused by oxidative stress in lupus T cells.
Peroxynitrite may alter protein structure and function by interacting with different amino acids. By oxidating cysteine residues (S-nitrosylation), peroxynitrite inactivates many enzymes involved in energetic processes in addition to protein tyrosine phosphatases that may enhance tyrosine phosphorylation-dependent signaling (20). ONOO− also oxidizes tyrosine residues (O-nitration) to form 3-nitrotyrosine derivatives (20). Tyrosine nitration is considered to be a major cause of peroxynitrite-mediated cytotoxicity because it affects protein structure and function, that may result in the generation of antigenic epitopes (47), changes in the catalytic activity of enzymes, and impaired cell signal transduction (48). Nitration is a highly selective process, limited to specific tyrosine residues on a limited number of proteins. In general, protein nitration is associated with loss of function (45). Others have reported an increased amount of NO-mediated oxidation products, such as 3-nitrotyrosine proteins, due to elevated levels of ONOO−in the serum of lupus patients (40). We found higher level of nitrotyrosine-containing proteins in T cells from patients with active lupus relative to patients with inactive lupus and to T cells from healthy controls. The levels of nitrated PKCδ were also greater in T cells from patients with active lupus, and PMA stimulated T505 phosphorylation of the nitrated proteins was impaired. Similar results were observed in control T cells pretreated with ONOO−. Therefore, the presence of significant levels of nitrated PKCδ in T cells from patients with active lupus may explain the decrease in PKCδ p-T505 observed in T cells from lupus patients, the consequent decrease in ERK signaling pathway and their correlation with disease severity. Protein nitration is often associated with pathological states related to inflammation, but is also recognized as a normal physiological process (20), suggesting an explanation for the presence of nitrated proteins in T cells from healthy donors and patients with inactive lupus. The relatively lower level of PKCδ phosphorylation that we found in normal T cells in the nitrated fraction could be due to a lesser degree of oxidation that normally takes place during physiological metabolism. However, our studies also demonstrate that human T cell PKCδ is directly modified by oxidation. The oxidation selectively decreases PMA-stimulated PKCδ T505 phosphorylation, and is directly related to a decrease in ERK signaling, suggesting that higher levels of nitration cause structural changes in PKCδ that make it catalytically inactive.
Our study reveals that the impaired T cell PKCδ kinase activation observed in patients with active lupus is likely due to the oxidative damage, and causes the impaired ERK pathway signaling in T cells from lupus patients. These studies point to PKCδ as a link between oxidative stress, caused by environmental agents, and the epigenetic changes observed in lupus T cells. Other poorly understood autoimmune diseases also result from gene-environment interactions, and involve epigenetic mechanisms that include not only DNA methylation but histone modifications and microRNA’s as well (49). Oxidative stress may also contribute to these disorders through mechanisms such as altered signaling.
Acknowledgments
We are grateful to Dr. George Tsokos, (Division of Rheumatology, Dept. of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA) for his kind donation of the PP2Ac constructs and siRNA PP2Ac.
Abbreviations
- SLE
systemic lupus erythematosus
- SLEDAI
SLE disease activity index
- PMA
phorbol 12-myristate 13-acetate
- ONOO−
peroxynitrite
- PDK-1
3'-Phosphoinositide-dependent protein kinase-1
Footnotes
This work was supported by NIH grants AR42525, P30AR048310, P30ES 017885, AG25877, ES015214, and a Merit grant from the Department of Veterans Affairs.
Financial conflict of interest: There is no financial conflict of interest to disclose.
References
- 1.Hewagama A, Richardson B. The genetics and epigenetics of autoimmune diseases. J Autoimmun. 2009;33(1):3–11. doi: 10.1016/j.jaut.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attwood JT, Yung RL, Richardson BC. DNA methylation and the regulation of gene transcription. Cell Mol Life Sci. 2002;59(2):241–57. doi: 10.1007/s00018-002-8420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bestor T, Laudano A, Mattaliano R, Ingram V. Cloning and sequencing of a cDNA encoding DNA methyltransferase of mouse cells. The carboxyl-terminal domain of the mammalian enzymes is related to bacterial restriction methyltransferases. J Mol Biol. 1988;203(4):971–83. doi: 10.1016/0022-2836(88)90122-2. [DOI] [PubMed] [Google Scholar]
- 4.Deng C, Yang J, Scott J, Hanash S, Richardson BC. Role of the ras-MAPK signaling pathway in the DNA methyltransferase response to DNA hypomethylation. Biol Chem. 1998;379(8–9):1113–20. doi: 10.1515/bchm.1998.379.8-9.1113. [DOI] [PubMed] [Google Scholar]
- 5.MacLeod AR, Rouleau J, Szyf M. Regulation of DNA methylation by the Ras signaling pathway. J Biol Chem. 1995;270(19):11327–37. doi: 10.1074/jbc.270.19.11327. [DOI] [PubMed] [Google Scholar]
- 6.Rouleau J, MacLeod AR, Szyf M. Regulation of the DNA methyltransferase by the Ras-AP-1 signaling pathway. J Biol Chem. 1995;270(4):1595–601. doi: 10.1074/jbc.270.4.1595. [DOI] [PubMed] [Google Scholar]
- 7.Deng C, Lu Q, Zhang Z, Rao T, Attwood J, Yung R, et al. Hydralazine may induce autoimmunity by inhibiting extracellular signal-regulated kinase pathway signaling. Arthritis Rheum. 2003;48(3):746–56. doi: 10.1002/art.10833. [DOI] [PubMed] [Google Scholar]
- 8.Yung RL, Quddus J, Chrisp CE, Johnson KJ, Richardson BC. Mechanism of drug-induced lupus. I. Cloned Th2 cells modified with DNA methylation inhibitors in vitro cause autoimmunity in vivo. J Immunol. 1995;154(6):3025–35. [PubMed] [Google Scholar]
- 9.Sawalha AH, Jeffries M, Webb R, Lu Q, Gorelik G, Ray D, et al. Defective T-cell ERK signaling induces interferon-regulated gene expression and overexpression of methylation-sensitive genes similar to lupus patients. Genes Immun. 2008;9(4):368–78. doi: 10.1038/gene.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng C, Kaplan MJ, Yang J, Ray D, Zhang Z, McCune WJ, et al. Decreased Ras-mitogen-activated protein kinase signaling may cause DNA hypomethylation in T lymphocytes from lupus patients. Arthritis Rheum. 2001;44(2):397–407. doi: 10.1002/1529-0131(200102)44:2<397::AID-ANR59>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 11.Lu Q, Wu A, Richardson BC. Demethylation of the Same Promoter Sequence Increases CD70 Expression in Lupus T Cells and T Cells Treated with Lupus-Inducing Drugs. J Immunol. 2005;174(10):6212–6219. doi: 10.4049/jimmunol.174.10.6212. [DOI] [PubMed] [Google Scholar]
- 12.Gorelik G, Fang JY, Wu A, Sawalha AH, Richardson B. Impaired T cell protein kinase C delta activation decreases ERK pathway signaling in idiopathic and hydralazine-induced lupus. J Immunol. 2007;179(8):5553–63. doi: 10.4049/jimmunol.179.8.5553. [DOI] [PubMed] [Google Scholar]
- 13.Amos S, Martin PM, Polar GA, Parsons SJ, Hussaini IM. Phorbol 12-myristate 13-acetate induces epidermal growth factor receptor transactivation via protein kinase Cdelta/c-Src pathways in glioblastoma cells. J Biol Chem. 2005;280(9):7729–38. doi: 10.1074/jbc.M409056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basu A, Tu H. Activation of ERK during DNA damage-induced apoptosis involves protein kinase Cdelta. Biochem Biophys Res Commun. 2005;334(4):1068–73. doi: 10.1016/j.bbrc.2005.06.199. [DOI] [PubMed] [Google Scholar]
- 15.Kuriyama M, Taniguchi T, Shirai Y, Sasaki A, Yoshimura A, Saito N. Activation and translocation of PKCdelta is necessary for VEGF-induced ERK activation through KDR in HEK293T cells. Biochem Biophys Res Commun. 2004;325(3):843–51. doi: 10.1016/j.bbrc.2004.10.102. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Q, Ye DQ, Chen GP, Zheng Y. Oxidative protein damage and antioxidant status in systemic lupus erythematosus. Clin Exp Dermatol. 2010;35(3):287–94. doi: 10.1111/j.1365-2230.2009.03437.x. [DOI] [PubMed] [Google Scholar]
- 17.Mansour RB, Lassoued S, Gargouri B, El Gaid A, Attia H, Fakhfakh F. Increased levels of autoantibodies against catalase and superoxide dismutase associated with oxidative stress in patients with rheumatoid arthritis and systemic lupus erythematosus. Scand J Rheumatol. 2008;37(2):103–8. doi: 10.1080/03009740701772465. [DOI] [PubMed] [Google Scholar]
- 18.Kurien BT, Scofield RH. Free radical mediated peroxidative damage in systemic lupus erythematosus. Life Sci. 2003;73(13):1655–66. doi: 10.1016/s0024-3205(03)00475-2. [DOI] [PubMed] [Google Scholar]
- 19.Ahmad R, Rasheed Z, Ahsan H. Biochemical and cellular toxicology of peroxynitrite: implications in cell death and autoimmune phenomenon. Immunopharmacol Immunotoxicol. 2009;31(3):388–96. doi: 10.1080/08923970802709197. [DOI] [PubMed] [Google Scholar]
- 20.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 22.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35(6):630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 23.Katsiari CG, Kyttaris VC, Juang YT, Tsokos GC. Protein phosphatase 2A is a negative regulator of IL-2 production in patients with systemic lupus erythematosus. J Clin Invest. 2005;115(11):3193–204. doi: 10.1172/JCI24895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein Kinase C Isotypes Controlled by Phosphoinositide 3-Kinase Through the Protein Kinase PDK1. Science. 1998;281(5385):2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 25.Casamayor A, Morrice NA, Alessi DR. Phosphorylation of Ser-241 is essential for the activity of 3-phosphoinositide-dependent protein kinase-1: identification of five sites of phosphorylation in vivo. Biochem J. 1999;342 ( Pt 2):287–92. [PMC free article] [PubMed] [Google Scholar]
- 26.Knight ZA. For a PDK1 inhibitor, the substrate matters. Biochem J. 2011;433(2):e1–2. doi: 10.1042/BJ20102038. [DOI] [PubMed] [Google Scholar]
- 27.Morgan PE, Sturgess AD, Davies MJ. Increased levels of serum protein oxidation and correlation with disease activity in systemic lupus erythematosus. Arthritis Rheum. 2005;52(7):2069–79. doi: 10.1002/art.21130. [DOI] [PubMed] [Google Scholar]
- 28.Steinberg SF. Distinctive activation mechanisms and functions for protein kinase Cdelta. Biochem J. 2004;384(3):449–459. doi: 10.1042/BJ20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kikkawa U, Matsuzaki H, Yamamoto T. Protein kinase C delta (PKC delta): activation mechanisms and functions. J Biochem. 2002;132(6):831–9. doi: 10.1093/oxfordjournals.jbchem.a003294. [DOI] [PubMed] [Google Scholar]
- 30.Wu F, Wilson JX. Peroxynitrite-dependent activation of protein phosphatase type 2A mediates microvascular endothelial barrier dysfunction. Cardiovasc Res. 2009;81(1):38–45. doi: 10.1093/cvr/cvn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srivastava J, Goris J, Dilworth SM, Parker PJ. Dephosphorylation of PKCdelta by protein phosphatase 2Ac and its inhibition by nucleotides. FEBS Lett. 2002;516(1–3):265–9. doi: 10.1016/s0014-5793(02)02500-0. [DOI] [PubMed] [Google Scholar]
- 32.Myles T, Schmidt K, Evans DR, Cron P, Hemmings BA. Active-site mutations impairing the catalytic function of the catalytic subunit of human protein phosphatase 2A permit baculovirus-mediated overexpression in insect cells. Biochem J. 2001;357(Pt 1):225–32. doi: 10.1042/0264-6021:3570225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang G, Pierangeli SS, Papalardo E, Ansari GA, Khan MF. Markers of oxidative and nitrosative stress in systemic lupus erythematosus: Correlation with disease activity. Arthritis Rheum. 2010;62(7):2064–2072. doi: 10.1002/art.27442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Avalos I, Chung CP, Oeser A, Milne GL, Morrow JD, Gebretsadik T, et al. Oxidative stress in systemic lupus erythematosus: relationship to disease activity and symptoms. Lupus. 2007;16(3):195–200. doi: 10.1177/0961203306075802. [DOI] [PubMed] [Google Scholar]
- 35.Nagy G, Koncz A, Perl A. T- and B-cell abnormalities in systemic lupus erythematosus. Crit Rev Immunol. 2005;25(2):123–40. doi: 10.1615/critrevimmunol.v25.i2.30. [DOI] [PubMed] [Google Scholar]
- 36.Khan F, Siddiqui AA, Ali R. Measurement and significance of 3-nitrotyrosine in systemic lupus erythematosus. Scand J Immunol. 2006;64(5):507–14. doi: 10.1111/j.1365-3083.2006.01794.x. [DOI] [PubMed] [Google Scholar]
- 37.Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9(7):484–96. [PubMed] [Google Scholar]
- 38.Steinberg SF. Structural Basis of Protein Kinase C Isoform Function. Physiological Reviews. 2008;88(4):1341–1378. doi: 10.1152/physrev.00034.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagy G, Koncz A, Telarico T, Fernandez D, Ersek B, Buzas E, et al. Central role of nitric oxide in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res Ther. 2010;12(3):210. doi: 10.1186/ar3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oates JC, Christensen EF, Reilly CM, Self SE, Gilkeson GS. Prospective measure of serum 3-nitrotyrosine levels in systemic lupus erythematosus: correlation with disease activity. Proc Assoc Am Physicians. 1999;111(6):611–21. doi: 10.1046/j.1525-1381.1999.99110.x. [DOI] [PubMed] [Google Scholar]
- 41.Rybin VO, Guo J, Sabri A, Elouardighi H, Schaefer E, Steinberg SF. Stimulus-specific differences in protein kinase C delta localization and activation mechanisms in cardiomyocytes. J Biol Chem. 2004;279(18):19350–61. doi: 10.1074/jbc.M311096200. [DOI] [PubMed] [Google Scholar]
- 42.Denning MF, Dlugosz AA, Threadgill DW, Magnuson T, Yuspa SH. Activation of the Epidermal Growth Factor Receptor Signal Transduction Pathway Stimulates Tyrosine Phosphorylation of Protein Kinase C [IMAGE] J Biol Chem. 1996;271(10):5325–5331. doi: 10.1074/jbc.271.10.5325. [DOI] [PubMed] [Google Scholar]
- 43.Konishi H, Yamauchi E, Taniguchi H, Yamamoto T, Matsuzaki H, Takemura Y, et al. Phosphorylation sites of protein kinase C Î′ in H2O2-treated cells and its activation by tyrosine kinase invitro. Proc Natl Acad Sci U S A. 2001;98(12):6587–6592. doi: 10.1073/pnas.111158798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kronfeld I, Kazimirsky G, Lorenzo PS, Garfield SH, Blumberg PM, Brodie C. Phosphorylation of protein kinase Cdelta on distinct tyrosine residues regulates specific cellular functions. J Biol Chem. 2000;275(45):35491–8. doi: 10.1074/jbc.M005991200. [DOI] [PubMed] [Google Scholar]
- 45.Kiroycheva M, Ahmed F, Anthony GM, Szabo C, Southan GJ, Bank N. Mitogen-activated protein kinase phosphorylation in kidneys of beta(s) sickle cell mice. J Am Soc Nephrol. 2000;11(6):1026–32. doi: 10.1681/ASN.V1161026. [DOI] [PubMed] [Google Scholar]
- 46.Wayne J, Sielski J, Rizvi A, Georges K, Hutter D. ERK regulation upon contact inhibition in fibroblasts. Mol Cell Biochem. 2006;286(1–2):181–9. doi: 10.1007/s11010-005-9089-z. [DOI] [PubMed] [Google Scholar]
- 47.Khan F, Ali R. Antibodies against nitric oxide damaged poly L-tyrosine and 3-nitrotyrosine levels in systemic lupus erythematosus. J Biochem Mol Biol. 2006;39(2):189–96. doi: 10.5483/bmbrep.2006.39.2.189. [DOI] [PubMed] [Google Scholar]
- 48.Webster RP, Roberts VH, Myatt L. Protein nitration in placenta - functional significance. Placenta. 2008;29(12):985–94. doi: 10.1016/j.placenta.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hedrich CM, Tsokos GC. Epigenetic mechanisms in systemic lupus erythematosus and other autoimmune diseases. Trends Mol Med. 2011;17(12):714–24. doi: 10.1016/j.molmed.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]