Abstract
Evidence suggests that NK and NKT cells contribute to inflammation and mortality during septic shock caused by cecal ligation and puncture (CLP). However, the specific contributions of these cell types to the pathogenesis of CLP-induced septic shock have not been fully defined. The goal of the present study was to determine the mechanisms by which NK and NKT cells mediate the host response to CLP. Control, NK cell-deficient, and NKT cell-deficient mice underwent CLP. Survival, cytokine production, and bacterial clearance were measured. NK cell trafficking and interaction with myeloid cells was also studied. Results show that mice treated with anti-asialoGM1 (NK cell deficient) or anti-NK1.1 (NK/NKT cell deficient) show less systemic inflammation and have improved survival compared with IgG-treated controls. CD1 knockout mice (NKT cell deficient) did not demonstrate decreased cytokine production or improved survival compared with wild type mice. Trafficking studies show migration of NK cells from blood and spleen into the inflamed peritoneal cavity where they appear to facilitate the activation of peritoneal macrophages (F4–80+GR-1−) and F4–80+Gr-1+ myeloid cells. These findings indicate that NK but not CD1-restricted NKT cells contribute to acute CLP-induced inflammation. NK cells appear to mediate their proinflammatory functions during septic shock, in part, by migration into the peritoneal cavity and amplification of the proinflammatory activities of specific myeloid cell populations. These findings provide new insights into the mechanisms used by NK cells to facilitate acute inflammation during septic shock.
Severe sepsis and septic shock are characterized by systemic inflammation, cardiovascular dysfunction, and end-organ derangements such as renal insufficiency, coagulopathy, and acute respiratory distress syndrome (1, 2). Although inflammation is believed to cause many of the physiological alterations associated with sepsis, treatment strategies aimed at attenuating inflammation have been largely unsuccessful in improving survival (3). This is likely due, in part, to an incomplete understanding of the mechanisms of sepsis-induced inflammation. Myeloid cell populations such as monocytes, macrophages, and neutrophils are known to be activated during sepsis and contribute to the sepsis-induced systemic inflammatory response (4). However, the contributions of lymphoid populations to the pathogenesis of sepsis are less well characterized. Several studies indicate that T lymphocyte dysfunction contributes to a state of immunosuppression during the later stages of sepsis, which may predispose the septic host to further infectious complications (5–7). However, the contributions of lymphoid cells to the inflammatory component of septic shock have not been determined.
Our studies show that mice depleted of CD8+ T and NK cells are resistant to mortality during septic shock caused by cecal ligation and puncture (CLP)3 (8–10). CD8+ T and NK cell-deficient mice show less systemic inflammation, less cardiovascular dysfunction, and fewer physiological derangements after CLP, compared with control mice (8, 10). It appears that these cell populations act additively or synergistically because depletion of both cell types provides a level of protection that is greater than that conferred by depletion of either cell type alone (8, 9). However, the mechanisms used by CD8+ T and NK cells to facilitate systemic inflammation and injury after CLP are not well understood. Furthermore, few studies have been performed to evaluate the individual impacts of each cell type in the pathogenesis of septic shock using clinically relevant models such as CLP.
The contribution of NKT cells to CLP-induced injury is even less clear. Our studies using β2-microglobulin knockout mice show decreased systemic inflammation, less cardiovascular dysfunction, and improved survival after CLP, compared with wild-type controls (11–13). However, β2-microglobulin knockout mice have multiple immunological defects in addition to NKT cell deficiency (14, 15). These deficiencies include deficient expression of the class I MHC and CD-1 Ag presentation molecules as well as markedly decreased numbers of CD8+ T and NKT cells. We found that adoptive transfer of NKT cells, along with CD8+ T cells and NK cells, restores CLP-induced mortality to β2-microglobulin knockout mice (12). These results suggest that NKT cells may participate in the CLP-induced inflammatory response. However, the specific contributions of NKT cells to CLP-induced inflammation and injury have not been independently evaluated.
The present study was designed to specifically define the contributions of NK and NKT cells to inflammation and mortality caused by CLP. To achieve this goal, inflammation, bacterial clearance, and survival were evaluated in mice that were depleted of NK and/or NKT cells. Additionally, NK cell trafficking, activation, and interaction with myeloid cell populations were examined to better define the mechanisms used by NK cells to facilitate systemic inflammation and injury during septic shock.
Materials and Methods
Mice
Female, 8–10-wk-old C57BL/6J, BALB/c, and CD1 knockout (CD1KO, strain C.129S2-Cd1tm1Gru/J) mice were purchased from The Jackson Laboratory. Selective depletion of NK cells was achieved by injection of polyclonal anti-asialoGM1 (50 μg i.p.; Cedarlane Laboratories) into C57BL/6J mice 24 h before CLP. Combined depletion of NK and NKT cells was achieved by injection of anti-NK1.1 (50 μg i.p.; eBioscience) into C57BL/6J mice 24 h before CLP. Control C57BL/6J mice were injected with nonspecific IgG (50 μg i.p.; Sigma-Aldrich) in the same regimen. Ab treatment did not induce a significant systemic inflammatory response. Plasma and peritoneal fluid IL-6 and MIP-2 concentrations were <10 pg/ml at 24 h after Ab treatment and before performing CLP in all groups. CD1KO mice were in the BALB/c background, so wild-type BALB/c mice served as controls. All studies were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch and met National Institutes of Health guidelines for the care and use of experimental animals.
Cecal ligation and puncture
Mice were anesthetized with 2% isoflurane in oxygen via face mask and presented to the surgeon in a blinded fashion to minimize experimental bias. A 1- to 2-cm midline incision was made through the abdominal wall. The cecum was identified and ligated 1.5 cm from the tip with a 3-0 silk tie. A double puncture of the cecum was performed using a 20-gauge needle. Great care was taken to avoid obstruction of flow between the ileum and colon. The cecum was returned to the abdominal cavity and the incision was closed with surgiclips. All mice were resuscitated by i.p. injection with 1 ml of lactated Ringer's solution alone or lactated Ringer's solution containing imipenem/cilistatin (25 mg/kg Primaxin; Merck & Co.) immediately after CLP and twice daily thereafter. Sham mice underwent anesthesia and midline laparotomy; the cecum was exteriorized, returned to the abdomen, and the wound was closed with surgiclips. Control mice did not receive surgical manipulation. Experimental samples were harvested at time points ranging from 6 to 18 h after CLP for evaluation. Survival was monitored twice daily for 14 days after CLP.
RNase protection assay
Heart, lung, and spleen were harvested and flash frozen in liquid nitrogen. Ileum and colon were harvested and fecal material was removed by gentle irrigation of the bowel lumen with RPMI 1640 medium. The resultant samples were flash frozen in liquid nitrogen. Samples were stored at −80°C until used. Total RNA was isolated using Tri-Reagent (Molecular Research Center). The RNase protection assay was performed using the Riboquant system (BD Pharmingen) per the manufacturer's protocol. Specific mRNAs on fluorographs were quantified by densitometry using Quantity One software (Bio-Rad).
ELISA
Heparinized blood was obtained by carotid laceration in mice anesthetized with 2% isoflurane, and plasma was harvested from centrifuged blood (1200 × g for 10 min). Peritoneal lavage was performed with 5 ml of RPMI 1640 medium. Peritoneal cells were removed by centrifugation, and the supernatant was harvested for analysis. IL-6 and MIP-2 concentrations in plasma and peritoneal lavage fluid were determined using an ELISA according to the manufacturer's protocol (eBioscience).
Microbiology
Bacterial counts were performed on aseptically harvested blood and peritoneal fluid. All fluid and tissues were harvested under 2% isoflurane anesthesia. Blood was obtained by carotid laceration. Peritoneal fluid was harvested by injection and aspiration of 5 ml of sterile PBS into the peritoneal cavity after aseptic preparation of the abdominal wall. Samples were serially diluted in sterile PBS and cultured on tryptic soy agar plates. Plates were incubated (37°C) for 48–72 h and colony counts were performed. Anaerobic conditions were achieved using an anaerobic chamber and the BBL GasPak Plus Anaerobic System (BD Biosciences).
Flow cytometry
Leukocytes were harvested from various tissue sources. Splenocytes and hepatic leukocytes were isolated as previously described (12). Peritoneal leukocytes were harvested by peritoneal lavage with PBS. Lung leukocytes were harvested by chopping excised lung into small pieces and incubating (37°C) in RPMI 1640 containing 5% FBS, collagenase (1 mg/ml), and DNase (30 μg/ml) for 1 h with agitation. The homogenate was passed through a 1 μm mesh, and leukocytes were isolated by centrifugation in 40% Percoll. Bone marrow cells were harvested by flushing the femur marrow cavity with RPMI 1640 medium. Blood was harvested by laceration of the carotid artery. Peripheral blood leukocytes were purified using Histopaque per the manufacturer's instructions. Cells (1 × 106/tube) were placed in polystyrene tubes containing labeling Abs or isotype controls (0.5–1 μg of Ab/tube), incubated (4°C) for 30 min, and washed with 2 ml of cold PBS. For intracellular staining, cells were incubated with Cytofix/Cytoperm buffer (BD Biosciences) before incubation with Abs. In most cases, cells were fixed with 250–500 μl of 1% paraformaldehyde. Assessment of splenocyte apoptosis and necrosis was performed using an annexin V/propidium iodide apoptosis detection kit (BD Biosciences) per the manufacturer's instructions.
NK cell proliferation was evaluated by evaluation of BrdU labeling. In brief, mice underwent sham or CLP procedures followed by i.p. injection of BrdU (1 mg/mouse) at 6 h after surgical manipulation. Nonsurgically manipulated mice served as controls. At 1 h after BrdU injection, peritoneal cells were harvested and surfaced stained with FITC-conjugated anti-NK1.1 followed by staining with allophycocyanin-conjugated anti-BrdU according to the manufacturer's protocol (BrdU Flow kit; BD Biosciences).
Samples were analyzed with a FACScan or FACSCanto flow cytometer (BD Biosciences). Data were analyzed using WinMDI and FlowJo software packages.
Statistics
All data were analyzed using GraphPad Prism software. Survival curves were compared using the logrank test. For temperature, acid-base, and cytokine measurements, the mean and SEM were calculated. Data from multiple group experiments were analyzed using one-way ANOVA followed by a post hoc Tukey's test to compare groups. For measurements of bacterial CFU, groups were compared using a nonparametric Kruskal-Wallis test followed by a post hoc Dunn's test. A value of p < 0.05 was considered statistically significant for all experiments. All values are presented as the mean ± SE except for bacterial counts that are presented as scatter plots and the median value is designated.
Results
NK cell depletion improves survival during CLP-induced sepsis
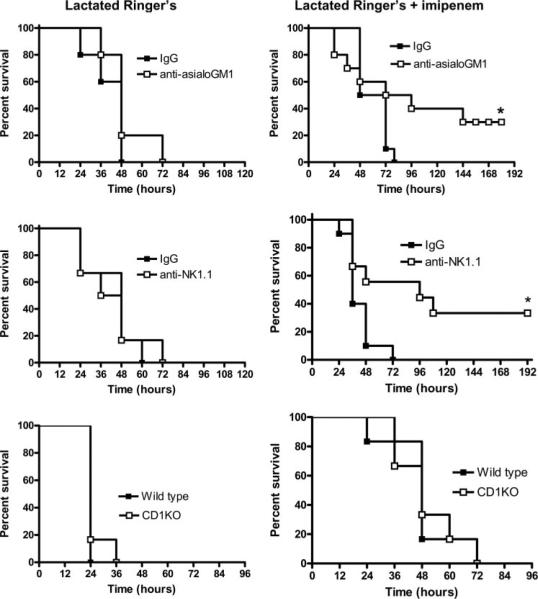
Mice depleted of NK cells by treatment with anti-asialoGM1 showed a significant improvement in long-term survival compared with IgG-treated controls when treated with the broad-spectrum antibiotic imipenem (Fig. 1). However, in the absence of imipenem treatment, survival was not significantly different between mice treated with nonspecific IgG or anti-asialoGM1. A similar survival profile was seen in mice depleted of NK and NKT cells by treatment with anti-NK1.1 (Fig. 1). Mice receiving anti-NK1.1 showed a significant survival benefit compared with IgG-treated controls when treated with imipenem but not when imipenem treatment was withheld. Survival was not significantly different between NKT cell-deficient CD1KO mice and wild-type control mice, regardless of whether imipenem treatment was given (Fig. 1).
FIGURE 1.
Survival of mice after CLP. WT mice were treated with nonspecific IgG, anti-asialoGM1 (NK cell-depleted), or anti-NK1.1 (NK/NKT cell-depleted) 24 h before CLP. NKT cell-deficient CD1KO mice and WT controls were also evaluated. Beginning immediately after CLP, all mice received i.p. injections of either lactated Ringer's solution (left column of graphs) or lactated Ringer's containing imipenem/cilistatin (Primaxin 25 mg/kg, right column of graphs). Antibiotic treatment was continued twice daily throughout the observation period. *, p < 0.05 compared with IgG control. n = 10 mice per group.
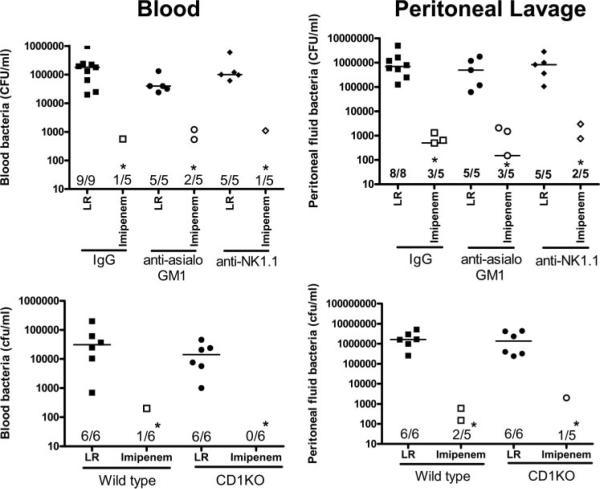
Bacterial counts in blood and peritoneal lavage fluid were measured at 18 h after CLP (Fig. 2). Bacterial counts were not significantly different between mice treated with anti-asialoGM1 or anti-NK1.1 compared with control mice treated with nonspecific IgG (Fig. 2). Bacterial counts in blood and the peritoneal cavity were also not significantly different between CD1KO mice and wild-type control mice. Treatment of mice with imipenem decreased bacterial counts by >99% in blood and peritoneal cavities of mice in all groups studied (Fig. 2).
FIGURE 2.
Bacterial counts in blood and peritoneal lavage fluid after CLP. Mice were treated as described in Materials and Methods. Blood and peritoneal lavage fluid were harvested at 18 h after CLP and cultured on tryptic soy agar plates. Bacterial counts represent the sum of aerobic and anaerobic cultures. Ratios reflect the number of positive cultures out of total cultures. *, p < 0.05 compared with lactated Ringer's solution (LR).
CLP-induced inflammation is attenuated in NK cell-depleted mice
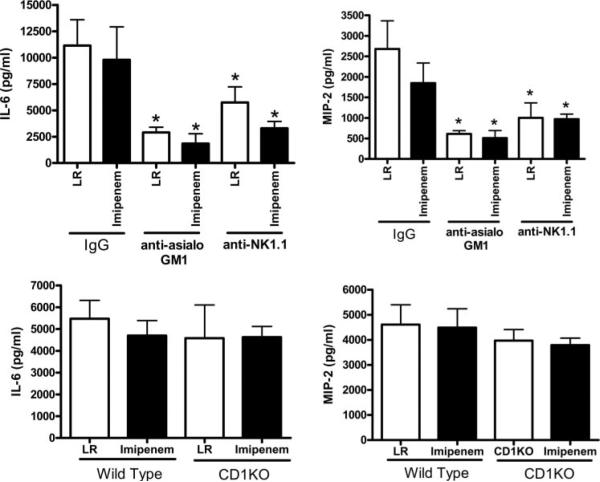
Evaluation of plasma cytokines at 18 h after CLP showed that IL-6 and MIP-2 concentrations were significantly lower in mice treated with anti-asialoGM1 or anti-NK1.1 compared with IgG-treated controls, regardless of whether mice received imipenem treatment (Fig. 3). Plasma IL-6 and MIP-2 concentrations were not significantly different between CD1KO and wild-type mice after CLP (Fig. 3).
FIGURE 3.
Plasma cytokine concentrations in control and NK cell-depleted mice. Mice were treated as described in Materials and Methods. Plasma was harvested 18 h after CLP. IL-6 and MIP-2 concentrations were measured by ELISA. *, p < 0.05 compared with IgG control. n = 6 mice per group.
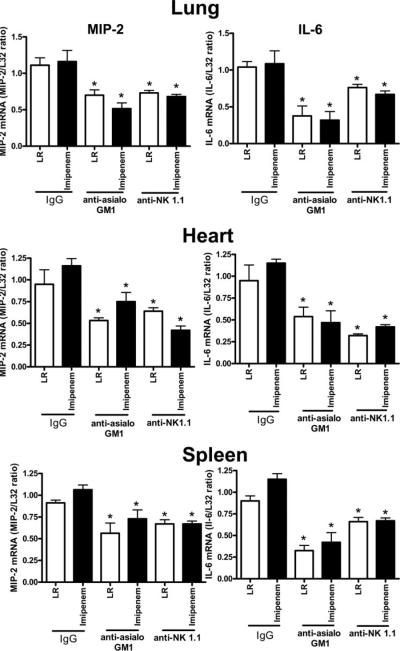
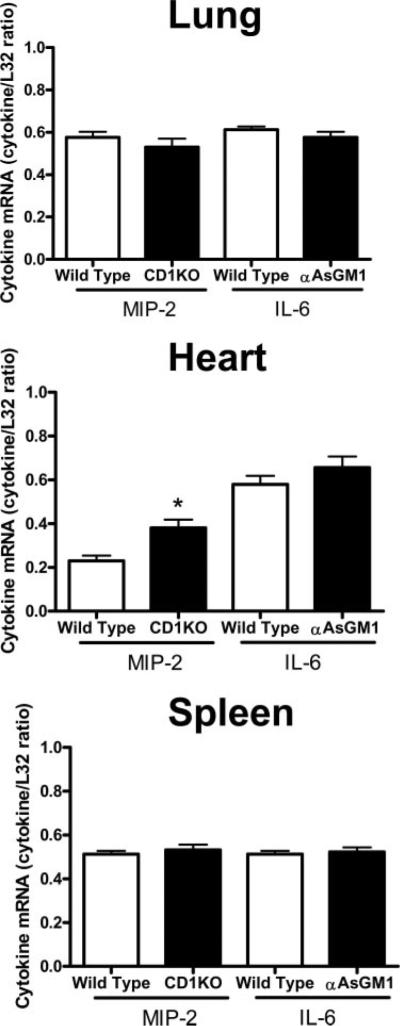
Evaluation of cytokine mRNA expression in heart, lung, and spleen at 18 h after CLP showed that IL-6 and MIP-2 expression was significantly lower in mice treated with anti-asialoGM1 or anti-NK1.1 compared with control mice treated with nonspecific IgG (Fig. 4). The decreased cytokine mRNA expression was observed regardless of whether or not mice received imipenem treatment.
FIGURE 4.
Cytokine mRNA expression in control and NK cell-depleted mice. Mice were treated as described in Materials and Methods. Tissues were harvested 18 h after CLP, total RNA was isolated, and cytokine mRNA expression was determined using an RNase protection assay. Graphs show densitometry results from all samples from each group. L32 was used as a loading control. *, p < 0.05 compared with IgG control. n = 8–10 mice per group.
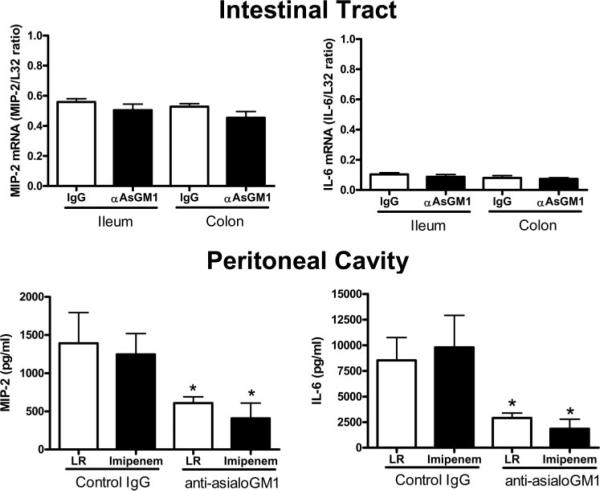
Cytokine production was also evaluated in the intestinal tract and peritoneum of control and NK cell-depleted mice (Fig. 5). MIP-2 mRNA was strongly expressed in ileum and colon after CLP, whereas IL-6 mRNA expression was relatively low. Expression of MIP-2 and IL-6 mRNAs in ileum and colon was not significantly different between mice treated with nonspecific IgG or anti-asialoGM1. Examination of MIP-2 and IL-6 concentrations in peritoneal lavage fluid showed lower amounts of both cytokines in mice treated with anti-asialoGM1 compared with IgG-treated controls (Fig. 5). Imipenem treatment did not affect the cytokine levels.
FIGURE 5.
Cytokine mRNA expression in intestine and peritoneal cavity of control and NK cell-depleted mice after CLP. Mice were treated as described in Materials and Methods. Tissues and peritoneal lavage fluid were harvested 18 h after CLP. Cytokine mRNA expression in colon and ileum was determined using an RNase protection assay. Graphs show densitometry results from all samples. L32 was used as a loading control. Cytokine concentrations in peritoneal lavage fluid were determined by ELISA. *, p < 0.05 compared with IgG control. n = 6 mice per group.
Evaluation of cytokine mRNA expression in wild-type and CD1KO mice showed that CLP-induced expression of IL-6 and MIP-2 mRNAs in lung and spleen were not significantly different in CD1KO mice compared with wild-type controls (Fig. 6). In the heart, MIP-2 expression was significantly higher in CD1KO mice compared with wild-type controls, whereas IL-6 mRNA expression was not significantly different between groups.
FIGURE 6.
Cytokine mRNA expression in WT and CD1KO mice. Tissues were harvested 18 h after CLP, total RNA was isolated, and cytokine mRNA expression was determined using an RNase protection assay. Graphs show densitometry results from all samples. L32 was used as a loading control. *, p < 0.05 compared with IgG control. n = 6 mice per group.
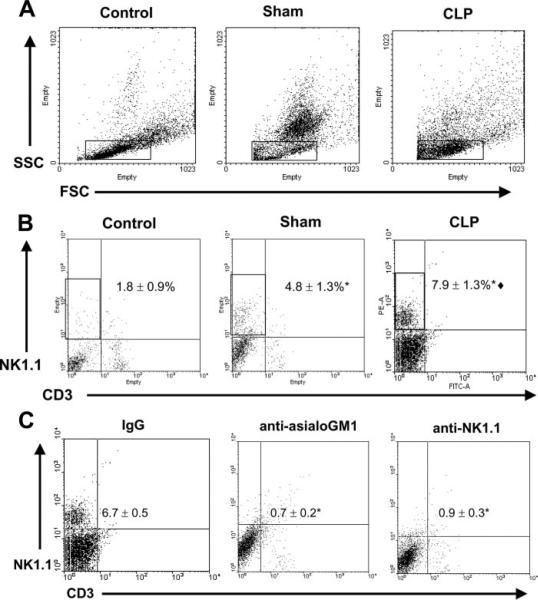
NK cell numbers and activation increase in the peritoneal cavity after CLP
Based on the decreased concentrations of CLP-induced proinflammatory cytokines observed in peritoneal lavage fluid from NK cell-depleted mice compared with control mice, studies were undertaken to assess NK cell infiltration into the peritoneal cavities of mice at 18 h after CLP (Fig. 7). The forward scatter and side scatter pattern of peritoneal cells from control, sham, and CLP mice is shown in Fig. 7A. The overall leukocyte populations in the peritoneal cavity varied widely between groups based on light-scattering properties, mainly due to leukocyte infiltration in the sham and CLP groups. Localization of NK1.1+ cells based on light-scattering properties showed that >90% of all NK1.1+ cells were within the rectangular gates shown in Fig. 7A. Lymphocytes and some granulocytes also localized within the gate (data not shown). This gate was used to evaluate NK1.1+ cell numbers in the peritoneal cavity as shown in Fig. 7, B and C. A significant increase in the numbers of NK (NK1.1+CD3−), but not NKT (NK1.1+CD3+), cells was observed in the peritoneal cavities of mice exposed to both sham (4.8 ± 1.3%) and CLP (7.9 ± 1.3%) procedures compared with control mice (1.8 ± 0.9%) (Fig. 7B). In addition, NK cell numbers in peritoneal lavage fluid from CLP mice were significantly ( p < 0.05) higher than in sham mice (Fig. 7B). Fig. 7C shows NK cell numbers in the peritoneal cavities of mice at 18 h after CLP in mice pretreated with nonspecific IgG, anti-asialoGM1, or anti-NK1.1. NK cell numbers were significantly lower in mice treated with anti-asialoGM1 or anti-NK1.1 compared with IgG-treated controls (Fig. 7C). To determine whether the increase in peritoneal NK cells might be due to local proliferation of resident NK cells after CLP, mice were subjected to CLP and received i.p. injection with BrdU at 6 h after CLP. BrdU incorporation was determined 1 h later. These studies showed that <2% of peritoneal NK cells were BrdU positive after CLP and that the number of BrdU-positive NK cells was not significantly different between control and CLP mice.
FIGURE 7.
NK cell numbers in peritoneal cavity after CLP. Peritoneal leukocytes were harvested from control, sham, and CLP mice at 18 h. Control mice did not receive surgical manipulation. Cells were analyzed by flow cytometry. A, Forward scatter (FSC) and side scatter (SSC) analysis of peritoneal leukocytes from control, sham, and CLP mice. Boxes show the analysis gates that were used to assess NK cell (NK1.1+CD3−) numbers in Fig. 7, B and C, Analysis of the light scatter properties of NK1.1+ cells showed that >90% of NK cells localized within this gate. B, Dot plots from peritoneal leukocytes surface stained with anti-CD3 and anti-NK1.1. Cells in the upper left quadrant designated by the rectangular box are NK cells (NK1.1+CD3−). C, Dot plots show peritoneal NK cell numbers 18 h after CLP in mice treated with nonspecific IgG, anti-asialoGM1, or anti-NK1.1. WT mice were treated with nonspecific IgG, anti-asialoGM1 (NK cell depleted), or anti-NK1.1 (NK/NKT cell depleted) 24 h before CLP. The numerical data (mean ± SE) were derived from 4 mice per group. *, p < 0.05 compared with control or IgG, **, p < 0.05 compared with sham.
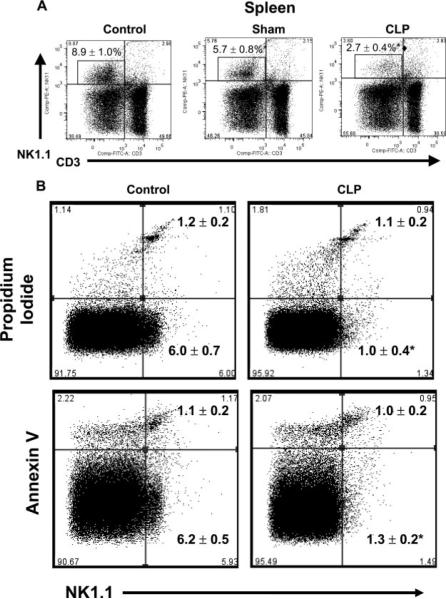
Further studies were undertaken to determine the numbers of NK cells in other tissues after CLP. Evaluation of NK cell numbers in the spleens of mice at 18 h after CLP showed a significant decrease of NK cells after CLP compared with control and sham mice (Fig. 8A). The percentages of NKT cells in the spleens of control and CLP mice (3.1 ± 0.6 vs 2.7 ± 0.5) were not significantly different. The possibility that the decrease in splenic NK cells was due to cell death was also evaluated (Fig. 8B). The number of NK1.1+ cells present in the spleen was significantly decreased at 18 h after CLP, compared with control mice, but the number of splenic NK cells that were positive for propidium iodide or annexin V was not significantly different (Fig. 8B). Splenic NK cell death was also evaluated at 8 h after CLP and showed that the percentages of NK cells in the spleens of control and CLP mice were not significantly different (5.9 ± 0.8% vs 4.5 ± 0.7%) nor were the percentage of propidium iodide- (2.0 ± 0.3% vs 2.4 ± 0.4%) or annexin V- (5.2 ± 0.5% vs 4.6 ± 1.0%) positive splenic NK cells.
FIGURE 8.
Splenic NK cells after CLP. A, Splenocytes were harvested from control, sham, and CLP mice at 18 h after CLP or sham procedures. Control mice did not receive surgical manipulation. Cells were analyzed by flow cytometry. Percentages are from pooled data, n = 4 mice per group. B, Evaluation of splenic NK cell death after CLP. Splenocytes were harvested at 18 h after CLP and stained with anti-NK1.1 and propidium iodide or annexin V. Cells were analyzed by flow cytometry. Numbers represent the percentages of NK1.1+PI+ and NK1.1+AV+ cells (mean ± SE). n = 4 mice per group.
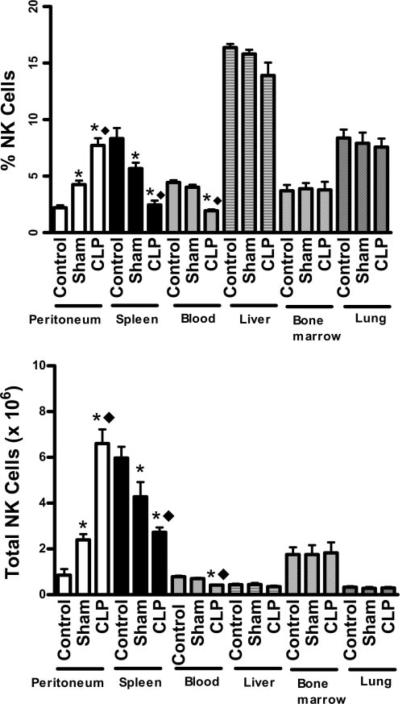
A quantitative analysis of the percentage and total numbers of NK cells in various tissues at 18 h after CLP is shown in Fig. 9. The percentage and total NK cell numbers significantly increased in the peritoneum of sham and CLP mice compared with control mice. Furthermore, the numbers of NK cells in the peritoneal cavities of CLP mice was significantly higher than in sham mice. The percentages and total numbers of NK cells in the spleen and blood of sham and CLP mice were significantly lower compared with control mice. In addition, blood and splenic NK cell numbers were significantly decreased in CLP mice compared with sham mice (Fig. 9). NK cell numbers in liver, bone marrow, and lung were not significantly different among experimental groups (Fig. 9).
FIGURE 9.
NK cell numbers in tissues and fluids after CLP. Leukocytes from peritoneum, spleen, blood, liver, bone marrow, and lung were harvested from control, sham, and CLP mice at 18 h after CLP or sham procedures. *, p < 0.05 compared with control. **, p < 0.05 compared with sham. n = 4 mice per group.
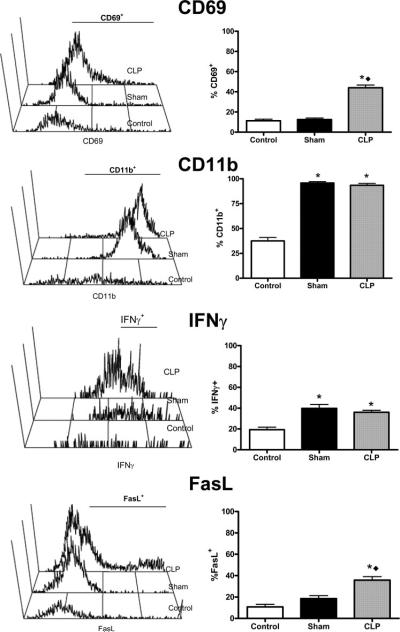
Activation of peritoneal NK cells after CLP was also examined (Fig. 10). NK cells isolated from mice after CLP showed increased expression of CD69, FasL, CD11b, and IFN-γ compared with control and sham mice. The percentages of CD69+ and FasL+ NK cells were significantly higher in the peritoneum of mice after CLP compared with control and sham mice. The percentages of CD11b+ cells were significantly increased in both sham and CLP mice compared with control mice. Although the percentages of CD11b+ NK cells were not significantly different between sham and CLP mice, the CD11b mean fluorescence intensity (MFI) was significantly higher in CLP mice compared with sham mice (196 ± 12 vs 90 ± 16, p < 0.05). The percentages of IFN-γ+ cells significantly increased in both sham and CLP mice compared with controls (Fig. 10). Because total NK cell numbers were significantly increased in mice subjected to CLP vs sham procedures, the absolute numbers of IFN+ and CD11b+ cells were likewise significantly increased.
FIGURE 10.
Activation marker expression on NK cells from the peritoneal cavities of control, sham, and CLP mice. Cells were harvested by peritoneal lavage at 18 h after sham or CLP procedures. Control mice did not undergo surgical manipulation. Activation marker expression was determined on NK1.1+CD3− cells by flow cytometry. n = 4 mice per group. *, p < 0.05 compared with control. **, p < 0.05 compared with sham.
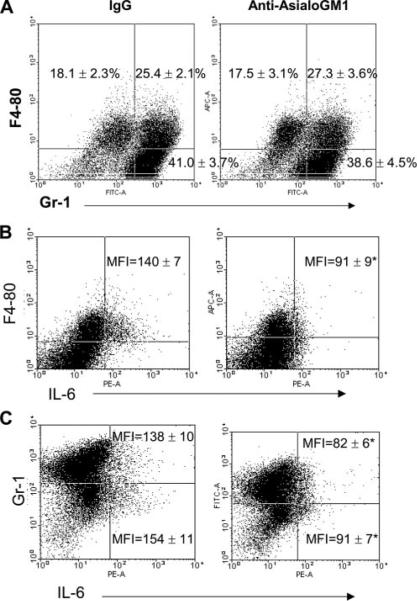
In additional experiments, the effect of NK cell depletion on the production of IL-6 by peritoneal myeloid cell populations was evaluated (Fig. 11). Peritoneal cells were harvested at 18 h after CLP, and IL-6 expression by myeloid cell populations was determined by intracellular staining and flow cytometry. Three myeloid cell populations could be distinguished in the peritoneal cavities of mice after CLP based on surface staining for F4-80 and Gr-1 (Fig. 11A). Both true macrophages (F4-80+Gr-1−) and neutrophils (F4-80−Gr-1+) were present. A third cell population was positive for both markers (F4-80+Gr-1+). The percentages of the respective cell populations were not significantly different between IgG-treated mice and mice treated with anti-asialoGM1, indicating that NK cell depletion did not significantly affect overall myeloid cell composition in the peritoneal cavity after CLP (Fig. 11A). Production of IL-6 by peritoneal myeloid cells after CLP was examined after NK cell depletion (Fig. 11, B and C). IL-6 was used as a marker of myeloid cell activation because this cytokine has been shown to be predictive of mortality after CLP. Examination of IL-6 production by peritoneal myeloid cell populations showed that virtually all IL-6 producing cells were F4-80+ (Fig. 11B). Furthermore, depletion of NK cells significantly decreased both the percentage of F4-80+IL-6+ cells (IgG = 9.4 ± 1.3% vs anti-asialoGM1 = 4.6 ± 0.7%) in the peritoneal cavity and the per cell production of IL-6 by F4-80+ cells as reflected by a significant decrease in IL-6 MFI (Fig. 11). Examination of IL-6 production by GR-1+ cells showed that both GR-1+ and GR-1− cells produce IL-6 after CLP. Treatment of mice with anti-asialoGM1 did not significantly change the percentage of GR1+IL-6+ cells compared with IgG-treated controls, but did significantly decrease the intensity of IL-6 production by GR-1+ cells as reflected by a significantly lower IL-6 MFI. Both the percentage of GR-1−IL-6+ cells in the peritoneum (IgG = 4.3 ± 0.6% vs anti-asialoGM1 = 2.1 ± 0.2%) and the IL-6 MFI for GR-1− cells were decreased by treatment with anti-asialoGM1 (Fig. 11). NK1.1+ cells did not produce IL-6 after CLP (data not shown).
FIGURE 11.
Effect of NK cell depletion on peritoneal myeloid cell recruitment and IL-6 production. Mice were treated with nonspecific IgG or anti-asialoGM1 24 h before CLP. At 18 h after CLP, peritoneal leukocytes were harvested for analysis. A, Characterization of surface F4-80 and Gr-1 expression by peritoneal myeloid cells after CLP in control (IgG) and NK cells depleted (anti-asialoGM1) mice. Values represent the proportion of cells in each quadrant as a percentage of all peritoneal leukocytes. B, IL-6 production by F4-80+ myeloid cells. Peritoneal leukocytes were stained with anti-F4-80 and anti-IL-6. The mean ± SE for IL-6 MFI is designated. C, IL-6 production by Gr-1+ myeloid cells. Peritoneal leukocytes were stained with anti-Gr-1 and anti-IL-6. The mean ± SE for IL-6 MFI for Gr-1+ and GR-1− cells is designated. Dot plots are representative of those obtained from 4 mice per group. The percentages and MFI are presented as the mean ± SE. n = 4 mice per group. p < 0.0.
Discussion
The present study shows that mice depleted of NK cells show improved survival after CLP when treated with the broad-spectrum antibiotic imipenem. Interestingly, systemic inflammation was attenuated in NK cell-deficient mice, regardless of whether imipenem was given. But, a survival benefit was only achieved with imipenem treatment, suggesting that NK cells contribute to CLP-induced septic shock by facilitating local and systemic inflammation. To assess this possibility, we evaluated CLP-induced inflammation within the small and large intestines, peritoneal cavity, plasma, and several remote tissues of control and NK cell-depleted mice. Our results show that NK cell depletion attenuated inflammation within the peritoneal cavity, blood, and distant tissues such as heart, lung, and spleen, but did not affect the CLP-induced inflammatory response within the intestinal tract. We found it surprising that NK cell depletion had a minimal effect on intestinal inflammation after CLP. It would seem logical that NK cells would contribute to inflammation within the intestinal tract during CLP-induced injury. The intestinal wall contains large numbers of NK cells in subepithelial regions as well as in the lamina propria (16). Several studies have shown that NK cells contribute to intestinal inflammation during enteric infections and inflammatory bowel disease (17–18). However, our study did not show differences in the production of proinflammatory mediators within the ileum and colon of control and NK cell-deficient mice after CLP. This finding suggests that intestinal inflammation caused by cecal ischemia and injury is independent of NK cells. Alternatively, Ab-mediated depletion of NK cells within the intestinal wall was not confirmed in this study, so it is possible that intestinal inflammation is dependent upon NK cells but could not be demonstrated due to incomplete depletion of NK cells. Nonetheless, NK cells appear to play an important role in i.p. inflammation after CLP. NK cell-deficient mice showed decreased production of CLP-induced proinflammatory mediators and a large influx of activated NK cells into the peritoneal cavity after CLP. Furthermore, NK cell depletion significantly decreased the activation of peritoneal macrophages and a myeloid cell population that is positive for both macrophage and granulocyte surface markers (F4-80+GR-1+). Taken together, these observations suggest that the peritoneal cavity is an important site of inflammation after CLP and that NK cells facilitate CLP-induced i.p. inflammation through interaction with myeloid populations. These findings provide new information regarding the mechanisms used by NK cells to facilitate the local inflammatory response during septic shock caused by CLP.
The mechanisms used by NK cells to potentiate i.p. inflammation after CLP have not been previously investigated. Results from the present study show that NK cell numbers increase in the peritoneal cavity and decrease in the spleen and blood after CLP. Our evaluation of NK cell trafficking showed accumulation of NK cells in the peritoneal cavity after CLP. The increase in peritoneal NK cells does not appear to be due to local proliferation because significant BrdU incorporation was not observed in peritoneal NK cells after CLP. Further studies showed that the decrease in splenic NK cell numbers does not appear to be caused by apoptosis or necrosis, because CLP did not cause a significant increase in the numbers of dead or apoptotic NK cells in the spleen. Taken together, these observations provide evidence that NK cells migrate from spleen and blood into the inflamed peritoneal cavity after CLP. The migration of NK cells from blood and accessory lymphoid organs into the site of acute injury after CLP had not been previously demonstrated and provides new information that may lead to a better understanding of the mechanisms used by NK cells to facilitate inflammation during CLP-induced septic shock. Previous studies, using other models of infection and inflammation, have shown the ability of NK cells to migrate to sites of inflammation. Prlic and colleagues (19) showed that NK cells migrate into the peritoneal cavity of mice after i.p. challenge with a Vaccinia virus. In another study, Wald et al. (20) observed migration of NK cells from the spleen and bone marrow into the liver in a model of Con A-induced hepatitis. Taken together, these findings indicate that NK cells can be rapidly mobilized to enter sites of injury or infection and that this process is activated by CLP. Currently, it is unclear whether CLP-induced i.p. inflammation potentiates inflammation in distant tissues. Our study shows that NK cell depletion decreases cytokine expression in lung, heart, and spleen after CLP. However, it is unclear whether NK cells amplify the inflammatory response within these tissues or whether the severe inflammatory response within the peritoneal cavity leads to systemic inflammation. In the present study, NK cell numbers did not change in the lungs or liver after CLP. Immunohistochemical analysis did not show the presence of NK cells in the hearts of mice subjected to CLP (data not shown). A previous study by Hirsh and colleagues (21) indicates that pulmonary NK cells contribute to CLP-induced lung injury. Seki et al. (22) have provided evidence that hepatic NK cells may facilitate local and systemic inflammation during sepsis. However, the importance of tissue-specific NK cells in facilitating CLP-induced inflammation remains to be fully delineated. These studies will require selective examination of NK cell activation and interaction with other cell populations within specific tissues during CLP.
The mechanisms by which NK cells facilitate local and systemic inflammation after CLP remain to be determined. Within the peritoneum, it is likely that NK cells interact with other infiltrating leukocyte populations as well as resident macrophages to facilitate leukocyte activation and inflammation. Our study shows that macrophages (F4-80+Gr-1−) as well as a myeloid cell population that coexpresses both F4-80 and Gr-1 (F4-80+Gr-1+) are the primary sources of IL-6 in the peritoneal cavity after CLP. Depletion of NK cells significantly decreased production of IL-6 by both of these myeloid cell populations. The infiltration of myeloid cells that coexpress surface markers that are typical of macrophages (F4-80) and granulocytes (Gr-1) into the peritoneal cavity after CLP is, to our knowledge, a novel finding. The interaction of NK cells with this unique myeloid cell population has not been previously reported. Further studies will need to be performed to characterize the functional properties of F4-80+Gr-1+ cells during sepsis. It appears that these cells migrate into the peritoneal cavity after CLP because they are not present in control mice (data not shown). It is possible that these cells are derived from resident peritoneal macrophages (F4-80+Gr-1−) or infiltrating granulocytes (F4-80−Gr-1+). A recent study by Sasmono and colleagues (23) showed the ability of mouse neutrophilic granulocytes to transdifferentiate into macrophages in the presence of CSF-1. Nevertheless, the source and functional characteristics of these cells is currently unclear and will require further investigation. However, the ability of the F4-80+Gr-1+ cell population to produce IL-6 and interact with NK cells is typical of macrophages. The ability of NK cells to amplify the proinflammatory capacity of macrophages after endotoxin challenge is well established (24, 25), an effect that appears to be mediated by the production of IFN-γ (24, 26, 27). However, an endotoxin challenge does not accurately emulate clinical sepsis. The CLP model causes bowel ischemia and peritonitis, which more accurately mimics clinical sepsis. Interestingly, IFN-γ production in tissues and plasma is much lower after CLP than after an endotoxin challenge, which brings into question the importance of IFN-γ in the pathogenesis of CLP-induced sepsis (8). Nevertheless, the present study shows that NK cells present in the peritoneal cavity of mice after CLP produce IFN-γ. In addition, our recent studies show that IFN-γ null mice treated with imipenem show a survival profile that is nearly identical with that seen in NK cell-deficient mice after CLP (unpublished observation). However, a cause and effect relationship between NK cell-derived IFN-γ and amplification of CLP-induced inflammation remains to be established. Scott et al. (28) have reported that the activation of peritoneal macrophages by splenic NK cells is independent of IFN-γ and requires CD40-CD154 interactions. Additional studies by Echtanacher and colleagues (29) show that IFN-γ neutralization does not improve survival after CLP. However, the latter studies did not treat mice with antibiotics and did not evaluate the proinflammatory response or bacterial clearance in IFN-γ-deficient mice after CLP. Therefore, the importance of IFN-γ in facilitating NK cell-facilitated inflammation after CLP remains to be fully ascertained and is being actively addressed in our laboratory.
Clearly, some degree of inflammation is beneficial during acute peritonitis. The acute inflammatory response functions to eliminate sources of injury, such as infection, and facilitate tissue repair. Without acute inflammation, the ability of the host to respond to infection would be greatly impaired. In the present study, NK cell depletion did not significantly alter bacterial counts in the perito-neal cavity or blood after CLP. This finding is in agreement with the studies of Godshall and colleagues (30). They showed that bacterial counts were higher in blood, liver, and peritoneum after NK cell depletion at 4 h after CLP, but that bacterial numbers were not different between control and NK cell-deficient mice at 18 h, the time point used in our studies. Other reports have shown that NK cells contribute to bacterial clearance in experimental models of E. coli, H. influenza, and L. monocytogenes infection, among others (31–32). Therefore, NK cells appear to be an important component of the host response to infection in many circumstances. However, in cases of severe systemic inflammation, as caused by CLP in the present study, NK cells appear to facilitate the systemic inflammatory response and contribute to inflammation-induced injury.
The present study shows that deficiency of CD1-restricted NKT cells does not significantly improve survival, decrease inflammation, or alter bacterial clearance in the CLP model of septic shock. This finding extends previous observations from our lab in which we showed that β2-microglobulin knockout mice exhibit resistance to CLP-induced injury (12). β2-microglobulin knockout mice have multiple immunological defects including deficiencies of NKT cells, CD8+ T cells, the class I MHC, and CD-1. The specific contribution of CD1-restricted NKT cell deficiency to CLP-induced septic shock had not been previously determined. The current study provides evidence that CD1-restricted NKT cells do not facilitate inflammation during septic shock caused by CLP. This finding is in agreement with results reported by Scott et al. (33) in which IL-10 neutralization facilitated NK, but not NKT, cell activation after CLP. Another study by Emoto and colleagues (34) showed that NK, but not NKT, cells contribute to endotoxin-induced shock. Taken together, these findings indicate that NKT cells do not facilitate the acute inflammatory response during sepsis. However, Tsujimoto and colleagues (35) recently reported that hepatic NKT cells are activated by bacterial CpG DNA during CLP-induced sepsis and postulate that NKT cells contribute to CLP-induced liver injury. Therefore, some controversy exists. Furthermore, some studies indicate that NKT cells may have anti-inflammatory effects during sepsis. A recent study by Sireci and colleagues (36) showed that activation of NKT cells with α-galactoceramide provides protection against endotoxin-induced shock. In other studies, Rhee and colleagues (37) reported that NKT cells contribute to CLP-induced immunosuppression rather than acute inflammation. Therefore, NKT cells may actually inhibit the acute inflammatory response during sepsis.
In conclusion, the present study demonstrates that NK cells but not CD1-restricted NKT cells contribute to inflammation and mortality during septic shock. This study provides new mechanistic insights into NK cell function during CLP-induced sepsis. Specifically, NK cells appear to rapidly migrate from the blood and spleen into the peritoneal cavity in response to CLP where they facilitate the proinflammatory functions of macrophages and a unique population of F4–80+Gr-1+ myeloid cells. This study also extends our understanding of the contributions, or lack thereof, of NKT cells to the acute inflammatory response during CLP-induced septic shock.
Footnotes
This work was supported by Grants R01 GM66885 from the National Institutes of Health and 8780 from the Shriners of North America.
Abbreviations used in this paper: CLP, cecal ligation and puncture; CD1KO, CD1 knockout; MFI, mean fluorescence intensity.
Disclosures The authors have no financial conflict of interest.
References
- 1.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis: The ACCP/SCCM Consensus Conference Committee: American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 2.Court O, Kumar A, Parrillo JE, Kumar A. Clinical review: myocardial depression in sepsis and septic shock. Crit. Care. 2002;6:500–508. doi: 10.1186/cc1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eichacker PQ, Parent C, Kalil A, Esposito C, Cui X, Banks SM, Gerstenberger EP, Fitz Y, Danner RL, Natanson C. Risk and the efficacy of antiinflammatory agents: retrospective and confirmatory studies of sepsis. Am. J. Respir. Crit. Care Med. 2002;166:1197–1205. doi: 10.1164/rccm.200204-302OC. [DOI] [PubMed] [Google Scholar]
- 4.Calandra T, Froidevaux C, Martin C, Roger T. Macrophage migration inhibitory factor and host innate immune defenses against bacterial sepsis. J. Infect. Dis. 2003;187(Suppl. 2):S385–S390. doi: 10.1086/374752. [DOI] [PubMed] [Google Scholar]
- 5.Lyons A, Kelly JL, Rodrick ML, Mannick JA, Lederer JA. Major injury induces increased production of IL-10 by cells of the immune system with a negative impact on resistance to infection. Ann. Surg. 1997;226:450–458. doi: 10.1097/00000658-199710000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung CS, Watkins L, Funches A, Lomas-Neira J, Cioffi WG, Ayala A. Deficiency of γδ T-lymphocytes contributes to mortality and immunosuppression in sepsis. Am. J. Physiol. 2006;291:R1338–R1343. doi: 10.1152/ajpregu.00283.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Coopersmith CM, Karl IE. Prevention of lymphocyte apoptosis: a potential treatment of sepsis? Clin. Infect. Dis. 2005;41(Suppl. 7):S465–S469. doi: 10.1086/431998. [DOI] [PubMed] [Google Scholar]
- 8.Sherwood ER, Enoh VT, Murphey ED, Lin CY. Mice depleted of CD8+ T and NK cells are resistant to injury caused by cecal ligation and puncture. Lab. Invest. 2004;84:1655–1665. doi: 10.1038/labinvest.3700184. [DOI] [PubMed] [Google Scholar]
- 9.Enoh VT, Fairchild CD, Lin CY, Varma TK, Sherwood ER. Differential effect of imipenem treatment on wild-type and NK cell-deficient CD8 knockout mice during acute intraabdominal injury. Am. J. Physiol. 2006;290:R685–R693. doi: 10.1152/ajpregu.00678.2005. [DOI] [PubMed] [Google Scholar]
- 10.Tao W, Enoh VT, Lin CY, Johnston WE, Li P, Sherwood ER. Cardiovascular dysfunction caused by cecal ligation and puncture is attenuated in CD8 knockout mice treated with anti-asialoGM1. Am. J. Physiol. 2005;289:R478–R485. doi: 10.1152/ajpregu.00081.2005. [DOI] [PubMed] [Google Scholar]
- 11.Enoh VT, Lin CY, Varma TK, Sherwood ER. Differential effect of imipenem treatment on injury caused by cecal ligation and puncture in wild-type and NK cell-deficient β2-microglobulin knockout mice. Am. J. Physiol. 2006;290:G277–G284. doi: 10.1152/ajpgi.00338.2005. [DOI] [PubMed] [Google Scholar]
- 12.Sherwood ER, Lin CY, Tao W, Hartmann CA, Dujon JE, French AJ, Varma TK. β2-microglobulin knockout mice are resistant to lethal intraabdominal sepsis. Am. J. Respir. Crit. Care Med. 2003;167:1641–1649. doi: 10.1164/rccm.200208-950OC. [DOI] [PubMed] [Google Scholar]
- 13.Tao W, Sherwood ER. β2-microglobulin knockout mice treated with anti-asialoGM1 exhibit improved hemodynamics and cardiac contractile function during acute intraabdominal sepsis. Am. J. Physiol. 2004;286:R569–R575. doi: 10.1152/ajpregu.00470.2003. [DOI] [PubMed] [Google Scholar]
- 14.Brutkiewicz RR, Bennink JR, Yewdell JW, Bendelac A. TAP-independent, β2-microglobulin-dependent surface expression of functional mouse CD1.1. J. Exp. Med. 1995;182:1913–1919. doi: 10.1084/jem.182.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rolph MS, Raupach B, Kobernick HH, Collins HL, Perarnau B, Lemonnier FA, Kaufmann SH. MHC class Ia-restricted T cells partially account for β2-microglobulin-dependent resistance to Mycobacterium tuberculosis. Eur. J. Immunol. 2001;31:1944–1949. doi: 10.1002/1521-4141(200106)31:6<1944::aid-immu1944>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 16.Cohavy O, Zhou J, Ware CF, Targan SR. LIGHT is constitutively expressed on T and NK cells in the human gut and can be induced by CD2-mediated signaling. J. Immunol. 2005;174:646–653. doi: 10.4049/jimmunol.174.2.646. [DOI] [PubMed] [Google Scholar]
- 17.Harrington L, Srikanth CV, Antony R, Shi HN, Cherayil BJ. A role for NK cells in intestinal inflammation caused by infection with Salmonella enterica serovar Typhimurium. FEMS Immunol. Med. Microbiol. 2007;51:372–380. doi: 10.1111/j.1574-695X.2007.00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egawa S, Hiwatashi N. NK cell activity in patients with inflammatory bowel disease. J. Clin. Lab. Immunol. 1986;20:187–192. [PubMed] [Google Scholar]
- 19.Prlic M, Gibbs J, Jameson SC. Characteristics of NK cell migration early after vaccinia infection. J. Immunol. 2005;175:2152–2157. doi: 10.4049/jimmunol.175.4.2152. [DOI] [PubMed] [Google Scholar]
- 20.Wald O, Weiss ID, Wald H, Shoham H, Bar-Shavit Y, Beider K, Galun E, Weiss L, Flaishon L, Shachar I, et al. IFN-γ acts on T cells to induce NK cell mobilization and accumulation in target organs. J. Immunol. 2006;176:4716–4729. doi: 10.4049/jimmunol.176.8.4716. [DOI] [PubMed] [Google Scholar]
- 21.Hirsh M, Kaplan V, Dyugovskaya L, Krausz MM. Response of lung NK1.1-positive NK cells to experimental sepsis in mice. Shock. 2004;22:40–45. doi: 10.1097/01.shk.0000129758.81361.45. [DOI] [PubMed] [Google Scholar]
- 22.Seki S, Habu Y, Kawamura T, Takeda K, Dobashi H, Ohkawa T, Hiraide H. The liver as a crucial organ in the first line of host defense: the roles of Kupffer cells, natural killer (NK) cells and NK1.1 Ag+ T cells in Th 1 immune responses. Immunol. Rev. 2000;174:35–46. doi: 10.1034/j.1600-0528.2002.017404.x. [DOI] [PubMed] [Google Scholar]
- 23.Sasmono R, Ehrsperger A, Cronau S, Ravasi T, Kandane R, Hickey M, Cook A, Himes S, Hamilton J, Hume D. Mouse neutrophilic granulocytes express mRNA encoding the macrophage CSF receptor (CSF-1R) as well as many other macrophage-specific transcripts and can transdifferentiate into macrophages in vitro in response to CSF-1. J. Leukocyte Biol. 2007;82:111–123. doi: 10.1189/jlb.1206713. [DOI] [PubMed] [Google Scholar]
- 24.Varma TK, Lin CY, Toliver-Kinsky TE, Sherwood ER. Endotoxin-induced γ IFN production: contributing cell types and key regulatory factors. Clin. Diagn. Lab. Immunol. 2002;9:530–543. doi: 10.1128/CDLI.9.3.530-543.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogasawara K, Takeda K, Hashimoto W, Satoh M, Okuyama R, Yanai N, Obinata M, Kumagai K, Takada H, Hiraide H, Seki S. Involvement of NK1+ T cells and their IFN-γ production in the generalized Shwartzman reaction. J. Immunol. 1998;160:3522–3527. [PubMed] [Google Scholar]
- 26.Heinzel FP. The role of IFN-γ in the pathology of experimental endotoxemia. J. Immunol. 1990;145:2920–2924. [PubMed] [Google Scholar]
- 27.Deriy LV, Beno DW, Uhing MR, Jiyamapa-Serna VA, Kimura RE. Splenectomy ablates endotoxin-induced IFNγ response in rats. Shock. 2002;17:312–315. doi: 10.1097/00024382-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Scott MJ, Hoth JJ, Stagner MK, Gardner SA, Peyton JC, Cheadle WG. CD40-CD154 interactions between macrophages and NK cells during sepsis are critical for macrophage activation and are not IFN γ dependent. Clin. Exp. Immunol. 2004;137:469–477. doi: 10.1111/j.1365-2249.2004.02547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Echtenacher B, Freudenberg MA, Jack RS, Mannel DN. Differences in innate defense mechanisms in endotoxemia and polymicrobial septic peritonitis. Infect. Immun. 2001;69:7271–7276. doi: 10.1128/IAI.69.12.7271-7276.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Godshall CJ, Scott MJ, Burch PT, Peyton JC, Cheadle WG. NK cells participate in bacterial clearance during septic peritonitis through interactions with macrophages. Shock. 2003;19:144–149. doi: 10.1097/00024382-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Miyazaki S, Ishikawa F, Shimizu K, Ubagai T, Edelstein P, Yamaguchi K. Gr-1 high polymorphonuclear leaukocytes and NK cells act via IL-15 to clear intracellular Hemophilus influenza in experimental murine peritonitis and pneumonia. J. Immunol. 2007;179:5407–5414. doi: 10.4049/jimmunol.179.8.5407. [DOI] [PubMed] [Google Scholar]
- 32.Ikuta S, Ono S, Kinoshita M, Seki S, Hiraide H, Mochizuki H. Enhanced IFN γ production and bacterial clearance in the liver of splenectomized mice in the models of Escherichia coli injection or intestinal obstruction. Shock. 2004;21:452–457. doi: 10.1097/00024382-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Scott MJ, Hoth JJ, Turina M, Woods DR, Cheadle WG. IL-10 suppresses NK cell but not NK T cell activation during bacterial infection. Cytokine. 2006;33:79–86. doi: 10.1016/j.cyto.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Emoto M, Miyamoto M, Yoshizawa I, Emoto Y, Schaible U, Kita E, Kauffman S. Critical role of NK cells rather than V α 14+ NKT cells in LPS-induced lethal shock in mice. J. Immunol. 2002;169:1426–1432. doi: 10.4049/jimmunol.169.3.1426. [DOI] [PubMed] [Google Scholar]
- 35.Tsujimoto H, Ono S, Matsumoto A, Kawabata T, Kinoshita M, Majima T, Hiraki S, Seki S, Moldawer LL, Mochizuki H. A critical role of CpG motifs in a murine peritonitis model by their binding to highly expressed TLR-9 on liver NKT cells. J. Hepatol. 2006;45:836–843. doi: 10.1016/j.jhep.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 36.Sireci G, La Manna M, Di Sano C, Di Liberto D, Porcelli S, Kronenberg M, Dieli F, Salerno A. Pivotal advance: α-galactoceramide induces protection against LPS-induced shock. J. Leukocyte Biol. 2007;81:607–622. doi: 10.1189/jlb.0506298. [DOI] [PubMed] [Google Scholar]
- 37.Rhee RJ, Carlton S, Lomas JL, Lane C, Brossay L, Cioffi WG, Ayala A. Inhibition of CD1d activation suppresses septic mortality: a role for NK-T cells in septic immune dysfunction. J. Surg. Res. 2003;115:74–81. doi: 10.1016/s0022-4804(03)00220-8. [DOI] [PubMed] [Google Scholar]